Abstract
Fructooligosaccharides (FOSs)—fructose-based oligosaccharides—are typical prebiotics with health-promoting effects in humans and animals. The trisaccharide 1-kestotriose is the most attractive inulin-type FOS. We previously reported a recombinant sucrose:sucrose 1-fructosyltransferase (1-SST, EC 2.4.1.99) from Schedonorus arundinaceus (Sa) that efficiently converts sucrose into 1-kestotriose. In this study, Pichia pastoris PGFT6x-308 constitutively expressing nine copies of the Sa1-SST gene displayed fructosyltransferase activity in undisrupted biomass (49.8 U/ml) and culture supernatant (120.7 U/ml) in fed-batch fermentation (72 hr) with sugarcane molasses. Toluene permeabilization increased 2.3-fold the Sa1-SSTrec activity of whole cells entrapped in calcium-alginate beads. The reaction with refined or raw sugar (600 g/l) yielded 1-kestotriose and 1,1-kestotetraose in a ratio of 8:2 with their sum representing above 55% (wt/wt) of total carbohydrates. The FOSs yield decreased to 45% (wt/wt) when sugarcane syrup and molasses were used as cheaper sucrose sources. The beads retained 80% residual Sa1-SSTrec activity after a 30-day batchwise operation with refined cane sugar at 30°C and pH 5.5. The immobilized biocatalyst is attractive for the continuous production of short-chain FOSs, most particularly 1-kestotriose.
Keywords: Calcium alginate, Cell immobilization, Fructooligosaccharides, Fructosyltransferase, Pichia pastoris
Introduction
Fructooligosaccharides (FOSs)—fructose-based oligosacchari-des—are prebiotic fibbers widely utilized as functional food ingredients and in the pharmaceutical industry. FOSs ingestion improves the composition of the resident intestinal microbiota by stimulating selectively the growth of probiotics (bifidobacteria and lactobacilli), which results in the competitive exclusion of pathogens. Other associated benefits are the reduction of serum cholesterol, the increase in calcium and magnesium absorption, the prevention of colon cancer, and the production of B-vitamins. Additionally, FOSs are noncariogenic, low-calorie sugars of sweet taste and exhibit antioxidant activity by free radical scavenging (Cunha et al., 2019; Faria et al., 2021; Kaplan & Hutkins, 2000; Maiorano et al., 2020; Pereira & Gibson, 2002; Perna et al., 2018).
Commercially available FOSs contain a terminal α-d-glucose residue (G) and two to four fructosyl units (F) linearly connected by β-(2→1) linkages. Among inulin-type FOSs, the trisaccharide 1-kestotriose (1-kestose, GF2) has a superior bifidus-stimulating effect and tastes sweeter than the tetrasaccharide 1,1-kestotetraose (nystose, GF3) and the pentasaccharide 1,1,1-kestopentaose (fructosyl-nystose, GF4) (Mendlik et al., 2012; Suzuki et al., 2006). With these attributes, 1-kestotriose when supplemented with a more intense low-calorie sweetener (e.g., stevia) is adequate for replacing table sugar in prebiotic diabetic recipes that reduce the risk of obesity (Mabel et al., 2008).
Current industrial systems for inulin-type FOSs production are based on the use of β-fructofuranosidases (EC 3.2.1.26) and β-fructosyltransferases (EC 2.4.1.9) from few species of filamentous fungi, mainly from the genera Aspergillus and Aureobasidium. The reaction needs to be conducted at high sucrose concentrations (600–700 g/l) to restrict substrate hydrolysis. Under this condition, the fungal enzymes produce a mixture of GF2, GF3, and GF4, with their sum representing around 55% (wt/wt) of total carbohydrates (Cunha et al., 2019; Fernandez et al., 2007; Sánchez-Martínez et al., 2020).
FOS-synthesizing enzymes of different origins have been produced in transgenic yeasts (Hernández et al., 2018; Rehm et al., 1998; Trujillo et al., 2001; Yang et al., 2016). Our group reported a recombinant sucrose:sucrose 1-fructosyltransferase (1-SST, EC 2.4.1.99) from the plant Schedonorus arundinaceus (Sa) with catalytic properties highly attractive for the production of short-chain inulin-type FOSs (Hernández et al., 2018). The enzyme produced in Pichia pastoris (Sa1-SSTrec) yielded 1-kestotriose and 1,1-kestotetraose in an invariable ratio of 9:1 and overall FOSs content above 55% (wt/wt) from sucrose at the wide concentration range of 100–800 g/l. Fructose release to water (sucrose hydrolysis) was negligible even at the lowest substrate concentration (Hernández et al., 2018). The P. pastoris multicopy clone PGFT6x-308 secreted Sa1-SSTrec as a glycoprotein into the periplasmic space (38%) and the extracellular medium (62%) in fed-batch fermentation using cane sugar for cell growth (Hernández et al., 2018). The yeast host holds the GRAS (generally recognized as safe) status and lacks endogenous sucrose- or fructan-transforming enzymes. Immobilization of whole PGFT6x-308 cells retaining N-glycosylated Sa1-SSTrec in the periplasmic space may result in an invertase-free biocatalyst appropriate for reuse in a cost-effective continuous process that efficiently converts sucrose into 1-kestotriose.
Cell immobilization processes often use insoluble calcium alginate as the entrapping matrix. The method is rapid, nontoxic, inexpensive, versatile, eco-friendly, and recommended for applications in the food and pharmaceutical industries (Smidsrød & Skja, 1990). In bioprocess, Ca-alginate entrapment of enzymes and cells offers relevant advantages, such as greater resistance to external aggressions, increased possibility of enzyme recovery and straightforward enzyme/product separation, reusable biocatalysts with operational stability, and reduction in operating costs (Gonçalves et al., 2019).
Several authors have successfully entrapped enzymes or fungal cells in Ca-alginate gels for continuous FOSs production from sucrose (Cheng et al., 1996; Fernandez-Arrojo et al., 2013; Hayashi et al., 1994; Park et al., 2005; Ganaie et al., 2014; Gonçalves et al., 2020). There are no previous reports on immobilized yeast cells expressing a plant fructosyltransferase. Ca-alginate entrapment of PGFT6x-308 cells may be advantageous over immobilization of the free enzyme. The whole-cell biocatalyst may prevent Sa1-SST from leaking out of the beads, but it may cause operational troubles if the confined yeast remains alive and fermentative metabolism of glucose releases the undesired products ethanol, acetate, and CO2.
Immobilized microorganisms are often treated with a membrane-permeabilizing agent in order to reduce diffusional barriers and accelerate biocatalytic reactions (He et al., 2015; Panesar et al., 2007, 2011; Park et al., 1994; Yun et al., 1994). Permeabilization treatments using toluene, a hydrophobic solvent, at appropriate levels dramatically decreased the viability of bacteria and yeasts without causing cell lysis (De Smet et al., 1978; Jackson & DeMoss, 1965; Murakami et al., 1980).
In this work, Ca-alginate entrapment of whole P. pastoris PGFT6x-308 cells was followed by a membrane-permeabilization treatment. The toluene-treated beads showed enhanced specific activity and a dramatic decrease in cell viability. The inactivation of the fermentative metabolism of glucose, a major product of the Sa1-SST reaction, in the permeabilized yeast avoided the undesired formation of ethanol, acetate, and CO2. The whole-cell biocatalyst was assayed for the production of short-chain FOSs, mainly 1-kestotriose, in repetitive cycles using different sucrose sources.
Materials and Methods
Reagents
Sucrose, glucose, fructose, 1-kestotriose, and 1,1-kestotetraose were purchased from Sigma Aldrich (USA). Toluene (≥99.5%) and sodium alginate (source Laminaria hyperborea) were purchased from BDH Chemicals (England). Refined sugar, raw sugar, sugarcane syrup, and molasses were collected at the sugar mill “Melanio Hernández,” Tuinucú, Sancti Spíritus, Cuba.
Strain
P. pastoris PGFT6x-308 constitutively expressing nine copies of the 1-SST gene from S. arundinaceus (Hernández et al., 2018) was maintained on agar plates containing YPG (Yeast extract 10 g/l, peptone 20 g/l, and glucose 20 g/l) with hygromycin (100 μg/ml) at 30°C.
Culture Conditions
One liter of MM-molasses medium for batch cultures of P. pastoris PGFT6x-308 contained 22 g NH4SO4, 18.2 g K2HPO4, 7.5 g MgSO4 7H2O, 0.5 g CaCl2 2H2O, 5 g yeast extract, and sugarcane molasses at 5ºBx. Vitamins and trace elements were prepared and used as recommended by d'Anjou and Daugulis (2001). The inoculum was cultured in a 500-ml shake flask with 100 ml of MM-molasses medium using a Minitron shaker (Infors, Switzerland) at 30°C with 250 rpm for 24 hr.
Fed-batch cultures of P. pastoris PGFT6x-308 were performed in a 5-l fermenter (Infors, Switzerland) with the temperature kept at 30°C and pH 5.5 controlled by the automatic addition of 40% (wt/vol) H3PO4 and 25% (wt/vol) NH3OH. The air flow rate was 3 l/min and the dissolved oxygen was maintained above 20% by increasing automatically the agitation speed from 500 rpm to 900 rpm. After 20 hr of batch cultivation, the air flow rate was adjusted to 6 l/min and the feeding phase was accomplished by the automatic addition of the carbon source at a rate range of 6–8 ml/l/hr according to the carbon demand, as monitored by the level of dissolved oxygen. The feeding solution was sugarcane molasses at 45°Bx. After 72 hr of cultivation, the cells were harvested by centrifugation (4°C, 3000 rpm).
Cell Immobilization
Whole cells (10, 20, 30, or 40 g, wet biomass) and 2 g of sodium alginate were suspended in water to a final volume of 100 ml and stirred thoroughly (100 rpm) at 25°C to ensure a homogenous distribution before extrusion in the CaCl2 solution. The alginate/cell mixtures were dropped through a fine needle into 1 l of 37 mM CaCl2 solution with constant stirring (100 rpm) using an impeller, type marine propeller, to avoid droplet aggregation. Gelation time was 1 hr and then the CaCl2 solution was discarded. The spherical alginate beads (diameter 2–3 mm) were hardened overnight in 67 mM CaCl2 at 4°C and stored in 1.46 M sucrose in 50 mM sodium acetate buffer (pH 5.5) at 4°C before use.
Permeabilization of Immobilized Cells
Beads (5 g, wet weight) were added to 10 ml of sodium acetate buffer (100 mM, pH 5.5) containing toluene at final concentrations of 3, 5, and 10% (vol/vol). The flasks were shaken at 100 rpm, 30°C for 1 hr to provide adequate contact between the entrapped cells and the solvent floating on the surface of the aqueous solution. After the membrane-permeabilization treatment, beads were recovered by filtration, washed twice with sodium acetate buffer (100 mM, pH 5.5) to remove residual toluene and stored in 1.46 M sucrose in 50 mM sodium acetate buffer (pH 5.5) at 4°C. Beads and solutions were assayed for sucrose-transforming activity.
To determine cell viability, beads were smashed in a liquid YPG medium and aliquots (0.1 ml) of sequential dilutions were plated on YPG agar. The number of colony-forming units (cfu) per gram of wet bead was determined on plates incubated at 30°C for 48 hr. Beads (5 g, wet weight) were incubated in 10 ml of sucrose solution (100 g/l) at 30°C for 12 hr to detect the release of CO2 as evidence of cell viability.
Enzyme Assay
Free or immobilized cells were reacted with 1.75 M sucrose in 100 mM sodium acetate buffer (pH 5.5) in a shaking water bath at 30°C with agitation (50 rpm). After 2 hr, the reaction was stopped by heating 20 min at 80°C. Glucose released to the solution was quantified by the dinitrosalicylic acid (DNSA) colorimetric method (Miller, 1959). One unit of Sa1-SSTrec is defined as the amount of enzyme releasing 1 µmol of glucose per minute at initial rates under the reaction conditions described above.
For evaluating the effect of toluene on the stability of free Sa1-SSTrec, the enzyme recovered from the culture supernatant of sucrose-grown P. pastoris PGFT6x-308 (Hernández et al., 2018) was preincubated with the organic solvent (0, 3, 5, and 10% vol/vol) in 100 mM sodium acetate buffer (pH 5.5) at 30°C. The residual Sa1-SSTrec activity after 30- and 60-min preincubations was measured with 1.75 M sucrose in 100 mM sodium acetate buffer (pH 5.5).
For assaying pH stability, toluene-treated beads (1 g, wet weight) were preincubated with 60% (wt/vol) sucrose in 100 mM sodium acetate/phosphate buffers of varying pH (4–8) at 30°C. For assaying thermal stability, the entrapped cells (1 g, wet weight) were preincubated with 60% (wt/vol) sucrose in 100 mM sodium acetate buffer (pH 5.5) at temperatures of 30, 40, 50, and 60°C. The residual Sa1-SSTrec activity of beads collected at time intervals was measured with 1.75 M sucrose in 100 mM sodium acetate buffer (pH 5.5).
FOSs Production and Operational Stability Assay
Beads (1 g, wet weight) were incubated in a 10-ml solution of the sucrose source (white refined sugar, raw sugar, sugarcane syrup, or molasses) adjusted to 50°Bx (around 600 g/l) in 100 mM sodium acetate buffer pH 5.5, at 30°C for 48 hr with agitation (100 rpm) in a stirred tank reactor. Samples were collected at regular intervals.
To evaluate the operational stability of the biocatalyst, 15 repetitive batches of 48 hr were performed under the conditions mentioned above. At the end of each cycle, the alginate beads were recovered by filtration and a fresh substrate solution was added. After 30 days, the beads were collected and assayed for Sa-1SSTrec activity. The residual activity was established by comparing with the activity of the beads stored in a sucrose solution (1.46 M) at 4°C.
Carbohydrate Analysis
Qualitative FOSs analysis was performed by thin layer chromatography (TLC) on TLC Silica gel 60 F254 plates (Merck, Germany) using acetone 95% (vol/vol) as a mobile phase. Detection was achieved by spraying with a saturated solution of 1-butanol 80% (vol/vol), urea 3% (wt/vol), H3PO4 6% (vol/vol), and absolute ethanol 4% (vol/vol) and heating for 5 min at 120°C.
Quantitative sugar analysis was performed by HPLC using an Aminex HPX-42C column (0.78 × 30 cm, BIORAD) equipped with a refractive index detector. The column temperature was kept at 85°C. Water was used as a mobile phase at a flow rate of 0.5 ml/min. Samples were appropriately diluted before injection. Solutions (20 mg/ml) of fructose, glucose, sucrose, 1-kestotriose, and 1,1-kestotetraose were used as standards. The program package ezData (www.chemilab.net) was used for sugars visualization.
Statistical Analysis
The statistical package for social sciences (SPSS) 15.0 was used for the data analyses. The data are presented as means ± standard deviation. The level of significance used in this study was p < 0.05.
Results and Discussions
Performance of Recombinant P. pastoris PGFT6x-308 in Fed-batch Fermentation With Molasses as Carbon Source
In a previous report, P. pastoris PGFT6x-308 constitutively expressing nine copies of the Sa1-SST was found to secrete the recombinant enzyme (Sa1-SSTrec), a fully active glycoprotein, to the cell periplasm and the extracellular medium in fed-batch fermentations using either glycerol or cane sugar as the carbon source (Hernández et al., 2018). The acquired ability of P. pastoris PGFT6x-308 to utilize sucrose prompted us to evaluate the use of sugarcane molasses as a cheaper substrate for cell growth.
Fig. 1 shows the behavior of P. pastoris PGFT6x-308 in a representative fed-batch fermentation using molasses as a carbon source. Cell density and Sa1-SSTrec production increased continuously over time (Fig. 1). After 72 hr of culture, Sa1-SSTrec activity was detected both in undisrupted biomass (49.8 U/ml of culture, equivalent to 0.48 U/mg of dry cell) and in extracellular medium (120.7 U/ml). The overall Sa1-SST activity (170.5 U/ml) was about 5.3- and 1.7-fold higher than the values reported for the carbon sources glycerol and cane sugar, respectively (Hernández et al., 2018). Previous cell fractionation experiments demonstrated that Sa1-SSTrec is not attached to membranes but secreted into the periplasmic space, where the substrate sucrose readily enters by diffusion (Hernández et al., 2018). The replacement of glycerol or cane sugar by molasses had no effect on the biomass yield, which remained above 100 g/l (dry weight). The volumetric productivity of P. pastoris PGFT6x-308 at the end of cell growth in the molasses-containing medium was 2368.0 U/l/hr.
Fig. 1.

Performance of Pichia pastoris PGFT6x-308 in fed-batch fermentation using molasses as a carbon source. Periplasmic (black bars) and extracellular (white bars) Sa1-SSTrec activities were measured in intact cells and the culture supernatant, respectively. Biomass yield (line) is expressed as dry weight.
Reducing sugars (glucose and fructose) and sucrose are the major carbohydrates in sugarcane molasses. Additional glucose is released from sucrose by the action of periplasmic and extracellular Sa1-SSTrec during cell growth. The continuous availability of the monosaccharide may have reinforced the transgene transcription. The GAP promoter, although constitutive, is known to behave stronger in glucose-grown cells (Waterham et al., 1997; Cereghino & Cregg, 2000).
Entrapment of P. pastoris PGFT6x-308 Cells in Calcium-Alginate Beads
Whole cells of P. pastoris PGFT6x-308 were entrapped in calcium-alginate gel using different biomass concentrations (100–400 g/l, wet weight) (Fig. 2). The highest recovery of Sa-1SSTrec activity was 98.9%, achieved in the beads prepared with the lowest biomass concentration (100 g/l). The immobilization yield decreased to 98, 97, and 95% in the treatment using 200, 300, and 400 g/l of wet biomass, respectively. Similarly, the immobilization yield dropped slightly with the increase in the biomass in the Ca-alginate entrapment process of heat-killed P. pastoris cells carrying a recombinant thermostable invertase in the periplasmic space (Martínez et al., 2014). We do not exclude the possibility that in both cases vigorous stirring may have caused some degree of mechanical damage to the cells releasing the soluble periplasmic enzymes to the external medium prior to bead formation.
Fig. 2.

Effect of biomass loading on cell entrapment efficiency (line) and Sa1-SSTrec activity (bars). Immobilization yield expresses the percentage of initial Sa1-SSTrec activity which was not recovered in the entrapped cells but remained in the CaCl2 solution. Data are averages of three experiments with standard deviation. Different letters show significant differences between enzyme activities per bead for each biomass loading (Student–Newman–Keuls test, α = 0.05).
The inferior Sa-1SSTrec activity (5.67 U/g) in the beads formed using the least concentrated biomass suspension (100 g/l) can be attributed to a suboptimal number of entrapped cells. The specific activity of the beads reached the maximum value (19.51 U/g wet bead) when biomass loading was 300 g/l. The treatment with the most concentrated cells (400 g/l) resulted in heavier beads of lower specific activity (13.11 U/g), suggesting a restricted internal diffusion of the substrate sucrose. Similarly, high biomass/alginate ratios failed to increase the activity of Ca-alginate entrapped cells of Saccharomyces cerevisiae (Polakovič et al., 2001) or P. pastoris with the recombinant periplasmic invertase (Martínez et al., 2014).
Scanning electron microscopy (SEM) studies are necessary to confirm the homogenous distribution of whole P. pastoris PGFT6x-308 cells inside the alginate spheres (diameter 2–3 mm) as well as to explore the 3D surface structure of the beads. SEM examinations of entrapped whole cells from different microorganisms have allowed a detailed characterization of the internal and external morphology of Ca-alginate beads (Van Neerven et al., 1990; Duarte et al., 2013; Gonçalves et al., 2020).
Permeabilization of Immobilized P. pastoris PGFT6x-308 Cells
The spherical Ca-alginate beads (diameter 2–3 mm) with specific sucrase activity of 19.51 U/g were incubated with toluene at three different concentrations. Sucrose conversion by the whole-cell biocatalyst was increased 2.2-, 2.3-, and 1.5-fold after the treatment with 3, 5, and 10% (vol/vol) of toluene, respectively (Fig. 3a). The membrane-permeabilization treatment enhanced the diffusion of the substrate sucrose into the cell increasing the catalytic action of the recombinant enzyme in the periplasmic space. Sa1-SSTrec is known to behave more active with the increase of sucrose concentration (Hernández et al., 2018). The activity decay observed when the toluene concentration was raised from 5 to 10% (vol/vol) may be attributed to the enzyme sensibility to the organic solvent. Preincubation of free Sa1-SSTrec with toluene in 100 mM sodium acetate buffer (pH 5.5) at 30°C for 1 hr decreased the enzyme stability in direct proportion to the solvent concentration (Fig. 3b). In another experiment, the stirring (100 rpm) of a whole-cell suspension in the presence of 10% (vol/vol) toluene at pH 5.5 and 30°C for 1 hr did not result in the release of N-glycosylated periplasmic Sa1-SSTrec to the external medium.
Fig. 3.
Permeabilization of immobilized Pichia pastoris PGFT6x-308 cells. (a) Effect of toluene concentration on the Sa1-SSTrec activity of the calcium-alginate beads. (b) Effect of toluene on the stability of free Sa1-SSTrec. (c) Reduction of CO2 formation by toluene-permeabilized cells during transformation of sucrose (1.75 M) at 30°C. Cut glasses contain beads previously incubated with 0, 3, or 5% (vol/vol) toluene. Data are the means of triplicate measurements ± standard deviation. Different letters are significant differences between relative activity at the three concentrations with respect to the control (Student–Newman–Keuls test, α = 0.05).
Beads that were not exposed to toluene (control beads) produced CO2 bubbles and floated up during incubation with 1.75 M sucrose at 30°C. Instead, the toluene-treated beads hardly released gas and sank to the bottom of the flask (Fig. 3c). Cell viability decreased from 109 in the control beads to 103 cfu/g of wet beads after the treatment with toluene at the optimal concentration of 5% (vol/vol). Toluene permeabilization elicited cell death, which is technically advantageous for biocatalyst operation. The reaction of Sa1-SSTrec on sucrose releases glucose, which is metabolized by living PGFT6x-308 cells producing the contaminants ethanol and acetate in the FOSs syrup. The glucose fermentation process generates CO2. The formation of CO2 bubbles by immobilized yeast increases the internal pressure of packed-bed columns which then tend to crack (Najafpour et al., 2004).
Toluene at the assayed concentrations (3, 5, and 10% vol/vol) did not provoke the leakage of periplasmic Sa1-SSTrec out of the Ca-alginate matrix, considering that no sucrose-transforming activity was detected in the solutions after beads recovery. We assume that the permeabilization treatment killed the yeast without causing cell disruption. Early microscopic examinations revealed that nonviable bacterial and yeast cells treated with toluene remain essentially intact (Jackson & DeMoss, 1965; De Smet et al., 1978; Murakami et al., 1980).
Effect of pH and Temperature on Sa1-SSTrec Activity and Stability in Immobilized Cells
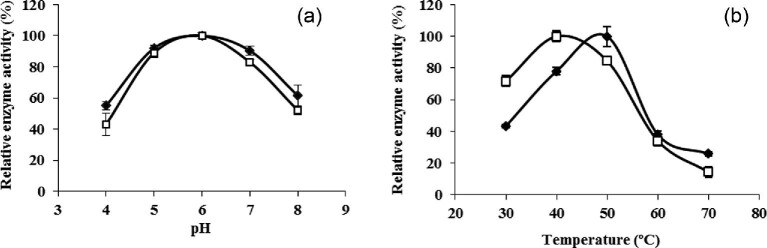
The effect of pH on the initial rates of Sa1-SSTrec reaction on sucrose (1.75 M) in free and immobilized P. pastoris PGFT6x-308 cells was evaluated in the range of 4–8 at 30°C (Fig. 4a). Free cells and toluene-treated beads showed similar pH-activity curves with the highest values at pH 6.0. The use of calcium alginate as the cell entrapment matrix caused no changes in the net charge of the biocatalyst.
Fig. 4.
Effect of pH (a) and temperature (b) on the initial rates of Sa1-SSTrec activity in free (□) and immobilized (◆) cells. Values are means of three experiments with standard deviation.
The influence of temperature (30–70°C) on the initial rates of Sa1-SSTrec reaction on sucrose (1.75 M) in free and immobilized cells was evaluated at pH 5.5 (Fig. 4b). A 10-degree higher optimal temperature (50°C) was observed for the immobilized cells. It is plausible to think that the increase of the reaction temperature reduced the viscosity of the concentrated sucrose solution (1.75 M) enhancing the substrate diffusion into the beads. Similarly, mycelial fructosyltransferases from Aspergillus flavus NFCCI 2364 and Aspergillus oryzae IPT-301 displayed a higher optimal temperature after cell entrapment in calcium-alginate beads (Ganaie et al., 2014; Gonçalves et al., 2020).
The effect of pH (4–8) and temperature (30–60°C) on Sa1-SSTrec stability in nonviable entrapped cells was assayed at a sucrose concentration of 600 g/l to mimic the operational conditions of FOSs synthesis. No significant variations in the enzyme stability were observed for the pH range 5–7 with remaining activities of 97 and 66% after preincubation for 2 and 30 days at 30°C, respectively. The half-life values of Sa1-SSTrec in the toluene-treated beads preincubated at 30, 40, and 50°C were 37 days, 8 days, and 18 hr, respectively. The enzyme was rapidly inactivated in the beads exposed to 60°C. The half-life of Sa1-SSTrec in the beads at 30°C is comparable to the values reported for immobilized fungal fructosyltransferases, which otherwise are often operated at 50°C due to their higher thermal stability (Chien et al., 2001; Nishizawa et al., 2001; Gonçalves et al., 2020).
Use of Different Sucrose Sources in FOSs Production by Immobilized Cells
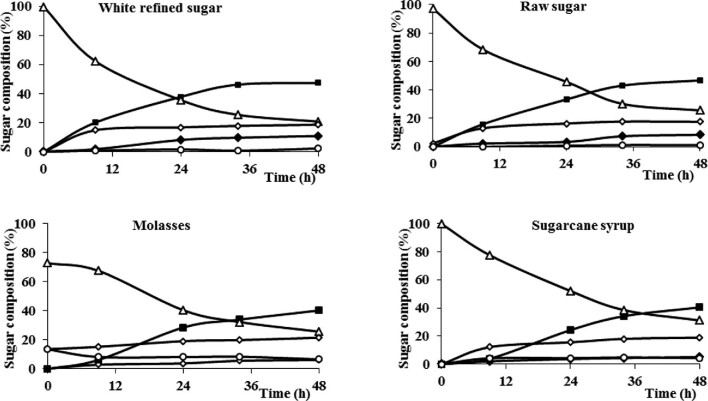
Toluene-treated beads were incubated with refined sugar, raw sugar, sugarcane syrup, and molasses. The reactions were conducted at pH 5.5 and 30°C, and the product composition was monitored for 48 hr (Fig. 5). In all sucrose sources, 1-kestotriose (GF2) was the main product, and 1,1-kestotetraose (GF3) appeared as the reactions progressed. At the end of the four reactions, the ratio of 1-kestotriose and 1,1-kestotetraose was above 8:2. The overall FOSs yield in the reactions with refined or raw sugar represented above 55% (wt/wt) of total carbohydrates, but it decreased to 45% (wt/wt) with sugarcane syrup and molasses (Table 1). The highest FOSs productivity (7.3 g/l/hr) was achieved when using refined sugar as the substrate. On the other hand, the use of an inexpensive sucrose source as molasses appears particularly attractive for applications that do not require FOSs purification, for instance, animal feed.
Fig. 5.
Fructooligosaccharides production by the immobilized whole-cell biocatalyst reacting with different sucrose sources in a stirred tank reactor. Fructose (○), glucose (◊), sucrose (∆), 1-kestotriose (■), and 1,1-kestotetraose (◆). Raw data are included as a supplementary table.
Table 1.
Carbohydrate Composition, Fructooligosaccharide (FOS) Yield and Productivity of the Biocatalyst Reaction With Different Sucrose Sources
| Sucrose source | Refined sugar | Raw sugar | Molasses | Sugarcane syrup |
|---|---|---|---|---|
| Carbohydrate composition (%, wt/wt) | ||||
| Glucose (G) | 18.7 | 17.7 | 21.4 | 18.9 |
| Fructose (F) | 2.3 | 1.3 | 6.6 | 4.4 |
| Sucrose (GF) | 20.8 | 25.7 | 25.6 | 31.1 |
| 1-Kestose (GF2) | 47.3 | 46.8 | 40.2 | 40.4 |
| Nystose (GF3) | 10.9 | 8.6 | 6.2 | 5.1 |
| YFOS/SUC a | 58.2 | 55.4 | 46.4 | 45.5 |
| YFOS/BC b | 3.5 | 3.3 | 2.8 | 2.7 |
| Ykestosa/FOS c | 81.2 | 84.5 | 86.7 | 88.7 |
| Productivity d | 7.3 | 6.9 | 5.7 | 5.8 |
aYield was calculated as FOS produced (g)/initial sucrose concentration (g) × 100.
bYield was calculated from FOS produced (g)/biocatalyst (g).
cYield was calculated from 1-kestose produced (g)/FOS produced (g) × 100.
dProductivity was calculated from FOS produced (g/l)/time of reaction (hr).
Reuse of Immobilized Cells in Semicontinuous FOSs Production
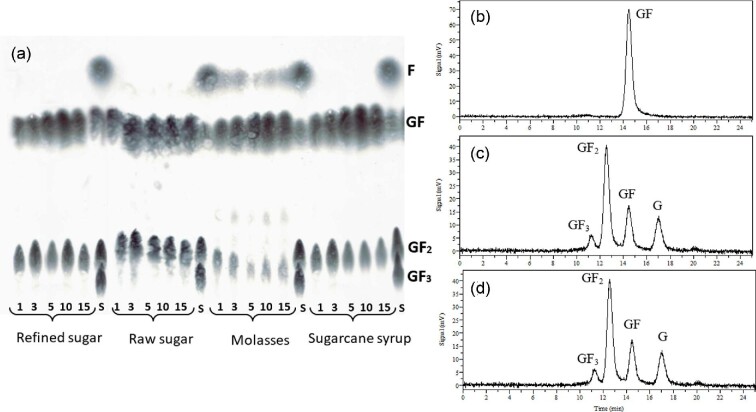
Semicontinous FOSs production was performed in stirred tanks at 30°C using refined sugar, raw sugar, sugarcane syrup, and molasses as substrates. The immobilized cells were reused in 15 repeated cycles of 48 hr, each for a total operation time of 30 days. TLC analysis of samples collected after cycles 1, 3, 5, 10, and 15 revealed the synthesis of 1-kestotriose (GF2) in the four sucrose sources (Fig. 6). After 5 cycles, the 1-kestotriose yield tended to decrease, particularly when using sugarcane syrup and molasses as the sucrose source.
Fig. 6.
Chromatographic analysis of fructooligosaccharides synthesized by immobilized cells from different sucrose sources in repetitive cycles. (a) TLC chromatograms of samples collected after batches 1, 3, 5, 10, and 15. S means mixture of standard sugars. (b–d) HPLC chromatograms. (b) Refined and raw sugar. (c) Refined sugar after the first batch. (d) Raw sugar after the first batch. GF3, 1,1-kestotetraose; GF2, 1-kestotriose; GF, sucrose; G, glucose; F, fructose.
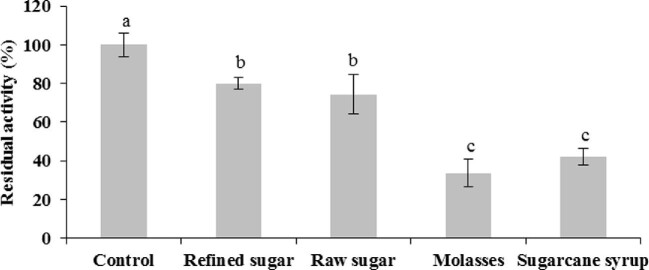
The stability of the biocatalyst was evaluated at the end of the 30-day batchwise operation at 30°C (Fig. 7). The residual activity of the beads incubated with sugarcane syrup and molasses was 41 and 32%, respectively. The continuous exposition with molasses softened and deformed the beads. Deterioration of the alginate matrix might have been caused by cations as manganese and zinc, with lower stabilizing power than calcium, as well as chelating agents as phosphates (Thu et al., 1996).
Fig. 7.
Sa1-SSTrec stability in immobilized cells after 15 cycles of batchwise operation. Different letters show significant differences between residual activities in each sucrose source (Student–Newman–Keuls test, α = 0.05).
More attractively, the beads reacted with refined or raw sugar retained 75–80% of the initial Sa1-SSTrec activity. The high enzyme stability in the immobilized biocatalyst allows producing repetitive FOSs batches and offers the possibility to increase the residence time in the last cycles during prolonged reuse.
To our knowledge, this work is the first report of immobilized yeast cells expressing a plant fructosyltranferase. While Sa1-SSTrec recovered from the culture supernatant (free enzyme) of P. pastoris PGFT6x-308 is appropriate for sucrose conversion into 1-kestotriose in one-cycle batch reactions (Hernández et al., 2018), the stable performance of periplasmic Sa1-SSTrec in Ca-alginate entrapped cells during repetitive operation cycles makes the immobilized whole-cell biocatalyst attractive for continuous FOSs production. The reduction of diffusional barriers in the toluene-treated beads not only accelerated the Sa1-SSTrec reaction but also induced cell death avoiding the formation of undesired metabolic by-products (ethanol, acetate, and CO2) during sucrose transformation. A heating treatment killed P. pastoris expressing a bacterial thermophilic invertase and enhanced sucrose hydrolysis in the cell periplasm (Martínez et al., 2014). Here, we show that membrane permeabilization is a valid option for the operational optimization of immobilized yeast cells expressing a mesophilic fructosyltransferase specialized in 1-kestotriose synthesis.
Conclusions
P. pastoris PGFT6x-308 cells constitutively expressing 1-SST from S. arundinaceus (Sa) were entrapped in calcium-alginate beads and exposed to a membrane-permeabilizing agent. In the toluene-treated beads, the enhancement of substrate and products diffusion through the outer membrane increased the catalytic action of periplasmic Sa1-SSTrec. The immobilized whole-cell biocatalyst yielded 55% (wt/wt) FOSs, 1-kestotriose, and 1,1-kestotetraose in a ratio above 8:2, when reacted with refined or raw sugar (600 g/l) in a stirred tank reactor at 30°C and pH 5.5. The beads retained 75–80% of the initial Sa1-SSTrec activity after 15 repetitive cycles of FOSs production in a 30-day batchwise operation. The immobilized biocatalyst is suitable for reuse and cost-effective production of 1-kestotriose, the most attractive commercial FOS.
Supplementary Material
Acknowledgments
The authors acknowledge the sugar mill “Melanio Hernández” (Tuinucú, Sancti Spíritus, Cuba) for providing the sucrose sources for the study.
Contributor Information
Enrique R Pérez, Departamento de Investigación-Desarrollo, Centro de Ingeniería Genética y Biotecnología de Sancti Spíritus (CIGBSS), Circunvalante Norte S/N, Olivos 3, Apartado Postal 83, Sancti Spíritus 60200, Cuba.
Duniesky Martínez, Departamento de Investigación-Desarrollo, Centro de Ingeniería Genética y Biotecnología de Sancti Spíritus (CIGBSS), Circunvalante Norte S/N, Olivos 3, Apartado Postal 83, Sancti Spíritus 60200, Cuba.
Carmen Menéndez, Grupo Tecnología de Enzimas, Dirección de Investigaciones Agropecuarias, Centro de Ingeniería Genética y Biotecnología (CIGB), Ave 31 entre 158 y 190, Apartado Postal 6162, Habana 10600, Cuba.
Dubiel Alfonso, Grupo Tecnología de Enzimas, Dirección de Investigaciones Agropecuarias, Centro de Ingeniería Genética y Biotecnología (CIGB), Ave 31 entre 158 y 190, Apartado Postal 6162, Habana 10600, Cuba.
Iván Rodríguez, Departamento de Ingeniería Química, Facultad de Química-Farmacia, Universidad Central “Marta Abreu” de Las Villas, Carretera a Camajuaní Km. 5 y 1/2, Santa Clara, Villa Clara 50100, Cuba.
Luis E Trujillo, Grupo Tecnología de Enzimas, Dirección de Investigaciones Agropecuarias, Centro de Ingeniería Genética y Biotecnología (CIGB), Ave 31 entre 158 y 190, Apartado Postal 6162, Habana 10600, Cuba.
Alina Sobrino, Departamento de Investigación-Desarrollo, Centro de Ingeniería Genética y Biotecnología de Sancti Spíritus (CIGBSS), Circunvalante Norte S/N, Olivos 3, Apartado Postal 83, Sancti Spíritus 60200, Cuba.
Ricardo Ramírez, Grupo Tecnología de Enzimas, Dirección de Investigaciones Agropecuarias, Centro de Ingeniería Genética y Biotecnología (CIGB), Ave 31 entre 158 y 190, Apartado Postal 6162, Habana 10600, Cuba.
Eulogio Pimentel, Grupo Tecnología de Enzimas, Dirección de Investigaciones Agropecuarias, Centro de Ingeniería Genética y Biotecnología (CIGB), Ave 31 entre 158 y 190, Apartado Postal 6162, Habana 10600, Cuba.
Lázaro Hernández, Grupo Tecnología de Enzimas, Dirección de Investigaciones Agropecuarias, Centro de Ingeniería Genética y Biotecnología (CIGB), Ave 31 entre 158 y 190, Apartado Postal 6162, Habana 10600, Cuba.
Funding
This work was financially supported by Centro de Ingeniería Genética y Biotecnología (CIGB), Cuba.
Conflict of Interest
The authors confirm that there is no conflict of interest regarding this article.
Data Availability
Experimental data are provided in the manuscript. Authors agree to provide any other data if requested.
References
- Cereghino J. L., Cregg J. M. (2000). Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiology Reviews, 24(1), 45–66. [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Duan K. J., Sheu D. C., Lin C. T., Li S. Y. (1996). Production of fructooligosaccharides by immobilized mycelium of Aspergillus japonicus. Journal of Chemical Technology & Biotechnology, 66(2), 135–138. [Google Scholar]
- Chien C. S., Lee W. C., Lin T. J. (2001). Immobilization of Aspergillus japonicus by entrapping cells in gluten for production of fructooligosaccharides. Enzyme and Microbial Technology, 29(4-5), 252–257. [Google Scholar]
- Cunha J. S., Ottoni C. A., Morales S. A. V., Silva E. S., Maiorano A. E., Perna R. F. (2019). Synthesis and characterization of fructosyltransferase from Aspergillus oryzae IPT-301 for high fructooligosaccharides production. Brazilian Journal of Chemical Engineering, 36(2), 657–668. [Google Scholar]
- d'Anjou M. C., Daugulis A. J. (2001). A rational approach to improving productivity in recombinant Pichia pastoris fermentation. Biotechnology and Bioengineering, 72(1), 1–11. [DOI] [PubMed] [Google Scholar]
- De Smet M. J., Kingma J., Witholt B. (1978). The effect of toluene on the structure and permeability of the outer and cytoplasmic membranes of Escherichia coli. Biochimica et Biophysica Acta (BBA) - Biomembranes, 506(1), 64–80. [DOI] [PubMed] [Google Scholar]
- Duarte J. C., Rodrigues J. A. R., Moran P. J. S., Valença G. P., Nunhez J. R. (2013). Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Express, 3(1), 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria L. L., Morales S. A. V., Prado J. P. Z., Dias G. S., de Almeida A. F., Xavier M. C. A., da Silva E. S., Maiorano A. E., Perna R. F. (2021). Biochemical characterization of extracellular fructosyltransferase from Aspergillus oryzae IPT-301 immobilized on silica gel for the production of fructooligosaccharides. Biotechnology Letters, 43(1), 43–59. [DOI] [PubMed] [Google Scholar]
- Fernandez R. C., Ottoni C. A., da Silva E. S., Matsubara R. M. S., Carter J. M., Magossi L. R., Wada M. A. A., de Andrade Rodrigues M. F., Maresma B. G., Maiorano A. E. (2007). Screening of β-fructofuranosidase-producing microorganisms and effect of pH and temperature on enzymatic rate. Applied Microbiology and Biotechnology, 75(1), 87–93. [DOI] [PubMed] [Google Scholar]
- Fernandez-Arrojo L., Rodriguez-Colinas B., Gutierrez-Alonso P., Fernandez-Lobato M., Alcalde M., Ballesteros A. O., Plou F. J. (2013). Dried alginate-entrapped enzymes (DALGEEs) and their application to the production of fructooligosaccharides. Process Biochemistry, 48(4), 677–682. [Google Scholar]
- Ganaie M. A., Rawat H. K., Wani O. A., Gupta U. S., Kango N. (2014). Immobilization of fructosyltransferase by chitosan and alginate for efficient production of fructooligosaccharides. Process Biochemistry, 49(5), 840–844. [Google Scholar]
- Gonçalves M. C. P., Kieckbusch T. G., Perna R. F., Fujimoto J. T., Morales S. A. V., Romanelli J. P. (2019). Trends on enzyme immobilization researches based on bibliometric analysis. Process Biochemistry, 76, 95–110. [Google Scholar]
- Gonçalves M. C. P., Morales S. A. V., Silva E. S., Maiorano A. E., Perna R. F., Kieckbusch T. G. (2020). Entrapment of glutaraldehyde crosslinked cells from Aspergillus oryzae IPT-301 in calcium alginate for high transfructosylation activity. Journal of Chemical Technology & Biotechnology, 95(9), 2473–2482. [Google Scholar]
- Hayashi S., Tubouchi Y., Takasaki Y., Imada K. (1994). Long-term continuous reaction of immobilized β-fructofuranosidase. Biotechnology Letters, 16(3), 227–228. [Google Scholar]
- He Y. C., Liu F., Zhang D. P., Gao S., Li Z. Q., Tao Z. C., Ma C. L. (2015). Biotransformation of 1,3-propanediol cyclic sulfate and its derivatives to diols by toluene-permeabilized cells of Bacillus sp. cczu11-1. Applied Biochemistry and Biotechnology, 175(5), 2647–2658. [DOI] [PubMed] [Google Scholar]
- Hernández L., Menéndez C., Pérez E. R., Martínez D., Alfonso D., Trujillo L. E., Ramírez R., Sobrino A., Mazola Y., Musacchio A., Pimentel E. (2018). Fructooligosaccharides production by Schedonorus arundinaceus sucrose:sucrose 1-fructosyltransferase constitutively expressed to high levels in Pichia pastoris. Journal of Biotechnology, 266, 59–71. [DOI] [PubMed] [Google Scholar]
- Jackson R. W., DeMoss J. A. (1965). Effects of toluene on Escherichia coli. Journal of Bacteriology, 90(5), 1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H., Hutkins R. W. (2000). Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Applied and Environmental Microbiology, 66(6), 2682–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabel M. J., Sangeetha P. T., Platel K., Srinivasan K., Prapulla S. G. (2008). Physicochemical characterization of fructooligosaccharides and evaluation of their suitability as a potential sweetener for diabetics. Carbohydrate Research, 343(1), 56–66. [DOI] [PubMed] [Google Scholar]
- Maiorano A. E., da Silva E. S., Perna R. F., Ottoni C. A., Piccoli R. A. M., Fernandez R. C., Maresma B. G., de Andrade Rodrigues M. F. (2020). Effect of agitation speed and aeration rate on fructosyltransferase production of Aspergillus oryzae IPT-301 in stirred tank bioreactor. Biotechnology Letters, 42(12), 2619–2629. [DOI] [PubMed] [Google Scholar]
- Martínez D., Menéndez C., Echemendia F. M., Pérez E. R., Trujillo L. E., Sobrino A., Ramírez R., Quintero Y., Hernández L. (2014). Complete sucrose hydrolysis by heat-killed recombinant Pichia pastoris cells entrapped in calcium alginate. Microbial Cell Factories, 13(1), 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlik K., Albrecht J. A., Schnepf M. (2012). Effects of fructooligofructoses chain length on the bifidobacteria of the human colon: A pilot study. Food and Nutrition Sciences, 3, 1615–1618. [Google Scholar]
- Miller G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428. [Google Scholar]
- Murakami K., Nagura H., Yoshino M. (1980). Permeabilization of yeast cells: Application to study on the regulation of AMP deaminase activity in situ. Analytical Biochemistry, 105(1), 407–413. [DOI] [PubMed] [Google Scholar]
- Najafpour G., Younesi H., Ismail K. S. K. (2004). Ethanol fermentation in an immobilized cell reactor using Saccharomyces cerevisiae. Bioresource Technology, 92(3), 251–260. [DOI] [PubMed] [Google Scholar]
- Nishizawa K., Nakajima M., Nabetani H. (2001). Kinetic study on transfructosylation by β-fructofuranosidase from Aspergillus niger ATCC 20611 and availability of a membrane reactor for fructooligosaccharide production. Food Science and Technology Research, 7, 39–44. [Google Scholar]
- Panesar P. S., Panesar R., Singh R. S., Bera M. B. (2007). Permeabilization of yeast cells with organic solvents for β-galactosidase activity. Research Journal of Microbiology, 2, 34–41. [Google Scholar]
- Panesar R., Panesar P. S., Singh R. S., Kennedy J. F. (2011). Hydrolysis of milk lactose in a packed bed reactor system using immobilized yeast cells. Journal of Chemical Technology and Biotechnology, 86(1), 42–46. [Google Scholar]
- Park M. C., Lim J. S., Kim J. C., Park S. W., Kim S. W. (2005). Continuous production of neo-fructooligosaccharides by immobilization of whole cells of Penicillium citrinum. Biotechnology Letters, 27(2), 127–130. [DOI] [PubMed] [Google Scholar]
- Park Y. M., Choi E. S., Rhee S. K. (1994). Effect of toluene-permeabilization on oxidation of D-sorbitol to L-sorbose by Gluconobacter suboxydans cells immobilized in calcium alginate. Biotechnology Letters, 16(4), 345–348. [Google Scholar]
- Pereira I. A., Gibson G. R. (2002). Effects of consumption of probiotics and prebiotics on serum lipid levels in humans. Critical Reviews in Biochemistry and Molecular Biology, 37(4), 259–281. [DOI] [PubMed] [Google Scholar]
- Perna R. F., Cunha J. S., Gonçalves M. C. P., Basso R. C., Silva E. S., Maiorano A. E. (2018). Microbial fructosyltransferase: Production by submerged fermentation and evaluation of pH and temperature effects on transfructosylation and hydrolytic enzymatic activities. International Journal of Engineering Research and Science, 4(3), 43–50. [Google Scholar]
- Polakovič M., Kudláčová G., Štefuca V., Báleš V. (2001). Determination of sucrose effective diffusivity and intrinsic rate constant of hydrolysis catalysed by Ca-alginate entrapped cells. Chemical Engineering Science, 56(2), 459–466. [Google Scholar]
- Rehm J., Willmitzer L., Heyer A. G. (1998). Production of 1-kestose in transgenic yeast expressing a fructosyltransferase from Aspergillus foetidus. Journal of Bacteriology, 180(5), 1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Martínez M. J., Soto-Jover S., Antolinos V., Martínez-Hernández G. B., López-Gómez A. (2020). Manufacturing of short-chain fructooligosaccharides: From laboratory to industrial scale. Food Engineering Reviews, 12(2), 149–172. [Google Scholar]
- Smidsrød O., Skja G. (1990). Alginate as immobilization matrix for cells. Trends in Biotechnology, 8, 71–78. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Aiba Y., Takeda H., Fukumori Y., Koga Y. (2006). Superiority of 1-kestose, the smallest fructo-oligosaccharide, to a synthetic mixture of fructo-oligosaccharides in the selective stimulating activity on bifidobacteria. Bioscience and Microflora, 25(3), 109–116. [Google Scholar]
- Thu B., Bruheim P., Espevik T., Smidsrød O., Soon-Shiong P., Skjåk-Braek G. (1996). Alginate polycation microcapsules: I. Interaction between alginate and polycation. Biomaterials, 17(10), 1031–1040. [DOI] [PubMed] [Google Scholar]
- Trujillo L. E., Arrieta J. G., Dafhnis F., García J., Tambara Y., Pérez M., Hernández L. (2001). Fructo-oligosaccharides production by the Gluconacetobacter diazotrophicus levansucrase expressed in the methilotrophic yeast Pichia pastoris. Enzyme and Microbial Technology, 28(2-3), 139–144. [DOI] [PubMed] [Google Scholar]
- Van Neerven A. R. W., Wijffels R. H., Zehnder A. J. B. (1990). Scanning electron microscopy of immobilized bacteria in gel beads: A comparative study of fixation methods. Journal of Microbiological Methods, 11(3-4), July 1990, 157–168. [Google Scholar]
- Waterham H. R., Digan M. E., Koutz P. J., Lair S. V., Cregg J. M. (1997). Isolation of the Pichia pastoris glyceraldehyde-3-phosphate dehydrogenase gene and regulation and use of its promoter. Gene, 186(1), 37–44. [DOI] [PubMed] [Google Scholar]
- Yang H., Wang Y., Zhang L., Shen W. (2016). Heterologous expression and enzymatic characterization of fructosyltransferase from Aspergillus niger in Pichia pastoris. New Biotechnology, 33(1), 164–170. [DOI] [PubMed] [Google Scholar]
- Yun J. W., Lee M. G., Song S. K. (1994). Continuous production of isomalto-oligosaccharides from maltose syrup by immobilized cells of permeabilized Aureobasidium pullulans. Biotechnology Letters, 16(11), 1145–1150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experimental data are provided in the manuscript. Authors agree to provide any other data if requested.