Abstract
Growth performance and immune systems of tilapias could be improved by Lactobacillus rhamnosus GG (LGG) and Jerusalem artichoke. This research aimed to determine the effects of Jerusalem artichoke on LGG viability after drying and pelleting and their subsequent exposure to simulated gastrointestinal conditions. Fresh LGG cells were added into wall material solutions, including alginate (AL), alginate + milk powder (AM), and alginate + milk powder + Jerusalem artichoke at different concentrations (AMJ). The solutions were then spray dried to obtain LGG powders. The powder with the highest cell number was then selected to mix with tilapia feed mash and pelleted using a nonthermal feed extruder to obtain pelleted feed containing LGG and Jerusalem artichoke. The LGG viability spray dried powders and pelleted feed were analyzed for their cell counts after drying and after exposure to simulated gastrointestinal conditions. The result showed that the number of viable cells in AMJ was significantly higher than AM and AL after drying. The number of viable cells under both simulated gastric and bile salt fluids was improved with the increasing of Jerusalem artichoke concentrations. The number of viable cells after pelleting process could be maintained. LGG in the pelleted feed could also survive under the simulated gastric and bile salt conditions. The study indicates that JA enhanced LGG viability after drying and exposure to simulated gastrointestinal conditions. The pelleted feed containing LGG and Jerusalem artichoke could be applied in tilapia farming, providing convenience to the farmers, and valuable effects to the fish.
Keywords: Probiotics, Prebiotics, Encapsulation, Fish, Viability
Introduction
Nile tilapia (Oreochromis niloticus) is globally popular farm-raised fish worldwide, and one of Thailand’s most important freshwater fish of economic importance. In 2018, global production of Nile tilapia was approximately 4.5 million metric tons and ranked as the one of top three finfish produced in the world (FAO 2020). With the increasing of intensification and commercialization of Nile tilapia, the fish has been cultured intensively. Undoubtedly, this has created new challenges, such as the surge of bacterial infections, which is rapidly becoming a pressing issue for tilapia farming industry. It has been reported that outbreaks of Streptococcus agalactiae infections in farm-raised tilapia operation has caused more than 75% of fish mortality (Amal and Zamri-Saad 2011). In the last decade, S. agalactiae infection has been regarded as the worst disease resulting in huge economic losses (Yi et al. 2019). It was reported that less than 10% of red tilapia could survive after two-day S. agalactiae infection (Sirimanapong et al. 2018), while the outbreak in tilapia farms in China directly caused their financial losses of 1.0–1.5 billion (Li et al. 2013). However, it has been well-documented that the use of antibiotics and vaccination in farm-raised freshwater fish results in the emergence of drug resistant microorganisms, which may affect the human population by transmitting their resistant genes to human pathogens. Antibiotics could harm to beneficial colonic microorganisms in the fish and leave the residues in the fish products, causing dangers to human consumption (Aliyu-A et al. 2019). Therefore, European Union started to ban the use of antibiotics in animal production as growth promoters since 2006 (Denev et al. 2009). This has recently created opportunities to develop alternative approaches in feeding and health management practices for Nile tilapia. The use and development of new dietary supplements as probiotics, prebiotics, synbiotics, and phytobiotics has emerged as a potential strategy that can be implemented to grow healthy farm-raised tilapia.
Probiotics are “live microorganisms in which an adequate consumption of probiotics confers many beneficial effects on the host” (FAO/WHO, 2001). There have been many studies reported that probiotics provided health benefits to a variety of fish species such as the common carp (Yanbo and Zirong 2006), rainbow trout (Denev et al. 2009), and Nile tilapia (Aliyu-A et al. 2019). Lactobacillus rhamnosus GG, called as LGG, a probiotic found in the human gut, could promote growth performance, healthy intestinal structures, villus height, and gut mucosal production of the Nile tilapia (Pirarat et al. 2015). LGG could increase the number of mucous cells, consequently promoting secretion of mucus. This could help protect the fish from pathogenic infection, such as Streptococcus agalactiae, contributing to reduction of fish mortality (Pirarat et al. 2015). Besides, viable LGG could stimulate the immune systems of rainbow trout, lead to induction of inflammatory response, and increase of IgG, IgA, and IgM production, affecting enhancement of nonspecific humoral response (Hirano et al. 2003). However, it has been well-known that probiotic microorganisms are susceptible to heat, ice crystals, acids, hydrogen peroxide, oxygen (Cook et al. 2012) and these have brought concerns to the applications of probiotics in Nile tilapias. The fish has the small and sac-like stomach, consisting different types of mucins include neutral mucin and acid mucin, but lack of pepsin (Klahan et al. 2009), causing a low pH (1.0–2.5) conditions (Phrompanya et al. 2019; Morrison and Wright 1999). In addition, the intestine morphology of Nile tilapias is an unadorned tube, with pyloric ceca entirely absent. The intestinal ceca have several functions, including absorption, fermentation, storage, and breeding sites for gut microbiota (Smith et al. 2000). The digestive enzyme system of tilapias includes protease, amylase, lipase, and bile salts which are mainly found and active in the intestine and alkaline conditions (Klahan et al. 2009). Hence, retaining the high cell viability of probiotics after consumption as well as after formulation, processing, and storage is challenged for commercial productions of probiotic products. To overcome the difficulties, a well-established spray drying, technology has been applied to incorporate beneficial probiotics into wall materials to prevent the probiotics from unfavorable conditions, which could be implemented on an industrial scale (Rezvankhah et al. 2020; Rodrigues et al. 2020; Misra et al. 2021).
Beside probiotics, prebiotics, nondigestible dietary substance that can enhance the growth of probiotic microorganism, such as inulin and frutooligosaccharides (FOS) has also been considered to promote healthy fish guts. Specifically, prebiotic was defined as “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora that confers benefits upon host well-being and health”(Gibson et al. 2004). Jerusalem artichoke (JA) is an inulin sources containing high level of short chain and medium chain fructan for 42–53% and 64–71%, respectively (Boonanuntanasarn et al. 2018). Although JA has been used for food or animal feed and for the past two decades, alternative uses as functional food ingredients have been discovered. JA may enhance animal growth performance, including fish, poultry, and swine, modulate their intestinal microbiota, and improve their hematological and immune systems (Mouriño et al. 2012). Some studies have mentioned that inulin could be used as a thermal protectant to prevent probiotics from thermal process. It could increase thermal resistance of Lactobacillus casei MTCC 1423 (Govind and Nithyalakshmi 2011). Moreover, when it was combined with high resistance maize starch, it could also improve heat resistivity to Lactobacillus casei (Doherty et al. 2011). As JA has a high level of both inulin and FOS, it would probably function as an effective protective agent for LGG during microencapsulation.
Probiotics and prebiotics for fish are commercially available as powders. They are generally added directly into the fish ponds or mixed them with feed ingredients. However, these approaches may drastically reduce the number of survival probiotic cells. Incorporation of probiotics and prebiotics into pelleted fish feed could better guarantee that the fish would obtain those functional ingredients. It was reported that the fingerlings fed with mixed probiotic pelletized feed had greater percentage of weight gain and specific growth rate, while feed conversion ratio was lower than mixed probiotic feed (Sivakumar et al. 2020). In addition, it could be more favorable for the farmers as the pelleted feed incorporated with probiotics and prebiotics would be convenient to use and help to reduce time and labor for feed preparation. Yuan et al. (2017) mentioned that the production costs for tilapia mainly comprised fish feed, rental fee, and labor cost accounted for approximately 66, 11, and 6% of the total cost, respectively. The changes of those costs including the fish price certainly affected the net profit of tilapia. Moreover, it was reported that feeding tilapia with pelleted feed diets could provide higher yields and greater profits than basal feeds due to increase of growth rates and feed conversion ratio improvement (Neira et al. 2009). Therefore, the aims of this research were to develop pelleted feed containing probiotic LGG, and prebiotic JA for Nile tilapia and to investigate effects of JA on LGG viable cells after drying and their subsequent exposure to simulated gastric and intestinal fluids.
Materials and methods
Preparation of L. rhamnosus GG
Frozen cultures of LGG in 20% glycerol was reactivated into de Man Rogosa Sharpe (MRS) broth (BD Difco™, Maryland, USA) and incubated at 37 °C for 24 h (BE 500, Schwa Bach, Germany). The bacteria (50 mL) was then subsequently re-inoculated into MRS broth (1000 mL) and incubated at 37 °C for 16 h to reach its stationary phase. The cell pellets were harvested by centrifugation at 7000 × g, for 10 min at 4 °C (Sigma 2-16PK, Sartorius, Germany).
Spray drying of L.rhamnosus GG
JA powders was kindly provided by Phetchabun Research Station, Agro-Ecological System Research and Development Institute, Kasetsart University, Thailand. It contained 28 g/100 g of sugar, 39 g/100 g of inulin, and 16 g/100 g of fructo-oligosaccharide (Tiengtam et al. 2017). Five feed solutions containing LGG were prepared including alginate solution (18 g/L), alginate solution (18 g/L) with milk powder (200 g/L), and alginate solutions (18 g/L) with milk powder (200 g/L) and JA at different concentrations (100 g/L, 150 g/L, and 200 g/L). First, JA solution were prepared at the concentration of 100 g/L, 150 g/L, and 200 g/L by dissolving the JA powder in distilled water until the total soluble solid of the solution reached 10, 15, or 20 ºBrix, respectively. The JA solutions were then centrifuged at 5000 × g for 5 min to remove the remained particles. After that, 200 g/L of milk powder (containing 10.8 g/100 g of protein, 25.3 g/100 g of fat, and 57 g/100 g of lactose) was then added and magnetically stirred at room temperature until homogenously. Food-grade alginate (18 g/L) was then added into the solutions prior to resuspend the LGG cell pellets (~ 109 CFU/mL). Only alginate solution and alginate with milk powder solution were used as controls. The alginate solution was prepared by dissolving the alginate in distilled water and subsequently used to resuspend the LGG cells, while alginate with milk powder solution was prepared by adding alginate into milk powder solution before resuspending LGG. Each feed solution was fed into in a laboratory scale spray dryer (Buchi B-290, Buchi, Germany) at inlet temperature of 125 °C and outlet temperature of 65 °C with the flow rate of 300 mL/h) to obtain encapsulated LGG powders, namely encapsulated LGG in alginate (AL), encapsulated LGG in alginate and milk powder (AM), encapsulated LGG in alginate, milk powder, and JA at 100, 150, and 200 g/L (AMJ10, AMJ15, and AMJ20, respectively). After spray drying, the number of viable LGG in the powders was enumerated as well as under the simulated gastrointestinal tract and the cell survival (%) was calculated by the following equation, Cell survival (%) = (N/N0) × 100, where N and N0 were the number of viable LGG in the spray dried powders (log CFU/g) and the number of viable cells before spray drying (log CFU/g), respectively.
Determination of LGG viability after drying processes and under simulated Nile tilapia gastrointestinal tract
After spray drying
One gram of each LGG powders, namely AL, AM, AMJ10, AMJ15, and AMJ20, were resuspended in 9 mL of normal saline solution (0.85 g/100 mL). The serial dilution from 10–1 to 10–8 was then conducted. A pour-plate method was used to enumerate the cell number of LGG powder on MRS agars. The samples were then incubated at 37 °C for 48 h.
In vitro simulated gastric conditions and bile salt solutions
Simulated gastric fluids (SGF) of Nile tilapia were prepared using normal saline solution, adjusted pH to 1.5, 2.0, and 3.0 using 5 M hydrochloric solution (Lab scan, Analytical, Thailand). Exactly 0.5 g of spray dried LGG powders (AL, AM, AMJ10, AMJ15, and AMJ20) were placed into separated test tubes containing 4.5 mL of SGF. One mL of each sample was taken after incubation at 25 °C in a water bath for 0, 1, and 2 h (JSGI-250 J, Schwa Bach, Germany) and enumerated for the cell counts on MRS agars using a pour-plate method.
For bile tolerance determination, 0.5 mL of LGG fresh cells as control and 0.5 g of LGG powders (AL, AM, AMJ10, AMJ15, and AMJ20) were added into 4.5 mL of SGF at pH 2.0 and incubated at 25 °C for 1 h. After the incubation, the samples were then centrifuged. SGF was removed and separately replaced with 4.5 mL of 3 mL/100 mL, 5 mL/100 mL, and 10 mL/100 mL of tilapia bile salt. One mL of each sample was taken after further incubations of 1, 2, and 3 h at 25 °C and enumerated on MRS agars using a pour-plate method to determine for the number of viable cells.
Production of Nile tilapias pelleted feed containing LGG
To prepare the pelleted feed, dry feed ingredients, including fish meal (200 g/kg of total weight), soybean meal (130 g/kg of total weight), rice bran (150 g/kg of total weight), corn meal (120 g/kg of total weight), cassava chips (120 g/kg of total weight), and premix (50 g/kg of total weight) were ground, sterilized at 121 °C for 15 min, and dried in a hot-air oven (Contherm, Thermotec 2000, Germany) at 55 °C until their moisture contents reached 12–13%. Dry feed ingredients were then mixed with starch binding agent (30 g/kg of total feed ingredients), dried coconut meal as a floating aid (200 g/kg of total feed ingredients), and the selected LGG powder, AMJ20 (100 g/kg of feed ingredients). To obtain a feed mash, tap water (300 g/kg of total feed ingredients) was then added according to a manual of fish feed formulations for local fish farmers suggested by Department of Fisheries. The mash was conveyed to a single screw extruder and proceeded at room temperature to obtain pelleted feed containing LGG (PFL). The pellets were then dried at 50 °C for 8 h. PFL was evaluated for proximate analysis and cell viability after drying and under simulated gastrointestinal tract.
Cell viability in Nile tilapia pelleted feeds containing LGG
Exactly one-gram PFL was disintegrated by soaking in 1 M phosphate buffer pH 7.0 in a stomacher bag and subsequently placing it in a stomacher (stomacher®400 Circulator, Seward, UK) for 3 min prior to perform serial dilutions in normal saline solution (0.85 g/100 mL). LGG fresh cells and the selected LGG power were used as controls. The cell counts of LGG in all samples were enumerated on MRS agars using a pour-plate method.
Proximate analysis of Nile tilapia pelleted feed containing LGG
Moisture and protein contents were analyzed using standard methods of ISO 6496:1999 and ISO 5983–2:2005, respectively. Ash and total fat were determined by following the methods of AOAC (2016) 942.05 and AOAC (2016) 954.02, respectively. The total carbohydrate was calculated by the following equation; Total carbohydrate content (%) = 100 − (moisture content + protein content + total fat content + ash content).
Statistical analysis
Means and standard deviations of all values were calculated. Statistical analysis was performed using SPSS Statistics software (version 24). An one-way ANOVA and Duncan test were conducted to indicate differences of the data at the significance level of P ≤ 0.05.
Results and discussion
Production of spray dried LGG
After spray drying, the viability of LGG was obviously reduced. The number of viable cells in AL (4.00 ± 0.54 log CFU/g) was significantly lower than that of AM (5.87 ± 0.50 log CFU/g) and AMJ samples (P ≤ 0.05). The cell survival of AL and AM was 42.4 ± 5.62% and 62.1 ± 5.31%, respectively. The number of viable cells in AMJ samples was not significantly different, which were 7.01 ± 0.11, 6.84 ± 0.36, and 6.31 ± 0.16 log CFU/g for AMJ20, AMJ15, and AMJ10, respectively (Fig. 1), which was followed the suggested concentration for probiotic bacteria which should have at least 106 CFU/g to have an impact on the host health (Shah 2000). When compared with AM, AMJ20, and AMJ15 had significantly higher number of viable cells (P ≤ 0.05), showing that appropriate JA concentrations were needed to enhance the cell viability. In addition, the cell survival was undergone for 74.3 ± 1.11%. 72.4 ± 3.86%, and 66.8 ± 1.65% for AMJ20, AMJ15, and AMJ10, respectively. The results thus indicated that milk powder and JA played important roles in the cell viability during spray drying. It has been suggested that during drying water was removed from the bacteria cells, leading to cell membrane transition, and leakage resulting in the cells death (Doherty et al. 2011). Milk protein is well known for its ability to protect the cells from dehydration processes. The proteins in skim milk accumulate within the cells, helping stabilize cell membrane constituents, and reducing the difference in osmotic pressure between the internal and external environments (Mille et al. 2004). In addition, leakage of the cell membrane could be avoided by interaction of lactose in milk with the phosphate groups of phospholipids as well as proteins located in the cell membrane, consequently minimizing during spray drying (Maciel et al. 2014). In addition, it was recently reported that milk calcium possibly interacted with the bacterial cell wall, while milk proteins were aggregated during heat treatment. These probably resulted in the formation of a protective barrier, providing the protection to probiotics (Zheng et al. 2016; Wang et al. 2016a). Sugars and some polysaccharides, such as fructooligosaccharides (FOS) and inulin were reported to be able to protect the cells from dehydration. JA typically contains 39 g/100 mL of inulin and 16 g/100 mL of fructo-oligosaccharide (Khuenpet et al. 2017). It was reported that FOS and inulin could function as a water replacer, helping stabilize the cell membrane during dehydration (Doherty et al. 2011). Romano et al. (2018) revealed that the amorphous structures of inulin could protect L. plantarum from spray drying and their cell stability during storage directly, resulted from stabilization of inulin structures. In contrast, it was found that co-encapsulation of LGG in acidified goat milk with agave tequilana fructans, fructose-based polymers, by spray drying did not help increase the number of viable LGG after drying. The total cell counts were from 7.7 to 7.9 log CFU/g and the survival rate was approximately 87%. However, the agave tequilana fructans could improve the cell survival during storage at – 20 °C for 14 days (Alvarado-Reveles et al. 2019). The effects of divalent cations (Ca2+) on LGG viability during spray drying were investigated. The cells were encapsulated with lactose and trehalose containing 1 mM CaCl2. The results showed that the number of viable cells in the lactose/Ca and trehalose/Ca powders were reduced for 5% and 30%, respectively, which was lower than the other results using reconstituted skim milk, whey protein isolate, and concentrated sweet whey as protective agents (Su et al. 2019). When compared our finding with these results, it could suggest that sources of fructans and milk types possibly played an important role in effectiveness of the protective agents affecting the viability of LGG after spray drying.
Fig. 1.
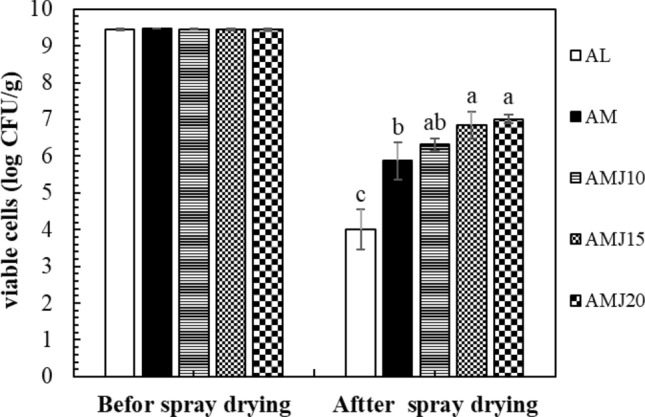
Viability of LGG powders before and after spray drying; AL LGG powder with only alginate, AM LGG powder with alginate and milk powder, AMJ10, AMJ15, and AMJ20 LGG powder with alginate, milk powder, and JA at 100, 150, and 200 g/L, respectively. a–bMeans ± standard deviation with different letters were significantly different (P ≤ 0.05)
Viability of spray dried LGG powders in simulated gastrointestinal conditions
In vitro simulated gastric conditions
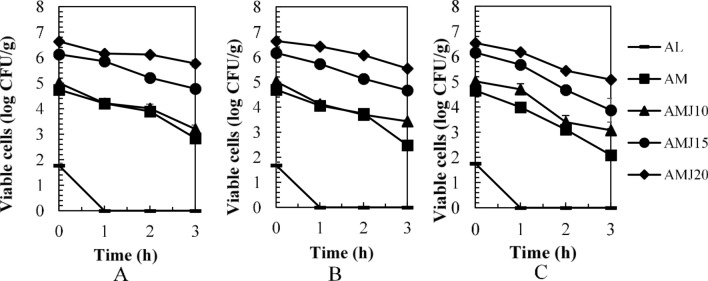
As well-known, pH in the medium played an important role in inhibiting bacterial activity by un-dissociated acid forms passing through the cell membrane, resulting in acidification of the cytoplasm and causing the cell death (Lund et al. 2014). The maintenance of probiotic viability throughout the stomach is crucial. Figure 2 showed the number of viable cells in LGG powders after 2-h exposure to simulated gastric fluids (SGF) at different pH. LGG survived in the SGF at pH 3.0 and 2.0. The number of viable cells of all treatments at pH 3.0 and 2.0 was not significantly different. However, remarkable decreases in the number of viable cells were significantly observed in SGF at pH 1.5. Regarding pH 1.5, the viable cell counts were enhanced by encapsulating LGG in milk powder and JA. The cell log reductions in AMJ20, AMJ15, and AMJ10 were 1.21, 1.67, and 2.31, respectively, while AM, having only milk powder, had 3.02 log reduction. No viable cells were detected in the control or in AL. AMJ20 and AMJ15 had survival rate at 84.10 ± 2.43% and 75.82 ± 4.76%, respectively (Fig. 3), which were significantly greater than that of AMJ10 (65.25 ± 6.76%) and AM (49.69 ± 3.48%) (P ≤ 0.05). This finding was similar with the results reported by Pirarat et al. (2015) mentioning that unencapsulated LGG failed to survive under the simulated gastric fluids at the pH of 1.5 (SGF). Besides, it was reported that the number of viable LGG encapsulated by alginate–skim milk gel extrusion method was reduced about 1 log cycle after exposure to the SGF for 3 h. When compared the results to our study, it was suggested that the encapsulation method could interfere with the cell viability when they were under a high acidic environment.
Fig. 2.
Viability of LGG powders in simulated gastric fluids (pH 1.5 (A), pH 2.0 (B), and pH 3.0 (C)); AL LGG powder with only alginate, AM LGG powder with alginate and milk powder, AMJ10, AMJ15, and AMJ20 LGG powder with alginate, milk powder, and JA at 100, 150, and 200 g/L, respectively
Fig. 3.

Survival rates of LGG powders after incubating in low pH conditions for 2 h; AL LGG powders with only alginate, AM LGG powder with alginate and milk powder, AMJ10, AMJ15, and AMJ20 LGG powder with alginate, milk powder, and JA at 100, 150, and 200 g/L respectively. a–cMeans ± standard deviation with different letter within the same color was significantly different (P ≤ 0.05). A,BMeans ± standard deviation with different letters within the same pH was significantly different (P ≤ 0.05)
The results also indicated that JA could protect LGG from high acid conditions. This was possibly attributed to chemical interactions between alginate and inulin of JA. It was reported that alginate was able to create interactions with inulin by the nucleophilic attack between the COO− group of alginate and OH− group of inulin, which leading to alginate–inulin matrix formation. The matrix probably improved the barrier protection to the bacterial cells by mitigating the migration of acid fluids to the cells. Moreover, the presence of inulin in alginate matrix could decrease the porosity of the alginate when it was in gastric environment with low pH (Atia et al. 2016).
In vitro tilapia bile salt conditions
The viability of LGG powders after incubating in bile salt conditions (3 mL/100 mL, 5 mL/100 mL, and 10 mL/100 mL of fluids) for 3 h was shown in Fig. 4. Bile salt is as an emulsifier and fat solubilizer that could hydrolyze plasma membranes of bacteria cells. This causes the cells to lose their cell wall integrity (Bustos et al. 2018). No viable cells were detected in the control (AL) for all bile salt conditions after exposure for 1 h. The cell losses were increased with the increasing bile salt concentrations in all treatments and control. Regardless of bile salt concentrations, after 3-h incubating in bile salts, AMJ20 had the lowest cell reductions, which were 0.86, 1.10, and 1.27 log reductions for 3 mL/100 mL, 5 mL/100 mL, and 10 mL/100 mL of tilapia bile salt, respectively, followed by AMJ15, AMJ10, and AM. In regard to survival rates of LGG, at 3 mL/100 mL of fluids and 5 mL/100 mL of fluids, AMJ20 had significantly greater survival rates than AMJ15 and AMJ10, while AM had the lowest survival rate (P ≤ 0.05). Similarly, the LGG survival rate of AMJ-20 in bile salt at the concentration of 10 mL/100 mL of fluids was 80.52 ± 0.46%, which was significantly higher than AMJ15 (62.78 ± 7.52%) and AMJ10 (61.26 ± 1.39%) and followed by AM (44.93 ± 2.74%) (P ≤ 0.05) (Fig. 5). Therefore, this indicated that milk powder could protect LGG in the powders from bile salt effects. JA had ability to enhance the protection of the cells during bile salt conditions. The results were compatible with those of Pirarat et al. (2015) mentioning that skim milk–alginate matrix could improve cell viability of LGG after being exposed in 10% tilapia bile when compared with only alginate matrix and fresh cells. This could be due to protein in skim milk possibly acting as an insoluble matrix protecting probiotics in the gastrointestinal tract (Ying et al. 2013). In addition, alginate and skim milk could structurally bind together increasing the matrix resistance to the effects of bile salt solutions. As mentioned earlier, JA was a rich source of inulin and FOS. After incubating in 1% bile salt solution for 2 h, L. plantarum that were encapsulated in skim milk coated with combined inulin and alginate beads by an extrusion method showed 1.21 log CFU/mL reduction (Wang et al. 2016b). The addition of inulin could enhance the acid and bile tolerance of encapsulated L. acidophilus and L. casei powders when they were under simulated gastric juice at pH 1.55 followed by intestinal juice containing 0.6% bile salt for 2 h and 2.5 h, respectively (Krasaekoopt and Watcharapoka 2014). Spray drying of L. acidophilus La-5 with 10% of inulin and 6% of FOS as coating agents could significantly reduce the number of cell reduction when they were in simulated intestinal phases. Moreover, Xavier dos Santos et al. (2019) noted that protection provided by the coating agents was further elevated by food products. This indicates another possible advantage of incorporating the probiotic into the fish feed.
Fig. 4.
Viability of LGG powders in bile salt conditions (bile salt 3 mL/10 mL (A), bile salt 5 mL/100 mL (B), and bile salt 10 mL/100 mL (C)); AL LGG powder with only alginate, AM LGG powder with alginate and milk powder, AMJ10, AMJ15, and AMJ20 LGG powder with alginate, milk powder, and JA at 100, 150, and 200 g/L, respectively
Fig. 5.

Survival rates of LGG powders after incubating in bile salt conditions for 3 h; AL LGG powder with only alginate, AM LGG powder with alginate and milk powder, AMJ10, AMJ15, and AMJ20 LGG powder with alginate, milk powder, and JA at 100, 150, and 200 g/L respectively. a–cMeans ± standard deviation with different letter within the same color was significantly different (P ≤ 0.05). A,BMeans ± standard deviation with different letters within the same pH was significantly different (P ≤ 0.05)
Cell viability in Nile tilapia pelleted feeds containing LGG
Nile tilapia pelleted feeds containing LGG (PFL) were composed of 55.35 g of carbohydrate/100 g dry feed matter, 24.54 g of protein/100 g dry feed matter, 8.66 g of fat/100 g dry feed matter, and 11.13 g of ash/100 g dry feed matter. The moisture content of PFL was 2.71 g/100 g of PFL after drying at 50 °C for 8 h. It was recommended that crude protein levels of tilapia feeds should not under 20 g/100 g due to poor palatability. Feed containing approximately 25 g/100 g of crude protein were commonly used in Thailand as it was cost effective (Bhujel 2013). After pelleting and drying, the final cell concentration of LGG in PFL was 7.08 ± 0.04 log CFU/g, which was not significantly different with the spray dried LGG powder (7.23 ± 0.06 log CFU/g). This indicated that the pelleting and drying processes did not affect the cell viability of LGG powder. The number of viable cells in probiotic products to provide benefits to the host should have at least 106 CFU/g (Anal and Singh 2007).
In the simulated gastrointestinal fluids, after incubating the PFL in a low pH condition (pH 2.0) for 1 h prior to expose to bile salt solution at 10 mL/100 mL for 3 h as shown in Fig. 6, the cell viability in PFL was reduced by 0.57 log CFU/g, which was not significantly different form the spray dried LGG (0.41 log reduction), while rapid loss of LGG fresh cells was detected (3.94 log reduction). The number of viable cells after 1 h incubation in SGF at pH 2.0 was 6.51 ± 0.02, 6.82 ± 0.02, and 5.83 ± 0.04 log CFU/g for PFL, spray dried LGG, and fresh cells, respectively. Regarding bile salt tolerance, the results showed that the number of viable cells in PFL was 5.04 ± 0.03 log CFU/g, followed by the spray dried LGG (5.22 ± 0.04 log CFU/g), which had undergone 2.04 and 2.01 log reduction, respectively. The results were not significantly different. In contrast, there was a massive decrease in viable cells of the fresh cell, which was 7.84 log cycles. There were only 1.93 ± 0.02 log CFU/g survived. The survival rates after 3 h in the bile salt solution were 77.42 ± 0.43%, 76.53 ± 0.44%, and 33.1 ± 0.05% for PFL, spray dried LGG, and fresh cells, respectively. The addition of spray dried LGG into the pelleted feed could potentially provide not only high nutritional and functional values, but also convenience of use as well as decrease of feed preparation times and labor.
Fig. 6.
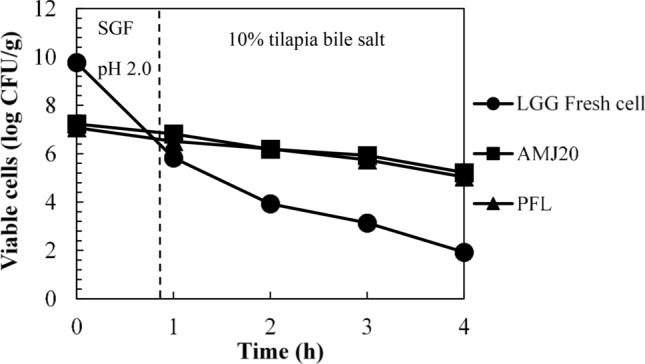
Viability of LGG in Nile tilapia pelleted feeds during exposure to simulated gastrointestinal condition; Fresh cell LGG grown in MRS broth for 24 h, AMJ20 LGG powder with alginate, milk powder, and JA at 200 g/L, PLF pelleted feed containing LGG
Conclusion
In conclusion, the results indicated that combination of JA with alginate and milk powder significantly improved viability of LGG under spray drying and under simulated gastrointestinal fluids. The spray dried LGG with JA could be incorporated into the feed ingredients to be pelleted and be maintained during nonthermal pelleting process and hot air drying. The result suggests that JA could be used as a potential protective agent for probiotic powders and pelleted LGG powder with feed ingredients could be applied in Nile tilapia farming, providing convenience to the farmers and valuable effects to the fish.
Acknowledgements
This work was financially supported by Thailand Research Fund (MRG6080108).
Declarations
Conflict of interest
The authors declared that there were no potential conflicts of interest with respect to authorship or research publication of this article.
References
- Aliyu-A A, Aliyu-Paiko M, Abafi J, Abdul-Malik A, Adamu K, King M. Fermentation of feed ingredients as potential strategy to improve health status and reduce opportunistic pathogens in fish farming. Asian J Biotechnol Bioresour Technol. 2019;5:1–17. [Google Scholar]
- Alvarado-Reveles O, Fernández-Michel S, Jiménez-Flores R, Cueto-Wong C, Vázquez-Moreno L, Montfort GR-C. Survival and goat milk acidifying activity of Lactobacillus rhamnosus GG encapsulated with agave fructans in a buttermilk protein matrix. Probiotics Antimicro. 2019;11(4):1340–1347. doi: 10.1007/s12602-018-9475-y. [DOI] [PubMed] [Google Scholar]
- Amal M, Zamri-Saad M. Streptococcosis in tilapia (Oreochromis niloticus): a review. Pertanika J Trop Agric Sci. 2011;34(2):195–206. [Google Scholar]
- Anal AK, Singh H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci Technol. 2007;18(5):240–251. doi: 10.1016/j.tifs.2007.01.004. [DOI] [Google Scholar]
- Atia A, Gomaa A, Fliss I, Beyssac E, Garrait G, Subirade M. A prebiotic matrix for encapsulation of probiotics: physicochemical and microbiological study. J Microencapsul. 2016;33(1):89–101. doi: 10.3109/02652048.2015.1134688. [DOI] [PubMed] [Google Scholar]
- Bhujel RC (2013) On-farm feed management practices for nile tilapia (Oreochromis niloticus) in thailand, On-farm feeding and feed management in aquaculture. FAO Fisheries and Aquaculture Technical Paper No. 038, Rome, Italy.
- Boonanuntanasarn S, Tiengtam N, Pitaksong T, Piromyou P, Teaumroong N. Effects of dietary inulin and jerusalem artichoke (Helianthus tuberosus) on intestinal microbiota community and morphology of nile tilapia (Oreochromis niloticus) fingerlings. Aquac Nutr. 2018;24(2):712–722. doi: 10.1111/anu.12600. [DOI] [Google Scholar]
- Bustos AY, de Valdez GF, Fadda S, Taranto MP. New insights into bacterial bile resistance mechanisms: the role of bile salt hydrolase and its impact on human health. Food Res Int. 2018;112:250–262. doi: 10.1016/j.foodres.2018.06.035. [DOI] [PubMed] [Google Scholar]
- Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Microencapsulation of probiotics for gastrointestinal delivery. J Control Release. 2012;162(1):56–67. doi: 10.1016/j.jconrel.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Denev S, Beev G, Staykov Y, Moutafchieva R. Microbial ecology of the gastrointestinal tract of fish and the potential application of probiotics and prebiotics in finfish aquaculture. Int Aquat Res. 2009;1(1):1–29. [Google Scholar]
- Doherty S, Gee V, Ross R, Stanton C, Fitzgerald G, Brodkorb A. Development and characterisation of whey protein micro-beads as potential matrices for probiotic protection. Food Hydrocoll. 2011;25(6):1604–1617. doi: 10.1016/j.foodhyd.2010.12.012. [DOI] [Google Scholar]
- FAO (2020) The state of world fisheries and aquaculture 2020. Sustainability in action. FAO, Rome, Italy. http://www.fao.org/documents/card/en/c/ca9229en/. Accessed 24 April 2021.
- Gibson GR, Probert HM, Van Loo J, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17(2):259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Govind B, Nithyalakshmi V. Influence of prebiotic composition on probiotic survivability in calcium alginate coated synbiotic microcapsules at thermal incubation. Agric Res J. 2011;6(5):231–236. [Google Scholar]
- Hirano J, Yoshida T, Sugiyama T, Koide N, Mori I, Yokochi T. The effect of Lactobacillus rhamnosus on enterohemorrhagic Escherichia coli infection of human intestinal cells in vitro. Med Microbiol. 2003;47(6):405–409. doi: 10.1111/j.1348-0421.2003.tb03377.x. [DOI] [PubMed] [Google Scholar]
- Khuenpet K, Jittanit W, Sirisansaneeyakul S, Srichamnong W. Inulin powder production from Jerusalem artichoke (Helianthus tuberosus l.) tuber powder and its application to commercial food products. J Food Process Preserv. 2017;41(4):e13097. doi: 10.1111/jfpp.13097. [DOI] [Google Scholar]
- Klahan R, Areechon N, Yoonpundh R, Engkagul A. Characterization and activity of digestive enzymes in different sizes of nile tilapia (Oreochromis niloticus l.) Agric Nat Resour. 2009;43(1):143–153. [Google Scholar]
- Krasaekoopt W, Watcharapoka S. Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT. 2014;57(2):761–766. doi: 10.1016/j.lwt.2014.01.037. [DOI] [Google Scholar]
- Li L, Wang R, Liang W, Gan X, Huang T, Huang Y, Li J, Shi Y, Chen M, Luo H. Rare serotype occurrence and pfge genotypic diversity of Streptococcus agalactiae isolated from tilapia in china. Vet Microbiol. 2013;167(3):719–724. doi: 10.1016/j.vetmic.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Lund P, Tramonti A, De Biase D. Coping with low ph: Molecular strategies in neutralophilic bacteria. FEMS Microbiol Rev. 2014;38(6):1091–1125. doi: 10.1111/1574-6976.12076. [DOI] [PubMed] [Google Scholar]
- Maciel G, Chaves K, Grosso C, Gigante M. Microencapsulation of Lactobacillus acidophilus LA-5 by spray-drying using sweet whey and skim milk as encapsulating materials. J Dairy Sci. 2014;97(4):1991–1998. doi: 10.3168/jds.2013-7463. [DOI] [PubMed] [Google Scholar]
- Mille Y, Obert JP, Beney L, Gervais P. New drying process for lactic bacteria based on their dehydration behavior in liquid medium. Biotechnol Bioeng. 2004;88(1):71–76. doi: 10.1002/bit.20211. [DOI] [PubMed] [Google Scholar]
- Misra S, Pandey P, Mishra HN. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: a review. Trends Food Sci Technol. 2021;109:340–351. doi: 10.1016/j.tifs.2021.01.039. [DOI] [Google Scholar]
- Morrison CM, Wright JR., Jr A study of the histology of the digestive tract of the nile tilapia. J Fish Biol. 1999;54(3):597–606. doi: 10.1111/j.1095-8649.1999.tb00638.x. [DOI] [Google Scholar]
- Mouriño J, Do Nascimento Vieira F, Jatobá A, Da Silva B, Jesus G, Seiffert W, Martins M. Effect of dietary supplementation of inulin and w. Cibaria on haemato-immunological parameters of hybrid surubim (Pseudoplatystoma sp) Aquac Nutr. 2012;18(1):73–80. doi: 10.1111/j.1365-2095.2011.00879.x. [DOI] [Google Scholar]
- Neira I, Engle CR, Ngugi C. Economic and risk analysis of tilapia production in kenya. J Appl Aquac. 2009;21(2):73–95. doi: 10.1080/10454430902892842. [DOI] [Google Scholar]
- Phrompanya P, Saenphet K, Saenphet S. Comparative histochemical study of the gastrointestinal tracts of the nile tilapia (Oreochromis niloticus) and the hybrid catfish (clarias batrachus x clarias gariepinus) Acta Histochem. 2019;121(3):261–267. doi: 10.1016/j.acthis.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Pirarat N, Pinpimai K, Rodkhum C, Chansue N, Ooi EL, Katagiri T, Maita M. Viability and morphological evaluation of alginate-encapsulated Lactobacillus rhamnosus GG under simulated tilapia gastrointestinal conditions and its effect on growth performance, intestinal morphology and protection against streptococcus agalactiae. Anim Feed Sci Technol. 2015;207:93–103. doi: 10.1016/j.anifeedsci.2015.03.002. [DOI] [Google Scholar]
- Rezvankhah A, Emam-Djomeh Z, Askari G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: a review. Dry Technol. 2020;38(1–2):235–258. doi: 10.1080/07373937.2019.1653906. [DOI] [Google Scholar]
- Rodrigues FJ, Cedran MF, Bicas JL, Sato HH. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications—a narrative review. Food Res Int. 2020;137:109682. doi: 10.1016/j.foodres.2020.109682. [DOI] [PubMed] [Google Scholar]
- Romano N, Mobili P, Zuñiga-Hansen ME, Gómez-Zavaglia A. Physico-chemical and structural properties of crystalline inulin explain the stability of Lactobacillus plantarum during spray-drying and storage. Food Res Int. 2018;113:167–174. doi: 10.1016/j.foodres.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Shah NP. Probiotic bacteria: selective enumeration and survival in dairy foods. J Dairy Sci. 2000;83(4):894–907. doi: 10.3168/jds.S0022-0302(00)74953-8. [DOI] [PubMed] [Google Scholar]
- Sirimanapong W, Thompson KD, Shinn AP, Adams A, Withyachumnarnkul B. Streptococcus agalactiae infection kills red tilapia with chronic francisella noatunensis infection more rapidly than the fish without the infection. Fish Shellfish Immunol. 2018;81:221–232. doi: 10.1016/j.fsi.2018.07.022. [DOI] [PubMed] [Google Scholar]
- Sivakumar K, Rama MS, Janani R, Muthupriya P, Magesh R. Effect of probiotic dietary on growth performances and feed utilization of cyprinus carpio fingerlings. Bull Pure Appl Sci Zool. 2020;39(2):463. doi: 10.5958/2320-3188.2020.00053.4. [DOI] [Google Scholar]
- Smith BJ, Smith SA, Tengjaroenkul B, Lawrence TA. Gross morphology and topography of the adult intestinal tract of the tilapian fish (Oreochromis niloticus) Cells Tissues Organs. 2000;166(3):294–303. doi: 10.1159/000016743. [DOI] [PubMed] [Google Scholar]
- Su Y, Zheng X, Zhao Q, Fu N, Xiong H, Wu WD, Chen XD. Spray drying of Lactobacillus rhamnosus GG with calcium-containing protectant for enhanced viability. Powder Technol. 2019;358:87–94. doi: 10.1016/j.powtec.2018.09.082. [DOI] [Google Scholar]
- Tiengtam N, Paengkoum P, Sirivoharn S, Phonsiri K, Boonanuntanasarn S. The effects of dietary inulin and Jerusalem artichoke (Helianthus tuberosus) tuber on the growth performance, haematological, blood chemical and immune parameters of nile tilapia (Oreochromis niloticus) fingerlings. Aquac Res. 2017;48(10):5280–5288. doi: 10.1111/are.13341. [DOI] [Google Scholar]
- Wang J, Huang S, Fu N, Jeantet R, Chen XD. Thermal aggregation of calcium-fortified skim milk enhances probiotic protection during convective droplet drying. J Agric Food Chem. 2016;64(30):6003–6010. doi: 10.1021/acs.jafc.6b02205. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu X, Xu H, Aguilar ZP, Wei H. Effect of skim milk coated inulin-alginate encapsulation beads on viability and gene expression of Lactobacillus plantarum during freeze-drying. LWT. 2016;68:8–13. doi: 10.1016/j.lwt.2015.12.001. [DOI] [Google Scholar]
- Xavier dos Santos D, Casazza AA, Aliakbarian B, Bedani R, Saad SMI, Perego P. Improved probiotic survival to in vitro gastrointestinal stress in a mousse containing Lactobacillus acidophilus LA-5 microencapsulated with inulin by spray drying. LWT. 2019;99:404–410. doi: 10.1016/j.lwt.2018.10.010. [DOI] [Google Scholar]
- Yanbo W, Zirong X. Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim Feed Sci Technol. 2006;127(3–4):283–292. doi: 10.1016/j.anifeedsci.2005.09.003. [DOI] [Google Scholar]
- Yi M, Wang M, Li Z, Liu Z, Song C, Zhang D, Gao F, Ke X, Cao J, Lu M. An investigation into the effects of streptococcus agalactiae on the 5-ht system and the behavior of gift tilapia (oreochromis niloticus) Aquac Rep. 2019;15:100232. doi: 10.1016/j.aqrep.2019.100232. [DOI] [Google Scholar]
- Ying D, Schwander S, Weerakkody R, Sanguansri L, Gantenbein-Demarchi C, Augustin MA. Microencapsulated lactobacillus rhamnosus gg in whey protein and resistant starch matrices: Probiotic survival in fruit juice. J Funct Foods. 2013;5(1):98–105. doi: 10.1016/j.jff.2012.08.009. [DOI] [Google Scholar]
- Yuan Y, Yuan Y, Dai Y, Gong Y. Economic profitability of tilapia farming in china. Aquac Int. 2017;25(3):1253–1264. doi: 10.1007/s10499-017-0111-8. [DOI] [Google Scholar]
- Zheng X, Fu N, Huang S, Jeantet R, Chen XD. Exploring the protective effects of calcium-containing carrier against drying-induced cellular injuries of probiotics using single droplet drying technique. Food Res Int. 2016;90:226–234. doi: 10.1016/j.foodres.2016.10.034. [DOI] [PubMed] [Google Scholar]