Abstract
Objective: To explore the effect of pantoprazole and somatostatin combined with thrombin in the treatment of non-esophagogastric varicosity upper gastrointestinal bleeding (UGB) as well as its influence on serum hs-CRP and coagulation function. Methods: From June 2016 to May 2018, patients with upper gastrointestinal hemorrhage due to non-esophagogastric varices in our hospital were selected as research subjects. After screening, they were randomly divided into the combined group (57 cases) and the control group (57 cases). After the two groups are treated, the therapeutic effect was observed. The two groups of patients were followed up for 6 consecutive months, and the data were statistically analyzed. Results: It was found that there wass no significant difference between the two groups in gender, age, amount of bleeding, and etiology (P > 0.05). It was found that the immediate hemostasis rate and the hemostasis rate within 24 hours in the combined group were distinctly higher compared to the control group. The difference has statistical significance (P < 0.05). The total effective rate of the combined group was distinctly higher compared to the control group (P < 0.05). By comparing the expression levels of hs-CRP and IL-6 protein in the serum of the two groups before and after treatment, it was found that there was no significant difference in the expression levels of hs-CRP and IL-6 protein before treatment. However, after treatment, it was found that the levels of hs-CRP and IL-6 protein in the combined group were distinctly lower compared to the control group (P < 0.05). By analyzing adverse reactions, it was found that the combined group had distinctly lower adverse reactions compared to the control group (P < 0.05). Conclusion: This work provides an experimental basis for the diagnosis and treatment of non-esophagogastric varicose UGB in the clinic.
Keywords: Pantoprazole, somatostatin, upper gastrointestinal hemorrhage, thrombin, hs-CRP
Introduction
With the acceleration of the process of social industrialization, living standards of people accordingly continue to improve [1]. However, with the improvement of life quality, health has become a main focus of attention. The mortality from upper gastrointestinal hemorrhage is up to 25%. In the analysis of its pathogenic factors, non-esophagogastric varices bleeding is the most common cause of cirrhosis and it is dangerous. The common ones are peptic ulcer, gastric and duodenal erosion, gastroesophageal reflux disease, and gastrointestinal tumor [2,3]. At present, the treatment plan for upper gastrointestinal hemorrhage has been improving at the clinical level. The most important thing is to control acute hemorrhage. However, if the patient does not receive long-term and effective continuous treatment after controlling the bleeding, the patient is likely to have upper gastrointestinal bleeding (UGB) again [4,5]. Therefore, it is important to study the continuous treatment of UGB and the types of drugs used.
Pantoprazole compound has a white solid form. As a proton pump inhibitor, it is often used clinically to inhibit gastric acid secretion and also to treat active peptic ulcer reflux esophagitis [6]. Upper gastrointestinal hemorrhage usually refers to the gastrointestinal tract above the troostal ligament, which is caused by esophageal, gastric, duodenal, or pancreaticobiliary lesions. The bleeding of jejunal lesions after gastrojejunostomy also belongs to this class. In the treatment of upper gastrointestinal hemorrhage, the treatment plan is constantly maturing. However, the highest mortality rate is still shock caused by hemorrhage [7,8]. If a healthy adult has less than 400 ml of blood loss at a single occurrence it will not lead to the occurrence of serious diseases, but it will rise due to the acceleration of blood circulation, the increase of heart rate and the storage of blood in the spleen [9]. However, if the amount of one-time bleeding is more than 1000 ml, it is likely to lead to shock and even be life-threatening [10]. Gastric and duodenal ulcers are mostly caused by excessive gastric acid secretion in the stomach or severe trauma and burns caused by HP infection and stress [11]. Bleeding is due to acid damage to the gastrointestinal mucosa and submucosal tissue protection. Therefore, in the treatment of bleeding symptoms, antacids and vasoactive drugs such as somatostatin play an important role in hemostasis [12,13]. In the treatment of non-esophagogastric varicosity UGB, the current clinical medication is different. Martínez-Alcalá et al. (2018) used endoscopy to observe non-varicose UGB and proposed a new treatment [14]. Lanas et al. (2017) paid close attention to the clinical non-variceal gastrointestinal bleeding and analyzed its bleeding characteristics and clinical treatment principles [15]. Yuan et al. (2019) analyzed the prognosis of ulcer related and non-ulcer related UGB and used somatostatin combined with pantoprazole for corresponding treatment. They observed the effect and found that the treatment effect of somatostatin combined with pantoprazole is good [16].
Through the analysis of the previous clinical data, it was found that the mechanism of somatostatin and pantoprazole in the treatment of non-esophagogastric varices upper gastrointestinal hemorrhage is different. At the same time, thrombin has an inhibitory effect on bleeding, and its combination may improve the clinical effect. Therefore, in this study, from June 2016 to May 2018, patients with non-esophagogastric variceal UGB were chosen as the research subjects. They were divided into the combined group and the control group, to observe the effect of pantoprazole, somatostatin and thrombin combined with pantoprazole alone. Furthermore, we provide a new idea for the diagnosis and treatment of non-esophagogastric variceal UGB in the later clinical stage.
Materials and methods
Research subjects
In this research, 108 patients with upper gastrointestinal hemorrhage of non-esophagogastric varices who were treated in our hospital from June 2016 to May 2018 were used as the research subjects. Among them, there were 67 males and 47 females, aged between 31 and 65 years old. All the experimental operations were approved by patients and their families, as well as approved by the ethics committee.
Inclusion criteria
Subjects signed an informed consent before the experiment. Subjects ranged in age from 21 to 67 years old. The clinical manifestations of the subjects were hematemesis with black stool, dizziness, asthenia, decreased blood pressure, and increased pulse rate. The amount of bleeding was more than 1000 ml. Other important organ functions of the subjects were normal.
Exclusion criteria
Patients who had combined thrombotic diseases. Patients who had combined immune system diseases. The age of the patients was less than 18 years old or more than 70 years old. The patients had been treated with drugs or endoscopy or had gastrointestinal bleeding symptoms for more than 48 hours. Patients with contraindications to the drugs needed in the course of the study. Patients who could not continue with treatment and quit.
According to the above criteria, the experiment excluded 4 patients and included 104 patients. They were randomly divided into the combined group (57 cases) and the control group (57 cases). The general data of the two groups were collected.
Treatment method
Routine treatment
First, the patients in the two groups were treated by general treatment. Patients maintained absolute bed rest and took oxygen. An electrocardiogram monitor was used to monitor heart rate, respiration, oxygen saturation, and arterial blood pressure of upper extremites. Effective venous access was established and maintained. Second, oral care was needed to keep the respiratory tract unobstructed and avoid asphyxia caused by hematemesis. Then, the patient’s blood volume needed to be replenished. When patients had hypotension symptoms (systolic blood pressure less than 90 mmHg or heart rate greater than 120 beats/min), they needed to expand blood volume with transfusion. The hematocrit should be between 25% and 30%. According to the monitoring results of central venous pressure, it was necessary to adjust the volume and speed of transfusion. Finally, when the bleeding was uncontrollable and life-threatening or bleeding again after hemostasis, it was necessary to perform endoscopic ligation or injection of sclerosing agent for hemostasis and transfer of the patient to surgery.
Group treatment
After routine treatment for all patients, the two groups of patients were treated separately. The patients in the combined group were given pantoprazole (approval number; gyzz H19900166; manufacturer: Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd.; drug characteristics: chemicals. 40 mg) 40 mg mixed with 0.9% sodium chloride injection, 100 ml intravenous drip twice a day for 2-4 days.
At the same time, the group was injected with 0.25 mg somatostatin and 0.9% sodium chloride injection for 1 ml. The slow intravenous injection was taken as a loading dose, which was finished within 3-5 minutes. After that, it needed to be continuously pumped into the vein according to 0.25 mg/h micro pump and treated with hemagglutinin (approval No.: gyzz H20041419; production unit: Penglai Nuokang Pharmaceutical Co., Ltd.; drug characteristics; chemical. 1 unit). After the bleeding stopped, the treatment was maintained for 5 days. Patients in the control group were given pantoprazole 40 mg mixed with 0.9% sodium chloride injection twice a day for two to four days. After bleeding control, the dosage was gradually reduced and the treatment was maintained for 5 days.
Observation index
After admission, it was necessary to observe whether the two groups of patients had active bleeding symptoms such as hematemesis and black stool. Moreover, the color and clarity of gastric drainage fluid were observed, as well as the changes in blood pressure, pulse, heart rate, and bowel sounds were checked. In addition, it was necessary to collect blood samples, detect the changes in red blood cell count and hemoglobin in blood samples, as well as estimate the amount of bleeding according to the symptoms, signs, and blood detection indicators of patients. Routine gastroscopy was used to make the diagnosis clear. After 72 hours of administration, the gastroscopy was used to determine if the bleeding stopped. When there was no improvement or aggravation, treatment such as ligation and sclerosing agent injection were carried out according to the conditions. The total amount of blood transfusion, hemostasis time (referring to the time from the beginning of treatment to the success of hemostasis), and the total days of hospitalization were monitored. The mean arterial pressure (MAP) of the two groups was monitored. The equation is: MAP = (systolic pressure + 2 × diastolic pressure)/3. The arterial blood pressure of the upper extremities in the patient’s quiet state was measured, calculated, and recorded. In addition, the adverse reactions of the two groups when taking drugs were observed.
Curative effect standard
Symptoms of successful hemostasis
Active bleeding stopped within 72 hours, symptoms such as hematemesis or black stool disappeared, stool color turned yellow, stool became dry, and stool blood test turned negative. The gastric drainage was colorless and clear. The bowel sounds were normal and inactive. Blood pressure was stable and pulse returned to normal (70-90 times/min). Hemoglobin level and red blood cell count were stable or increased. Gastroscopy confirmed that active bleeding stopped.
Symptoms of ineffective hemostasis
After 72 hours of treatment, clinical symptoms and signs were not improved and bleeding was not controlled. Hemoglobin and red blood cell count showed a continuous downward trend, and they needed to be treated by gastroscopy, ligation, hemostasis, and surgical disconnection.
Follow-up
After discharge, the patients needed to be followed up for 6 months, and the outpatient re-examination was carried out once a month. In re-examination, endoscopy was used to check the degree of non-esophageal and gastric varices. Moreover, the incidence of re-bleeding within 6 months should be counted. The criterion of re-bleeding was the occurrence of active bleeding such as hematemesis, black stool, or hematochezia was assessed after the control of bleeding. Verification that the increase in heart rate was more than 20 times/min, or the decrease of systolic pressure was more than 20 mmhg. In the absence of blood transfusion, hemoglobin decreased by more than 30 mg/L. If there was no re-bleeding within 6 months and the degree of the varicose veins was not increased or reduced, it was determined that varicose vein treatment was effective.
Statistical analysis
The experimental results were expressed as mean ± standard deviation (x ± s). SPSS 22.0 was used to analyze the statistical results. Also, the rank-sum test was used to compare the two groups. In addition, the T test was used to compare the experimental results between the two groups. Before and after treatment, the paired design t-test was adopted. The percentage (%) was used for the expression of the counting data, and chi-square test was adopted for the analysis of the qualitative data. P < 0.05 indicated the difference had statistical significance. P < 0.01 was considered to have obvious statistical significance.
Results
Comparative analysis of basic data of patients in each group
The basic data of the 104 selected cases were collected and compared in terms of gender, age, bleeding volume, and etiology in research, as shown in Table 1. It was found that there was no significant difference between the two groups in gender, age, amount of bleeding, and etiology (P > 0.05).
Table 1.
Comparison and analysis of basic data of two groups of patients
| Index | Combined group | Control group | χ2 | P | |
|---|---|---|---|---|---|
| Gender (cases) | Male | 34 (59.65%) | 33 (57.89%) | 0.037 | 0.859 |
| Female | 23 (40.35%) | 24 (42.11%) | |||
| Age (years old) | 42.79±7.18 | 43.16±6.92 | 0.356 | 0.728 | |
| Bleeding volume (ML) | 2551±613 | 2604±605 | 0.314 | 0.697 | |
| Etiology (case) | Peptic ulcer | 26 (45.61%) | 25 (43.86%) | 0.039 | 0.861 |
| Acute and chronic gastritis | 18 (31.58%) | 17 (29.82%) | 0.036 | 0.842 | |
| Esophageal injury | 8 (14.04%) | 9 (15.79%) | 0.063 | 0.795 | |
| Gastric polyps | 1 (1.75%) | 1 (1.75%) | 0.211 | 0.714 | |
| Duodenal ulcer | 2 (3.51%) | 3 (5.27%) | 0.213 | 0.693 | |
| Gastric mucosa injury | 2 (3.51%) | 2 (3.51%) | 0.215 | 0.653 | |
Results of clinical efficacy analysis of the patients in each group
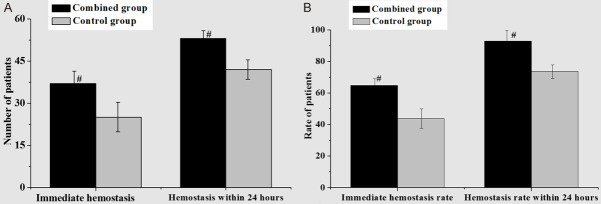
As shown in Figure 1, through the comparative analysis of hemostasis between the two groups, it was found that the immediate hemostasis rate and the hemostasis rate within 24 hours in the combined group were distinctly higher than those in the control group. Also, there was a statistically significant difference (P < 0.05). After a further comparative analysis of the total effective rate, the results were shown in Table 2. It can be found that the number of basically cured cases in the combined group was distinctly higher than that in the control group. The sum of basically cured and improved cases, that was, the total effective cases in the combined group was distinctly higher than that in the control group. For the total effective rate, the difference had statistical significance (P < 0.05).
Figure 1.
Comparative analysis of hemostasis between the two groups (A. comparison of hemostasis; B. comparison of hemostasis rate (Compared to the control group, #P < 0.05 was statistically significant, ##P < 0.01 had significant statistical significance)).
Table 2.
Comparison and analysis of the total effective rate of the two groups of patients
| Group | Basic cure | Improved | Invalid | Total effective rate |
|---|---|---|---|---|
| Combined group | 35 (61.4%) | 21 (36.84%) | 1 (1.75%) | 56 (98.25%)# |
| Control group | 24 (42.11%) | 27 (47.37%) | 6 (10.53%) | 51 (89.47%) |
| P | 0.0396 | 0.0410 | 0.0256 | 0.0372 |
| T | 2.331 | 2.014 | 2.962 | 2.413 |
Note: compared to the control group;
P < 0.05 had statistical significance.
Comparative analysis of the levels of hs-CRP and IL-6 protein in serum between the cases of the two groups before and after treatment
For the two groups, the expression levels of hs-CRP and IL-6 protein in serum before and after treatment were compared and analyzed, as shown in Table 3. It was found that the two groups had no distinct difference in the expression level of IL-6 before treatment. However, the expression level of IL-6 in the combined group was distinctly higher than that in the control group after treatment (P < 0.01). By comparing the hs-CRP protein expression level, the two groups had no statistical difference before treatment. The serum hs-CRP expression level in the combined group after treatment was distinctly higher than that in the control group (P < 0.01).
Table 3.
Comparison of the levels of hs-CRP and IL-6 protein in serum of the two groups of cases before and after treatment (x ± s)
| Group | IL-6 (pg/mL) | Hs-CRP (mg/L) | ||
|---|---|---|---|---|
|
|
|
|||
| Before treatment | After treatment | Before treatment | After treatment | |
| Combined group | 183.52±23.19 | 99.65±11.68 | 40.16±2.53 | 5.69±0.31 |
| Control group | 181.67±27.43 | 146.59±13.97 | 39.62±4.75 | 8.62±0.68 |
| P | 0.874 | < 0.001 | 0.756 | < 0.001 |
| T | 1.544 | 4.381 | 1.651 | 4.755 |
Comparative analysis of adverse reactions between the two groups of patients
By comparing and analyzing adverse reactions and incidence, the results were shown in Table 4. It was found that the combined group had dizziness, drowsiness, and other adverse symptoms. After two days of recovery, the adverse symptoms disappeared. The control group had dizziness, muscle pain, edema, and other adverse symptoms. The adverse symptoms disappeared after two days of drug recovery. For the two groups, their incidences of adverse reactions were compared. It was found that the incidence of adverse reactions in the combined group was significantly lower compared to the control group (P < 0.05).
Table 4.
Comparison and analysis of adverse reactions between the two groups of patients
| Group | Dizzy | Muscle pain | Drowsiness | Edema | Incidence rate |
|---|---|---|---|---|---|
| Combined group | 1 | 0 | 1 | 0 | 2 (3.51%) |
| Control group | 1 | 1 | 0 | 1 | 3 (5.26%) |
| P | 0.0312 | ||||
| T | 2.332 |
Discussion
Upper gastrointestinal hemorrhage is very common clinically, mainly including peptic ulcer, rupture of esophageal and gastric varices caused by portal hypertension, acute gastric mucosal damage and gastric cancer [17]. Among them, the incidence of non-esophagogastric varices UGB is highest, and the pathogenesis is mostly gastric acid damage. Generally, the hemostasis function of platelet aggregation and plasma coagulation can only play an effective role after the pH ≥ 6.0. Proton pump inhibitors can improve the pH value in the stomach and promote the formation of platelet aggregation and fibrin clot, thereby avoiding the early dissolution of the blood clot. It is conducive to hemostasis and prevention of re-bleeding [18]. Pantoprazole, as a proton pump inhibitor, can effectively bind to the ATPase in the tissue cells, thereby inhibiting the secretion of gastric acid and achieving the effect of increasing the pH value [19]. By analyzing the basic data of the two groups, there was no significant difference between the two groups in gender, age, amount of bleeding, and etiology (P > 0.05). By analyzing the curative effect, it was found that the immediate hemostasis rate and the hemostasis rate within 24 hours in the combined group are distinctly higher than those in the control group. Also, the difference has statistical significance (P < 0.05). Regarding the total effective rate, the number of cases that were basically cured in the combined group was distinctly higher than compared to the control group. The sum of basic cure and improvement, that is, the total effective number of cases in the combined group is distinctly higher than compared to the control group. For the total effective rate, the difference has statistical significance (P < 0.05).
Somatostatin is a synthetic cyclic 14 amino acid peptide, which has the same effect as the secretion of related peptide hormones from the gastric mucosa, posterior pituitary, and human islets. It can inhibit the secretion of glucagon, insulin, vasoactive intestinal peptide, pepsin, and gastrin. Also, it has a hemostatic effect [20]. CRP is a kind of acute-phase reaction protein, which is synthesized by the liver. The level of CRP in the physiological state is relatively low, with an average of 3.5 mg/l. When the body is in a state of stress, CRP can rise rapidly in a short period of time and can rise to 10 times, 100 times, or even 1000 times of the normal level. After the state of stress relieve, the level of stress will decrease significantly. Finally, it will return to the normal level. Hs-CRP is a more sensitive and accurate quantitative measure than CRP [21]. In this study, through the comparative analysis of the expression levels of hs-CRP and IL-6 protein in the serum of the two groups before and after treatment, it was found that there was no significant difference between the expression levels of hs-CRP and IL-6 protein before treatment. However, after treatment, it was found that the levels of hs-CRP and IL-6 protein in the combined group were distinctly lower compared to the control group (P < 0.05). By analyzing adverse reactions, it was found that the combined group was distinctly lower compared to the control group (P < 0.05). Therefore, it is conjectured that the effect of the combined group is better than that of pantoprazole alone.
In conclusion, through this study, we found that pantoprazole combined with somatostatin and thrombin in the treatment of non-esophagogastric varicosity UGB and the recovery of serum hs-CRP are better than pantoprazole alone. It provides an experimental basis for the diagnosis and treatment of later stage clinical UGB. This is almost consistent with the research results of scholars such as Wang (2009). The research results show that after the endoscopic treatment of peptic ulcer bleeding, intravenous pantoprazole can reduce the re-bleeding rate of ulcers [22]. However, there are some shortcomings in the process of the experiment, such as being only one sampling group selected in the experiment. In the follow-up research, the range of subjects can be further selected, and the operation process will be more rigorous, providing a more reliable basis for the clinical treatment of non-esophagogastric varicosity UGB.
Disclosure of conflict of interest
None.
References
- 1.Bilal M, Samuel R, Murtaza M, Khalil MK, Parupudi S, Abougergi MS. Non-variceal upper gastrointestinal hemorrhage is a rare complication after percutaneous coronary intervention for myocardial infarction, but has a detrimental effect on mortality: a national analysis over 11 months: 532. Am J Gastroenterol. 2018;113:S304. [Google Scholar]
- 2.Ghassemi KA, Jensen DM. What does lesion blood flow tell us about risk stratification and successful management of non-variceal UGI bleeding? Curr Gastroenterol Rep. 2017;19:17. doi: 10.1007/s11894-017-0556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush DA, Hillebrand DJ, Slater JM, Slater JD. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127:S189–S193. doi: 10.1053/j.gastro.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Lin TC, Chang WL. Multifocal gastrointestinal varices: a rare manifestation of immunoglobulin G4-related disease. Postgrad Med. 2019;131:176–181. doi: 10.1080/00325481.2019.1568018. [DOI] [PubMed] [Google Scholar]
- 5.Fouad TR, Abdelsameea E, Abdel-Razek W, Attia A, Mohamed A, Metwally K, Naguib M, Waked I. Upper gastrointestinal bleeding in Egyptian patients with cirrhosis: post-therapeutic outcome and prognostic indicators. J Gastroenterol Hepatol. 2019;34:1604–1610. doi: 10.1111/jgh.14659. [DOI] [PubMed] [Google Scholar]
- 6.Chang-Xue J, Ying L, Shuai J, Jin-Wei Q. Percutaneous transsplenic varices embolization in treatment of upper gastrointestinal hemorrhage of schistosomiasis cirrhosis. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2017;29:349–351. doi: 10.16250/j.32.1374.2016257. [DOI] [PubMed] [Google Scholar]
- 7.Alhazzani W, Guyatt G, Alshahrani M, Deane AM, Marshall JC, Hall R, Muscedere J, English SW, Lauzier F, Thabane L, Arabi YM, Karachi T, Rochwerg B, Finfer S, Daneman N, Alshamsi F, Zytaruk N, Heel-Ansdell D, Cook D Canadian Critical Care Trials Group. Withholding pantoprazole for stress ulcer prophylaxis in critically ill patients: a pilot randomized clinical trial and meta-analysis. Crit Care Med. 2017;45:1121–1129. doi: 10.1097/CCM.0000000000002461. [DOI] [PubMed] [Google Scholar]
- 8.Selvanderan SP, Summers MJ, Finnis ME, Plummer MP, Ali Abdelhamid Y, Anderson MB, Chapman MJ, Rayner CK, Deane AM. Pantoprazole or placebo for stress ulcer prophylaxis (POP-UP): randomized double-blind exploratory study. Crit Care Med. 2016;44:1842–1850. doi: 10.1097/CCM.0000000000001819. [DOI] [PubMed] [Google Scholar]
- 9.Hansen AR, Tannock IF, Templeton A, Chen E, Evans A, Knox J, Prawira A, Sridhar IF, Tan S, Vera-Badillo F, Wang L, Wouters BG, Joshua AM. Pantoprazole affecting docetaxel resistance pathways via autophagy (PANDORA): phase II trial of high dose pantoprazole (autophagy inhibitor) with docetaxel in metastatic castration-resistant prostate cancer (mCRPC) Oncologist. 2019;24:1188–1194. doi: 10.1634/theoncologist.2018-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shakhnovich V, Smith PB, Guptill JT, James LP, Collier DN, Wu H, Livingston CE, Zhao J, Kearns GL, Cohen-Wolkowiez M Best Pharmaceuticals for Children Act-Pediatric Trials Network. A population-based pharmacokinetic model approach to pantoprazole dosing for obese children and adolescents. Paediatr Drugs. 2018;20:483–495. doi: 10.1007/s40272-018-0305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akyuz L, Sargin I, Kaya M, Ceter T, Akata I. A new pollen-derived microcarrier for pantoprazole delivery. Mater Sci Eng C Mater Biol Appl. 2017;71:937–942. doi: 10.1016/j.msec.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Edlund A, Wennmalm A. Endothelin does not affect aggregation of human platelets. Clin Physiol. 1990;10:585–590. doi: 10.1111/j.1475-097x.1990.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb JE, Menashe PI, Cruz E. Gastrointestinal complications in critically ill patients: the intensivists’ overview. Am J Gastroenterol. 1986;81:227–238. [PubMed] [Google Scholar]
- 14.Martínez-Alcalá A, Mönkemüller K. Emerging endoscopic treatments for nonvariceal upper gastrointestinal hemorrhage. Gastrointest Endosc Clin N Am. 2018;28:307–320. doi: 10.1016/j.giec.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Lanas A, Dumonceau JM, Hunt RH, Fujishiro M, Scheiman JM, Gralnek IM, Campbell HE, Rostom A, Villanueva C, Sung JJY. Non-variceal upper gastrointestinal bleeding. Nat Rev Dis Primers. 2018;4:18020. doi: 10.1038/nrdp.2018.20. [DOI] [PubMed] [Google Scholar]
- 16.Yuan Y, Leontiadis GI. Editorial: ulcer-related vs non-ulcer-nonvariceal upper gastrointestinal bleeding-which has worse outcomes? Aliment Pharmacol Ther. 2019;49:818–819. doi: 10.1111/apt.15144. [DOI] [PubMed] [Google Scholar]
- 17.Stanley AJ, Laine L, Dalton HR, Ngu JH, Schultz M, Abazi R, Zakko L, Thornton S, Wilkinson K, Khor CJ, Murray IA, Laursen SB International Gastrointestinal Bleeding Consortium. Comparison of risk scoring systems for patients presenting with upper gastrointestinal bleeding: international multicentre prospective study. BMJ. 2017;356:i6432. doi: 10.1136/bmj.i6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachs G, Shin JM, Howden CW. Review article: the clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther. 2006;23(Suppl 2):2–8. doi: 10.1111/j.1365-2036.2006.02943.x. [DOI] [PubMed] [Google Scholar]
- 19.Shih PC, Liu SJ, Li ST, Chiu AC, Wang PC, Liu LY. Weekend effect in upper gastrointestinal bleeding: a systematic review and meta-analysis. Peer J. 2018;6:e4248. doi: 10.7717/peerj.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins SA, Poulianos G, Coraggio F, Rotondano G. Somatostatin in the treatment of non-variceal upper gastrointestinal bleeding. Dig Dis. 1998;16:214–224. doi: 10.1159/000016869. [DOI] [PubMed] [Google Scholar]
- 21.Kalkan Ç, Soykan I, Karakaya F, Tüzün A, Gençtürk ZB. Comparison of three scoring systems for risk stratification in elderly patients wıth acute upper gastrointestinal bleeding. Geriatr Gerontol Int. 2017;17:575–583. doi: 10.1111/ggi.12757. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Yang K, Ma B, Tian J, Liu Y, Bai Z, Jiang L, Sun S, Liu R, Hao X, He X. Intravenous pantoprazole as an adjuvant therapy following successful endoscopic treatment for peptic ulcer bleeding. Can J Gastroenterol. 2009;23:287–299. doi: 10.1155/2009/191706. [DOI] [PMC free article] [PubMed] [Google Scholar]