Abstract
Introduction and Aim: There is a growing need for better, cheaper and faster histopathological diagnostic. The authors reviewed the main steps of the efforts towards the improvement of the pre-analytical phase of tissue processing for histological examination. Results: Since their introduction decades ago tissue microarrays (TMAs) proved their value by increasing efficiency, standardization and accuracy of many histological techniques, such as histochemistry, histoenzymology, immunohistochemistry, in situ hybridization, etc. By allowing the simultaneous analysis and comparison of multiple different tissues on a single histology slide (up to 1000 individual samples), TMAs are also having a significant economic advantage (consumables and labor). From its first description until recent years, the TMA techniques have evolved steadily but slowly despite many attempts to adapt it for clinical diagnostics. In this paper, we are reviewing the main techniques of obtaining TMA blocks from the beginning to the present day, as well as recent developments that are expanding their scope into high accuracy/efficiency clinical diagnostics. Conclusions: Considering recent developments, we believe that the prospect of high-throughput histology might be achievable in the not-so-distant future.
Keywords: paraffin tissue block, tissue microarray, immunohistochemistry, in situ hybridization, clinical diagnostic
⧉ Introduction
Tissue microarray (TMA) technology is contributing to the rapid expansion of current studies of molecular in situ analysis and its integration with clinical and pathological data. In conventional methods, the tissue samples are extracted from archived “donor” paraffin blocks and then inserted into a “recipient” paraffin block. Sections from TMA blocks are used in several types of assays, such as immunohistochemistry, in situ hybridization, histoenzymology, histochemistry, etc., and it has been shown in numerous experiments that these arrays can be representative of tissues in donor blocks, although the samples used are sometimes only 0.6 mm in diameter. This method will be the basis of multiple experiments in different fields of research because it has the power to speed up the transition of in-depth research results towards clinical applications [1].
TMAs facilitate the analysis of molecular changes for thousands of tissue samples in a parallel manner. A single TMA block contains information on the molecular aspects of up to 1000 tissue fragments that are analyzed simultaneously. This is in contrast with the classical, conventional method, in which each histological slide belongs to a single tissue sample. In the end, it would mean analyzing and staining 1000 individual sections [2].
Aim
Given all this, in view of all this, we have reviewed some of the most relevant milestones that have marked the long road of efforts to improve one of the crucial stages of the pre-analytical phase of tissue processing for histological examination.
⧉ Paraffin technique TMAs versus support technique TMAs
After the introduction of the method, several questions were asked about its accuracy, such as:
▪ Is the size of the tissue fragment extracted from the donor block sufficiently representative for the entire tumor?
▪ Do molecular analyzes on histological sections have the same results on TMA sections?
▪ How many fragments of tissue extracted from the same donor block are needed to build a TMA?
▪ What is the optimal diameter for a piece of tissue extracted from a donor block [3]?
During the numerous studies performed until the present day, it is known that the quality of a TMA is variable and proportionally reflects the execution of the entire obtaining process [4].
With the rapid development of staining methods and molecular analysis, new techniques for obtaining efficient TMA were increasingly required, and since the discovery of the method in 1965 [5] and until now new techniques are being tried that can be applied in both fields, research and diagnosis. Below are shown the most important techniques, TMA obtained from donor tissue fragments inserted in the paraffin block are compared with TMA obtained using support materials embedded in the receptor paraffin block having the role of identifying and supporting tissue fragments.
Paraffin tissue-based TMAs
Battifora method
In 1986, H. Battifora proposed a method by which, in the first step, tissue fragments were extracted with a knife from paraffin blocks, deparaffined and rehydrated with decreasing ethanol solutions (100% ethanol to 50% ethanol). In the second step, after rehydration, the tissues were cut with a razor blade into rods, 10 mm long and 1 mm2 in diameter. Finally, about 100 such rods were placed in mammalian small intestine segments and then the paraffin block was made [6].
Wan method
In 1987, W.H. Wan et al. designed a method that consisted first of manufacturing of a special sampling tool, obtained from a 16-gauge syringe needle that was then fixed to a syringe. In the first step, tissues could be removed from the donor paraffin blocks, then extruded from the needle using a wire. In the second step, the tissues were placed in small pieces of plastic tubes (drinking straws) as a coating. In the third step, the tissue fragments inside a straw were melted together to obtain a perfect fusion of paraffin between them. Finally, after the solidification of the paraffin inside the straw, it was cut and removed, and the obtained assembly was placed in a receiving paraffin block [7].
Kononen method
In 1998, J. Kononen et al. developed a device, the manual tissue arraying machine. This device consists of two perforators of different sizes mounted on a movable vertical arm to move on a horizontal X–Y route. Tissue specimens extracted from the donor blocks using the donor perforator are transferred to the holes of the recipient paraffin block.
With this method, approximately 1000 perforated donor tissues with diameters that can vary (0.6 mm, 0.8 mm, 1.00 mm, 2.00 mm) can be embedded in a receiver block, with a size of 44×20 mm [8,9,10].
Mengel method
Mengel et al. developed, in 2003, a two-step process. In the first step, between 60 and 120 metal pins with a thickness of 1.5 mm were fixed on a conventional modified metal mold for paraffin embedding. These pins were placed on the bottom of the mold over which they poured liquid paraffin. After solidification of the paraffin, the pins were removed resulting in a receptor paraffin block for a tissue arrayer with up to 120 holes. In the second step, the blank block was introduced into a second conventional embedding mold in order to fill holes with donor tissue fragments. The resulted assembly was heated through the bottom of the mold, allowing the paraffin block to melt up to 80% of its height for perfect fusion [11,12].
Vogel method
F. Vogel et al. proposed and then improved from 2004 till 2010 another protocol that began with the process of prefabrication of paraffin blocks from usual histological cassettes and paraffin. In the first step, the paraffin was placed in the regular steel molds, over which the histological cassette was placed and allowed to cool at room temperature. The holes could have several dimensions (from 0.6 mm to 1 mm) and were performed by drilling the paraffin block fixed on a horizontal support (MB140/S, Proxxon) using a milling machine (e.g., Proxxon K70). To ensure the cooling of the cutter used during the drilling operation, the paraffin blocks were submerged in a polyvinyl chloride (PVC) container with deionized water. Prior to filling the holes with donor tissues, the paraffin blocks were left at room temperature to evaporate the water from the holes. In the second step, donor tissues were obtained by manual drilling of donor paraffin blocks using modified conventional needles and a manual milling machine equipped with a cutting disc. To ensure fusion between the inserted donor tissues and the surrounding paraffin, the receptor blocks with the formed tissue matrices were placed in an incubator at 50°C for at least 15 minutes [13,14,15,16,17,18].
LeBaron method
J. LeBaron et al. developed, between 2005 and 2010, a new method based on a construction that involves sequential gluing of cross sections of specimen stacks. To obtain such many specimens, LeBaron et al. avoided the perforation technique. Instead, in the first step, the tissue specimens were cut in the form of plates with a knife or a microtome, having a thickness of about 100 μm. In the second step, these tissue plates were melted or glued together to form a primary stack and, finally, sectioned again until three-dimensional (3D) secondary stacks resulted [19,20].
Support matrix TMAs
Battifora & Mehta method
In 1990, H. Battifora & P. Mehta have developed a new method that consisted of using a knife made of several single-use microtome blades, evenly spaced apart. Sticks of raw or paraffined tissues of similar thickness were cut with this knife. The rods were then placed in aluminum molds with rectangular grooves covered with 3% molten fluid agar at 60°C. After solidification, the agar-embedded rod assemblies were stacked and placed in perforated metal boxes for paraffin embedding [21].
Musat method
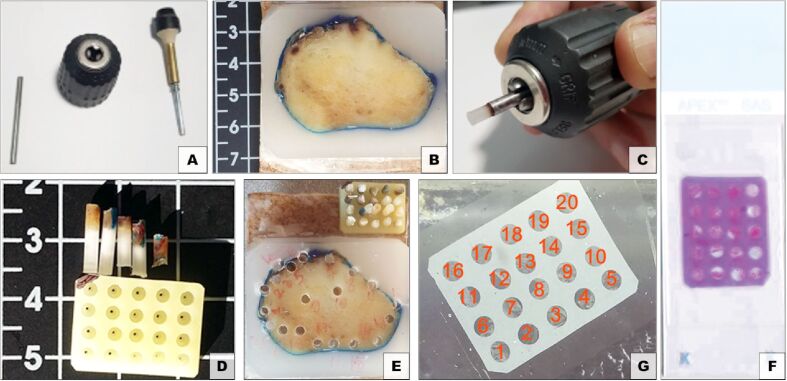
Musat has completed designing, in 2002, a new method that involved a plant-based support for TMA, starting from an earlier version of a matrix array he used in 1999 when he inserted liver tissue [22]. To obtain the supporting plant matrices, sweet potatoes (Ipomoea batatas) were used, previously peeled and cut into 10 cm slices, placed in containers with 4% buffered formaldehyde solution for the fixation step. After fixation, the potato slices were dehydrated with ethanol solutions (progressive concentrations, the last one being absolute ethanol), then cleared with transitional solvent, followed by paraffin embedding under vacuum. The resulting support matrices were then planed and drilled to form receptacles where donor tissues/cells were placed (Figure 1). After inserting the samples, the obtained TMA was embedded in paraffin to obtain the blocks which, then, have been sectioned at the microtome.
Figure 1.
Vegetal TMA – Muşat method: (A) Paraffin donor tissue sampling device; (B) Donor paraffin block; (C) Removing the donor tissue from the sampling device; (D) Empty vegetal tissue microarray and donor tissue samples of different sizes; (E) Loaded TMA block; (F) TMA section; (G) HE-stained slide of a vegetal TMA section. HE: Hematoxylin–Eosin; TMA: Tissue microarray.
Chen method
Chen & Q. Zhou developed a method, in 2005, based on the use of an adhesive platform for fixing donor tissues without the need to pre-manufacture the receptor paraffin blocks. A photographic plate (a piece of X-ray film) cut to the size of the paraffin embedding mold was used, over which a piece of double-sided adhesive tape was attached. The protective paper of the adhesive tape was removed on the area where the donor tissues were glued. The donor tissues with a size of 3–4 mm were extracted from the paraffin blocks using a biopsy needle and removed from the needle directly on the adhesive tape. This subassembly was placed in a mold over which hot paraffin, at 70°C, was poured until it was filled, then the resulting block was cooled to 4°C for 10 minutes. After obtaining the TMA block, the adhesive tape and the photographic plate were removed [23,24,25,26,27,28,29,30].
Song method
M. Song et al. proposed, in 2006, another two steps technique for obtaining TMA blocks. The first step represents the construction of the support matrices from agarose solution by the following process: a quantity of 2% molten agarose is poured into a mold followed by solidification of the agarose at room temperature; then, the agarose block is dehydrated in successive solutions of ethanol 30%, 50%, 70%, 80%, 95%, 100%; then, it is clarified in three xylene solutions, followed by infiltration of the support blocks in melted paraffin, at 64°C, after which it is left at room temperature for solidification. In the second step, TMA block formation, a Beecher tool was used to build in order to form holes in the receptor block with agarose-paraffin support. The needles used for perforations had an inner diameter of 0.6 mm, 1 mm and 2 mm and were guided on the axis with precision on the X–Y axes. Cylindrical donor tissues of different diameters were extracted from paraffin tissue donor blocks and inserted into recipient block with defined matrix coordinates. For the complete integration of the donor tissues into the receptor blocks, they were heated for 10 minutes at 42°C and then flattened [31,32,33,34,35,36].
Musat method
This method designed in 2013 is directly represented by the BxChip™ product, a support matrix for processing and parallel sectioning of small diameter tissue (biopsies), placed horizontally by the surgeon performing the procedure (Figure 2). Unlike all other techniques, used strictly for research purposes or in the production of control preparations, these matrices have been used from the beginning in routine clinical diagnosis, mainly in human medicine. This support matrix is a biomimetic colloid with tunable properties. In the first step, the support matrix is obtained by preparing a mix having a protein component, a hydrophobic component, a polymerizable carbohydrate, an aqueous phase, a polymerizing agent, a plasticizing agent, and, optionally, a pigment [37,38,39,40,41].
Figure 2.
BxChip™ TMA: (A and B) Loading the BxChip™ with biopsies; (C) Placing the BxChip™ in a histological cassette between two reticulated sponges; (D) BxChip™ paraffin block; (E) HE-stained slide of a BxChip™ section. HE: Hematoxylin–Eosin; TMA: Tissue microarray
Williamson method
P. Williamson IV et al. designed, in 2014, a method that is represented by tissue orientation gels (Tissue-Tek® Paraform® from Sakura Finetek, USA), where pre-shaped gels approximately 2 mm thick are used in human clinical diagnosis. This support matrix is obtained by mixing corn starch, gelatin, methyl paraben and borax with deionized water. The aqueous mixture is heated until it starts to boil then poured in a non-stick pan, leaving the material to flow into all edges and set to a uniform thickness. The gel is removed from the pan in one piece after more than two hours. Specific holes or shapes in the gel can be obtained using molds or die cutting machines. This product is a geometric shape support used for orienting of at least one tissue sample during a histopathology process (processing, embedding and microtome slicing) [42].
⧉ TMA techniques applied for research or clinical diagnosis
The methods for obtaining TMAs mentioned above aim at maximizing the use of the very small fragment tissues, saving time and materials used in the execution of numerous molecular studies, genetic studies, etc. Processing and performing analyzes simultaneously on tissues eliminate the inter-experimental variability of some extremely capricious analysis methods. Additionally, this tissue multiplexing allows the upgrading of qualitative methods into quantitative methods. Since in recent years the trend is to save on the execution of histological processes (consumables) but also of expensive molecular tests, there is a major interest in finding methods to maintain the quality of clinical diagnosis while decreasing laboratory costs.
Table 1 shows the advantages and disadvantages of using various methods (in chronological order) to obtain TMAs in the field of research and their translation into human diagnosis.
Table 1.
Comparison of TMA techniques (research or clinical diagnosis)
|
Method [Reference(s)] |
Donor tissue |
TMA support matrix or codification system |
Ease of TMA manufacturing |
TMA sectioning |
Research or clinical diagnostic |
|
(I) Battifora [6] |
P-TSs |
None |
Very laborious, especially the stage of dewaxing the tissues or wrapping the baguette. |
Easy |
Research |
|
(I) Wan et al. [7] |
P-TSs |
None |
Laborious method due to the use of plastic straws. |
Easy |
Research |
|
(II) Battifora & Mehta [21] |
Raw or P-TSs |
Agar-embedded assemblies |
Laborious method due to need for deparaffinizing and agar embedding. |
Easy |
Research |
|
P-TSs (up to 1000) |
Precise alignment and easy relocation of tissue specimens |
Time-consuming method due to the use of manual tissue arrayer. |
Rolling and folding of the P-TSs because of lack of fusion between tissue samples and paraffin in the recipient block |
Research |
|
|
(II) Musat-Marcu et al. [22] |
P-TSs |
Vegetable material embedded support with few receptacles that made the traceability process easy |
Laborious method due to obtaining the plant-based support after a lengthy process of dehydration, paraffin embedding and leveling the surface. |
Easy sectioning |
Research |
|
P-TSs |
Precise alignment and easy relocation of tissue specimens |
Laborious technique because of the two-step method: (i) obtaining the receptor block with metal pins; (ii) manual insertion of the samples. |
Samples can be lost in the sectioning process because of uneven height |
Research |
|
|
P-TSs |
Precise alignment and easy relocation of tissue specimens |
Laborious technique because of the two-step method: (i) obtaining the receptor paraffin block with a drilling machine; (ii) tissue samples insertion and TMA incubation at 50°C. |
Rolling and folding of the P-TSs in sectioning process |
Research |
|
|
P-TSs |
Precise arrangement of stack plates |
Laborious method (melting/gluing the stacks until obtaining a 3D stack paraffin block. |
Cracking of P-TSs |
Research |
|
|
P-TSs |
Precise alignment of tissue specimens |
Laborious method because of the three-step process: (i) cutting a double-sided adhesive tape; (ii) extracting the tissues samples from donor blocks; (iii) putting the tape in upright position in embedding mold filled with melted paraffin. |
Rolling and folding of the P-TSs during sectioning |
Research |
|
|
P-TSs |
Precise alignment of tissue specimens |
Time consuming method (obtaining the agar stabilization bodies through a long process). |
P-TSs are in good contact with the surrounding paraffin |
Research |
|
|
F-Ts |
Precise alignment and easy relocation of tissue specimens by unique codification |
A TMA support matrix with similar properties to human tissue that fuses with the tissue samples. |
Easy sectioning with fast stretching of the sections on the water bath and clear identification of each biopsy |
Clinical diagnosis |
|
|
(II) Williamson et al. [42] |
F-Ts |
Perfect orientation of tissue specimens but without precise identification of patients or the number of excised biopsies |
Getting a fast TMA with a stable support for specimens during the dehydration process but without concomitant shrinking with the inserted tissue samples. |
Capricious sectioning |
Clinical diagnosis |
3D: Three-dimensional; (I): Paraffin tissue-based TMAs; (II): Support matrix TMAs; F-Ts: Fresh tissue samples; P-TSs: Paraffined tissue sections; TMA: Tissue microarray
As can be seen in Table 1, all methods of obtaining TMAs are laborious, except the Musat (BxChip™) and the Williamson (tissue orientation gel) methods, which enable the incorporation of multiple fresh biopsies. These methods streamline traceability, facilitate handling of the resulting matrices and result in expeditious sectioning, which makes them suitable for routine diagnosis.
All the other methods, whether using plain paraffin or other support materials, require a large number of already paraffined biopsies to be meticulously aligned within the recipient blocks. This is invariably creating problems during sectioning.
Throughout the history of TMAs, from the discovery of the method of obtaining a TMA to the present day, the techniques have been directed towards clinical diagnosis. Either techniques have been developed to incorporate as many tissue specimens as possible to save solvents, molecular tests, etc., or to improve the quality of diagnosis by eliminating variability from the many stages of the histological process.
Only in recent years has been a massive focus on improving the histological process from the first stage – tissue sampling. The two methods, Williamson (2014) [42] and Musat (2016) [38] come with a new approach that favors an accurate diagnosis. Although both methods incorporate fresh tissue specimens and facilitate laboratory work, only the Musat method preserves accurate and crucial information due to the coding of the support matrices.
By this aspect, the traceability of the information cannot be lost at any stage (regardless of the positioning of the support matrix in the histological cassette, during paraffin embedding or when placing the section on the slide), therefore the order or area from which the biopsy was excised is identifiable by its unique location within the support matrix [43].
⧉ Conclusions
Traditional histopathological techniques, such as histochemistry, immunohistochemistry, in situ molecular techniques, etc., are benefiting greatly from the rapid development of TMAs with low cost and ease of use. Most importantly, TMAs have the potential to improve routine clinical diagnostic, particularly using support matrices at the point of care.
Conflict of interest
Alina Elena Ştefan, Daniela Gologan and Sorin Muşat are employed by LUMEA Inc., Lehi, Utah, USA, who are manufacturing BxChip™ (Patent US D836796 S, 2017).
Authors’ contribution
The first and the second authors had equal contribution to the achievement of this paper
References
- 1.Bubendorf L, Nocito A, Moch H, Sauter G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol. 2001;195(1):72–79. doi: 10.1002/path.893. [DOI] [PubMed] [Google Scholar]
- 2.Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet. 2001;10(7):657–662. doi: 10.1093/hmg/10.7.657. [DOI] [PubMed] [Google Scholar]
- 3.Sauter G. Representativity of TMA studies. Methods Mol Biol. 2010;664:27–35. doi: 10.1007/978-1-60761-806-5_3. [DOI] [PubMed] [Google Scholar]
- 4.Tennstedt P, Sauter G. Quality aspects of TMA analysis. Methods Mol Biol. 2010;664:17–26. doi: 10.1007/978-1-60761-806-5_2. [DOI] [PubMed] [Google Scholar]
- 5.Lillie RD. 3. New York: Blakiston Division, McGraw–Hill Book Company; 1965. Histopathologic technic and practical histochemistry.https://www.worldcat.org/title/histopathologic-technic-and-practical-histochemistry/oclc/14500572/editions?start_edition=21&sd=desc&referer=di&se=yr&editionsView=true&fq= [Google Scholar]
- 6.Battifora H. The multitumor (sausage) tissue block: novel method for immunohistochemical antibody testing. Lab Invest. 1986;55(2):244–248. [PubMed] [Google Scholar]
- 7.Wan WH, Fortuna MB, Furmanski P. A rapid and efficient method for testing immunohistochemical reactivity of monoclonal antibodies against multiple tissue samples simultaneously. J Immunol Methods. 1987;103(1):121–129. doi: 10.1016/0022-1759(87)90249-3. [DOI] [PubMed] [Google Scholar]
- 8.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 9.Schraml P, Kononen J, Bubendorf L, Moch H, Bissig H, Nocito A, Mihatsch MJ, Kallioniemi OP, Sauter G. Tissue microarrays for gene amplification surveys in many different tumor types. Clin Cancer Res. 1999;5(8):1966–1975. [PubMed] [Google Scholar]
- 10.Gillett CE, Springall RJ, Barnes DM, Hanby AM. Multiple tissue core arrays in histopathology research: a validation study. J Pathol. 2000;192(4):549–553. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH721>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Mengel M, Kreipe H, von Wasielewski R. Rapid and large-scale transition of new tumor biomarkers to clinical biopsy material by innovative tissue microarray systems. Appl Immunohistochem Mol Morphol. 2003;11(3):261–268. doi: 10.1097/00129039-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Hoos A, Cordon-Cardo C. Tissue microarray profiling of cancer specimens and cell lines: opportunities and limitations. Lab Invest. 2001;81(10):1331–1338. doi: 10.1038/labinvest.3780347. [DOI] [PubMed] [Google Scholar]
- 13.Vogel UF, Bueltmann BD. Simple, inexpensive, and precise paraffin tissue microarrays constructed with a conventional microcompound table and a drill grinder. Am J Clin Pathol. 2006;126(3):342–348. doi: 10.1309/F2Q38DXN1V1V4GQM. [DOI] [PubMed] [Google Scholar]
- 14.Mirlacher M, Simon R. Recipient block TMA technique. Methods Mol Biol. 2010;664:37–44. doi: 10.1007/978-1-60761-806-5_4. [DOI] [PubMed] [Google Scholar]
- 15.Vogel UF. Inexpensive and precise paraffin tissue microarrays constructed with a computer numerical control (CNC) drilling machine. Histopathology. 2007;51(1):136–137. doi: 10.1111/j.1365-2559.2007.02713.x. [DOI] [PubMed] [Google Scholar]
- 16.Vogel UF, Bode J, Bueltmann B. Increasing the efficiency of paraffin tissue microarrays by packing the paraffin tissue core biopsies in a honeycomb pattern. Appl Immunohistochem Mol Morphol. 2007;15(3):343–345. doi: 10.1097/01.pai.0000213140.47277.f6. [DOI] [PubMed] [Google Scholar]
- 17.Vogel UF. One-step complete melting of paraffin tissue microarrays using stabilization bodies. Appl Immunohistochem Mol Morphol. 2008;16(4):382–386. doi: 10.1097/PAI.0b013e318158ec68. [DOI] [PubMed] [Google Scholar]
- 18.Vogel UF, Bültmann B. Application of a novel and low-cost technique to construct paraffin tissue microarrays out of paraffinised needle biopsy specimens from patients with breast cancer. J Clin Pathol. 2010;63(7):640–643. doi: 10.1136/jcp.2010.076356. [DOI] [PubMed] [Google Scholar]
- 19.LeBaron MJ, Crismon HR, Utama FE, Neilson LM, Sultan AS, Johnson KJ, Andersson EC, Rui H. Ultrahigh density microarrays of solid samples. Nat Methods. 2005;2(7):511–513. doi: 10.1038/nmeth772. [DOI] [PubMed] [Google Scholar]
- 20.Tran TH, Lin J, Sjolund AB, Utama FE, Rui H. Protocol for constructing tissue arrays by cutting edge matrix assembly. Methods Mol Biol. 2010;664:45–52. doi: 10.1007/978-1-60761-806-5_5. [DOI] [PubMed] [Google Scholar]
- 21.Battifora H, Mehta P. The checkerboard tissue block. An improved multitissue control block. Lab Invest. 1990;63(5):722–724. [PubMed] [Google Scholar]
- 22.Musat-Marcu S, Gunter HE, Jugdutt BI, Docherty JC. Inhibition of apoptosis after ischemia-reperfusion in rat myocardium by cycloheximide. J Mol Cell Cardiol. 1999;31(5):1073–1082. doi: 10.1006/jmcc.1999.0939. [DOI] [PubMed] [Google Scholar]
- 23.Chen N, Zhou Q. Constructing tissue microarrays without prefabricating recipient blocks: a novel approach. Am J Clin Pathol. 2005;124(1):103–107. doi: 10.1309/LHCJRFBUH8Q6QD3N. [DOI] [PubMed] [Google Scholar]
- 24.Hsu FD, Nielsen TO, Alkushi A, Dupuis B, Huntsman D, Liu CL, van de Rijn M, Gilks CB. Tissue microarrays are an effective quality assurance tool for diagnostic immunohistochemistry. Mod Pathol. 2002;15(12):1374–1380. doi: 10.1097/01.MP.0000039571.02827.CE. [DOI] [PubMed] [Google Scholar]
- 25.Packeisen J, Buerger H, Krech R, Boecker W. Tissue microarrays: a new approach for quality control in immunohistochemistry. J Clin Pathol. 2002;55(8):613–615. doi: 10.1136/jcp.55.8.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Packeisen J, Korsching E, Herbst H, Boecker W, Buerger H. Demystified... tissue microarray technology. Mol Pathol. 2003;56(4):198–204. doi: 10.1136/mp.56.4.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hidalgo A, Piña P, Guerrero G, Lazos M, Salcedo M. A simple method for the construction of small format tissue arrays. J Clin Pathol. 2003;56(2):144–146. doi: 10.1136/jcp.56.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, Ghahary A, Moqbel R. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173(10):5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 29.Jugdutt BI, Musat-Marcu S. Opposite effects of amlodipine and enalapril on infarct collagen and remodelling during healing after reperfused myocardial infarction. Can J Cardiol. 2000;16(5):617–625. [PubMed] [Google Scholar]
- 30.Stenton GR, Ulanova M, Déry RE, Merani S, Kim MK, Gilchrist M, Puttagunta L, Musat-Marcu S, James D, Schreiber AD, Befus AD. Inhibition of allergic inflammation in the airways using aerosolized antisense to Syk kinase. J Immunol. 2002;169(2):1028–1036. doi: 10.4049/jimmunol.169.2.1028. [DOI] [PubMed] [Google Scholar]
- 31.Song YM, Jeong HJ, Jang SC. Recipient block and method for preparation thereof. United States Patent No. US20050176088. 2006 https://patentscope.wipo.int/search/en/detail.jsf?docId=US41124107&_fid=WO2004111614 [Google Scholar]
- 32.Yan P, Seelentag W, Bachmann A, Bosman FT. An agarose matrix facilitates sectioning of tissue microarray blocks. J Histochem Cytochem. 2007;55(1):21–24. doi: 10.1369/jhc.6A6987.2006. [DOI] [PubMed] [Google Scholar]
- 33.Hewitt SM. Design, construction, and use of tissue microarrays. Methods Mol Biol. 2004;264:61–72. doi: 10.1385/1-59259-759-9:061. [DOI] [PubMed] [Google Scholar]
- 34.Howat WJ, Warford A, Mitchell JN, Clarke KF, Conquer JS, McCafferty J. Resin tissue microarrays: a universal format for immunohistochemistry. J Histochem Cytochem. 2005;53(10):1189–1197. doi: 10.1369/jhc.5C6659.2005. [DOI] [PubMed] [Google Scholar]
- 35.Matysiak BE, Brodzeller T, Buck S, French A, Counts C, Boorsma B, Datta MW, Kajdacsy-Balla AA. Simple, inexpensive method for automating tissue microarray production provides enhanced microarray reproducibility. Appl Immunohistochem Mol Morphol. 2003;11(3):269–273. doi: 10.1097/00129039-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Pan CC, Chen PCH, Chiang H. An easy method for manual construction of high-density tissue arrays. Appl Immunohistochem Mol Morphol. 2004;12(4):370–372. doi: 10.1097/00129039-200412000-00015. [DOI] [PubMed] [Google Scholar]
- 37.Musat S. Matrix for receiving a tissue sample and use thereof. United States Patent No. US 9851349 B2. 2013 https://patentimages.storage.googleapis.com/f1/ff/26/89c5552d6aa206/US9851349.pdf [Google Scholar]
- 38.Musat S. Biomimetic gellable colloid for encoding, processing and sectioning histological samples. International Patent Application No. WO/2016/013949. 2016 https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016013949 [Google Scholar]
- 39.Jinga V, Petrescu A, Berdan G, Tanase F, Braticevici B, Amuzescu B, Musat S, Moisanu D, Radavoi D. C174 A comparison between classical and simultaneous processing of prostate core biopsies using Histobest Biopsy Chip™. Eur Urol Suppl. 2012;11(4):124–124. [Google Scholar]
- 40.Farcaş CP, Spînu AD, Petrescu A, Dumitrescu M, Vasilescu F, Muşat S, Dinu M, Mischianu D. High throughput processing and analysis of prostate core biopsies for research applications. Rev Rom Urol (Rom J Urol) 2014;13(3):9–14. http://revista-urologia.ro/wp-content/uploads/2014/11/High-throughput-processing-and-analysis-of-prostate-core-biopsies-for-research-applications.pdf [Google Scholar]
- 41.Murugan P, Shukla D, Morocho J, Smith D, Sciacca D, Pickard M, Wahlsten M, Gunderson A, Konety B, Khalifa MA, Warlick C. Prostate biopsy processing: an innovative model for reducing cost, decreasing test time, and improving diagnostic material. Am J Clin Pathol. 2019;152(6):757–765. doi: 10.1093/ajcp/aqz101. [DOI] [PubMed] [Google Scholar]
- 42.Williamson WP IV, Whitlatch SP, Saez CA. Method for orienting tissue samples on a microtome sectionable biopsy support. United States Patent No. US 8796038 B2. 2014 https://patentimages.storage.googleapis.com/9d/10/85/c652f4bd18910c/US8796038.pdf [Google Scholar]
- 43.Leavitt MO, Musat S. Tissue sample receptacle. United States Design Patent No. US D836796 S. 2017 https://patentimages.storage.googleapis.com/a6/8c/65/af2a244bd5321e/USD836796.pdf [Google Scholar]