Abstract
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), the responsible agent for the coronavirus disease 2019 (Covid-19), has its entry point through interaction with angiotensin converting enzyme 2 (ACE2) receptors, highly expressed in lung type II alveolar cells and other tissues, like heart, pancreas, brain, and vascular endothelium. This review aimed to elucidate the potential role of leukotrienes (LTs) in the pathogenesis and clinical presentation of SARS-CoV-2 infection, and to reveal the critical role of LT pathway receptor antagonists and inhibitors in Covid-19 management. A literature search was done in PubMed, Scopus, Web of Science and Google Scholar databases to find the potential role of montelukast and other LT inhibitors in the management of pulmonary and extra-pulmonary manifestations triggered by SARS-CoV-2. Data obtained so far underline that pulmonary and extra-pulmonary manifestations in Covid-19 are attributed to a direct effect of SARS-CoV-2 in expressed ACE2 receptors or indirectly through NF-κB dependent induction of a cytokine storm. Montelukast can ameliorate extra-pulmonary manifestations in Covid-19 either directly through blocking of Cys-LTRs in different organs or indirectly through inhibition of the NF-κB signaling pathway.
Keywords: SARS-CoV-2, Leukotriene, Montelukast, Extrapulmonary manifestations
1. Introduction
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) is the responsible infectious agent for the coronavirus disease 2019 (Covid-19), whose entry-point is through interaction with angiotensin converting enzyme 2 (ACE2) receptors, highly expressed in lung type II alveolar cells, but also in heart, pancreas, brain, vascular endothelium and testis (Al-Kuraishy et al., 2020a). In addition, the SARS-CoV-2 spike protein, furin and cell transmembrane serine protease 2 facilitate the entry to the target cells (Al-Kuraishy et al., 2020b).
In lungs, the SARS-CoV-2 binding to the ACE2 receptors expressed in alveolar cells, airway epithelial cells, macrophages and endothelial cells triggers down-regulation of these receptors with consequent deregulation of the renin-angiotensin system (RAS) (Mascolo et al., 2020). Then, dysfunction of RAS with high angiotensin II is associated with acute lung injury (ALI) through augmentation of inflammatory changes and vascular permeability in the lung (Al-Kuraishy et al., 2020c). Together, SARS-CoV-2 and injured lung cells activate a local immune response, recruiting monocytes and macrophages to the site of infection provoking adaptive B and T cells immune response to resolve infection (Lugnier et al., 2021). However, an abnormal immune response and high viral replication may cause pyroptosis (inflammatory programmed cell death) of lung cell and a systemic disease. Thereby, pyroptosis activates more inflammatory reactions via induction of interleukin (IL)-1β release (Freeman et al., 2020). In addition, severe SARS-CoV-2 and abnormal immune response may propagate to induce the development of cytokine storm (CS) (Al-Kuraishy et al., 2021).
At molecular level, SARS-CoV-2 infection is closely similar to that of SARS-CoV, characterized by a strong inflammatory response causing airway damage, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (Cadegiani et al., 2020). However, the disease severity is not only linked to the viral infection but to an exaggerated immune response, similarly to that stated in previous SARS-CoV and Middle East Respiratory Syndrome coronavirus (Zou et al., 2020). Indeed, it has been shown that ARDS is associated with death in 70% of Covid-19 cases, while CS and secondary bacterial co-infections lead to 28%, due to development of multi-organ failure (Khan et al., 2020). Moreover, pulmonary SARS-CoV-2 infection stimulates mucus secretion in acute infection and interstitial pulmonary fibrosis in chronic infection due to activation of mast cells and release of pro-inflammatory cytokines (Al-kuraishy et al., 2020d). Besides, the recovered Covid-19 patients may develop interstitial pulmonary fibrosis (Wang et al., 2020). In fact, it has been shown that activation of the leukotriene (LT) pathway is linked Covid-19 severity (Funk et al., 2020). In this sense, this review aims to elucidate the potential role of LTs in the pathogenesis and clinical presentation of SARS-CoV-2 infection, and to clarify the critical role of LT pathway antagonists or inhibitors in the management of Covid-19.
2. Method and search strategy
A literature search was done on PubMed, Scopus, Web of Science and Google Scholar databases by using string keywords, including “SARS-CoV-2 OR Covid-19” AND “acute respiratory syndrome OR acute lung injury”, “SARS-CoV-2 OR Covid-19” AND “leukotriene pathway OR leukotriene synthesis”, “SARS-CoV-2 OR Covid-19” AND “leukotriene receptor antagonist OR leukotriene synthesis inhibitors”, “SARS-CoV-2 OR Covid-19” AND “acute kidney injury OR brain injury OR cardiac injury”, “SARS-CoV-2 OR Covid-19” AND “pulmonary manifestations OR extra-pulmonary manifestations”, “leukotriene receptor antagonist OR leukotriene synthesis inhibitors” AND “pulmonary manifestations OR extra-pulmonary manifestations”. The literature search was done following the guidelines for systematic review, and was done independently by all authors through searching the titles and abstracts of retrieved articles. All published and pre-printed studies were included in this study without applying any language restriction. Following preliminary search and screening, the selected articles were tested for eligibility and summarized in a mini-review.
3. Leukotriene pathway
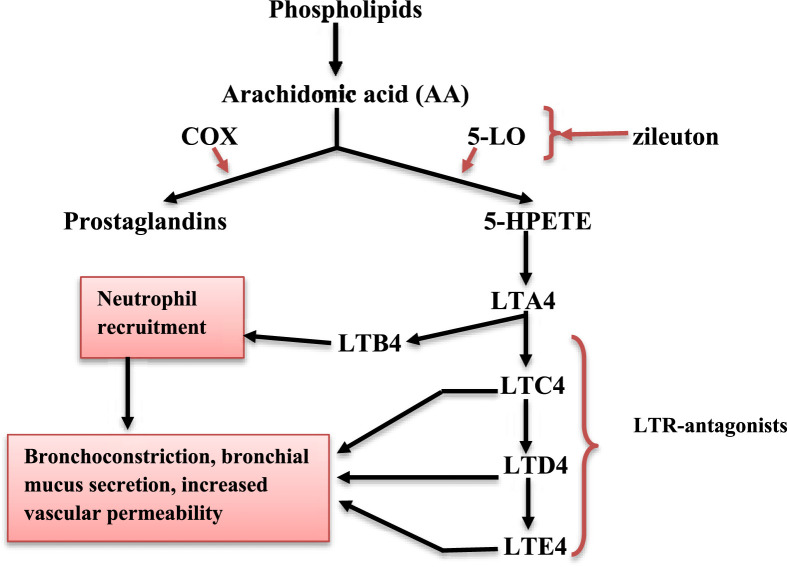
LTs are synthesized by 5-lipoxygenase from arachidonic acid (AA) in immune-competent cells, including mast cells, neutrophils, eosinophils, basophils and monocytes (Gelfand et al., 2017). The activation of these cells leads to AA release from cell membrane phospholipid by action of phospholipase A2. Then, AA is converted by two pathways into prostaglandin via cyclooxygenase, and 5-hydroperoxyeicosatetraenoic acid via 5-lipoxygenase (5-LO). 5-hydroperoxyeicosatetraenoic acid is then converted to leukotriene A4 (LTA4) (Fig. 1 ) (Gautier-Veyret et al., 2018). In monocytes and neutrophils, LTA4 is converted to leukotriene B4 (LTB4) by LTA4 hydrolase. LTB4 is involved in the recruitment of neutrophils and production of inflammatory cytokines from immune cells. LTB4 antagonists may reduce neutrophil-induced inflammatory disorders. Alternatively, LTA4 is converted to leukotriene C4 (LTC4) through the action of LTC4 synthase which is highly expressed in eosinophil and mast cells (Fig. 1). Outside the cell membrane, LTC4 is converted to leukotriene D4 (LTD4) and leukotriene E4 (LTE4). LTC4, LTD4 and LTE4 are named cysteinyl-leukotrienes (Cys-LTs). They act on specific receptors (Cys-LTR1 and Cys-LTR2) leading to bronchoconstriction, bronchial mucus secretion and increasing the permeability of vascular endothelium. In addition to the G-protein coupled LT receptors mentioned above, some LTs may also activate peroxisome proliferator activated nuclear receptors (Göbel et al., 2019).
Fig. 1.
Leukotriene pathway. COX: cyclooxygenase, 5-LO: 5-lipoxygenase, 5-HPETE: 5-hydroperoxyeicosatetraenoic acid, LTA4: leukotriene A4, LTB4: leukotriene B4, LTC4: leukotriene C4, LTD4: leukotriene D4, LTE4: leukotriene E4.
4. Montelukast and pulmonary manifestations of Covid-19
Cys-LTs are mainly involved in several respiratory illnesses, including allergic bronchitis and asthma. LTs are widely formed in inflammatory and immune cells, notably monocytes, macrophages, basophils, eosinophil, dendritic cells, mast cells, T and B cells as well as platelets and endothelial cells (Zhang et al., 2004). Specifically, Cys-LTR1 is mainly expressed in the neutrophils and to a lesser extent in lung, liver and brain, while Cys-LTR2 is chiefly expressed in neutrophils and brain. LTE4 receptor is mainly found in lung epithelial cells involved in mucosal swelling and mucin production. Cys-LTs also act on P2Y12 receptors, widely found in platelets (Yokomizo et al., 2018).
As stated above, LTs play a critical role in the pathogenesis of ALI, ARDS and other respiratory disorders, like asthma and pulmonary fibrosis by modulating cell and molecular responses (Jo-Watanabe et al., 2019). Particularly, the role of LTs in Covid-19 is not well-elucidated; however, it seems that LTs are involved in the pathogenesis of viral pneumonia, ALI and ARDS that are common manifestations in acute SARS-CoV-2 infections (Lazarinis et al., 2018; Davino-Chiovatto et al., 2019). Also, LTs augment the recruitment of innate immune response cells, such as neutrophils and macrophages, which consequently improve the ability of immune system to eradicate microbial pathogens through modulation and boosting of cytokine release in a paracrine and autocrine manner (Le Bel et al., 2014). In previous SARS-CoV outbreaks, different pathological analysis confirmed that LTs are intricate in the pathogenesis of alveolar damage, pneumocyte hyperplasia and interstitial fibrosis in dying patients (Nicholls et al., 2003). Specifically, in SARS-CoV-2 infection, the formation of LTs is evident by high neutrophil infiltration, and microangiopathy in the lung with high blood neutrophil-lymphocyte ratios which is a risk factor associated with Covid-19 severity (Schwerd et al., 2017; Kong et al., 2020). In addition, activation of LTs in SARS-CoV-2 infections is linked to a high level of pro-inflammatory cytokines and poor clinical outcomes in patients with severe Covid-19. Thereby, the inhibition of LT pathway may mitigate immune response, pulmonary inflammation and Covid-19 severity (Copertino et al., 2021). Hence, Cys-LTR1 antagonists, like montelukast and zafirlukast, able to block the synthesis and release of pro-inflammatory cytokines and production of reactive oxygen species via inhibition of nuclear factor kappa-B (NF-κB) and mitogen activated protein kinase (MAPK) P38 of activated macrophages may reduce the likelihood of develop different inflammatory disorders (Citron et al., 2020). Thereby, Cys-LTR1 antagonists may reduce SARS-CoV-2 infections-induced pulmonary hyper-inflammation. Also, Cys-LTR1 antagonists may have antiviral effects. For example, Chen et al. (2019) illustrated that montelukast inhibits both proliferation and infectivity of Zika virus and other enveloped RNA viruses, despite revealed to be ineffective for non-enveloped virus, like RNA enterovirus 71. A molecular docking study illustrated that SARS-CoV-2 membrane protein protease is essential for viral pathogenesis. Montelukast inhibits proliferation of SARS-CoV-2 through inhibition of a membrane protein protease, thereby reducing the SARS-CoV-2 viral load and infectivity (Wu et al., 2020). Therefore, using Cys-LTR1 antagonists is linked to a reduction in viral infectivity and pro-inflammatory cytokines-induced ALI (Davino-Chiovatto et al., 2019). At present, there is not sufficient data supporting the effect of Cys-LTR1 antagonists in Covid-19-induced ALI.
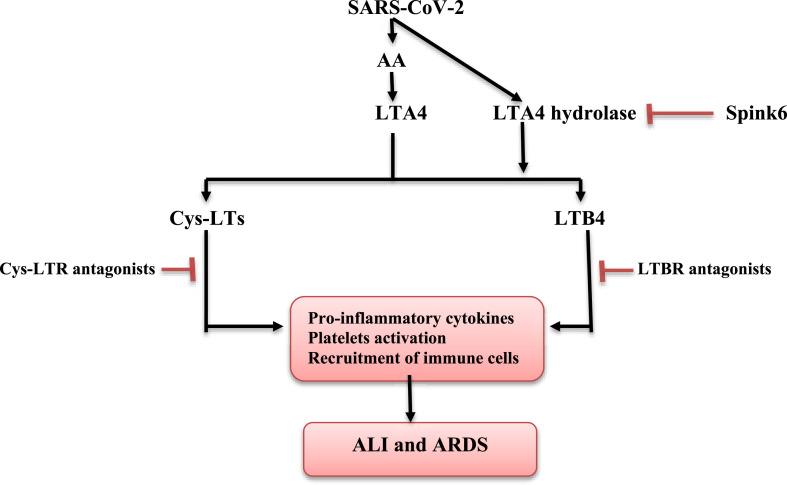
It has been increasingly evident that SARS-CoV-2 infection is linked to platelet activation and risk of thrombosis. Indeed, activated platelets contribute to lung inflammation through release of inflammatory molecules and recruitment of immune cells that together increase the vascular permeability and trigger the development of interstitial pulmonary edema (Zaid et al., 2020). Likewise, Zhang et al. (2020) illustrated that SARS-CoV-2 leads to direct activation of platelet through platelet ACE2 binding with subsequent raise of immune hyperactivation and thrombosis. Diverse studies confirmed that LT activate platelet through platelets Cys-LTR1 and Cys-LTR2 receptors leading to upregulation of P-selectin, which is involved in neutrophil recruitment, chemokines and thromboxane A2 release (Cummings et al., 2013; Liu et al., 2019). Thus, LT antagonists, such as montelukast and zafirlukast or inhibition of LT biosynthesis by zileuton may reduce the hyperinflammatory response, lung inflammation and thrombotic complications in Covid-19. However, the potential effect of zileuton might be more effective due to suppression of neutrophil recruitment (Chauhan et al., 2020). Moreover, LTA4 hydrolase serum level is elevated while Spink6 (an endogenous inhibitor of LTA4 hydrolase) serum level is reduced in patients with severe Covid-19 at intensive care unit. This effect is due to stimulation of LTA4 hydrolase by SARS-CoV-2 spike protein. Therefore, inhibition of LTA4 hydrolase or activation of Spink6 may be viewed as a targeting therapy limiting LTB4 biosynthesis and overactivation in SARS-CoV-2 infection (Vorobjeva et al., 2021). For instance, YC Li et al. (2020) stated that LTA4 hydrolase inhibitors, such as compound 26 and hydroxamic acid, alleviate ALI and pulmonary fibrosis through reduction of LTB4 biosynthesis. Taken together, data obtained in these studies suggest that SARS-CoV-2 infection boosts ALI and ARDS partly through LT pathway activation (Fig. 2 ).
Fig. 2.
The interaction between SARS-CoV-2 and leukotriene pathway in Covid-19. AA: Arachidonic acid; ALI: acute lung injury; ARDS: acute respiratory distress syndrome; Cys-LTs: cysteinyl-leukotrienes; Cys-LTR: cysteinyl-leukotriene receptor; LTBR: leukotriene B receptor.
5. Montelukast and extra-pulmonary manifestations of Covid-19
Besides pulmonary manifestations, acute SARS-CoV-2 infection may lead to extra-pulmonary manifestations, like acute brain injury and cardiovascular complications. Indeed, SARS-CoV-2 binds to neuronal ACE2 receptors which are expressed in both neurons and glial cells. In brain, SARS-CoV-2 infection may lead to encephalitis and damage to the respiratory center with development of respiratory failure (X Li et al., 2020). Amid a large body of evidence, montelukast has also shown neuroprotective effects, being able to restore blood brain barrier, induce neurogenesis, reduce neuroinflammations, improve neurological functions and renovate neuronal conductivity in different neurological disorders (Mansour et al., 2018; Gelosa et al., 2019). Therefore, montelukast may attenuate SARS-CoV-2-induced acute brain injury and associated neuroinflammations. In Covid-19 cases, SARS-CoV-2 also binds to endothelial and cardiomyocyte ACE2 receptor leading to endothelial dysfunction and acute cardiac injury. Also, SARS-CoV-2 infection-induced hypercytokinemia and CS may contribute to cardiovascular complications and acute cardiac injury (Annamaria et al., 2020). Montelukast attenuates endothelial dysfunction by inhibiting the expression of adhesion molecules (Rundell et al., 2010). In addition, montelukast reduces acute cardiomyocyte injury through its anti-inflammatory and antioxidant effects (Khodir et al., 2016). Previously, Gonca (2013) showed that zileuton reduces cardiac ischemic/reperfusion-induced arrhythmias. Similarly, montelukast has a cardioprotective effect against doxorubicin-induced cardiotoxicity (Hafez and Hassanein, 2020). Thus, anti-inflammatory and cardioprotective effects of montelukast may reduce the risk of cardiovascular complications in Covid-19.
Likewise, SARS-CoV-2 infection is associated with acute kidney injury (AKI) through direct binding to renal ACE2, expressed in larger amounts than lung (Joseph et al., 2020). Kidney ACE2 is co-localized with ACE and expressed mainly on the brush border of proximal renal tubules and to lesser extent on podocytes (Ali et al., 2018). At first, SARS-CoV-2 invades podocytes and then enters to the tubular fluid to bind ACE2 at the apical side of proximal renal tubules (Trimarchi, 2020). Moreover, transmembrane serine protease 2 is highly expressed in distal nephrons and involved in activating and facilitating SARS-CoV-2 entry. Replication of SARS-CoV-2 within the podocytes initiates inflammatory changes and cellular damage leading to proteinuria (Chen et al., 2020). However, Covid-19-induced CS and microangiopathy could contribute into podocyte damage, collapsing glomerulopathy and induction of AKI. Besides, high angiotensin II levels due to dysregulation of RAS in SARS-CoV-2 infection may cause AKI (Bhaskar et al., 2020). On the other hand, it has been shown that activation of the LT pathway, both 5-LO and Cys-LTs, is evident and mediate cisplatin-induced nephrotoxicity through induction of inflammation and apoptosis. Thus, montelukast and zileuton mitigate nephrotoxicity and AKI (Malek and Nematbakhsh, 2015). Similarly, montelukast reverses doxorubicin-induced AKI and ischemic reperfusion renal injury through modulation of neutrophil recruitment and oxidative stress (Kose et al., 2019, Şener et al., 2006). Barré and his colleagues (2020) reported that montelukast may play a key role in Covid-19 by mitigating ischemic/reperfusion injury, CS, endothelitis, vascular inflammation, endothelial dysfunction and oxidative stress, as all of these pathophysiological changes are involved in the development and progression of AKI (Kim et al., 2017).
Different studies have also shown that activation of NF-κB signaling pathway is associated with the development of AKI. Thereby, the inhibition of NF-κB signaling pathway may attenuate the development of AKI (Zhang et al., 2020; Huang et al., 2017). Bapputty et al. (2018) illustrated that montelukast has potent anti-inflammatory effects, being able to alleviate diabetic retinopathy through suppression of NF-κB signaling. In Covid-19, NF-κB signaling pathway is overactivated by SARS-CoV-2 viroporins in different organs, including kidney. As a consequence, the activated NF-κB signaling pathway provokes release of pro-inflammatory cytokines and chemokines with induction of CS-induced multi-organ failure (Hariharan et al., 2020). A systematic review including 17,391 patients with severe Covid-19 showed that 11% of them have AKI due to activation of the NF-κB signaling pathway (Kunutsor and Laukkanen, 2020). Activation of renal NF-κB signaling also appeared to be due to high circulating angiotensin II and TNF-α serum levels in Covid-19. So, stimulated NF-κB signaling is associated with the development of AKI through initiation of inflammatory-induced proximal renal tubules necrosis and thrombotic microangiopathy (Hirsch et al., 2020). Hence, montelukast via inhibition of the NF-κB signaling pathway reduces the release of TNF-α and IL-6 and associated systemic inflammatory changes.
Also noteworthy, SARS-CoV-2 infection may affect gastrointestinal tract (GIT) since this virus can be transmitted through feces by inhalation of infected droplet. However, GIT manifestations of SARS-CoV-2 are not correlated with Covid-19-induced ALI and some patients with positive stool test for SARS-CoV-2 did not have GIT symptoms (Gu et al., 2020). Among GIT manifestations, the most common in Covid-19 are diarrhea (10%), nausea and vomiting (3%), and abdominal pain (5%); however, these symptoms are increased in cases of high viral load (Kopel et al., 2020). The pathogenesis of SARS-CoV-2 enteropathy is due to its binding to ACE2 of enterocytes, and in the same way SARS-CoV-2 can be transmitted to other organs of GIT, like liver (Mandal et al., 2020). A meta-analysis study by Cheung et al. (2020) illustrated that 17.6% of Covid-19 patient's present GIT symptoms, with the SARS-CoV-2 RNA being detected in 48.1% of stools samples, following a negative test of respiratory samples. Other studies illustrated that Covid-19-induced CS may be linked with GIT manifestations, as high levels of pro-inflammatory cytokines are associated with disturbances in the contraction of intestinal smooth muscles through up-regulation of G protein-coupled receptors and down-regulation of L-Ca2+ channels (Russell et al., 2020; Samanta et al., 2020). At present, apart from antiviral agents used in Covid-19 and symptomatic therapy, there are no effective therapy against SARS-CoV-2-induced GIT manifestations. Moreover, it has been reported that Cys-LT antagonists have anti-inflammatory and anti-emetic effect, mainly zafirlukast, montelukast and pranlukast that are effective against chemotherapy-induced vomiting (Darmani et al., 2017). De Maeyer et al. (2011) demonstrated that montelukast alleviates intestinal inflammation induced by eosinophils in eosinophilic gastroenteritis. Also, both 5-LO and Cys-LT play a crucial role in the pathophysiology of intestinal ischemic reperfusion injury, thereby montelukast and other Cys-LT inhibitors attenuate intestinal ischemic reperfusion injury and gastric mucosal damage (Wu et al., 2015). Recently, Marian et al. (2019) revealed that montelukast has secondary anti-inflammatory effects independent of Cys-LTR antagonist by inhibiting histone acetyltransferase, phosphodiesterase and purinergic receptors. Nonetheless, the intestinal LTB4 pathway has a protective effect against intestinal injury; briefly, it improves intestinal healing by activating epithelial cells (Matsumoto et al., 2020). In Covid-19, AA release with subsequent formation of LTs in various tissues leads to induction of inflammatory reactions (Hoxha, 2020). Hence, montelukast may reduce Covid-19-induced GIT disorders through modulation of Cys-LT formation in enterocytes during SARS-CoV-2 infections.
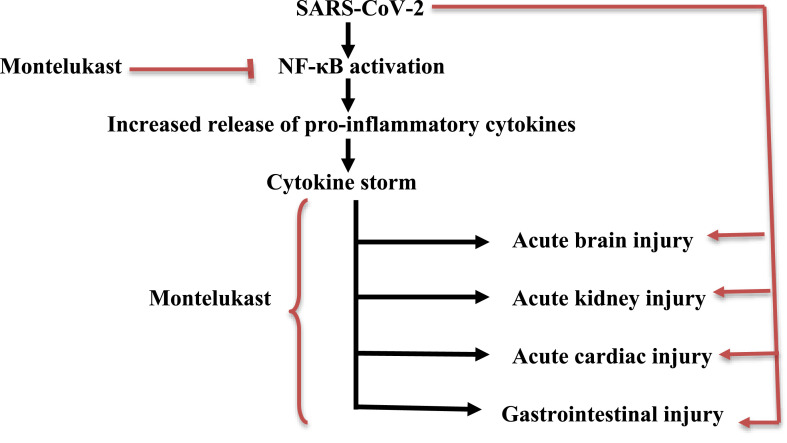
In summary, the extra-pulmonary manifestations in Covid-19 appear to be due to the direct effect of SARS-CoV-2 on ACE2 or indirectly through induction of NF-κB-dependent cytokine storm. Regarding LT inhibitors, montelukast is able to ameliorate the extra-pulmonary manifestations in Covid-19 patients, either directly by blocking Cys-LTs in different organs or indirectly by inhibiting the NF-κB signaling pathway (Fig. 3 ).
Fig. 3.
Role of the Cys-LTR antagonist montelukast in the mitigation of extrapulmonary manifestations in Covid-19.
6. Conclusion
In summary, this review sheds light on the potential role of the LT pathway and Cys-LTR antagonists on the pulmonary and extra-pulmonary manifestations in Covid-19. Montelukast may attenuate pulmonary and extra-pulmonary manifestations in Covid-19 by antagonizing Cys-LTRs and the NF-κB signaling pathway, and thus exert wide-spread mitigation of SARS-CoV-2 infection and associated complications. These observations mandate further clinical trials and prospective studies to confirm the protective effect of montelukast and 5-LO inhibitors in the management of Covid-19.
Funding and sponsorship
None.
Declaration of competing interest
None.
Acknowledgements
N.C.-M. acknowledges the Portuguese Foundation for Science and Technology under the Horizon 2020 Program (PTDC/PSI-GER/28076/2017).
References
- Al-Kuraishy H.M., Al-Naimi M.S., Lungnier C.M., Al-Gareeb A.I. Macrolides and COVID-19: an optimum premise. Biomedical and Biotechnology Research Journal (BBRJ) 2020;4(3):189–197. 1. [Google Scholar]
- Al-Kuraishy H.M., Hussien N.R., Al-Naimi M.S., Al-Buhadily A.K., Al-Gareeb A.I., Lungnier C. Renin–Angiotensin system and fibrinolytic pathway in COVID-19: one-way skepticism. Biomedical and Biotechnology Research Journal (BBRJ) 2020;4(5):33–39. [Google Scholar]
- Al-Kuraishy H.M., Al-Niemi M.S., Hussain N.R., Al-Gareeb A.I., Al-Harchan N.A., Al-Kurashi A.H. In: Kibel A., editor. vol. 2. IntechOpen; London: 2020. The potential role of renin angiotensin system (RAS) and dipeptidyl peptidase-4 (DPP-4) in COVID-19: navigating the uncharted; pp. 151–165. (Selected Chapters from the Reninangiotensin System). [Google Scholar]
- Al-kuraishy H.M., Al-Maiahy T.J., Al-Gareeb A.I., Musa R.A., Ali Z.H. COVID-19 pneumonia in an Iraqi pregnant woman with preterm delivery. Asian Pacific Journal of Reproduction. 2020;9(3):156. [Google Scholar]
- Al-Kuraishy H.M., Al-Gareeb A.I., Cruz-Martins N., Batiha G.E. Hyperbilirubinemia in Gilbert syndrome attenuates Covid-19 induced-metabolic disturbances: a case-report study. Frontiers in Cardiovascular Medicine. 2021;8:71–75. doi: 10.3389/fcvm.2021.642181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali R.M., Al-Shorbagy M.Y., Helmy M.W., El-Abhar H.S. Role of Wnt4/β-catenin, Ang II/TGFβ, ACE2, NF-κB, and IL-18 in attenuating renal ischemia/reperfusion-induced injury in rats treated with Vit D and pioglitazone. Eur. J. Pharmacol. 2018;831:68–76. doi: 10.1016/j.ejphar.2018.04.032. [DOI] [PubMed] [Google Scholar]
- Annamaria V., Rosetta R., Chiara C., Giuseppina B. COVID-19 and cardiovascular consequences: is the endothelial dysfunction the hardest challenge? Thromb. Res. 2020;196:143–151. doi: 10.1016/j.thromres.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapputty R.M., Talahalli R., Gubitosi-Klug R.A. Montelukast modulates the NF-kB inflammatory cascade in mouse retinal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2018;59(9):190–198. [Google Scholar]
- Barré J., Sabatier J.M., Annweiler C. Montelukast drug may improve COVID-19 prognosis: a review of evidence. Front. Pharmacol. 2020;11:1344. doi: 10.3389/fphar.2020.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar S., Sinha A., Banach M., Mittoo S., Weissert R., Kass J.S., Rajagopal S., Pai A.R., Kutty S. Cytokine storm in COVID-19 - immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front. Immunol. 2020;11:1648–1661. doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadegiani F.A., Wambier C.G., Goren A. Spironolactone: an anti-androgenic and anti-hypertensive drug that may provide protection against the novel coronavirus (SARS-CoV-2) induced acute respiratory distress syndrome (ARDS) in COVID-19. Front. Med. 2020;28(7):453. doi: 10.3389/fmed.2020.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A.J., Wiffen L.J., Brown T.P. COVID‐19: a collision of complement, coagulation and inflammatory pathways. J. Thromb. Haemostasis. 2020;18(9):2110–2117. doi: 10.1111/jth.14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li Y., Wang X., Zou P. Montelukast, an anti-asthmatic drug, inhibits zika virus infection by disrupting viral integrity. Front. Microbiol. 2019;10:3079. doi: 10.3389/fmicb.2019.03079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.L., Li J.Q., Xiang Z.D., Lang Y., Guo G.J., Liu Z.H. Localization of cell receptor-related genes of SARS-CoV-2 in the kidney through single-cell transcriptome analysis. Kidney Dis. 2020;6(4):258–270. doi: 10.1159/000508162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K.S., Hung I.F., Chan P.P., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T.W., Tam A.R., Yip C.C. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron F., Perelli L., Deem A.K., Genovese G., Viale A. Prostaglandins, leukotrienes, and essential fatty acids; 2020 Oct 1. Leukotrienes, a Potential Target for Covid-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copertino D.C., Duarte R.R., Powell T.R., de Mulder Rougvie M., Nixon D.F. Montelukast drug activity and potential against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) J. Med. Virol. 2021;93(1):187–189. doi: 10.1002/jmv.26299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings H.E., Liu T., Feng C., Laidlaw T.M., Conley P.B., Kanaoka Y., Boyce J.A. Cutting edge: leukotriene C4 activates mouse platelets in plasma exclusively through the type 2 cysteinyl leukotriene receptor. J. Immunol. 2013;191(12):5807–5810. doi: 10.4049/jimmunol.1302187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani N.A., Chebolu S., Zhong W., Kim W.D., Narlesky M., Adams J., Dong F. The anti-asthmatic drug pranlukast suppresses the delayed-phase vomiting and reverses intracellular indices of emesis evoked by cisplatin in the least shrew (Cryptotis parva) Eur. J. Pharmacol. 2017;809:20–31. doi: 10.1016/j.ejphar.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Davino-Chiovatto J.E., Oliveira-Junior M.C., MacKenzie B., Santos-Dias A., Almeida-Oliveira A.R., Aquino-Junior J.C., Brito A.A., Rigonato-Oliveira N.C., Damaceno-Rodrigues N.R., Oliveira A.P., Silva A.P. Montelukast, leukotriene inhibitor, reduces LPS-induced acute lung inflammation and human neutrophil activation. Arch. Bronconeumol. 2019;55(11):573–580. doi: 10.1016/j.arbres.2019.05.003. [DOI] [PubMed] [Google Scholar]
- De Maeyer N., Kochuyt A.M., Van Moerkercke W., Hiele M. Montelukast as a treatment modality for eosinophilic gastroenteritis. Acta Gastro-Enterol. Belg. 2011;74(4):570–575. [PubMed] [Google Scholar]
- Freeman T.L., Swartz T.H. Targeting the NLRP3 inflammasome in severe COVID-19. Front. Immunol. 2020;23(11):1518–1523. doi: 10.3389/fimmu.2020.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C.D., Ardakani A. A novel strategy to mitigate the hyperinflammatory response to COVID-19 by targeting leukotrienes. Front. Pharmacol. 2020;11:1214–1219. doi: 10.3389/fphar.2020.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier-Veyret E., Bäck M., Arnaud C., Belaïdi E., Tamisier R., Lévy P., Arnol N., Perrin M., Pépin J.L., Stanke-Labesque F. Cysteinyl-leukotriene pathway as a new therapeutic target for the treatment of atherosclerosis related to obstructive sleep apnea syndrome. Pharmacol. Res. 2018;134:311–319. doi: 10.1016/j.phrs.2018.06.014. [DOI] [PubMed] [Google Scholar]
- Gelfand E.W. Importance of the leukotriene B4-BLT1 and LTB4-BLT2 pathways in asthma. InSeminars in immunology. 2017 Oct 1;33:44–51. doi: 10.1016/j.smim.2017.08.005. Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelosa P., Bonfanti E., Castiglioni L., Delgado-Garcia J.M., Gruart A., Fontana L., Gotti M., Tremoli E., Lecca D., Fumagalli M., Cimino M. Improvement of fiber connectivity and functional recovery after stroke by montelukast, an available and safe anti-asthmatic drug. Pharmacol. Res. 2019;142:223–236. doi: 10.1016/j.phrs.2019.02.025. [DOI] [PubMed] [Google Scholar]
- Göbel T., Diehl O., Heering J., Merk D., Angioni C., Wittmann S.K., Buscato E., Kottke R., Weizel L., Schader T., Maier T.J. Zafirlukast is a dual modulator of human soluble epoxide hydrolase and peroxisome proliferator-activated receptor γ. Front. Pharmacol. 2019;10:263–269. doi: 10.3389/fphar.2019.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonca E. The effects of zileuton and montelukast in reperfusion-induced arrhythmias in anesthetized rats. Curr. Ther. Res. 2013;75:27–32. doi: 10.1016/j.curtheres.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez H.M., Hassanein H. Montelukast ameliorates doxorubicin-induced cardiotoxicity via modulation of p-glycoprotein and inhibition of ROS-mediated TNF-α/NF-κB pathways. Drug Chem. Toxicol. 2020;27:1–2. doi: 10.1080/01480545.2020.1730885. [DOI] [PubMed] [Google Scholar]
- Hariharan A., Hakeem A.R., Radhakrishnan S., Reddy M.S., Rela M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology. 2020;7 doi: 10.1007/s10787-020-00773-9. 1-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J.S., Ng J.H., Ross D.W., Sharma P., Shah H.H., Barnett R.L., Hazzan A.D., Fishbane S., Jhaveri K.D., Abate M., Andrade H.P. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxha M. What about COVID-19 and arachidonic acid pathway? Eur. J. Clin. Pharmacol. 2020;76(11):1501–1504. doi: 10.1007/s00228-020-02941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Ma L., Fu P. Mp239 effect of macrophage tlr4/nf-kb pathway on rhabodmyolysis-induced acute kidney injury. Nephrol. Dial. Transplant. 2017;32(3):iii514–i519. [Google Scholar]
- Jo-Watanabe A., Okuno T., Yokomizo T. The role of leukotrienes as potential therapeutic targets in allergic disorders. Int. J. Mol. Sci. 2019;20(14):3580–3586. doi: 10.3390/ijms20143580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A., Zafrani L., Mabrouki A., Azoulay E., Darmon M. Acute kidney injury in patients with SARS-CoV-2 infection. Ann. Intensive Care. 2020;10(1):1–8. doi: 10.1186/s13613-020-00734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan J.R., Awan N., Islam M., Muurlink O. Healthcare capacity, health expenditure, and civil society as predictors of COVID-19 case fatalities: a global analysis. Frontiers in public health. 2020;8:347–356. doi: 10.3389/fpubh.2020.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodir A.E., Ghoneim H.A., Rahim M.A., Suddek G.M. Montelukast attenuates lipopolysaccharide-induced cardiac injury in rats. Hum. Exp. Toxicol. 2016;35(4):388–397. doi: 10.1177/0960327115591372. [DOI] [PubMed] [Google Scholar]
- Kim H.Y., Yoo T.H., Hwang Y., Lee G.H., Kim B., Jang J., Yu H.T., Kim M.C., Cho J.Y., Lee C.J., Kim H.C. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD) Sci. Rep. 2017;7(1):1–6. doi: 10.1038/s41598-017-03130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M., Zhang H., Cao X., Mao X., Lu Z. Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiol. Infect. 2020;148:E139. doi: 10.1017/S0950268820001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopel J., Perisetti A., Gajendran M., Boregowda U., Goyal H. Digestive Diseases and Sciences; 2020 May 23. Clinical Insights into the Gastrointestinal Manifestations of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kose E., Oguz F., Vardi N., Sarihan M.E., Beytur A., Yucel A., Polat A., Eki̇nci̇ N. Therapeutic and protective effects of montelukast against doxorubicin-induced acute kidney damage in rats. Iranian journal of basic medical sciences. 2019;22(4):407–412. doi: 10.22038/ijbms.2019.33493.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunutsor S.K., Laukkanen J.A. Renal complications in COVID-19: a systematic review and meta-analysis. Ann. Med. 2020;52(7):345–353. doi: 10.1080/07853890.2020.1790643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarinis N., Bood J., Gomez C., Kolmert J., Lantz A.S., Gyllfors P., Davis A., Wheelock C.E., Dahlén S.E., Dahlén B. Leukotriene E4 induces airflow obstruction and mast cell activation through the cysteinyl leukotriene type 1 receptor. J. Allergy Clin. Immunol. 2018;142(4):1080–1089. doi: 10.1016/j.jaci.2018.02.024. [DOI] [PubMed] [Google Scholar]
- Le Bel M., Brunet A., Gosselin J. Leukotriene B4, an endogenous stimulator of the innate immune response against pathogens. Journal of innate immunity. 2014;6(2):159–168. doi: 10.1159/000353694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Xie M., Lu C., Mao J., Cao Y., Yang Y., Wei Y., Liu X., Cao S., Song Y., Peng J. Design and synthesis of Leukotriene A4 hydrolase inhibitors to alleviate idiopathic pulmonary fibrosis and acute lung injury. Eur. J. Med. Chem. 2020;203:112614–112619. doi: 10.1016/j.ejmech.2020.112614. [DOI] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J. Med. Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Barrett N.A., Kanaoka Y., Buchheit K., Laidlaw T.M., Garofalo D., Lai J., Katz H.R., Feng C., Boyce J.A. Cysteinyl leukotriene receptor 2 drives lung immunopathology through a platelet and high mobility box 1-dependent mechanism. Mucosal Immunol. 2019;12(3):679–690. doi: 10.1038/s41385-019-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugnier C., Al-Kuraishy H.M., Rousseau E. PDE4 inhibition as a therapeutic strategy for improvement of pulmonary dysfunctions in Covid-19 and cigarette smoking. Biochem. Pharmacol. 2021;28:114431–114438. doi: 10.1016/j.bcp.2021.114431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek M., Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Ren. Inj. Prev. 2015;4(2):20–25. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A., Konala V.M., Adapa S., Naramala S., Gayam V. Gastrointestinal manifestations in COVID-19 infection and its practical applications. Cureus. 2020;12(6):33–39. doi: 10.7759/cureus.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour R.M., Ahmed M.A., El-Sahar A.E., El Sayed N.S. Montelukast attenuates rotenone-induced microglial activation/p38 MAPK expression in rats: possible role of its antioxidant, anti-inflammatory and antiapoptotic effects. Toxicol. Appl. Pharmacol. 2018;358:76–85. doi: 10.1016/j.taap.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Marian Y.W., Ghada F.S., Morsi M. Montelukast efficiency in improving the deleterious gastrointestinal effects of dexamethasone in rats. Med. J. Cairo Univ. 2019;87:4303–4313. [Google Scholar]
- Mascolo A., Scavone C., Rafaniello C., Ferrajolo C., Racagni G., Berrino L., Paolisso G., Rossi F., Capuano A. Renin-angiotensin system and Coronavirus disease 2019: a narrative review. Frontiers in cardiovascular medicine. 2020;7 doi: 10.3389/fcvm.2020.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Matsuya Y., Nagai K., Amagase K., Saeki K., Matsumoto K., Yokomizo T., Kato S. Leukotriene B4 receptor type 2 accelerates the healing of intestinal lesions by promoting epithelial cell proliferation. J. Pharmacol. Exp. Therapeut. 2020;373(1):1–9. doi: 10.1124/jpet.119.263145. [DOI] [PubMed] [Google Scholar]
- Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W., Yan K.W. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361(9371):1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K.W., Steigerwald M.D., Fisk M.Z. Montelukast prevents vascular endothelial dysfunction from internal combustion exhaust inhalation during exercise. Inhal. Toxicol. 2010;22(9):754–759. doi: 10.3109/08958371003743254. [DOI] [PubMed] [Google Scholar]
- Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta J., Dhar J., Khaliq A., Kochhar R. 2019 novel coronavirus infection: gastrointestinal manifestations. J. Dig. Endosc. 2020;11(1):13–19. [Google Scholar]
- Schwerd T., Twigg S.R., Aschenbrenner D., Manrique S., Miller K.A., Taylor I.B., Capitani M., McGowan S.J., Sweeney E., Weber A., Chen L. A biallelic mutation in IL6ST encoding the GP130 co-receptor causes immunodeficiency and craniosynostosis. J. Exp. Med. 2017;214(9):2547–2562. doi: 10.1084/jem.20161810. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şener G., Ö Şehirli, Velioğlu-Öğünç A., Çetinel Ş., Gedik N., Caner M., Sakarcan A., Yeğen B.Ç. Montelukast protects against renal ischemia/reperfusion injury in rats. Pharmacol. Res. 2006;54(1):65–71. doi: 10.1016/j.phrs.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Trimarchi H. SARS-CoV-2 and Fabry nephropathy: potential risks and the pathophysiological perspective. Journal of Nephropathology. 2020;9(4):56–65. [Google Scholar]
- Vorobjeva N.V., Sud'ina G.F., Chernyak B.V. Mitochondria are potential targets for the development of new drugs against neutrophilic inflammation in severe pneumonia including COVID-19. Front. Pharmacol. 2021;(11):12–19. doi: 10.3389/fphar.2021.609508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang B.J., Yang J.C., Wang M.Y., Chen C., Luo G.X., He W.F. Advances in the research of mechanism of pulmonary fibrosis induced by Corona Virus Disease 2019 and the corresponding therapeutic measures. Zhonghua Shao Shang za zhi= Zhonghua Shaoshang Zazhi= Chinese Journal of Burns. 2020;16(36):E006–E011. doi: 10.3760/cma.j.cn501120-20200307-00132. [DOI] [PubMed] [Google Scholar]
- Wu S., Zhu X., Jin Z., Tong X., Zhu L., Hong X., Zhu X., Liu P., Shen W. The protective role of montelukast against intestinal ischemia-reperfusion injury in rats. Sci. Rep. 2015;5:15787. doi: 10.1038/srep15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo T., Nakamura M., Shimizu T. Leukotriene receptors as potential therapeutic targets. J. Clin. Invest. 2018;128(7):2691–2701. doi: 10.1172/JCI97946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid Y., Puhm F., Allaeys I., Naya A., Oudghiri M., Khalki L., Limami Y., Zaid N., Sadki K., Ben El Haj R., Mahir W. Platelets can associate with SARS-Cov-2 RNA and are hyperactivated in COVID-19. Circ. Res. 2020;127(11):1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.P., Hu H., Zhang L., Ding W., Yao H.T., Chen K.D., Sheng W.W., Chen Z., Wei E.Q. Expression of cysteinyl leukotriene receptor 1 in human traumatic brain injury and brain tumors. Neurosci. Lett. 2004;363(3):247–251. doi: 10.1016/j.neulet.2004.03.088. [DOI] [PubMed] [Google Scholar]
- Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., Liu M., Zhao X., Xie Y., Yang Y., Zhang S. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020;13(1):1–22. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Tang P.M., Niu Y., Córdoba G.A., Alexandra C., Huang X.R., Yu C., Lan H.Y. Long non-coding RNA LRNA9884 promotes acute kidney injury via regulating NF-kB-Mediated transcriptional activation of MIF. Front. Physiol. 2020;11:1399–1405. doi: 10.3389/fphys.2020.590027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Dai L., Zhang Y., Fu W., Gao Y., Zhang Z., Zhang Z. Clinical characteristics and risk factors for disease severity and death in patients with Coronavirus Disease 2019 in Wuhan, China. Front. Med. 2020;13(7):532–538. doi: 10.3389/fmed.2020.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]