INTRODUCTION
Historically, fungal infections were viewed as a relatively uncommon cause of clinically relevant disease compared with other bacterial and viral pathogens.1 This trend shifted in the second half of the 20th century, as the number of immunocompromised patients susceptible to opportunistic fungal infections increased, following advancements in medical treatment and the HIV/AIDS epidemic.1,2 Fungi previously assumed to be rare causes of infection, such as Cryptococcus species, emerged as substantial causes of invasive disease in hosts with impaired immunity.3 The emergence of these opportunistic fungal infections, which caused increasing morbidity and mortality, introduced notable diagnostic and therapeutic challenges in health care and resulted in increased epidemiologic attention on fungal diseases.4
Over the past decade, the variety of fungi identified causing human disease and the spectrum of clinical presentations associated with these infections has increased.5,6 With the evolution of antiretroviral therapy (ART), HIV-associated cases of cryptococcosis and other opportunistic fungal infections declined in North America, yet diseases caused by health care–associated fungal pathogens, including Candida species, Aspergillus species, and other molds, increased, to a large extent owing to substantial increases in at-risk populations.3,6-8 Although traditionally transmission between humans was rare, with only sporadic reports of fungal outbreaks, transmission of certain fungi between patients has increasingly been reported in clinical settings, causing numerous health care–associated outbreaks.2,9 Molds, including mucoromycetes, Fusarium species, Scedosporium species, and dimorphic fungi specific to certain geographic regions such as Blastomyces, Coccidioides, and Histoplasma, have also grown in importance.4,6 For some, environmental changes have contributed to geographic expansion.4,6,10 Furthermore, epidemiologic trends demonstrate dramatic increases in the incidence of resistant infections and emergence of novel multidrug-resistant fungi.9,11 The frequency of fungal infections continues to increase; according to some global estimates, more than 300 million people are affected by serious fungal disease each year.5 Worldwide, mortality estimates exceed 1.5 million deaths annually, with the death rate for certain invasive infections in some populations surpassing 50%.1,5,12 In the United States, more than 75,000 hospitalizations and nearly $7.2 billion in medical care costs were attributed to fungal infections in 2017.13
Many health care–related, environmental, and socioeconomic factors have influenced these recent epidemiologic shifts, introducing considerable challenges to decreasing disease burden.2,12,14 The expansion of prophylactic antifungal use has resulted in a decline in candidemia incidence within certain populations, yet such use also contributed to the growing threat of increasing resistance.15 Advances in health care practices and medical procedures have resulted in new risk factors and an overall increase in the number of susceptible hosts.6,14 For instance, a dramatic expansion in the use of immunosuppressive medications, chemotherapeutic regimens, and antibiotics have resulted in new and expanding patient populations at risk.2,4,14 Advances in therapeutics that have prolonged survival in patients with previously fatal conditions have allowed more time for infection by opportunistic pathogens.8 Furthermore, although some fungi are commensal organisms that live in the gastrointestinal tract and on the skin, the increased use of invasive medical devices and procedures (eg, catheters and hematopoietic transplantation modalities) provides additional opportunities for these fungi to reach tissues and blood and cause invasive disease.1,6,16 Increased global travel and environmental changes also interplay to increase the intensity of interactions between humans and the environment, ultimately extending geographic disease ranges.17-19 In recent years, major health events not typically associated with mycoses, such as seasonal influenza epidemics and the severe acute respiratory syndrome novel coronavirus pandemic, resulted in larger proportions of the population becoming critically ill and susceptible to secondary fungal infections.20
Early diagnosis, intervention, and appropriate antifungal treatment are the keys to decreasing the burden of fungal diseases.4,14 Yet, despite developments in diagnostic techniques, antifungal options are still limited, and morbidity and mortality remain high, while awareness remains low.2 Understanding, the epidemiology and emerging trends in fungal infections remains critical for prevention, diagnosis, management of care, and improvement in patient outcomes. In this review, we aim to summarize recent updates to the epidemiologic profiles of clinically significant fungal pathogens in North America (Table 1).
Table 1.
Summary of top trends in the epidemiology of clinically significant fungal pathogens in North America
| Fungal Pathogen | Top Epidemiologic Trends |
|---|---|
| Candida | Injection drug use has emerged as a risk factor and is becoming more common in the context of the opioid epidemic. Although C albicans remains the leading cause of invasive candidiasis, a growing proportion of diagnoses have been attributed C glabrata and C parapsilosis. C auris, a multidrug-resistant organism shown to cause outbreaks in clinical settings, has rapidly emerged since first identified in 2009. |
| Mold (Aspergillus and non-Aspergillus) | Influenza-associated pulmonary aspergillosis and coronavirus disease 2019–associated pulmonary aspergillosis are emerging coinfections. Azole-resistant strains of A fumigatus are increasing in prevalence. Reports point to a potential increase in non-Aspergillus invasive mold infections among patients with hematologic malignancies and transplant recipients. |
| Blastomyces | The US annual incidence remains consistent at 1–2 cases per 100,000 population. Recent cases have been reported in areas not known to be endemic. |
| Coccidioides | US cases have increased in recent years, reaching >15,000 in 2018. Recently reported cases beyond the traditional geographic areas indicate a northward expansion into Northern California, Utah, and Washington. |
| Histoplasma | Evidence suggests that the occurrence of histoplasmosis may extend beyond the already broadly defined historical region. Histoplasmosis-associated hospitalizations nearly doubled from 2001 to 2012. |
| Cryptococcus | As the rate of disease among people living with HIV has decreased, the rate among those living without HIV has not. Approximately 20% of non-HIV patients who develop Cryptococcus disease have no known immune impairments. Understanding of C gattii's geographic distribution expanded during an outbreak in Canada and a recent uptick in cases in the US Pacific Northwest. |
| Pneumocystis jirovecii | Among people living with HIV in the United States and Canada, Pneumocystis pneumonia is the most common opportunistic infection. Populations at risk have shifted toward HIV-uninfected immunocompromised groups owing to immunosuppressive regimens that weaken the immune system. |
| Sporothrix | The US incidence is estimated to be 2 cases per 1 million people, with the highest incidence in southern and south-central states. S brasiliensis recently emerged in South America and is associated with zoonotic transmission, spreading via animal scratches and bites. |
Candida
Candida bloodstream infection, known as candidemia, is the most common invasive Candida infection; at the genus level, Candida ranks as one of the most prevalent causes of health care–associated infections in North America.21,22 Data from a nationally representative US-based surveillance system indicated a decline in incidence from 2009 to 2013, which stabilized at approximately 9 cases per 100,000 population from 2013 to 2017.16,23 This decrease occurred primarily among patients with health care exposure, specifically those with central venous catheters and, therefore, may be related to increased infection control practices in catheter care.23 Of particular note, a large proportion of cases historically occurred in children less than 1 year of age, yet candidemia incidence among this age group sharply decreased from 2009 to 2012, likely because of increased prophylaxis and improved catheter-related care among neonates.23,24 In the United States, neonatal incidence decreased from approximately 32 cases per 100,000 births in 2009 to less than 12 cases per 100,000 births by 2012, and has remained stable since then.24 Despite overall declines through 2013 in the United States across all age groups, incidence remains the highest among those 65 years and older. Large racial disparities persist across all age groups (the incidence among Blacks is 2.3 times higher than among non-Blacks). All-cause hospital mortality among all persons infected remains high at approximately 25% (yet varies by age group ranging from 10% among 1- to 18-year-olds to 32% among those ≥65 years old).16,23
In contrast with the United States, Canada’s estimated incidence of candidemia is less than 3 cases per 100,000, with little change over the past 15 years.25-27 Similar to the United States, incidence is highest among those 65 years and older, followed by those less than 1 year old.27 Few studies have examined trends in Mexico; 1 study using demographic data and population-based surveys estimated national incidence to be 5 cases per 100,000 population, with the intensive care unit (ICU) incidence 10-fold higher than the non-ICU incidence.28 In 14 medical centers in Mexico from 2010 to 2011, Candida species accounted for nearly all (98%) fungal bloodstream infections among pediatric patients.29
The major risk factors for invasive Candida infections have varied little over the past decade.16 These include the presence of indwelling catheters (mainly central venous catheters) and other medical devices, hematologic or solid organ malignancies, recent abdominal surgeries, hemodialysis, diabetes, receipt of systemic antibiotics or immunosuppressive medications including steroids, and receipt of total parenteral nutrition.16,30 Recently, injection drug use emerged as a risk factor; it is becoming more common in the context of the opioid epidemic.31,32 In the United States, approximately 10% of candidemia cases identified through surveillance in 2017 occurred in patients with recent injection drug use.31 Patients with injection drug use tend to be younger, non-Hispanic Whites, and the disease is often community-associated rather than health care-associated.31,32
Five species account for most candidemia infections worldwide: C albicans, C glabrata, C tropicalis, C parapsilosis, and C krusei.16,33 Worldwide, these 5 species are estimated to account for more than 90% of all infections; however, the precise distribution and rank order of Candida species differ by geographic area, health care unit, underlying conditions, and patient demographic characteristics.16,33,34 Although C albicans remains the most common Candida species causing invasive infection in most clinical settings in North America, an increasing proportion of diagnoses in recent years have been attributed to non-albicans species, particularly C glabrata and C parapsilosis. In some settings, these species have even surpassed C albicans.28,35 The increased proportion of C parapsilosis infections is concerning because this species can colonize health care workers’ hands, ultimately causing outbreaks.9 In one US surveillance site, the proportion of cases caused by C albicans decreased from 52% (1992–1993) to 41% (2008–2011), whereas C glabrata cases increased from 12% to 27% over the same time period.35 Similar trends have been documented in Canada, where in 1 multicenter study the proportion of C albicans cases declined from 61% to 42% and C glabrata cases increased from 17% to 22% from 2011 to 2016.36
Furthermore, non-albicans species often have decreased susceptibility to first-line antifungal therapies used to treat candidemia, including azoles and echinocandins.37 Approximately 10% of C glabrata isolates in the United States are resistant to fluconazole.35,38 Yet similar to species distributions, resistance patterns vary geographically and by medical institution (eg, a Canadian study found fluconazole resistance in only 1% among C glabrata isolates, whereas susceptibility testing from 2 Mexican tertiary care hospitals revealed fluconazole resistance to be 11% among C glabrata isolates).28,36 However, multidrug resistance remains uncommon among these top 5 species.34
Although some Candida species are intrinsically resistant to certain antifungals (eg, C lusitaniae and amphotericin B, C krusei and fluconazole), of increasing concern is acquired resistance, particularly among C glabrata isolates.37,39-43 These genomic and epidemiologic shifts in species and resistance patterns have been attributed to frequent prophylactic antifungal use, favoring less susceptible species.44 These changes have introduced new challenges to patient care and management.
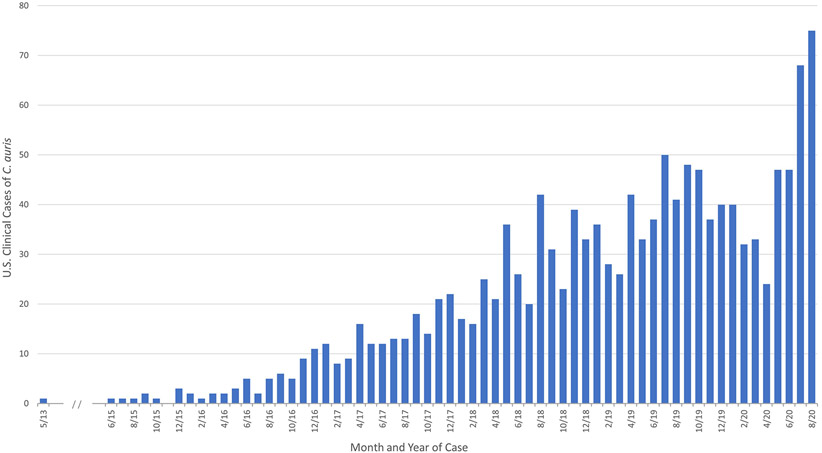
The recent emergence of C auris represents a paradigm shift in Candida epidemiology.9 First described in 2009 in Japan and first reported in North America in 2016 (with the earliest reported isolate from 2013), C auris differs from other Candida species, behaving more like a bacterium than a fungus.9,45 By late 2019, more than 1000 cases had been reported to the US Centers for Disease Control and Prevention (Fig. 1). Multidrug resistance is common in C auris, and unlike other Candida species, some isolates are resistant to all 3 major antifungal classes, presenting substantial challenges to successful treatment.46 In the United States, approximately 90% of C auris isolates are resistant to fluconazole, 30% to amphotericin B, and 5% to echinocandins.9 Furthermore, like C glabrata, acquired resistance in patients undergoing treatment for C auris infection has been documented.9,47 Unlike most Candida species, C auris is commonly transmitted among patients in the health care environment, and outbreaks have been reported worldwide.48,49 Further complicating the control of transmission, patients can become colonized on skin, nares, groin, or axilla and spread C auris in the health care environment, yet remain asymptomatic.48,50 In 2019, the US Centers for Disease Control and Prevention classified C auris as an urgent threat.51
Fig. 1.
Reported U.S. Clinical Cases of C auris from 2013 to 2020. C auris began spreading in the United States in 2015; the earliest reported US cases were identified through a retrospective review. (From Centers for Disease Control and Prevention [CDC] unpublished data.)
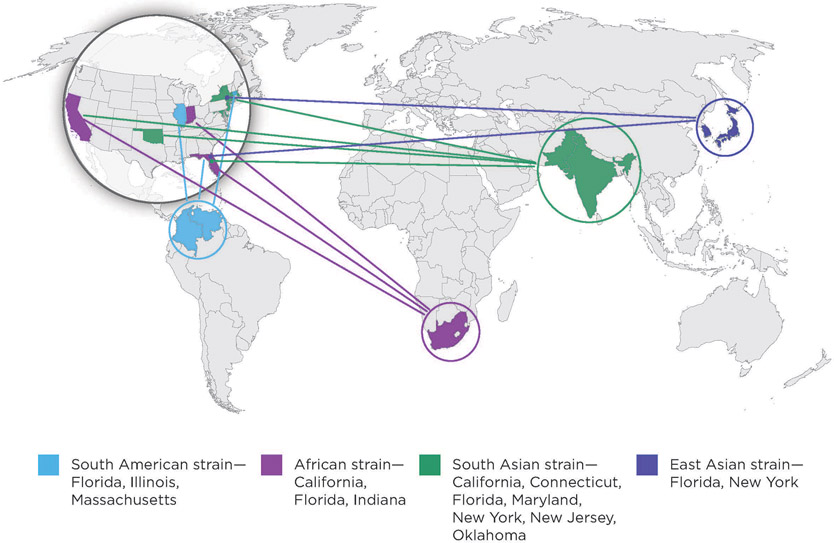
Most perplexing is the simultaneous emergence of C auris within different geographic regions of the world, with whole genome sequencing revealing 4 distinct geographic clades: South Asia, South Africa, South America, and East Asia (Fig. 2).19,52-54 To date, C auris cases have been reported in dozens of countries, yet the prevalence is likely underestimated owing to limited diagnostic capacities to accurately identify the species.55,56 Clinically, C auris causes invasive infection, and mortality estimates range from 30% to 60%; among recovered patients, indefinite colonization can occur.46 Identified risk factors are similar to those of other invasive Candida infections.9,46,55 Additional risk factors specific to C auris include the receipt of antifungals at or near the time of diagnosis and, unlike other Candida infections often associated with ICU settings, patients tend to have exposure to long-term care or skilled nursing facilities.57 The rapid emergence of C auris indicates the need for improved diagnostics and a broader range of treatments for multidrug-resistant fungal pathogens.9
Fig. 2.
Geographic representation of the 4 major C auris clades identified in the United States through 2019. Shading of certain countries is used to represent the geographic regions that the specific C auris clades are associated with, and does not represent direct introduction from these countries to the United States. (From Centers for Disease Control and Prevention [CDC]. Antibiotic Resistance Threats in the United States. 2019. Available at: http://www.cdc.gov/drugresistance/Biggest-Threats.html. Accessed February 23, 2021.)
MOLD
Aspergillus
More than 180 species make up the Aspergillus genus, although only a subset have been tied to human disease. A fumigatus has historically been responsible for most aspergillosis-related conditions, though infections are increasingly associated with non-fumigatus species, including A flavus, A terreus, and A niger.58-60
Aspergillosis remains a substantial risk for persons with weakened immune systems. Illness severity ranges from mild to serious, and invasive infection substantially increases the risk of death.61 Invasive aspergillosis, although relatively rare, is the most common type of invasive mold infection and can cause severe health problems, particularly among immunocompromised populations.62,63
Invasive fungal disease (IFD), including invasive aspergillosis, is classified according to consensus definitions of the Mycoses Study Group (MSG) and the European Organization for Research and Treatment of Cancer (EORTC). Criteria include a strict set of host factors and clinical features comprising classic symptoms and radiologic findings.64 The definitions reflect persons particularly at risk for invasive aspergillosis, including those with hematologic malignancies, a recent history of neutropenia, and hematopoietic transplant or solid organ transplant recipients. Similarly, patients receiving immunosuppressive therapies or high doses of corticosteroids may be more susceptible to opportunistic infection.65 A trend analysis is difficult because invasive aspergillosis is not a reportable disease. A combination of literature reviews and modeling have been used to assess the burden of disease in Canada and Mexico, where incidence is estimated to be 1.6 per 100,000 population and 4.6 per 100,000 population, respectively.25,28 Validation of these estimates through formal epidemiologic studies is needed.
Hospitalization data can provide valuable insight into the epidemiology of this disease because most people with invasive aspergillosis require hospitalization. In a nationally representative database of US hospitalizations, invasive aspergillosis-associated hospitalizations increased by 57% from 2000 to 2013, and the overall rate of invasive aspergillosis-associated hospitalizations increased by 3% during the same timeframe.66
The increase in US hospitalizations may reflect a number of factors. The susceptible population has likely grown owing to an increase in the number of stem cell and organ transplantations in recent years, as well as the more widespread use of immunosuppressive agents.2,67,68 Additionally, diagnostic advancements may have contributed to increased detection of invasive aspergillosis, particularly in patients who present without classic symptoms. Despite the increased hospitalization numbers, decreases in crude mortality rates and excess attributable length of stay suggest that invasive aspergillosis outcomes have improved.69 The increased survival of invasive aspergillosis is likely attributable in part to the development of newer azole antifungal medications, including voriconazole and posaconazole.70
However, azole-resistant strains of A fumigatus have been discovered, with serious implications for the management of aspergillosis. Azole resistance was first identified in Europe, but has since been detected worldwide.71-73 These strains present a clear challenge to treatment and are associated with high mortality rates.74
Of note, the main resistance mechanism found in Europe has been detected in azole-naïve patients and not in those who have undergone long-term azole therapy, where other mutations were detected.75 This finding has led researchers to suspect an environmental source, and reports have confirmed that resistance may develop as a result of exposure to azole fungicides used for agricultural purposes.75,76 This finding is of particular concern given the widespread use of azoles as crop pesticides.71,77,78
The epidemiologic characteristics of recently described patients coinfected with aspergillus and influenza are distinct from the classic MSG/EORTC criteria, which may lead physicians to forgo testing for these infections. A substantial proportion of patients with influenza-associated pulmonary aspergillosis (IAPA) may be immunocompetent or may not present with classic IFD host factors.79,80 Clinical and radiologic findings are not necessarily indicative of IFD; lesions on a computed tomography scan with halo signs are typically absent in ICU patients. A consensus case definition has been proposed to account for IAPA’s epidemiologic differences from the MSG/EORTC classifications.80 IAPA is associated with severe outcomes among critically ill patients in the ICU.79,81,82 Results from a multicenter study in the Netherlands and Belgium showed that the 90-day mortality rate among ICU patients with IAPA (51%) was nearly double that of ICU patients with influenza without invasive aspergillosis (28%).79 It is suggested that physicians consider IAPA in selected ICU patients with influenza, especially those with ventilator-associated pneumonia, yet physician awareness of IAPA remains low, particularly in the United States.83,84
The advent of the severe acute respiratory syndrome novel coronavirus, that causes the coronavirus disease 2019 (COVID-19), has posed a similar challenge. Critically ill patients with COVID-19 present with clinical characteristics comparable with patients with severe influenza and are likewise susceptible to secondary infection.85 Early reports of COVID-19–associated pulmonary aspergillosis (CAPA) show that patients do not generally meet MSG/EORTC criteria, prompting the need for specific diagnostic and screening criteria to classify cases of CAPA.85
Evidence to date suggests that critically ill IAPA and CAPA patient symptoms may be notably different from classic IFD features. However, much remains to be learned about these emerging infections.80,85,86
Non–Aspergillus Molds
Recent reports show a potential increase in non-Aspergillus invasive mold infections (NAIMIs) among patients with hematologic malignancies and transplant recipients.87-89 These molds, such as mucoromycetes, Fusarium, and Scedosporium spp., are rare but extremely hazardous to the health of those infected. Mucormycosis is typically the most common NAIMI, followed by fusariosis and scedosporiosis; prominence has been shown to vary geographically.90-92
These infections are characterized by high mortality rates, resistance to multiple antifungal drugs, and dissemination to multiple organs. Analyses showed the proportion of HSCT patients who contracted a NAIMI was 1% or less, but the alarming 1-year survival rate ranged from 6% to 22%.62,93 These findings are consistent with previous reports, suggesting that problems associated with disease management and treatment have persisted.94
The increased in NAIMIs could be a result of expanded immunosuppressive therapy use and anti-Aspergillus prophylaxis,95 or it could stem from the increase in susceptible patients. Additional NAIMI risk factors such as neutropenia, extended corticosteroid use, and diabetes align with established risk factors for all fungal infections,96,97 although variations across pathogens have been reported.97
The early detection of NAIMIs is essential to determine the optimal clinical course and most appropriate antifungal therapy. A nonspecific clinical presentation and limited diagnostics present challenges to diagnosis, although advancements in the identification of mold pathogens through molecular techniques is promising.80,89,98 The infection may not become evident until later stages of disease when illness is more severe.88 Although host factors such as immune system recovery or progression of underlying conditions play a substantial role in patient prognosis, continued advancements in diagnostics and therapeutic approaches will be important to increase survival from these deadly diseases.
ENDEMIC MYCOSES
Geographically restricted fungal diseases, commonly referred to as endemic, namely, blastomycosis, coccidioidomycosis, and histoplasmosis, present a growing public health concern. Preventing exposure to the causative fungi may be difficult in areas where they are prevalent in the environment. Infection can result in serious illness or death, particularly among immunocompromised populations. However, public and physician awareness of these diseases remains low.99 Surveillance and diagnostic challenges limit the known epidemiology of these mycoses.
Blastomycosis, coccidioidomycosis, histoplasmosis, and other dimorphic fungi are often collectively referred to as endemic mycoses, although the term “endemic” may be misrepresentative. These diseases have historically been associated with specific geographic regions.100,101 However, infections and outbreaks acquired outside of traditional locations indicate that geographic distribution is wider than previously recognized.102 Within the classic regions, there are areas of hyperendemicity and hypoendemicity as well as seasonal trends that are not fully captured by the classification of endemic versus nonendemic.103
Estimates of case counts and incidence are likely subject to under-reporting and misdiagnosis, masking the true burden of disease. These fungal infections are often clinically indistinguishable from other respiratory illnesses such as community-acquired pneumonia.104 Nonspecific symptoms such as cough, fever, and shortness of breath may lead persons with mild cases not to seek care. Many cases may go undetected by surveillance efforts as blastomycosis, coccidioidomycosis, and histoplasmosis are each only reportable in a subset of US states (5, 26 plus the District of Columbia, and 12, respectively).105 Only coccidioidomycosis is nationally notifiable in the United States.
An innovative approach toward understanding the current distribution is multidisciplinary modeling of environmental characteristics. These models leverage environmental data to detect areas with risk of exposure based on the suitability of the surrounding conditions for the causative fungi.10 Such techniques are important to appreciate the expanding geographic range of regional fungal diseases and tailor public health efforts accordingly. However, more robust and widespread surveillance for these diseases is needed to better understand their changing geographic distribution and epidemiologic trends.
Blastomyces
Rates of blastomycosis seem to be stable, although the rarity of the disease complicates assessment. In US states where it is reportable, the annual incidence rates have been consistent at approximately 1 to 2 cases per 100,000 population,106,107 and evidence from Canada indicates a similar rate of 0.62 per 100,000 population.108 Wisconsin classically has the highest rate of any state, ranging from 10 to 40 cases per 100,000 persons in some counties.101
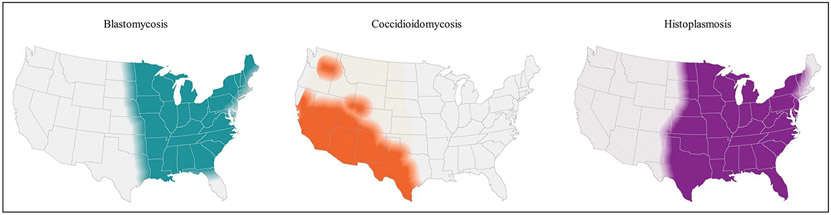
Blastomyces primarily lives in regions surrounding the Ohio and Mississippi River valleys, the Great Lakes, the Saint Lawrence River, and southern Canada. Although the disease is relatively uncommon, it covers a wide geographic area, as shown in Fig. 3. Several US states with comparatively high rates of blastomycosis-associated hospitalizations, including Illinois, Kentucky, and Tennessee, do not mandate reporting.109 Recent cases have been reported in areas not known to be endemic for blastomycosis.110 It is unclear whether environmental factors such as fluctuations in temperature or precipitation may have contributed to this spread as the ecology of Blastomyces is not well-understood.111
Fig. 3.
Geographic distribution of blastomycosis, coccidioidomycosis and histoplasmosis in the United States. (From Centers for Disease Control and Prevention [CDC]. More information about the estimated areas with blastomycosis, coccidioidomycosis (Valley fever), and histoplasmosis in the United States. 2020. Available at: https://www.cdc.gov/fungal/pdf/more-information-about-fungal-maps-508.pdf. Accessed February 23, 2021.)
Anyone can contract blastomycosis in areas where the fungus lives; hospitalization and death are more likely among individuals with immunocompromised status or other underlying medical conditions.102,109 Outdoor activities such as boating, fishing, and hiking may put people at greater risk of infection.110 The disease is more prevalent among older populations, although finding this could be a factor of weakening immune systems.112 Historically, the higher proportion of blastomycosis-related morbidity and mortality among males was thought to be a consequence of occupational differences or varied recreational activities.113 However, it is possible that the discrepancy is due to hormonal distinctions between the sexes, as suggested for other fungal diseases.114,115
Coccidioides
The number of coccidioidomycosis cases reported to the US Centers for Disease Control and Prevention has increased consistently in recent years. This increase follows a downward trend from 2011 (22,634 cases) to 2014 (8232 cases), after which the total number of cases increased each year, reaching 15,611 in 2018.116 Arizona and California account for more than 95% of the reported cases. The incidence in California more than tripled from 6.0 per 100,000 population in 2014 to 18.8 per 100,000 population in 2018.117 In recent years, the total number of cases reported in California surpassed those reported in Arizona.116 Arizona’s incidence also increased from 2014 (84.4 per 100,000 population) to 2018 (105.7 per 100,000 population), although it remained well below the 2011 peak (255.8 per 100,000 population).118
Reasons for the decrease and subsequent increase in reported cases are likely multifaceted. The year-to-year changes may have been influenced by environmental or climate changes, increases to the susceptible population based on travel or new residence in endemic areas, revised reporting and testing practices, or changes in land use.119-121
The presence of Coccidioides in the southwestern United States as well as parts of Mexico and Central and South America is well-established. Recently reported cases beyond these traditional areas indicate a northward expansion of the geographic range (see Fig. 3), stretching to Northern California, Utah, and Washington.103,122-124 In Washington, whole genome sequencing determined that infection was locally acquired rather than a result of travel.125,126
Histoplasma
Evaluating the true burden of histoplasmosis is a continuing challenge, though the disease is likely more common than currently appreciated. In 2016, the US Council of State and Territorial Epidemiologists approved a standardized surveillance case definition for histoplasmosis. Previously, US states in which the disease was reportable used varying definitions, limiting the ability to make comparisons across states or evaluate overall trends in incidence using public health surveillance data.
US histoplasmosis-associated hospitalizations nearly doubled from 0.9 per 100,000 persons (2604 hospitalizations) in 2001 to 1.7 per 100,000 persons (5175 hospitalizations) in 2012.11 Considerable increases observed across certain patient populations suggest the emergence of new high-risk groups, specifically transplant recipients and patients whose conditions warrant treatment with biologic agents.127 These increases correspond with the proliferation of new biologic treatments and the increase in solid organ and hematopoietic stem cell transplants. Notably, the proportion of histoplasmosis-associated hospitalizations among HIV/AIDS patients decreased, which can likely be attributed to the availability of ART.127
In the United States, Histoplasma generally lives in the central and eastern states, especially in areas surrounding the Ohio and Mississippi River valleys (see Fig. 3). Surveillance data and outbreak investigations suggest that the occurrence of histoplasmosis extends well beyond the already broadly defined historical region.11,106 A geographic suitability model suggests that preferred soil environments for Histoplasma have extended to the upper Missouri River basin, possibly owing to environmental changes.108
CRYPTOCOCCUS
Cryptococcosis is an opportunistic infection that typically presents as meningitis or meningoencephalitis and emerged as a predominant cause of disease during the HIV/AIDS epidemic in the 1980s.128,129 With the increase in immunocompromised susceptible hosts, cases of cryptococcosis increased dramatically worldwide throughout the early years of the epidemic.129,130 The incidence of cryptococcosis has decreased since the implementation of ART and other improvements to the early detection and treatment of HIV/AIDS.129 Most cryptococcosis cases are due to 2 species complexes: C neoformans, which comprises the vast majority of isolated species, and C gattii.131 We continue to learn about the ecological and environmental niches of Cryptococcus, but C gattii has been mainly associated with certain trees and soil debris and may be more limited in distribution. C neoformans is cosmopolitan and can be associated with soil contaminated by bird, particularly pigeon, excrements.12,132,133
Recent data show approximately 15% of AIDS-related deaths are attributed to cryptococcal meningitis.134 Worldwide cryptococcal meningitis estimates exceed 220,000 cases annually, with most geographically concentrated in sub-Saharan Africa.134,135 North America experienced the most dramatic decreases in HIV-associated cryptococcosis incidence in recent decades.129 Advanced modeling methods estimate 3700 people are cryptococcal antigenemia positive in the region; the annual burden of cryptococcal meningitis and cryptococcal meningitis-related deaths are estimated at 3000 and 700 cases, respectively.134
In the United States, cryptococcosis incidence decreased by approximately 90% in the 1990s among people living with HIV, and a recent study using sera collected from 1986 to 2012 estimates that approximately 3% of individuals living with advanced HIV with CD4 counts of less than 100 cells/μL are cryptococcal antigen positive.130,136,137 Estimated incidence of cryptococcal meningitis ranges from 2 to 7 cases per 1000 person years among individuals living with HIV in the United States, with mortality ranging from 12% to 25%.130,138 The US cryptococcal infection burden is estimated to be between 2500 and 5000 cases.134 In Mexico, cryptococcal infection annual burden is estimated to be less than 1000 cases. In Canada, the burden is estimated to be less than 500 cases.134
Although ART has contributed to overall decreases in disease burden, ART initiation may not directly correlate with decreased infection rates; additional factors including continuous care and high ART adherence also contribute to preventing infection.139,140 Recent studies have also identified individuals with subclinical meningitis, meaning that Cryptococcus species is present in the central nervous system yet the patient remains asymptomatic, shifting the understanding of the burden of disease.128
The fungus also infects persons with impaired immunity owing to non-HIV conditions, including those with lymphoproliferative disorders, sarcoidosis, malignancies, and diabetes; those who have received immunosuppressive therapies or solid organ transplants; and those with no identified underlying immunodeficiencies.141,142 In a recent US-based retrospective analysis, only 36% of patients with cryptococcosis were HIV positive, with the remainder including solid organ transplant recipients (28%) or other non-HIV/nontransplant patients.138 Cryptococcosis ranked as the third most common invasive fungal infection among solid organ transplant recipients in a review of transplant infections in the United States.143 Approximately 25% to 54% of transplant patients who develop cryptococcosis have pulmonary disease.144 The cumulative lifetime risk of disease among solid organ transplant recipients ranges from 1% to 2%.129
As the rate of disease among people living with HIV in North America has decreased, the rate among those living without HIV has not, resulting in an increased proportion of non–HIV-associated cases.129 HIV-negative patients tend to be older and have lower rates of meningitis, although other forms of disseminated disease are still common.129,138 Furthermore, although immunodeficiencies remain a significant risk factor, approximately 20% of non-HIV patients who develop Cryptococcus disease have no known immune impairments.142 Notably, non-HIV/nontransplant patients experience poorer clinical outcomes compared with both HIV-positive and solid organ transplant patients, with higher rates of morbidity and mortality (46% mortality in nonimmunosuppressed vs 19% among those immunosuppressed and 15% among HIV-positive patients), potentially related to delayed detection and an overly reactive immune response among immunocompetent individuals.129,145
Similar to other fungi, antifungal resistance remains a challenge. There are no breakpoints for Cryptococcus, so the burden of resistance is relatively unknown and additional studies are needed. In a recent systematic review that included nearly 5000 isolates of Cryptococcus species, the majority being C neoformans, across 29 studies (1988–2017), the average prevalence with elevated fluconazole minimum inhibitory concentration values was found to be 10.6% among incident isolates.135,146
Cryptococcus gattii Species Complex
In contrast with C neoformans, historically C gattii was limited to the tropical and subtropical regions of South America, Asia, Australia, and parts of Africa.12,131,133,147 Yet in 1999, C gattii’s known geographic distribution expanded when the species caused an unprecedented outbreak on Vancouver Island in British Columbia, Canada.132 An increasing number of cases have since been reported on mainland Canada and the US Pacific Northwest, with many of those infected lacking any immunocompromising condition.12,141 In the Vancouver Island outbreak, only 40% of cases had any immunocompromising disorder.142 Recently, sporadic cases of C gattii have also been reported in the Southeastern United States, and in Mexico C gattii comprises 12% to 20% of all cryptococcal isolates.132,148,149 Genetically, the C gattii species complex is divided into 5 different species: C gattii sensu stricto, C deuterogattii, C bacillisporus, C tetragattii, and C decagattii. All species have been detected in Mexico, yet most isolates in Canada and the northwestern US region belong to C deuterogattii.150 Nonetheless, environmental distributions have not been fully characterized owing to limited environmental sampling.150
OTHER FUNGAL INFECTIONS
Pneumocystis jirovecii
Pneumocystis pneumonia (PCP) is an infection that affects persons with weakened immune systems. Although uncommon before the HIV/AIDS epidemic in the 1980s,151 PCP quickly became one of the leading AIDS-defining illnesses.152 The introduction of ART and treatment with trimethoprim and sulfamethoxazole corresponded with a considerable decrease in PCP among people living with HIV/AIDS.153-155 However, PCP remains an important cause of morbidity and mortality worldwide.
A study of HIV-infected patients in the United States and Canada found PCP to be the most common opportunistic infection, despite a decrease in incidence from 0.92 cases per 100 person-years to 0.39 cases per 100 person-years from 2000 to 2010.156 Among persons living with HIV/AIDS, the risk of PCP persists for those who do not receive or do not respond to ART.
Populations at risk for PCP have shifted toward HIV-uninfected immunocompromised groups owing to immunosuppressive regimens that weaken the immune system. Risk factors include stem cell transplants or solid organ transplants, cancer, chronic lung disease, and autoimmune diseases.112,157,158 The clinical presentation can vary by HIV status, and HIV-uninfected persons with PCP may experience a greater diagnostic delays and increased risk of mortality compared with HIV-infected counterparts.113 The changing epidemiology of PCP as a result of therapeutic developments highlights the continued threat of the disease.
Sporothrix
Sporotrichosis, typically associated with contact with plant matter including sphagnum moss, rose bushes, and hay, is rare in North America.159,160 Sporothrix schenckii has been found worldwide, with most US cases occurring in southern coastal regions and river valleys.159 Using a large commercial health insurance database, US sporotrichosis incidence was estimated to be 2 cases per 1 million people from 2012 to 2018; the incidence was highest in Oklahoma, Michigan, Kansas, and Kentucky.161 S schenckii outbreaks in the United States have been associated with forestry, gardening, and exposure to farms.159,160,162
In Mexico, where the incidence peaks during the cold and dry seasons, S schenckii is the most prevalent species. In some areas, the incidence reaches 25 cases per 1000 population, suggesting high endemicity within some regions.162 In the state of Puebla, Mexico, 53% of residents were reactive to sporotrichin on intradermal skin tests.160 Differences by age and sex are typically related to occupational exposures.163
The species S brasiliensis recently emerged in South America and is associated with zoonotic transmission, spreading via animal scratches and bites.159 Brazil is currently experiencing an unprecedented outbreak of S brasiliensis with more than 4500 human cases reported; sporadic cases and smaller outbreaks have been reported in Argentina and other South American countries.159 Although S brasiliensis has not been reported in North America, there is concern the species could spread through the movement of infected animals.
DISCUSSION
The epidemiology of fungal diseases is constantly evolving. Environmental changes, medical advancements, emerging species, and diagnostic and therapeutic developments have all contributed to meaningful shifts in the geographic distribution, population risk, pathogen virulence, and disease progression in recent years. Increasingly widespread use of immunosuppressive agents among patients with underlying conditions has contributed to increasing opportunistic infections and emerging pathogenic fungi.1-3 Although Candida and Aspergillus spp. remain the most common causes of IFD, the increase in regional fungal diseases and NAIMIs is of concern. Even within the Candida and Aspergillus genera, changes in the distribution of disease reflect the growing prevalence of non-C albicans and non-A fumigatus species.28,35,58
Large-scale health events such as seasonal influenza epidemics and the COVID-19 pandemic merge ongoing with new public health challenges in the context of fungal disease. Recent resurgence of C auris in health care facilities where its spread had previously been controlled highlights the importance of infection control measures. The emergence of IAPA and CAPA stress the need for standardized case definitions and IFD testing to detect secondary infection.86,164
It is critical to consider that documented updates regarding fungal diseases only paint part of the picture. To advance the field of mycotic epidemiology, more robust surveillance is needed to monitor long-term trends and identify the emergence of new infections. Because most fungal diseases are not nationally notifiable, this lack of surveillance often leads to underestimation of disease burden. As a result of generally nonspecific symptoms associated with fungal infections, patients may not seek care or physicians may not test appropriately, leading to underdiagnosis or delayed diagnosis.
An increased awareness at both the patient and physician levels is needed to avoid delays in diagnosis and proper treatment.99,113,165 Given the expanding geographic boundaries of regional fungal diseases and the potential for travel-related infections, it is important that physicians outside of the traditional endemic areas are familiar with these mycoses and do not discount them solely on the basis of location.
Continued improvements in laboratory diagnostics are also essential for early diagnosis and appropriate treatment. Advancements in molecular techniques and lateral flow assays show progress toward testing ease and efficiency.166,167 Additionally, the use of whole genome sequencing is valuable to understand disease exposure, transmission, and overall genetic composition.
SUMMARY
The epidemiology of fungal infections is complex and multifaceted. Limitations with surveillance, diagnostics, and overall awareness demonstrate the interdisciplinary challenges facing current efforts to curtail the burden of disease. As the field continues to evolve, it will be necessary to effectively address these shortcomings to decrease morbidity and mortality from infection.
KEY POINTS.
Environmental changes, medical advancements, and diagnostic developments have all contributed to meaningful shifts in at-risk populations, geographic distribution, and fungal disease progression in recent years.
Although Candida and Aspergillus spp. remain the most common causes of invasive fungal disease, the increase in regional fungal diseases and non-Aspergillus invasive mold infections is of concern.
Early intervention and appropriate treatment are key to reducing disease burden.
Yet, despite developments in diagnostic techniques, there are limited antifungal options, mortality remains high, and awareness remains low.
Large-scale health events such as seasonal influenza epidemics and the coronavirus disease 2019 pandemic have introduced new public health challenges in the control of fungal disease.
CLINICS CARE POINTS.
Physicians may not consider fungal diseases in the differential diagnosis owing to low awareness and nonspecific clinical presentation.
Adherence to infection control practices is important to the prevention of health care–associated infection and likely contributed to a recent decrease in the incidence of candidemia in the United States.
Diagnosis of fungal disease is further complicated by patients who present with atypical host characteristics. Resulting misdiagnosis and diagnostic delays may lead to unnecessary antibiotic use and increased morbidity and mortality.
Large-scale health events such as seasonal influenza epidemics and the COVID-19 pandemic may lead to an increase in the proportions of the population susceptible to secondary fungal infection.
Footnotes
DISCLAIMER
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
DISCLOSURE
The authors have nothing to disclose.
REFERENCES
- 1.Richardson MD. Changing patterns and trends in systemic fungal infections. J Antimicrob Chemother 2005;56(Suppl 1):i5–11. [DOI] [PubMed] [Google Scholar]
- 2.Casadevall A. Fungal diseases in the 21st century: the near and far horizons. Pathog Immun 2018;3(2):183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michael A, Pfaller PGP, John R, et al. Invasive fungal pathogens: current epidemiological trends. Clin Infect Dis 2006;43(Supplement_1):S3–14. [Google Scholar]
- 4.Webb BJ, Ferraro JP, Rea S, et al. Epidemiology and clinical features of invasive fungal infection in a US Health Care Network. Open Forum Infect Dis 2018;5(8):ofy187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bongomin F, Gago S, Oladele RO, et al. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J Fungi (Basel) 2017;3(4). 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh N. Trends in the epidemiology of opportunistic fungal infections: predisposing factors and the impact of antimicrobial use practices. Clin Infect Dis 2001;33(10):1692–6. [DOI] [PubMed] [Google Scholar]
- 7.Pyrgos V, Seitz AE, Steiner CA, et al. Epidemiology of cryptococcal meningitis in the US: 1997-2009. PLoS One 2013;8(2):e56269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson M, Lass-Florl C. Changing epidemiology of systemic fungal infections. Clin Microbiol Infect 2008;14(Suppl 4):5–24. [DOI] [PubMed] [Google Scholar]
- 9.Forsberg K, Woodworth K, Walters M, et al. Candida auris: the recent emergence of a multidrug-resistant fungal pathogen. Med Mycol 2019;57(1):1–12. [DOI] [PubMed] [Google Scholar]
- 10.Maiga AW, Deppen S, Scaffidi BK, et al. Mapping histoplasma capsulatum exposure, United States. Emerg Infect Dis 2018;24(10):1835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong PA, Jackson BR, Haselow D, et al. Multistate Epidemiology of Histoplasmosis, United States, 2011-2014. Emerg Infect Dis 2018;24(3):425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown GD, Denning DW, Gow NA, et al. Hidden killers: human fungal infections. Sci Transl Med 2012;4(165):165rv13. [DOI] [PubMed] [Google Scholar]
- 13.Benedict K, Jackson BR, Chiller T, et al. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis 2019;68(11):1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallabhaneni S, Mody RK, Walker T, et al. The Global Burden of Fungal Diseases. Infect Dis Clin North Am 2016;30(1):1–11. [DOI] [PubMed] [Google Scholar]
- 15.Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis 2017;17(12):e383–92. [DOI] [PubMed] [Google Scholar]
- 16.Toda M, Williams SR, Berkow EL, et al. Population-based active surveillance for culture-confirmed candidemia - four sites, United States, 2012-2016. MMWR Surveill Summ 2019;68(8):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benedict K, Park BJ. Invasive fungal infections after natural disasters. Emerg Infect Dis 2014;20(3):349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez H, Martinez LR. Relationship of environmental disturbances and the infectious potential of fungi. Microbiology (Reading) 2018;164(3):233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chow NA, Gade L, Tsay SV, et al. Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: a molecular epidemiological survey. Lancet Infect Dis 2018;18(12):1377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rijnders BJA, Schauwvlieghe A, Wauters J. Influenza-associated pulmonary aspergillosis: a local or global lethal combination? Clin Infect Dis 2020. 10.1093/cid/ciaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiner-Lastinger LM, Abner S, Edwards JR, et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol 2020;41(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsay SV, Mu Y, Williams S, et al. Burden of Candidemia in the United States, 2017. Clin Infect Dis 2020. 10.1093/cid/ciaa193. [DOI] [PubMed] [Google Scholar]
- 23.Cleveland AA, Harrison LH, Farley MM, et al. Declining incidence of candidemia and the shifting epidemiology of Candida resistance in two US metropolitan areas, 2008-2013: results from population-based surveillance. PLoS One 2015;10(3):e0120452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benedict K, Roy M, Kabbani S, et al. Neonatal and pediatric candidemia: results from population-based active laboratory surveillance in four US locations, 2009-2015. J Pediatr Infect Dis Soc 2018;7(3):e78–85. [DOI] [PubMed] [Google Scholar]
- 25.Dufresne SF, Cole DC, Denning DW, et al. Serious fungal infections in Canada. Eur J Clin Microbiol Infect Dis 2017;36(6):987–92. [DOI] [PubMed] [Google Scholar]
- 26.Lamoth F, Lockhart SR, Berkow EL, et al. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother 2018;73(suppl_1):i4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laupland KB, Gregson DB, Church DL, et al. Invasive Candida species infections: a 5 year population-based assessment. J Antimicrob Chemother 2005;56(3):532–7. [DOI] [PubMed] [Google Scholar]
- 28.Corzo-Leon DE, Armstrong-James D, Denning DW. Burden of serious fungal infections in Mexico. Mycoses 2015;58(Suppl 5):34–44. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez GM, Trevino-Rangel Rde J, Palma-Nicolas JP, et al. Species distribution and antifungal susceptibility of bloodstream fungal isolates in paediatric patients in Mexico: a nationwide surveillance study. J Antimicrob Chemother 2013;68(12):2847–51. [DOI] [PubMed] [Google Scholar]
- 30.Poissy J, Damonti L, Bignon A, et al. Risk factors for candidemia: a prospective matched case-control study. Crit Care 2020;24(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang AY, Shrum S, Williams S, et al. The changing epidemiology of candidemia in the united states: injection drug use as an increasingly common risk factor - active surveillance in selected sites, United States, 2014-17. Clin Infect Dis 2019. 10.1093/cid/ciz1061. [DOI] [PubMed] [Google Scholar]
- 32.Rossow JA, Gharpure R, Brennan J, et al. Injection drug use-associated candidemia: incidence, clinical features, and outcomes, East Tennessee, 2014-2018. J Infect Dis 2020;222(Supplement_5):S442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 2014;20(Suppl 6):5–10. [DOI] [PubMed] [Google Scholar]
- 34.Wiederhold NP. Antifungal resistance: current trends and future strategies to combat. Infect Drug Resist 2017;10:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lockhart SR, Iqbal N, Cleveland AA, et al. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol 2012;50(11):3435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuller J, Dingle TC, Bull A, et al. Species distribution and antifungal susceptibil-ity of invasive Candida isolates from Canadian hospitals: results of the CANWARD 2011-16 study. J Antimicrob Chemother 2019;74(Suppl 4):iv48–54. [DOI] [PubMed] [Google Scholar]
- 37.Jensen RH, Johansen HK, Soes LM, et al. Posttreatment antifungal resistance among colonizing Candida isolates in candidemia patients: results from a systematic multicenter study. Antimicrob Agents Chemother 2015;60(3):1500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vallabhaneni S, Cleveland AA, Farley MM, et al. Epidemiology and risk factors for echinocandin nonsusceptible Candida glabrata bloodstream infections: data from a large multisite population-based candidemia surveillance program, 2008-2014. Open Forum Infect Dis 2015;2(4):ofv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett JE, Izumikawa K, Marr KA. Mechanism of increased fluconazole resistance in Candida glabrata during prophylaxis. Antimicrob Agents Chemother 2004;48(5):1773–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borst A, Raimer MT, Warnock DW, et al. Rapid acquisition of stable azole resistance by Candida glabrata isolates obtained before the clinical introduction of fluconazole. Antimicrob Agents Chemother 2005;49(2):783–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapeland-Leclerc F, Hennequin C, Papon N, et al. Acquisition of flucytosine, azole, and caspofungin resistance in Candida glabrata bloodstream isolates serially obtained from a hematopoietic stem cell transplant recipient. Antimicrob Agents Chemother 2010;54(3):1360–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cleary JD, Garcia-Effron G, Chapman SW, et al. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob Agents Chemother 2008;52(6):2263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corcione S, D’Avolio A, Pasero D, et al. Acquisition of FKS2 mutation after echinocandin treatment of infective endocarditis by Candida glabrata. Infez Med 2019;27(3):328–31. [PubMed] [Google Scholar]
- 44.Vermitsky JP, Edlind TD. Azole resistance in Candida glabrata: coordinate upregulation of multidrug transporters and evidence for a Pdr1-like transcription factor. Antimicrob Agents Chemother 2004;48(10):3773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satoh K, Makimura K, Hasumi Y, et al. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 2009;53(1):41–4. [DOI] [PubMed] [Google Scholar]
- 46.Lone SA, Ahmad A. Candida auris-the growing menace to global health. Mycoses 2019;62(8):620–37. [DOI] [PubMed] [Google Scholar]
- 47.Ostrowsky B, Greenko J, Adams E, et al. Candida auris isolates resistant to three classes of antifungal medications - New York, 2019. MMWR Morb Mortal Wkly Rep 2020;69(1):6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeffery-Smith A, Taori SK, Schelenz S, et al. Candida auris: a review of the literature. Clin Microbiol Rev 2018;31(1). 10.1128/CMR.00029-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biswal M, Rudramurthy SM, Jain N, et al. Controlling a possible outbreak of Candida auris infection: lessons learnt from multiple interventions. J Hosp Infect 2017;97(4):363–70. [DOI] [PubMed] [Google Scholar]
- 50.Tsay S, Kallen A, Jackson BR, et al. Approach to the investigation and management of patients with Candida auris, an emerging multidrug-resistant yeast. Clin Infect Dis 2018;66(2):306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. 2019. Available at: http://www.cdc.gov/drugresistance/Biggest-Threats.html. Accessed October 5, 2020.
- 52.Lockhart SR, Etienne KA, Vallabhaneni S, et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin Infect Dis 2017; 64(2):134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson BR, Chow N, Forsberg K, et al. On the origins of a species: what might explain the rise of Candida auris? J Fungi (Basel) 2019;5(3). 10.3390/jof5030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casadevall A, Kontoyiannis DP, Robert V. On the emergence of Candida auris: climate change, azoles, swamps, and birds. mBio 2019;10(4). 10.1128/mBio.01397-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cortegiani A, Misseri G, Fasciana T, et al. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J Intensive Care 2018; 6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention. Tracking Candida auris. 2020. Available at: https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html. Accessed October 5, 2020. [Google Scholar]
- 57.Tsay S, Welsh RM, Adams EH, et al. Notes from the field: ongoing transmission of candida auris in health care facilities - United States, June 2016-May 2017. MMWR Morb Mortal Wkly Rep 2017;66(19):514–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morgan J, Wannemuehler KA, Marr KA, et al. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol 2005;43(Suppl 1):S49–58. [DOI] [PubMed] [Google Scholar]
- 59.Steinbach WJ, Benjamin DK Jr, Kontoyiannis DP, et al. Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin Infect Dis 2004;39(2):192–8. [DOI] [PubMed] [Google Scholar]
- 60.Lionakis MS, Lewis RE, Torres HA, et al. Increased frequency of non-fumigatus Aspergillus species in amphotericin B- or triazole-pre-exposed cancer patients with positive cultures for aspergilli. Diagn Microbiol Infect Dis 2005;52(1):15–20. [DOI] [PubMed] [Google Scholar]
- 61.Barnes PD, Marr KA. Aspergillosis: spectrum of disease, diagnosis, and treatment. Infect Dis Clin North Am 2006;20(3):545–61, vi. [DOI] [PubMed] [Google Scholar]
- 62.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis 2010;50(8):1091–100. [DOI] [PubMed] [Google Scholar]
- 63.Pfaller MA, Pappas PG, Wingard JR. Invasive fungal pathogens: current epidemiological trends. Clin Infect Dis 2006;43(Supplement_1):S3–14. [Google Scholar]
- 64.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020;71(6):1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baddley JW. Clinical risk factors for invasive aspergillosis. Med Mycol 2011;49(Suppl 1):S7–12. [DOI] [PubMed] [Google Scholar]
- 66.Vallabhaneni S, Benedict K, Derado G, et al. Trends in Hospitalizations Related to Invasive Aspergillosis and Mucormycosis in the United States, 2000-2013. Open Forum Infect Dis 2017;4(1):ofw268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Health Resources and Services Administration USDoHHS. Organ Procurement and Transplantation Network: view data reports. Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/. Accessed October 5, 2020.
- 68.Centers for International Blood and Marrow Transplant Research. Center for International Blood and Marrow Transplant Research Transplant activity report covering 2009–2013. Available at: http://bloodcell.transplant.hrsa.gov/research/transplant_data/transplant_activity_report/summary-total_tx_by_year.pdf. Accessed October 5, 2020. [Google Scholar]
- 69.Zilberberg MD, Nathanson BH, Harrington R, et al. Epidemiology and outcomes of hospitalizations with invasive aspergillosis in the United States, 2009-2013. Clin Infect Dis 2018;67(5):727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;63(4):e1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beer KD, Farnon EC, Jain S, et al. Multidrug-resistant Aspergillus fumigatus carrying mutations linked to environmental fungicide exposure - three states, 2010-2017. MMWR Morb Mortal Wkly Rep 2018;67(38):1064–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vermeulen E, Lagrou K, Verweij PE. Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr Opin Infect Dis 2013;26(6):493–500. [DOI] [PubMed] [Google Scholar]
- 73.van der Linden JW, Arendrup MC, Warris A, et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis 2015;21(6):1041–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Linden JW, Snelders E, Kampinga GA, et al. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007-2009. Emerg Infect Dis 2011;17(10):1846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snelders E, Huis In’t Veld RA, Rijs AJ, et al. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 2009;75(12):4053–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snelders E, van der Lee HA, Kuijpers J, et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med 2008;5(11):e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Price CL, Parker JE, Warrilow AG, et al. Azole fungicides - understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag Sci 2015;71(8):1054–8. [DOI] [PubMed] [Google Scholar]
- 78.Lockhart SR, Beer K, Toda M. Azole-resistant Aspergillus fumigatus: what you need to know. Clin Microbiol Newsl 2020;42(1):1–6. [Google Scholar]
- 79.Schauwvlieghe A, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med 2018;6(10):782–92. [DOI] [PubMed] [Google Scholar]
- 80.Verweij PE, Rijnders BJA, Bruggemann RJM, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med 2020;46(8):1524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wauters J, Baar I, Meersseman P, et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med 2012;38(11):1761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van de Veerdonk FL, Kolwijck E, Lestrade PP, et al. Influenza-Associated Aspergillosis in Critically Ill Patients. Am J Respir Crit Care Med 2017;196(4):524–7. [DOI] [PubMed] [Google Scholar]
- 83.Toda M, Beekmann SE, Polgreen PM, et al. Knowledge of Infectious Disease Specialists Regarding Aspergillosis Complicating Influenza, United States. Emerg Infect Dis 2020;26(4):809–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thevissen K, Jacobs C, Holtappels M, et al. International survey on influenza-associated pulmonary aspergillosis (IAPA) in intensive care units: responses suggest low awareness and potential underdiagnosis outside Europe. Crit Care 2020;24(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Armstrong-James D, Youngs J, Bicanic T, et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur Respir J 2020;56(4). 10.1183/13993003.02554-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.White PL, Dhillon R, Cordey A, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis 2020. 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bitar D, Van Cauteren D, Lanternier F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997-2006. Emerg Infect Dis 2009;15(9):1395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lamoth F, Kontoyiannis DP. Therapeutic challenges of non-Aspergillus invasive mold infections in immunosuppressed patients. Antimicrob Agents Chemother 2019;63(11). 10.1128/AAC.01244-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Douglas AP, Chen SC, Slavin MA. Emerging infections caused by non-Aspergillus filamentous fungi. Clin Microbiol Infect 2016;22(8):670–80. [DOI] [PubMed] [Google Scholar]
- 90.Lewis RE, Cahyame-Zuniga L, Leventakos K, et al. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: a 20-year autopsy study. Mycoses 2013;56(6):638–45. [DOI] [PubMed] [Google Scholar]
- 91.Park BJ, Pappas PG, Wannemuehler KA, et al. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001-2006. Emerg Infect Dis 2011;17(10):1855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Slavin M, van Hal S, Sorrell TC, et al. Invasive infections due to filamentous fungi other than Aspergillus: epidemiology and determinants of mortality. Clin Microbiol Infect 2015;21(5):490.e1–10. [DOI] [PubMed] [Google Scholar]
- 93.Riches ML, Trifilio S, Chen M, et al. Risk factors and impact of non-Aspergillus mold infections following allogeneic HCT: a CIBMTR infection and immune reconstitution analysis. Bone Marrow Transplant 2016;51(2):322. [DOI] [PubMed] [Google Scholar]
- 94.Marr KA, Carter RA, Crippa F, et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 2002;34(7):909–17. [DOI] [PubMed] [Google Scholar]
- 95.Lamoth F, Chung SJ, Damonti L, et al. Changing Epidemiology of Invasive Mold Infections in Patients Receiving Azole Prophylaxis. Clin Infect Dis 2017;64(11):1619–21. [DOI] [PubMed] [Google Scholar]
- 96.Fukuda T, Boeckh M, Carter RA, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood 2003;102(3):827–33. [DOI] [PubMed] [Google Scholar]
- 97.Garcia-Vidal C, Upton A, Kirby KA, et al. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis 2008;47(8):1041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Halliday CL, Kidd SE, Sorrell TC, et al. Molecular diagnostic methods for invasive fungal disease: the horizon draws nearer? Pathology 2015;47(3):257–69. [DOI] [PubMed] [Google Scholar]
- 99.Benedict K, Molinari NAM, Jackson BR. Public awareness of invasive fungal diseases - United States, 2019. MMWR Morb Mortal Wkly Rep 2020;69(38):1343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Edwards PQ, Palmer CE. Prevalence of sensitivity to coccidioidin, with special reference to specific and nonspecific reactions to coccidioidin and to histoplasmin. Dis Chest 1957;31(1):35–60. [DOI] [PubMed] [Google Scholar]
- 101.Benedict K, Roy M, Chiller T, et al. Epidemiologic and ecologic features of blastomycosis: a review. Curr Fungal Infect Rep 2012;6(4):327–35. [Google Scholar]
- 102.Khuu D, Shafir S, Bristow B, et al. Blastomycosis mortality rates, United States, 1990-2010. Emerg Infect Dis 2014;20(11):1789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Benedict K, Thompson GR 3rd, Deresinski S, et al. Mycotic Infections Acquired outside Areas of Known Endemicity, United States. Emerg Infect Dis 2015;21(11):1935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hage CA, Knox KS, Wheat LJ. Endemic mycoses: overlooked causes of community acquired pneumonia. Respir Med 2012;106(6):769–76. [DOI] [PubMed] [Google Scholar]
- 105.Centers for Disease Control and Prevention. Reportable fungal diseases by state. Available at: https://www.cdc.gov/fungal/fungal-disease-reporting-table.html. Accessed October 5, 2020.
- 106.Herrmann JA, Kostiuk SL, Dworkin MS, et al. Temporal and spatial distribution of blastomycosis cases among humans and dogs in Illinois (2001-2007). J Am Vet Med Assoc 2011;239(3):335–43. [DOI] [PubMed] [Google Scholar]
- 107.Centers for Disease Control and Prevention. Blastomycosis–Wisconsin, 1986-1995. MMWR Morb Mortal Wkly Rep 1996;45(28):601–3. [PubMed] [Google Scholar]
- 108.Litvinjenko S, Lunny D. Blastomycosis hospitalizations in northwestern Ontario: 2006-2015. Can Commun Dis Rep 2017;43(10):200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seitz AE, Younes N, Steiner CA, et al. Incidence and trends of blastomycosis-associated hospitalizations in the United States. PLoS One 2014;9(8):e105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ireland M, Klumb C, Smith K, et al. Blastomycosis in Minnesota, USA, 1999-2018. Emerg Infect Dis 2020;26(5):866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reed KD, Meece JK, Archer JR, et al. Ecologic niche modeling of Blastomyces dermatitidis in Wisconsin. PLoS One 2008;3(4):e2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morris A, Lundgren JD, Masur H, et al. Current epidemiology of Pneumocystis pneumonia. Emerg Infect Dis 2004;10(10):1713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gold JAW, Jackson BR, Benedict K. Possible diagnostic delays and missed prevention opportunities in pneumocystis pneumonia patients without HIV: analysis of commercial insurance claims data-United States, 2011-2015. Open Forum Infect Dis 2020;7(7):ofaa255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shankar J, Restrepo A, Clemons KV, et al. Hormones and the resistance of women to paracoccidioidomycosis. Clin Microbiol Rev 2011;24(2):296–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guess TE, Rosen JA, McClelland EE. An overview of sex bias in C. neoformans infections. J Fungi (Basel) 2018;4(2). 10.3390/jof4020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Centers for Disease Control and Prevention. Valley Fever Statistics. Available at: https://www.cdc.gov/fungal/diseases/coccidioidomycosis/statistics.html. Accessed October 5, 2020.
- 117.California Department of Public Health. Epidemiologic summary of coccidioidomycosis in California 2018. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/CocciEpiSummary2018.pdf. Accessed October 5, 2020.
- 118.Arizona Department of Health. Valley fever 2018 annual report. Available at: https://vfce.arizona.edu/sites/default/files/valley-fever-2018.pdf. Accessed October 5, 2020.
- 119.McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the Epidemiology of coccidioidomycosis in the United States. Med Mycol 2019;57(Supplement_1):S30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cooksey GS, Nguyen A, Knutson K, et al. Notes from the field: increase in coccidioidomycosis - California, 2016. MMWR Morb Mortal Wkly Rep 2017; 66(31):833–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bezold CP, Khan MA, Adame G, et al. Notes from the field: increase in coccidioidomycosis - Arizona, October 2017-March 2018. MMWR Morb Mortal Wkly Rep 2018;67(44):1246–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marsden-Haug N, Goldoft M, Ralston C, et al. Coccidioidomycosis acquired in Washington State. Clin Infect Dis 2013;56(6):847–50. [DOI] [PubMed] [Google Scholar]
- 123.Werner SB, Pappagianis D, Heindl I, et al. An epidemic of coccidioidomycosis among archeology students in northern California. N Engl J Med 1972;286(10): 507–12. [DOI] [PubMed] [Google Scholar]
- 124.Centers for Disease Control and Prevention. Coccidioidomycosis in workers at an archeologic site–Dinosaur National Monument, Utah, June-July 2001. MMWR Morb Mortal Wkly Rep 2001;50(45):1005–8. [PubMed] [Google Scholar]
- 125.Litvintseva AP, Marsden-Haug N, Hurst S, et al. Valley fever: finding new places for an old disease: Coccidioides immitis found in Washington State soil associated with recent human infection. Clin Infect Dis 2015;60(1):e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oltean HN, Springer M, Bowers JR, et al. Suspected Locally Acquired Coccidioidomycosis in Human, Spokane, Washington, USA. Emerg Infect Dis 2020;26(3):606–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martinez R. Epidemiology of Paracoccidioidomycosis. Rev Inst Med Trop Sao Paulo 2015;57(Suppl 19):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Greene G, Lawrence DS, Jordan A, et al. Cryptococcal meningitis: a review of cryptococcal antigen screening programs in Africa. Expert Rev Anti Infect Ther 2020;1–12. 10.1080/14787210.2020.1785871. [DOI] [PubMed] [Google Scholar]
- 129.O’Halloran JA, Powderly WG, Spec A. Cryptococcosis today: it is not all about HIV infection. Curr Clin Microbiol Rep 2017;4(2):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McKenney J, Bauman S, Neary B, et al. Prevalence, correlates, and outcomes of cryptococcal antigen positivity among patients with AIDS, United States, 1986-2012. Clin Infect Dis 2015;60(6):959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hurtado JC, Castillo P, Fernandes F, et al. Mortality due to Cryptococcus neoformans and Cryptococcus gattii in low-income settings: an autopsy study. Sci Rep 2019;9(1):7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Diaz JH. The disease ecology, epidemiology, clinical manifestations, and management of emerging Cryptococcus gattii complex infections. Wilderness Environ Med 2020;31(1):101–9. [DOI] [PubMed] [Google Scholar]
- 133.Vanhove M, Beale MA, Rhodes J, et al. Genomic epidemiology of Cryptococcus yeasts identifies adaptation to environmental niches underpinning infection across an African HIV/AIDS cohort. Mol Ecol 2017;26(7):1991–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017;17(8):873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bongomin F, Oladele RO, Gago S, et al. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses 2018;61(5):290–7. [DOI] [PubMed] [Google Scholar]
- 136.Rajasingham R, Boulware DR. Cryptococcal Antigen Screening and Preemptive Treatment-How Can We Improve Survival? Clin Infect Dis 2020;70(8):1691–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mirza SA, Phelan M, Rimland D, et al. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992-2000. Clin Infect Dis 2003;36(6):789–94. [DOI] [PubMed] [Google Scholar]
- 138.Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PLoS One 2013;8(3):e60431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Osler M, Hilderbrand K, Goemaere E, et al. The continuing burden of advanced HIV disease over 10 years of increasing antiretroviral therapy coverage in South Africa. Clin Infect Dis 2018;66(suppl_2):S118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tenforde MW, Mokomane M, Leeme T, et al. Advanced human immunodeficiency virus disease in Botswana following successful antiretroviral therapy rollout: incidence of and temporal trends in cryptococcal meningitis. Clin Infect Dis 2017;65(5):779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cogliati M. Global Molecular Epidemiology of Cryptococcus neoformans and Cryptococcus gattii: an atlas of the molecular types. Scientifica (Cairo) 2013;2013:675213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc 2013;124:61–79. [PMC free article] [PubMed] [Google Scholar]
- 143.George IA, Santos CAQ, Olsen MA, et al. Epidemiology of Cryptococcosis and Cryptococcal Meningitis in a Large Retrospective Cohort of Patients After Solid Organ Transplantation. Open Forum Infect Dis 2017;4(1):ofx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Singh N, Alexander BD, Lortholary O, et al. Pulmonary cryptococcosis in solid organ transplant recipients: clinical relevance of serum cryptococcal antigen. Clin Infect Dis 2008;46(2):e12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nguyen MH, Husain S, Clancy CJ, et al. Outcomes of central nervous system cryptococcosis vary with host immune function: results from a multi-center, prospective study. J Infect 2010;61(5):419–26. [DOI] [PubMed] [Google Scholar]
- 146.Nascimento E, Vitali LH, Kress M, et al. Cryptococcus neoformans and C. gattii isolates from both HIV-infected and uninfected patients: antifungal susceptibility and outcome of cryptococcal disease. Rev Inst Med Trop Sao Paulo 2017;59:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev 2014;27(4):980–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Byrnes EJ 3rd, Li W, Lewit Y, et al. First reported case of Cryptococcus gattii in the Southeastern USA: implications for travel-associated acquisition of an emerging pathogen. PLoS One 2009;4(6):e5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bruner KT, Franco-Paredes C, Henao-Martinez AF, et al. Cryptococcus gattii complex infections in HIV-infected patients, southeastern United States. Emerg Infect Dis 2018;24(11):1998–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Julie Harris SL, Chiller Tom. Cryptococcus gattii: where do we go from here? Med Mycol 2012;50:113–29. [DOI] [PubMed] [Google Scholar]
- 151.Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States: epidemiologic, diagnostic, and clinical features. Natl Cancer Inst Monogr 1976;43:55–63. [PubMed] [Google Scholar]
- 152.Hay JW, Osmond DH, Jacobson MA. Projecting the medical costs of AIDS and ARC in the United States. J Acquir Immune Defic Syndr 1988;1(5):466–85. [PubMed] [Google Scholar]
- 153.Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis 2000;30(Suppl 1):S5–14. [DOI] [PubMed] [Google Scholar]
- 154.Buchacz K, Baker RK, Palella FJ Jr, et al. AIDS-defining opportunistic illnesses in US patients, 1994-2007: a cohort study. AIDS 2010;24(10):1549–59. [DOI] [PubMed] [Google Scholar]
- 155.Kelley CF, Checkley W, Mannino DM, et al. Trends in hospitalizations for AIDS-associated Pneumocystis jirovecii pneumonia in the United States (1986 to 2005). Chest 2009;136(1):190–7. [DOI] [PubMed] [Google Scholar]
- 156.Buchacz K, Lau B, Jing Y, et al. Incidence of AIDS-defining opportunistic infections in a multicohort analysis of HIV-infected persons in the United States and Canada, 2000-2010. J Infect Dis 2016;214(6):862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev 2012;25(2):297–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gerhart JL, Kalaaji AN. Development of Pneumocystis carinii pneumonia in patients with immunobullous and connective tissue disease receiving immunosuppressive medications. J Am Acad Dermatol 2010;62(6):957–61. [DOI] [PubMed] [Google Scholar]
- 159.Sizar O, Talati R. Sporotrichosis. [Updated 2020 Jun 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available at: https://www.ncbi.nlm.nih.gov/books/NBK532255. [Google Scholar]
- 160.Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, et al. Global epidemiology of sporotrichosis. Med Mycol 2015;53(1):3–14. [DOI] [PubMed] [Google Scholar]
- 161.Benedict KJ, Jackson BR. Sporotrichosis cases in commercial insurance data, United States, 2012–2018. Emerg Infect Dis 2020;26(11):2783–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mora-Montes HM. Special Issue "Sporothrix and Sporotrichosis. J Fungi (Basel) 2018;4(4). 10.3390/jof4040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and Sporotrichosis. Clin Microbiol Rev 2011;24(4):633–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.California Department of Health. Health advisory: resurgence of Candida auris in healthcare facilities in the setting of COVID-19. Available at: https://www.smchealth.org/sites/main/files/file-attachments/2020-08-19_mdroandcovid-19containmentcahan081920.pdf?1597881499. Accessed October 5, 2020.
- 165.Benedict K, Derado G, Mody RK. Histoplasmosis-associated hospitalizations in the United States, 2001-2012. Open Forum Infect Dis 2016;3(1):ofv219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lamoth F, Calandra T. Early diagnosis of invasive mould infections and disease. J Antimicrob Chemother 2017;72(suppl_1):i19–28. [DOI] [PubMed] [Google Scholar]
- 167.Sanguinetti M, Posteraro B, Beigelman-Aubry C, et al. Diagnosis and treatment of invasive fungal infections: looking ahead. J Antimicrob Chemother 2019;74(Suppl 2):ii27–37. [DOI] [PubMed] [Google Scholar]