Abstract
Background
The novel Coronavirus (SARS‐CoV‐2) has caused almost 2 million deaths worldwide. Both Food and Drug Administration and European Medicines Agency have recently approved the first COVID‐19 vaccines, and a few more are going to be approved soon.
Methods
Several different approaches have been used to stimulate the immune system in mounting a humoral response. As more traditional approaches are under investigation (inactivated virus vaccines, protein subunit vaccines, recombinant virus vaccines), more recent and innovative strategies have been tried (non‐replicating viral vector vaccines, RNA based vaccines, DNA based vaccines).
Results
Since vaccinations campaigns started in December 2020 in both the US and Europe, gastroenterologists will be one of the main sources of information regarding SARS‐CoV 2 vaccination for patients in their practice, including vulnerable patients such as those with Inflammatory Bowel Disease (IBD), patients with chronic liver disease, and GI cancer patients.
Conclusions
Thus, we must ourselves be well educated and updated in order to provide unambiguous counseling to these categories of vulnerable patients. In this commentary, we aim to provide a comprehensive review of both approved COVID‐19 vaccines and the ones still under development, and explore potential risks, benefits and prioritization of vaccination.
Keywords: Coronavirus, endoscopy, prevention, public health, vaccine
INTRODUCTION
Since December 2019, when the World Health Organization (WHO) was informed of the first cases of pneumonia of unknown etiology,1 the novel Coronavirus (SARS‐CoV‐2) has caused more than 94,000,000 cases and almost 2 million deaths worldwide, as of 16th January.2 The world community has responded to the deadly challenge of Coronavirus‐related disease (COVID‐19) by relying on several public containment measures in order to slow down the spread of the virus.3, 4 As of today, no drug has been proved to be a game‐changer in the fight against the COVID‐19,5, 6 and our hope for an end to this pandemic led to an unprecedented fast track path for developing a reliable vaccine. (Table 1)
TABLE 1.
Developed and developing COVID‐19 vaccines. EUA: emergency use authorization; MHRA: Medicines and Healthcare Products Regulatory Agency; DCGI: Drugs Controller General of India
| Category | Name | Developer | Target | Schedule | Phase | Comments |
|---|---|---|---|---|---|---|
| mRNA | BNT162b2 | BioNTech–Pfizer | Prefusion stabilized, membrane‐anchored, full‐length spike protein | Two doses (30 μg; day 0, day 21) | Post‐EUA | 95% efficacy. Protection against severe disease. No differences in subgroups. |
| Cold chain logistic difficulties. | ||||||
| Anaphylaxis incidence: approx. 1 in 100′000. | ||||||
| mRNA | mRNA‐1273 | Moderna | Prefusion stabilized, full‐length spike protein | Two doses (100 μg; day 0, day 28) | Post‐EUA | 94% efficacy. Protection against severe disease. No differences in subgroups. |
| Similar excipient composition to BNT162b2 | ||||||
| Nonreplicating adenovirus | ChAdOx1 nCoV‐19 (AZD1222) | AstraZeneca and University of Oxford | Full length spike protein | Two doses (4 weeks apart) | Phase 3 | Nonreplicating simian adenovirus vector ChAdOx1. |
| MHRA and DCGI EUA. | ||||||
| No profit. | ||||||
| Nonreplicating adenovirus | Ad26.COV2.S | Janssen | Stabilized prefusion spike protein | Single dose | Phase 3 | Nonreplicating adenovirus serotype 26 vector. |
| Phase 3 enrollment completed in Dec 2020. Interim data available by late January. | ||||||
| Protein subunit | NVX‐CoV2373 | Novavax | Stable prefusion protein antigen of the spike protein | Two doses (day 0, day 21) | Phase 3 | Glycoprotein nanoparticle with Matrix M1 adjuvant. |
All trials compared the safety and efficacy of the vaccine against normal saline, except for ChAdOx1 nCoV‐19 that was compared to Meningococcal group A, C, W, and Y conjugate vaccine or normal saline. All vaccines are administered intramuscularly.
Though primarily considered as a respiratory disease, gastroenterologists had to face the SARS‐CoV 2 pandemic in different ways in their everyday practice. First, COVID‐19 may affect various systems including the digestive tract, causing gastrointestinal (GI) symptoms such as diarrhea, nausea, and abdominal pain in around 12% of patients.7 Furthermore, the risk of exposure of health care workers has been relevant in endoscopy units, considering that COVID‐19 is spread via an airborne route. Indeed, endoscopy demands short physical distance from patients to personnel and endoscopists are exposed to various biological material.8, 9, 10 This risk could be even more relevant considering the detection of SARS‐CoV 2 in biopsy specimens and stool, suggesting a possible faecal–oral transmission.7 However, adequate use of personal protective equipment and other infection control measures11 seemed to lead to a low risk of COVID‐19 transmission in GI endoscopy units.12, 13, 14
After Food and Drug Administration (FDA) and European Medicines Agency (EMA) approval, vaccinations campaigns started in December 2020 in both the US and Europe. Gastroenterologists will be one of the main sources of information regarding SARS‐CoV 2 vaccination for patients in their practice, including vulnerable patients such as those with Inflammatory Bowel Disease (IBD),15 patients with chronic liver disease, and GI cancer patients.16 Thus, we must ourselves be well educated and updated in order to provide unambiguous counseling to these categories of vulnerable patients.
In this commentary, we provide a comprehensive review of both approved COVID‐19 vaccines and the ones still under development, and explore potential risks, benefits and prioritization of vaccination in order to properly guide patients' awareness and choices.
SARS‐CoV 2 VACCINES
Coronaviruses are single‐stranded, positive‐sense RNA enveloped viruses.17, 18 Two Alphacoronaviruses (229E and NL63) and two Betacoronaviruses lineage A (OC43 and HKU1) are known to elicit common cold symptoms and are endemic.19 Severe acute respiratory syndrome coronavirus (SARS‐CoV) and Middle East respiratory syndrome coronavirus (MERS‐CoV) are Betacoronaviruses with epidemic spread and may cause lethal infection in humans. SARS‐CoV‐2 shares 50% genome sequence identity with MERS‐CoV and 79% with SARS‐CoV.20
The first attempts to develop a vaccine against SARS‐CoV began in the early 2000s,21, 22 but trials were stopped because of the disappearance of the disease. MERS‐CoV vaccines are under active development.
Receptor binding and membrane fusion of the virion are mediated by a single surface protein, the spike protein. SARS‐CoV‐2 shares more than 90% amino acid identity with SARS‐CoV, with the most differences in the spike protein.20, 23 The receptor‐binding domain of the spike protein of SARS‐CoV‐2 binds to angiotensin‐converting enzyme 2 (ACE2)24 receptors in the nasal cavity, the respiratory tract and other ACE2 receptor locations including intestinal enterocytes,25 leading to endocytosis and release of the viral genome after fusion of membranes. As antibodies directed against the spike receptor‐binding domain inhibit the entrance of the virion in SARS‐CoV and in MERS‐CoV, the spike protein was identified as the main target of SARS‐CoV‐2 vaccines.
A cause of concern is the evidence for some betacoronaviruses of antibody‐dependent enhancement (ADE) of the virus.26 Instead of neutralizing the virions, antibodies may promote viral invasion in some types of cell. In particular, SARS‐CoV viruses enter macrophages through the antibody Fc portion and skew macrophage function.27 In MERS‐CoV, the interaction of a monoclonal antibody induces conformational changes and causes viral entry through the Fc portion.26 This phenomenon is partly dependent on the concentration of the antibody, so a high titer of neutralizing antibodies may prevent ADE of the virus.26 Further, the introduction of an adjuvant may promote Th2‐type immunity and reduce the immunopathology.28
There is no clear evidence of ADE of the virus in SARS‐CoV‐2, but it may explain conflicting results in the use of convalescent plasma.29 Also, it mandates to evaluate candidate vaccines for prevention of either mild or severe COVID‐19. To date, no approved vaccine for SARS‐CoV‐2 demonstrated ADE of the virus.30, 31, 32
In phase 1/2 trials, efficacy was measured as immunogenicity33, 34 defined as antibody titer against the defined antigen. In phase 3 trials, the efficacy was defined as difference in number of symptomatic infections.30, 31 Up to date, no test is recommended to assess vaccine efficacy after the scheduled doses; it seems reasonable, though, to measure antibody titer in selected patients.
Also, as no trial published results for asymptomatic carriage, it is not yet known if vaccinated people may still carry and transmit the virus (i.e., “sterilizing immunity”).35 Therefore, prevention strategies should be maintained in the vaccinated population.
Vaccine platforms
Up to January 16, 2021, 237 vaccine candidates for COVID‐19 had been reported, and 64 of them were in human clinical trials.36 Three of them have completed phase 3 trials, and reports are published30, 31, 32; 19 more vaccines are in phase 3 studies.
Several different approaches have been used to stimulate the immune system in mounting a humoral response.37 As more traditional approaches are under investigation (inactivated virus vaccines, protein subunit vaccines, recombinant virus vaccines), more recent and innovative strategies have been tried (Non‐replicating viral vector vaccines, RNA based vaccines, DNA based vaccines; Figure 1). See Appendix for an insight on different vaccine platforms.39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61
FIGURE 1.
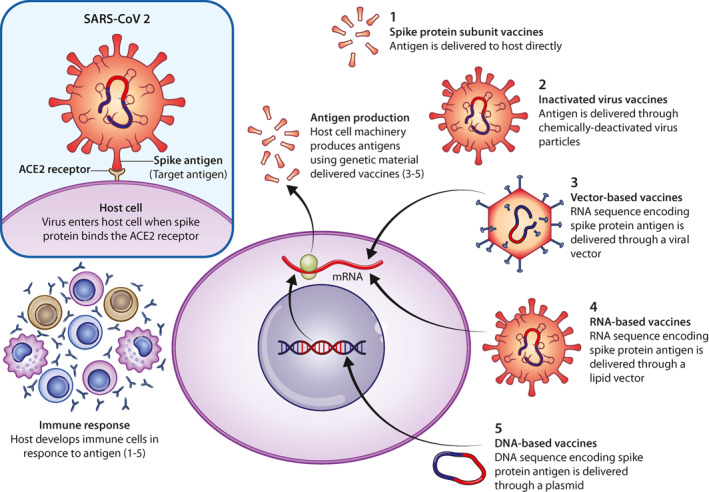
COVID‐19 vaccines mechanisms
SPECIFIC CONSIDERATIONS FOR VULNERABLE PATIENTS WITH DIGESTIVE DISORDERS
Oncological patients
Cancer patients have been shown to be at higher risk of severe COVID‐19, with mortality rates ranging from 5% to 61%.62, 63 Of note, this increased risk appears to be higher for patients with a recent diagnosis of both solid and hematologic tumors.64, 65 Moreover, substantial increases in the number of avoidable cancer‐related morbidity and mortality may be expected as a result of diagnostic delays due to the COVID‐19 pandemic.66, 67
Studies on immune response to antiviral vaccination in neoplastic patients are scarce, and most of them are related to the influenza vaccination.68 Nevertheless, based on the mechanism of action of the vaccines against COVID‐19, along with data extrapolation from other vaccines, COVID‐19 vaccine may be estimated to have similar efficacy and safety to those patients without cancer. As a matter of fact, patients receiving chemotherapy are expected to show lower rates of seroconversion and seroprotection compared to general population.69 However, observational clinical studies investigating the impact of influenza vaccination showed lower infection and mortality rates due to influenza in cancer patients receiving the vaccine,70 implying an efficient immune response, which was proved to be higher in patients with solid tumors.71 According to the recent European Society for Medical Oncology guideline on COVID‐19 vaccination and the National Comprehensive Cancer Network,62, 72 the vaccine should be administered before initiation of chemotherapy whenever possible. On the other hand, in patients who have already initiated systemic therapies, specific timing of administration is not supported by current evidence.72, 73 In those patients, multiple doses of vaccine might help to reach adequate efficacy.73
In summary, although conclusive data regarding vaccination in patients with cancer are still lacking, there is enough evidence to support the administration of COVID‐19 vaccine, even in patients with cancer undergoing immunosuppressive therapy.74, 75, 76 Indeed, according to the Advisory Committee on Immunization Practices within the Centers for Disease Cisontrol and Prevention and the American Association for Cancer Research COVID‐19 and Cancer Task Force, patients with an active cancer should be considered at a higher risk for severe COVID‐19, and should be considered for early COVID‐19 vaccination.77
IBD patients
Unlike cancer patients, there is no evidence suggesting any increased susceptibility to COVID‐19 for IBD patients.78 Indeed, although the persistent concerns about patients on high and prolonged dose of steroids,79, 80 the range of immune‐active therapies (biologicals, JAK inhibitors or other immunosuppressive agents) used in IBD management, have been proposed to have some beneficial effects considering the theoretical role in preventing the cytokine storm which is thought to be the main cause of COVID‐19‐related acute respiratory distress syndrome.81
After having overcome the fear of being high‐risk targets for COVID‐19, IBD patients have to face the feeling of being excluded from the common hope brought by the vaccines. From patients' point of view, the main concerns imply vaccine safety, and the risk of triggering disease flares. In this regard, previous experiences coming from influenza, HBV and pneumococcal vaccinations, do not suggest any association between vaccines and IBD exacerbation. Even in IBD patients receiving immunosuppressive therapies, the main concerns are related to the theoretical risk of sub‐optimal vaccine responses rather than vaccine side effects. Several studies have evaluated the impact of immunosuppressants on the efficacy of vaccines among patients with IBD,82 , 83 confirming a blunted immune response. Further, rapid decline of protective antibody titers may be expected.84, 85 However, a lower response does not imply vaccine inefficacy, and COVID‐19 vaccine should be strongly suggested even in these patients, whichever approved vaccination is offered to them.82, 86 In addition, for some infections like hepatitis B, it might be appropriate to assess antibodies titers and consider possible additional booster dosing.84
In summary, we may reassure our patients about the safety and the efficacy of the major candidate vaccine mechanisms. This recommendation is consistent with current knowledge and first statements of national and international societies.87, 88 However, we should continue to evaluate the SARS‐CoV‐2 vaccine research and outcomes with a particular focus on immunosuppressed patients.
Liver diseases
Liver diseases of viral etiology are not per se associated with COVID‐19 disease severity or outcome and the same holds true for liver transplanted patients89, 90, 91, 92).
Importantly, COVID‐19 has been demonstrated to be associated with liver function deterioration and elevated mortality in patients with cirrhosis in studies from Europe and the US.93, 94 However, the main determinant of mortality in both studies was the Charlson Comobidity Index score, highlighting that frailty often found in patients with advanced cirrhosis rather than liver disease is the main determinant of mortality. These findings although derived from small studies cohort studies, are consistent and have been replicated also in patients from Asia.
Recent data form the European Reference Network for Rare Liver Diseases show that autoimmune liver diseases are not a specific risk factor for COVID19, and similar to any other etiologies, the risk is determined by the stage of cirrhosis.
With regards to Covid‐19 vaccination, subjects with liver diseases were excluded from most clinical trials evaluating the new COVID‐19 vaccines. When included, the criteria used to define liver disease and classify its severity remain unclear.95 A detailed understanding of SARS‐CoV‐2 vaccine safety and the immunological response in patients with liver disease may have to wait for post‐licensing, real‐world investigation. However, considering previous experience with other vaccinations,96, 97 there are no data suggesting that any of the available and developing COVID‐19 vaccines would not be safe and effective in protecting these patients. Thus, these categories, including liver transplant recipients,98 should be strongly encouraged to get vaccinated against COVID‐19 with any of the approved vaccines when one is offered to them, as recently recommended by national and international societies99, 100
Finally, we would like to emphasize that other recommended vaccinations for patients with chronic diseases and/or immunosuppression (i.e., influenza, pneumococcal vaccines) should be administered during this pandemic just as they would have in pre‐COVID era.
Health care workers
As the current critical pressures squeeze health‐care systems worldwide, frontline health care workers (HCW) have been unanimously considered as a priority in the vaccination strategy101, 102, 103, 104, 105, 106, 107, 108, 109, 110 (Figure 2). If a significant number of HCWs were unable to work due to COVID exposure of illness, this could be devastating considering the problems health care facilities are already struggling with regarding staff shortages. Furthermore, this would also result in slowing down the proper rollout of the vaccine itself, which needs to be administered by healthcare staff. In the area of gastroenterology and endoscopy, HCWs are considered potentially more exposed and at higher risk of developing COVID infection because of the multiple issues related to aerosol‐generating endoscopic procedures as well as potential persistence of viral components in the stool of infected patients with GI symptoms. This is why all HCWs involved in the practice of gastroenterology and endoscopy should be regarded as a priority in the vaccination list of the medical facilities around the world.
FIGURE 2.
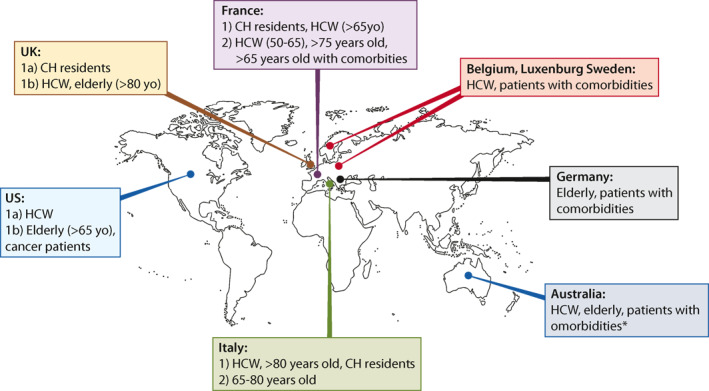
High priority patients. CH: care home. HCW: health care workers. *immunocompromised, multiple comorbidities, chronic lung disease, diabetes, cardiovascular disease and severe obesity
Finally, we should consider that HCW attitude and utilization of vaccines is a positive example for others and an accepted factor for reducing patient's hesitation and improving adherence to vaccination schedules.111 Thus, HCWs should be informed, prioritized and encouraged to undergo COVID‐19 vaccination (see the American Collage of Gastroenterology‐ACG‐virtual Grand Round at https://urldefense.proofpoint.com/v2/url?u=https‐3A__webfiles.gi.org_videos_media_vgr‐5Fcovid.mp4&d=DwMFAg&c=shNJtf5dKgNcPZ6Yh64b‐A&r=tiVVdsJMY‐BPrudjrMffg9U_QLXXl_XIAwOFM7Y6EYU&m=‐WpF8q7Q8BISrq33llUX2ck1L‐2bt6uZsl_5Amv04OI&s=62Z6Ch‐LY4I7j9oisKNCggyLbIlVGzLcZ8k1CCh04H8&e=).
CONCLUSIONS
As Gastroenterologists, patients will be looking to us for guidance regarding vaccination, especially those patients who may be more vulnerable to COVID‐19 due to their underlying digestive diseases. Thus, we must be ready to advocate for our patients and ourselves regarding SARS‐CoV‐2 vaccines promoting scientific research and rigor.
Different guidelines for vaccination strategies have been published worldwide in order to prioritize different populations.101, 102, 103, 104, 105, 106, 107, 108, 109, 110 However, our patients with digestive disorders do not represent a homogeneous population and different recommendations will be applied. Importantly, most of these categories were excluded from clinical trials evaluating SARS‐CoV 2 vaccines, and therefore recommendations are not based on evidence form the current RCTs.
To the best of our knowledge, we recommend COVID‐19 vaccination for our patients, even for fragile, immunosuppressed patients such as IBD patients, GI cancer patients or patients with chronic liver disease. For these categories, lower rates of complete immune response are expected, however a partial protection may still have a relevant clinical role. Nevertheless, considering that any vaccine will not be 100% effective in preventing SARS‐CoV‐2 transmission, we should still highlight the need of maintaining prevention strategies including hygienic practices such as handwashing, face masking and social distancing. Of note, data from clinical trials indicate that COVID‐19 vaccines can safely be given to persons with prior SARS‐CoV‐2 infection.30, 31 However, since vaccine supply are limited, they may temporarily delay vaccination.
DISCLAIMER
This review is based on the available evidence at the time of its preparation and may not apply in all situations. Recommendations should be interpreted in light of specific clinical situations and resource availability.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTION
Marco Spadaccini, Lorenzo Canziani, Alessio Aghemo and Alessandro Repici drafted the manuscript. All the Authors revised and approved the final manuscript.
Supporting information
Supplementary Material S1
ACKNOWLEDGMENTS
Thanks to Dr Bruno Borgiani for image editing.
Spadaccini M, Canziani L, Aghemo A, Lleo A, Maselli R, Anderloni A, et al. What gastroenterologists should know about SARS–CoV 2 vaccine: World Endoscopy Organization perspective. United European Gastroenterol J. 2021;9(7):787–796. 10.1002/ueg2.12103
DATA AVAILABILITY STATEMENT
Data sharing not applicable – no new data generated.
REFERENCES
- 1.World Health Organization . Pneumonia of unknown caused China. https://www.who.int/csr/don/05-january-2020-pneumoniaof-unkown-cause-china/en/. 14 February 2020. [Google Scholar]
- 2.COVID‐19 CORONAVIRUS PANDEMIC . Last updated: January 16, 2021, 13:03 GMT. https://www.worldometers.info/coronavirus/.
- 3.Legido‐Quigley H, Asgari N, Teo YY, Leung GM, Oshitani H, Fukuda K , et al. Are high‐performing health systems resilient against the COVID‐19 epidemic? Lancet. 2020;395:848–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arun TK. Coronavirus: is there an alternative to lockdowns? Economic Times. WHO. 2020. Coronavirus Disease (COVID‐19) Advice for the Public. Geneva, Switzerland; 2020. Available from: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/advice‐forpublic. Updated WHO Recommendations for International Traffic in Relation to the COVID‐19 Outbreak. Geneva, Switzerland; 2020 [Feb 29th 2020]. Available from: https://www.who.int/newsroom/articles-detail/updated-who-recommendations-forinternational-traffic-in-relation-to-covid-19-outbreak [Google Scholar]
- 5.Canziani LM, Trovati S, Brunetta E, Testa A, De Santis M, Bombardieri E, et al. Humanitas and Gavazzeni/Castelli COVID‐19 Task Forces. Interleukin‐6 receptor blocking with intravenous tocilizumab in COVID‐19 severe acute respiratory distress syndrome: a retrospective case‐control survival analysis of 128 patients. J Autoimmun. 2020;114:102511. 10.1016/j.jaut.2020.102511. Epub 2020 Jul 8. PMID: 32713677; PMCID: PMC7342030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thoguluva Chandrasekar V, Venkatesalu B, Patel HK, Spadaccini M, Manteuffel J, Ramesh M. Systematic review and meta‐analysis of effectiveness of treatment options against SARS‐CoV‐2 infection. J Med Virol, 93–775, 85. 2020. 10.1002/jmv.26302. Epub ahead of print. PMID: 32667699; PMCID: PMC7404948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parasa S, Desai M, Thoguluva Chandrasekar V, Patel HK, Kennedy KF, Roesch T, et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta‐analysis. JAMA Netw Open. 2020;3:e2011335. 10.1001/jamanetworkopen.2020.11335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang JW, Li Y, Eames I, Chan PKS, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64:100–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston ER, Habib‐Bein N, Dueker JM, Quiroz B, Corsaro E, Ambrogio M, et al. Risk of bacterial exposure to the endoscopists face during endoscopy. Gastrointest Endosc. 2019;89:818–24. [DOI] [PubMed] [Google Scholar]
- 10.Wong TW, Lee CK, Tam W, Lau JT‐f, Yu T‐s, Lui S‐f, et al. Cluster of SARS amongmedical students exposed to single patient, Hong Kong. Emerg Infect Dis. 2004;10:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Repici A, Maselli R, Colombo M, Gabbiadini R, Spadaccini M, Anderloni A, et al. Coronavirus (COVID‐19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020;92:192–7. 10.1016/j.gie.2020.03.019. Epub 2020 Mar 14. PMID: 32179106; PMCID: PMC7102667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wichmann D, Atique NB, Stüker D, Fusco S, Schempf U, Grottenthaler JM, et al. Impact of the COVID‐19 pandemic on an interdisciplinary endoscopy unit in a German "hotspot" area: a single center experience. Surg Endosc. 2020:1–8. 10.1007/s00464-020-08119-w. Epub ahead of print. PMID: 33140149; PMCID: PMC7605334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Repici A, Aragona G, Cengia G, Cantù P, Spadaccini M, Maselli R, et al. Low risk of COVID‐19 transmission in GI endoscopy. Gut. 2020;69:1925–7. 10.1136/gutjnl-2020-321341. Epub 2020 Apr 22. PMID: 32321857. [DOI] [PubMed] [Google Scholar]
- 14.Repici A, Pace F, Gabbiadini R, Colombo M, Hassan C, Dinelli M, et al. Endoscopy units and the coronavirus disease 2019 outbreak: a multicenter experience from Italy. Gastroenterology. 2020;159:363–6.e3. 10.1053/j.gastro.2020.04.003. Epub 2020 Apr 10. PMID: 32283102; PMCID: PMC7151374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeung JH, Goodman KJ, Fedorak RN. Inadequate knowledge of immunization guidelines: a missed opportunity for preventing infection in immunocompromised IBD patients. Inflamm Bowel Dis. 2012;18:34–40. [DOI] [PubMed] [Google Scholar]
- 16.Han HJ, Nwagwu C, Anyim O, Ekweremadu C, Kim S. COVID‐19 and cancer: from basic mechanisms to vaccine development using nanotechnology. Int Immunopharm. 2020;90:107247. 10.1016/j.intimp.2020.107247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krammer F. SARS‐CoV‐2 vaccines in development. Nature. 2020;586:516–27. [DOI] [PubMed] [Google Scholar]
- 18.Hu B, Guo H, Zhou P, et al. Characteristics of SARS‐CoV‐2 and COVID‐19. Nat Rev Microbiol. 2020;19:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung TS, Liu DX. Human coronavirus: host‐pathogen interaction. Annu Rev Microbiol. 2019;73:529–57. 10.1146/annurev-micro-020518-115759. Epub 2019 Jun 21. PMID: 31226023. [DOI] [PubMed] [Google Scholar]
- 20.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395 (10224):565–74. 10.1016/S0140-6736(20)30251-8. Epub 2020 Jan 30. PMID: 32007145; PMCID: PMC7159086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin JT, Zhang JS, Su N, Xu JG, Wang N, Chen JT, et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther. 2007;12:1107–13. PMID: 18018769. [PubMed] [Google Scholar]
- 22.Yong CY, Ong HK, Yeap SK, Ho KL, Tan WS. Recent advances in the vaccine development against Middle East respiratory syndrome‐coronavirus. Front Microbiol. 2019;10:1781. 10.3389/fmicb.2019.01781. PMID: 31428074; PMCID: PMC6688523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plante JA, Liu Y, Liu J, Xia H, Johnson BA, Lokugamage KG, et al. Spike mutation D614G alters SARS‐CoV‐2 fitness. Nature. 2020;592:116–21. 10.1038/s41586-020-2895-3. Epub ahead of print. PMID: 33106671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, et al. SARS‐CoV‐2 productively infects human gut enterocytes. Science. 2020;369:50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen J, Cheng Y, Ling R, Dai Y, Huang B, Huang W, et al. Antibody‐dependent enhancement of coronavirus. Int J Infect Dis. 2020;100:483–9. 10.1016/j.ijid.2020.09.015. Epub 2020 Sep 11. PMID: 32920233; PMCID: PMC7483033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, et al. Anti‐spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS‐CoV infection. JCI Insight. 2019;4:e123158. 10.1172/jci.insight.123158. PMID: 30830861; PMCID: PMC6478436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotez PJ, Corry DB, Bottazzi ME. COVID‐19 vaccine design: the Janus face of immune enhancement. Nat Rev Immunol. 2020 Jun;20:347–8. 10.1038/s41577-020-0323-4. Epub 2020 Apr 28. PMID: 32346094; PMCID: PMC7187801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A randomized trial of convalescent plasma in covid‐19 severe pneumonia. N Engl J Med. 2020:NEJMoa2031304. 10.1056/NEJMoa2031304. Epub ahead of print. PMID: 33232588; PMCID: PMC7722692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. C4591001 clinical trial group. Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N Engl J Med. 2020;383:2603–15. 10.1056/NEJMoa2034577. Epub 2020 Dec 10. PMID: 33301246; PMCID: PMC7745181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2020. 10.1056/NEJMoa2035389. Epub ahead of print. PMID: 33378609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet. 2020:S0140‐6736(20)32661‐1. 10.1016/S0140-6736(20)32661-1. Epub ahead of print. PMID: 33306989; PMCID: PMC7723445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN , et al. mRNA‐1273 study group. An mRNA vaccine against SARS‐CoV‐2 ‐ preliminary report. N Engl J Med. 2020;383:1920–31. 10.1056/NEJMoa2022483. Epub 2020 Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Phase I/II study of COVID‐19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–93. 10.1038/s41586-020-2639-4. Epub 2020 Aug 12. Erratum in: Nature. 2021 Feb;590(7844):E26. [DOI] [PubMed] [Google Scholar]
- 35. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 36.Enjuanes L, Zuñiga S, Castaño‐Rodriguez C, Gutierrez‐Alvarez J, Canton J, Sola I. Molecular basis of coronavirus virulence and vaccine development. Adv Virus Res. 2016;96:245–86. 10.1016/bs.aivir.2016.08.003. Epub 2016 Aug 30. PMID: 27712626; PMCID: PMC7112271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines ‐ a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–79. 10.1038/nrd.2017.243. Epub 2018 Jan 12. PMID: 29326426; PMCID: PMC5906799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li B, Zhang X, Dong Y. Nanoscale platforms for messenger RNA delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11:e1530. 10.1002/wnan.1530. Epub 2018 May 4. PMID: 29726120; PMCID: PMC6443240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baric RS. Emergence of a highly fit SARS‐CoV‐2 variant. N Engl J Med. 2020;383:2684–6. 10.1056/NEJMcibr2032888. Epub 2020 Dec 16. PMID: 33326716. [DOI] [PubMed] [Google Scholar]
- 40.Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking changes in SARS‐CoV‐2 spike: evidence that D614G increases infectivity of the COVID‐19 virus. Cell. 2020;182:812–27. 10.1016/j.cell.2020.06.043. Epub 2020 Jul 3. PMID: 32697968; PMCID: PMC7332439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castells MC, Phillips EJ. Maintaining safety with SARS‐CoV‐2 vaccines. N Engl J Med. 2020. 10.1056/NEJMra2035343. Epub ahead of print. PMID: 33378605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.StoneCA, Jr, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7:1533–40. 10.1016/j.jaip.2018.12.003. Epub 2018 Dec 14. PMID: 30557713; PMCID: PMC6706272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matz KM, Marzi A, Feldmann H. Ebola vaccine trials: progress in vaccine safety and immunogenicity. Expert Rev Vaccines. 2019;18:1229–42. 10.1080/14760584.2019.1698952. PMID: 31779496. [DOI] [PubMed] [Google Scholar]
- 44.Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type‐5 vectored COVID‐19 vaccine: a dose‐escalation, open‐label, non‐randomised, first‐in‐human trial. Lancet. 2020;395:1845–54. 10.1016/S0140-6736(20)31208-3. Epub 2020 May 22. PMID: 32450106; PMCID: PMC7255193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. https://clinicaltrials.gov/ct2/show/NCT04540419
- 46.Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, Dzharullaeva AS, et al. Safety and immunogenicity of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine in two formulations: two open, non‐randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–97. 10.1016/S0140-6736(20)31866-3. Epub 2020 Sep 4. PMID: 32896291; PMCID: PMC7471804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. https://clinicaltrials.gov/ct2/show/NCT04530396
- 48.Mercado NB, Zahn R, Wegmann F, et al. Single‐shot Ad26 vaccine protects against SARS‐CoV‐2 in rhesus macaques. Nature. 2020;586:583–588. 10.1038/s41586-020-2607-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. https://clinicaltrials.gov/ct2/show/NCT04505722
- 50.Chen J, Miao L, Li JM, Li YY, Zhu QY, Zhou CL, et al. Receptor‐binding domain of SARS‐Cov spike protein: soluble expression in E. coli, purification and functional characterization. World J Gastroenterol. 2005;11:6159–64. 10.3748/wjg.v11.i39.6159. PMID: 16273643; PMCID: PMC4436633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsieh CL, Goldsmith JA, Schaub JM, DiVenere AM, Kuo HC, Javanmardi K, et al. Structure‐based design of prefusion‐stabilized SARS‐CoV‐2 spikes. Science. 2020;369:1501–5. 10.1126/science.abd0826. Epub 2020 Jul 23. PMID: 32703906; PMCID: PMC7402631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1‐2 trial of a SARS‐CoV‐2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383 (24):2320–32. 10.1056/NEJMoa2026920. Epub 2020 Sep 2. PMID: 32877576; PMCID: PMC7494251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. https://clinicaltrials.gov/ct2/show/NCT04611802
- 54. https://clinicaltrials.gov/ct2/show/NCT04646590
- 55.Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Development of an inactivated vaccine candidate for SARS‐CoV‐2. Science. 2020;369 (6499):77–81. 10.1126/science.abc1932. Epub 2020 May 6. PMID: 32376603; PMCID: PMC7202686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palacios R, Patiño EG, de Oliveira Piorelli R, Conde MTRP, Batista AP, Zeng G, et al. Double‐Blind, randomized, placebo‐controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID‐19 (inactivated) vaccine manufactured by sinovac ‐ profiscov: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:853. 10.1186/s13063-020-04775-4. PMID: 33059771; PMCID: PMC7558252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS‐CoV‐2 vaccine, BBIBP‐CorV: a randomised, double‐blind, placebo‐controlled, phase 1/2 trial. Lancet Infect Dis. 2021 Jan;21(1):39–51. 10.1016/S1473-3099(20)30831-8. Epub 2020 Oct 15. PMID: 33069281; PMCID: PMC7561304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. https://clinicaltrials.gov/ct2/show/NCT04510207
- 59.Krammer F. SARS‐CoV‐2 vaccines in development. Nature. 2020;586:516–27. [DOI] [PubMed] [Google Scholar]
- 60. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=45306
- 61.Garassino MC, Giesen N, Grivas P, et al. COVID‐19 vaccination in cancer patients: ESMO statements; 2020. Epub Jan. https://www.esmo.org/covid‐19‐and‐cancer/covid‐19‐vaccination. [Google Scholar]
- 62.Rüthrich MM, Giessen‐Jung C, Borgmann S, et al. COVID‐19 in cancer patients: clinical characteristics and outcome‐an analysis of the LEOSS registry. Ann Hematol. 2020. 10.1007/s00277-020-04328-4. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020; 584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zorzi M, Hassan C, Capodaglio G, Baracco M, Antonelli G, Bovo E, et al. Colonoscopy later than 270 days in a fecal immunochemical test‐based population screening program is associated with higher prevalence of colorectal cancer. Endoscopy. 2020;52(10):871–6. 10.1055/a-1159-0644. Epub 2020 Apr 30. PMID: 32356282. [DOI] [PubMed] [Google Scholar]
- 65.Desai A, Gupta R, Advani S, Ouellette L, Kuderer NM, Lyman GH, et al. Mortality in hospitalized patients with cancer and coronavirus disease 2019: a systematic review and meta‐analysis of cohort studies. Cancer. 2020. 10.1002/cncr.33386. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 66.Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modelling study. Lancet Oncol. 2020;21(8):1023–34. 10.1016/S1470-2045(20)30388-0. Epub 2020 Jul 20. Erratum in: Lancet Oncol. 2021 Jan;22(1):e5. PMID: 32702310; PMCID: PMC7417808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ward EM, Flowers CR, Gansler T, et al. The importance of immunization in cancer prevention, treatment, and survivorship. CA Cancer J Clin. 2017;67:398–410. [DOI] [PubMed] [Google Scholar]
- 68.Loulergue P, Alexandre J, Iurisci I, et al. Low immunogenicity of seasonal trivalent influenza vaccine among patients receiving docetaxel for a solid tumour: results of a prospective pilot study. Br J Canc. 2011;104:1670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bitterman R, Eliakim‐Raz N, Vinograd I, et al. Influenza vaccines in immunosuppressed adults with cancer. Cochrane Database Syst Rev. 2018; 2:CD008983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nordøy T, Aaberge IS, Husebekk A, et al. Cancer patients undergoing chemotherapy show adequate serological response to vaccinations against influenza virus and Streptococcus pneumoniae. Med Oncol. 2002;19:71–8. [DOI] [PubMed] [Google Scholar]
- 71.Rubin LG, Levin MJ, Ljungman P, Infectious Diseases Society of America , et al. IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2013; 58:309–18. [DOI] [PubMed] [Google Scholar]
- 72.Rousseau B, Loulergue P, Mir O, et al. Immunogenicity and safety of the influenza A H1N1v 2009 vaccine in cancer patients treated with cytotoxic chemotherapy and/or targeted therapy: the VACANCE study. Ann Oncol. 2012;23:450–7. [DOI] [PubMed] [Google Scholar]
- 73.Cordonnier C, Einarsdottir S, Cesaro S, et al. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019; 19:e200–e212. [DOI] [PubMed] [Google Scholar]
- 74.Mikulska M, Cesaro S, de Lavallade H, et al. Vaccination of patients with haematological malignancies who did not have transplantations: guidelines from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019; 19:e188–e199. [DOI] [PubMed] [Google Scholar]
- 75.Rieger CT, Liss B, Mellinghoff S, et al. Anti‐infective vaccination strategies in patients with hematologic malignancies or solid tumors‐guideline of the infectious diseases working party (AGIHO) of the German society for hematology and medical Oncology (DGHO). Ann Oncol. 2018; 29:1354–65. 10.1093/annonc/mdy117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dooling K, McClung N, Chamberland M, Marin M, Wallace M, Bell BP, et al. The advisory committee on immunization practices' interim recommendation for allocating initial supplies of COVID‐19 vaccine ‐ United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69 (49):1857–9. 10.15585/mmwr.mm6949e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ribas A, Sengupta R, Locke T, Zaidi SK, Campbell KM, Carethers JM, et al. Priority COVID‐19 vaccination for patients with cancer while vaccine supply is limited. Canc Discov. 2021;11(2):233–6. 10.1158/2159-8290.CD-20-1817. Epub 2020 Dec 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brenner EJ, Ungaro R, Colombel JF, Kappelman MD. Secure‐IBD database public data update; 2020. https://covidibd.org/ [Google Scholar]
- 79.Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous‐Hunt M, Lewis JD, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID‐19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2):481–91. e3. 10.1053/j.gastro.2020.05.032. Epub 2020 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395 (10223):497–506. 10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neurath MF. COVID‐19 and immunomodulation in IBD. Gut. 2020;69(7):1335–42. 10.1136/gutjnl-2020-321269. Epub 2020 Apr 17. PMID: 32303609; PMCID: PMC7211083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Melmed GY, Agarwal N, Frenck RW, et al. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:148–54. [DOI] [PubMed] [Google Scholar]
- 83.Cullen G, Bader C, Korzenik JR, et al. Serological response to the 2009 H1N1 influenza vaccination in patients with inflammatory bowel disease. Gut. 2012;61:385–391. [DOI] [PubMed] [Google Scholar]
- 84.Gisbert JP, Villagrasa JR, Rodriguez‐Nogueiras A, et al. Kinetics of anti‐hepatitis B surface antigen titers after hepatitis B vaccination in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:554–558. [DOI] [PubMed] [Google Scholar]
- 85.Cleveland NK, Rodriquez D, Wichman A, et al. Many inflammatory bowel disease patients are not immune to measles or pertussis. Dig Dis Sci. 2016;61:2972–29766. [DOI] [PubMed] [Google Scholar]
- 86.Alexanderc JL, Gordon Moran G, Gaya DR, Raine T, Hart A, Kennedy NA. British society of gastroenterology inflammatory Bowel disease section and IBD clinical research group position statement on SARS‐CoV2 vaccination. First published on 04 Jan 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alexander JL, Moran G, Gaya DR, et al. British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement on SARS‐CoV2 Vaccination. https://www.bsg.org.uk/covid-19-advice/british-society-of-gastroenterology-inflammatory-bowel-disease-section-and-ibd-clinical-research-group-position-statement-on-sars-cov2-vaccination/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raccomandazioni congiunte IG‐IBD – AMICI Italia Onlus per la vaccinazione anti‐SARS‐CoV‐2 nei pazienti affetti da malattie infiammatorie croniche intestinali (MICI/IBD). https://igibd.it/assets/media/Raccomandazioni_congiunte_.pdf [Google Scholar]
- 89.Härmälä S, Parisinos CA, Shallcross L, OBrien A, Hayward A. Effectiveness of influenza vaccines in adults with chronic liver disease: a systematic review and meta‐analysis. BMJ Open. 2019;9(9):e031070. 10.1136/bmjopen-2019-031070. PMID: 31494620; PMCID: PMC6731888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu R, Zhao L, Cheng X, Huan H, Li C, Li D, et al. Clinical characteristics of COVID‐19 patients with hepatitis B virus infection ‐ a retrospective study. Liver Int. 2020. 10.1111/liv.14774. [DOI] [PubMed] [Google Scholar]
- 91.Butt A, et al. Hospitalization and mortality in SARS‐CoV‐2 infected persons with and without hepatitis C virus infection liver international in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Becchetti C, Zambelli MF, Pasulo L, Donato MF, Invernizzi F, Detry O, et al. COVID‐19 in an international European liver transplant recipient cohort. Gut. 2020;69 (10):1832–40. 10.1136/gutjnl-2020-321923. Epub 2020 Jun 22. PMID: 32571972; PMCID: PMC7335697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, et al. High rates of 30‐day mortality in patients with cirrhosis and COVID‐19. J Hepatol. 2020;73 (5):1063–71. 10.1016/j.jhep.2020.06.001. Epub 2020 Jun 9. PMID: 32526252; PMCID: PMC7280108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bajaj JS, Garcia‐Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, et al. Comparison of mortality risk in patients with cirrhosis and COVID‐19 compared with patients with cirrhosis alone and COVID‐19 alone: multicentre matched cohort. Gut. 2020:gutjnl‐2020‐322118. 10.1136/gutjnl-2020-322118. Epub ahead of print. PMID: 32660964; PMCID: PMC7371484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marjot T, Webb GJ, Barritt AS, Ginès P, Lohse AW, Moon AM, et al. SARS‐CoV‐2 vaccination in patients with liver disease: responding to the next big question. Lancet Gastroenterol Hepatol. 2021:S2468‐1253(21)00008‐X. 10.1016/S2468-1253(21)00008-X. Epub ahead of print. PMID: 33444545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valour F, Conrad A, Ader F, Launay O. Vaccination in adult liver transplantation candidates and recipients. Clin Res Hepatol Gastroenterol. 2020;44(2):126–34. 10.1016/j.clinre.2019.08.007. Epub 2019 Oct 10. PMID: 31607643. [DOI] [PubMed] [Google Scholar]
- 97.Helanterä I, Janes R, Anttila VJ. Clinical efficacy of seasonal influenza vaccination: characteristics of two outbreaks of influenza A(H1N1) in immunocompromised patients. J Hosp Infect. 2018;99 (2):169–74. 10.1016/j.jhin.2017.12.003. Epub 2017 Dec 8. PMID: 29225054. [DOI] [PubMed] [Google Scholar]
- 98.British transplantation society position statement on vaccination for COVID‐19 in solid organ transplant recipients (adults, children and young people) [Google Scholar]
- 99.Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID‐19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021:S0168‐8278(21)00081‐7. 10.1016/j.jhep.2021.01.032. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fix OK, Blumberg EA, Chang KM, et al. AASLD expert panel consensus statement: vaccines to prevent covid‐19 infection in patients with liver disease. https://www.aasld.org/sites/default/files/2021-02/AASLD-COVID19-VaccineDocument-February2021-FINAL.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.World Health Organization . WHO SAGE values framework for the allocation and prioritization of COVID‐19 vaccination, 14 September 2020. World Health Organization. 2020. 19th December 2020, date last accessed. [Google Scholar]
- 102.Evidence to recommendations for COVID‐19 vaccines: evidence framework. World Health Organization. 2020. (19th December 2020, date last accessed). [Google Scholar]
- 103.Dooling K, ACIP COVID‐19 Vaccines Work Group . Phase 1 allocation COVID‐19 vaccine: work Group considerations ACIP COVID‐19 Vaccines Work Group. 2020. 19th December 2020, date last accessed. [Google Scholar]
- 104.Australian Technical Advisory Group on Immunisation (ATAGI) . Preliminary advice on general principles to guide the prioritisation of target populations in a COVID‐19 vaccination program in Australia 19th December 2020, date last accessed. [Google Scholar]
- 105.Independent report. JCVI: updated interim advice on priority groups for COVID‐19 vaccination. JCI, 2020. (19th December 2020, date last accessed). [Google Scholar]
- 106.Stratégie de vaccination contre le Sars‐Cov‐2 ‐ recommandations préliminaires sur la stratégie de priorisation des populations à vacciner. HAS, 2020. (19th December 2020, date last accessed). [Google Scholar]
- 107.Conseil supérieur de la santé . Stratégie de vaccination contre le Covid‐19 en Belgique; 2020. 19th December 2020, date last accessed. [Google Scholar]
- 108.RECOMMANDATIONS générales du CONSEIL SUPERIEUR des MALADIES INFECTIEUSES, concernant la stratégie vaccinale contre la COVID‐19. Conseil Superieur Des Maladies Infectieuses. 2020. (19th December 2020, date last accessed). [Google Scholar]
- 109.Nationell plan för vaccination mot covid‐19. (19th December 2020, date last accessed). [Google Scholar]
- 110.How should access to a COVID‐19 vaccine be regulated? Position paper of the joint working group of members of the standing committee on vaccination, THE GERMAN ETHICS COUNCIL AND THE NATIONAL ACADEMY OF SCIENCES LEOPOLDINA. (19th December 2020, date last accessed). [Google Scholar]
- 111.Asma S, Akan H, Uysal Y, et al. Factors effecting influenza vaccination uptake among health care workers: a multi‐center cross‐sectional study. BMC Infect Dis. 2016:1–9. 10.1186/s12879-016-1528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1
Data Availability Statement
Data sharing not applicable – no new data generated.


