Abstract
Purpose
The biologic behavior and the therapeutic resistance of diffuse and anaplastic gliomas varies greatly. This may be explained by differences in cell-to-cell communication, determined by the Cx43-associated junctional activity and the microtubules-defined network, in which GAP-43 is the dominant structural component. We assessed the expression of these crucial communication proteins in samples of patients harboring WHO°II and III gliomas, graded according to the current 4th revised WHO classification.
Methods
Tissue of adult patients with WHO°II and III gliomas, who underwent surgery between 2014 and 2018, were selected from our institutional biobank. GAP-43 and Cx43 expression was analyzed using IHC. Routine clinical and neuropathological findings were additionally retrieved from our institutional prospective database.
Results
43 (57%) males and 33 (43%) females with a median age of 47 (IqR: 35–61) years were selected. IDH1 wildtype tumors showed a significantly higher expression of Cx43 (p = 0.014) and a tendency for increased GAP-43 production. Advanced Cx43 expression significantly correlated with lower mitosis rate (p = 0.014): more in IDH1 wildtype (r = − 0.57, p = 0.003) than in mutated gliomas (r = − 0.37, p = 0.019). There was no difference in Cx43 or GAP-43 expression in relation to anaplastic phenotype, Gadolinum-contrasted enhancement (CE) on MRI and advanced EGFR or p53 expression.
Conclusions
Intercellular communication tends to be more relevant in slower proliferating, e.g. lower malignant tumors. They could have more time to establish this network, providing longitudinally acquired resistance against specific oncological therapy. This feature matches the unfavorable IDH1 wildtype status of glioma and supports the noted malignant behavior of these tumors in the upcoming 5th WHO classification of gliomas.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-021-03711-6.
Keywords: Glioma, IDH1 wildtype, Cx43, GAP-43, Intercellular communication
Introduction
The prognosis of diffuse and anaplastic glioma varies according to neuropathological features (Claus et al. 2015): tumors with wildtype isocitrate dehydrogenase 1 (IDH1) (Cohen et al. 2013; Sanson et al. 2009; Leeper et al. 2015) and lost nuclear expression of X-linked ATP-dependent helicase (ATRX) (Leeper et al. 2015; Nandakumar et al. 2017; Wiestler et al. 2013; Weller et al. 2020) are considered to be unfavorable. New considerations considering the role of genetic factors are going to be implemented into upcoming 5th WHO classification of gliomas (Weller et al. 2020; Rushing 2021) as IDH1 wildtype diffuse or anaplastic gliomas with EGFR amplification, combined whole chromosome 7 gain and 10 loss or TERT promoter mutation, that were categorized as WHO °II or III according to the 4th revised classification of central nervous system tumors (CNS) (Louis et al. 2016a, b), show actually biologically behavior of °IV glioma (Brat et al. 2018; Hartmann et al. 2010). Unfortunately, the mechanisms that lead to different behavior of gliomas in underlying these biological differences are poorly understood.
Changes in junctional intercellular communication have been associated with tumor aggression (Aasen et al. 2019; Sinyuk et al. 2018). Connexin-43 (Cx43, GJA1) is the main gap junction protein, that is expressed in normal astrocytes (Giaume et al. 1991; Zhao et al. 2018) as well as neural precursors (Marins et al. 2009). It allows metabolic and electrophysiologic intercellular cooperation and, thus, maintains tissue homeostasis (Zhao et al. 2018). The role of junctional intercellular communication for expansion of glioma has been described to be complex and is still controversial (Sin et al. 2012).
Another way for glioma growth is malignant intercellular networking using tumor microtubes (TMs), in which growth associated protein 43 (GAP-43) is the dominant structural protein (Osswald et al. 2015, 2016; Weil et al. 2017). Normally, like Cx43, GAP-43 plays a significant role in phylogenesis providing migration and integration of neural progenitor cells (Haag et al. 2012). Similarly, gliomas create a united structural and functional syncytium. GAP-43 supports malignant cell migration and invasion and provides functional intercellular communication, which is believed to support tumor resistance against radio- and chemotherapy (Osswald et al. 2015; Weil et al. 2017; Sontheimer 2015). Although Cx43 is expressed on TMs as well (Osswald et al. 2015), its input has not been entirely assessed (Sin et al. 2012; Weil et al. 2017).
The aim of this study therefore was to analyze the expression of GAP-43 and Cx43 diffuse and anaplastic gliomas in relation to actual WHO genetical aspects as well as epidemiological and clinical features.
Materials and methods
Consecutive adult patients with neuropathologically proven intracranial diffuse and anaplastic glioma, operated at our institution according to routine clinical indications between 2014 and 2018, were selected from a prospective neurooncological database and tissue bank, which are approved by the institutional ethics committee (AN5220 329/4.4). Informed consent was obtained from the subjects before the surgery. The neuropathological examination was routinely performed after surgery on FFP-embedded tissue. Integrated neuropathological reports were based on the WHO classification system (Louis et al. 2016a; Daumas-Duport et al. 1988). The WHO grade of diffuse and anaplastic gliomas in our cohort was assessed according to phenotypical features as defined in the current 4th revised WHO classification of CNS tumors (Louis et al. 2016a, b). Presence of IDH1 mutation in the R132H position was tested with IHC and in case of a negative result DNA sequencing was performed for patients under 40 years to approve the IDH1 wildtype status. Nuclear ATRX, p53, EGFR expression and MiB as proliferation marker and were also assessed by IHC.
A total of 83 patients from our database were found eligible for further investigation. While seven patients had to be excluded due to a low amount of FFPE tissue left to perform IHC, analysis was performed for the remaining 76 patients. The HE slides were used to control tumor position in the tissue and representability. GAP-43 and Cx43 IHC processing was performed automatically with commercial specific antibodies (Abcam P17302 anti-GAP43 and Abcam P17302 anti-Connexin-43). IHC processing was checked using negative and positive tissue controls. The interpretation was performed by an experienced neuropathologist. The expression of Cx43 and GAP-43 was semiquantitatively ranked in glioma cellular fractions: 0 = no expression at all, 1 = minimal expression, 2 = moderate expression (more than minimal but less than strong), 3 = strong expression (comparable to cortex); see Supplement 1 and 2. Anaplasia was defined according to WHO °III criteria and has been detected either in the entire tumor or only focally (focal anaplasia).
The epidemiological and routine neuropathological findings have been gathered prospectively in our database. Preoperative MRI including T1-weighted Gadolinum-contrasted imaging was performed in all cases as local standard protocol and standard of care in patients harboring intracranial tumors (Freyschlag et al. 2018). Time interval between imaging-based diagnosis and histological diagnosis was assessed.
Statistical analysis was processed using IBM SPSS Statistics (IBM SPSS Statistics for Mac OS, Version 26.0. Armonk, NY: IBM Corp.). Normal distribution of scale parameters was checked by the Kolmogorov–Smirnov test. Correlations for non-parametric data were exposed according to Spearman’s method. Mann–Whitney U-test for rank or scale parameters lacking normal distribution and Chi2-test comparing two binominal parameters were applied. The Benjamini–Hochberg (B–H) correction was respected for multiple hypothesis (Benjamini and Yosef 1995; Hochberg and Benjamini 1990). The confidence interval and α were defined as 95%.
Results
43 (57%) males and 33 (43%) females with a median age of 47 years (IqR 35–61, range 20–89) were selected in our series. In 15 patients (20%) a diffuse glioma and in 46 patients (60%) gliomas with anaplasia in all exposed tumor tissue were diagnosed. Further 15 patients (20%) were identified with a diffuse glioma showing focal anaplasia only. The detailed distribution of neuropathological parameters according to the level of anaplasia is listed in Table 1.
Table 1.
Distribution of neuropathological markers in relation to anaplastic signs in histomorphology
| Total | No anaplasia | Focal anaplasia | Anaplasia | |||
|---|---|---|---|---|---|---|
| Marker | N | 76 | 15 | 15 | 46 | |
| IDH1 | 69 | Mutated | 43 (62%) | 9 (69%) | 11 (79%) | 23 (55%) |
| Wild | 26 (38%) | 4 (31%) | 3 (21%) | 19 (41%) | ||
| ATRX | 66 | Loss | 26 (39%) | 5 (38%) | 10 (71%) | 11 (28%) |
| Expression | 40 (61%) | 8 (62%) | 4 (29%) | 28 (72%) | ||
| EGFR | 67 | Loss | 25 (37%) | 4 (31%) | 5 (38%) | 16 (39%) |
| Expression | 42 (63%) | 9 (69%) | 8 (62%) | 25 (61%) | ||
| p53 | 67 | Loss | 8 (12%) | 4 (31%) | 1 (7%) | 3 (7%) |
| Expression | 59 (88%) | 9 (69%) | 13 (93%) | 37 (93%) | ||
| MiB | 73 | Expression |
14% (IqR: 8–29%) |
8% (IqR: 5–9%) |
12% (IqR: 8–16%) |
21% (IqR: 14–34%) |
Cx43
The score distribution of the Cx43 expression is provided in Fig. 1a and examples in Fig. 2. The IDH1 wildtype tumors showed significantly higher expression of Cx43 (p = 0.003, B–H 0.014). The IDH1 wildtype tumors demonstrated a trend for even higher Cx43 expression in patients with longer intervals (median 1.5 months, range 0–265) between imaging-based diagnosis and actual biopsy (r = 0.43; p = 0.032, B–H 0.072), whereas this association was absent in IDH1 mutated gliomas (p—n.s.). Advanced Cx43 expression significantly correlated with lower proliferation rate (p = 0.003, B–H 0.014) in both IDH1 wildtype (r = − 0.57, p = 0.003) and mutated gliomas (r = − 0.37, p = 0.019). The presence of nuclear ATRX was trended with higher Cx43 expression (p = 0.029, B–H 0.072). EGFR and p53 expression did not related to Cx43 (p—n.s.). There was no correlation between Cx43 expression and anaplasia or contrast enhancement rate on MRI (p—n.s.).
Fig. 1.
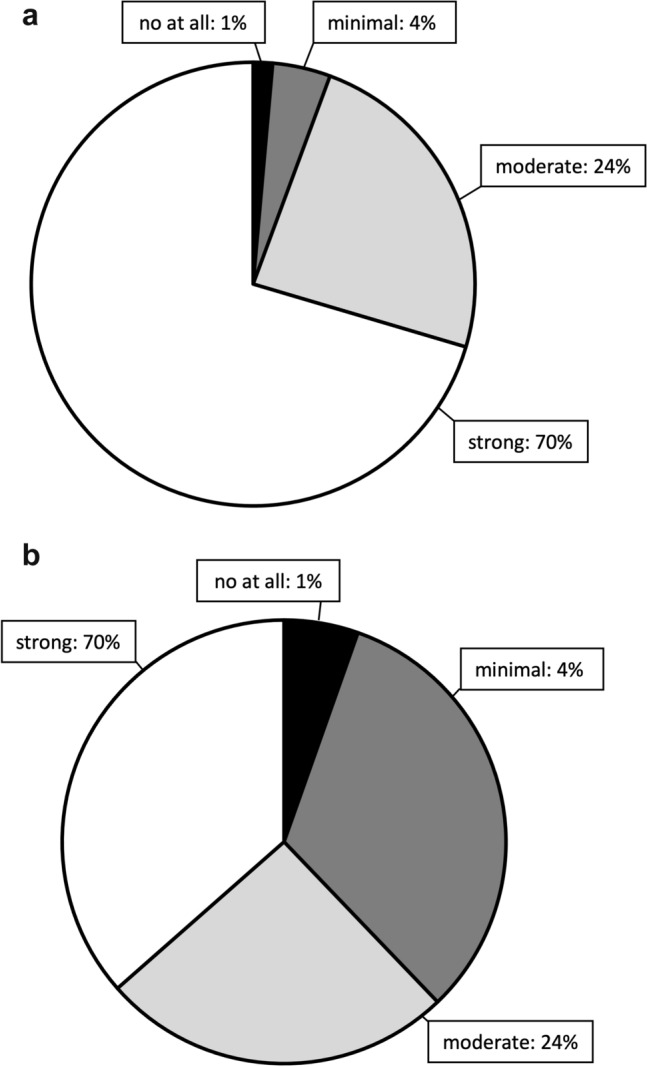
Score distribution of the Cx-43 (a) and GAP-43 (b) expression in IHC is provided as the pie chart
Fig. 2.
Examples of consecutive histological (HE) and immunohistological sections for Cx-43 and GAP-43. The expression of Cx-43 and GAP-43 is advanced in diffuse WHO °II glioma (b), compared to anaplastic glioma (a). Tissue Philips IntelliSite Ultra Fast Scanner, macroscopical view and metric reference of digital magnification is showed on the figure
GAP-43
The score distribution of the GAP-43 expression is provided in Fig. 1b and examples in Fig. 2. GAP-43 expression did not demonstrate any statistically significant differences in our series of diffuse and anaplastic gliomas. There was a trend for maintained nuclear ATRX expression in case of advanced GAP-43 expression (p = 0.022), which failed to reach significance after statistical correction by multiple hypothesis. IDH1-wildtype gliomas also trended to show more GAP-43 expression (p = 0.075). Common neuropathological findings like proliferation rate, anaplasia, EGFR or p53 status as well as contrast enhancement on MRI and time interval between imaging-based diagnosis and actual biopsy did not demonstrate any differences in GAP-43 expression (p—n.s.).
Discussion
Based on our data wildtype IDH-1 diffuse and anaplastic gliomas, which were correspondingly graded as II and III according to their phenotype as defined in the current 4th revised WHO classification, demonstrated more expression of networking proteins: higher Cx43 expression and a tendency towards advanced GAP-43 production. These tumors also, tended to show a longitudinal increase of Cx43, based on time between imaging-based diagnosis and actual biopsy. Interestingly, gliomas with a lower mitosis rate produced more Cx43.
According to the literature, Cx43 expression is decreased in malignant gliomas (McDonough et al. 1999; Aftab et al. 2015; Sin et al. 2016): this feature is reversely dependent on the proliferation rate and WHO grade (Pu et al. 2004). However, the inhibition of Cx43 results in favorable sensitizing to TMZ chemotherapy (Grek et al. 2018; Potthoff et al. 2019). At the same time, the more promising outcome of oligodendroglioma has been showed to be the result of poor networking via TMs: the reduced syncytial construction is caused by the unbalanced deficiency of core neurotrophic factors, which drive GAP-43 expression (Osswald et al. 2015, 2016; Weil et al. 2017). These factors are coded on the 1p/19q chromosomal regions, which are co-deleted, e.g. absent, in case of oligodendroglial tumors (Osswald et al. 2015). The canonical IDH-mutation did not influence this feature (Osswald et al. 2016).
For the group of diffuse and anaplastic gliomas, on the other hand, there is insufficient information regarding the TMs network and their relation to important neuropathological parameters, used in clinical routine. Previous studies were mainly performed in-vitro and used mostly glioblastoma multiforme clonal cells, that have another etiology and consequently taxonomic belonging. Moreover, in vitro tests dismiss possible interactions with other brain cells in-vivo, which could be important in establishing intercellular communication. It has been described, that glioma syncytia have connections with normal astrocytes (Sin et al. 2016; Chen et al. 2015; Lin et al. 2016). A similar interaction was described in brain metastasis (Chen et al. 2016). This lead to the conclusion that physiological brain cells supported the tumor. It is uncertain, if the isolated clonal cells are able to produce the representative syncytial networking, e.g. if the results would be translatable to clinical cases. To review the inhomogeneity within the heterogenous group of diffuse and anaplastic gliomas larger studies are required.
We could not verify Cx43 or GAP-43 correlation with the established phenotypical WHO grading according to the 4th classification. Moreover, Cx43 or GAP-43 expression did not differ in relation to other typical signs of malignancy like presence of anaplastic features, Gadolinum-contrasted enhancement in T1 MRI, p53 or EGFR expression. However, even if the glioma did not show high mitosis rates, it may acquire intercellular networking options with time: unfavorable wild-type IDH glioma trended to produce longitudinally more Cx43 and GAP-43. This could be explained with the longer interphase in slower proliferating cells—they have more time for transcription and translation, thus, protein synthesis. This dependency correlated more with IDH1 wildtype gliomas compared to mutated gliomas. That matches to the fact, that wildtype IDH1 glioma has more aggressive behavior and poorer outcome, which is going to be integrated in the 5th WHO classification of gliomas. The loss of ATRX nuclear expression is typical for diffuse IDH1 mutated astrocytomas, so this could be the reason for their tendency to show less Cx43 and GAP-43 expression. These results are concordant to literature data demonstrating that Cx43 expression is decreased in malignant gliomas with higher mitosis rate (McDonough et al. 1999; Aftab et al. 2015; Sin et al. 2016). At the same time, the increase of Cx43 expression in slower proliferating tumors was not associated with advanced GAP-43 production. That could represent the advanced ability for junctional intercellular networking even without TMs networking.
It was proposed, that Cx43-mediated gap intercellular communication allows to keep the Ca2+ homeostasis, thus, working against TMZ (Osswald et al. 2015; Weil et al. 2017) and radiotherapy (Osswald et al. 2015) for which a high intracellular Ca2+ concentration is required. Thus, less proliferating tumors take more time to grow, but during it they obtain defense mechanisms against our current treatment options. That could have crucial clinical impact, as adjuvant radiotherapy and TMZ chemotherapy is frequently first applied in cases when diffuse glioma show progression after surgical resection and a wait-and-scan period (Picca et al. 2018).
Our study has limitations. Despite trends, we were unable to find significant differences for some parameters related to Cx43 and GAP-43. That could be explained with the precise conservative statistic methods with corrections for multiple hypothesis (Benjamini and Yosef 1995; Hochberg and Benjamini 1990). It was not possible to detect 1p/19q co-deletion in our series, as it was routinely performed only after 2016, respecting the updated WHO classification. However, the primary factor that lead to 1p/19q co-deletion or competitive ATRX nuclear expression is IDH1 mutation, which was analyzed in our series (Louis et al. 2016b). The IHC analysis has restrictions by itself: semi-quantitative and examiner-dependent assessment is performed and smaller differences in expression between samples could be abandoned. There is no worldwide standardization of IHC stains, especially in the scientific field. Still, IHC is able to provide confident ranged information about protein expression inside the cohort and detect the location of proteins in tissue sections, which was used in this study (Abcam 2021; Hofman 2002; Kim et al. 2016).
Conclusions
Intercellular communication is more relevant in slower proliferating, e.g. lower malignant tumors, as they have more time to establish this networking. It possibly provides acquired pathogenicity and resistance against later oncological therapy. This feature depends on genetic features, directly matching the unfavorable IDH1 wildtype status of glioma, showing a potential pathway of its unfavorable outcome. Our data supports the 5th upcoming WHO classification of gliomas that specifies these tumors as WHO °IV in behavior. Anaplasia or other typical signs of malignancy like MR CE, advanced EGFR or p53 expression did not predict advanced intercellular interaction in our series. Thus, diffuse and anaplastic gliomas are not homogenic and need to be carefully evaluated regarding to intercellular communication, their genetic profile and not primarily the WHO grading or phenotype.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1. References of semi-quantitative scoring of Cx43 expression in IHC testing of gliomas: 0 = no expression at all, 1 = minimal expression, 2= moderate expression, 3 = strong expression. 400x, M8 Slide-scanner (PreciPoint, Germany) (TIFF 6708 KB)
Supplementary file 2. References of semi-quantitative scoring of GAP-43 expression in IHC testing of gliomas: 0 = no expression at all, 1 = minimal expression, 2= moderate expression, 3 = strong expression. 400x, M8 Slide-scanner (PreciPoint, Germany) (TIFF 6946 KB)
Author contributions
Conceptualization, AK and CFF; methodology, AK, PM and CFF; validation, AK, PM and HF; formal analysis, AK and PM; investigation, AK and PM; resources, PM, HF, KB, CT and CFF.; data curation, AK and MD; writing—original draft preparation, AK; writing—review and editing, HF, CFF and CT; supervision, CFF and CT; project administration, AK. All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive support from any organization for the submitted work. Only the internal resources of our institution were used.
Availability of data and material
The raw data was generated in authors’ institution. The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due their containing information that could compromise the privacy of research participants.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no direct or indirect financial or non-financial conflict of interest or competing interests.
Ethics approval
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The database and tissue bank are approved by the ethics committee of Medical University Innsbruck (AN5220 329/4.4).
Consent to participate
Written informed content was acquired from the participants.
Consent to publication
No individual data are showed separately in the manuscript. All data is used only after anonymized statistical processing and is described with pool results.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aasen T, Leithe E, Graham SV, Kameritsch P, Mayan MD, Mesnil M, Pogoda K, Tabernero A (2019) Connexins in cancer: bridging the gap to the clinic. Oncogene 38(23):4429–4451. 10.1038/s41388-019-0741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abcam (2021) Immunohistochemistry (IHC): the complete guide. https://www.abcam.com/content/immunohistochemistry-the-complete-guide. Accessed 30 May 2021
- Aftab Q, Sin WC, Naus CC (2015) Reduction in gap junction intercellular communication promotes glioma migration. Oncotarget 6(13):11447–11464. 10.18632/oncotarget.3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Yosef H (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300 [Google Scholar]
- Brat DJ, Aldape K, Colman H, Holland EC, Louis DN, Jenkins RB, Kleinschmidt-DeMasters BK, Perry A, Reifenberger G, Stupp R, von Deimling A, Weller M (2018) cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV.” Acta Neuropathol 136(5):805–810. 10.1007/s00401-018-1913-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Wang D, Du X, He Y, Chen S, Shao Q, Ma C, Huang B, Chen A, Zhao P, Qu X, Li X (2015) Glioma cells escaped from cytotoxicity of temozolomide and vincristine by communicating with human astrocytes. Med Oncol 32(3):43. 10.1007/s12032-015-0487-0 [DOI] [PubMed] [Google Scholar]
- Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, Jacob L, Patwa R, Shah H, Xu K, Cross JR, Massague J (2016) Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533(7604):493–498. 10.1038/nature18268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus EB, Walsh KM, Wiencke JK, Molinaro AM, Wiemels JL, Schildkraut JM, Bondy ML, Berger M, Jenkins R, Wrensch M (2015) Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus 38(1):E6. 10.3171/2014.10.FOCUS12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AL, Holmen SL, Colman H (2013) IDH1 and IDH2 mutations in gliomas. Curr Neurol Neurosci Rep 13(5):345. 10.1007/s11910-013-0345-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumas-Duport C, Scheithauer B, O’Fallon J, Kelly P (1988) Grading of astrocytomas. A simple and reproducible method. Cancer 62(10):2152–2165. 10.1002/1097-0142(19881115)62:10%3c2152::aid-cncr2820621015%3e3.0.co;2-t [DOI] [PubMed] [Google Scholar]
- Freyschlag CF, Krieg SM, Kerschbaumer J, Pinggera D, Forster MT, Cordier D, Rossi M, Miceli G, Roux A, Reyes A, Sarubbo S, Smits A, Sierpowska J, Robe PA, Rutten GJ, Santarius T, Matys T, Zanello M, Almairac F, Mondot L, Jakola AS, Zetterling M, Rofes A, von Campe G, Guillevin R, Bagatto D, Lubrano V, Rapp M, Goodden J, De Witt Hamer PC, Pallud J, Bello L, Thome C, Duffau H, Mandonnet E (2018) Imaging practice in low-grade gliomas among European specialized centers and proposal for a minimum core of imaging. J Neurooncol 139(3):699–711. 10.1007/s11060-018-2916-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Fromaget C, el Aoumari A, Cordier J, Glowinski J, Gros D (1991) Gap junctions in cultured astrocytes: single-channel currents and characterization of channel-forming protein. Neuron 6(1):133–143. 10.1016/0896-6273(91)90128-m [DOI] [PubMed] [Google Scholar]
- Grek CL, Sheng Z, Naus CC, Sin WC, Gourdie RG, Ghatnekar GG (2018) Novel approach to temozolomide resistance in malignant glioma: connexin43-directed therapeutics. Curr Opin Pharmacol 41:79–88. 10.1016/j.coph.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag D, Zipper P, Westrich V, Karra D, Pfleger K, Toedt G, Blond F, Delhomme N, Hahn M, Reifenberger J, Reifenberger G, Lichter P (2012) Nos2 inactivation promotes the development of medulloblastoma in Ptch1(+/−) mice by deregulation of Gap43-dependent granule cell precursor migration. PLoS Genet 8(3):e1002572. 10.1371/journal.pgen.1002572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T, Reifenberger G, Weller M, Loeffler M, von Deimling A (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120(6):707–718. 10.1007/s00401-010-0781-z [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y (1990) More powerful procedures for multiple significance testing. Stat Med 9(7):811–818. 10.1002/sim.4780090710 [DOI] [PubMed] [Google Scholar]
- Hofman F (2002) Immunohistochemistry. Curr Protoc Immunol. 10.1002/0471142735.im2104s49 [DOI] [PubMed] [Google Scholar]
- Kim SW, Roh J, Park CS (2016) Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J Pathol Transl Med 50(6):411–418. 10.4132/jptm.2016.08.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeper HE, Caron AA, Decker PA, Jenkins RB, Lachance DH, Giannini C (2015) IDH mutation, 1p19q codeletion and ATRX loss in WHO grade II gliomas. Oncotarget 6(30):30295–30305. 10.18632/oncotarget.4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Liu Z, Ling F, Xu G (2016) Astrocytes protect glioma cells from chemotherapy and upregulate survival genes via gap junctional communication. Mol Med Rep 13(2):1329–1335. 10.3892/mmr.2015.4680 [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler O, Cavenee WK (2016a) WHO Classification of tumours of the central nervous system, revised 4th edition, vol 1. International Agency for Research on Cancer [Google Scholar]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016b) The 2016 World Health Organization Classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- Marins M, Xavier AL, Viana NB, Fortes FS, Froes MM, Menezes JR (2009) Gap junctions are involved in cell migration in the early postnatal subventricular zone. Dev Neurobiol 69(11):715–730. 10.1002/dneu.20737 [DOI] [PubMed] [Google Scholar]
- McDonough WS, Johansson A, Joffee H, Giese A, Berens ME (1999) Gap junction intercellular communication in gliomas is inversely related to cell motility. Int J Dev Neurosci 17(5–6):601–611. 10.1016/s0736-5748(99)00024-6 [DOI] [PubMed] [Google Scholar]
- Nandakumar P, Mansouri A, Das S (2017) The role of ATRX in glioma biology. Front Oncol 7:236. 10.3389/fonc.2017.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M, Huang L, Ratliff M, Karimian Jazi K, Kurz FT, Schmenger T, Lemke D, Gommel M, Pauli M, Liao Y, Haring P, Pusch S, Herl V, Steinhauser C, Krunic D, Jarahian M, Miletic H, Berghoff AS, Griesbeck O, Kalamakis G, Garaschuk O, Preusser M, Weiss S, Liu H, Heiland S, Platten M, Huber PE, Kuner T, von Deimling A, Wick W, Winkler F (2015) Brain tumour cells interconnect to a functional and resistant network. Nature 528(7580):93–98. 10.1038/nature16071 [DOI] [PubMed] [Google Scholar]
- Osswald M, Solecki G, Wick W, Winkler F (2016) A malignant cellular network in gliomas: potential clinical implications. Neuro Oncol 18(4):479–485. 10.1093/neuonc/now014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picca A, Berzero G, Sanson M (2018) Current therapeutic approaches to diffuse grade II and III gliomas. Ther Adv Neurol Disord 11:1756285617752039. 10.1177/1756285617752039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff AL, Heiland DH, Evert BO, Almeida FR, Behringer SP, Dolf A, Guresir A, Guresir E, Joseph K, Pietsch T, Schuss P, Herrlinger U, Westhoff MA, Vatter H, Waha A, Schneider M (2019) Inhibition of gap junctions sensitizes primary glioblastoma cells for temozolomide. Cancers (basel). 10.3390/cancers11060858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu P, Xia Z, Yu S, Huang Q (2004) Altered expression of Cx43 in astrocytic tumors. Clin Neurol Neurosurg 107(1):49–54. 10.1016/j.clineuro.2004.03.006 [DOI] [PubMed] [Google Scholar]
- Rushing EJ (2021) WHO classification of tumors of the nervous system: preview of the upcoming 5th edition. Mag Eur Med Oncol. 10.1007/s12254-021-00680-x [Google Scholar]
- Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JY (2009) Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 27(25):4150–4154. 10.1200/JCO.2009.21.9832 [DOI] [PubMed] [Google Scholar]
- Sin WC, Crespin S, Mesnil M (2012) Opposing roles of connexin43 in glioma progression. Biochim Biophys Acta 1818(8):2058–2067. 10.1016/j.bbamem.2011.10.022 [DOI] [PubMed] [Google Scholar]
- Sin WC, Aftab Q, Bechberger JF, Leung JH, Chen H, Naus CC (2016) Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene 35(12):1504–1516. 10.1038/onc.2015.210 [DOI] [PubMed] [Google Scholar]
- Sinyuk M, Mulkearns-Hubert EE, Reizes O, Lathia J (2018) Cancer connectors: connexins, gap junctions, and communication. Front Oncol 8:646. 10.3389/fonc.2018.00646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H (2015) Brain cancer: tumour cells on neighbourhood watch. Nature 528(7580):49–50. 10.1038/nature15649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil S, Osswald M, Solecki G, Grosch J, Jung E, Lemke D, Ratliff M, Hanggi D, Wick W, Winkler F (2017) Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro Oncol 19(10):1316–1326. 10.1093/neuonc/nox070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Ruda R, Short S, Smits M, Taphoorn MJB, von Deimling A, Westphal M, Soffietti R, Reifenberger G, Wick W (2020) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 10.1038/s41571-020-00447-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiestler B, Capper D, Holland-Letz T, Korshunov A, von Deimling A, Pfister SM, Platten M, Weller M, Wick W (2013) ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol 126(3):443–451. 10.1007/s00401-013-1156-z [DOI] [PubMed] [Google Scholar]
- Zhao Y, Xin Y, He Z, Hu W (2018) Function of connexins in the interaction between glial and vascular cells in the central nervous system and related neurological diseases. Neural Plast 2018:6323901. 10.1155/2018/6323901 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1. References of semi-quantitative scoring of Cx43 expression in IHC testing of gliomas: 0 = no expression at all, 1 = minimal expression, 2= moderate expression, 3 = strong expression. 400x, M8 Slide-scanner (PreciPoint, Germany) (TIFF 6708 KB)
Supplementary file 2. References of semi-quantitative scoring of GAP-43 expression in IHC testing of gliomas: 0 = no expression at all, 1 = minimal expression, 2= moderate expression, 3 = strong expression. 400x, M8 Slide-scanner (PreciPoint, Germany) (TIFF 6946 KB)
Data Availability Statement
The raw data was generated in authors’ institution. The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due their containing information that could compromise the privacy of research participants.
Not applicable.



