Abstract
We wish to highlight that on encountering a suspicious hepatic and a colonic lesion, the possibility of TB should also be kept in mind apart from the obvious possibility of metastasis of a colonic cancer especially in an endemic country like Tunisia.
Keywords: case report, colonic cancer, gastrointestinal tuberculosis, ileocecal tuberculosis
We wish to highlight that on encountering a suspicious hepatic and a colonic lesion, the possibility of TB should also be kept in mind apart from the obvious possibility of metastasis of a colonic cancer especially in an endemic country like Tunisia.

1. INTRODUCTION
Tuberculosis (TB) remains a serious health concern in developing countries. Tunisia is identified as having a high TB burden. Extrapulmonary tuberculosis occurs in about 20% of tuberculosis 1 ; among them, abdominal tuberculosis constitutes about 10%. 2
The involvement of the gastrointestinal tract is rare, while the ileocecal region is the most affected site. 3 The diagnosis of tuberculosis in this part of the body is challenging since there are no specifics symptoms and signs. Ileocecal TB is a great mimicker; it may sometimes be misdiagnosed as Crohn's disease, colon cancer, ischemic colitis, or amebiasis. 4 Radiologic investigations play an important role in establishing the diagnosis, but the definite diagnosis is still based on histological findings.
We herein report a rare case of ileocecal and hepatic tuberculosis mimicking a metastatic cecal tumor.
2. CASE PRESENTATION
We present the case of a 42‐year‐old woman, with a family history of colon cancer (her father died of left‐sided colon cancer at the age of 58 years old), who presented to the emergency room with a 2‐week history of abdominal pain, fever, and weight loss of 8 kg. During the physical examination, the patient was presented with fever (38.3°), a normal blood pressure of 120/75 mm Hg, and a pulse rate of 86/min. She appeared pale and had abdominal tenderness and a 6 cm firm palpable mass in the right iliac fossa. Laboratory data found a high level of white blood cells (WBC: 8090 Ele/mm3) and C‐reactive protein (CRP: 153 mg/L). Complete blood count revealed severe microcytic hypochromic anemia (hemoglobin = 6.8 g/dL).
An enhanced CT scan of the abdomen showed a circumferential wall thickening of the cecum with fat infiltration and numerous enlarged mesenteric and retroperitoneal lymph nodes, measuring up to 19 mm in short axis. The appendix appeared also involved (wall thickness 8 mm) (Figure 1). Furthermore, the abdominal computed tomography (CT) demonstrated three hypodense low attenuation lesions (Figures 2 and 3) in segment IV and VIII of the liver. Given her oncological history, the diagnosis of an infected cecal tumor associated with liver metastasis was considered.
FIGURE 1.

Computed tomography of the abdomen showing circumferential wall thickening of the cecum (red arrow) with fat infiltration and enlarged mesenteric lymph nodes
FIGURE 2.
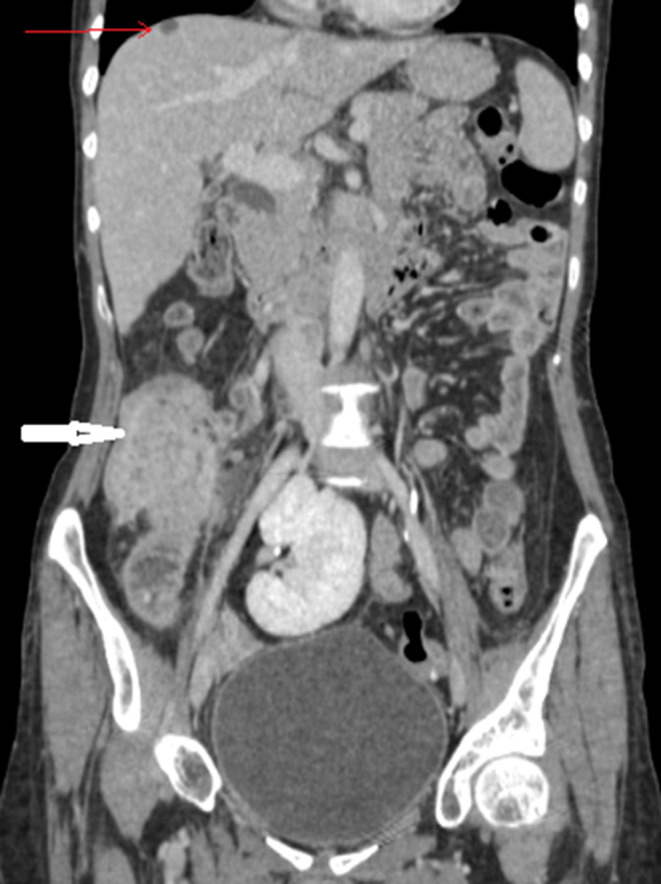
Hypodense lesion in segment VIII of the liver suggestive of metastasis (red arrow) with the ileocoecal mass (white arrow)
FIGURE 3.

Hypodense lesions in segment VIII and IV of the liver suggestive of metastasis (red arrows)
As the suspected tumor was doubly complicated by infection and bleeding, the patient was transfused with two units of packed red blood cells and was operated on without any delay via a midline incision. Intraoperatively, we found a wall thickening of the cecum with enlarged lymphadenopathies in the right iliac fossa with no abscess formation. Exploration revealed also multiple subscapular hepatic lesion of both the right and left lobe ranging in size from 0.5 cm to 2 cm suggestive of metastasis. There was no evidence of any free fluid or peritoneal deposits. A carcinologic right hemicolectomy with ileotransverse anastomosis was performed. The postoperative course was uneventful, and the patient was discharged on the fourth operative day.
Pathological examination of the resected specimen showed epithelioid and gigantocellular granulomas with caseating necrosis (Figure 4). The final diagnosis of ileocecal tuberculosis was then made. Based on the histopathology findings, anti‐tuberculous treatment was initiated with rifampicin, isoniazid, pyrazinamide, and ethambutol. Ultrasound of the abdomen done after 2 months showed the absence of any lesions in the liver which enables us to conclude that the previously described hepatic lesions are consistent with hepatic tuberculosis. Thus, the patient responded well to anti‐tubercular treatment and is still continuing with the treatment.
FIGURE 4.

Cut section of a lymph node showing white necrosis
3. DISCUSSION
Tuberculosis is a major public health problem in undeveloped countries; it affects mainly lungs. Many extrapulmonary localizations are reported in the literature. The gastrointestinal tract is the sixth most common site of dissemination, and the ileocecal region is known to be the most involved area accounting for 64% of cases of gastrointestinal TB. 5 , 6
The diagnosis of ileocecal tuberculosis is tricky as the latter can mimic other disorders like Crohn's disease, amebiasis, diverticulitis, and neoplasms of the colon. 7 This localization has no specific symptoms and may manifest as abdominal pain, fever, distention, vomiting, night sweats, weight loss, diarrhea, and lower gastrointestinal bleeding. 5
In our case, the ileocecal involvement was primary, and the patient had no evidence of pulmonary TB clinically and radiologically.
Colonoscopy has a major role in the diagnosis and management of ileocecal tuberculosis. The communist colonoscopy features are ulceration, nodular appearance, cecal mass, and ileocecal valve deformation. 8 As the diagnosis of ileocecal tuberculosis is based on histopathological findings, biopsies should be systematically performed. Anatomopathologists describe three histologic forms: ulcerative form (60%), hypertrophic form (10%), and ulcerohypertrophic form (30%). The latter mostly mimics malignancies. 9
In our case, colonoscopy was not performed because it was not available and the diagnosis of complicated carcinoma of the cecum was made based on the clinical and the radiological findings especially the coexistence of hepatic lesions. In such cases, CT scan can be useful showing thickening of the cecum and terminal ileum and abdominal lymph nodes. However, these features are not pathognomonic and the diagnosis of cecal carcinoma cannot be ruled out.
The conservative management is the cornerstone treatment of ileocecal tuberculosis. Anti‐tuberculous therapy should be started as soon as possible with a four‐drug regimen for 6 to 9 months. 10 Surgery should be considered only in complicated cases with intestinal obstruction, gastrointestinal fistula, perforation, and gastrointestinal hemorrhage.
If the diagnosis of ileocecal tuberculosis seems to be doubtful, further endoscopic and radiological investigations should be carried out to avoid unnecessary colonic resection.
Hepatic TB associated with ileocecal TB is a rare association that has very rarely been reported in the literature. This case illustrates the diagnostic difficulties of such an association. Hepatic TB is an extremely rare entity. It is classified into three types which include the miliary TB derived from generalized infection, the primary hepatic miliary TB, and the rarest nodular lesion named tuberculoma. 11
Hepatic TB does not have any pathognomonic clinical presentation, and its diagnosis requires liver specimen acquired by laparotomy, US, or CT‐guided biopsy. As far as the imaging methods are concerned, the imaging findings are generally not conclusive. The US can demonstrate mostly hypoechoic lesions, while the typical CT finding is the heterogeneity of the lesions which vary from hypodense to hyperdense. 12 It may sometimes be difficult to differentiate them from metastases or lymphoma involvement. 13
We wish to highlight that on encountering a suspicious hepatic and a colonic lesion, the possibility of TB should also be kept in mind apart from the obvious possibility of metastasis of colonic cancer especially in an endemic country like Tunisia.
4. CONCLUSION
Surgeons practicing in high tuberculosis prevalence countries should keep in mind the differential diagnosis of ileocecal tuberculosis to avoid unnecessary surgery.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
IBI: involved in Manuscript writing; IBI, HBC: involved in study concepts; SR: helped in data interpretation and manuscript evaluation; ZH: involved in data acquisition; AZ: involved in critical revision.
ETHICAL APPROVAL
Ethical approval was not required, and patient identifying knowledge was not presented in the report.
ACKNOWLEDGMENTS
Published with written consent of the patient.
Ben Ismail I, Rebii S, Ben Chaabane H, Hakim Z, Zoghlami A. Ileocecal and hepatic tuberculosis mimicking a metastatic cecal malignancy: Case report. Clin Case Rep. 2021;9:e04101. 10.1002/ccr3.4101
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Yang Z, Kong Y, Wilson F, et al. Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis. 2004;38:199‐205. [DOI] [PubMed] [Google Scholar]
- 2. Rathi P, Gambhire P. Abdominal tuberculosis. J Assoc Physicians India. 2016;64:38‐47. [PubMed] [Google Scholar]
- 3. Ben Ismail I, Zenaidi H, Rebii S, Zoghlami A. A pitfall in the diagnosis of ileocaecal tuberculosis. Int J Infect Dis. 2020;96:671‐672. [DOI] [PubMed] [Google Scholar]
- 4. Kawaratani H, Moriya K, Ishida K, et al. Cecal tuberculosis mimicking submucosal tumor. Intern Med. 2016;55(14):1859‐1863. [DOI] [PubMed] [Google Scholar]
- 5. Michalopoulos A, Papadopoulos VN, Panidis S, et al. Cecal obstruction due to primary intestinal tuberculosis: a case series. J Med Case Rep. 2011;5:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma R. Abdominal tuberculosis. Imaging Sci Today. 2009;14:6. [Google Scholar]
- 7. Niaz K, Shraf M. Intestinal tuberculosis: diagnostic dilemma. Professional Med J. 2010;17:532‐537. [Google Scholar]
- 8. Ergün M, Tunç B, Ülker A, Şaşmaz N. Doktorlar için bir klinik zorluk: abdominal tüberküloz 24 olgunun derlemesi. Endoskopi Dergisi. 2012;20:72‐76. [Google Scholar]
- 9. Chong VH, Lim KS. Gastrointestinal tuberculosis. Singapore Med J. 2009;50(6):638‐645. [PubMed] [Google Scholar]
- 10. Blumberg HM, Burman WJ, Chaisson RE, et al. American thoracic society/centers for disease control and prevention/infectious diseases society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603‐662. [DOI] [PubMed] [Google Scholar]
- 11. Spiegel CT, Tuazon CU. Tuberculous liver abscess. Tubercle. 1984;65(2):127‐131. [DOI] [PubMed] [Google Scholar]
- 12. Yu RS, Zhang SZ, Wu JJ, Li RF. Imaging diagnosis of 12 patients with hepatic tuberculosis. World J Gastroenterol. 2004;10(11):1639‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kakkar C, Polnaya AM, Koteshwara P, Smiti S, Rajagopal KV, Arora A. Hepatic tuberculosis: a multimodality imaging review. Insights Imaging. 2015;6(6):647‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


