Abstract
A 23-year-old man presented with severe hypertension. Based on his history of minocycline treatment for over three years and clinical symptoms, such as myalgias and renovascular hypertension with multiple intrarenal aneurysms, he was diagnosed with minocycline-induced renal polyarteritis nodosa (PAN). After minocycline treatment cessation and management of the hypertension, his blood pressure, renin-aldosterone levels, and urinary protein levels gradually improved. Seven and a half years later, repeated angiography found that the aneurysms had resolved. This is the first report in English describing a case of minocycline-induced renal PAN that was reversed functionally and morphologically without steroids or immunosuppressive drugs.
Keywords: aneurysms, angiography, drug-induced vasculitis, minocycline, polyarteritis nodosa, renovascular hypertension
Introduction
Polyarteritis nodosa (PAN) is a rare, systemic, necrotizing vasculitis that predominantly affects medium- and small-sized arteries (1). Inflammation of the arteries causes tissue ischemia or hemorrhaging in various organs, such as the kidneys, skin, nerves, and gastrointestinal tract (2-4). Renal involvement, which is present in 8-66% of PAN cases (5), is characterized by stenosis or occlusion from the renal arcuate to the interlobular arteries and formation of multiple, intrarenal aneurysms. These lesions lead to clinical manifestations, such as renovascular hypertension (RVH), hematuria, renal infarction, and hematoma (5).
Although most PAN cases are idiopathic, there are reports of secondary PAN associated with viral infections, malignancies, and medications (6). Minocycline, a semisynthetic tetracycline derivative that is widely used for acne vulgaris, has been associated with the development of secondary PAN (6-14). However, due to its relatively low incidence and prevalence, the clinical course and treatment of minocycline-induced PAN are not fully understood.
We herein report a 23-year-old man with angiographically proven renal PAN associated with minocycline use. Unlike in cases of idiopathic PAN, minocycline cessation and antihypertensive drug administration alone resulted in RVH remission and the disappearance of multiple aneurysms on bilateral renal arteriography. We also performed a literature review of minocycline-induced PAN and summarized the characteristics of this disease. These findings reveal unique traits in the clinical course of minocycline-induced PAN and provide new insight into its treatment and pathogenesis.
Case Report
A 23-year-old man was diagnosed with severe hypertension and ocular fundus hemorrhaging at a medical checkup and admitted to our hospital for a further examination and management. He also had symmetric polyarthralgia in the ankles and knees, myalgias in the upper arms, forearms, thighs, and lower legs, malaise, a low-grade fever, and eyesight deterioration for the past roughly one year. His medical history included acne vulgaris that had been treated with minocycline (100 mg/day) for more than 3 years. Before minocycline treatment, he had had no history of diseases, such as renal disease or hypertension. Regarding his family history, his paternal grandfather and grandmother had angina pectoris and rheumatoid arthritis, respectively, and his maternal aunt had hypertension. His maternal uncle had died at 36 years old from pontine hemorrhaging. He had no history of smoking, and he consumed alcohol socially.
On admission, his height and weight were 173.5 cm and 61.0 kg, respectively. His blood pressure, pulse, and body temperature were 230/130 mmHg, 94 beats per minute, and 36.9 °C, respectively. A physical examination revealed acne vulgaris on his cheeks; however, no skin purpura or livedo reticularis were present on the extremities. Neither significant lymphadenopathy nor arthritis was observed. Manual muscle testing (MMT) revealed almost 5/5 in all muscle groups. There was mild tenderness in the lower legs. No abdominal vascular murmurs were heard.
The laboratory data on admission (Table 1) revealed hypokalemia, a mildly impaired renal function, an increased plasma active renin concentration (ARC), and increased plasma and urine aldosterone levels. A urinalysis revealed a urine protein excretion of 3.95 g/day; however, the urine was negative for both occult blood and casts. The antinuclear antibody (ANA) test was positive (×320, speckled), and the erythrocyte sedimentation rate was mildly elevated. In contrast, the white blood cell (WBC) count and C-reactive protein (CRP), C3, C4, and D-dimer levels were within their normal ranges, and the double-stranded DNA antibody test was negative. Both the myeloperoxidase- and proteinase-3-antineutrophil cytoplasmic antibody tests were negative. Furthermore, both the hepatitis B surface antigen and anti-hepatitis C virus antibody tests were negative. Anti-hepatitis B surface antibody was measured 5 years after the first hospitalization and was negative.
Table 1.
The Patient’s Laboratory Findings at the First Admission.
| Urinalysis | Blood chemistry | Serology | Blood Hormone | |||||||||||||||
| pH | 7.0 | TP | 7.2 | g/dL | ESR | 22 | mm | ACTH | 44 | pg/mL | ||||||||
| protein | 3+ | Alb | 3.8 | g/dL | CRP | 0.06 | mg/dL | Cortisol | 16.8 | μg/dL | ||||||||
| glucose | negative | AST | 27 | U/L | IgG | 1,694 | mg/dL | ARC | 96.7 | pg/mL | ||||||||
| blood | negative | ALT | 17 | U/L | IgA | 209 | mg/dL | Aldosterone | 407 | pg/mL | ||||||||
| CAST | negative | LDH | 271 | U/L | IgM | 57 | mg/dL | Adrenaline | <0.01 | ng/mL | ||||||||
| UN | 19.2 | mg/dL | C3 | 112 | mg/dL | Noradrenaline | 0.19 | ng/mL | ||||||||||
| Hematology and coagulation | Cr | 1.1 | mg/dL | C4 | 25 | mg/dL | Dopamine | <0.02 | ng/mL | |||||||||
| WBC | 5,900 | /μL | eGFR | 70.2 | mL/min/1.73m2 | CH-50 | 58.9 | U/mL | ||||||||||
| Neutrophil | 60 | % | UA | 5.6 | mg/dL | IC | 2.0 | μg/mL | 24-hour urine analysis (Volume: 2500 mL) | |||||||||
| Lymphocyte | 29 | % | Na | 139.3 | mEq/L | ANA | ×320 | speckled | Cr | 1.71 | g/day | |||||||
| Eosinophil | 6 | % | K | 2.9 | mEq/L | Anti-dsDNA Ab | 9 | IU/mL | Protein | 3.95 | g/day | |||||||
| Monocyte | 5 | % | Cl | 100 | mEq/L | PR3-ANCA | <10 | EU | Aldosterone | 33.3 | μg/day | |||||||
| RBC | 467×104 | /μL | TC | 189 | mg/dL | MPO-ANCA | <10 | EU | Free cortisol | 89.1 | μg/day | |||||||
| Hb | 13.1 | g/dL | TG | 253 | mg/dL | Anti-GBM Ab | <10 | EU | Adrenaline | 12.2 | μg/day | |||||||
| Ht | 38.2 | % | BS | 109 | mg/dL | RF | <10 | IU/mL | Noradrenaline | 590 | μg/day | |||||||
| Plt | 20.6×104 | /μL | CK | 122 | U/L | HBs Ag | negative | Dopamine | 240 | μg/day | ||||||||
| D-dimer | 0.6 | μg/mL | HCV Ab | negative | Metanephrine | 0.14 | mg/day | |||||||||||
| Lupus anticoagulant | negative | RPR | negative | Normetanephrine | 0.28 | mg/day | ||||||||||||
| Cryoglobulin | negative | |||||||||||||||||
Alb: albumin, ACTH: adrenocorticotropic hormone, ALT: alanine aminotransferase, ANA: anti-nuclear antibody, anti-dsDNA Ab: anti-double stranded DNA antibody, anti-GBM Ab: anti-glomerular basement membrane antibody, ARC: active renin concentration, AST: aspartate aminotransferase, BS: blood sugar, Cl: serum chloride, CK: creatine kinase, Cr: creatinine, CRP: C-reactive protein, eGFR: estimated glomerular filtration rate, ESR: erythrocyte sedimentation rate, Hb: hemoglobin, HBs Ag: hepatitis B surface antigen, HCV Ab: anti-hepatitis C virus antibody, Ht: hematocrit, IC: immune complex, IgA: immunoglobulin A, IgG: immunoglobulin G, IgM: immunoglobulin M, K: serum potassium, LDH: lactate dehydrogenase, MPO-ANCA: myeloperoxidase-antineutrophil cytoplasmic antibody, Na: serum sodium, Plt: platelets, PR3-ANCA: proteinase-3-antineutrophil cytoplasmic antibody, RBC: red blood cells, RF: rheumatoid factor, RPR: rapid plasma regain, TC: total cholesterol, TG: triglyceride, TP: total protein, UA: serum uric acid, UN: urine nitrogen, WBC: white blood cells
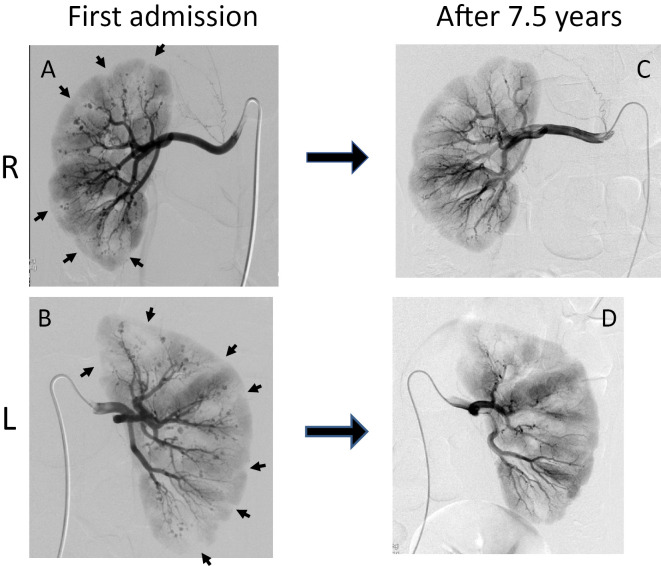
Although an electrocardiogram showed left ventricular hypertrophy according to the Cornell voltage-duration criteria, the echocardiography findings were almost normal. Contrast-enhanced computed tomography showed multiple wedge-shaped areas of decreased enhancement in both kidneys, suggesting multiple renal infarctions (Fig. 1). Although a renal echo Doppler study showed a normal bilateral renal blood flow with a peak systolic velocity of 73.8 cm/s on the right and 114.2 cm/s on the left, captopril renography revealed right-dominant, bilaterally delayed excretion rates (Fig. 2). The captopril challenge test demonstrated excessive renin secretion after captopril loading (Table 2), suggesting RVH (15). Bilateral renal angiography revealed multiple intrarenal aneurysms as well as multiple areas of decreased subcapsular enhancement that were suggestive of infarctions (Fig. 3A, B). Regarding the renal vein renin measurements, both renal vein ARCs were high, and the renal vein renin ratio was less than 1.5 (Table 3). When considered concurrently, these results led to a diagnosis of bilateral RVH.
Figure 1.

Contrast-enhanced computed tomography showed multiple wedge-shaped areas of decreased enhancement in both kidneys.
Figure 2.
Captopril renography showed right-dominant, bilaterally delayed excretion rates. The curve of the right kidney was transiently elevated between 15 and 20 minutes due to measurement of the transient accumulation of RI from the renal pelvis to the proximal tubule. Taking this into account, the T1/2 was estimated to be 16 minutes. L: left, R: right.
Table 2.
Captopril Challenge Test Showing Excessive Renin Secretion after Captopril Loading.
| 0 min | 60 min | 90 min | ||||
|---|---|---|---|---|---|---|
| ARC (pg/mL) | 47.6 | 255 | 254 | |||
| Aldosterone (pg/mL) | 373 | 149 | 119 |
ARC: active renin concentration
Figure 3.
On initial admission, bilateral renal angiography revealed multiple, intrarenal aneurysms as well as multiple subcapsular areas of decreased enhancements that were suggestive of infarctions (A, B). After seven and a half years, the multiple, intrarenal aneurysms had almost completely resolved (C, D). L: left kidney, R: right kidney. arrow: multiple subcapsular areas of decreased enhancements.
Table 3.
The Active Renin Concentrations (ARCs: pg/mL) According to the Renal Vein Renin Measurements.
| First admission | After 7.5 years | |||
|---|---|---|---|---|
| R-RV | 105 | 18.3 | ||
| L-RV | 117 | 18.6 | ||
| Upper IVC | 71.7 | 13.2 | ||
| Lower IVC | 78.9 | 13.8 |
On initial admission, both renal vein ARCs were high, and the renal vein renin ratio was <1.5. After seven and a half years, there was significant improvement in the renal vein ARCs bilaterally. IVC: inferior vena cava, L-RV: left renal vein, R-RV: right renal vein
Our patient's clinical manifestations met the American College of Rheumatology 1990 criteria for the classification of PAN (at least 3 out of the 10 parameters): (i) the development of hypertension, (ii) myalgia, and (iii) an arteriogram demonstrating visceral artery aneurysms or occlusions (16). In particular, his multiple, distinctive, bilateral, intrarenal aneurysms were consistent with findings related to PAN. In the setting of minocycline use, these findings strongly suggested minocycline-induced renal PAN.
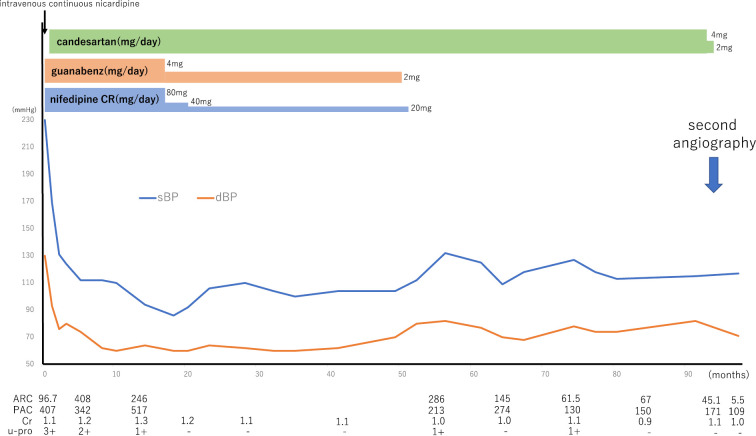
Fig. 4 shows the patient’s major clinical course thereafter. Initially, minocycline was discontinued, and intravenous continuous nicardipine administration was started at 1 μg/kg/min, being titrated up to 5 μg/kg/min. When the systolic blood pressure improved to 180 mmHg, he was switched to oral antihypertensive agents with nifedipine controlled release (80 mg/day) and guanabenz (4 mg/day). He was administered this regimen for 1.5 months, but due to the uncontrolled hypertension, candesartan was added. Thereafter, his blood pressure markedly improved. At that time, his renal function transiently worsened; however, it later recovered, and his proteinuria gradually resolved (Fig. 4). His symptoms, such as symmetric polyarthralgia, malaise, myalgia, and a low-grade fever, also progressively improved over time.
Figure 4.
Clinical course. ARC: active renin concentration (pg/mL), Cr: creatinine (mg/dL), dBP: diastolic blood pressure, nifedipine CR: nifedipine controlled-release, PAC: plasma aldosterone concentration (pg/mL), sBP: systolic blood pressure, u-pro: urinary protein
From five years after the treatment, the patient’s plasma renin and aldosterone levels and blood pressure slowly began to decrease. This led to a second evaluation of the renal lesions. Seven and a half years after the first angiography session, the patient underwent repeat angiography when the ARC and plasma aldosterone levels had improved to 45.1 pg/mL and 171 pg/mL, respectively, while he was being treated with 2 mg/day of candesartan. On repeat angiography, the multiple, bilateral, intrarenal aneurysms had almost disappeared (Fig. 3C, D). Furthermore, the renal vein renin measurements demonstrated a significant improvement in the bilateral renal vein ARC compared to previous measurements (Table 3). Based on these results, the decision was made to discontinue candesartan. The ARC and plasma aldosterone levels decreased to 5.5 and 109 pg/mL, respectively, with a blood pressure of 117/71 mmHg.
Discussion
Minocycline is a semisynthetic tetracycline derivative that is used to manage various infectious diseases. For acne vulgaris cases in young people, it is often prescribed for months to years. However, in recent years, studies have found that long-term minocycline use was associated with the development of autoimmune disorders, including autoimmune hepatitis, drug-induced lupus, and vasculitis (17). The pathogenesis of minocycline-induced PAN remains unclear. Schaffer et al. have suggested that processing of minocycline by neutrophils or hepatocytes may produce reactive metabolites that stimulate the immune response (18). Recently, Kawahara et al. reported the presence of human leukocyte antigen (HLA)-DRB1*09: 01 allele in cases of minocycline-induced vasculitis (19), indicating that the genetic background is also involved in the pathogenesis.
Although there have been several literature reviews of minocycline-induced polyarteritis nodosa (14,20,21), we performed a newer and larger web-based literature review with PubMed using the key terms “minocycline” and “polyarteritis nodosa” or “vasculitis.” Twenty-six peer-reviewed case reports with a total of 41 individual cases were identified in the English or Japanese-language literature from 1996 to 2020 (7-14,18,20,22-35), and the present case was added to the database, bringing the total to 42 individual cases. The following data were collected from the reports: the patient age and sex, target diseases for minocycline, duration of minocycline use, dosage of minocycline, manifestation type, presence or absence of ANA, perinuclear-anti-neutrophil cytoplasmic antibody (p-ANCA) and cytoplasmic-ANCA (c-ANCA), diagnosis, treatment, and outcome. A summary of the reported cases is presented in Table 4. Twenty-nine (69.0%) cases were women. In contrast to idiopathic PAN, which has a peak incidence in patients 50-60 years old (4,36-38), minocycline-induced PAN occurred in younger patients (average age, 30.0 years old). These findings may be related to the fact that the target diseases for minocycline use in most patients are acne vulgaris. It typically occurred after a substantial period of minocycline use ranged from a minimum of 2 weeks to a maximum of 68 months, with a mean of 25.9 months. The dosage of minocycline was 100 or 200 mg/day.
Table 4.
Clinical Manifestations in Patients with Minocycline-induced PAN.
| Age/ Sex |
Disease | Duration of MINO (mo) | Dosage of MINO (mg/day) | Type | ANA | p-ANCA | specificity for MPO | c-ANCA (PR3) | Diagnosis | Treatment | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19/F | Acne vulgaris | 48 | 100 | Skin | - | + | + | - | Skin Bx | Only cessation of MINO | R | 26 |
| 20/F | Acne vulgaris | 36 | N/A | Skin | + | + | + | - | ACR | PDN | R | |
| 22/F | Acne vulgaris | 48 | N/A | Systemic | + | + | + | - | ACR | Only cessation of MINO | R | |
| 15/F | Acne vulgaris | 9 | 100–200 | Skin | - | - | / | N/A | Skin Bx | PDN | R | 12 |
| 35/F | Acne vulgaris | 24 | 100–200 | Skin | + | - | / | - | Skin Bx | Only cessation of MINO | R | 33 |
| 20/F | Acne vulgaris | 44 | 100–200 | Skin | + | + | N/A | - | Skin Bx | PDN | R | 18 |
| 19/F | Acne vulgaris | 65 | 100–200 | Skin | + | + | + | - | Skin Bx | PDN | R | |
| 27/F | Eosinophilic pustular folliculitis | 68 | N/A | Skin | + | ± | N/A | ± | Skin Bx | Only cessation of MINO | R | 32 |
| 31/M | Chronic pyoderma | 24 | N/A | Skin | + | ± | ± | - | Skin Bx | Only cessation of MINO | R | |
| 23/F | Acne vulgaris | 24 | N/A | Skin | + | + | + | - | Skin Bx | PDN | R | 31 |
| 19/F | Acne vulgaris | 15 | 200 | Skin | + | + | - | - | Skin Bx | PDN | R | 7 |
| 47/M | Palmoplantar pustulosis | 36 | 100 | Skin | + | - | / | - | ACR | Only cessation of MINO | R | 9 |
| 40/F | Acne vulgaris | 6 | 100 | Skin | N/A | + | - | - | Skin Bx | Only cessation of MINO | R | 22 |
| 18/F | Acne vulgaris | >12 | 100 | Skin | + | N/A | N/A | N/A | Skin Bx | Only cessation of MINO | R | 14 |
| 30/F | Acne vulgaris | 24 | 100 | Skin | - | + | - | - | Skin Bx | Only cessation of MINO | R | 27 |
| 70/F | Infection after surgery | 8 | 100 | Nerve | + | - | / | - | Nerve Bx | PSL | R | 30 |
| 28/F | Acne vulgaris | 0.5 | N/A | Nerve | + | - | / | - | Nerve Bx | mPSL | R | 35 |
| 21/F | Acne vulgaris | 36 | N/A | Kidney | + | + | ± | - | Renal Angiogram | PDN, CPA, ACEI | R | 13 |
| 18/F | N/A | 24 | N/A | Skin | - | + | - | - | Skin Bx | PDN, SSZ | R | 20 |
| 38/F | N/A | 18 | N/A | Skin | + | + | + | - | Skin Bx | Only cessation of MINO | R | |
| 22/F | N/A | N/A | N/A | Skin | - | + | + | - | Skin Bx | Only cessation of MINO | R | |
| 30/F | N/A | 12 | N/A | Skin | - | + | - | - | Skin Bx | PDN, dapsone | R | |
| 40/F | N/A | N/A | N/A | Nerve | + | + | - | - | Nerve Bx | PDN, CPA, AZA | R | |
| 36/F | N/A | 18 | N/A | Nerve | N/A | + | N/A | - | Pathology of gall bladder | Only cessation of MINO | R | |
| 55/M | N/A | 48 | N/A | Systemic | - | + | - | PR3+ | Mesenteric angiogram | PDN | R | |
| 19/M | N/A | 30 | N/A | Kidney | - | + | - | - | Sinus Bx, renal angiogram | PDN, CPA | R | |
| 23/M | N/A | 24 | N/A | Kidney | - | + | ± | - | Renal angiogram | PDN, MMF | R | |
| 53/F | Palmoplantar pustulosis | 5 | 100 | Skin | + | - | / | - | Skin Bx | Only cessation of MINO | R | 21 |
| 19/M | Acne vulgaris | 36 | 200 | Systemic | + | + | + | - | ACR | Only cessation of MINO | R | 23 |
| 26/F | Acne vulgaris | >24 | N/A | Skin, nerve | ± | + | - | - | Skin Bx | Only cessation of MINO | R | 10 |
| 18/F | Acne vulgaris | 25 | N/A | Skin | + | N/A | N/A | N/A | Skin Bx | PDN | R | 11 |
| 27/M | Acne vulgaris | 12 | N/A | Nerve | - | + | + | - | Muscle Bx, vertebral angiogram | mPSL, PSL, CPA, AZA | R | 28 |
| 17/F | Acne vulgaris | 36 | N/A | Nerve | + | N/A | N/A | N/A | Nerve Bx | mPSL, AZA | R | 24 |
| 33/M | Acne vulgaris | 24 | N/A | Nerve | + | N/A | N/A | N/A | Muscle Bx | PSL, CPA | R | |
| 40s/F | Rosacea | >12 | N/A | Skin | N/A | + | - | - | Skin Bx | PDN | R | 25 |
| 17/M | Acne vulgaris | 18 | N/A | Nerve | + | - | / | - | Nerve Bx | mPSL, PDN, MTX | R | 29 |
| 47/F | Acne vulgaris | 3 | N/A | Nerve | - | - | / | - | Nerve Bx, muscle Bx | PDN | R | 8 |
| 37/M | Acne vulgaris | N/A | N/A | Nerve | + | - | / | - | Nerve Bx, muscle Bx | PSL, CPA | R | |
| 17/M | Acne vulgaris | 30 | 200 | Nerve | N/A | + | - | - | ACR | PDN | R | 34 |
| 47/M | Palmoplantar pustulosis | 24 | 100 | Skin | - | + | + | - | Skin Bx | PSL | R | 19 |
| 53/F | Palmoplantar pustulosis | 24 | N/A | Skin | + | + | + | - | - | Only cessation of MINO | R | |
| 23/M | Acne vulgaris | >36 | 100 | Kidney | + | - | - | - | Renal angiogram | Only cessation of MINO | R | The present case |
ACEI: angiotensin converting enzyme inhibitor, ACR: American College of Rheumatology 1990 criteria, ANA: antinuclear antibody, AZA: azathioprine, BX: biopsy, c-ANCA: cytoplasmic-antineutrophil cytoplasmic antibody, CPA: cyclophosphamide, F: female, M: male, mPSL: methylprednisolone, MINO: minocycline, MMF: mycophenolate mofetil, MPO: myeloperoxidase, MTX: methotrexate, N/A: not available, p-ANCA: perinuclear-antineutrophil cytoplasmic antibody, PDN: prednisone, PR3: proteinase 3, PSL: prednisolone, R: remission, Ref.: reference, SSZ: sulfasalazine
The most commonly affected sites in cases of minocycline-induced PAN were the skin (24 cases: 57.1%) and nerves (12 cases: 28.6%), with kidney involvement, such as that seen in our case, being relatively rare (4 cases: 9.5%). The 4 patients with renal PAN were relatively young (average age, 21.5 years old). Among those four cases, two were ANA-positive, and three were p-ANCA-positive, with some overlap. The previously reported three cases were treated with steroids or immunosuppressive drugs to achieve remission, while our patient is the first case wherein minocycline cessation and antihypertensive drug administration alone resulted in remission.
Overall, antinuclear antibodies were equivocal or positive in 26 cases (68.4%). In the 2012 International Chapel Hill Consensus Conference, PAN was defined as arteritis that is not associated with ANCAs (1), but our analysis identified 28 cases (73.7%) with the presence of p-ANCA serologies among minocycline-induced PAN patients. In contrast, c-ANCA was negative in most cases. Interestingly, 11 cases were positive for p-ANCA and negative for an enzyme-linked immunosorbent assay for myeloperoxidase, suggesting the existence of other antigens that are not measured clinically.
Twenty-five patients (59.5%) were treated with steroids or immunosuppressive drugs, such as cyclophosphamide and azathioprine, while 17 patients (40.5%) had only discontinuation of minocycline, and all patients eventually went into remission.
In RVH, the intraglomerular pressure of the affected kidney is maintained through efferent arteriole contraction, which is mediated in part by angiotensin II. Therefore, in cases with bilateral RVH, angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors (ACE-Is) are either contraindicated or must be administered with caution. In the present case, since the hypertension and urinary protein levels were not controlled, candesartan was initiated carefully. As a result, candesartan effectively lowered the blood pressure and reduced urinary protein excretion without causing severe renal dysfunction (Fig. 4).
We speculated that the pathophysiology of RVH in our case of minocycline-induced PAN might be different from that of RVH due to atherosclerosis or fibromuscular dysplasia (FMD). Unlike RVH due to atherosclerosis or FMD, wherein the renal artery main stem is narrowed, PAN only affects some-not all-intrarenal arteries (Fig. 1, 3). Therefore, although ARBs or ACE-Is may further exacerbate kidney ischemia in lesions that are already partially ischemic, they can also reduce the blood pressure and act in a renoprotective manner in unaffected areas, resulting in reduced urinary protein excretion.
In general, steroids and immunosuppressive agents, such as cyclophosphamide, methotrexate, and azathioprine, are used to manage patients with idiopathic PAN. Kermani et al. reported that, in cases of minocycline-induced PAN, 67% of the patients required treatment with immunosuppressive agents in addition to minocycline discontinuation (20). In the present case, steroid treatment was initially considered due to concerns about aneurysm rupture. However, the levels of inflammatory markers, such as CRP and erythrocyte sedimentation rate (ESR), were not considerably elevated. In addition, in cases of minocycline-induced cutaneous PAN, several studies found that minocycline cessation alone was enough to induce remission (Table 4). Furthermore, there was some concern that steroid treatment might exacerbate hypertension, which in turn might aggravate the fundus lesions. We therefore treated the patient through minocycline discontinuation and antihypertensive agent administration. As a result, we were able to achieve disease remission.
In summary, among case reports written in English, this is the first to describe a case of minocycline-induced renal PAN wherein minocycline cessation and antihypertensive drug administration alone resulted in functional and morphological remission. In cases of renal PAN, antihypertensive agents, especially ARBs, might be effective without impairing the renal function. Our literature review revealed the general characteristics of minocycline-induced PAN. Young women are more likely to be affected than the general population. This disease may be related to the long-term administration of minocycline. The cutaneous and neurological types are common, while the renal type is rare. ANA and p-ANCA are often positive. Steroids or immunosuppressive drugs are effective, but in some cases, remission is achieved through the discontinuation of minocycline alone. Through these treatments, all patients went into remission. Our literature review is limited due to selection bias and the small sample size. The roles of ANA and p-ANCA in pathophysiology and indicators of the need for steroids or immunosuppressive agents remain unclear and require further investigation.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65: 1-11, 2013. [DOI] [PubMed] [Google Scholar]
- 2. Lhote F, Cohen P, Guillevin L. Polyarteritis nodosa, microscopic polyangiitis and Churg-Strauss syndrome. Lupus 7: 238-258, 1998. [DOI] [PubMed] [Google Scholar]
- 3. Pagnoux C, Seror R, Henegar C, et al. Clinical features and outcomes in 348 patients with polyarteritis nodosa: a systematic retrospective study of patients diagnosed between 1963 and 2005 and entered into the French Vasculitis Study Group Database. Arthritis Rheum 62: 616-626, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Selga D, Mohammad A, Sturfelt G, Segelmark M. Polyarteritis nodosa when applying the Chapel Hill nomenclature--a descriptive study on ten patients. Rheumatology (Oxford) 45: 1276-1281, 2006. [DOI] [PubMed] [Google Scholar]
- 5. Hernández-Rodríguez J, Alba MA, Prieto-González S, Cid MC. Diagnosis and classification of polyarteritis nodosa. J Autoimmun 48-49: 84-89, 2014. [DOI] [PubMed] [Google Scholar]
- 6. Karadag O, Jayne DJ. Polyarteritis nodosa revisited: a review of historical approaches, subphenotypes and a research agenda. Clin Exp Rheumatol 36 (Suppl 111): 135-142, 2018. [PubMed] [Google Scholar]
- 7. Culver B, Itkin A, Pischel K. Case report and review of minocycline-induced cutaneous polyarteritis nodosa. Arthritis Rheum 53: 468-470, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Kang MK, Gupta RK, Srinivasan J. Peripheral vasculitic neuropathy associated with minocycline use. J Clin Neuromuscul Dis 19: 138-141, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Katada Y, Harada Y, Azuma N, et al. Minocycline-induced vasculitis fulfilling the criteria of polyarteritis nodosa. Mod Rheumatol 16: 256-259, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Klaas JP, Matzke T, Makol A, Fulgham JR. Minocycline-induced polyarteritis nodosa-like vasculitis presenting as brainstem stroke. J Clin Neurosci 22: 904-907, 2015. [DOI] [PubMed] [Google Scholar]
- 11. Odhav A, Odhav C, Dayal NA. Rare adverse effect of treatment with minocycline. Minocycline-induced cutaneous polyarteritis nodosa. JAMA Pediatr 168: 287-288, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Schrodt BJ, Callen JP. Polyarteritis nodosa attributable to minocycline treatment for acne vulgaris. Pediatrics 103: 503-504, 1999. [DOI] [PubMed] [Google Scholar]
- 13. Tabriziani H, Wilcox CS, Gilbert ON, Lipkowitz MS. Minocycline-induced renal polyarteritis nodosa. BMJ Case Rep 2012: bcr2012006503, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tehrani R, Nash-Goelitz A, Adams E, Dahiya M, Eilers D. Minocycline-induced cutaneous polyarteritis nodosa. J Clin Rheumatol 13: 146-149, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Muller FB, Sealey JE, Case DB, et al. The captopril test for identifying renovascular disease in hypertensive patients. Am J Med 80: 633-644, 1986. [DOI] [PubMed] [Google Scholar]
- 16. Lightfoot RW Jr, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum 33: 1088-1093, 1990. [DOI] [PubMed] [Google Scholar]
- 17. Elkayam O, Yaron M, Caspi D. Minocycline-induced autoimmune syndromes: an overview. Semin Arthritis Rheum 28: 392-397, 1999. [DOI] [PubMed] [Google Scholar]
- 18. Schaffer JV, Davidson DM, McNiff JM, Bolognia JL. Perinuclear antineutrophilic cytoplasmic antibody-positive cutaneous polyarteritis nodosa associated with minocycline therapy for acne vulgaris. J Am Acad Dermatol 44: 198-206, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Kawahara H, Nakashima A, Zoshima T, Kawano M. Contribution of HLA-DRB1*09: 01 allele to development of minocycline induced antineutrophil cytoplasmic antibody (ANCA)-associated cutaneous vasculitis: report of two cases. Mod Rheumatol Case Rep 4: 267-271, 2020. [DOI] [PubMed] [Google Scholar]
- 20. Kermani TA, Ham EK, Camilleri MJ, Warrington KJ. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum 42: 213-221, 2012. [DOI] [PubMed] [Google Scholar]
- 21. Nakamura J, Sugawara H, Ishii A, et al. Case report; a case of minocycline-induced polyarteritis nodosa with fever, arthralgia, and erythema on bilateral lower extremities. Nihon Naika Gakkai Zasshi 102: 2053-2056, 2013(in Japanese). [DOI] [PubMed] [Google Scholar]
- 22. Abad S, Kambouchner M, Nejjari M, Dhote R. Additional case of minocycline-induced cutaneous polyarteritis nodosa: comment on the article by Culver et al. Arthritis Rheum 55: 831-832, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Agur T, Levy Y, Plotkin E, Benchetrit S. Minocycline-induced polyarteritis nodosa-like vasculitis. Isr Med Assoc J 16: 322-323, 2014. [PubMed] [Google Scholar]
- 24. Baratta JM, Dyck PJ, Brand P, et al. Vasculitic neuropathy following exposure to minocycline. Neurol Neuroimmunol Neuroinflamm 3: e180, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barnes P, Chapman C, Fett N. Painful subcutaneous nodules in a patch of livedo reticularis. Int J Dermatol 56: e44-e46, 2017. [DOI] [PubMed] [Google Scholar]
- 26. Elkayam O, Yaron M, Caspi D. Minocycline induced arthritis associated with fever, livedo reticularis, and pANCA. Ann Rheum Dis 55: 769-771, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gait RC, Affleck AG, Leach IH, Varma S. Perinuclear antineutrophilic cytoplasmic antibody-positive polyarteritis nodosa secondary to minocycline treatment for acne vulgaris. J Am Acad Dermatol 58: S123-S124, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Garg N, Altowaijri GH, Nesbit GM, Gultekin SH, Bourdette DN. Minocycline-associated vasculitis of extracranial branches of vertebral arteries presenting as myelopathy. Neurol Neuroimmunol Neuroinflamm 1: e7, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McMillan HJ, Jansen GH, Koujok K, Milman N, Duffy CM, Watanabe Duffy K. Mononeuritis multiplex associated with minocycline in an adolescent. Muscle Nerve 56: e33-e35, 2017. [DOI] [PubMed] [Google Scholar]
- 30. Ogawa N, Kawai H, Yamakawa I, Sanada M, Sugimoto T, Maeda K. Case of minocycline-induced vasculitic neuropathy. Rinsho Shinkeigaku 50: 301-305, 2010(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 31. Pelletier F, Puzenat E, Blanc D, Faivre B, Humbert P, Aubin F. Minocycline-induced cutaneous polyarteritis nodosa with antineutrophil cytoplasmic antibodies. Eur J Dermatol 13: 396-398, 2003. [PubMed] [Google Scholar]
- 32. Sakai H, Komatsu S, Matsuo S, Iizuka H. Two cases of minocycline-induced vasculitis. Arerugi 51: 1153-1158, 2002(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 33. Schrodt BJ, Kulp-Shorten CL, Callen JP. Necrotizing vasculitis of the skin and uterine cervix associated with minocycline therapy for acne vulgaris. South Med J 92: 502-504, 1999. [DOI] [PubMed] [Google Scholar]
- 34. Starr MR, Tillema JM, Ytterberg SR, Bhatti MT, Chen JJ. Minocycline-induced vasculitis presenting as a third nerve palsy. J Neuroophthalmol 39: 240-241, 2019. [DOI] [PubMed] [Google Scholar]
- 35. Thaisetthawatkul P, Sundell R, Robertson CE, Dyck PJ. Vasculitic neuropathy associated with minocycline use. J Clin Neuromuscul Dis 12: 231-234, 2011. [DOI] [PubMed] [Google Scholar]
- 36. Gonzalez-Gay MA, Garcia-Porrua C, Guerrero J, Rodriguez-Ledo P, Llorca J. The epidemiology of the primary systemic vasculitides in Northwest Spain: implications of the Chapel Hill Consensus Conference definitions. Arthritis Rheum 49: 388-393, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Mahr A, Guillevin L, Poissonnet M, Aymé S. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener's granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheum 51: 92-99, 2004. [DOI] [PubMed] [Google Scholar]
- 38. Mohammad AJ, Jacobsson LT, Mahr AD, Sturfelt G, Segelmark M. Prevalence of Wegener's granulomatosis, microscopic polyangiitis, polyarteritis nodosa and Churg-Strauss syndrome within a defined population in southern Sweden. Rheumatology (Oxford) 46: 1329-1337, 2007. [DOI] [PubMed] [Google Scholar]