Abstract
The repetitive restorative cycle should be avoided, aiming at the smallest number of restorations’ replacements to ensure greater tooth longevity. Antibacterial materials associated with the control of caries etiological factors can help improve restoration's durability. This review aimed to analyze the results of in vitro studies that added Dimethylaminohexadecyl methacrylate (DMAHDM), an antibacterial monomer, to restorative materials. The PubMed, SCOPUS, Web of Science, and Biblioteca Virtual em Saúde databases were screened for studies published between 2015 and 2020. After full-text reading, 24 articles were included in the final sample. DMAHDM has demonstrated antibacterial efficacy against several bacteria related to dental caries and periodontal diseases, causing a transition in the biofilm balance without inducing resistance. When DMAHDM was included in acrylic resin, the material cytotoxicity increased, and changes in mechanical properties were observed. In contrast, resin composites had their mechanical properties maintained in most studies; however, toxicity was not examined. The association between DMAHDM and 2-methacryloyloxyethyl phosphorylcholine or silver nanoparticles improved the antibacterial effect. Besides, the association with nanoparticles of amorphous calcium phosphate or nanoparticles of calcium fluoride can provide remineralization capacity. There is a lack of information on the cytotoxicity and bacteria resistance induction, and further studies are needed to address this.
Keywords: Quaternary ammonium compounds, Dental caries, Antibacterial agents
1. Introduction
Dental caries is one of the most common bacterial infections in the human body. It represents a global public health problem with serious economic consequences, affecting an estimated 2.3 billion of the world's population [1], [2]. The disease's underlying mechanism consists of demineralization caused by the acid attack of bacteria present in the dental biofilm [3], [4], [5]. The growth of such acidogenic bacteria in the biofilm is possible from a diet rich in fermentable carbohydrates and poor oral hygiene [6], [7].
The advance of the carious process may require the restoration of lost dental tissues so that resin composites are widely used with this aim due to their esthetics and ability to fill tooth preparations directly [8], [9]. Much effort has been made to decrease polymerization contraction stresses and improve surface polishing to provide greater restoration longevity. However, factors, such as restoration's surface location, patient's caries susceptibility, socioeconomic status or age, operator's skill and the criteria for lesion detection, can still contribute to the occurrence of recurrent caries [10], [11], [12].
Several studies report the frequent presence of caries lesions at the restorations’ margins, so that a high occurrence of restoration replacement is observed [8], [13], [14], [15], [16], [17]. From this perspective, a composite resin with antibacterial properties could help in this process and reduce caries lesions at the restoration margins by interfering with the restorations’ adjacent dental biofilm. An antimicrobial composite resin, associated with the control of caries’ etiological factors, would be of great value in this process.
In this context, the association of resin composites with quaternary ammonium monomers (QAM) would inhibit biofilm growth due to this monomer's capacity to cause bacterial lysis by breaking the cellular membrane [18]. QAM's antimicrobial activity's exact mechanism consists of its binding to bacterial cell membranes, causing cytoplasmic leaks by disturbing the electrical balance and consequent bacterial lysis due to osmotic pressure. This process is made possible by encountering the negative bacterial cell and positive charge sites in the QAM, causing death by contact [19], [20].
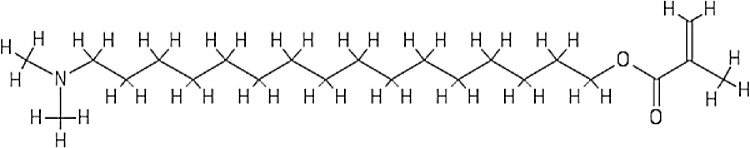
Several studies have evaluated the antibacterial effect of dimethylaminohexadecyl methacrylate (DMAHDM) (Fig. 1), a recently synthesized QAM [21], [22], [23], [24], [25]. Thus, this paper aims to discuss the role of DMAHDM in resin-based dental materials concerning its immediate and long-term antibacterial activity, cytotoxicity, and how it interacts with other chemical components in dental resins. A bibliographic search was performed on PubMed, Web of Science, SCOPUS, and Biblioteca Virtual em Saúde (BVS) research databases, using only “DMAHDM” as a descriptor due to the scarcity of studies. We considered papers published from 2015 to 2020, and all types of study designs were included. The results of our research can be found in Table 1.
Fig. 1.
Chemical structure of the DMAHDM monomer.
Table 1.
Data extracted from primary studies.
| Authors | Material used together | Mechanical properties analyzed | Antibacterial properties |
|---|---|---|---|
| Wu et al. (2015) [25] | 20% nanoparticles of amorphous calcium phosphate (NACP) | • 3.75% DMAHDM (by wt%): Flexural strength: similar values to control. Modulus of elasticity: similar values to control. |
3.75% DMAHDM (by wt%): • Dead/alive bacteria: increased adherent compromised bacteria. • Metabolic activity: values between 0 and 0.2 A540/cm2 (control between 1 and 1.2 A540/cm2). • Lactic acid production: approximate value 0 mmol/L (control between 20 and 25 mmol/L). • CFU: reduced the CFU between 3 and 4 log. Values between 104 and 107 (control between 107 and 1010 CFU) in a biofilm of S. mutans, total Streptococci, and total microorganisms. |
| Wu et al. (2015) [45] | 30% NACP | • 0.75–3% DMAHDM (by wt%): Fracture toughness: similar values to control. |
3% DMAHDM (by wt%): • Dead/alive bacteria: increased adherent compromised bacteria as the percentage of DMAHDM increased from 0.75–3%. The area of live bacteria with 3% DMAHDM was close to 10% (control between 90 and 100%). • Metabolic activity: approximate value 0 A540/cm2 (control 1 A540/cm2). • Lactic acid production: approximate value 0 mmol/L (control 24 mmol/L). • CFU: Reduced the CFU by 3 log. Values between 104 and 108 (control between 107 and 1011), in a biofilm of S. mutans, total Streptococci, and total microorganisms. |
| Zhang et al. (2015) [46] | 3% MPC | • 3% DMAHDM (by wt%): Flexural strength: similar values to control. Modulus of elasticity: similar values to control With 3% MPC: it could only be added up to 1.5% DMAHDM without changing the mechanical properties; |
1.5% DMAHDM (by wt%): • Dead/alive bacteria: increased adherent compromised bacteria. Values close to 20% coverage of the area with live bacteria (controls close to 100%). |
| Wang et al. (2016) [47] | 3% MPC | • 3% DMAHDM (by wt%): Flexural strength: similar values to control. Modulus of elasticity: similar values to control. 4.5% DMAHDM changed the properties. |
3% DMAHDM (by wt%): • Dead/alive bacteria: increased adherent compromised bacteria. • CFU: Reduced the CFU by 4 log. Values between 105 and 107 (control between 109 and 1010) in biofilms of P. gingivalis, P. intermedia, A. actinomycetemcomitans, and F. nucleatum. • Metabolic activity: approximate value 0.2OD540/cm2 (control between 0.8 and 0.9OD540/cm2). • Polysaccharide production: values between 20 and 30 mg/L (control between 60 and 80 mg/L). |
| Wang et al. (2016) [49] | 20% NACP | • 3% DMAHDM (by wt%): Flexural strength: similar values to control. Modulus of elasticity: similar values to control. |
3% DMAHDM (by wt%): • Dead/alive bacteria: increased adherent compromised bacteria. • CFU: reduced the CFU by 3 log. Values between 105 and 107 (control between 108 and 1010), in a biofilm of P. gingivalis, P. intermedia, P. nigrescens, A. actinomycetemcomitans, F. nucleatum, and E. faecalis. • Biofilm biomass: values between 0.3 and 0.6OD600nm (control between 0.9 and 1.8 OD600nm) • Polysaccharide production: values between 10 and 20 mg/L (control between 40 and 100 mg/L). |
| Xie et al. (2016) [48] | 3%MPC and 30%NACP | • 3% DMAHDM (by wt%): Flexural Strength: similar values to control. Modulus of elasticity: values lower than control. |
3% DMAHDM (by wt%): • Dead/alive bacteria: increased adherent compromised bacteria. • Metabolic activity: approximate value 0A540/cm2 (control between 0.6 and 0.7A540/cm2). • CFU: reduced the CFU by 3 log. Values between 104 and 107 (control between 107 and 1010), a biofilm of S. mutans, total Streptococci, and total microorganisms. |
| Zhang et al. (2017) [50] | 3%MPC | • 1.5% DMAHDM (by wt%) after 180 days of immersion in water: Flexural strength: similar values to control. Modulus of elasticity: similar values to control. |
1.5% DMAHDM (by wt%): • Dead/alive bacteria: increased adherent compromised bacteria. • Metabolic activity: on day 1 and after 180 days the values remained between 0.1 and 0A540/cm2 (control between 0.8 and 1A540/cm2). • Lactic acid production: on day 1 and after 180 days, the values remained between 0 and 2 mmol/L (control between 17 and 20 mmol/L). • CFU: reduced the CFU by 3 log. Values between 10 5and 107 (control between 107 and 1010), a biofilm of S. mutans, total Streptococci, and total microorganisms. |
| Al-Dulaijan et al. (2018) [24] | 20% NACP | • 3% DMAHDM (by wt%): Flexural strength: similar values to control. Modulus of elasticity: similar Values to control. |
3% DMAHDM (by wt%): • Dead/alive bacteria: increased adherent compromised bacteria. • Metabolic activity: approximate value 0.05 A540/cm2 (control between 0.2 and 0.25 A540/cm2). • Lactic acid production: values between 0 and 1 mmol/L (control 5 mmol/L). • CFU: reduced the CFU between 3 and 4 log. Values between 103 and 107 (control between 105 and 109), in a biofilm of S. mutans, total Streptococci and total microorganisms. |
| Cao et al. (2018) [35] | 3% MPC | • 1.5% DMAHDM (by wt%): Flexural strength: similar values to control. Modulus of elasticity: similar values to control. 2.25% DMAHDM (by wt%): Flexural strength: similar values to control. Modulus of elasticity: similar values to control. The use of MPC did not change the properties when incorporated up to a maximum of 3%; |
1.5 DMAHDM (by wt%): • Dead/alive bacteria: increased adherent compromised bacteria. • Metabolic activity: values between 0.05 and 0.1A540/cm2 (contro l0.25A540/cm2). Combined use with MPC resulted in approximately 0.05A540/cm2. |
| Wang et al. (2018) [51] | None | • Not analyzed | 3% DMAHDM (by wt%): • Dead/alive bacteria: increased adherent compromised bacteria as the percentage of DMAHDM increased from 0.75–3%. • Metabolic activity: (48 h) Approximate value 0.4 OD540/cm2 (control 1.5OD540/cm2). (72 h). Approximate value 0.6 OD540/cm2 (control 1.6OD540/cm2). • Lactic acid production: (48 h) Approximate value 5 mmol/L (control 15 mmol/L). (72 h) approximate value 8 mmol/L (control 17 mmol/L). • Polysaccharide production: (48 h) Approximate value is 60 mg/mL (control 160 mg/mL). (72 h) approximate value is 100 mg/mL (control 230 mg/mL). • CFU: (48 h) Reduced CFU between 3 and 4 log. Values between 105 and 106. (72 h) Reduced CFU by 2 log. Approximate value 107 (control 109). |
| Wang et al. (2018) [60] | None | • Not analyzed | 3% DMAHDM (by wt%): • S. mutans, S. sanguinis, and S. gordonii in planktonic forms did not develop resistance to DMAHDM dissolved in distilled water. • S. mutans, S. sanguinis, and S. gordonii in biofilm did not develop resistance to 3% DMAHDM incorporated into the resin. • CFU: reduced the CFU by 3 log. Approximate value 107 (control 1010) in S. mutans biofilm. Reduced the CFU by 4 log. Values between 104 and 105 (control between 108 and 109) in S. sanguinis biofilm. Reduced the CFU by 4 log. The approximate value is 106 (control 1010) in S. gordonii biofilm. |
| Chen et al. (2019) [44] | 30% NACP | • 3% DMAHDM (by wt%): Flexural strength: similar values to control Modulus of elasticity: similar values to control • After 30 days of acid attack of the biofilm, composite with 3DMAHDM + 30NACP reached hardness values close to 2 GPa (control 1 GPa). |
3% DMAHDM (by wt%): • Dead/alive bacteria: increased adherent compromised bacteria. • Metabolic activity: approximate value 0.1 OD540/cm2 (control 0.5 OD540/cm2). • Polysaccharide production: approximate value 0.1 OD490/cm2 (control 0.4 OD490/cm2). • Lactic acid production: approximate value 3 mmol/L (control18mmol/L). • CFU: reduced CFU by 4 log. Values between 104 and 105 (control between108 and109), S. mutans biofilm. |
| Wang et al. (2019) [37] | 3%MPC + 20%NACP | • 3%DMAHDM (by wt%): Surface roughness: similar to control. The surface load density with the addition of DMAHDM was approximately 3 times higher than the controls. |
3% DMAHDM (by wt%): • Dead/alive bacteria increased adherent compromised bacteria. DMAHDM isolated in a biofilm of 9 species resulted in more live bacteria compared to isolated species. • CFU: antibacterial efficacy of DMAHDM decreased as the number of species increased. Reduced CFU by 2 log. Values between 107 and 109 (control between 109 and 1010). Biofilm of P. gingivalis, S. gordonii, F. nucleatum, A. naeslundii, P. intermedia, A. actinomycetemcomitans, P. nigrensens, T. forsythia, and P. micra. • Metabolic activity: value between0.4 and 0.8 OD492nm/cm2, (control between 1 and 1.4OD492nm/cm2). • Polysaccharide production: value between 20 and 60 mg/mL, (control between 60 and 80 mg/mL). |
| Xiao et al. (2019) [23] | 0.12% silver nanoparticles (nAg); 3%MPC and 30%NFCA. | • 3% DMAHDM (by wt%): Flexural strength: similar values to control. Modulus of elasticity: similar values to control. Dentin shear bond strength: similar values to control. |
3% DMAHDM (by wt%): • Dead/live bacteria: increased adherent compromised bacteria. • Metabolic activity: values between 0 and 0.2 OD540/cm2 (control between 0.8 and 1 OD540/cm2). • Polysaccharide production: values between 10 and 20 mg/L (control between 60 and 80 mg/L). • CFU: reduce the CFU between 3 and 5 log. Values between 105 and 106 (control between 108 and1010), Biofilm of P. gingivalis, A. actinomycetemcomitans and F. nucleatum. |
| Balhaddad et al., 2020 [43] | 20% NACP | • 3% DMAHDM (by wt%): Flexural strength: superior values to commercial control and similar values to experimental control. Modulus of elasticity: values lower than controls Surface roughness: Similar values to controls. • 5% DMAHDM (by wt%): Flexural strength: similar values to commercial control. Modulus of elasticity: values lower than commercial control. |
5% DMAHDM and 3%DMAHDM (by wt%): • Live/Dead: increased adherent compromised bacteria. • Metabilic activity: (3%DMAHDM) values between 0.05 and 0.1 A540/cm2. (5%DMAHDM) approximate value 0.05 A540/cm2 (control between 0.2 and 0.25 A540/cm2). • CFU: (3% DMAHDM) Reduced the CFU by 2 log. Values between 105 and 109. 5% DMAHDM reduced the CFU by 3 log. Values between 103 and 108 (control between 107 and 1010) Biofilm of S. mutans, total Streptococci, total microorganisms, and total Lactobacilli. • Lactic acid: (3%DMAHDM) Approximate value 5 mmol/L (5%DMAHDM) Approximate value 1 mmol/L (Control 10 mmol/L). |
| Bhadila et al. (2020) [40] | 20% NACP; | • 3% DMAHDM (by wt%): Flexural strengths: similar values to control. Modulus of elasticity: similar values to control. Surface roughness: similar values to control. |
3% DMAHDM (by wt%): • Live/dead: increased adherent compromised bacteria. • CFU: reduced the CFU between 3 and 4 log. Values between 104 and 105 (Control 107) in S. mutans biofilm. • Lactic acid: approximate value is 2 mmol/L (control 10 mmol/L). |
| Bhadila et al. (2020) [64] | 20% NACP; Low shrinkage-stress composite. |
• 3% DMAHDM (by wt%): Flexural strengths: similar values to control. Modulus of elasticity: similar values to control. Hardness: similar values to control. Cytotoxicity: similar to the BisGMA monomer. Enamel hardness after an acid attack: the UV + 3DMAHDM + 20NACP + 43glass group had the highest values, followed by 3% DMAHDM alone. |
3% DMAHDM (by wt%): • Not rated. |
| Bhadila et al. (2020) [41] | 20% NACP Low shrinkage-stress composite. |
• 3% DMAHDM (by wt%): Flexural strength: similar values to control. Modulus of elasticity: similar values to control. Dentin hardness: the group with DMAHDM + NACP + UV show 1.5 times greater than the control. • After 200 days of aging: Flexural strength: values lower than control. Modulus of elasticity: values lower than control. |
3% DMAHDM (by wt%): • Live/Dead: increased adherent compromised bacteria. • CFU: reduced the CFU by 5 log. Approximate value 104 (Control 109) in S. mutans biofilm. • Lactic acid production: approximate value 2 mmol/L (control between 30 and 40 mmol/L). • Biomass: approximate value 1 OD = 570 (control 3.5 OD = 570). |
| Campos et al. (2020) [52] | dCHX | • Cytotoxicity at 1, 3, and 7 days: (5% DHAMDM) showed cytotoxicity values higher than chlorhexidine, exceeding the value of combined use on day 3. Flexural strength: values lower than control. Surface roughness: values lower than control. |
5% DMAHDM (by wt%): • Metabolic activity: values between 0.11 A620/cm2 and 0.05 A620/cm2 (control between 0.36 A620/cm2 and 0.23 A620/cm2); biofilm of S. mutans and C. albicans, respectively. |
| Chen et al. (2020) [36] | None | Not rated | 1.5% e 3% DMAHDM (by wt%): • Live/dead: increased adherent compromised bacteria. • Biomass: (1.5%DMAHDM) Values between 0.6 and 0.9 OD570nm. (3%DMAHDM) Values between 0.3 and 0.6 OD570nm (control between 1.5 and 1.8 OD570nm). • Polysaccharide production: (1.5% DMAHDM) Approximate value 0.2 OD490 nm. (3%DMAHDM) values between 0.1 and 0.2 OD490nm (control between 0.5 and 0.6 OD490nm). • CFU: (1.5% DMAHDM) Reduced the CFU between 3 and 4 log. (3% DMAHDM) reduced the CFU between 5 and 6 log (control 109) in S. mutans biofilm. • Metabolic activity: (1.5% DMAHDM) 0.4 OD540 nm. (3%DMAHDM) 0.2 OD540nm (control values between 1 e 1.2 OD540nm). • Lactic acid: (1.5%DMAHDM) values between 4 and 8 mmol/L. (3%DMAHDM) approximately 2 mmol/L (Control 20 mmol/L). |
| Mitwalli et al., 2020 [53] | 3% MPC e 15% nanoparticles of calcium fluoride (nCaF2) | • 3%DMAHDM (by wt%): Flexural strength: similar values to control. Modulus of elasticity: similar values to control. |
3% DMAHDM (by wt%): • Live/dead: increased adherent compromised bacteria. • CFU: reduced the CFU by 6 log. Values between 102 and 104. (Control between 104 and 1010) biofilm of S. mutans, total Streptococci and total microrganisms. • Metabolic activity: approximate value 0 A540/cm2 (control between 0.15 and 0.2 A540/cm2). • Lactic acid: approximate value 0 mmol/L (control between 1 and 10 mmol/L). |
| Zhou et al. (2020) [38] | 30% NACP | • 3%DMAHDM (by wt%): Flexural strength: similar values to control. Modulus of elasticity: similar values to control. |
3% DMAHDM (by wt%): • Live/dead: increased adherent compromised bacteria. • Metabolic activity: approximate value 0.1 OD490/cm2 (Control between 0.1 and 0.5 OD490/cm2). • CFU: reduced the CFU by 5 log. Approximate value 104 (control 109) for biofilm of S.mutans and multispecies. • Polysaccharide production: approximate value 0.1 OD450/cm2 (Control between 0.1 and 0.6 OD450/cm2. • Latic acid: approximate value 0 mmol/L (Control between 1 e 25 mmol/L). |
| Zhou et al. (2020) [39] | 30% NACP | • 3%DMAHDM (by wt%): Flexural strengths: similar values to control Modulus of elasticity: similar values to control |
3% DMAHDM (by wt%): • Live/dead: increased adherent compromised bacteria. • Metabolic activity: approximate value 0.10 OD450/cm2 (Control between 0.15 and 0.17 OD450/cm2). • CFU: reduced the CFU by 2 log. Approximate value 106 (Control between 108 and 109) in saliva-derived biofilm. • Lactic acid: values between 2 and 3 mmol/L (control 7 mmol/L). • Polysaccharide production: approximate value 0.2 OD490/cm2 (control 0.5 OD490/cm2). |
| Zhou et al. (2020) [42] | 30% NACP | • 3%DMAHDM (by wt%): Flexural strengths: similar values to control. Modulus of elasticity: similar values to control. Enamel hardness: highest values for NACP + DMAHDM, followed by the group with only DMAHDM (1.5 times greater than the control). |
3% DMAHDM (by wt%): • Live/Dead 48 h: increased adherent compromised bacteria. • Metabolic activity: DMAHDM greatly reduced the metabolic activity of biofilms. Approximate value 0.1 OD450/cm2 (control close to 0.5 OD450/cm2). • CFU: reduced the CFU by 5 log. Values between 103 and 104 (Control 108). S. mutans biofilm. • Lactic acid: approximate value 0 mmol/L (control 12 mmol/L). • Polysaccharides: values between 30 and 40 mg/mL (Control 230 mg/mL). |
2. Mechanism of action
Quaternary ammonium monomers have mechanisms of action similar to quaternary ammonium salts (QAS). Long-chain quaternary ammonium compounds can penetrate the bacterial cell membrane, disturbing the lipid layers’ balance, causing the leaking of the bacterial cytoplasmic content [26], [27]. Another possible mechanism occurs from the contact between the positively charged N+ quaternary amine in the structure of the QAS with the negatively charged bacterial membrane. This contact causes the loss in the balance of essential ions, disturbance in the membrane functions, such as respiration, transport of solutes, and biosynthesis of the cellular wall [22], [28] be seen in Fig. 2.
Fig. 2.
Mechanism of action of DMAHDM. (a) The contact between the negatively charged bacteria and long-chained positively charged quaternary ammonium compounds can lead to (b) Penetration of bacterial membrane, causing cytoplasmatic leakage; (c) Disruption of membrane functions, such as solute transportation, respiration, and cell wall biosynthesis; and (d) Alteration on the superficial electrostatic charge from the contact with the positive charge on quaternary amine N+ of the monomer, collapsing proton-motive force and ATP production, leading to (e) Loss of DNA multiplication capability and (f) Interruption of protein synthesis.
It is also suggested that quaternary ammonium surfactant, a sub-group of the quaternary ammonium compounds, may alter the superficial electrostatic charge in contact with the bacterial cell membrane. The cell membrane would become more positive, causing the collapse of the proton-motive force (PMF), an electrochemical gradient of protons critical for ATP production [29], [30]. Thus, it is possible to suggest that the loss of the capacity of multiplication of bacterial DNA and protein activity in the cell pointed out in previous studies is associated with the collapse of the PMF and consequent loss of the production of ATP, considering that protein synthesis and replication of DNA require ATP [22], [31], [32], [33].
3. Antibacterial properties
As highlighted above, from contact between the negatively charged bacterial cell wall and the positive charge of the quaternary amine present in the monomer, it becomes possible to disturb the cell wall's electrical balance, leading to bacterial death [34]. This mechanism allowed a good bactericidal efficacy to be achieved in several resin compositions, bringing benefits that can be further enhanced by the association with different components.
Regarding the antibacterial potential of DMAHDM monomer, all studies included in this review used several tests to verify this property: Colony Formation Units (CFU), live/dead biofilm staining assay, the phenol-sulfuric acid method to evaluate polysaccharide production, enzymatic (lactate dehydrogenase) method to measure lactic acid production, and MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay of metabolic activity of biofilms. The comparison between studies should be made with caution since there is great heterogeneity due to variations in the use of DMAHDM alone or associated with other components, tests of different concentrations, and biofilms of various species. Firstly, it is remarkable in all 25 studies that the monomer's antibacterial efficacy has been confirmed through the adopted tests. All of them found a better performance of DMAHDM than the control.
The live/dead biofilm staining assay demonstrated that the experimental resins were mainly covered with dead bacteria [23], [24], [25], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], with live bacteria covering 10% to 20% of the surface of the resins, while controls ranged from 50% to 100% [46], [48]. Polysaccharide production showed reduction values between 2 and 6 times higher than the control [23], [36], [37], [38], [39], [42], [44], [47], [49], [51]. There was also a reduction in metabolic activity [23], [24], [25], [35], [36], [37], [38], [39], [42], [43], [44], [45], [46], [47], [48], [50], [51], [52], [53], biofilm biomass [36], [41], and lactic acid production [24], [25], [36], [38], [39], [40], [41], [42], [43], [44], [45], [46], [50], [51], [53] indicated in the studies, demonstrating that the monomer can inhibit the bacterial activity responsible for the appearance of caries lesions. Another important point is the evaluation of the CFU count in experimental resins. There was a great variation in the composition of the biofilms analyzed. However, DMAHDM reduced CFU formation in all studies, ranging from a minimum of 3 to a maximum of 6 orders of magnitude [23], [24], [25], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [53].
The pathogenesis of caries involves lowering the local pH due to acids’ production from the metabolism of fermentable carbohydrates by bacterial colonies present in dental biofilm [2]. The evidence pointed out above for the inhibition of polysaccharide production, lactic acid, bacterial metabolism, and CFU count suggests that the DMAHDM monomer's use may directly promote bacterial death, avoiding the successive events necessary for the formation of caries lesions.
In addition to reducing bacterial microbiota, DMAHDM can also alter the biofilm balance to increase the number of commensal species, such as S. sanguinis and S. gordonii [51]. Previous studies state that the commensal species mentioned are primary colonizers. They can antagonize S. mutans, producing hydrogen peroxide and neutralizing the acids in the biofilm [54], [55], [56], [57]. This finding justifies a higher proportion of S. sanguinis and S. gordonii in the biofilm of healthy tooth surfaces and caries-free individuals. Conversely, a higher proportion of S. mutans indicates acidogenicity associated with individuals with caries [55], [58], [59].
Adding the evidence of inhibition of the mechanisms of caries pathogenesis to the change in biofilm balance shown above, we can strongly suggest that the DMAHDM monomer incorporated in restorative materials represents a possibility of an advantage to act in the carious process when compared to conventional resin composites. Therefore, the use of such a component can be seen as a preventive tool to avoid recurrent carious lesions, given that it would act early in the mechanism of the disease.
Still, the studies analyzed did observe not only cariogenic species but also periodontopathogenic species such as P. gingivalis, P. intermedia, A. actinomycetemcomitans, and F. nucleatum. Three studies [37], [47], [49] evaluated the inhibition of such species provided by the DMAHDM and found excellent results compared with the control evidenced by the reduction in UFC count, bacterial metabolism, polysaccharide production, and biofilm biomass. Thus, it is likely that DMAHDM has the potential to prevent the occurrence of dental caries and be an important tool in the prevention of gingival and periodontal diseases.
3.1. Bacterial resistance
Wang et al. (2018) [60] evaluated the development of resistance of S. mutans, S. sanguinis, and S. gordonii in planktonic forms and biofilm against a mass fraction of 3%DMAHDM dissolved in sterile distilled water or incorporated in resin composites also containing bisphenol A glycidyl dimethacrylate (BisGMA) and triethylene glycol dimethacrylate (TEGDMA). The results showed that none of the species studied developed resistance in the planktonic form of biofilm, even after 20 passages of tests that took about four months.
One of the main concerns when using antibacterial compounds in resin-based dental materials is bacterial resistance induction since the material should remain in the oral cavity long-lastly. Wang et al. (2017) [61] dissolved DMAHDM in sterile distilled water at 200 g/mL and examined the induction of drug resistance in eight species of cariogenic, endodontic, and periodontal bacteria (S. mutans, S. sanguis, S. gordonii, E. faecalis, A. actinomycetemcomitans, F. nucleatum, P. gingivalis, and P. intermedia). The results showed that after ten passages, the bacterial strains did not develop resistance to the monomer in contrast with chlorhexidine that induced resistance in four species, indicating a low risk for DMAHDM induce resistance.
However, as highlighted by the authors in the study of Wang et al. (2018) [60], only Streptococcal species were examined, against a mass fraction of 3% DMAHDM, incorporated in a resin composite. Therefore, allied to the fact that only that study was identified in our search, it remains unclear if the monomer stimulates resistance in different conditions against other species used in a resin composite for dental restoration.
4. Cytotoxicity
Campos et al. (2019) [52] synthesized a self-curing experimental acrylic resin and analyzed the toxicity against rat fibroblasts by adding eluted resin samples in the medium of Dulbecco Modified Eagle Medium with fibroblast culture. The DMAHDM was incorporated in the PMMA acrylic resin (5 wt%), along with polymethyl methacrylate polymer, benzoyl peroxide (initiator), pigments, methyl methacrylate monomer, cross-linking agent (EDMA), DMT, inhibitor, and fluorescent. The authors observed morphological changes in the cells and higher cytotoxicity than chlorhexidine diacetate (dCHX). Similarly, Regis et al. (2012) [62] showed higher cytotoxicity values for MUPB (methacryloyloxyloxyurelpyridinium bromide), a QAM also used in acrylic resin, compared to the control. The authors highlighted that the cytotoxicity values found for MUPB are close to the results for HEMA and UDMA, monomers widely used in restorative dental materials [63].
5. Mechanical properties
Resin-based dental materials are used in the oral cavity for restorative applications, subjected to occlusal forces, temperature cycles, and erosive challenges from the diet and biofilm. Thus, tooth restorations’ long-lasting clinical behavior depends on the obtention of material with satisfactory mechanical strength. Most of the studies analyzed [23], [24], [25], [35], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [52], [53], [64] have verified properties of experimental composites, such as flexural strength (FS), modulus of elasticity (ME), fracture toughness (FT), surface roughness (SR), hardness (HC), dentin shear bond strength (DSBS) and color stability (CS).
The majority of studies that analyzed resin composites observed no change in FS with an addition of 1.5 to 3.75 wt% DMAHDM [23], [24], [25], [38], [39], [40], [41], [42], [43], [44], [46], [47], [48], [49], [50], [53]. However, Balhaddad et al. (2020) [43] observed a reduction of FS by the addition of 5 wt% DMAHDM compared to a resin composite with 3 wt% DMAHDM and experimental control, although it remained superior to the commercial control.
For ME, the experimental resin composites showed a reduction in the studies of Xie et al. (2016) [48], Mitwalli et al. (2020) [53], and Balhaddad et al. (2020) [43]. Xie et al. (2016) [48] worked with DMAHDM at a mass fraction of 1.5% to 3%, observing a significant reduction compared to the control. The work of Mitwalli et al. (2020) [53] showed a reduction with 3% (by wt%) DMAHDM in comparison with the experimental control, but it remained similar to the commercial control. Balhaddad et al. (2020) [43] obtained results indicating ME significantly lower than the control by adding 3% and 5%DMAHDM mass fractions. These results contrast with other studies in which there was no change [23], [24], [25], [38], [39], [40], [41], [42], [44], [46], [47], [49], [50], [64].
Surface roughness was analyzed in three studies [37], [40], [43] by the addition of 3 wt% DMAHDM [37], [40], [43] and 5 wt% [43]. All results demonstrated no alterations in SR, even after biofilm challenges [43], showing that adding up to 5 wt% did not alter that property. DSBS, FT, and HC of experimental resin composites were also verified by Xiao et al. [23], Wu et al. [45], and Bhadila et al. (2020) [64], respectively. The results showed that 3 wt% DMAHDM in the experimental resin composites did not change the properties tested.
In comparison, the studies that examined acrylic resins [35], [52] observed decreased FS, ME, SR, and color stability by the addition of 2.25 wt% DMAHDM [35] and 5 wt% DMAHDM [52]. It is interesting to highlight the fact that the addition of 1.5% (by wt%) DMAHDM resulted in similar FS and ME compared to control in the work of Cao et al. [35].
The resin composites’ physical and mechanical properties can critically affect performance in the oral cavity [65]. The properties investigated in this review's studies may influence the ability to resist occlusal forces, the rigidity of the material, and the ability to accumulate biofilm, thus resulting in better or worse longevity [65], [66], [67]. The results suggest that these properties were maintained for composite resins in most studies and are comparable to commercial control materials. Therefore, the use of DMAHDM does not appear to weaken the restorative material, keeping its benefit to impair bacterial growth.
6. Association with other chemical components
The use of DMAHDM monomer generally occurred in conjunction with other anti-adherent, antibacterial, and remineralize components: 2-methacryloyloxyethyl phosphorylcholine (MPC), nanoparticles of amorphous calcium phosphate (NACP), and nanoparticles of calcium fluoride (nCaF2), chlorhexidine diacetate (dCHX), silver nanoparticles (Ag), and nanoparticles of calcium fluoride [23], [52], [53].
6.1. MPC
Special attention should be given to MPC in such an analysis, considering that several studies have shown better antibacterial performance using DMAHDM + MPC (Table 1). Wang et al. (2019) [37], using multispecies periodontal biofilm, demonstrated that the efficacy of DMAHDM alone was reduced as the biofilm changed from single to multiple species. However, the compound with DMAHDM + MPC maintained its efficacy, even with an increase in pathogens, suggesting that the combined use is promising.
These can be explained initially by MPC's hydrophilic nature since most proteins adsorb preferably to hydrophobic surfaces [68], [69], [70]. Moreover, it is known that the abundance of free but not water-bound water around MPC polymers is capable of detaching proteins on the surface, preventing adsorption [71], [72], [73]. Thus, by repelling the layer of salivary proteins that naturally deposits on the resin-based material's surface, MPC contributes to increasing the interaction between DMAHDM and bacteria, which reinforces the antibacterial effect [19], [74], [75], [76], [77].
6.2. NACP
Considering NACP, it is remarkable that Ca and P ions’ release provides a mechanism to inhibit caries lesions, especially during an acidic cariogenic challenge, helping to maintain the pH to avoid demineralization and facilitating remineralization [48], [78], [79]. The combination of NACP and DMAHDM was examined in several studies [23], [24], [25], [37], [38], [39], [40], [41], [42], [43], [44], [45], [48], [49], [64], and the combined use did not impair Ca and P ions release. Thus, the association between DMAHDM and NACP can be beneficial due to the synergistic effect of antibacterial activity, remineralization, and acid neutralization. Some studies demonstrated changes in enamel hardness [42], [64] and dentin hardness [41] in resin composites with 3 wt% DMAHDM and 20 wt% or 30 wt% NACP, showing hardness 1.5 [41] and two times [42], [64] greater in comparison to the control. However, an observation should be made, because according to the data collected, the concentration of NACP should not exceed 30% due to the risk of damaging mechanical properties, as was demonstrated by Chen et al. (2019) [44].
6.3. nAg
The nAg nanoparticles have antibacterial activity due to their ability to increase cytoplasmic permeability, which facilitates bacterial’ cell envelope breaking [71]. Therefore, dental materials containing Ag nanoparticles can interfere with biofilm formation to prevent dental caries [80]. Xiao et al., 2019 [23] analyzed the antibacterial activity of 3%DMAHDM + 0.12%nAg + 3%MPC and obtained increased antibacterial effect through the reduction in polysaccharide production, metabolism, adhered live bacteria, and CFU count, without harming mechanical properties such as modulus of elasticity, flexural strength, and shear bond strength in dentin. However, as it is known that MPC improves the ability of DMAHDM to inhibit bacterial activity [19], [74], [75], [76], [77], we cannot rule out the possibility that MPC contributed to increasing the antibacterial effect of the blend.
6.4. dCHX
The combined use of DMAHDM with dCHX [52] in PMMA acrylic resin showed no benefit compared to DMAHDM alone. dCHX has been widely used in adhesive systems to improve the restoration's longevity by inhibiting matrix metalloproteinases (MMPs) and having antimicrobial efficacy [81], [82], [83]. However, this component has some flaws, such as increased water sorption [84]. Campos et al. [52] observed that the combined use resulted in worse surface roughness values, so it does not seem as if this approach was more beneficial than DMAHDM alone.
6.5. Nanoparticles of calcium fluoride (nCaF2)
Topical fluoride can stabilize the pH of saliva, reduce cariogenic bacteria, remineralize dental structure, and strengthen enamel, minimalizing demineralization because of fluorapatite crystals formation [85], [86], [87]. Calcium fluoride nanoparticles would enforce the enamel structure by promoting remineralization and forming fluorapatite [53].
Our research identified a single study regarding the combined use of nCaF2 and DMAHDM. Mitwalli et al. (2020) [53] observed flexural strength and modulus of elasticity similar to control by adding 15% nCaF2 + 3%DMAHDM mass fraction. However, the 15%nCaF2+ 3%DMAHDM + 3%MPC group showed a significant reduction in FS compared to the control. The association achieved great antibacterial activity evidenced by the reduction in CFU count, metabolic activity, and lactic acid production, allied with fluoride and calcium ion release, further potentialized by association with 3%MPC mass fraction. The results indicate the potential to use such a combination in dental restorative to prevent recurrent carious lesions. However, more studies must analyze the alliance to understand the effects of long-term ions release and mechanical properties.
7. Limitations and future perspectives
It was not possible to identify clinical studies using any material containing DMAHDM, so the notes made here are limited to in vitro studies. Moreover, only the papers by Zhang et al. (2017) [50] and Bhadila et al. (2020) [41] evaluated the long-term efficacy of 1.5 wt% to 3 wt% DMAHDM, obtaining consistent results for antibacterial activity after 90 [41] and 180 days [50]. However, regarding mechanical properties, Zhang et al. (2017) [50] obtained results similar to the control for FS and ME after 180 days, contrasting with Bhadila et al. (2020) [41] that observed a similar reduction for FS, but worse values for ME compared to the control after 200 days. Thus, the results presented in this review were analyzed only for the monomer's immediate efficacy because other long-term studies could not be identified.
Future studies are needed to obtain further clarification on the development of oral cell cytotoxicity and bacterial resistance. Materials with different concentrations of DMAHDM exposed to more bacterial species should be analyzed, as the oral environment is composed of a very diverse microbiota, consisting of more than 700 bacterial species [88]. It is pertinent to recommend evaluating the bactericidal capacity of DMAHDM and the possibility to favor commensal species over cariogenic species. For better safety in maintaining mechanical properties, analysis of other resin composites’ characteristics such as CS, FT, and SR should be carried out, considering that most studies have observed only FS and ME.
8. Conclusion
The DMAHDM monomer has demonstrated short-time antibacterial efficacy against several oral pathogenic species and its benefits by association with MPC. The experimental resin composites containing DMAHDM showed satisfactory FS and ME, but this monomer worsened acrylic resins’ mechanical properties. DMAHDM presents itself as a promising antibacterial monomer for association with components such as MPC, nCaF2 and NACP in resin-based dental materials. Future research is still needed to provide more information regarding cytotoxicity, the development of bacterial resistance, the capability of favoring commensal species over cariogenic, and the analysis of mechanical properties, such as fracture toughness, surface roughness, and color stability.
Role of the funding source
No funding.
Conflicts of interest
None declared.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(November (10159)):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. PMID: 30496104; PMCID: PMC6227754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selwitz R.H., Ismail A.I., Pitts N.B. Dental caries. Lancet. 2007;369(January (9555)):51–59. doi: 10.1016/S0140-6736(07)60031-2. PMID: 17208642. [DOI] [PubMed] [Google Scholar]
- 3.Featherstone J.D. The science and practice of caries prevention. J Am Dent Assoc. 2000;131(July (7)):887–899. doi: 10.14219/jada.archive.2000.0307. PMID: 10916327. [DOI] [PubMed] [Google Scholar]
- 4.Featherstone J.D. The continuum of dental caries—evidence for a dynamic disease process. J Dent Res. 2004;83 Spec No C:C39–C42. doi: 10.1177/154405910408301s08. PMID: 15286120. [DOI] [PubMed] [Google Scholar]
- 5.van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73(March (3)):672–681. doi: 10.1177/00220345940730031301. PMID: 8163737. [DOI] [PubMed] [Google Scholar]
- 6.Deng D.M., ten Cate J.M. Demineralization of dentin by Streptococcus mutans biofilms grown in the constant depth film fermentor. Caries Res. 2004;38(January–February (1)):54–61. doi: 10.1159/000073921. PMID: 14684978. [DOI] [PubMed] [Google Scholar]
- 7.Totiam P., González-Cabezas C., Fontana M.R., Zero D.T. A new in vitro model to study the relationship of gap size and secondary caries. Caries Res. 2007;41(6):467–473. doi: 10.1159/000107934. Epub 2007 Sep 7. PMID: 17827964. [DOI] [PubMed] [Google Scholar]
- 8.Drummond J.L. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87(August (8)):710–719. doi: 10.1177/154405910808700802. PMID: 18650540; PMCID: PMC2561305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferracane J.L. Resin composite—state of the art. Dent Mater. 2011;27(January (1)):29–38. doi: 10.1016/j.dental.2010.10.020. Epub 2010 Nov 18. PMID: 21093034. [DOI] [PubMed] [Google Scholar]
- 10.Naoum S.J., Mutzelburg P.R., Shumack T.G., Thode D., Martin F.E., Ellakwa A.E. Reducing composite restoration polymerization shrinkage stress through resin modified glass-ionomer based adhesives. Aust Dent J. 2015;60(December (4)):490–496. doi: 10.1111/adj.12265. PMID: 25476699. [DOI] [PubMed] [Google Scholar]
- 11.Deljoo Z., Sadeghi M., Azar M.R., Bagheri R. The effect of different polishing methods and storage media on discoloration of resin composites. J Dent Biomater. 2016;3(June (2)):226–232. PMID: 28959747; PMCID: PMC5608056; 3: 226–232. [PMC free article] [PubMed] [Google Scholar]
- 12.Askar H., Krois J., Göstemeyer G., Bottenberg P., Zero D., Banerjee A. Secondary caries: what is it, and how it can be controlled, detected, and managed? Clin Oral Investig. 2020;24(May (5)):1869–1876. doi: 10.1007/s00784-020-03268-7. Epub 2020 Apr 17. PMID: 32300980. [DOI] [PubMed] [Google Scholar]
- 13.Lynch C.D., Frazier K.B., McConnell R.J., Blum I.R., Wilson N.H. Minimally invasive management of dental caries: contemporary teaching of posterior resin-based composite placement in U.S. and Canadian dental schools. J Am Dent Assoc. 2011;142(June (6)):612–620. doi: 10.14219/jada.archive.2011.0243. PMID: 21628682. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi R.L. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Summary of discussion from the Portland Composites Symposium (POCOS) June 17–19, 2004, Oregon Health and Science University, Portland, Oregon. Dent Mater. 2005;21(January (1)):3–6. doi: 10.1016/j.dental.2004.10.008. PMID: 15680996. [DOI] [PubMed] [Google Scholar]
- 15.Bohaty B.S., Ye Q., Misra A., Sene F., Spencer P. Posterior composite restoration update: focus on factors influencing form and function. Clin Cosmet Investig Dent. 2013;5(May):33–42. doi: 10.2147/CCIDE.S42044. PMID: 23750102; PMCID: PMC3666491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mjör I.A., Toffenetti F. Secondary caries: a literature review with case reports. Quintessence Int. 2000;31(March (3)):165–179. PMID: 11203922. [PubMed] [Google Scholar]
- 17.Frost P.M. An audit on the placement and replacement of restorations in a general dental practice. Prim Dent Care. 2002;9(January (1)):31–36. doi: 10.1308/135576102322547548. PMID: 11901789. [DOI] [PubMed] [Google Scholar]
- 18.Zhang K., Melo M.A., Cheng L., Weir M.D., Bai Y., Xu H.H. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent Mater. 2012;28(August (8)):842–852. doi: 10.1016/j.dental.2012.04.027. Epub 2012 May 14. PMID: 22592165; PMCID: PMC3393841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyth N., Yudovin-Farber I., Bahir R., Domb A.J., Weiss E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27(July (21)):3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. Epub 2006 Mar 27. PMID: 16564083. [DOI] [PubMed] [Google Scholar]
- 20.Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19(September (6)):449–457. doi: 10.1016/s0109-5641(02)00102-1. PMID: 12837391. [DOI] [PubMed] [Google Scholar]
- 21.He J., Söderling E., Österblad M., Vallittu P.K., Lassila L.V. Synthesis of methacrylate monomers with antibacterial effects against S. mutans. Molecules. 2011;16(November (11)):9755–9763. doi: 10.3390/molecules16119755. PMID: 22113583; PMCID: PMC6264393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou H., Li F., Weir M.D., Xu H.H. Dental plaque microcosm response to bonding agents containing quaternary ammonium methacrylates with different chain lengths and charge densities. J Dent. 2013;41(November (11)):1122–1131. doi: 10.1016/j.jdent.2013.08.003. Epub 2013 Aug 13. PMID: 23948394; PMCID: PMC3845448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao S., Wang H., Liang K., Tay F., Weir M.D., Melo M.A.S. Novel multifunctional nanocomposite for root caries restorations to inhibit periodontitis-related pathogens. J Dent. 2019;81(February):17–26. doi: 10.1016/j.jdent.2018.12.001. Epub 2018 Dec 12. PMID: 30552930. [DOI] [PubMed] [Google Scholar]
- 24.Al-Dulaijan Y.A., Cheng L., Weir M.D., Melo M.A.S., Liu H., Oates T.W. Novel rechargeable calcium phosphate nanocomposite with antibacterial activity to suppress biofilm acids and dental caries. J Dent. 2018;72(May):44–52. doi: 10.1016/j.jdent.2018.03.003. Epub 2018 Mar 8. PMID: 29526668. [DOI] [PubMed] [Google Scholar]
- 25.Wu J., Weir M.D., Melo M.A., Xu H.H. Development of novel self-healing and antibacterial dental composite containing calcium phosphate nanoparticles. J Dent. 2015;43(March (3)):317–326. doi: 10.1016/j.jdent.2015.01.009. Epub 2015 Jan 24. PMID: 25625674; PMCID: PMC4559273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melo M.A., Guedes S.F., Xu H.H., Rodrigues L.K. Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013;31(August (8)):459–467. doi: 10.1016/j.tibtech.2013.05.010. Epub 2013 Jun 28. PMID: 23810638; PMCID: PMC3845439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert P.A., Hammond S.M. Potassium fluxes, first indications of membrane damage in micro-organisms. Biochem Biophys Res Commun. 1973;54(September (2)):796–799. doi: 10.1016/0006-291x(73)91494-0. PMID: 4585693. [DOI] [PubMed] [Google Scholar]
- 28.Salt W.G., Wiseman D. The relation between the uptake of cetyltrimethylammonium bromide by Escherichia coli and its effects on cell growth and viability. J Pharm Pharmacol. 1970;22(April (4)):261–264. doi: 10.1111/j.2042-7158.1970.tb08516.x. PMID: 4392782. [DOI] [PubMed] [Google Scholar]
- 29.Inácio Â.S., Domingues N.S., Nunes A., Martins P.T., Moreno M.J., Estronca L.M. Quaternary ammonium surfactant structure determines selective toxicity towards bacteria: mechanisms of action and clinical implications in antibacterial prophylaxis. J Antimicrob Chemother. 2016;71(March (3)):641–654. doi: 10.1093/jac/dkv405. Epub 2015 Dec 17. PMID: 26679255. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. 1966. Biochim Biophys Acta. 2011;1807(December (12)):1507–1538. doi: 10.1016/j.bbabio.2011.09.018. PMID: 22082452. [DOI] [PubMed] [Google Scholar]
- 31.Marini M., Bondi M., Iseppi R., Toselli M., Pilati F. Preparation and antibacterial activity of hybrid materials containing quaternary ammonium salts via sol–gel process. Eur Polym J. 2007;43(August (8)):3621–3628. doi: 10.1016/j.eurpolymj.2007.06.002. [DOI] [Google Scholar]
- 32.Pontes M.H., Groisman E.A. Protein synthesis controls phosphate homeostasis. Genes Dev. 2018;32(January (1)):79–92. doi: 10.1101/gad.309245.117. Epub 2018 Feb 1. PMID: 29437726; PMCID: PMC5828397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skarstad K., Katayama T. Regulating DNA replication in bacteria. Cold Spring Harb Perspect Biol. 2013;5(April (4)):a012922. doi: 10.1101/cshperspect.a012922. PMID: 23471435; PMCID: PMC3683904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiao Y., Niu L.N., Ma S., Li J., Tay F.R., Chen J.H. Quaternary ammonium-based biomedical materials: state-of-the-art, toxicological aspects and antimicrobial resistance. Prog Polym Sci. 2017;71(August):53–90. doi: 10.1016/j.progpolymsci.2017.03.001. Epub 2017 Mar 12. PMID: 32287485; PMCID: PMC7111226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao L., Xie X., Wang B., Weir M.D., Oates T.W., Xu H.H.K. Protein-repellent and antibacterial effects of a novel polymethyl methacrylate resin. J Dent. 2018;79(December):39–45. doi: 10.1016/j.jdent.2018.09.007. Epub 2018 Sep 21. PMID: 30248381. [DOI] [PubMed] [Google Scholar]
- 36.Chen H., Zhang B., Weir M.D., Homayounfar N., Fay G.G., Martinho F. mutans gene-modification and antibacterial resin composite as dual strategy to suppress biofilm acid production and inhibit caries. J Dent. 2020;93(February):103278. doi: 10.1016/j.jdent.2020.103278. Epub 2020 Jan 13. PMID: 31945398. [DOI] [PubMed] [Google Scholar]
- 37.Wang L., Xie X., Qi M., Weir M.D., Reynolds M.A., Li C. Effects of single species versus multispecies periodontal biofilms on the antibacterial efficacy of a novel bioactive Class-V nanocomposite. Dent Mater. 2019;35(June (6)):847–861. doi: 10.1016/j.dental.2019.02.030. Epub 2019 Mar 13. PMID: 30878285. [DOI] [PubMed] [Google Scholar]
- 38.Zhou W., Zhou X., Huang X., Zhu C., Weir M.D., Melo M.A.S. Antibacterial and remineralizing nanocomposite inhibit root caries biofilms and protect root dentin hardness at the margins. J Dent. 2020;97(June):103344. doi: 10.1016/j.jdent.2020.103344. Epub 2020 Apr 18. PMID: 32315666. [DOI] [PubMed] [Google Scholar]
- 39.Zhou W., Peng X., Zhou X., Bonavente A., Weir M.D., Melo M.A.S. Novel nanocomposite inhibiting caries at the enamel restoration margins in an in vitro saliva-derived biofilm secondary caries model. Int J Mol Sci. 2020;21(September (17)):6369. doi: 10.3390/ijms21176369. PMID: 32887330; PMCID: PMC7503730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhadila G., Baras B.H., Weir M.D., Wang H., Melo M.A.S., Hack G.D. Novel antibacterial calcium phosphate nanocomposite with long-term ion recharge and re-release to inhibit caries. Dent Mater J. 2020;39(August (4)):678–689. doi: 10.4012/dmj.2019-203. Epub 2020 Apr 16. PMID: 32295987. [DOI] [PubMed] [Google Scholar]
- 41.Bhadila G., Filemban H., Wang X., Melo M.A.S., Arola D.D., Tay F.R. Bioactive low-shrinkage-stress nanocomposite suppresses S. mutans biofilm and preserves tooth dentin hardness. Acta Biomater. 2020 Sep 15;114:146–157. doi: 10.1016/j.actbio.2020.07.057. Epub 2020 Aug 6. PMID: 32771591. [DOI] [PubMed] [Google Scholar]
- 42.Zhou W., Peng X., Zhou X., Weir M.D., Melo M.A.S., Tay F.R. In vitro evaluation of composite containing DMAHDM and calcium phosphate nanoparticles on recurrent caries inhibition at bovine enamel-restoration margins. Dent Mater. 2020;36(October (10)):1343–1355. doi: 10.1016/j.dental.2020.07.007. Epub 2020 Aug 13. PMID: 32800353. [DOI] [PubMed] [Google Scholar]
- 43.Balhaddad A.A., Ibrahim M.S., Weir M.D., Xu H.H.K., Melo M.A.S. Concentration dependence of quaternary ammonium monomer on the design of high-performance bioactive composite for root caries restorations. Dent Mater. 2020;36(August (8)):e266–e278. doi: 10.1016/j.dental.2020.05.009. Epub 2020 Jun 8. PMID: 32527499. [DOI] [PubMed] [Google Scholar]
- 44.Chen H., Tang Y., Weir M.D., Lei L., Masri R., Lynch C.D. Effects of S. mutans gene-modification and antibacterial calcium phosphate nanocomposite on secondary caries and marginal enamel hardness. RSC Adv. 2019;9(December):41672–41683. doi: 10.1039/C9RA09220J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J., Zhou H., Weir M.D., Melo M.A., Levine E.D., Xu H.H. Effect of dimethylaminohexadecyl methacrylate mass fraction on fracture toughness and antibacterial properties of CaP nanocomposite. J Dent. 2015;43(December (12)):1539–1546. doi: 10.1016/j.jdent.2015.09.004. Epub 2015 Sep 25. PMID: 26404407; PMCID: PMC5001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang N., Ma J., Melo M.A., Weir M.D., Bai Y., Xu H.H. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J Dent. 2015;43(February (2)):225–234. doi: 10.1016/j.jdent.2014.11.008. Epub 2014 Dec 3. PMID: 25478889; PMCID: PMC4321720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L., Xie X., Imazato S., Weir M.D., Reynolds M.A., Xu H.H.K. A protein-repellent and antibacterial nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Mater Sci Eng C Mater Biol Appl. 2016;67(October):702–710. doi: 10.1016/j.msec.2016.05.080. Epub 2016 May 19. PMID: 27287170. [DOI] [PubMed] [Google Scholar]
- 48.Xie X., Wang L., Xing D., Arola D.D., Weir M.D., Bai Y. Protein-repellent and antibacterial functions of a calcium phosphate rechargeable nanocomposite. J Dent. 2016;52(September):15–22. doi: 10.1016/j.jdent.2016.06.003. Epub 2016 Jun 17. PMID: 27327110. [DOI] [PubMed] [Google Scholar]
- 49.Wang L., Melo M.A., Weir M.D., Xie X., Reynolds M.A., Xu H.H. Novel bioactive nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Dent Mater. 2016;32(December (12)):e351–e361. doi: 10.1016/j.dental.2016.09.023. Epub 2016 Sep 23. PMID: 27671471. [DOI] [PubMed] [Google Scholar]
- 50.Zhang N., Zhang K., Melo M.A., Weir M.D., Xu D.J., Bai Y. Effects of long-term water-aging on novel anti-biofilm and protein-repellent dental composite. Int J Mol Sci. 2017;18(January (1)):186. doi: 10.3390/ijms18010186. PMID: 28106774; PMCID: PMC5297818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H., Wang S., Cheng L., Jiang Y., Melo M.A.S., Weir M.D. Novel dental composite with capability to suppress cariogenic species and promote non-cariogenic species in oral biofilms. Mater Sci Eng C Mater Biol Appl. 2019;94(January):587–596. doi: 10.1016/j.msec.2018.10.004. Epub 2018 Oct 2. PMID: 30423744; PMCID: PMC6239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campos K.P.L., Viana G.M., Cabral L.M., Portela M.B., Hirata Junior R., Cavalcante L.M. Self-cured resin modified by quaternary ammonium methacrylates and chlorhexidine: cytotoxicity, antimicrobial, physical, and mechanical properties. Dent Mater. 2020;36(January (1)):68–75. doi: 10.1016/j.dental.2019.10.007. Epub 2019 Nov 14. PMID: 31735423. [DOI] [PubMed] [Google Scholar]
- 53.Mitwalli H., Balhaddad A.A., AlSahafi R., Oates T.W., Melo M.A.S., Xu H.H.K. Novel CaF2 nanocomposites with antibacterial function and fluoride and calcium ion release to inhibit oral biofilm and protect teeth. J Funct Biomater. 2020;11(August (3)):56. doi: 10.3390/jfb11030056. PMID: 32752248; PMCID: PMC7564802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolenbrander P.E., Palmer R.J., Jr., Rickard A.H., Jakubovics N.S., Chalmers N.I., Diaz P.I. Bacterial interactions and successions during plaque development. Periodontol. 2000. 2006;42:47–79. doi: 10.1111/j.1600-0757.2006.00187.x. PMID: 16930306. [DOI] [PubMed] [Google Scholar]
- 55.Ge Y., Caufield P.W., Fisch G.S., Li Y. Streptococcus mutans and Streptococcus sanguinis colonization correlated with caries experience in children. Caries Res. 2008;42(6):444–448. doi: 10.1159/000159608. Epub 2008 Oct 3. PMID: 18832831; PMCID: PMC2680676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J., Wu C., Huang I.H., Merritt J., Qi F. Differential response of Streptococcus mutans towards friend and foe in mixed-species cultures. Microbiology (Reading) 2011;157(September (Pt 9)):2433–2444. doi: 10.1099/mic.0.048314-0. Epub. 2011 May 12. PMID: 21565931; PMCID: PMC3352174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burne R.A., Marquis R.E. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett. 2000;193(December (1)):1–6. doi: 10.1111/j.1574-6968.2000.tb09393.x. PMID: 11094270. [DOI] [PubMed] [Google Scholar]
- 58.Marchant S., Brailsford S.R., Twomey A.C. The predominant microflora of nursing caries lesions. Caries Res. 2001;35:397–406. doi: 10.1159/000047482. [DOI] [PubMed] [Google Scholar]
- 59.Agnello M., Marques J., Cen L., Mittermuller B., Huang A., Chaichanasakul Tran N. Microbiome associated with severe caries in Canadian first nations children. J Dent Res. 2017;96(November (12)):1378–1385. doi: 10.1177/0022034517718819. Epub 2017 Jul 14. PMID: 28709393; PMCID: PMC5652857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang S., Wang H., Ren B., Li X., Wang L., Zhou H. Drug resistance of oral bacteria to new antibacterial dental monomer dimethylaminohexadecyl methacrylate. Sci Rep. 2018;8(April (1)):5509. doi: 10.1038/s41598-018-23831-3. PMID: 29615732; PMCID: PMC5882658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S., Wang H., Ren B., Li H., Weir M.D., Zhou X. Do quaternary ammonium monomers induce drug resistance in cariogenic, endodontic and periodontal bacterial species? Dent Mater. 2017;33(October (10)):1127–1138. doi: 10.1016/j.dental.2017.07.001. Epub 2017 Jul 26. PMID: 28755761. [DOI] [PubMed] [Google Scholar]
- 62.Regis R.R., Della Vecchia M.P., Pizzolitto A.C., Compagnoni M.A., Souza P.P., de Souza R.F. Antimicrobial properties and cytotoxicity of an antimicrobial monomer for application in prosthodontics. J Prosthodont. 2012;21(June (4)):283–290. doi: 10.1111/j.1532-849X.2011.00815.x. Epub 2012 Feb 19. PMID: 22339776. [DOI] [PubMed] [Google Scholar]
- 63.Geurtsen W., Lehmann F., Spahl W., Leyhausen G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res. 1998;41(September (3)):474–480. doi: 10.1002/(sici)1097-4636(19980905)41:3<474::aid-jbm18>3.0.co;2-i. PMID: 9659618. [DOI] [PubMed] [Google Scholar]
- 64.Bhadila G., Wang X., Zhou W., Menon D., Melo M.A.S., Montaner S. Novel low-shrinkage-stress nanocomposite with remineralization and antibacterial abilities to protect marginal enamel under biofilm. J Dent. 2020;99(August):103406. doi: 10.1016/j.jdent.2020.103406. Epub 2020 Jun 8. PMID: 32526346. [DOI] [PubMed] [Google Scholar]
- 65.Mese A., Ea Palamara J., Bagheri R., Fani M., Burrow M.F. Fracture toughness of seven resin composites evaluated by three methods of mode I fracture toughness (KIc) Dent Mater J. 2016;35(December (6)):893–899. doi: 10.4012/dmj.2016-140. Epub 2016 Sep 29. PMID: 27680036. [DOI] [PubMed] [Google Scholar]
- 66.Rodrigues Junior S.A., Zanchi C.H., Carvalho R.V., Demarco F.F. Flexural strength and modulus of elasticity of different types of resin-based composites. Braz Oral Res. 2007;21(January–March (1)):16–21. doi: 10.1590/s1806-83242007000100003. PMID: 17384850. [DOI] [PubMed] [Google Scholar]
- 67.Isabel C.A.C., Dominguette A.A.S., Santos S.G., Ribeiro J.C., Moysés M.R. Rugosidade superficial de resina composta. Rev Gaúcha Odontol. 2016;64(March (1)):50–55. doi: 10.1590/1981-863720160001000072929. [DOI] [Google Scholar]
- 68.Ishihara K., Ueda T., Nakabayashi N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polym J. 1990;22:355–360. doi: 10.1295/polymj.22.355. [DOI] [Google Scholar]
- 69.An Y.H., Friedman R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res. 1998;43(Fall (3)):338–348. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. PMID: 9730073. [DOI] [PubMed] [Google Scholar]
- 70.Katsikogianni M., Missirlis Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater. 2004;8(December):37–57. doi: 10.22203/ecm.v008a05. PMID: 15593018. [DOI] [PubMed] [Google Scholar]
- 71.Ishihara K., Nomura H., Mihara T., Kurita K., Iwasaki Y., Nakabayashi N. Why do phospholipid polymers reduce protein adsorption? J Biomed Mater Res. 1998;39(February (2)):323–330. doi: 10.1002/(sici)1097-4636(199802)39:2<323::aid-jbm21>3.0.co;2-c. PMID: 9457564. [DOI] [PubMed] [Google Scholar]
- 72.Yamasaki A., Imamura Y., Kurita K., Iwasaki Y., Nakabayashi N., Ishihara K. Surface mobility of polymers having phosphorylcholine groups connected with various bridging units and their protein adsorption-resistance properties. Colloids Surf B Biointerfaces. 2003;28(1):53–62. doi: 10.1016/S0927-7765(02)00130-3. [DOI] [Google Scholar]
- 73.Goda T., Konno T., Takai M., Ishihara K. Photoinduced phospholipid polymer grafting on Parylene film: advanced lubrication and antibiofouling properties. Colloids Surf B Biointerfaces. 2007;54(January (1)):67–73. doi: 10.1016/j.colsurfb.2006.09.006. Epub 2006 Sep 14. PMID: 17137760. [DOI] [PubMed] [Google Scholar]
- 74.Namba N., Yoshida Y., Nagaoka N., Takashima S., Matsuura-Yoshimoto K., Maeda H. Antibacterial effect of bactericide immobilized in resin matrix. Dent Mater. 2009;25(April (4)):424–430. doi: 10.1016/j.dental.2008.08.012. Epub 2008 Nov 18. PMID: 19019421. [DOI] [PubMed] [Google Scholar]
- 75.Müller R., Eidt A., Hiller K.A., Katzur V., Subat M., Schweikl H. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 2009;30(October (28)):4921–4929. doi: 10.1016/j.biomaterials.2009.05.079. Epub 2009 Jul 9. PMID: 19545893. [DOI] [PubMed] [Google Scholar]
- 76.Li F., Weir M.D., Fouad A.F., Xu H.H. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent Mater. 2014;30(February (2)):182–191. doi: 10.1016/j.dental.2013.11.004. Epub 2013 Dec 11. PMID: 24332270; PMCID: PMC4023513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imazato S., Ebi N., Takahashi Y., Kaneko T., Ebisu S., Russell R.R. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials. 2003;24(September (20)):3605–3609. doi: 10.1016/s0142-9612(03)00217-5. PMID: 12809790. [DOI] [PubMed] [Google Scholar]
- 78.Xu H.H., Moreau J.L., Sun L., Chow L.C. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011;27(August (8)):762–769. doi: 10.1016/j.dental.2011.03.016. Epub 2011 Apr 22. PMID: 21514655; PMCID: PMC3125490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moreau J.L., Sun L., Chow L.C., Xu H.H. Mechanical and acid neutralizing properties and bacteria inhibition of amorphous calcium phosphate dental nanocomposite. J Biomed Mater Res B Appl Biomater. 2011;98(July (1)):80–88. doi: 10.1002/jbm.b.31834. Epub 2011 Apr 18. PMID: 21504057; PMCID: PMC3375606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin I.X., Zhang J., Zhao I.S., Mei M.L., Li Q., Chu C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int J Nanomed. 2020;15(April):2555–2562. doi: 10.2147/IJN.S246764. PMID: 32368040; PMCID: PMC7174845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stanislawczuk R., Pereira F., Muñoz M.A., Luque I., Farago P.V., Reis A. Effects of chlorhexidine-containing adhesives on the durability of resin-dentine interfaces. J Dent. 2014;42(January (1)):39–47. doi: 10.1016/j.jdent.2013.11.002. Epub 2013 Nov 16. PMID: 24252801. [DOI] [PubMed] [Google Scholar]
- 82.Kim D.S., Kim J., Choi K.K., Kim S.Y. The influence of chlorhexidine on the remineralization of demineralized dentine. J Dent. 2011;39(December (12)):855–862. doi: 10.1016/j.jdent.2011.09.010. Epub 2011 Oct 7. PMID: 22001065. [DOI] [PubMed] [Google Scholar]
- 83.Lindblad R.M., Lassila L.V., Salo V., Vallittu P.K., Tjäderhane L. One year effect of chlorhexidine on bonding of fibre-reinforced composite root canal post to dentine. J Dent. 2012;40(September (9)):718–722. doi: 10.1016/j.jdent.2012.05.002. Epub 2012 May 11. PMID: 22580353. [DOI] [PubMed] [Google Scholar]
- 84.Riggs P.D., Braden M., Patel M. Chlorhexidine release from room temperature polymerising methacrylate systems. Biomaterials. 2000;21(February (4)):345–351. doi: 10.1016/s0142-9612(99)00187-8. PMID: 10656315. [DOI] [PubMed] [Google Scholar]
- 85.Dholam K.P., Somani P.P., Prabhu S.D., Ambre S.R. Effectiveness of fluoride varnish application as cariostatic and desensitizing agent in irradiated head and neck cancer patients. Int J Dent. 2013;2013:824982. doi: 10.1155/2013/824982. Epub 2013 Jun 13. PMID: 23843793; PMCID: PMC3697298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Recommendations for using fluoride to prevent and control dental caries in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2001;50(Aug (RR-14)):1–42. PMID: 11521913. [PubMed] [Google Scholar]
- 87.Santos Lde M., Reis J.I., Medeiros M.P., Ramos S.M., Araújo J.M. In vitro evaluation of fluoride products in the development of carious lesions in deciduous teeth. Braz Oral Res. 2009;23(July–September (3)):296–301. doi: 10.1590/s1806-83242009000300012. PMID: 19893965. [DOI] [PubMed] [Google Scholar]
- 88.Dziedzic A., Wojtyczka R.D., Kubina R. Inhibition of oral streptococci growth induced by the complementary action of berberine chloride and antibacterial compounds. Molecules. 2015;20(July (8)):13705–13724. doi: 10.3390/molecules200813705. PMID: 26225951; PMCID: PMC6332409. [DOI] [PMC free article] [PubMed] [Google Scholar]