Abstract
Gangliosides are sialic acid-containing glycosphingolipids that have been found in the cell membranes of all vertebrates. Their important biological functions are contributed by both the glycan and the ceramide lipid components. GM3 is a major ganglioside and a precursor for many other more complex gangliosides. To obtain structurally diverse GM3 gangliosides containing various sialic acid forms and different fatty acyl chains in low cost, an improved process was developed to chemically synthesize lactosyl sphingosine from an inexpensive L-serine derivative. It was then used to obtain GM3 sphingosines from diverse modified sialic acid precursors by an efficient one-pot multienzyme sialylation system containing Pasteurella multocida sialyltransferase 3 (PmST3) with in situ generation of sugar nucleotides. A highly effective chemical acylation and facile C18-cartridge purification process was then used to install fatty acyl chains of varying lengths and different modifications. The chemoenzymatic method represents a powerful total synthetic strategy to access a library of structurally defined GM3 gangliosides to explore their functions.
Keywords: Chemoenzymatic synthesis, GM3, ganglioside, glycosphingolipid, sialic acid, sialylation
Graphical Abstract

INTRODUCTION
Gangliosides are sialic acid-containing glycosphingolipids (GSLs). They have been found in the cell plasma membranes of all vertebrates and are the most abundant in their central nervous systems.1 Similar to other GSLs, gangliosides are structurally diverse with differences in both the glycan and the lipid components. Compare to other GSLs, gangliosides have additional structural variations on the sialic acid forms. In addition to N-acetylneuraminic acid (Neu5Ac) which is the most common sialic acid form, 9-O-acetyl Neu5Ac (Neu5,9Ac2),2 7-O-acetyl Neu5Ac (Neu5,7Ac2),3 and non-human sialic acid N-glycolylneuraminic acid (Neu5Gc)4–5 have been found in gangliosides. Ceramide, the unique lipid component of GSLs including gangliosides, consists of a sphingoid base and a single acyl chain.6 In mammals, a sphingosine with 18 or 20 carbons7 is the most common sphingoid base while the fatty acyl component varies significantly in lengths, degrees of unsaturation (16:0 to 26:0/26:18 or longer6), and with or without additional hydroxylation.9 The combinations of different ceramides, the glycan structures, and sialic acid forms result in a significantly large number of structurally diverse gangliosides.
GM3 is a major ganglioside and the precursor for the formation of other more complex major ganglio-series gangliosides.10 Overexpression of GM3 has been connected to cancer11–12 and GM3 is among the four gangliosides in a 75-prioritized-cancer-antigen list.11 The levels of human serum GM3 gangliosides containing various acyl chains have been shown to correlate with risk factors for metabolic diseases.9 Increased GM3 synthesis has been shown to associate with insulin resistance and impaired wound healing in patients with type 2 diabetes or in related mouse models.13–15 GM3 has also been shown to inhibit the activities of receptor tyrosine kinases such as epidermal growth factor receptor (EGFR),16–18 and such activity varies according to the sialic acid forms (Neu5Ac or Neu5Gc).19 GM3 containing the Neu5Gc sialic acid form (Neu5Gc-GM3) has been found in different cancers20 and Neu5Gc-GM3 extracted from horse erythrocytes has been used to form very-small-size-proteoliposomes (VSSP) with Neisseria meningitidis outer membrane protein complex21 in clinical trials for breast cancer patients.21–26
Due to the influence of both sialic acid forms and the ceramide structures on the functions of GM3, developing efficient methods for synthesizing structurally diverse GM3 gangliosides is urgently needed. Among various methods developed for GM3 synthesis,27–34 the glycosyltransferase-based one-pot multienzyme (OPME) chemoenzymatic strategy that we developed31 has distinct advantages. In this method, lactosyl sphingosine (LacβSph) was chemically synthesized and used as a water-soluble acceptor substrate for the sialyltransferase-catalyzed synthesis of GM3 sphingosine with in situ generation of cytidine 5’-monophosphate (CMP)-sialic acid, the sugar nucleotide donor of the sialyltransferase. Here we explore the efficiency of the approach in accessing a versatile library of GM3 gangliosides containing different sialic acid forms and various fatty acyl chains. An efficient chemical synthetic process for accessing LacβSph from less expensive starting materials is also developed. Different from previous chemoenzymatic synthetic strategies for GM3 sphingosine which used Neu5Ac or a modified sialic acid as a sialyltransferase donor precursors,31–32 our current approach uses ManNAc and its chemically modified derivatives as readily accessible donor precursors.
RESULTS AND DISCUSSION
Efficient Chemical Synthesis of Lactosyl Sphingosine (LacβSph) from Inexpensive N-Boc L-Serine Methyl Ester.
Obtaining LacβSph is an essential step for OPME chemoenzymatic total synthesis of GM3. Previously we used commercially available phytosphingosine31, 35 as a starting material. In order to further decrease the cost to access the critical intermediate LacβSph in large scales more economically, N-Boc L-serine methyl ester 1 (e.g. $10 for 5 grams and $44 for 100 grams from Aaron Chemicals )36 (Scheme 1), a compound that is much less expensive than phytosphingosine ($276 for 5 grams from TCI Chemical), was identified as a well suited starting material.
Scheme 1.
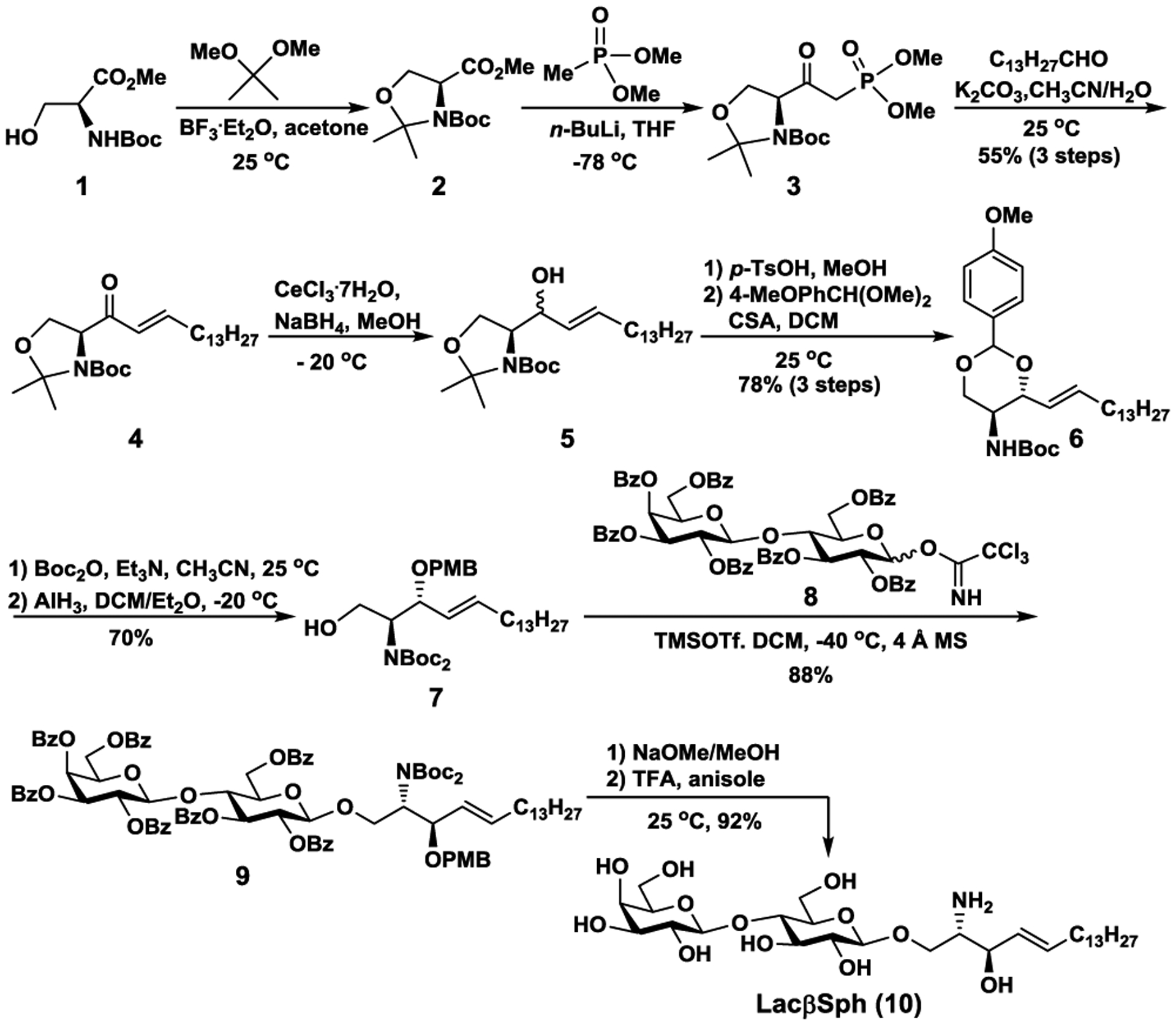
Efficient chemical synthesis of LacβSph (10) from commercially available inexpensive N-Boc L-serine methyl ester (1) with purification of the desired D-erythro isomer 6 by crystallization.
As shown in Scheme 1, the acetonide protection of N(Boc)-protected amino alcohol 1 using 2,2-dimethoxypropane in the presence of BF3.Et2O provided acetonide 2. In order to minimize the purification steps to develop an economic and efficient approach towards the synthesis of LacβSph, compound 2 was used directly for the next step to form the corresponding β-ketophosphonate 3 by treating it with an excess amount of lithium dimethyl methylphosphonate at −78 °C. The critical step to establish the E olefin geometry in the sphingoid base skeleton was achieved using the Horner-Wadsworth-Emmons reaction.37 The β-ketophosphonate 3 reacted with myristyl aldehyde in the presence of K2CO3 in CH3CN/H2O to form exclusively the E-olefin derivative 438 in a good yield (55% over three steps). It was then necessary to carry out column purification at this stage to increase the diastereomeric excess of the product in the next reaction. Subsequently, compound 4 was subjected to diastereoselective Luche reduction with CeCl3/NaBH4 to produce a mixture of R and S diastereomers 5 with the desired R isomer as the major product (9:1 R/S). It is worth noting that many of the previous methods reported used chromatographic purification to separate the diastereomers. Such a purification approach in a large-scale synthesis is laborious and often results in a mixture. Therefore, a more reliable and straightforward large-scale approach to purify the diastereomers is necessary.39–41 In this context, we aimed to develop a more efficient purification strategy. It was rationalized that the open-chain forms of compound 5 diastereomers would be conformationally more flexible and be more difficult to separate.42 In contrast, forming cyclic structures encompassing the chiral centers by simultaneously protecting the diols as an acetal would make the separation easier. Indeed, deprotection of the oxazolidine moiety in compound 5 diastereomers using p-TsOH/MeOH followed by the formation of a six-membered ring acetal using anisaldehyde dimethyl acetal led to a clean separation of the isomers as shown by thin-layer chromatography (Fig S1). Furthermore, the desired acetal protected D-erythro isomer was readily crystallized out from the mixture in hexane/EtOAc to provide the enantiomerically pure compound 6 (78%), thus making the synthetic route more applicable for large-scale production of sphingosine. Later, compound 6 was converted to a diBoc-protected sphingosine 7 in good yield (70%) by reacting with Boc2O followed by selective reductive opening of p-methoxybenzylidene acetal with AlH3. Compound 7 served as a novel acceptor for glycosylation, which was coupled with lactosyl trichloroacetimidate 843 using catalytic TMSOTf as the promoter to produce the β-linked product 9 in an excellent yield (88%). Subsequent global deprotection produced LacβSph 10 (92%) on a 5.2-gram scale, which served as the acceptor substrate for enzymatic synthesis of GM3 glycosphingosines and their derivatives.
One-Pot Multienzyme (OPME) Synthesis of GM3 Sphingosines Containing Different Sialic Acid Forms.
To introduce diverse sialic acid forms to LacβSph to form GM3 sphingosines, the one-pot multienzyme (OPME) sialylation system containing a sialyltransferase and enzymes for in situ generation of its sugar nucleotide CMP-sialic acid or derivatives has significant advantages. While GM3 synthases responsible for the formation of GM3 from lactosyl ceramide have been cloned from human (ST3GAL V)44 and mouse (ST3Gal V),45 they have so far been expressed in mammalian cell culture expression systems.44–47 Expression of active mammalian GM3 synthases as soluble and active enzymes in Escherichia coli has not been reported. For ganglioside enzymatic synthesis purpose, obtaining glycosyltransferases and related sugar nucleotide biosynthetic enzymes in Escherichia coli expression systems will allow an easier access to these robust biocatalysts at lower costs, which is highly desirable. While bacterial α2–3-sialyltransferases from Pasteurella multocida PmST248 and PmST331 can both use LacβSph as an acceptor substrate for the synthesis of GM3 sphingosine (GM3βSph), PmST3 has a higher expression level in E. coli and can use oligosaccharides and glycopeptides in addition to glycolipids as acceptor substrates.49–50 The previous application of PmST3 in synthesizing GM3βSph from LacβSph was limited to the introduction of Neu5Ac with in situ generation of CMP-Neu5Ac from commercially available Neu5Ac with N. meningitidis CMP-sialic acid synthetase (NmCSS).31 Here we report the exploration of the donor substrate promiscuity of PmST3 in synthesizing GM3βSph containing different sialic acid forms and derivatives. The PmST3 donor substrates were generated in situ from six-carbon precursors (11–16, Table 1) of sialic acids and derivatives with the combined functions of Pasteurella multocida sialic acid aldolase (PmNanA)51 and NmCSS,52 two enzymes both with substrate promiscuity as shown previously.
Table 1.
Production of GM3 gangliosides containing various sialic acid forms (23–28) using one-pot multienzyme (OPME) synthesis of GM3 sphingosines (17–22) followed by chemical acylation of the sphingosine chain.

|
As shown in Table 1, GM3 sphingosines Neu5Acα2–3LacβSph (17) and Neu5Gcα2–3LacβSph (18) containing natural sialic acid forms Neu5Ac and Neu5Gc, respectively, were readily synthesized from LacβSph as the acceptor substrate and the corresponding sialic acid precursors ManNAc (11) and N-glycolylmannosamine (ManNGc, 12)52 in very good yields (95% and 87%, respectively) using the OPME sialylation system containing PmNanA, NmCSS, and PmST3.
Next, the synthesis of GM3 sphingosine containing an azido group- or a fluorine-substituted terminal sialic acid was carried out. Neu5Ac9N3α2–3LacβSph (19, 86%), Neu5AcN3α2–3LacβSph (20, 90%), and Neu5AcFα2–3LacβSph (21, 91%) were obtained in excellent yields from ManNAc6N3 (13), ManNAcN3 (14),52 and ManNAcF (15),53 respectively. The gangliosides containing the azido group can be used as important chemical glycobiological tools for bioorthogonal chemoselective ligation reactions54–55 or for azide reduction followed by further conjugation to form an amide bond.56–59 Attachment of the electronegative fluorine on sialic acid moiety may lead to compounds with novel properties. For instance, the introduction of a sialic acid analogue bearing a fluorine atom at C-7 to the N-glycans of a therapeutic glycoprotein was shown to enhance its serum half-life in a mouse model.60
Glycolipids are vital mediators of cell-cell, cell-virus, and cell-ligand interactions, but these interactions are often difficult to study due to their fast off rates and low affinities. Diazirine has been widely used for photo-labelling to capture the highly interesting glycan-protein interactions by forming a covalent bond upon irradiation, and different “photo-sugars” that contain a diazirine moiety on the N-acyl side chain have been developed.61–62 5-SiaDAz-modified gangliosides produced by metabolic cell surface glycan engineering have been confirmed as new partners of CD22 using cholera toxin subunit B (CTxB).63 We were interested in synthesizing new photo-active GM3 ganglioside derivatives with a diazirine-modified sialic acid. To our delight, the six-carbon sialic acid precursor ManNDAz (16)62 was well tolerated by all enzymes in the OPME sialylation system and Neu5DAzα2–3LacβSph (22) was obtained in 85% yield. We envision compound 22 as an important molecular tool for discovering novel GM3-binding proteins.
Facile Synthesis and Purification of GM3 Gangliosides with Different Fatty Acyl Chains.
The synthesis of GM3 gangliosides is completed by installing a fatty acyl chain to GM3βSph. We tested the method that we developed previously for glycosphingosine acylation by coupling palmitoyl chloride with the amine in sphingosine using THF/sat. aq. NaHCO3.31 To our delight, as shown in Scheme 2, the method was confirmed to be robust and the acylation reactions of 17–22 with stearoyl (18:0) chloride in a mixture of tetrahydrofuran (THF) and saturated aqueous NaHCO3 solution (1:1, v/v) reached completion in 2 h. GM3 gangliosides 23–28 containing different sialic acid forms were produced in nearly quantitative yields. Furthermore, the installation of fatty acyl chains of different lengths and with or without a cis- or trans-double bond was succeeded in high yields. Reactions of GM3βSph 17 with myristoyl chloride, palmitoyl chloride, 10-undecenoyl chloride, and (Z)-9-octadecenoyl chloride in a mixture of THF and saturated aqueous NaHCO3 solution (1:1, v/v) produced GM3 gangliosides 29 (100%), 30 (99%), 31 (98%), and 32 (99%), respectively.
Scheme 2.

Synthesis of GM3 gangliosides with different fatty acyl chains.
CONCLUSIONS
In conclusion, an improved process which requires minimal column purification steps for large-scale chemical synthesis of LacβSph from inexpensive L-serine derivative has been developed. Importantly, D-erythro and D-threo sphingosine derivatives were separated cleanly by forming six-membered acetals followed by recrystallization of the desired isomer from the mixture which made the process more reliable and more adaptable for large-scale synthesis. The sphingosine hydrophobic tag in water-soluble LacβSph facilitates the product purification from the enzymatic reaction mixture by using a simple C18-cartridge purification process. The OPME sialylation system with in situ generation of CMP-sialic acids and derivatives from their six-carbon precursors allows the easy introduction of different sialic acid forms. The installation of a fatty acyl chain of choice in the last step in high yields with a streamlined synthesis and purification procedure makes the production of gangliosides with diverse fatty acyl chains easy. The high efficiency of the chemoenzymatic method is demonstrated by the total synthesis of a diverse library of GM3 gangliosides containing different sialic acid residues, including natural sialic acids Neu5Ac and Neu5Gc, and sialic acid derivatives with a F, N3, or diazirine substitution. The method described here using a highly efficient OPME sialylation system with a downstream chemical acylation process to install the desired fatty acid chains to the sphingosine moiety can be a general strategy for producing diversified GM3 gangliosides in preparative scales.
EXPERIMENTAL SECTION
Materials and General Methods.
Chemicals were purchased and used without further purification. 1H NMR (800 MHz) and 13C NMR (200 MHz) spectra were recorded on a Bruker Avance-800 NMR spectrometer and 1H NMR (600 MHz) and 13C NMR (150 MHz) spectra were recorded on a Bruker Avance-III HD 600 NMR spectrometer. High resolution electrospray ionization (ESI) mass spectra were obtained using Thermo Electron LTQ-Orbitrap Hybrid Mass Spectrometer or a Thermo Scientific Q Exactive HF Orbitrap Mass Spectrometer at the Mass Spectrometry Facilities in the University of California, Davis. Silica gel 60 Å (230–400 mesh, Sorbent Technologies) was used for flash column chromatography. Thin-layer chromatography (TLC, Sorbent Technologies) was performed on silica gel plates using anisaldehyde sugar stain for detection. Melting point was recorded on a Stuart SMP10 instrument. Recombinant enzymes Pasteurella multocida sialic acid aldolase (PmAldolase),51 Neisseria meningitidis CMP-sialic acid synthetase (NmCSS),52 and Pasteurella multocida multifunctional α2–3-sialyltransferase 3 (PmST3)49 were expressed and purified as described previously. Compounds 12–16 were synthesized as described previously.52–53, 62, 64
Chemical Synthesis of Lactosyl Sphingosine (10).
3-(tert-Butyl) 4-methyl (S)-2,2-dimethyloxazolidine-3,4-dicarboxylate (2).
To the commercially available methyl(tert-butoxycarbonyl)-L-serinate 1 (43 g, 196.1 mmol) in acetone (300 mL) at room temperature, 2,2-dimethoxypropane (217.2 mL, 1.77 mol) was added. To the above reaction mixture, BF3.OEt2 (1.40 mL, 11.2 mmol) was added at the same temperature and stirred for 6 h. The reaction mixture was concentrated under reduced pressure and dissolved in dichloromethane (100 mL) was washed with sat. aq. NaHCO3 solution (500 mL), brine (500 mL), and water (500 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated to dryness to obtain compound 2 as a liquid, which was used in the next step without further purification. A small portion of the crude compound was purified using flash column chromatography using hexanes and EtOAc , and the data were in agreement with the reported literature.65–66 1H NMR (600 MHz, CDCl3) δ 4.43 (ddd, J = 10.1, 6.9, 2.8 Hz, 1H), 4.14 (ddd, J = 13.8, 9.2, 7.0 Hz, 1H), 4.04 (ddd, J = 15.5, 9.2, 2.8 Hz, 1H), 3.76 (d, J = 1.9 Hz, 3H), 1.66 (d, J = 19.9 Hz, 3H), 1.52 (d, J = 22.4 Hz, 7H), 1.41 (s, 5H).
tert-Butyl (S,E)-4-(hexadec-2-enoyl)-2,2-dimethyloxazolidine-3-carboxylate (4).
To a solution of dimethyl methyl phosphonate in anhydrous THF (150 mL) was added n-BuLi (237.6 mL, 1.6 M in hexanes, 380.6 mmol) slowly over 30 min at −78 °C. After stirring for 1 h at the same temperature, crude serine derivative 2 in anhydrous THF (40 mL) was added slowly at the same temp and stirred for 10 min. The reaction was slowly warmed to 0 °C for a 1 h-period, stirred at the same temperature for 30 min, and quenched with THF/water mixture (5:1, 6 mL) at 0 °C. The pH was adjusted to 6.0–7.0 with 20% citric acid and extracted with EtOAc (3 × 300 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain crude compound 3, which was used further without purification.
The crude compound 3 and potassium carbonate (54.2 g, 392.2 mmol.) was dissolved in 1 L of acetonitrile. To the above solution, 1-tetradecanal (33.2 g, 156.8 mmol, 0.8 eq) and 12 mL of water were added to adjust the pH to 9. The reaction was stirred at room temperature for 12 hours. The reaction mixture was filtered, and the solid was washed with 1 L of hexanes. The organic phases were combined and evaporated to dryness under reduced pressure. The crude concentrate was dissolved in 1 L of hexanes and washed with of sat. aq. brine solution (500 mL). The organic layer was collected and dried over anhydrous sodium sulfate. The organic layer was filtered, concentrated under reduced pressure, and purified by flash column chromatography using hexanes/EtOAc to obtain 47.2 g (55% yield over three steps) of compound 4 and the data were in agreement with the reported literature.67 1H NMR (600 MHz, CDCl3) δ 7.0–6.94 (m, 1H), 6.28 (dd, J = 27.1, 15.7 Hz, 1H), 4.73–4.48 (m, 1H), 4.21–4.13 (m, 1H), 3.93 (ddd, J = 12.8, 9.0, 3.0 Hz, 1H), 2.23 (dt, J = 13.5, 6.8 Hz, 2H), 1.68 (d, J = 34.2 Hz, 3H), 1.57–1.44 (m, 9H), 1.37 (s, 6H), 1.26 (s, 22H), 0.88 (t, J = 7.0 Hz, 3H).
tert-Butyl ((4R, 5S)-2-(4-methoxyphenyl)-4-((E)-pentadec-1-en-1-yl)-1,3-dioxan-5-yl)carbamate (6).
Compound 4 (30 g, 68.6 mmol) and cerium chloride heptahydrate (38.3 g, 102.9 mmol) were stirred in methanol in a 500 mL round bottom flask over 30 min at rt and cooled to −20 °C. A sodium borohydride solution (3.8 g, 102.9 mmol) in 30% caustic soda (15.5 mL) was cooled to 0 °C and then carefully added to the allylic ketone solution dropwise over 2 h. After the complete addition, the reaction was allowed to stir at −20 °C for 30min and allowed to reach the rt over 2 h. Methanol was evaporated at 40 °C under reduced pressure and diluted with Et2O (300 mL), and filtered. The precipitate was washed with 300 mL Et2O over portions, and the organic layer was washed with sat. aq. NaHCO3, dried over anhydrous sodium sulphate, filtered, and concentrated to dryness. The crude compound 5 was used further without purification. The ratio of diastereomers was found to be on an average of 9:1 (D-erythro/L-erythro-sphingosine) using 1H crude NMR of 5 and 6.
To the crude solution of compound 5 in anhydrous methanol (300 mL), was added p-TsOH (2.3 g, 13.7 mmol) at 0 °C and allowed to reach the rt over 1 h. The reaction was stirred until completion and quenched with sat. aq. NaHCO3 (10 mL). Methanol was evaporated under reduced pressure, and the crude was dissolved in Et2O (300 mL). The organic layer was washed with water (300 mL), and the organic layer was collected, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to dryness. The crude was dissolved in anhydrous dichloromethane (300 mL). To the reaction mixture, anisaldehyde dimethyl acetal (15.0 g, 82.3 mmol) and camphor sulfonic acid (3.17 g, 13.7 mmol) were added successively at room temperature. The reaction was stirred over 2 h and quenched with triethylamine (13.7 mmol). The reaction mixture was diluted with dichloromethane (300 mL) and washed with sat. aq. NaHCO3 (300 mL), brine (300 mL) and water (300 mL). The organic layer was dried over anhydrous sodium sulfate and concentrated to dryness under reduced pressure. D-Erythro isomer was crystallized using 15% hexanes/EtOAc to obtain a pure compound 6 (27.6 g, 78% yield), leaving the other diastereomer in the crude solution. MP 112–114 °C. 1H NMR (600 MHz, CD3OD/CDCl3 = 9:1, by volume) δ 7.38 (d, J = 8.4 Hz, 2H, Ar), 6.88 (d, J = 9.0 Hz, 2H, Ar), 5.86–5.81 (m, 1H, HC=C), 5.50 (dd, J = 7.2, 15.6 Hz, 1H, C=CH), 5.46 (S, 1H, acetal), 4.17–4.14 (m, 1H), 4.02 (t, J = 8.4 Hz, 1H, HC-C=C), 3.79 (s, 3H, OMe), 3.62–3.59 (m, 2H), 2.05 (q, J = 7.2 Hz, 2H, C=C-CH2), 1.43 (s, 9H), 1.28 (s, 22H), 0.89 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (150 MHz, CD3OD/CDCl3 = 9:1, by volume) δ 161.4, 157.6, 136.8, 131.8, 128.5, 127.9, 114.4, 162.1, 82.9, 70.6, 55.7, 33.3, 32.9, 30.6, 30.5, 30.4, 30.2, 30.1, 30.0, 28.8, 23.5, 14.3. HRMS (ESI-Orbitrap) m/z: [M + Na]+ Calcd for C31H51NNaO5 540.3665; found 540.3660.
Di-tert-butyl ((2S, 3R, E)-1-hydroxy-3-((4-methoxybenzyl)oxy)octadec-4-en-2-yl)iminodicarbonate (7).
To a solution of compound 6 (20 g, 38.6 mmol) in anhydrous acetonitrile, Et3N (3.9 g, 38.6 mmol) and Boc2O (10.1 g, 46.3 mmol) were added successively at room temperature and stirred over 12 h (0.2 eq of DMAP was added to increase the rate of the reaction). The solvent was evaporated and dissolved in dichloromethane (500 mL) and washed with 1N HCl (250 mL), sat. aq. NaHCO3 (250 mL) and water (250 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated to dryness under reduced pressure. The crude was taken forward without purification.
The crude was co-evaporated with anhydrous toluene (2 × 50 mL) and dissolved in 1:1 anhydrous Et2O/dichloromethane (200 mL). The reaction was cooled to −20 °C and LiAlH4 (6.6 g, 173.7 mmol) was added. To the above reaction mixture, AlCl3 (4M in anhydrous Et2O, 7.7 g, 57.9 mmol) was added slowly at −20 °C. The reaction was allowed to reach 0 °C and stirred at the same temperature for 3 h. After completion, the reaction was quenched by adding EtOAc (50 mL) and water (50 mL) slowly at 0 °C. The reaction mixture was extracted with EtOAc (650 mL) and washed with 1N HCl (350 mL), sat. aq. NaHCO3 (350 mL) and water (350 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated to dryness under reduced pressure. The crude was purified using flash column chromatography over silica gel using hexanes/EtOAc to obtain compound 7 (16.7 g, 70% yield). 1H NMR (600 MHz, CDCl3) δ 7.22 (d, J = 8.5 Hz, 2H, Ar), 6.88 (d, J = 8.5 Hz, 2H, Ar), 5.83–5.71 (m, 1H, HC=C), 5.43 (dd, J = 15.4, 7.9 Hz, 1H, C=CH), 4.56 (d, J = 11.5 Hz, 1H), 4.25 (d, J = 11.5 Hz, 1H), 4.00 (s, 1H), 3.95 (d, J = 9.4 Hz, 1H), 3.81 (s, 3H, OMe), 3.62 (d, J = 6.8 Hz, 2H), 2.89 (s, 1H), 2.10 (dd, J = 14.2, 7.0 Hz, 2H, C=C–CH2), 1.44 (s, 9H), 1.28 (s, 21H), 0.90 (t, J = 7.0 Hz, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 159.2, 155.8, 136.6, 129.9, 129.3, 126.6, 113.8, 81.4, 79.3, 70.1, 62.3, 54.1, 54.8, 32.2, 31.8, 29.6, 29.6, 29.5, 29.4, 29.3, 29.1, 29.0, 28.3, 22.6, 14.0.. HRMS (ESI-Orbitrap) m/z: [M + H]+ Calcd for C36H62NO7 620.4526; found 620.4533.
(2R,3S,4S,5R,6S)-2-((Benzoyloxy)methyl)-6-(((2R,3R,4S,5R,6R)-4,5-bis(benzoyloxy)-2-((benzoyloxy)methyl)-6-(((2S,3R,E)-2-(bis(tert-butoxycarbonyl)amino)-3-((4-methoxybenzyl)oxy)octadec-4-en-1-yl)oxy)tetrahydro-2H-pyran-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl tribenzoate (9).
To solution of glycosyl donor 843 (23.5 g, 19.3 mmol) and acceptor 7 (10 g, 16.13 mmol)in anhydrous dichloromethane was added activated 4 Å MS (25 g) at room temperature and the solution was stirred at the same temperature for 2 h. The reaction mixture was cooled to −40 °C using acetonitrile and dry ice. After 10 min, TMSOTf (0.36 mL, 1.6 mmol) was added dropwise at −40 °C. The reaction was allowed to stir at the same temperature for 1 h and quenched with triethylamine (400 μL) after confirming the reaction with TLC. The solution was filtrated to remove 4 Å MS and the filtrate was diluted with dichloromethane (300 mL) and washed by sat. aq. NaHCO3 (200 mL) and brine (200 mL). The organic layer was dried with anhydrous Na2SO4, filtered, and purified over silica gel using hexanes and EtOAc to obtain compound 9 (23.7 g, 88%) as a white foam. 1H NMR (600 MHz, CDCl3) δ 8.05–7.99 (m, 10H, Ar), 7.92 (dd, J = 8.3, 1.2 Hz, 2H, Ar), 7.75 (dd, J = 8.4, 1.2 Hz, 2H, Ar), 7.67–7.63 (m, 1H, Ar), 7.63– 7.59 (m, 1H, Ar), 7.59–7.55 (m, 1H, Ar), 7.55–7.49 (m, 5H, Ar), 7.45–7.40 (m, 6H, Ar), 7.40–7.32 (m, 4H, Ar), 7.26–7.22 (m, 2H, Ar), 7.19 (t, J = 7.8 Hz, 2H, Ar), 7.13 (d, J = 8.6 Hz, 2H, Ar), 6.77 (d, J = 8.6 Hz, 2H, Ar), 5.85–5.80 (m, 1H), 5.78–5.72 (m, 2H), 5.48 (dd, J = 9.9, 7.9 Hz, 1H), 5.39 (dd, J = 10.3, 3.4 Hz, 1H), 5.35 (t, J = 6.9 Hz, 1H, HC=C), 5.24 (dd, J = 15.4, 8.3 Hz, 1H, C=CH), 4.89 (d, J = 7.9 Hz, 1H, anomeric), 4.71 (d, J = 7.9 Hz, 1H, anomeric), 4.58 (dd, J = 16.0, 5.6 Hz, 2H), 4.53 (dd, J = 12.1, 4.3 Hz, 1H), 4.37 (d, J = 11.0 Hz, 1H), 4.27 (dd, J = 19.5, 10.0 Hz, 2H), 4.20 (d, J = 10.9 Hz, 1H), 3.90 (t, J = 6.7 Hz, 1H), 3.84–3.81 (m, 1H), 3.79–3.74 (m, 4H), 3.71 (dd, J = 11.3, 7.0 Hz, 2H), 3.65 (t, J = 8.2 Hz, 1H), 3.56 (dd, J = 9.7, 3.0 Hz, 1H), 1.95–1.89 (m, 2H), 1.34 (s, 9H), 1.28 (s, 22H), 0.91 (t, J = 7.0 Hz, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 165.8, 165.5, 165.4, 165.3, 165.2, 164.8, 159.0, 155.2, 136.7, 133.5, 133.4, 133.3, 133.2, 130.5, 130.0, 129.8, 129.7, 129.6, 129.4, 129.2, 128.8, 128.6, 128.5, 128.5, 128.2, 127.2, 113.6, 101.5, 100.9, 79.2, 79.0, 75.9, 73.0, 72.8, 72.0, 71.8, 71.3, 70.1, 69.8, 68.6, 67.5, 62.5, 61.0, 55.2, 53.1, 32.2, 31.9, 29.7, 29.6, 29.5, 29.3, 29.2, 29.1, 28.3, 22.7, 14.1. HRMS (ESI-Orbitrap) m/z: [M + Na]+ Calcd for C97H109NNaO24 1694.7237; found 1694.7245.
(2S,3R,4S,5R,6R)-2-(((2R,3S,4R,5R,6R)-6-(((2S,3R,E)-2-Amino-3-hydroxyoctadec-4-en-1-yl)oxy)-4,5-dihydroxy-2-(hydroxymethyl)tetrahydro-2H-pyran-3-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol (10).
To a solution of compound 9 (15 g, 8.9 mmol) in anhydrous methanol (200 mL), NaOMe was added until the pH reached 9. The pH was maintained at 9 over 2 h and stirred at room temperature over 12 h. The reaction was neutralized with Dowex 50WX8 acid resin and filtered over celite. The filtrated was concentrated to dryness under reduced pressure. Hexanes (2 × 50 mL) was added and decanted to remove the nonpolar side products. The crude was dried under vacuum over 6 h and dissolved in 9:1 TFA/Anisole (100 mL) and stirred over 3 h. The solvent was evaporated, and under reduced pressure, the crude was purified using water and acetonitrile over the C18 reverse phase column to obtain LacβSph 10 (5.2 g, 92%) as a white solid. The data is in agreement with the reported literature.31
General Procedure of One-Pot Three-Enzyme Preparative-Scale Synthesis of GM3 Sphingosines 17–22.
Lactosyl sphingosine (LacβSph, 10 mM), sialic acid precursor (11–16, 15 mM), and CTP (20 mM) were incubated at 30 °C in a Tris-HCl buffer (100 mM, pH 8.5) containing MgCl2 (20 mM), PmAldolase (0.2 mg/mL), NmCSS (0.1 mg/mL), PmST3 (0.3 mg/mL). The reaction was incubated in an incubator shaker at 30 °C for 24 h with agitation at 100 rpm. The product formation was monitored by mass spectrometry. Upon completion, the same volume of cold ethanol was added and the mixture was incubated at 4 °C for 30 min before it was centrifuged to remove precipitates. The supernatant was concentrated and the residue was dissolved in 20 mL water. The sample was purified using a 51 g ODS-SM column (50 μM, 120 Å, Yamazen) on a CombiFlash® Rf 200i system. After loading the sample, the column was washed with water for 5 min then with gradient acetonitrile in water (0 to 100%). Products 17–22 were eluted with 60% acetonitrile in water (v/v). The fractions containing the product were collected to give the target glycolipid.
(5-Acetamido-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-amino-4-octadecene-1,3-diol (17).
White powder, 142 mg, 95% yield; 1H NMR (800 MHz, CD3OD) δ 5.75 (dt, J = 14.4, 6.4 Hz, 1H, = CH), 5.39 (dd, J = 15.2, 7.2 Hz, 1H, = CH), 4.32 (d, J = 8.0 Hz, 1H, anomeric), 4.26 (d, J = 8.0 Hz, 1H, anomeric), 4.16 (t, J = 6.4 Hz, 1H), 3.96 (dd, J = 9.6, 3.2 Hz, 1H), 3.88–3.35 (m, 18H), 3.20 (t, J = 9.6 Hz, 1H), 2.77 (dd, J = 12.0, 4.0 Hz, 1H, H3eq), 2.03–1.99 (m, 2H), 1.92 (s, 3H, CH3), 1.62 (t, J = 12.0 Hz, 1H), 1.35–1.15 (m, 22 H), 0.80 (t, J = 7.2 Hz, 3H, CH3); 13C{1H} NMR (200 MHz, CD3OD) δ 174.1, 173.4, 135.1, 127.2, 103.6, 102.4, 99.6, 79.1, 76.2, 75.7, 75.1, 74.8, 73.5, 73.0, 71.5, 70.1, 69.3, 68.7, 67.8, 67.5, 66.5, 63.2, 61.3, 60.2, 55.2, 52.5, 40.7, 31.9, 31.6, 29.3, 29.3, 29.3, 29.3, 29.2, 29.0, 28.9, 28.7, 22.3, 21.1, 13.0. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C41H73N2O20 913.4762; found 913.4766.
(3,5-Dideoxy-5-glycolamido-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-amino-4-octadecene-1,3-diol (18).
White powder, 67 mg, 87% yield; 1H NMR (800 MHz, CD3OD) δ 5.85 (dt, J = 14.4, 6.4 Hz, 1H, = CH), 5.46 (dd, J = 15.2, 7.2 Hz, 1H, = CH), 4.40 (d, J = 8.0 Hz, 1H, anomeric), 4.35 (d, J = 8.0 Hz, 1H, anomeric), 4.06–4.00 (m, 3H), 3.96–3.37 (m, 22H), 3.29 (t, J = 8.0 Hz, 1H), 2.85 (dd, J = 12.0, 4.0 Hz, 1H, H3eq), 2.10–2.16(m, 2H), 1.72 (t, J = 12.0 Hz, 1H), 1.42–1.24 (m, 22H), 0.87 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (200 MHz, CD3OD) δ 176.0, 173.6, 135.3, 127.0, 103.8, 102.4, 99.7, 79.3, 76.3, 75.7, 75.2, 74.8, 73.4, 73.1, 71.7, 69.6, 69.4, 68.8, 67.7, 67.7, 66.0, 63.4, 61.4, 61.3, 60.3, 55.4, 52.3, 40.8, 32.0, 29.4, 29.4, 29.3, 29.2, 29.1, 29.0, 28.8, 22.3, 13.0. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C41H73N2O21 929.4711; found 929.4713.
(5-Acetamido-9-azido-3,5,9-trideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-amino-4-octadecene-1,3-diol (19).
White powder, 66 mg, 86% yield; 1H NMR (600 MHz, CD3OD) δ 5.79 (dt, J = 14.4, 6.6 Hz, 1H, = CH), 5.41 (dd, J = 15.4, 6.8 Hz, 1H, = CH), 4.33 (d, J = 7.8 Hz, 1H, anomeric), 4.29 (d, J = 7.8 Hz, 1H, anomeric), 4.24 (t, J = 6.0 Hz, 1H), 3.98–3.24 (m, 22H), 2.78 (dd, J = 12.0, 4.2 Hz, 1H, H3eq), 2.02 (p, J = 6.6, 6.0 Hz, 2H), 1.94 (s, 3H), 1.65 (t, J = 11.4 Hz, 1H), 1.35 (q, J = 7.2 Hz, 2H), 1.22–1.17 (m, 22H), 0.82 (t, J = 6.6 Hz, 3H); 13C{1H} NMR (150 MHz, CD3OD) δ 172.7, 172.0, 133.7, 125.3, 102.2, 100.8, 98.3, 77.9, 74.8, 74.1, 73.7, 73.3, 71.8, 71.5, 69.2, 68.0, 67.9, 66.3, 66.2, 64.3, 59.8, 59.0, 53.8, 51.9, 51.0, 39.0, 30.5, 30.4, 30.1, 27.9, 27.8, 27.7, 27.7, 27.5, 27.5, 27.4, 27.3, 20.8, 19.8, 11.6. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C41H72N5O19 938.4827; found 938.4823.
[5-(2-Azidoacetamido)-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid]-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-amino-4-octadecene-1,3-diol (20).
White powder, 70 mg, 90% yield; 1H NMR (600 MHz, CD3OD) δ 5.75 (dt, J = 14.4, 6.8 Hz, 1H, = CH), 5.40 (dd, J = 15.4, 6.9 Hz, 1H, = CH), 4.33 (d, J = 7.8 Hz, 1H, anomeric), 4.27 (d, J = 7.8 Hz, 1H, anomeric), 4.16 (t, J = 6.0 Hz, 1H), 3.96 (dd, J = 9.6, 3.0 Hz, 1H), 3.91–3.36 (m, 22H), 2.76 (dd, J = 12.4, 4.2 Hz, 1H, H3eq), 2.01 (q, J = 7.2 Hz, 2H), 1.66 (t, J = 11.6 Hz, 1H), 1.33–1.16 (m, 22H), 0.80 (t, J = 7.0 Hz, 3H); 13C{1H} NMR (150 MHz, CD3OD) δ 172.0, 169.2, 133.5, 126.0, 102.1, 101.0, 98.2, 77.7, 74.7, 74.1, 73.6, 73.2, 71.7, 71.6, 70.3, 68.8, 67.9, 67.1, 66.4, 66.1, 65.3, 61.8, 59.8, 58.8, 53.7, 51.0, 49.9, 39.1, 30.5, 30.1, 27.9, 27.8, 27.8, 27.7, 27.5, 27.5, 27.3, 20.8, 11.5. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C41H72N5O20 954.4776; found 954.4781.
[3,5-Dideoxy-5-(2-fluoroacetamido)-D-glycero-α-D-galacto-2-nonulopyranosylonic acid]-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-amino-4-octadecene-1,3-diol (21).
White powder, 71 mg, 91% yield; 1H NMR (800 MHz, CD3OD) δ 5.76 (dt, J = 14.4, 6.4 Hz, 1H, = CH), 5.40 (dd, J = 15.2, 7.2 Hz, 1H, = CH), 4.33 (d, J = 8.0 Hz, 1H, anomeric), 4.27 (d, J = 8.0 Hz, 1H, anomeric), 4.20 (t, J = 6.4 Hz, 1H), 3.96 (dd, J = 9.6, 3.2 Hz, 1H), 3.88–3.21 (m, 23H), 2.76 (dd, J = 12.0, 4.0 Hz, 1H, H3eq), 2.04–1.98 (m, 2H), 1.65 (t, J = 12.0 Hz, 1H), 1.36–1.13 (m, 22H), 0.80 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (200 MHz, CD3OD) δ 173.6, 170.9, 170.8, 135.2, 127.1, 103.7, 102.4, 99.7, 80.0, 79.2, 79.1, 76.3, 75.7, 75.2, 74.8, 73.1, 73.0, 71.8, 69.8, 69.4, 68.6, 67.7, 67.6, 66.2, 63.3, 61.4, 60.3, 55.3, 52.1, 40.7, 32.0, 31.7, 29.4, 29.4, 29.3, 29.1, 29.0, 28.8, 22.4. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C41H72FN2O20 931.4668; found 931.4662.
[3,5-Dideoxy-5-[3-(3-methyl-3H-diazirin-3-yl)-1-oxopropyl]amido-D-glycero-α-D-galacto-2-nonulopyranosylonic acid]-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-amino-4-octadecene-1,3-diol (22).
White powder, 68 mg, 85% yield; 1H NMR (800 MHz, CD3OD) δ 5.75 (dt, J = 14.4, 7.2 Hz, 1H, = CH), 5.45 (dd, J = 15.2, 7.2 Hz, 1H, = CH), 4.42 (d, J = 8.0 Hz, 1H, anomeric), 4.30 (d, J = 8.0 Hz, 1H, anomeric), 4.04 (dd, J = 9.6, 4.0 Hz, 1H), 4.01 (J = 8.0 Hz, 1H), 3.92–3.41 (m, 18H), 3.28 (t, J = 8.0 Hz, 1H), 2.96–2.93 (m, 1H), 2.84 (dd, J = 12.0, 4.0 Hz, 1H, H3eq), 2.20–2.11 (m, 2H), 2.08–2.06 (m, 2H), 1.71 (t, J = 12.0 Hz, 1H), 1.67–1.65 (m, 2H), 1.42–1.23 (m, 22H), 1.00 (s, 3H), 0.81 (t, J = 7.2 Hz, 3H); 13C{1H} NMR (200 MHz, CD3OD) δ 175.3, 173.5, 134.3, 129.1, 103.7, 102.8, 99.7, 79.5, 76.2, 75.7, 75.1, 74.7, 73.6, 73.3, 72.8, 71.7, 69.8, 69.4, 68.8, 67.9, 67.6, 63.4, 61.4, 60.5, 55.0, 52.5, 43.9, 40.8, 32.0, 31.7, 29.9, 29.8, 29.4, 29.4, 29.4, 29.4, 29.4, 29.2, 29.1, 29.0, 29.0, 25.0, 22.3, 18.4, 13.1. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C44H77N4O20 981.5137; found 981.5131.
General Procedures for Converting GM3 Sphingosine to GM3 Gangliosides 23–32 with Different Fatty Acyl Chains.
To a solution of GM3 sphingosines (10–20 mg) in sat. aq. NaHCO3-THF (3 mL, 1:1), acyl chloride (1.5–2.0 eq) in 1 mL of THF was added. The resulting mixture was stirred vigorously at room temperature for 2 h. The solution was adjusted to pH 7 using 2N HCl and then concentrated. The residue was dissolved in 2 mL of water and loaded to a preconditioned Discovery® C18 cartridge (5 G) through a 10 mL plastic syringe and washed with water (10 mL). The ganglioside products were eluted from the C18 cartridge using a solution of 50 to 100% acetonitrile in water and fractions of 2 mL each were collected. The fractions contained pure target compounds were collected, concentrated and lyophilized.
(5-Acetamido-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-octadecanamino-4-octadecene-1,3-diol (Neu5Ac_GM3Cer, 23).
White powder, 64 mg, 99% yield. 1H NMR (800 MHz, CD3OD) δ 5.58 (dt, J = 14.4, 7.2 Hz, 1H, = CH), 5.35 (dd, J = 15.2, 7.2 Hz, 1H, = CH), 4.33 (d, J = 8.0 Hz, 1H, anomeric), 4.21 (d, J = 8.0 Hz, 1H, anomeric), 4.08 (dd, J = 9.6, 4.0 Hz, 1H), 3.97 (t, J = 8.0 Hz, 1H), 3.95 (dd, J = 9.6, 3.2 Hz, 1H), 3.89–3.31 (m, 18H), 3.19 (t, J = 8.0 Hz, 1H), 2.76 (dd, J = 12.0, 4.0 Hz, 1H, H3eq), 2.06 (t, J = 8.0 Hz, 2H), 1.96–1.91 (m, 2H), 1.91 (s, 3H), 1.65–1.61 (m, 1H), 1.52–1.46 (m, 2H), 1.33–1.14 (m, 52 H), 0.81 (t, J = 7.2 Hz, 6H); 13C{1H} NMR (200 MHz, CD3OD) δ 174.5, 174.1, 173.5, 133.6, 130.0, 103.7, 103.1, 99.7, 79.4, 76.2, 75.7, 75.1, 74.8, 73.5, 73.4, 71.6, 69.4, 68.7, 68.6, 68.0, 67.6, 63.2, 61.3, 60.4, 53.3, 52.5, 40.7, 37.9, 36.0, 32.1, 31.7, 31.7, 31.7, 29.5, 29.5, 29.4, 29.4, 29.4, 29.4, 29.3, 29.3, 29.3, 29.1, 29.1, 29.1, 29.0, 26.4, 25.8, 22.4, 22.4, 22.3, 21.2, 21.2, 13.1, 13.0. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C59H107N2O21 1179.7372; found 1179.7369.
(3,5-Dideoxy-5-glycolamido-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-octadecanamino-4-octadecene-1,3-diol (Neu5Gc_GM3Cer, 24).
White powder, 21 mg, 98% yield. 1H NMR (800 MHz, CD3OD) δ 5.78 (dt, J = 14.4, 6.4 Hz, 1H, = CH), 5.54 (dd, J = 15.2, 7.2 Hz, 1H, = CH), 4.52 (d, J = 8.0 Hz, 1H, anomeric), 4.39 (d, J = 8.0 Hz, 1H, anomeric), 4.28 (dd, J = 10.4, 4.8 Hz, 1H), 4.17 (d, J = 8.0 Hz, 1H), 4.16 (s, 2H), 4.12–3.50 (m, 19H), 3.41 (t, J = 8.0 Hz, 1H), 2.95 (dd, J = 12.0, 4.0 Hz, 1H, H3eq), 2.27–2.25 (m, 2H), 2.12–2.09 (m, 2H), 1.85 (t, J = 12.0 Hz, 1H), 1.71–1.30 (m, 54H), 0.97 (t, J = 7.2 Hz, 6H); 13C{1H} NMR (200 MHz, CD3OD) δ 176.0, 174.7, 173.6, 134.3, 129.5, 103.8, 102.9, 99.6, 79.5, 76.0, 75.5, 74.9, 74.5, 73.3, 71.7, 71.5, 69.2, 68.8, 68.6, 67.6, 61.4, 60.3, 53.2, 52.2, 36.3, 32.3, 31.8, 31.8, 31.8, 29.6, 29.6, 29.6, 29.5, 29.5, 29.5, 29.5, 29.4, 29.4, 29.3, 29.3, 29.2, 29.2, 29.2, 29.2, 26.3, 25.9, 23.3, 22.5, 13.6, 13.6. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C59H107N2O22 1195.7321; found 1195.7329.
(5-Acetamido-9-azido-3,5,9-trideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-octadecanamino-4-octadecene-1,3-diol (Neu5Ac9N3_GM3Cer, 25).
White powder, 25 mg, 100% yield. 1H NMR (600 MHz, CDCl3/CD3OD/D2O) δ 5.67 (dt, J = 15.0, 6.6 Hz, 1H, = CH), 5.30 (dd, J = 15.6, 7.8 Hz, 1H, = CH), 4.42 (d, J = 7.8 Hz, 2H, anomeric), 4.17 (dd, J = 10.2, 4.2 Hz, 1H), 4.06–3.29 (m, 21H), 2.76 (dd, J = 12.6, 4.2 Hz, 1H, H3eq), 2.14 (t, J = 7.2 Hz, 2H), 2.01(s, 3H), 2.00–1.97 (m, 2H), 1.74–1.70 (m, 1H), 1.56–1.21 (m, 54H), 0.86 (t, J = 7.2 Hz, 6H); 13C{1H} NMR (150 MHz, DMSO-d6/D2O) δ 173.0, 172.5, 171.2, 132.0, 131.7, 104.5, 103.8, 100.0, 81.2, 76.2, 75.4, 74.8, 73.5, 73.3, 71.0, 70.4, 69.6, 69.0, 67.6, 67.0, 60.8, 53.6, 53.3, 53.2, 36.0, 32.2, 31.8, 31.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.4, 29.2, 29.2, 29.1, 25.9, 22.9, 22.6, 22.5, 14.4. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C59H106N5O20 1204.7437; found 1204.7439.
[5-(2-Azidoacetamido)-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid]-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-octadecanamino-4-octadecene-1,3-diol (Neu5AcN3_GM3Cer, 26).
White powder, 28 mg, 99% yield. 1H NMR (600 MHz, CD3OD) δ 5.68 (dt, J = 13.2, 6.6 Hz, 1H, = CH), 5.44 (dd, J = 15.6, 7.8 Hz, 1H, = CH), 4.42 (d, J = 7.8 Hz, 1H, anomeric), 4.30 (d, J = 7.8 Hz, 1H, anomeric), 4.19 (dd, J = 10.2, 4.8 Hz, 1H), 4.07 (t, J = 8.4 Hz, 1H), 4.03 (dd, J = 9.6, 3.0 Hz, 1H), 3.98–3.40 (m, 18H), 3.30 (t, J = 8.4 Hz, 1H), 2.84 (dd, J = 12.6, 4.2 Hz, 1H, H3eq), 2.18–2.13 (m, 4H), 2.03–1.99 (m, 2H), 1.74 (t, J = 10.8 Hz, 1H), 1.65–1.16 (m, 54H), 0.88 (t, J = 7.0 Hz, 6H); 13C{1H} NMR (150 MHz, CD3OD) δ 174.7, 173.6, 170.8, 134.0, 129.7, 103.71, 103.0, 99.6, 79.5, 76.2, 75.6, 75.0, 74.7, 73.4, 73.2, 71.7, 69.4, 68.7, 68.6, 67.9, 67.4, 63.3, 61.4, 60.4, 53.3, 52.5, 51.6, 48.8, 40.7, 38.1, 36.2, 32.2, 31.8, 31.7, 31.7, 29.6, 29.6, 29.5, 29.5, 29.5, 29.5, 29.5, 29.4, 29.4, 29.4, 29.4, 29.3, 29.2, 29.2, 29.2, 29.1, 29.1, 26.5, 25.9, 22.4, 22.4, 22.4, 13.4, 13.4, 13.4. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C59H106N5O21 1220.7386; found 1220.7387.
[3,5-Dideoxy-5-(2-fluoroacetamido)-D-glycero-α-D-galacto-2-nonulopyranosylonic acid]-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-octadecanamino-4-octadecene-1,3-diol (Neu5AcF_GM3Cer, 27).
White powder, 26 mg, 100% yield. 1H NMR (800 MHz, CD3OD) δ 5.59 (dt, J = 14.4, 7.2 Hz, 1H, = CH), 5.35 (dd, J = 15.2, 7.2 Hz, 1H, = CH), 4.34 (d, J = 8.0 Hz, 1H, anomeric), 4.22 (d, J = 8.0 Hz, 1H, anomeric), 4.09 (dd, J = 9.6, 4.0 Hz, 1H), 4.00–3.96 (m, 2H), 3.90–3.32 (m, 20H), 3.20 (t, J = 8.0 Hz, 1H), 2.77 (dd, J = 12.0, 4.0 Hz, 1H, H3eq), 2.08 (t, J = 8.0 Hz, 2H), 1.96–1.92 (m, 2H), 1.65 (t, J = 12.0 Hz, 1H), 1.52–1.12 (m, 54 H), 0.81 (t, J = 7.2 Hz, 6H); 13C{1H} NMR (200 MHz, CD3OD) δ 181.7, 174.6, 173.6, 133.7, 130.0, 103.7, 103.1, 99.7, 79.4, 76.2, 75.6, 75.1, 74.8, 73.4, 73.0, 71.8, 71.6, 69.4, 68.6, 67.8, 67.5, 63.1, 61.3, 60.4, 53.3, 52.1, 40.7, 38.0, 36.0, 32.1, 31.7, 31.7, 31.7, 29.5, 29.5, 29.5, 29.4, 29.4, 29.4, 29.4, 29.4, 29.3, 29.3, 29.3, 29.1, 29.1, 29.1, 29.0, 26.5, 25.8, 22.4, 22.3, 13.1, 13.1, 13.1. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C59H106FN2O21 1197.7278; found 1197.7275.
[3,5-Dideoxy-5-[3-(3-methyl-3H-diazirin-3-yl)-1-oxopropyl]amido-D-glycero-α-D-galacto-2-nonulopyranosylonic acid]-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-octadecanamino-4-octadecene-1,3-diol (Neu5DAz_GM3Cer, 28).
White powder, 24 mg, 98% yield. 1H NMR (600 MHz, CDCl3/CD3OD/D2O) δ 5.66 (dt, J = 15.0, 7.2 Hz, 1H, = CH), 5.40 (dd, J = 15.0, 7.8 Hz, 1H, = CH), 4.29 (d, J = 7.8 Hz, 2H, anomeric), 4.18 (dd, J = 10.2, 4.2 Hz, 1H), 4.05–3.21 (m, 21H), 2.76 (dd, J = 12.6, 4.2 Hz, 1H, H3eq), 2.18–1.93 (m, 5H), 1.73–1.65 (m, 3H), 1.61–1.13 (m, 54 H), 0.99 (s, 3H), 0.86 (t, J = 7.2 Hz, 6H); 13C{1H} NMR (150 MHz, DMSO-d6/D2O) δ 174.3, 172.6, 171.3, 132.2, 131.7, 104.3, 103.8, 99.9, 80.6, 76.1, 75.3, 74.7, 73.5, 73.4, 71.6, 71.0, 69.6, 69.0, 68.9, 67.5, 67.0, 63.7, 60.9, 60.5, 53.3, 53.1, 36.0, 32.2, 31.7, 31.7, 30.2, 30.1, 29.6, 29.5, 29.5, 29.5, 29.4, 29.4, 29.2, 29.2, 29.1, 26.2, 25.9, 22.5, 22.5, 19.7, 14.4. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C62H111N4O21 1247.7746; found 1247.7734.
(5-Acetamido-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-tetradecanamino-4-octadecene-1,3-diol (29).
White powder, 25 mg, 100% yield. 1H NMR (800 MHz, CD3OD) δ 5.59 (dt, J = 14.4, 7.2 Hz, 1H, = CH), 5.36 (dd, J = 15.2, 7.2 Hz, 1H, = CH), 4.34 (d, J = 8.0 Hz, 1H, anomeric), 4.21 (d, J = 8.0 Hz, 1H, anomeric), 4.08 (dd, J = 9.6, 4.0 Hz, 1H), 3.98 (t, J = 8.0 Hz, 1H), 3.96 (dd, J = 9.6, 3.2 Hz, 1H), 3.89–3.32 (m, 18H), 3.19 (t, J = 8.0 Hz, 1H), 2.77 (dd, J = 12.0, 4.0 Hz, 1H, H3eq), 2.08 (t, J = 8.0 Hz, 2H), 1.96–1.92 (m, 2H), 1.92 (s, 3H), 1.65–1.62 (m, 1H), 1.52–1.46 (m, 2H), 1.33–1.14 (m, 44 H), 0.81 (t, J = 7.2 Hz, 6H); 13C{1H} NMR (200 MHz, CD3OD) δ 174.6, 174.1, 173.5, 133.6, 130.0, 103.7, 103.1, 99.7, 79.4, 76.2, 75.6, 75.1, 74.7, 73.5, 73.4, 71.6, 69.4, 68.7, 68.6, 68.0, 67.6, 63.1, 61.3, 60.4, 53.3, 52.5, 40.7, 37.9, 36.0, 32.1, 31.7, 29.5, 29.5, 29.4, 29.4, 29.4, 29.3, 29.3, 29.3, 29.1, 29.1, 29.1, 29.1, 29.0, 26.4, 25.8, 22.4, 21.2, 13.1. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C55H99N2O21 1123.6746; found 1123.6750.
(5-Acetamido-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)-(2→3)−β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-hexadecanamino-4-octadecene-1,3-diol (30).
White powder, 24 mg, 99% yield. 1H NMR (800 MHz, CD3OD) δ 5.60 (dt, J = 14.4, 7.2 Hz, 1H, = CH), 5.36 (dd, J = 15.2, 7.2 Hz, 1H, = CH), 4.35 (d, J = 8.0 Hz, 1H, anomeric), 4.23 (d, J = 8.0 Hz, 1H, anomeric), 4.10 (dd, J = 9.6, 4.0 Hz, 1H), 4.00–3.33 (m, 19H), 3.20 (t, J = 8.0 Hz, 1H), 2.77 (dd, J = 12.0, 4.0 Hz, 1H, H3eq), 2.09 (t, J = 8.0 Hz, 2H), 1.96–1.93 (m, 2H), 1.93 (s, 3H), 1.65–1.63 (m, 1H), 1.51–1.48 (m, 2H), 1.32–1.14 (m, 48 H), 0.82 (t, J = 7.2 Hz, 6H); 13C{1H} NMR (200 MHz, CD3OD) δ 174.5, 174.0, 173.4, 133.5, 129.9, 103.6, 103.0, 99.6, 79.3, 76.2, 75.6, 75.0, 74.7, 73.4, 73.3, 71.5, 69.3, 68.6, 68.4, 67.9, 67.5, 63.1, 61.2, 60.3, 53.2, 52.5, 40.6, 35.9, 32.0, 31.6, 31.6, 29.4, 29.4, 29.4, 29.4, 29.3, 29.3, 29.3, 29.3, 29.2, 29.2, 29.0, 29.0, 29.0, 29.0, 29.0, 25.7, 22.3, 21.1, 13.0. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C57H103N2O21 1151.7059; found 1151.7046.
(5-Acetamido-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-(10-undecanamino-4-octadecene-1,3-diol (31).
White powder, 23 mg, 98% yield. 1H NMR (800 MHz, CD3OD) δ 5.74–5.69 (m, 1H, = CH), 5.60 (dt, J = 14.4, 7.2 Hz, 1H, = CH), 5.36 (dd, J = 15.2, 7.2 Hz, 1H, = CH), 4.91–4.87 (m, 1H, = CH), 4.83–4.81 (m, 1H, = CH), 4.33 (d, J = 8.0 Hz, 1H, anomeric), 4.21 (d, J = 8.0 Hz, 1H, anomeric), 4.08 (dd, J = 9.6, 4.0 Hz, 1H), 3.97 (t, J = 8.0 Hz, 1H), 3.96 (dd, J = 9.6, 3.2 Hz, 1H), 3.90–3.32 (m, 18H), 3.19 (t, J = 8.0 Hz, 1H), 2.76 (dd, J = 12.0, 4.0 Hz, 1H, H3eq), 2.08 (t, J = 8.0 Hz, 2H), 1.97–1.92 (m, 2H), 1.92 (s, 3H), 1.65–1.61 (m, 1H), 1.52–1.47 (m, 2H), 1.33–1.14 (m, 34 H), 0.81 (t, J = 7.2 Hz, 6H); 13C{1H} NMR (200 MHz, CD3OD) δ 174.6, 174.1, 173.5, 138.7, 133.6, 129.9, 113.4, 113.3, 103.7, 103.1, 99.7, 79.4, 76.2, 75.7, 75.1, 74.8, 73.5, 73.4, 71.6, 71.6, 69.4, 68.7, 68.6, 67.9, 67.6, 63.2, 61.3, 60.4, 53.3, 52.5, 40.7, 37.9, 36.0, 33.5, 33.5, 32.1, 31.7, 29.5, 29.4, 29.4, 29.4, 29.2, 29.2, 29.1, 29.1, 29.0, 29.0, 28.9, 28.8, 28.8, 28.7, 25.8, 22.4, 21.2, 13.1. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C52H91N2O21 1079.6120; found 1079.6113.
(5-Acetamido-3,5-dideoxy-D-glycero-α-D-galacto-2-nonulopyranosylonic acid)-(2→3)-β-D-galactopyranosyl-(1→4)-β-D-glucopyranosyl-(1→1)-(2S, 3R, 4E)-2-[(Z)-9-octadecanamino]-4-octadecene-1,3-diol (32).
White powder, 25 mg, 99% yield. 1H NMR (800 MHz, CD3OD) δ 5.59 (dt, J = 14.4, 7.2 Hz, 1H, = CH), 5.35 (dd, J = 15.2, 7.2 Hz, 1H, = CH), 5.27–5.22 (m, 2H, CH=CH), 4.33 (d, J = 8.0 Hz, 1H, anomeric), 4.21 (d, J = 8.0 Hz, 1H, anomeric), 4.08 (dd, J = 9.6, 4.0 Hz, 1H), 3.97 (t, J = 8.0 Hz, 1H), 3.95 (dd, J = 9.6, 3.2 Hz, 1H), 3.89–3.31 (m, 18H), 3.18 (t, J = 8.0 Hz, 1H), 2.76 (dd, J = 12.0, 4.0 Hz, 1H, H3eq), 2.06 (t, J = 8.0 Hz, 2H), 1.96–1.91 (m, 2H), 1.92 (s, 3H), 1.65–1.61 (m, 1H), 1.52–1.46 (m, 2H), 1.33–1.14 (m, 44 H), 0.80 (t, J = 7.2 Hz, 6H); 13C{1H} NMR (200 MHz, CD3OD) δ 181.5, 174.5, 174.1, 173.5, 133.6, 130.0, 129.5, 129.5, 129.4, 103.7, 103.1, 99.7, 79.4, 76.2, 75.6, 75.1, 74.7, 73.5, 73.4, 71.6, 71.6, 69.4, 68.7, 68.6, 67.9, 67.6, 63.1, 61.3, 60.4, 56.2, 56.1, 56.0, 53.3, 52.5, 40.7, 37.8, 36.0, 32.1, 31.7, 31.7, 31.7, 29.5, 29.5, 29.5, 29.5, 29.5, 29.4, 29.4, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 29.1, 29.1, 29.1, 29.1, 29.1, 29.1, 29.0, 29.0, 29.0, 28.9, 28.9, 26.8, 26.7, 26.4, 25.8, 25.2, 22.4, 22.3, 21.2, 13.1, 13.1, 13.1. HRMS (ESI-Orbitrap) m/z: [M - H]− Calcd for C59H105N2O21 1177.7215; found 1177.7220.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the United States National Institutes of Health (NIH) Common Fund Glycoscience Program grant U01GM120419. The Thermo Scientific Q Exactive HF Orbitrap Mass Spectrometer was purchased with a United States NIH Shared Instrumentation Grant (grant no. S10OD025271). The Bruker Avance-800 NMR spectrometer was purchased with a grant funded by the United States National Science Foundation (grant no. DBI-0722538).
Footnotes
Supporting Information
TLC analysis of compounds 5 and 6 as well as NMR spectra of the products. The Supporting Information is available free of charge on the ACS Publications website.
The authors declare no competing financial interest.
REFERENCES
- (1).Yu RK; Nakatani Y; Yanagisawa M The Role of Glycosphingolipid Metabolism in The Developing Brain. J. Lipid Res 2009, 50 Suppl, S440–S445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Cavdarli S; Dewald JH; Yamakawa N; Guerardel Y; Terme M; Le Doussal JM; Delannoy P; Groux-Degroote S Identification of 9-O-Acetyl-N-acetylneuraminic Acid (Neu5,9Ac2) as Main O-Acetylated Sialic Acid Species of GD2 in Breast Cancer Cells. Glycoconj. J 2019, 36, 79–90. [DOI] [PubMed] [Google Scholar]
- (3).Wipfler D; Srinivasan GV; Sadick H; Kniep B; Arming S; Willhauck-Fleckenstein M; Vlasak R; Schauer R; Schwartz-Albiez R Differentially Regulated Expression of 9-O-Acetyl GD3 (CD60b) and 7-O-Acetyl-GD3 (CD60c) During Differentiation and Maturation of Human T and B Lymphocytes. Glycobiology 2011, 21, 1161–1172. [DOI] [PubMed] [Google Scholar]
- (4).Scursoni AM; Galluzzo L; Camarero S; Lopez J; Lubieniecki F; Sampor C; Segatori VI; Gabri MR; Alonso DF; Chantada G; de Dávila MT Detection of N-Glycolyl GM3 Ganglioside in Neuroectodermal Tumors by Immunohistochemistry: An Attractive Vaccine Target for Aggressive Pediatric Cancer. Clin. Dev. Immunol 2011, 2011, 245181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).D’Angelo G; Capasso S; Sticco L; Russo D Glycosphingolipids: Synthesis and Functions. FEBS J 2013, 280, 6338–6353. [DOI] [PubMed] [Google Scholar]
- (6).Schnaar RL; Kinoshita T Glycosphingolipids. In Essentials of Glycobiology, rd; Varki A; Cummings RD; Esko JD; Stanley P; Hart GW; Aebi M; Darvill AG; Kinoshita T; Packer NH; Prestegard JH; Schnaar RL; Seeberger PH Eds. Cold Spring Harbor (NY), 2015; pp 125–135. [PubMed] [Google Scholar]
- (7).Sonnino S; Chigorno V Ganglioside Molecular Species Containing C18- and C20-Sphingosine in Mammalian Nervous Tissues and Neuronal Cell Cultures. Biochim. Biophys. Acta 2000, 1469, 63–77. [DOI] [PubMed] [Google Scholar]
- (8).Li Y; Arigi E; Eichert H; Levery SB Mass Spectrometry of Fluorocarbon-Labeled Glycosphingolipids. J. Mass Spectrom 2010, 45, 504–519. [DOI] [PubMed] [Google Scholar]
- (9).Veillon L; Go S; Matsuyama W; Suzuki A; Nagasaki M; Yatomi Y; Inokuchi J Identification of Ganglioside GM3 Molecular Species in Human Serum Associated with Risk Factors of Metabolic Syndrome. PLoS One 2015, 10, e0129645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Park EJ; Suh M; Ramanujam K; Steiner K; Begg D; Clandinin MT Diet-Induced Changes in Membrane Gangliosides in Rat Intestinal Mucosa, Plasma and Brain. J. Pediatr. Gastroenterol. Nutr 2005, 40, 487–495. [DOI] [PubMed] [Google Scholar]
- (11).Cheever MA; Allison JP; Ferris AS; Finn OJ; Hastings BM; Hecht TT; Mellman I; Prindiville SA; Viner JL; Weiner LM; Matrisian LM The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for The Acceleration of Translational Research. Clin. Cancer Res 2009, 15, 5323–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Zheng C; Terreni M; Sollogoub M; Zhang Y Ganglioside GM3 and Its Role in Cancer. Curr. Med. Chem 2019, 26, 2933–2947. [DOI] [PubMed] [Google Scholar]
- (13).Dam DHM; Paller AS Gangliosides in Diabetic Wound Healing. Prog. Mol. Biol. Transl. Sci 2018, 156, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Tagami S; Inokuchi J.-i.; Kabayama K; Yoshimura H; Kitamura F; Uemura S; Ogawa C; Ishii A; Saito M; Ohtsuka Y; Sakaue S; Igarashi Y Ganglioside GM3 Participates in the Pathological Conditions of Insulin Resistance. J. Biol. Chem 2002, 277, 3085–3092. [DOI] [PubMed] [Google Scholar]
- (15).Inamori KI; Inokuchi JI Roles of Gangliosides in Hypothalamic Control of Energy Balance: New Insights. Int. J. Mol. Sci 2020, 21, 5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Bremer EG; Hakomori S; Bowen-Pope DF; Raines E; Ross R Ganglioside-Mediated Modulation of Cell Growth, Growth Factor Binding, and Receptor Phosphorylation. J. Biol. Chem 1984, 259, 6818–6825. [PubMed] [Google Scholar]
- (17).Cutillo G; Saariaho AH; Meri S Physiology of Gangliosides and The Role of Antiganglioside Antibodies in Human Diseases. Cell Mol. Immunol 2020, 17, 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Bremer EG; Schlessinger J; Hakomori S Ganglioside-Mediated Modulation of Cell Growth. Specific Effects of GM3 on Tyrosine Phosphorylation of The Epidermal Growth Factor Receptor. J. Biol. Chem 1986, 261, 2434–2440. [PubMed] [Google Scholar]
- (19).Hayashi N; Chiba H; Kuronuma K; Go S; Hasegawa Y; Takahashi M; Gasa S; Watanabe A; Hasegawa T; Kuroki Y; Inokuchi J; Takahashi H Detection of N-Glycolyated Gangliosides in Non-Small-Cell Lung Cancer Using GMR8 Monoclonal Antibody. Cancer Sci 2013, 104, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Labrada M; Dorvignit D; Hevia G; Rodriguez-Zhurbenko N; Hernandez AM; Vazquez AM; Fernandez LE GM3(Neu5Gc) Ganglioside: An Evolution Fixed Neoantigen for Cancer Immunotherapy. Semin. Oncol 2018, 45, 41–51. [DOI] [PubMed] [Google Scholar]
- (21).Estevez F; Carr A; Solorzano L; Valiente O; Mesa C; Barroso O; Sierra GV; Fernandez LE Enhancement of The Immune Response to Poorly Immunogenic Gangliosides After Incorporation into Very Small Size Proteoliposomes (VSSP). Vaccine 1999, 18, 190–197. [DOI] [PubMed] [Google Scholar]
- (22).de la Torre A; Pérez K; Vega AM; Santiesteban E; Ruiz R; Hernández L; Durrutí D; Viada CE; Sánchez L; Álvarez M; Durán Y; Moreno YG; Arencibia M; Cepeda M; Domecq M; Cabrera L; Sánchez JL; Hernández JJ; Valls AR; Fernández LE Superior Efficacy and Safety of a Nonemulsive Variant of the NGcGM3/VSSP Vaccine in Advanced Breast Cancer Patients. Breast Cancer (Auckl) 2016, 10, 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Fernandez LE; Gabri MR; Guthmann MD; Gomez RE; Gold S; Fainboim L; Gomez DE; Alonso DF NGcGM3 Ganglioside: A Privileged Target for Cancer Vaccines. Clin. Dev. Immunol 2010, 2010, 814397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Carr A; Rodríguez E; Arango Mdel C; Camacho R; Osorio M; Gabri M; Carrillo G; Valdés Z; Bebelagua Y; Pérez R; Fernández LE Immunotherapy of Advanced Breast Cancer with A Heterophilic Ganglioside (NeuGcGM3) Cancer Vaccine. J. Clin. Oncol 2003, 21, 1015–1021. [DOI] [PubMed] [Google Scholar]
- (25).Mulens V; de la Torre A; Marinello P; Rodríguez R; Cardoso J; Díaz R; O’Farrill M; Macias A; Viada C; Saurez G; Carr A; Crombet T; Mazorra Z; Perez R; Fernandez LE Immunogenicity and Safety of A NeuGcGM3 Based Cancer Vaccine: Results from A Controlled Study in Metastatic Breast Cancer Patients. Hum. Vaccin 2010, 6, 736. [DOI] [PubMed] [Google Scholar]
- (26).de la Torre A; Hernandez J; Ortiz R; Cepeda M; Perez K; Car A; Viada C; Toledo D; Guerra PP; García E; Arboláez M; Fernandez LE NGlycolylGM3/VSSP Vaccine in Metastatic Breast Cancer Patients: Results of Phase I/IIa Clinical Trial. Breast Cancer (Auckl) 2012, 6, 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Liu Y; Wen L; Li L; Gadi MR; Guan W; Huang K; Xiao Z; Wei M; Ma C; Zhang Q; Yu H; Chen X; Wang PG; Fang J A General Chemoenzymatic Strategy for the Synthesis of Glycosphingolipids. Eur. J. Org. Chem 2016, 2016, 4315–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Ito Y; Paulson JC A Novel Strategy for Synthesis of Ganglioside GM3 Using An Enzymically Produced Sialoside Glycosyl Donor. J. Am. Chem. Soc 1993, 115, 1603–1605. [Google Scholar]
- (29).Duclos RI The Total Synthesis of Ganglioside GM3. Carbohydr. Res 2000, 328, 489–507. [DOI] [PubMed] [Google Scholar]
- (30).Murase T; Ishida H; Kiso M; Hasegawa A A Facile, Regio- and Stereo-Selective Synthesis of Ganglioside GM3. Carbohydr. Res 1989, 188, 71–80. [DOI] [PubMed] [Google Scholar]
- (31).Yu H; Santra A; Li Y; McArthur JB; Ghosh T; Yang X; Wang PG; Chen X Streamlined Chemoenzymatic Total Synthesis of Prioritized Ganglioside Cancer Antigens. Org. Biomol. Chem 2018, 16, 4076–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Rich JR; Cunningham A-M; Gilbert M; Withers SG Glycosphingolipid Synthesis Employing A Combination of Recombinant Glycosyltransferases and An Endoglycoceramidase Glycosynthase. Chem. Commun 2011, 47, 10806–10808. [DOI] [PubMed] [Google Scholar]
- (33).Takahashi M; Shirasaki J; Komura N; Sasaki K; Tanaka H-N; Imamura A; Ishida H; Hanashima S; Murata M; Ando H Efficient Diversification of GM3 Gangliosides via Late-Stage Sialylation and Dynamic Glycan Structural Studies with 19F Solid-State NMR. Org. Biomol. Chem 2020, 18, 2902–2913. [DOI] [PubMed] [Google Scholar]
- (34).Li Q; Jaiswal M; Rohokale RS; Guo Z A Diversity-Oriented Strategy for Chemoenzymatic Synthesis of Glycosphingolipids and Related Derivatives. Org. Lett 2020, 22, 8245–8249. [DOI] [PubMed] [Google Scholar]
- (35).Santra A; Li Y; Yu H; Slack TJ; Wang PG; Chen X Highly Efficient Chemoenzymatic Synthesis and Facile Purification of alpha-Gal Pentasaccharyl Ceramide Galalpha3nLc4betaCer. Chem. Commun 2017, 53, 8280–8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Koskinen PM; Koskinen AM Total Synthesis of Sphingosine and Its Analogs. Methods Enzymol 2000, 311, 458–479. [DOI] [PubMed] [Google Scholar]
- (37).Wadsworth WS Jr. Synthetic Applications of Phosphoryl-Stabilized Anions. In Organic Reactions, pp 73–253. [Google Scholar]
- (38).Koolath S; Murai Y; Suga Y; Monde K Chiral Combinatorial Preparation and Biological Evaluation of Unique Ceramides for Inhibition of Sphingomyelin Synthase. Chirality 2020, 32, 308–313. [DOI] [PubMed] [Google Scholar]
- (39).Garner P; Park JM; Malecki E A Stereodivergent Synthesis of D-Erythro-Sphingosine and D-Threo-Sphingosine from L-Serine. J. Org. Chem 1988, 53, 4395–4398. [Google Scholar]
- (40).Azuma H; Tamagaki S; Ogino K Stereospecific Total Syntheses of Sphingosine and Its Analogues from L-Serine. J. Org. Chem 2000, 65, 3538–3541. [DOI] [PubMed] [Google Scholar]
- (41).Gao Y; He X; Ding F; Zhang Y Recent Progress in Chemical Syntheses of Sphingosines and Phytosphingosines. Synthesis 2016, 48, 4017–4037. [Google Scholar]
- (42).Hafner A; Duthaler RO; Marti R; Rihs G; Rothe-Streit P; Schwarzenbach F, Enantioselective Syntheses with Titanium Carbohydrate Complexes. Part 7. Enantioselective Allyltitanation of Aldehydes with Cyclopentadienyldialkoxyallyltitanium Complexes. J. Am. Chem. Soc 1992, 114, 2321–2336. [Google Scholar]
- (43).Boons GJPH; van Delft FL; van der Klein PAM; van der Marel GA; van Boom JH Synthesis of ld-Hepp and KDO Containing Di- and Tetrasaccharide Derivatives of Neisseria meningitidis Inner-Core Region via Iodonium Ion Promoted Glycosidations. Tetrahedron 1992, 48, 885–904. [Google Scholar]
- (44).Ishii A; Ohta M; Watanabe Y; Matsuda K; Ishiyama K; Sakoe K; Nakamura M; Inokuchi J; Sanai Y; Saito M Expression Cloning and Functional Characterization of Human cDNA for Ganglioside GM3 Synthase. J. Biol. Chem 1998, 273, 31652–31655. [DOI] [PubMed] [Google Scholar]
- (45).Kono M; Takashima S; Liu H; Inoue M; Kojima N; Lee YC; Hamamoto T; Tsuji S Molecular Cloning and Functional Expression of A Fifth-Type alpha 2,3-Sialyltransferase (mST3Gal V: GM3 Synthase). Biochem. Biophys. Res. Commun 1998, 253, 170–175. [DOI] [PubMed] [Google Scholar]
- (46).Zava S; Milani S; Sottocornola E; Berra B; Colombo I Two Active and Differently N-Glycosylated Isoforms of Human ST3Gal-V Are Produced from The Placental mRNA Variant by A Leaky Scanning Mechanism. FEBS Lett. 2010, 584, 1476–1480. [DOI] [PubMed] [Google Scholar]
- (47).Berselli P; Zava S; Sottocornola E; Milani S; Berra B; Colombo I Human GM3 Synthase: A New mRNA Variant Encodes An NH2-terminal Extended form of The Protein. Biochim. Biophys. Acta 2006, 1759, 348–358. [DOI] [PubMed] [Google Scholar]
- (48).Thon V; Lau K; Yu H; Tran BK; Chen X PmST2: A Novel Pasteurella multocida Glycolipid alpha2-3-Sialyltransferase. Glycobiology 2011, 21, 1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Thon V; Li Y; Yu H; Lau K; Chen X PmST3 from Pasteurella multocida Encoded by Pm1174 Gene Is A Monofunctional alpha2-3-Sialyltransferase. Appl. Microbiol. Biotechnol 2012, 94, 977–985. [DOI] [PubMed] [Google Scholar]
- (50).Malekan H; Fung G; Thon V; Khedri Z; Yu H; Qu J; Li Y; Ding L; Lam KS; Chen X One-Pot Multi-Enzyme (OPME) Chemoenzymatic Synthesis of Sialyl-Tn-MUC1 and sialyl-T-MUC1 Glycopeptides Containing Natural or Non-Natural Sialic Acid. Bioorg. Med. Chem 2013, 21, 4778–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Li Y; Yu H; Cao H; Lau K; Muthana S; Tiwari VK; Son B; Chen X Pasteurella multocida Sialic Acid Aldolase: A Promising Biocatalyst. Appl. Microbiol. Biotechnol 2008, 79, 963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Yu H; Yu H; Karpel R; Chen X Chemoenzymatic Synthesis of CMP-Sialic Acid Derivatives by a one-pot two-Enzyme System: Comparison of Substrate Flexibility of Three Microbial CMP-Sialic Acid Synthetases. Bioorg. Med. Chem 2004, 12, 6427–6435. [DOI] [PubMed] [Google Scholar]
- (53).Cao H; Li Y; Lau K; Muthana S; Yu H; Cheng J; Chokhawala HA; Sugiarto G; Zhang L; Chen X Sialidase Substrate Specificity Studies Using Chemoenzymatically Synthesized Sialosides Containing C5-Modified Sialic Acids. Org. Biomol. Chem 2009, 7, 5137–5145. [DOI] [PubMed] [Google Scholar]
- (54).Saxon E; Bertozzi CR Cell Surface Engineering By A Modified Staudinger Reaction. Science 2000, 287, 2007–2010. [DOI] [PubMed] [Google Scholar]
- (55).Agard NJ; Prescher JA; Bertozzi CR A Strain-Promoted [3 + 2] Azide-alkyne Cycloaddition For Covalent Modification of Biomolecules In Living Systems. J. Am. Chem. Soc 2004, 126, 15046–15047. [DOI] [PubMed] [Google Scholar]
- (56).Blixt O; Han S; Liao L; Zeng Y; Hoffmann J; Futakawa S; Paulson JC Sialoside Analogue Arrays For Rapid Identification of High Affinity Siglec Ligands. J. Am. Chem. Soc 2008, 130, 6680–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Prescher H; Schweizer A; Kuhfeldt E; Nitschke L; Brossmer R Discovery of Multifold Modified Sialosides As Human CD22/Siglec-2 Ligands with Nanomolar Activity on B-Cells. ACS Chem. Biol 2014, 9, 1444–1450. [DOI] [PubMed] [Google Scholar]
- (58).Wang X; Lang S; Tian Y; Zhang J; Yan X; Fang Z; Weng J; Lu N; Wu X; Li T; Cao H; Li Z; Huang X Glycoengineering of Natural Killer Cells with CD22 Ligands for Enhanced Anticancer Immunotherapy. ACS Cent. Sci 2020, 6, 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Komura N; Suzuki KG; Ando H; Konishi M; Koikeda M; Imamura A; Chadda R; Fujiwara TK; Tsuboi H; Sheng R; Cho W; Furukawa K; Furukawa K; Yamauchi Y; Ishida H; Kusumi A; Kiso M Raft-Based Interactions of Gangliosides with A GPI-Anchored Receptor. Nat. chem. Biol 2016, 12, 402–410. [DOI] [PubMed] [Google Scholar]
- (60).Geissner A; Baumann L; Morley TJ; Wong AKO; Sim L; Rich JR; So PPL; Dullaghan EM; Lessard E; Iqbal U; Moreno M; Wakarchuk WW; Withers SG 7-Fluorosialyl Glycosides Are Hydrolysis Resistant but Readily Assembled by Sialyltransferases Providing Easy Access to More Metabolically Stable Glycoproteins. ACS Cent. Sci 2021, 7, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Tanaka Y; Kohler JJ Photoactivatable Crosslinking Sugars for Capturing Glycoprotein Interactions. J. Am. Chem. Soc 2008, 130, 3278–3279. [DOI] [PubMed] [Google Scholar]
- (62).Bond MR; Zhang HC; Vu PD; Kohler JJ Photocrosslinking of Glycoconjugates Using Metabolically Incorporated Diazirine-Containing Sugars. Nat. Protoc 2009, 4, 1044–1063. [DOI] [PubMed] [Google Scholar]
- (63).Bond MR; Whitman CM; Kohler JJ Metabolically Incorporated Photocrosslinking Sialic Acid Covalently Captures A Ganglioside–Protein Complex. Mol. BioSyst 2010, 6, 1796–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Yu H; Huang S; Chokhawala H; Sun M; Zheng H; Chen X Highly Efficient Chemoenzymatic Synthesis of Naturally Occurring and Non-Natural alpha-2,6-Linked Sialosides: A P. damsela alpha-2,6-Sialyltransferase with Extremely Flexible Donor-Substrate Specificity. Angew. Chem. Int. Ed 2006, 45, 3938–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Dondoni A; Perrone D Synthesis of 1,1-Dimethylethyl (S)-4-Formyl-2,2-Dimethyl-3-Oxazolidinecarboxylate by Oxidation of The Alcohol. Org. Synth 2000, 77, 64–77. [Google Scholar]
- (66).Yoshida M; Saito K; Kato H; Tsukamoto S; Doi T Total Synthesis and Biological Evaluation of Siladenoserinol A and Its Analogues. Angew. Chem. Int. Ed 2018, 57, 5147–5150. [DOI] [PubMed] [Google Scholar]
- (67).Ghosh A; Chattopadhyay SK A Diversity Oriented Synthesis of D-Erythro-Sphingosine and Siblings. Tetrahedron Asymm 2017, 28, 1139–1143. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


