Abstract
Introduction
Maternal Cytomegalovirus (CMV) infection in the first trimester (T1) of pregnancy is a public health concern, as it increases the risk of severe neurodevelopmental outcomes associated with congenital infection compared to infections occurring later during pregnancy.
Objectives
To determine CMV seroprevalence in T1 of pregnancy, its trend, risk factors and the incidence rate of primary infection during pregnancy.
Methods
Using the biobank of the prospective cohort “Grossesse en Santé de Québec” collected between April 2005 and March 2010 at the Québec-Laval Hospital, Québec, Canada, maternal CMV serology was determined using Abbott Architect Chemiluminescence microparticle immunoassays for immunoglobulin G(IgG), immunoglobulin M(IgM) titration and IgG avidity testing. Changepoint detection analysis was used to assess temporal trends. Risk factors associated with seropositivity were determined by multivariable logistic regression.
Results
CMV seroprevalence in T1 of pregnancy was 23.4% (965/4111, 95% CI, 22.1–24.7%). The incidence rate for CMV primary infection during pregnancy was 1.8 (95% CI, 1.2–2.6) per 100 person-years. No changepoint was identified in the maternal CMV-seroprevalence trend. Multivariable analyses showed that T1 maternal CMV seropositivity was associated with having one child OR 1.3 (95% CI, 1.10–1.73) or two or more children OR 1.5 (95%CI, 1.1–2.1), ethnicity other than Caucasian OR 2.1 (95% CI, 1.1–3.8) and country of birth other than Canada and the USA OR 2.8 (95% CI, 1.5–4.9).
Conclusions
In this cohort, maternal seroprevalence in T1 of pregnancy and seroconversion rate were low. This information and identified risk factors could help guide the development and implementation of preventive actions and evidence-based health policies to prevent CMV infection during pregnancy.
Introduction
Cytomegalovirus (CMV) is the most common congenital infection and a major cause of childhood disability worldwide. Although post-natal infection is largely benign, congenital CMV infection causes sequelae in approximately 20% of infected children, including deafness, blindness and neurodevelopmental delay [1]. Congenital CMV infection can occur either in primary maternal infection during pregnancy, or as a result of “non-primary” infection, which refers to either reactivation of a pre-existing latent CMV strain, or reinfection of the pregnant woman with a new viral strain [2, 3].
The adult seroprevalence of CMV varies widely between countries, from <50% to nearly 100% [4–6], and the prevalence of congenital CMV infection in a population is positively correlated with the CMV seroprevalence among women of childbearing age [7]. Thus, in low- and middle-income countries, where CMV infects >90% of the population during early childhood [7], non-primary maternal infection accounts for most congenital infections. In contrast, the prevalence of CMV infection is approximately 50% in most high-income countries, where a larger proportion of congenital cases are due to primary maternal infection during pregnancy.
The risk of congenital CMV infection associated with primary maternal infection during pregnancy has been estimated to be between 30 and 40% [7]. However, primary CMV infection during the first trimester of pregnancy is associated with more severe symptoms of congenital infection and worse neurological outcomes, compared to primary maternal CMV infection later in pregnancy [8–10]. Thus, determining maternal CMV seroprevalence and the rate of primary infection during pregnancy is essential for understanding the drivers of congenital CMV infection in a population. As few data are available from Canada, we undertook this study in Quebec to estimate the seroprevalence for CMV among pregnant women in the first trimester and the incidence of primary infection during pregnancy, as well as their associated risk factors.
Materials and methods
Study population
This is a secondary analysis using the biobank of the prospective cohort “Grossesse en Santé de Quebec”, which took place from April 2005 to March 2010 at the Centre Hospitalier Universitaire (CHU) de Québec-Laval, Canada to study complications of pregnancy [11, 12]. A total of 7,855 women were enrolled at their first antenatal visit and provided samples and questionnaire data at enrollment and at delivery. The inclusion criteria for the cohort were age 18 years or older; gestational age of at least 10 weeks; and no chronic liver or kidney disease. Exclusion criteria were pregnancies with major fetal abnormalities and those ending in termination, miscarriage or fetal death before 24 weeks of gestation (n = 142). The population of interest for the current study consists of pregnant women who had available specimens at the first trimester (T1) and at delivery (T3), which represents 52% of the total Grossesse en Santé cohort (4,111/7,855)- see S1 Table. Relevant de-identified clinical and socio-demographic variables were extracted from the “Grossesse en Santé” database, including age, marital status, ethnicity, parity, level of education, annual household income, work status and country of birth.
Ethical approval for data collection and biobanking of the initial cohort, as well as use of the data and samples for subsequent studies, was granted by the Research Ethics Board of the Quebec-Laval Hospital. In addition, all aspects of this study were approved by the ethics committee of the CHU Sainte-Justine and the Science and Health Research Ethics Committee of the University of Montréal.
Serological testing
Serological tests were performed on T1 and T3 maternal plasma samples at the CHU Sainte-Justine virology laboratory. All samples were tested using Abbott reagents of the same reference number. Plasma were tested for CMV IgG and, if positive, for CMV IgM, using the Abbott Architect Platform by Chemiluminescence microparticle immunoassays [13]. Health Canada-approved positivity limits are for IgG ≥6.0 AU/ml (arbitrary units per ml) and IgM ≥0.85 s/co (sample to cutoff). Baseline maternal CMV seropositivity was defined as the presence of a positive IgG result, regardless of IgG avidity results or IgM serologies. CMV IgG avidity testing was performed with the Abbott Architect when both IgG and IgM were positive on T1 samples to estimate the chronicity of infection (10). The thresholds for avidity were <50% (low avidity, indicating infected ≤4 months prior), between 50% and <60% (intermediate avidity, indeterminate timing), and ≥ 60% (high avidity, infected >4 months), as described by the manufacturer’s instructions. Primary CMV infection was defined as seroconversion from IgG-negative to -positive from T1 to T3.
Statistical analysis
Baseline CMV seroprevalence was defined as the proportion of participants who were CMV IgG seropositive in T1, out of the total number of participants. Changepoint detection analysis was performed to determine if there was a significant change in the trend of baseline seroprevalence over the study period [14]. A time series of monthly seroprevalence was created and the cpt.meanvar function in R software [14] was used for the changepoint detection.
Study data were subjected to bivariate analysis (= unadjusted analysis) using the Pearson chi-square test or Fisher exact test in order to assess associations between risk factors and maternal seropositivity and between risk factors and the incidence of primary CMV infection and then, to multivariable logistic regression (= adjusted analysis) analyses to examine adjusted associations between risk factors and maternal seropositivity.
The variables used in the multivariable logistic regression models to assess risk factors for CMV seropositivity in the first trimester were selected from the literature. They included age, marital status, ethnicity, parity, level of education, annual household income, work status and country of birth. The conditions applied to the logistic regression model were verified for goodness of fit using the Hosmer and Lemeshow test and the “linktest” to check whether the model specification was good. The absence of collinearity between the variables included in the model was verified by the variance inflation factors and the absence of extreme values (outliers) by graphical analysis of the scatter plot of residual deviance versus predicted values.
The incidence of primary CMV infection during pregnancy was determined by the number of primary infections between the T1 and T3 samples among participants that were seronegative at T1. This incidence rate was expressed out of 10000 person-days at risk. Uncontrolled analysis by confounding factors, i.e. unadjusted, was performed to determine the risk of primary infection by Incidence Rate Ratio. The small number of participants with primary infection precluded a multivariable analysis for this dependent variable.
Statistical analyses were performed with the statistical software STATA version 12.1 and R version 3.6.1
Results
Study cohort
The flow of the 4,111 study participants is illustrated in Fig 1. Most participants were Caucasian (96.7%), born in Canada or in the United States (96.2%).
Fig 1. Flowchart of study to detect cytomegalovirus seroprevalence and primary infection among pregnant women of the cohort "Grossesse en santé".
The figure illustrates the flowchart and breakdown of the study population. During T1, 23% (n = 965) of women were CMV IgG positive. Of these, 113 were also IgM positive: 4 were low IgG avidity, 5 were intermediate avidity, and 104 were high avidity. Of the 3146 women participants who were IgG-negative at T1 (after excluding one patient with insufficient plasma from T3), 28 women were IgG positive at T3, indicating that they had a primary infection between T1 and T3.
Seroprevalence (Fig 2)
Fig 2. Seroprevalence of CMV in the first trimester of pregnancy over year.
The seroprevalence at T1 was 23.47% (965/4111; 95% CI 22.18–24.79%). Among seropositive patients who had both IgG and IgM positive in first trimester samples (n = 113), 4 seropositive patients had a low avidity, indicating infection ≤4 months prior, 5 had intermediate avidity and 104 had high avidity, indicating infection >4 months.
As shown in Fig 2, apart from the year 2005 when only 185 women had been enrolled in the cohort, annual IgG seroprevalence at T1 was stable and ranged between 21.2% and 24.7% over the duration of the study period.
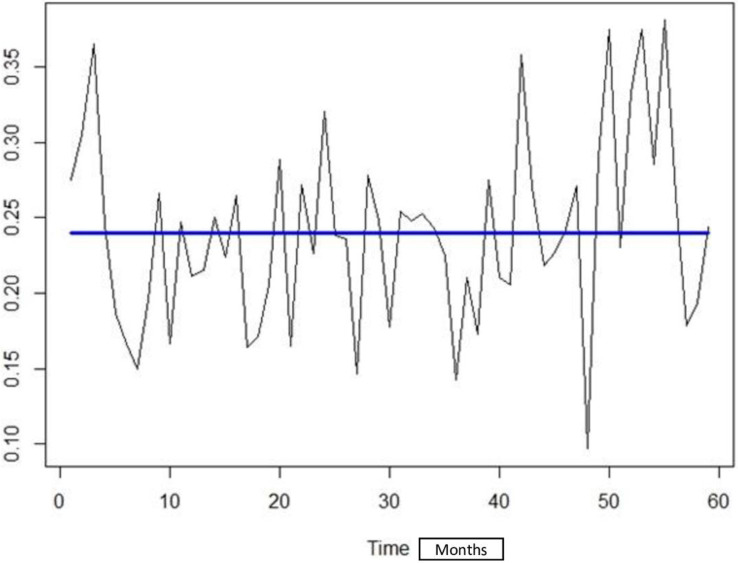
No change in baseline maternal CMV seroprevalence over the period of study was detected with the changepoint detection analysis using the Binary Segmentation “Binseg” method with ’Exponential’ as test statistic, and Schwarz Information Criterion “SIC” as penalty (Fig 3).
Fig 3. Maternal CMV seroprevalence across all cohort participants from April 2005 to March 2010.
Fig 3 shows the trend of seroprevalence by CMV over the study period. No significant point of change in seroprevalence has been objectified during the study period presented by the straight line of the trend. Seroprevalence rates were analyzed on a monthly basis, with 0 being the study start month of April 2005 and 60 being the study end month of March 2010. The y-axis (0.10–0.35) represents the seroprevalence scale.
The risk factors for CMV seropositivity in the first trimester are shown in Table 1. In the multivariable analysis, only parity, ethnicity and country of birth remained significantly associated with baseline CMV seropositivity. Of note, among women born in Canada or in the USA, 98.1% were Caucasian, while among those born in other countries, 60.6% were Caucasian (P-value <0.001). This association did not affect the stability of the multivariable model.
Table 1. Association between CMV IgG seropositivity and socio-demographic factors.
| Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | N total | n among IgG positive | % of IgG positive | OR* | IC 95% | OR | IC 95% | P-value |
| Age | 4111 | 0.6583 | ||||||
| 35–45 | 433 | 127 | 29.3 | 1.6 | 1.2—2.1 | 1.1 | 0.6—1.7 | |
| 30–34 | 1322 | 322 | 24.3 | 1.2 | 1.0—1.6 | 1.0 | 0.7—1.5 | |
| 25–29 | 1749 | 393 | 22.4 | 1.1 | 0.9—1.4 | 1.1 | 0.8–1.6 | |
| 18–24 | 607 | 123 | 20.2 | 1 | 1 | |||
| Parity | 4111 | 0.006 | ||||||
| 2 children or more | 529 | 145 | 27.4 | 1.4 | 1.1–1.7 | 1.5 | 1.0–2.1 | |
| 1 child | 1610 | 402 | 24.9 | 1.2 | 1.0–1.4 | 1.3 | 1.1—1.7 | |
| 0 (nulliparous) | 1972 | 418 | 21.2 | 1 | 1 | |||
| Annual household income | 3528 | 0.690 | ||||||
| Less than 15,499 $ | 124 | 38 | 30.6 | 1.5 | 1.0—2.2 | 0.9 | 0.4—1.6 | |
| 15,500 $- 24,999 $ | 201 | 55 | 27.3 | 1.2 | 0.9—1.7 | 0.9 | 0.5—1.6 | |
| 25,000 $- 39,999 $ | 466 | 116 | 24.8 | 1.1 | 0.8—1.4 | 1.1 | 0.8—1.5 | |
| 40,000 $- 59,999 $ | 777 | 166 | 21.3 | 0.9 | 0.7—1.1 | 0.8 | 0.6—1.1 | |
| 60,000 $ or above | 1960 | 445 | 22.7 | 1 | 1 | |||
| Ethnicity | 3675 | 0.017 | ||||||
| Other* | 121 | 71 | 58.6 | 5.0 | 3.4—7.3 | 2.0 | 1.1—3.8 | |
| Caucasian | 3554 | 180 | 21.9 | 1 | 1 | |||
| Marital status | 3793 | 0.1155 | ||||||
| Single | 263 | 62 | 23.5 | 1.0 | 0.7—1.4 | 1.4 | 0.9—2.2 | |
| Married | 837 | 225 | 26.8 | 1.2 | 1.0—1.5 | 0.8 | 0.6—1.0 | |
| Separated/Divorced | 26 | 6 | 23.5 | 1.0 | 0.4—2.6 | 1.1 | 0.2—4.2 | |
| Common-law partner | 2667 | 594 | 22.2 | 1 | 1 | |||
| Country of birth | 3809 | <0.001 | ||||||
| Other countries | 144 | 95 | 65.9 | 6.9 | 4.8—9.9 | 2.8 | 1.5—4.9 | |
| Canada and USA | 3665 | 798 | 21.7 | 1 | 1 | |||
| Work | 2573 | 0.795 | ||||||
| Yes | 2347 | 496 | 21.1 | 0.7 | 0.5—1.3 | 0.9 | 0.6—1.3 | |
| No | 226 | 60 | 26.5 | 1 | 1 | |||
| Level of education | 0.759 | |||||||
| None (high school not completed) | 147 | 52 | 35.3 | 1.8 | 1.2—2.6 | 1.3 | 0.7—2.5 | |
| Secondary (including professional) | 894 | 205 | 22.9 | 1.0 | 0.8—1.2 | 1.0 | 0.8—1,4 | |
| College (CEGEP) | 1280 | 296 | 23.1 | 1.0 | 0.8—1.2 | 1.0 | 0.8—1.3 | |
| University level | 1473 | 336 | 35.3 | 1 | 1 | |||
*OR. Odds ratio
Primary CMV infection
As shown in Fig 1, among the 3145 pregnant women who were seronegative in T1 of pregnancy, 28 seroconverted, indicating a primary CMV infection rate of 0.9% (95% CI, 0.6–1.3%) during pregnancy. By adding the 4 cases who were seropositive- low avidity (recent infection in T1) to the 28 seroconverted cases, the incidence proportion of primary infection (32/3149) was 1.0% (95% CI, 0.7–1.4).
The total incidence density of primary CMV infection during pregnancy was 1.8 (95% CI,1.9–2.7) per 100 person years or 0.5 (95% CI, 0.3–0.7) per 10,000 person days at risk. No variables were associated with the risk of primary infection in the unadjusted analysis except for parity. As demonstrated in Table 2, higher CMV incidence density was found in the following groups of women: age between 35 and 45 years, parity of at least one, annual household income ≥ $60,000, celibate, birth in Canada or the USA, having at least part-time work and having a college education.
Table 2. Incidence of cytomegalovirus seroconversion in seronegative participants from the “Grossesse en santé” cohort study, Québec, 2005–2010 (n = 3145).
| Variables | Person-time (days) | No of Seroconversion | Incidence Rate* | Unadjusted Incidence Rate Ratio (95%CI**) |
|---|---|---|---|---|
| Age | ||||
| 35–45 | 53021 | 3 | 0.5 | 1.1 (0.1–7.0) |
| 30–34 | 173987 | 9 | 0.5 | 1.0 (0.3–4.8) |
| 25–29 | 236790 | 12 | 0.5 | 1.0 (0.3–4.5) |
| 18–24 | 84580 | 4 | 0.4 | 1 |
| Parity | ||||
| 2 children or more | 66651 | 1 | 0.1 | 0.5 (0.01–3.8) |
| 1 child | 209993 | 19 | 0.9 | 3.0 (1.2–8.1) |
| 0 (nulliparous) | 271734 | 8 | 0.2 | 1 |
| Annual household income | ||||
| Less than 15,499 $ | 14,600 | 0 | 0 | - |
| 15 500 $ - 24 999 $ | 25 239 | 1 | 0.3 | 0.6 (0.01–3.9) |
| 25 000 $ - 39 999 $ | 60 535 | 1 | 0.1 | 0.2 (0.01–1.6) |
| 40 000 $ - 59 999 $ | 105 841 | 3 | 0.2 | 0.4 (0.1–1.5) |
| 60 000 $ or above | 265110 | 17 | 0.4 | 1 |
| Ethnicity | ||||
| Other (African Canadian, Asian, Latin-Canadian, Canadian First Nation and other) | 8557 | 1 | 1.1 | 2.5 (0.1–15.8) |
| Caucasian | 482 806 | 22 | 0.4 | 1 |
| Marital status | ||||
| Single | 34 652 | 3 | 0.8 | 1.9 (0.3–6.8) |
| Married | 105946 | 6 | 0.5 | 1.2 (0.4–3.4) |
| Separated/Divorced | 3361 | 0 | 0 | - |
| Common-law partner | 361926 | 16 | 0.4 | 1 |
| Country of birth | ||||
| Other countries | 8569 | 0 | - | - |
| Canada or USA | 499104 | 21 | 0.5 | 1 |
| Work (part-time or full-time) | ||||
| Yes | 322672 | 16 | 0.4 | - |
| No | 28841 | 0 | - | - |
| Level of education | ||||
| None (high school not completed)3 | 16426 | 0 | - | - |
| Secondary (including professional)1 | 119393 | 7 | 0.5 | 1.6 (0.4–5.5) |
| College (CEGEP) | 170864 | 11 | 0.6 | 1.8 (0.6–5.5) |
| University level | 199061 | 7 | 0.3 | 1 |
* /10.000 person-days at risk;
** CI = confidence interval
Discussions
In this prospective cohort study, we found a relatively low CMV seroprevalence in the first trimester (23.47%) and a low incidence rate of primary infection (1.86 per 100 person years). To our knowledge, this seroprevalence is the lowest reported to date. Previous Canadian studies reported estimated maternal CMV seroprevalences in Quebec of 40% [15] and 54% [16], and 55% in Alberta [17]. Previous reports from France, Europe, USA and Asia reported estimates that were all superior to 50% [4, 18]. The low estimate in our cohort could be explained by the almost homogeneous composition of the “Grossesse en Santé” cohort, with most participants being of Caucasian ethnicity and born in Canada or in the USA, whereas the composition of the previous Quebec cohort was more cosmopolitan [15, 19].
Parity, country of birth, and ethnicity were the risk factors significantly associated with maternal CMV seropositivity. Maternal seroprevalence of CMV in African-American and non-Hispanic black women is often reported to be higher than in Caucasian women [20–22]. In this study, most women who were born outside of Canada and the United States were from low- and middle-income countries, where the prevalence of CMV among children can reach 90% at age 2 years [23]. In contrast, in high-income countries, seroprevalence is lower and infections tend to occur later in life [23]. Women from non-Caucasian ethnicity born in Canada or in the USA may remain in a community with high CMV transmission rates, thereby increasing their risk of infection early in life [24, 25]. Given that parity is a proxy variable for the number of children at home, our finding is similar to previous reports [26] in that seroprevalence is positively correlated with the number of close child contacts (Table 1). Exposure to young children, who shed CMV at high viral loads in saliva and urine for prolonged periods when infected, is a known risk factor for CMV infection [27].
The primary infection rate in our study is similar to the estimates of annual seroconversion during pregnancy in other high-income countries, which range from 1 to 7% [28], but it is 3 times lower than the seroconversion estimated at 1.4 per 10,000 person-days in the previous Quebec study from Lamarre et al. [15]. Of note, the proportion of women with low avidity rate (3.5%) among Ig M-positive women in our cohort was low compared to other studies where the rates of low avidity were found to be greater than 20% [29–31]. These findings support the positive association between baseline CMV seroprevalence in a population and the rate of virus transmission in this same population.
Compared to studies conducted in Canada and other settings, this large prospective study allowed for precise determination of first trimester maternal CMV seroprevalence and the incidence of primary infection in pregnant women. However, this study has some limitations. First, the “Grossesse en Santé” cohort may not be generalizable to the entire Quebec population, as they tended to come from a high socioeconomic level (see Table 1) and therefore may underestimate maternal CMV seroprevalence in the wider Québec population [22, 32, 33]. This could also explain the lack of significant association between low socioeconomic status and CMV seropositivity in our study. Commonly-used indicators for measuring social status are education, occupation, and income, although these are limited in different contexts [34]. In our database, only education and income were available, and these two factors are often associated proportionately [35]. Second, the number of non-Caucasian subjects was small compared to Caucasians and may not be representative. Third, the small number of participants with primary infection in the cohort precluded a multivariate analysis to determine the risk factors associated with primary infection. This also precluded time series analyses of seroconversion rates over time. Fourth, some variables of interest, such as risk factors for CMV, were not present in the “Grossesse en Santé” cohort database, including occupation or preventive job withdrawal or reassignment job which is recommended in Quebec for prevention of CMV infection [36]. Finally, the rate of congenital CMV infection in this cohort was not available.
Conclusions
In conclusion, the low first trimester CMV seroprevalence and low frequency of CMV primary infection found in this study, when compared to estimates from a different cohort from Quebec [15, 19], show that first trimester CMV seroprevalence and primary infection incidence rate are heterogeneous within the province of Quebec. The identified risk factors should be considered when devising interventions to prevent congenital infection. In addition, despite the low maternal CMV seroprevalence, the results of this study show that maternal CMV infection during pregnancy remains a public health priority in order to prevent congenital CMV infection. Hygiene precautions and eventually vaccination have the potential to prevent maternal and congenital CMV infection but may also have differential efficacy against primary and non-primary infections. Thus, in the future, it will be important to conduct studies to regularly assess the maternal incidence and seroprevalence of CMV infection on a longitudinal basis using large population-based samples, and to assess the risk factors for infection among different ethnic groups.
Supporting information
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Acknowledgments
The authors thank Dr. Christian Band for his critical appraisal and editing of the manuscript.
Data Availability
Data cannot be shared publicly because some data are confidential. Data are available from the CHU de Québec-Université Laval Ethics Review Board (ethiquedelarecherche@chudequebec.ca) for researchers who meet the criteria for access to confidential data.
Funding Statement
This work is funded by Canadian Institutes of Health Research (CIHR) Operating Grant (grand award number 386200). Dr Boucoiran is a recipient of a salary award (chercheur-boursier) from FRQ-S (Quebec’s Health research fund). Dr Safari Joseph Balegamire is a recipient from a scholarship from Altona and FRQ-S.
References
- 1.Schleiss MR. Congenital cytomegalovirus: Impact on child health. Contemporary pediatrics. 2018;35(7):16. [PMC free article] [PubMed] [Google Scholar]
- 2.Bonalumi S, Trapanese A, Santamaria A, D’Emidio L, Mobili L. Cytomegalovirus infection in pregnancy: review of the literature. Journal of prenatal medicine. 2011;5(1):1. [PMC free article] [PubMed] [Google Scholar]
- 3.Alford CA, Stagno S, Pass RF, Britt WJ. Congenital and perinatal cytomegalovirus infections. Reviews of infectious diseases. 1990;12(Supplement_7):S745–S53. doi: 10.1093/clinids/12.supplement_7.s745 [DOI] [PubMed] [Google Scholar]
- 4.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Reviews in medical virology. 2007;17(4):253–76. doi: 10.1002/rmv.535 [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim S, Siddiqui AA, Siddiqui AR, Ahmed W, Moss PA, Lalani E-NM. Sociodemographic factors associated with IgG and IgM seroprevalence for human cytomegalovirus infection in adult populations of Pakistan: a seroprevalence survey. BMC public health. 2016;16(1):1112. doi: 10.1186/s12889-016-3772-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanzieri TM, Dollard SC, Bialek SR, Grosse SD. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. International Journal of Infectious Diseases. 2014;22:44–8. doi: 10.1016/j.ijid.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britt W. Controversies in the natural history of congenital human cytomegalovirus infection: the paradox of infection and disease in offspring of women with immunity prior to pregnancy. Medical microbiology and immunology. 2015;204(3):263–71. doi: 10.1007/s00430-015-0399-9 [DOI] [PubMed] [Google Scholar]
- 8.Pass RF, Fowler KB, Boppana SB, Britt WJ, Stagno S. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. Journal of clinical virology. 2006;35(2):216–20. doi: 10.1016/j.jcv.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 9.Leruez-Ville M, Foulon I, Pass R, Ville Y. Cytomegalovirus infection during pregnancy: State of the science. American journal of obstetrics and gynecology. 2020. doi: 10.1016/j.ajog.2020.02.018 [DOI] [PubMed] [Google Scholar]
- 10.Buxmann H, Hamprecht K, Meyer-Wittkopf M, Friese K. Primary human cytomegalovirus (HCMV) infection in pregnancy. Deutsches Ärzteblatt International. 2017;114(4):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giguere Y, Masse J, Theriault S, Bujold E, Lafond J, Rousseau F, et al. Screening for pre-eclampsia early in pregnancy: performance of a multivariable model combining clinical characteristics and biochemical markers. BJOG: An International Journal of Obstetrics & Gynaecology. 2015;122(3):402–10. doi: 10.1111/1471-0528.13050 [DOI] [PubMed] [Google Scholar]
- 12.Tancrède S, Bujold E, Giguère Y, Renald M-H, Girouard J, Forest J-C. Mid-trimester maternal serum AFP and hCG as markers of preterm and term adverse pregnancy outcomes. Journal of Obstetrics and Gynaecology Canada. 2015;37(2):111–6. doi: 10.1016/s1701-2163(15)30331-5 [DOI] [PubMed] [Google Scholar]
- 13.Lapierre SG, Vallières E, Rabaamad L, Labrecque M, Chartrand C, Renaud C. Evaluation of the Abbott ARCHITECT™ cytomegalovirus IgM/IgG, rubella IgM/IgG, and syphilis treponemal antibodies enzyme immunoassays in a mother and child health center population. Diagnostic microbiology and infectious disease. 2019;94(3):231–5. doi: 10.1016/j.diagmicrobio.2018.12.017 [DOI] [PubMed] [Google Scholar]
- 14.Killick R, Eckley I. changepoint: An R package for changepoint analysis. Journal of statistical software. 2014;58(3):1–19. [Google Scholar]
- 15.Lamarre V, Gilbert N, Rousseau C, Gyorkos TW, Fraser W. Seroconversion for cytomegalovirus infection in a cohort of pregnant women in Québec, 2010–2013. Epidemiology & Infection. 2016;144(8):1701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wizman S, Lamarre V, Coic L, Kakkar F, Le Meur J-B, Rousseau C, et al. Awareness of cytomegalovirus and risk factors for susceptibility among pregnant women, in Montreal, Canada. BMC pregnancy and childbirth. 2016;16(1):54. doi: 10.1186/s12884-016-0844-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaudry W, Rosychuk RJ, Lee BE, Cheung PY, Pang X, Preiksaitis JK. Congenital cytomegalovirus infection in high-risk Canadian infants: Report of a pilot screening study. Canadian Journal of Infectious Diseases and Medical Microbiology. 2010;21. doi: 10.1155/2010/942874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leruez-Ville M, Sellier Y, Salomon LJ, Stirnemann JJ, Jacquemard F, Ville Y. Prediction of fetal infection in cases with cytomegalovirus immunoglobulin M in the first trimester of pregnancy: a retrospective cohort. Clinical infectious diseases. 2013;56(10):1428–35. doi: 10.1093/cid/cit059 [DOI] [PubMed] [Google Scholar]
- 19.Fraser WD, Shapiro GD, Audibert F, Dubois L, Pasquier JC, Julien P, et al. 3D Cohort Study: the integrated research network in perinatology of Quebec and Eastern Ontario. Paediatric and perinatal epidemiology. 2016;30(6):623–32. doi: 10.1111/ppe.12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC infectious diseases. 2007;7(1):71. doi: 10.1186/1471-2334-7-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clinical infectious diseases. 2010;50(11):1439–47. doi: 10.1086/652438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Reviews in medical virology. 2010;20(4):202–13. doi: 10.1002/rmv.655 [DOI] [PubMed] [Google Scholar]
- 23.Imbert-Marcille B-M. Histoire naturelle des infections à cytomégalovirus. Médecine thérapeutique. 2001;7(8):577–84. [Google Scholar]
- 24.Asanuma H, Numazaki K, Nagata N, Hotsubo T, Horino K, Chiba S. Role of milk whey in the transmission of human cytomegalovirus infection by breast milk. Microbiology and immunology. 1996;40(3):201–4. doi: 10.1111/j.1348-0421.1996.tb03335.x [DOI] [PubMed] [Google Scholar]
- 25.Boucoiran I, Mayer BT, Krantz EM, Marchant A, Pati S, Boppana S, et al. Non-primary maternal CMV infection following viral shedding in infants. The Pediatric infectious disease journal. 2018;37(7):627. doi: 10.1097/INF.0000000000001877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pass RF, Anderson B. Mother-to-child transmission of cytomegalovirus and prevention of congenital infection. Journal of the Pediatric Infectious Diseases Society. 2014;3(suppl_1):S2–S6. doi: 10.1093/jpids/piu069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starke KR, Kofahl M, Freiberg A, Schubert M, Groß ML, Schmauder S, et al. The risk of cytomegalovirus infection in daycare workers: a systematic review and meta-analysis. International archives of occupational and environmental health. 2020:1–18. doi: 10.1007/s00420-019-01464-x [DOI] [PubMed] [Google Scholar]
- 28.Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Reviews in medical virology. 2010;20(5):311–26. doi: 10.1002/rmv.659 [DOI] [PubMed] [Google Scholar]
- 29.Kaneko M, Sameshima H, Minematsu T, Kusumoto K, Yamauchi A, Ikenoue T. Maternal IgG avidity, IgM and ultrasound abnormalities: combined method to detect congenital cytomegalovirus infection with sequelae. Journal of Perinatology. 2013;33(11):831–5. doi: 10.1038/jp.2013.87 [DOI] [PubMed] [Google Scholar]
- 30.Revello MG, Fabbri E, Furione M, Zavattoni M, Lilleri D, Tassis B, et al. Role of prenatal diagnosis and counseling in the management of 735 pregnancies complicated by primary human cytomegalovirus infection: a 20-year experience. Journal of clinical virology. 2011;50(4):303–7. doi: 10.1016/j.jcv.2010.12.012 [DOI] [PubMed] [Google Scholar]
- 31.Revello MG, Gerna G. Diagnosis and implications of human cytomegalovirus infection in pregnancy. Fetal and Maternal Medicine Review. 1999;11(3):117–34. [Google Scholar]
- 32.Britt WJ. Maternal immunity and the natural history of congenital human cytomegalovirus infection. Viruses. 2018;10(8):405. doi: 10.3390/v10080405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowd J, Aiello AE, Alley D. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiology & Infection. 2009;137(1):58–65. doi: 10.1017/S0950268808000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galobardes B, Morabia A, Bernstein M. Statut socio-économique: un facteur de risque indépendant. Médecine et hygiène. 2000;58(2316):1910–3. [Google Scholar]
- 35.Jallade LA. Niveau d’instruction et salaires. Revue française de pédagogie. 1972:40–66. [Google Scholar]
- 36.Bouchard P, Turcotte G. La maternité en milieu de travail ou pourquoi les Québécoises sont-elles si nombreuses à demander un retrait préventif? Sociologie et sociétés. 1986;18(2):113–28. [Google Scholar]