Abstract
Premacular membranes developing following pars plana vitrectomy (PPV) can cause significant anatomical and functional deficits to the macula. Recent reports showed that postoperative premacular membranes are a localized presentation of macular proliferative vitreoretinopathy (mPVR). Here, we report retrospectively a case series of 5 patients with severe mPVR which developed following uneventful PPV and were followed up to 32 months in the Department of Ophthalmology, Hadassah-Hebrew University Medical Center, Jerusalem, between October 2016 and February 2020. All patients underwent primary repair of rhegmatogenous retinal detachment (RRD) before mPVR developed. Mean best-corrected visual acuity (BCVA) at presentation was 20/76 Snellen (0.58 LogMAR). Median duration of the retinal detachment time until surgery was 1.5 days (range 1–21 days). Mean interval time from last normal follow-up exam to diagnosis of mPVR was 19 days (range 10–28). BCVA dropped from a mean of 20/38 Snellen (0.28 LogMAR) prior to mPVR development to 20/166 Snellen (0.92 LogMAR) following its development, recovering to 20/57 Snellen (0.45 LogMAR) after peeling of membranes. Mean central macular thickness measured by optical coherence tomography decreased from 711 to 354 μm postsurgery. In conclusion, short-term mPVR is a different entity from macular pucker in terms of rapid development, structural distortion, and visual compromise. Surgical treatment significantly restores macular function and anatomy.
Keywords: Epiretinal membrane, Macular proliferative vitreoretinopathy, Macular pucker, Premacular membrane, Post-operative
Introduction
Premacular membranes can cause devastating anatomical damages to the macula, leading to functional deficits including decreased visual acuity, central scotoma, color and contrast abnormalities, and metamorphopsia [1]. The traction effect created by these membranes leads to retinal wrinkling, schesis, detachment, macular edema, photoreceptor atrophy, and even macular holes. For a very long time, postoperative premacular membranes were considered to be macular pucker, known also as epiretinal membrane (ERM). Alternatively, recent reports showed membranes with extreme presentation are proliferative vitreoretinopathy (PVR) [2, 3]. Specific risk factors for PVR have been identified including uveitis, large, giant, or multiple tears, vitreous hemorrhage, preoperative or postoperative choroidal detachments, aphakia, multiple previous surgeries, and large detachments involving >2 quadrants of the eye [4].
The molecular composition of the premacular membranes was discovered several decades ago [5, 6, 7, 8]. Interestingly, both ERM and PVR membranes express integrins, α-SMA, galectin, EMMPRIN, RCS, IBA1, and collagen-type I. Idiopathic membranes are composed of myofibroblasts, glial cells, and fibroblasts, while PVR membranes are composed predominantly of retinal pigment epithelial (RPE) cells, glial cells, and myofibroblasts [3, 7, 9].
Over the past few decades, many attempts have been made to develop various PVR inhibitors but with unsatisfactory efficacy including anti-inflammatory components [10, 11], anti-growth factors [12, 13, 14], antiproliferative components, and others [15, 16, 17]. Surgical peeling is considered the accepted treatment following significant advancements in vitrectomy techniques. However, it is highly important to accurately differentiate between ERM and PVR, as this may have implications in case management and prognosis. Here, we report 5 unrelated cases with severe macular PVR (mPVR) which developed following uneventful pars plana vitrectomy (PPV) for rhegmatogenous retinal detachment (RRD) repair and were followed up to 32 months in the Department of Ophthalmology, Hadassah-Hebrew University Medical Center, between October 2016 and February 2020.
The mean best-corrected visual acuity (BCVA) at presentation of the 5 cases was 20/76 Snellen (0.58 LogMAR) which dropped from a mean of 20/38 Snellen (0.28 LogMAR) prior to mPVR development to 20/166 Snellen (0.92 LogMAR) following its development, recovering to 20/57 Snellen (0.45 LogMAR) after peeling of the mPVR membranes.
The median duration of the retinal detachment time until surgery was 1.5 days (range 1–21 days). Following primary RRD repair, mPVR developed between 10 and 28 days (mean 19 days). Mean central macular thickness (CMT) measured by spectral-domain optical coherence tomography (SD-OCT) (Spectralis; Heidelberg Engineering, Inc., Carlsbad, CA, USA), decreased from 711 μm at mPVR diagnosis to 354 μm post-mPVR peeling surgery. mPVR development in this series appeared much faster than idiopathic ERM and was treated with good results.
All the PPV surgeries were operated on by 3 vitreoretinal surgeons, T.J., S.K., and M.H. using 23 g Stellaris Elite PPV system (Bausch & Lomb, 400 Somerset Corporate Blvd., Bridgewater, NJ, USA). Following PPV, the membranes were stained using dual blue dye and peeled-off from the entire posterior pole and the periphery when present.
Cases Presentation
Patient I
A 47-year-old (YO) male, known to have myopia (spherical equivalent −2.5 D), presented 3 months following cataract surgery with localized RRD and mild vitreous hemorrhage in the left eye (LE). BCVA at presentation was 20/25 Snellen (0.10 LogMAR). Ocular examination showed unremarkable anterior segment and in fundoscopy 2 superio-temporal retinal breaks associated with macula-on RRD were found. He underwent laser retinopexy to the localized detachment. At the 1-month follow-up visit, his BCVA remained stable, while a significant progression of the retinal detachment was observed. SD-OCT images confirmed sparing of the macula and absence of any macular pathology. He underwent uneventful 23 g PPV with air tamponade leading to successful anatomical repair while BCVA remained unchanged (Table 1).
Table 1.
Basic characteristics of patients described in the case series including demographics, RRD details, and surgical procedure performed
| Name | Age | Gender | Previous ocular history | Previous medical history | Eye | Duration of retinal detachment, days | Time between last normal retinal exam to mPVR diagnosis, days | Lens status | Location of retinal breaks, n | Macular status | Vitreous hemorrhage | Repair procedure, s | Intraocular tamponade | Fibrotic membrane thickness, µm* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient I | 47 | M | Laser retinopexy, cataract extraction | − | LE | 21 | 14 | PCIOL | 2- temporal and supero-temporal | On | + | PPV, EL, AFEx | Air | 19 |
| Patient II | 53 | F | Laser retinopexy | Microcystic meningioma [grade I] | LE | 1 | 21 | NS + 2 | 1 long horseshoe break at 12 o'clock and 2 small holes nasally | Off | - | Combined Phaco IOL, PPV, EL, AFEx, Gas | 15 % C3 F8 | 19 |
| Patient III | 64 | M | − | Diabetes, hypertension, ischemic heart disease, renal failure, obstructive sleep apnea, pulmonary hypertension, obesity | LE | 3 | 21 | NS + 2 | 1 retinal break at 12 o'clock | Split macula | + | Combined Phaco IOL, PPV, EL, AFEx | 20% SF6 | 50 |
| Patient IV | 47 | M | High myopia, BE refractive surgery 15 years ago | − | RE | 1 | 28 | Clear | Multiple holes at 6 o'clock | On | - | PPV, EL, AFEx, Gas | 20% SF6 | 25 |
| Patient V | 51 | M | − | Mild right thoracic outlet syndrome | LE | 2 | 10 | Clear | Superior retinal breaks | On | - | SB, Cryo followed by PPV, EL, AFEx | 20% SF6 | 42 |
AFEx, air-fluid exchange; EL, endolaser; F, female; M, male; NS, nuclear sclerosis; RE, right eye; LE, left eye; PCIOL, posterior chamber intraocular lens; PPV, pars plana vitrectomy; SB, scleral buckle; C3F8, perfluoropropane; SF6, sulfur hexafluoride; RRD, rhegmatogenous retinal detachment; OCT, optical coherence tomography; mPVR, macular proliferative vitreoretinopathy.
The thickness of the mPVR membranes was measured using Heidelberg Eye Explorer, version 1.9.10.0 (Heidelberg Engineering) for 1:1 µm OCT follow-up horizontal cross-section passing in the fovea.
Eight weeks post-PPV, he presented with blurred vision and a BCVA of 20/200 Snellen (1.00 LogMAR), while his last postoperative BCVA at 6-week visit was 20/20 Snellen (0 LogMAR) (Fig. 1a). Anterior segments remained unremarkable but fundoscopy showed flat retina and premacular membranes, confirmed by SD-OCT with CMT of 613 μm (Fig. 1b). He underwent another PPV, membranes peel, air tamponade, and intravitreal injection of 0.4 mg Triamcinolone acetonide. The BCVA recovered to 20/32 Snellen (0.2 LogMAR), and CMT decreased to 411 μm 8 months later (Fig. 1c, d, 2a).
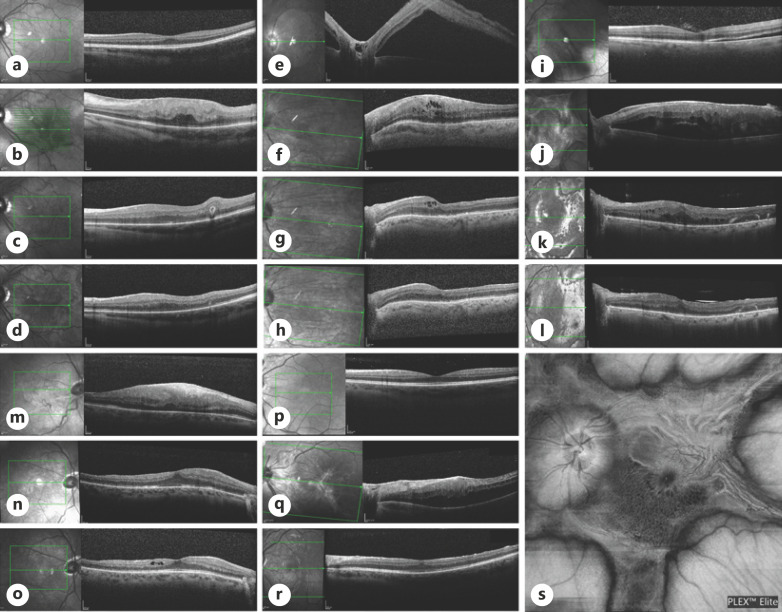
Fig. 1.
Horizontal cross-sections and en face scan of SD-OCT of the macular area for the 5 patients included in the case series: Patient I (a–d), patient II (e–h), patient III (i–l, s), patient IV (m–o), and patient V (p–r). Horizontal OCT cross-sections demonstrate macula-on RRD at presentation (a), developing mPVR leading to macular thickening and disruption of the inner retinal layers (b). Panels (c, d) show restoration of the macular structure following membranes peeling 1 month and in the last follow-up visits, respectively. Patient II presented with macula-off RRD initially (e) followed by mPVR development after 2 PPV surgeries (f). Scans (g, h) show decrease in the macular thickening and restoration of the retinal layers 1 month and as seen in the last follow-up visits post-peeling. Patient III presented with split macula RRD (i), underwent surgical repair followed by severe mPVR (j) seen as macular thickening, hypo-reflective intraretinal cysts, and thick hyper-reflective premacular fibrotic tissue. Surgical repair led to remodeling of the foveal structure as observed 1 month (k) and in the last follow-up visit (l). Patient IV had Macula-on RRD (no scan at presentation) and developed thick mPVR membrane disrupting the inner retinal layers 6 weeks later (m), which resolved almost completely 1 month (n) and 5 months (o) post-op. s En face scan of the macula of patient IV demonstrating unique star-like shape of the PVR membrane encroaching on the posterior pole. Patient V presented macula-on RRD (p) in his LE underwent SB followed by PPV. Three weeks later presented with mPVR (q) causing thickening of the macula without hydration. Membranes peeling restored the macular structure as seen 7 weeks later (r). PVR, proliferative vitreoretinopathy; BCVA, best-corrected visual acuity; SD-OCT, spectral-domain optical coherence tomography; PPV, pars plana vitrectomy; RRD, rhegmatogenous retinal detachment; mPVR, macular proliferative vitreoretinopathy; LE, left eye.
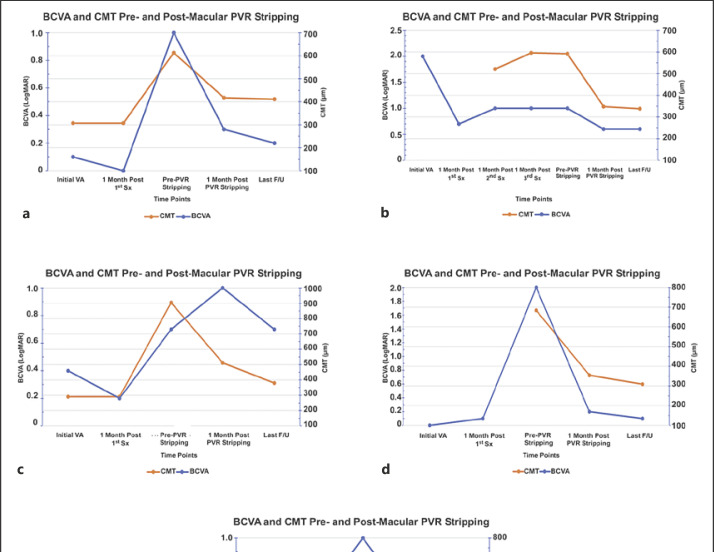
Fig. 2.
BCVA and CMT progression at critical time points of the PVR development and removal. BCVA is presented in LogMAR (blue line) and CMT in µm (orange line). a–e represents patients I–V, respectively. Overall, some inverse correlation between BCVA and CMT was observed, indicating to some extent the importance of membrane stripping in order to improve BCVA. PVR, proliferative vitreoretinopathy; BCVA, best-corrected visual acuity; CMT, central macular thickness.
Patient II
A 53 YO female, with a history of microcystic meningioma World Health Organization (WHO) grade I, presented complaining of reduced vision in the LE of 20/2000 Snellen equivalent visual acuity (2.00 LogMAR). On examination, unremarkable anterior segment, superior-temporal horseshoe at 12 o'clock, and 2 small holes nasally associated with macula-off RRD were observed (Fig. 1e; Table 1). Two days later, she underwent uneventful combined cataract extraction and 23 g PPV including 15% perfluoropropane (C3F8) gas tamponade. Postoperative BCVA of 20/100 Snellen (0.70 LogMAR) dropped to 20/2000 Snellen equivalent (2.00 LogMAR) about 2-month post-PPV. Anterior segment was still unremarkable, while fundoscopy showed premacular fibrosis confirmed by SD-OCT (Fig. 1f, 2b).
A second PPV, membrane peel, and silicone oil tamponade were performed resulting in BCVA of 20/200 Snellen (1.00 LogMAR). Subsequent examination showed macular pucker associated with thickening of 521 μm measured by SD-OCT and stable BCVA; hence, a third PPV, membrane peel, and air tamponade surgery were performed. The last follow-up examination revealed BCVA of 20/80 Snellen (0.60 LogMAR), CMT of 338 μm, and intact macular structure (Fig. 1g, h, 2b).
Patient III
Patient III is a 64 YO male suffering from multiple systemic diseases including diabetes, hypertension, ischemic heart disease, chronic renal failure, obstructive sleep apnea, pulmonary hypertension, and obesity, with no previous eye morbidity. He presented complaining of floaters and blurred vision in the LE for 3 days. BCVA measured 20/50 Snellen (0.40 LogMAR). Eye examination showed a mild nuclear sclerosis cataract. Funduscopic exam showed signs of mild nonproliferative diabetic retinopathy and a RRD due to a superior horseshoe retinal tear (12–2 clock hours) (Fig. 1i; Table 1). Preoperative SD-OCT showed the presence of split macula RRD and absence of diabetic macular edema.
He underwent uneventful combined cataract extraction and 23 g PPV including 20% sulfur hexafluoride (SF6) gas tamponade with a postoperative BCVA of 20/32 Snellen (0.20 LogMAR) that dropped to 20/100 Snellen (0.70 LogMAR) 3 months postsurgery. The anterior segment examination was unremarkable while his fundus exam showed premacular fibrotic tissue (Fig. 1j, s) causing significant macular thickening with a CMT of 904 μm (Fig. 2c).
A second PPV, membrane peel, and silicone oil tamponade were performed resulting in BCVA of 20/200 Snellen (1.00 LogMAR) with CMT of 512 μm 1 month following the surgery (Fig. 1k). The last follow-up examination 6 months after the first surgery revealed BCVA of 20/100 Snellen (0.70 LogMAR), CMT of 380 μm, and intact macular structure (Fig. 2c).
Patient IV
Patient IV is a 47 YO male with unremarkable systemic medical history and 15 years after refractive surgery due to high myopia. He presented complaining of superior visual field defect for 1 day. On examination, BCVA was 20/20 Snellen (0 LogMAR). The anterior segment was within normal limits, while the funduscopic exam showed an inferior retinal horseshoe tear associated with localized macula-on inferior RRD. He underwent uneventful 23 g PPV, endolaser, and intravitreal tamponade of 20% SF6 gas (Table 1). His postoperative BCVA dropped from 20/25 Snellen (0.10 LogMAR) to 20/2000 Snellen equivalent visual acuity (2.00 LogMAR) 6-week post-PPV. Fundoscopy showed premacular membranes confirmed by SD-OCT with CMT 684 μm (Fig. 1m, 2d). Nine-week post-PPV, he underwent 23 g PPV, membrane stripping, and air tamponade. The BCVA recovered nicely to 20/32 Snellen (0.20 LogMAR) and CMT to 355 μm at 6-week follow-up (Fig. 1n, 2d). Five months later, his cataract was extracted and BCVA of 20/25 Snellen (0.10 LogMAR) was measured, while SD-OCT showed intact macular structure and CMT of 309 μm (Fig. 1o, 2d).
Patient V
Patient V is a 52 YO male with unremarkable previous medical or ocular history presented complaining of inferior visual field defect for 2 days in the LE. On examination, BCVA was 20/32 Snellen (0.20 LogMAR). The anterior segment was within normal limits in both eyes, while his funduscopic exam showed 3 superior retinal tears associated with localized macula-on superior RRD in the LE. SD-OCT confirmed sparing of the macula and excluded the presence of any macular pathology (Fig 1p; Table 1). He underwent uneventful scleral buckle and cryopexy surgery. During the 1-month follow-up visit after the scleral buckle surgery, a persistent superior retinal detachment was noticed while the SD-OCT confirmed absence of any macular involvement or pathology.
He underwent a combined cataract surgery with 23 g PPV, endolaser, and intravitreal tamponade of 20% SF6 (Table 1). Three-week post-PPV, BCVA dropped from 20/63 Snellen (0.50 LogMAR) to 20/200 Snellen (1.00 LogMAR). The anterior segment was unremarkable while fundoscopy showed premacular membranes confirmed by SD-OCT with CMT 691 µm (Fig. 1q). He underwent PPV, membrane stripping, and silicone oil tamponade. The BCVA recovered to 20/40 Snellen (0.30 LogMAR), and CMT decreased to 321 μm at 7-week follow-up (Fig. 1r, 2e).
Discussion
Premacular fibrosis can be caused either by unknown reasons (idiopathic/primary) or secondary to diverse ocular conditions following multiple surgical procedures including cataract extraction, trabeculectomy, scleral buckle, PPV, laser and cryoretinopexy, and also due to nonsurgical causes including blunt trauma, uveitis, diabetes, posterior vitreous detachment, and other retinal vascular diseases. The incidence is 7–12% for idiopathic epiretinal membrane, with similar rates observed following retinal detachment repair, but this is expected to be even higher in the era of OCT imaging due to the significantly increased ability to identify subtle changes such as thin membranes. Older age, increasing myopic refraction and narrower retinal arteriolar diameter, was highly associated with increased primary ERM prevalence. Previous histologic studies showed various components of ERM, including hypocellular extracellular matrix components, monocytes, gliotic tissue elements and collagen, and RPE cells [18, 19]. The ocular implications, both functional and structural, can vary from no loss to severe loss, while the latter is uncommon and affects visual acuity, color vision, metamorphopsia, diplopia, and central scotoma. For years, post-vitrectomy premacular fibrosis was categorized as part of macular pucker spectrum [20]. Recently, few reports distinguished a unique subgroup with premacular fibrosis to be mPVR rather than ERM [2, 3]. Macular PVR was highly associated with retinal breaks compared to idiopathic ERM [2]. Here, we report a series of 5 cases who, following PPV, developed severe premacular fibrosis over a very short-term compatible with mPVR. In general, PVR is considered the most common cause of retinal detachment repair failure accounting for 10% of cases. Macular PVR is a specific condition which should be differentiated from the general process. The pathophysiology of the PVR involves migration of RPE and inflammatory cells to the retinal surface [2]. It was shown that mPVR patients present with worse visual acuity compared to idiopathic ERM patients. Our patients presented here showed significant decrease in their mean BCVA 20/38 Snellen (0.28 LogMAR) prior to mPVR development to 20/166 Snellen (0.92 LogMAR) following its development with very good improvement of 4 ETDRS lines following membrane stripping surgery (BCVA of 20/57 Snellen, 0.45 LogMAR). Of note, a previous report showed greater BCVA improvement for patients undergoing mPVR stripping compared to idiopathic ERM peeling (0.31 ± 0.23 vs. 0.09 ± 0.42) [2]. One main clinical difference is that mPVR seems to be progressive, while macular pucker remains stable and does not become progressively worse. OCT is highly helpful in characterizing these different subgroups. Idiopathic ERM was found to be globally adherent to the retinal surface while post-retinal detachment repair ERM is more focally adherent [21]. On the other hand, mPVR cases demonstrated fingerlike projections that extend from the inner retinal surface to the posterior vitreous cortex. Macular PVR tissue can span the retinal surface and the posterior border of silicone oil or can extend from the retinal surface into optically empty vitreous cavity in previously vitrectomized eyes [2]. Our patients presented in this case series showed thick fibrotic membranes tightly and diffusely adhered to the retina, lacking these fingerlike projections. A possible explanation for the difference in membrane appearance may be presence of posterior vitreous cortex. In our series, this posterior interphase was absent as posterior vitreous detachment is induced at the beginning of PPV and the vitreous is removed, while in the series published by Sigler et al. [2], patients developed mPVR secondary to retinal break while the posterior vitreous cortex still exists which constitutes a scaffold to the RPE and glial cells to create these fingerlike structures. Also, previous reports showed that epimacular membranes after retinal detachment repair have a focal adherence pattern rather than global adherence seen in idiopathic ERM patients [21]. These different features indicate a wider spectrum of tomographic presentations seen by the OCT. The membranes were more prominent and presented dramatically compared to idiopathic ERM. In addition, the SD-OCT demonstrated a significant decrease in the macular thickness post-membrane stripping (711–354 μm) which was inversely related to BCVA (Fig. 2), emphasizing the importance of mPVR peeling to achieve a better visual prognosis. Histological studies demonstrated that both idiopathic ERM and PVR membranes share similar proteinaceous components but differ in the cellular components [3].
To conclude, here, we present a case series of 5 patients who developed mPVR following PPV surgery to repair RRD. Our patients demonstrate unique clinical presentation and OCT features spanning the clinical spectrum known to-date, which distinguish mPVR from idiopathic ERM. Surgical removal of mPVR should be considered at an early stage and seems to improve BCVA significantly.
Statement of Ethics
The research was approved by the institutional review board at the Hadassah-Hebrew University Medical Center. The tenets of the Declaration of Helsinki were followed and prior to donation, a written informed consent for publication of this case report was obtained from individuals who participated in this study after explanation of the nature and possible consequences of the study.
Conflict of Interest Statement
The authors have no conflicts of interest.
Funding Sources
The authors did not receive any funding.
Author Contributions
S.K. and T.J. were responsible for planning the project and recruiting the patients. M.H. recruited 1 patient. S.K. and H.A. collected and analyzed the data. S.K. and H.A. drafted the manuscript. S.K., H.A., and T.J. reviewed and edited the manuscript.
Acknowledgments
The authors thank Ms. Nina Schneider for the English editing and all the patients and family members for their participation in this study.
References
- 1.Jones MA, Bhide S, Chin E, Ng BG, Rhodenizer D, Zhang VW, et al. Targeted polymerase chain reaction-based enrichment and next generation sequencing for diagnostic testing of congenital disorders of glycosylation. Genet Med. 2011;13((11)):921–32. doi: 10.1097/GIM.0b013e318226fbf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sigler EJ, Randolph JC, Calzada JI. Comparison of morphologic features of macular proliferative vitreoretinopathy and idiopathic epimacular membrane. Retina. 2014;34((8)):1651–7. doi: 10.1097/IAE.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 3.Guenther SR, Schumann RG, Hagenau F, Wolf A, Priglinger SG, Vogt D. Comparison of surgically excised premacular membranes in eyes with macular pucker and proliferative vitreoretinopathy. Curr Eye Res. 2019;44((3)):341–9. doi: 10.1080/02713683.2018.1542006. [DOI] [PubMed] [Google Scholar]
- 4.Pastor JC, De La Rúa ER, Martín F. Proliferative vitreoretinopathy: risk factors and pathobiology. Prog Retin Eye Res. 2002;21:127–44. doi: 10.1016/s1350-9462(01)00023-4. [DOI] [PubMed] [Google Scholar]
- 5.Michels RG. A clinical and histopathologic study of epiretinal membranes affecting the macula and removed by vitreous surgery. Trans Am Ophthalmol Soc. 1982;80:580–656. [PMC free article] [PubMed] [Google Scholar]
- 6.Machemer R, Laqua H. Pigment epithelium proliferation in retinal detachment (massive periretinal proliferation) Am J Ophthalmol. 1975;80((1)):1–23. doi: 10.1016/0002-9394(75)90862-4. [DOI] [PubMed] [Google Scholar]
- 7.Van Horn DL, Aaberg TM, Machemer R, Fenzl R. Glial cell proliferation in human retinal detachment with massive periretinal proliferation. Am J Ophthalmol. 1977;84((3)):383–93. doi: 10.1016/0002-9394(77)90684-5. [DOI] [PubMed] [Google Scholar]
- 8.Jerdan JA, Pepose JS, Michels RG, Hayashi H, de Bustros S, Sebag M, et al. Proliferative vitreoretinopathy membranes: an immunohistochemical study. Ophthalmology. 1989;96((6)):801–10. doi: 10.1016/s0161-6420(89)32818-1. [DOI] [PubMed] [Google Scholar]
- 9.Wiedemann P. Growth factors in retinal diseases: proliferative vitreoretinopathy, proliferative diabetic retinopathy, and retinal degeneration. Surv Ophthalmol. 1992;36((5)):373–84. doi: 10.1016/0039-6257(92)90115-a. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadieh H, Feghhi M, Tabatabaei H, Shoeibi N, Ramezani A, Mohebbi MR, Triamcinolone acetonide in silicone-filled eyes as adjunctive treatment for proliferative vitreoretinopathy. A randomized clinical trial. Ophthalmology. 2008;115((11)):1938–43. doi: 10.1016/j.ophtha.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Rubsamen PE, Cousins SW. Therapeutic effect of periocular corticosteroids in experimental proliferative vitreoretinopathy. Retina. 1997;17((1)):44–50. doi: 10.1097/00006982-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Nassar K, Grisanti S, Tura A, Lüke J, Lüke M, Soliman M, et al. A TGF-β receptor 1 inhibitor for prevention of proliferative vitreoretinopathy. Exp Eye Res. 2014;123:72–86. doi: 10.1016/j.exer.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Velez G, Weingarden AR, Lei H, Kazlauskas A, Gao G. SU9518 inhibits proliferative vitreoretinopathy in fibroblast and genetically modified Müller cell-induced rabbit models. Invest Ophthalmol Vis Sci. 2013;54((2)):1392–7. doi: 10.1167/iovs.12-10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, Ikuno Y, Ohj M, Kusaka S, Jiang R, Cekiç O, et al. Platelet-derived growth factor receptor kinase inhibitor AG1295 and inhibition of experimental proliferative vitreoretinopathy. Jpn J Ophthalmol. 2003;47((2)):158–65. doi: 10.1016/s0021-5155(02)00698-6. [DOI] [PubMed] [Google Scholar]
- 15.Charteris DG, Aylward GW, Wong D, Groenewald C, Asaria RHY, Bunce C, et al. A randomized controlled trial of combined 5-fluorouracil and low-molecular-weight heparin in management of established proliferative vitreoretinopathy. Ophthalmology. 2004;111((12)):2240–5. doi: 10.1016/j.ophtha.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa K, He S, Terasaki H, Nazari H, Zhang H, Spee C, et al. Resveratrol inhibits epithelial-mesenchymal transition of retinal pigment epithelium and development of proliferative vitreoretinopathy. Sci Rep. 2015;5:16386. doi: 10.1038/srep16386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peyman GA, Schulman J. Proliferative vitreoretinopathy and chemotherapeutic agents. Surv Ophthalmol. 1985;29:434–42. doi: 10.1016/0039-6257(85)90208-5. [DOI] [PubMed] [Google Scholar]
- 18.Clarkson JG, Green WR, Massof D. A histopathologic review of 168 cases of preretinal membrane. Am J Ophthalmol. 1977;84((1)):1–17. [PubMed] [Google Scholar]
- 19.Tang SB, Scheiffarth OF. An immunohistochemical study of vitreal and epiretinal membranes in human eyes. Zhonghua Yan Ke Za Zhi. 1990;26((5)):282–5. [PubMed] [Google Scholar]
- 20.Appiah AP, Hirose T, Kado M. A review of 324 cases of idiopathic premacular gliosis. Am J Ophthalmol. 1988;106((5)):533–5. doi: 10.1016/0002-9394(88)90581-8. [DOI] [PubMed] [Google Scholar]
- 21.Mori K, Gehlbach PL, Sano A, Deguchi T, Yoneya S. Comparison of epiretinal membranes of differing pathogenesis using optical coherence tomography. Retina. 2004;24((1)):57–62. doi: 10.1097/00006982-200402000-00009. [DOI] [PubMed] [Google Scholar]