Abstract
Background
Studies have shown that lung inflammation affects lung function, with life-threatening results. Vitamin D may play an important role in inhibiting inflammatory cytokines. Vitamin D deficiency is related to several lung problems, including respiratory distress syndrome, alveolar inflammation, epithelial damage, and hypoxia. Few studies have evaluated the benefits of vitamin D in preventing inflammation in alveolar cells.
Material/Methods
We developed a cell inflammation model induced by lipopolysaccharide (LPS) treatment. The effects of vitamin D on LPS-induced inflammation in A549 cells were examined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and the anti-inflammatory mechanism of vitamin D was evaluated using western blot analysis.
Results
Our results indicated that vitamin D promoted A549 cell survival following LPS-induced inflammation by downregulating nuclear factor nuclear factor kappa light chain enhancer of activated B cells, tumor necrosis factor-α, interleukin (IL)-1β, IL-6, and IL-12.
Conclusions
Our results indicated that vitamin D has the potential to manage lung inflammation, although further studies are needed.
Keywords: 1,25-dihydroxyvitamin D; Cytokines; Inflammation; Lipopolysaccharides; Lung
Background
Neutrophil-dependent failure of the endothelial and epithelial barriers of the lung is the primary cause of lung inflammation [1]. Impaired endothelial and epithelial integrity induced by indirect inflammatory cell influx and cytokine release worsens this condition, resulting in proteinaceous exudate/surfactant deficiency and respiratory distress syndrome [2].
Lung inflammation develops as a reaction to viruses and bacteria. As part of the respiratory system, the lungs are not always protected from exposure to bacteria. Bacteria can absorb nutrients provided by the lungs, which restricts lung health and promotes inflammation [3]. Lipopolysaccharide (LPS) from the outer membrane of gram-negative bacteria has been used as a toxic agent to study bacteria-induced lung inflammation. This molecule produces inflammation by stimulating the host innate immune response [4,5].
To manage respiratory distress syndrome associated with inflammation, the development of new and effective drugs and treatments is needed. Common drugs, such as dexamethasone and external pulmonary surfactants, require evaluation regarding adverse effects and cost-effectiveness, especially for middle-income to low-income families. Vitamin D has recently been proposed as a treatment because of its potential roles in the musculoskeletal, hormone, and immune systems and in cell proliferation and differentiation [6]. Epidemiological studies have demonstrated that low vitamin D levels are correlated with lung diseases, such as infection, chronic rhinitis, asthma, and, in particular, respiratory distress syndrome in preterm neonates. Vitamin D is involved in lung maturation, and its deficiency promotes respiratory distress syndrome [6–12].
The benefits of vitamin D in lowering inflammation associated with cell-mediated immunity may include regulating the immune system and reducing the inflammation response by decreasing cytokine levels. The innate immune system produces both proinflammatory and anti-inflammatory cytokines in reaction to viral and bacterial infections. LPS is an endotoxin derived from the membrane of gram-negative bacteria, which produces a strong inflammatory response in animal and cancer cell models [13–15].
Only a few studies have considered the benefits of vitamin D in cell viability and inflammatory systems, particularly involving cytokines in LPS-induced A549 cells. In the present study, the effects of vitamin D in LPS-induced inflammation of A549 cells were examined along with its putative mechanism of action.
Material and Methods
Cell Culture
A549 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Roswell Park Memorial Institute 1640 (Gibco, Grand Island, NY, USA) medium containing 10% (v/v) fetal bovine serum (Gibco), 100 units/mL of penicillin, and 0.1 mg/mL of streptomycin (Beyotime, Shanghai, China). After reaching 70% confluence, 30 μM Escherichia coli-derived LPS (Sigma-Aldrich, St. Louis, MO, USA) alone or different concentrations (1 μM, 0.1 μM, and 0.01 μM) of vitamin D (1,25(OH)2D3; Sigma-Aldrich, St. Louis, MO, USA) alone or combinations of 30 μM LPS and 1 μM, 0.1 μM, and 0.01 μM of vitamin D were tested by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and western blot analysis.
Cell Viability Assay
The viability of cells was determined by MTT colorimetric assay. After treatment, the cells were plated into 24-well plates at a density of 2×104 cells/well and then cultured for 1 day. Next, LPS or vitamin D were added alone or in combination for 24 h. Subsequently, 20 μL of MTT (Sigma, St. Louis, MO, USA) was added to each well and incubated for 4 h at 37°C. Next, 50 μL of hydrochloric acid was added for 10 min. The absorption was measured using a microtiter plate reader (BD Biosciences, Franklin Lakes, NJ, USA) at a wavelength of 450 nm. All experiments were performed in triplicate.
Western Blot Analysis
The protein samples were prepared using radioimmunoprecipitation assay lysis and extraction buffer (catalog no. 89900, Thermo Scientific, IL, USA), sample buffer, protease inhibitor cocktail (Roche, Basel, Switzerland), and dithiothreitol. Next, the samples were heated for 5 min at 96°C. Protein quantitation was done using a bicinchoninic acid protein assay kit (Pierce, Appleton, WI, USA). A549 cell protein extracts (10 μg/lane) were separated on 12% polyacrylamide gels (Invitrogen; Thermo Fisher Scientific, Inc.), transferred to nitrocellulose membranes (GE Healthcare) at room temperature for 1 h, and then incubated in 2% blocking reagent (GE Healthcare) in tris-buffered saline buffer containing Tween 20 (0.1%) (TBST) overnight at 4°C. Next, the membranes were incubated in 5% blocking buffer containing diluted primary antibody (Supplementary Table 1) at 4°C overnight. The membranes were washed and incubated with a secondary antibody near-infrared system (LiCOR, USA) at a 1: 10 000 dilution in TBST at room temperature for 90 min. The membranes were imaged (LI-COR Odissey Clx Western Blot Imager, USA), and the intensity of the bands was quantified using LiCOR quantification software.
Statistical Analysis
Data are presented as the mean±standard error, and were analyzed using SPSS version 20.0 (SPSS Inc., Armonk, NY, USA). The significance between groups was determined with one-way ANOVA, and P values <0.05 were considered statistically significant.
Results
Effect of Vitamin D on Cell Viability
We evaluated the effect of different concentrations of vitamin D and LPS treatment on the viability of A549 cells using the MTT assay. A549 cells were co-treated with 30 μM LPS and various concentrations of vitamin D (1 μM, 0.1 μM, and 0.01 μM) for 24 h. The MTT assay results revealed that cell viability in the LPS group decreased by 88.5% compared with that in the vitamin D group (P<0.05; Figure 1). In the vitamin D+LPS groups, a significant increase (89.36% and 90.11%) in cell viability was observed in response to 0.1 μM vitamin D +30 μM LPS and 0.01 μM vitamin D+30 μM LPS, respectively, compared with that of LPS alone (P<0.05; Figure 1). The results indicated that vitamin D protected epithelial cells against the effects of LPS exposure.
Figure 1.
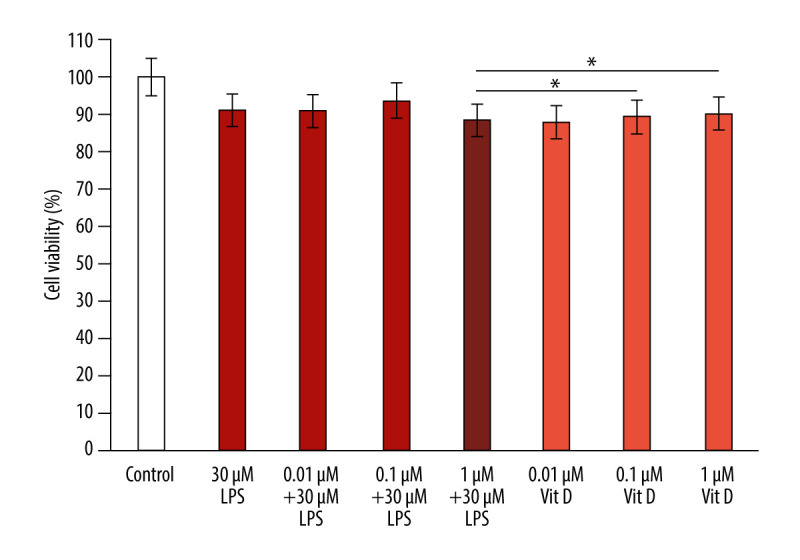
Vitamin D inhibits LPS-induced cytotoxicity in A549 cells. The data are presented as the mean±SD error bars of the mean (n=3). * P<0.05 vs LPS. Vit D – vitamin D; LPS – lipopolysaccharide.
Vitamin D Inhibited LPS-Induced Inflammation by Downregulating Inflammatory Cytokines
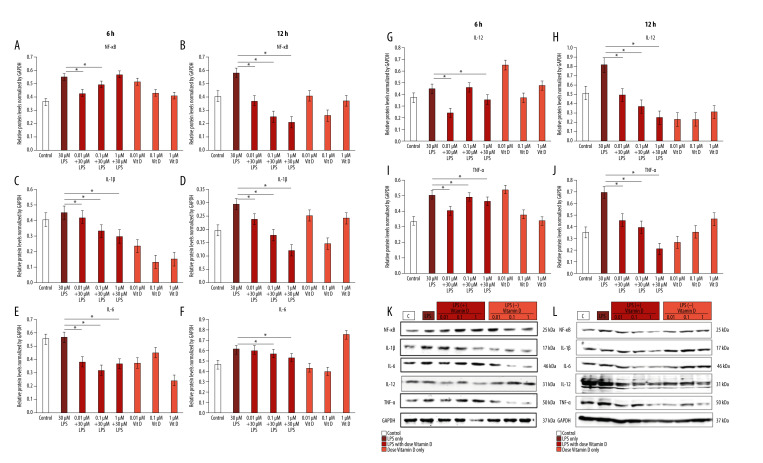
To understand the mechanism through which vitamin D inhibits the inflammatory effect of LPS in A549 cells, we examined the effect of co-treatment with small doses of vitamin D after 6 and 12 h of treatment with 30 μM LPS. We observed that LPS upregulated several inflammatory proteins, including nuclear factor nuclear factor kappa light chain enhancer of activated B cells (NF-κB) (55%, 57.9%), interleukin (IL)-1β (45%, 29.4%), IL-6 (56.8%, 61.2%), IL-12 (44.4%, 81.8%), and tumor necrosis factor (TNF)-α (50.5%, 69.5%) at 6 h and 12 h, respectively, compared with the control group. Western blot analysis revealed that treatment with LPS induced the expressions of the NF-κB, TNF-α, IL-1β, IL-6, and IL-12 inflammatory cytokines, which were inhibited in a time-independent manner with small doses of vitamin D. Time-dependent downregulation of inflammatory cytokines occurred at 6 h, and with a larger decrease at 12 h (Figure 2).
Figure 2.
Vitamin D decreased lipopolysaccharide (LPS)-induced inflammatory cytokine expression. A549 cells were treated with 1 μM, 0.1 μM, and 0.01 μM of vitamin D with and without 30 μM of LPS for 6 h and 12 h. (A, B) Relative protein levels of NF-κB; (C, D) IL-1β; (E, F) IL-6; (G, H) IL-12; and (I, J) TNF-α. (K, L) Expression of all proteins as measured using western blot with an internal control (GAPDH). Data are shown as mean±SD and * P<0.05 indicates a significant difference relative to LPS treatment. Vit D – vitamin D; LPS – lipopolysaccharide; GADPH – glyceraldehyde-3-phosphate dehydrogenase; NF – nuclear factor kappa light chain enhancer of activated B cells; IL – interleukin; TNF – tumor necrosis factor.
Discussion
This study examined the effects of vitamin D in an A549 lung epithelial cell model. The results indicated that LPS-induced inflammation in A549 cells was inhibited by treatment with vitamin D. This was confirmed by an increase in cell viability and a decrease in the production of the inflammatory cytokines NF-κB, TNF-α IL-1β, IL-6, and IL-12 in a time-dependent manner. As an inflammation model in other studies, LPS stimulated various inflammatory cytokines in many cell types. Therefore, LPS was selected as the inflammation trigger model in this study. Cell survival, inflammatory response, and cytokine levels are critical stages in lung inflammation, are common to the immaturity of the lungs, and can cause damage to the respiratory system [16,17].
Studies have demonstrated the influence of vitamin D on cell viability. Narvaez et al showed that vitamin D contributes to the regulation of cell differentiation [18]. In the present study, vitamin D impaired LPS-induced cell inflammation in a concentration-dependent manner. In this study, 0.1 μM and 0.01 μM of vitamin D increased cell viability compared with LPS-treated cells. Faridvand et al in found that the optimal doses of vitamin D ranged from 0.05 μM to 0.1 μM [19]. Moreover, Bischoff-Ferarri et al determined that the optimal concentrations of vitamin D3 ranged from 0.07 μM to 0.01 μM in serum [20]. These results indicate that vitamin D significantly decreases LPS-induced inflammation in A549 cells.
The upregulation of cell viability observed following treatment of A549 cells with vitamin D in the present study prompted us to examine LPS-induced inflammatory cytokines. LPS induces inflammation by stimulating the host innate immune response [4,5]. LPS binds to a pair of Toll-like receptor-4 molecules on macrophages. Then, the regulatory molecules in cells, namely Mal, MyD88, Tram, and Trip, activate the master regulator of inflammation, NF-κB. Then, NF-κB enters the nucleus and upregulates the production of cytokines, including TNF-α, IL-1β, IL-6, and IL-12 [21,22].
Our results indicated that vitamin D caused the sustained downregulation of NF-κB, TNF-α, IL-1β, IL-6, and IL-12 in A549 cells co-treated with vitamin D and LPS compared with those treated with LPS alone. Several studies have shown a significant correlation between vitamin D and the inflammatory response. Vitamin D suppresses inflammatory cytokines by reducing the production of hypoxia-inducible factor (HIF)-1α, NF-κB, IL-6, and TNF-α. Vitamin D inactivates the NF-κB pathway and upregulates VHL levels, thereby promoting a reduction in HIF-1α. Moreover, Ge et al confirmed that IL-6 and TNF-α levels are mediated by the NF-κB pathway and vitamin D reduces inflammatory cytokine production [23]. Vitamin D increases IκBα levels and this decreases nuclear translocation of NF-κB. This is the primary transcription factor for inflammatory cytokines, as described by Cohen-Lahav et al, and it is associated with the effects of vitamin D on inflammation [24]. Similar to transcription factors, vitamin D can interact with the glucocorticoid receptor as a secosteroid to exert anti-inflammatory effects [25].
The role of vitamin D in the inflammatory response after NF-κB activation and the release of cytokines in the nucleus has been explained in previous studies. Chen et al showed that vitamin D reduces the release of cytokines, such as IL-4, IL-13, OVA IgE, and IL-4, and enhances IL-12 and INF-γ levels in blood supernatants as well as BALF protein levels [26]. Vitamin D protection against LPS-induced inflammation may occur through its ability to increase vitamin D receptor expression and downregulate NF-κB regulation by inhibiting IL-6 in OKF6/TERT-2 cells. Furthermore, vitamin D associates with epithelial and endothelial cells through inflammatory cell reflux and cytokine release [27,28].
In the present study, LPS-induced inflammation was inhibited by vitamin D (Figure 1), which is consistent with the results of Lee et al, who showed that LPS-induced injury in cells was reversed and cell viability was increased by vitamin D treatment [29]. Moreover, Khare et al confirmed that treatment of A549 cells with vitamin D before infection and after infection did not alter cell viability that developed in the absence of vitamin D exposure [30]. The present study revealed the potential anti-inflammatory properties of vitamin D by showing a decrease in LPS-induced inflammatory proteins TNF-α, IL-1β, IL-6, IL-12, and NF-κB in cultured A549 cells (Figure 2). This suggests that the effects of vitamin D can be attributed to its ability to disrupt the NF-κB pathway.
NF-κB is a well-known transcription factor implicated in the inflammatory response [31,32]. Studies have shown that NF-κB plays a key role in tissue regulation and generation [33,34]. Furthermore, overexpression of NF-κB in the lung epithelium increases inflammation and injury in response to LPS, whereas downregulation of NF-κB decreases lung inflammation [35]. Li et al used siRNA against NF-κB p65 delivered through an intratracheal route to significantly decrease the proinflammatory response and TNF-α levels and ameliorate lung injury caused by LPS [36]. Huang et al showed that the downregulation of NF-κB p65 signaling successfully decreased LPS-induced lung injury and prevented the release of inflammatory factors, including IL-1β and TNF-α [37]. Hence, these reports indicate that NF-κB p65 is involved in lung inflammatory injury during acute respiratory distress syndrome.
In the context of the current COVID-19 pandemic, acute respiratory distress syndrome as a complication of SARS-CoV-2 infection induces neutrophil aggregation, pulmonary edema, and vascular permeability. These conditions increase the inflammatory response of the alveolar epithelial barrier, and thus the alveolus is loaded with cytokines and chemokines. This condition can be exacerbated by a deficiency in vitamin D [38,39].
Vitamin D has the potential to decrease the levels of proinflammatory cytokines that lead to lung inflammation. These mechanisms include enhancing immune system function by decreasing cytokine production. Proinflammatory and anti-inflammatory cytokines are triggered by the innate immune system in reaction to bacterial and viral infections, a response that occurs in patients with COVID-19 [40]. In several in vitro studies, vitamin D has shown positive benefits by inhibiting the production of cytokines, including IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF-α, TGFβ, and NF-κB [41,42]. The results of these studies are consistent with our results, in which we observed a decrease in several of these cytokines (IL-1β, IL-6, IL-12, TNF-α, NF-κB) (Figure 2).
The correlation between vitamin D and SARS-CoV-2 infection has been explored in several studies. For example, in a study in the United States, Jain et al found that Black patients with COVID-19 and vitamin D deficiency had relatively poor clinical outcomes [43]. A review by Rhodes et al revealed that there was significant ecological evidence of an association between the morbidity of patients with COVID-19 and vitamin D deficiency [44]. However, Raisi-Estabragh et al concluded that there was not sufficient evidence to declare vitamin D deficiency the cause of COVID-19 morbidity in specific populations [45]. Therefore, the association between vitamin D and COVID-19 requires further study to confirm the above associations.
Our study has some limitations. First, this was an in vitro study and the results should be confirmed in vivo and in clinical studies. Second, we explored the effect of vitamin D only at 12 h and 24 h after LPS treatment. The effects of long-term exposure need to be determined. In vivo experiments with longer vitamin D exposures are ongoing in our laboratory.
Conclusions
In the present study, we revealed several potential direct outcomes of vitamin D in preventing the inflammatory response in A549 cells, including the inhibition of the proinflammatory proteins NF-κB, TNF-α, IL-1β, IL-6, and IL-12 in a time-dependent manner. Our results show that the effects of vitamin D have the potential to manage lung inflammation, although further studies are needed.
Supplementary Data
Supplementary Table 1.
List of antibodies used in the study.
| No. | Antibody | Catalog No. | Company | Dilution |
|---|---|---|---|---|
| 1 | GAPDH | 102910192 | Sigma | 1: 1000 |
| 2 | NF-κB | 13586S | Sigma | 1: 1000 |
| 3 | TNF-α | 3707S | Sigma | 1: 1000 |
| 4 | IL-1β | 12242S | Sigma | 1: 1000 |
| 5 | IL-6 | AB9324 | Sigma | 1: 1000 |
| 6 | IL-12 | AB106270 | Sigma | 1: 1000 |
GADPH – glyceraldehyde-3-phosphate dehydrogenase; NF – nuclear factor kappa light chain enhancer of activated B cells; IL – interleukin; TNF – tumor necrosis factor.
Acknowledgments
We thank the Ministry of Research, Technology and Higher Education Republic of Indonesia for grant-in-aids for this research.
Footnotes
Conflicts of Interest
None.
Source of support: This work was financially supported by a grant-in-aid from the Ministry of Research and Technology for RA and RL
References
- 1.He B, Geng S, Zhou W, et al. MMI-0100 ameliorates lung inflammation in a mouse model of acute respiratory distress syndrome by reducing endothelial expression of ICAM-1. Drug Des Devel Ther. 2018;12:4253–60. doi: 10.2147/DDDT.S188095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Locci G, Fanos V, Gerosa C, Faa G. Hyaline membrane disease (HMD) : The role of the perinatal pathologist. J Pediatr Neonatal Individ Med. 2014;3(2):1–9. [Google Scholar]
- 3.Huffnagle GB, Dickson RP, Lukacs NW. The respiratory tract microbiome and lung inflammation: A two-way street. Mucosal Immunol. 2017;10(2):299–306. doi: 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueta M, Hamuro J, Kiyono H, Kinoshita S. Triggering of TLR3 by polyI: C in human corneal epithelial cells to induce inflammatory cytokines. Biochem Biophys Res Commun. 2005;331(1):285–94. doi: 10.1016/j.bbrc.2005.02.196. [DOI] [PubMed] [Google Scholar]
- 5.Chow JC, Young DW, Golenbock DT, et al. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 274(16):10689–92. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 6.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94(1):26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abuzeid WM, Akbar NA, Zacharek MA. Vitamin D and chronic rhinitis. Curr Opin Allergy Clin Immunol. 2012;12(1):13–17. doi: 10.1097/ACI.0b013e32834eccdb. [DOI] [PubMed] [Google Scholar]
- 9.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120(5):1031–35. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Niruban SJ, Alagiakrishnan K, Beach J, Senthilselvan A. Association between Vitamin D and respiratory outcomes in Canadian adolescents and adults. J Asthma. 2015;52(7):653–61. doi: 10.3109/02770903.2015.1004339. [DOI] [PubMed] [Google Scholar]
- 11.Herr C, Greulich T, Koczulla RA, et al. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res. 2011;12(1):1–9. doi: 10.1186/1465-9921-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatera VA, Abdulah R, Musfiroh I, et al. Updates on the status of vitamin D as a risk factor for respiratory distress syndrome. Adv Pharmacol Sci. 2018;2018:8944816. doi: 10.1155/2018/8494816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emiola A, Andrews SS, Heller C, George J. Crosstalk between the lipopolysaccharide and phospholipid pathways during outer membrane biogenesis in Escherichia coli. Proc Natl Acad Sci USA. 2016;113(11):3108–13. doi: 10.1073/pnas.1521168113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao Y, Chen YC, Lan T, et al. LPS-induced nuclear translocation of RhoA is dependent on NF-κB in the human lung cancer cell line A549. Oncol Lett. 2012;3(6):1283–87. doi: 10.3892/ol.2012.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sirota M, Willemsen G, Sundar P, et al. Effect of genome and environment on metabolic and inflammatory profiles. PLoS One. 2015;10(4):1–19. doi: 10.1371/journal.pone.0120898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narvaez CJ, Matthews D, LaPorta E, et al. The impact of vitamin D in breast cancer: Genomics, pathways, metabolism. Front Physiol. 2014;5(June):1–10. doi: 10.3389/fphys.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faridvand Y, Bagherpour-Hassanlouei N, Nozari S, et al. 1, 25-Dihydroxyvitamin D3 activates Apelin/APJ system and inhibits the production of adhesion molecules and inflammatory mediators in LPS-activated RAW264.7 cells. Pharmacol Reports. 2019;71(5):811–17. doi: 10.1016/j.pharep.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Erratum: Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(5):1253. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 21.Kumagai N, Fukuda K, Fujitsu Y, et al. Lipopolysaccharide-induced expression of intercellular adhesion molecule-1 and chemokines in cultured human corneal fibroblasts. Investig Ophthalmol Vis Sci. 2005;46(1):114–20. doi: 10.1167/iovs.04-0922. [DOI] [PubMed] [Google Scholar]
- 22.Song PI, Abraham TA, Park Y, et al. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Investig Ophthalmol Vis Sci. 2001;42(12):2867–77. [PubMed] [Google Scholar]
- 23.Ge X, Wang L, Li M, et al. Vitamin D/VDR signaling inhibits LPS-induced IFNγ and IL-1β in Oral epithelia by regulating hypoxia-inducible factor-1α signaling pathway. Cell Commun Signal. 2019;17(1):1–10. doi: 10.1186/s12964-019-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen-Lahav M, Shany S, Tobvin D, et al. Vitamin D decreases NFκB activity by increasing IκBα levels. Nephrol Dial Transplant. 2006;21(4):889–97. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 25.Karin M, Lin A. NF-κB at the crossroads of life and death. Nat Immunol. 2002;3(3):221–27. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Rao SQ, Gao BH, Jiang ZQ. Effect of early vitamin D supplementation on asthma and the possible mechanisms. Genet Mol Res. 2015;14(4):14136–43. doi: 10.4238/2015.October.29.35. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Li W, Wang Q. production through aryl hydrocarbon receptor/nuclear factor-κB signaling in oral epithelial cells. 2019;19(236):1–9. doi: 10.1186/s12903-019-0935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou W, Yuan G, Wang Q. Vitamin D attenuates lipopolysaccharide-induced inflammatory response in endothelial cells through inhibition of PI3K/Akt/NF-κB signaling pathway. Pharmazie. 2019;74(7):412–17. doi: 10.1691/ph.2019.9373. [DOI] [PubMed] [Google Scholar]
- 29.Lee C, Lau E, Chusilp S, et al. Protective effects of vitamin D against injury in intestinal epithelium. Pediatr Surg Int. 2019;35(12):1395–401. doi: 10.1007/s00383-019-04586-y. [DOI] [PubMed] [Google Scholar]
- 30.Khare D, Godbole NM, Pawar SD, et al. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr. 2013;52(4):1405–15. doi: 10.1007/s00394-012-0449-7. [DOI] [PubMed] [Google Scholar]
- 31.Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21(1):146–58. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadlonek N, Lee JH, Reece TB, et al. Interleukin-1 beta induces an inflammatory phenotype in human aortic valve interstitial cells through nuclear factor kappa beta. Ann Thorac Surg. 2013;96(1):155–62. doi: 10.1016/j.athoracsur.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karra R, Knecht AK, Kikuchi K, Poss KD. Myocardial NF-κB activation is essential for zebrafish heart regeneration. Proc Natl Acad Sci USA. 2015;112(43):13255–60. doi: 10.1073/pnas.1511209112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proto JD, Tang Y, Lu A, et al. NF-κB inhibition reveals a novel role for HGF during skeletal muscle repair. Cell Death Dis. 2015;6(4):1–13. doi: 10.1038/cddis.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez B, Maisonet TM, Londhe VA. Alveolar NF-κB signaling regulates endotoxin-induced lung inflammation. Exp Lung Res. 2015;41(2):103–14. doi: 10.3109/01902148.2014.977461. [DOI] [PubMed] [Google Scholar]
- 36.Li N, Song Y, Zhao W, et al. Small interfering RNA targeting NF-κB attenuates lipopolysaccharide-induced acute lung injury in rats. BMC Physiol. 2016;16(1):1–8. doi: 10.1186/s12899-016-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang J, Li L, Yuan W, et al. NEMO-binding domain peptide attenuates lipopolysaccharide-induced acute lung injury by inhibiting the NF-κB signaling pathway. Mediators Inflamm. 2016;2016:349603. doi: 10.1155/2016/7349603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quesada-gomez JM, Entrenas-castillo M, Bouillon R. Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections Revised Ms SBMB 2020_166. J Steroid Biochem Mol Biol. 2020;202:1–8. doi: 10.1016/j.jsbmb.2020.105719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Z, Shi L, Wang Y, et al. The Lancet Respiratory Medicine. Vol. 8. Elsevier Ltd; 2020. Pathological findings of COVID-19 associated with acute respiratory distress syndrome [Internet] pp. 420–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and covid-19 infections and deaths. Nutrients. 2020;12(4):1–19. doi: 10.3390/nu12061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–39. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohan M, Cherian JJ, Sharma A. Exploring links between Vitamin D deficiency and COVID-19. PLoS Pathog. 2020;16(9):1–6. doi: 10.1371/journal.ppat.1008874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain SK, Parsanathan R. Can vitamin D and L-cysteine co-supplementation reduce 25(oh)-vitamin D deficiency and the mortality associated with COVID-19 in African Americans? J Am Coll Nutr. 2020;39(8):1–6. doi: 10.1080/07315724.2020.1789518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhodes JM, Subramanian S, Laird E, et al. Perspective: Vitamin D deficiency and COVID-19 severity – plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med. 2021;289(1):97–115. doi: 10.1111/joim.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raisi-Estabragh Z, McCracken C, Bethell MS, et al. Greater risk of severe COVID-19 in black, asian and minority ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: Study of 1326 cases from the UK biobank. J Public Heal (United Kingdom) 2020;42(3):451–60. doi: 10.1093/pubmed/fdaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
List of antibodies used in the study.
| No. | Antibody | Catalog No. | Company | Dilution |
|---|---|---|---|---|
| 1 | GAPDH | 102910192 | Sigma | 1: 1000 |
| 2 | NF-κB | 13586S | Sigma | 1: 1000 |
| 3 | TNF-α | 3707S | Sigma | 1: 1000 |
| 4 | IL-1β | 12242S | Sigma | 1: 1000 |
| 5 | IL-6 | AB9324 | Sigma | 1: 1000 |
| 6 | IL-12 | AB106270 | Sigma | 1: 1000 |
GADPH – glyceraldehyde-3-phosphate dehydrogenase; NF – nuclear factor kappa light chain enhancer of activated B cells; IL – interleukin; TNF – tumor necrosis factor.