Abstract

End-stage renal disease (ESRD) is gradually becoming a major public healthcare burden worldwide. Post-translational modifications carrying epigenetic information play a crucial role in the pathogenesis of many chronic diseases. We performed lysine crotonylation (KCr) and lysine 2-hydroxyisobutyrylation (Khib) analyses with liquid chromatography–tandem mass spectrometry to obtain a comprehensive profile and reveal the specific pathogenesis of peripheral blood mononuclear cells in ESRD patients. 218 overlap proteins among differentially modified proteins (DMPs) of both 2-hydroxyisobutyrylation and crotonylation were identified. KEGG analysis enriched pathways of protein processing in endoplasmic reticulum (ER) and glycolysis/gluconeogenesis which is closely related with cell apoptosis. In Bip, a master regulator in the ER, eight sites were identified as having both KCr and Khib modifications. Five differentially KCr modification sites and three differentially Khib-modified sites were detected between ESRD patients and normal controls. Besides Bip, other proteins (GRP94, CNX, CRT, PDIs, GlcII, ERP57, Bap31, Hsp70, and Hsp90) happened both KCr and Khib modifications. Nine DMPs having both KCr and Khib modifications were related to the glycolysis/gluconeogenesis pathway containing two key regulatory enzymes of hexokinase-1 and pyruvate kinase. The two most abundant dual modification proteins were ENO1 and PGK1 with 15 sites and 8 sites, respectively. Lysine residue K228 with both KCr and Khib modifications in ENO1 was on its surface and made it accessible for p300 mediating dynamic modifications. Overall, we hypothesize that KCr and Khib comodifications may influence the number of immunocytes and further induce immune senescence in ESRD patients through the glycolysis/gluconeogenesis pathway and protein processing in the ER process, which may be a potential therapeutic direction in the future.
1. Introduction
The prevalence of end-stage renal disease (ESRD) is gradually increasing and becoming a major public healthcare burden worldwide. Due to insufficient supply of transplanted kidneys, hemodialysis (HD) is the main replacement therapy for ESRD patients worldwide. Immune cell dysfunction and systemic inflammation are the main features in patients under HD,1,2 which contribute to HD complications in patients with atherosclerosis, cardiovascular disease, cachexia, and anemia.3 However, the potential mechanisms/factors of immune deficiency in HD patients are not yet clear.
Post-translational modifications (PTMs) of a target protein are crucial to numerous biological processes and disease states.4−6 At present, more than 400 types of PTMs have been found.7−10 The center of regulation of different PTMs is found in the reversibility of the modifications that specifically add or remove a modification in a targeted (modified) amino acid residue, and the process is carried out by “writer” and “eraser.”11 Khib, a novel PTM, is closely related to the processes of transcription, protein degradation, and energy metabolism.12,13 Previous studies suggested that Khib plays a crucial role in cellular glucose metabolism, which in turn affects cell survival.14 It is known that crotonylation modification in histone might positively affect acute kidney injury by regulating inflammatory cytokine.6,15 Both Khib and Kcr modification marks are preferentially positioned in gene promoters, which are closely related to gene transcriptional activation.16,17 Khib is structurally different from lysine Kcr that the molecular formula of Khib is C4H7O2 and induces a mass shift of +86.0368 Da, while the molecular formula of Kcr is C4H5O with an accurate mass shift of + 68.0230 Da.16,17 The two PTMs share similar ε-amine linkage with lysine acetylation, yet Khib has bigger size and less hydrophobicity than Kcr.16,18 Both Khib and Kcr levels are directly regulated by the crotonyl-CoA and 2-hydroxyisobutyryl-CoA, respectively, which act as cofactors preferred by p300.16,19 It is reported that p300, a member of lysine acetyltransferases, has the potential of regulating crotonylation and 2-hydroxyisobutyrylation.14,20 The aliphatic portions of p300-bound diverse short-chain acyl-CoAs are accommodated in the substrate-binding tunnel of p300, sterically clashing with the lysine substrate binding following by the lysine substrates remodeling the acyl-CoAs into a conformation compatible with the acyl-chain transfer.21 It is also reported that histone deacetylases2 (HDAC2) and HDAC3 are identified as “eraser” to remove Khib and Kcr in vitro and in vivo.12,22,23 The treatment of suberoylanilide hydroxamic acid (SAHA), a common HDAC inhibitor, significantly elevated the levels of Khib and Kcr in most core histone sites. The fold changes upon SAHA of 2-hydroxyisobutyrylation and crotonylation on the nonhistone scale were highly correlated, although the exact mechanism is unclear.24 CKD is closely associated with inflammation, cell death, and diverse degrees of fibrosis, and all these biological processes could be regulated21 by epigenetic modifications.25 Whether these two epigenetic processes take part in the development of ESRD is unknown at present. In order to arrive at a better understanding of the impact of the two PTMs on the ESRD state, our study performed liquid chromatography–tandem mass spectrometry (LC–MS/MS) methods to investigate the key features of crotonylation and 2-hydroxyisobutyrylation modifications in the peripheral blood mononuclear cells (PBMCs) of ESRD patients under HD.
2. Results
2.1. Protein Identification
In our study, a total of 3139 quantifiable lysine 2-hydroxyisobutyrylation sites across 891 quantifiable proteins were identified in the PBMCs of ESRD patients and normal controls. In our previous study, 1109 lysine crotonylation sites across 347 proteins were detected among these two groups.26 Generally, the lysine 2-hydroxyisobutyrylation modification was more abundant than crotonylation in human PBMCs.
Differentially modified protein (DMP) differential expression standard as follows: a fold change of >1.2 is considered as an upregulation, while <1/1.2 is considered as a downregulation. In this study, differential Khib modification protein expression between ESRD patients and normal controls was observed at 1904 sites for 709 proteins, while differential KCr modification protein expression was observed at 860 sites for 295 proteins. Venn diagram (Figure 1a,b) provides the data indicating the number of overlapping upregulated/downregulated proteins and sites with KCr/Khib modifications. Then, we sorted out 218 overlap proteins among DMPs of both 2-hydroxyisobutyrylation and crotonylation modifications. The proportion of both 2-hydroxyisobutyrylation and crotonylation proteins was 62.8 in KCr DMPs and 24.5% in Khib DMPs.
Figure 1.
Qualitative analysis of lysine crotonylation and 2-hydroxyisobutyrylation between ESRD patients and normal controls. (a) Venn graph representing the overlap proteins modified by dual lysine crotonylation and 2-hydroxyisobutyrylation. (b) Venn graph showing the overlap modified sites between lysine crotonylation and 2-hydroxyisobutyrylation. (c) Subcellular location of the overlap DMPs of dual KCr and Khib. (d) Functional category of the overlap DMPs of dual KCr and Khib in GO terms.
2.2. Functional Characterization of Overlap DMPs of Dual KCr and Khib
The subcellular localization of the overlap DMPs of dual KCr and 2-hydroxyisobutyrylation modifications was distributed in the cytoplasm (55.96%), nucleus (10.09%), mitochondria (10.09%), extracellular region (9.63%), and endoplasmic reticulum (ER) (4.13%) (Figure 1c).
We investigated the functional category distribution of the 218 overlap DMPs of dual KCr and Khib utilizing the gene ontology (GO) analysis tool. Thirty groups of related biological functions were established for the overlap DMPs and further separated into three classifications: cellular component (30.0%), molecular function (23.7%), and biological process (46.7%) (Figure 1d).
2.3. Functional Enrichment of Overlap DMPs of Dual Kcr and Khib
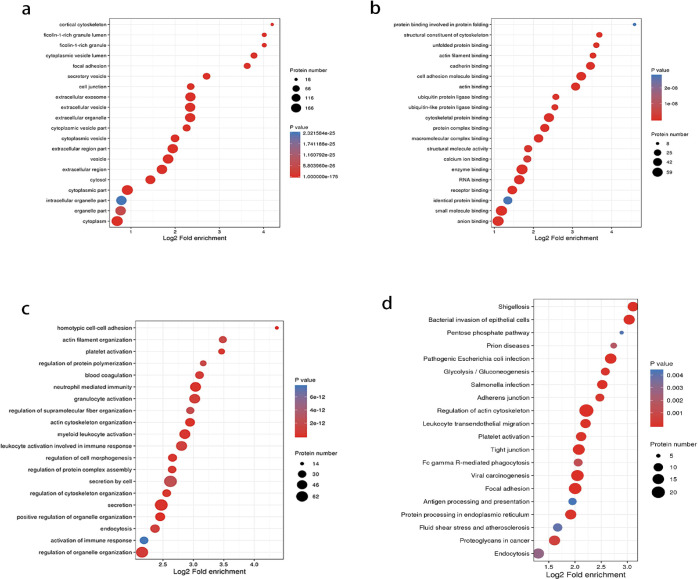
GO enrichment analysis was performed to further understand the functions of 218 overlap DMPs. The cellular component in the GO enrichment analysis showed significant enrichment of focal adhesion, cytoplasmic vesicle lumen, extracellular exosome, and extracellular vesicle (Figure 2a). According to molecular function enrichment classification, the cell adhesion molecule binding, structural constituent of cytoskeleton, unfolded protein binding, and protein complex binding were significantly enriched (Figure 2b). The overlap DMPs in the biological process were highly enriched in homotypic cell–cell adhesion, positive regulation of organelle organization, actin cytoskeleton organization, and secretion (Figure 2c).
Figure 2.
GO enrichment analysis and KEGG pathway enrichment analysis of overlap DMPs of dual Kcr and Khib. (a) Cellular component in the GO enrichment analysis. (b) Molecular function in the GO enrichment analysis. (c) Biological process in the GO enrichment analysis. (d) KEGG analysis of overlap DMPs of dual Kcr and Khib.
Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis revealed that the overlap DMPs were significantly related to the regulation of actin cytoskeleton, bacterial invasion of epithelial cells, and shigellosis. Besides, glycolysis/gluconeogenesis and protein processing in ER pathways were significantly enriched (Figure 2d).
2.4. Analysis of Functional Modules of Protein Interaction Network
To better understand the biological function of the 218 overlapped DMPs, we constructed a protein–protein interaction (PPI) network using the search tool for the retrieval of interacting genes (STRING) database (version 10.5). Cytoscape software was used for visualizing the results (Smoot et al. 2011). We fetched all interactions that had a combined score ≥0.9 and identified 183 Khib and KCr DMPs as nodes connected by 849 interactions (Figure 3). Nine clusters were detected in the network by molecular complex detection (MCODE). The clusters of top 2 MCODE score included regulation of actin cytoskeleton and vesicle-mediated transport. Then, we constructed PPI networks to show the interaction between the involved proteins of glycolysis/gluconeogenesis (9 nodes and 36 interactions) and protein processing in the ER pathway (14 nodes and 71 interactions) (Figure 4a and 5a).
Figure 3.
PPI network analysis of DMPs was performed, and two most significant modules were yielded with MCODE. One hundred and eighty three proteins from 218 overlapped DMPs of dual Khib and KCr were fetched according to the standard of combined score ≥0.9. Module 1 named regulation of actin cytoskeleton (score = 14.5) was constructed with 41 nodes and 290 edges. Module 2 named vesicle-mediated transport (score = 7.538) was constructed with 14 nodes and 49 edges.
Figure 4.
DMPs of dual Kcr and Khib in protein processing in the ER signal pathway. (a) PPI network of DMPs of dual Kcr and Khib enriched in protein processing in the ER pathway. (b) Number of modified sites of Kcr and Khib in the ER signal pathway. (c) Overview of DMPs of dual Kcr and Khib in the ER signal pathway. (d) Three-dimensional structure of Bip protein constructed using SWISS MODEL. Comodification happened in eight lysine sites in Bip in the same site of crotonylation and 2-hydroxyisobutyrylation, which are labeled yellow.
Figure 5.
DMPs of dual Kcr and Khib in protein processing in the glycolysis/gluconeogenesis signal pathway. (a) PPI network of DMPs of dual Kcr and Khib enriched glycolysis/gluconeogenesis pathway. (b) Number of modified sites of Kcr and Khib in the glycolysis/gluconeogenesis pathway. (c) Overview of DMPs of dual Kcr and Khib in the glycolysis/gluconeogenesis signal pathway.
2.5. Characteristic of Protein Processing in ER Pathway
Fourteen nodes, including CANX, HSP90,B1, CALR, HSPA5, P4HB, HSPA8, HSP90AB1, HSP90AA1, PDIA3/4/6, PRKCSH, HSPA1B, and BCAP31, and 71 interactions were involved in the network (Figure 4c). CANX, HSP90B1, and HSPA5 together with CALR and P4HB were the top three3 hub genes. HSPA5, coding for Bip, is a master regulator in the ER. Nine KCr sites and 18 Khib sites were identified in Bip. Eight sites (K96, K154, K163, K352, K446, K523, K553, and K579) in Bip were identified as having both KCr and Khib modifications (Figure 5). Five differential KCr modification sites (K118, K446, K523, K553, and K579) and three differentially Khib modification sites (K185, K213, and K164) were identified between ESRD patients and normal controls. The comodified sites among other proteins are shown in Table S1.
2.6. Characteristic of Glycolysis/Gluconeogenesis Pathway
Nine nodes, including PGAM1, PGK1, HK1, GPI, ALDOA, GAPDH, TPI1, ENO1, and PKM, and 36 interactions were involved in the network (Figure 5c). PGK1, HK1, and PGAM1 were the top three hub genes. Hexokinase-1 (HK1) and pyruvate kinase (PKM) are the two key regulatory enzymes in the glycolysis process. Lysine K315 in HK1 happened co-occurring KCr and Khib in the same site. One differential KCr modification site (K346) and seven differentially Khib modification sites (K187, K315, K353, K418, K501, K738, and K777) in HK1 were identified between ESRD patients and normal controls (Table S2). Lysine K62, K135, K261, K270, and K498 in PKM were identified as having both KCr and Khib modifications. Four differential KCr modification sites (K62, K135, K261, and K498) and 10 differentially Khib modification sites (K62, K89, K115, K125, K135, K207, K261, K305, K311, and K498) were identified between ESRD patients and normal controls (Table S3).
ENO1 and PGK1 were the two most abundantly dual modification proteins in the glycolysis/gluconeogenesis pathway. Sixteen6 KCr sites and 21 Khib sites were identified in ENO1. KCr and Khib coregulating in the same site in ENO1 were detected among 16 sites, 7 sites of which are differentially modified sites containing K64, K92, K221, K262, K330, K335, and K420 (Table S4). Nine KCr sites and 15 Khib sites were identified in PGK1. Co-occurring modification of KCr and Khib in the same site in PGK1 was identified among eight sites, four of which are differentially modified sites containing K131, K141, K146, and K191 (Table S5).
3. Discussion
Increasing evidence has revealed the close association of PTMs.27−29 With the recent explosion in protein discovery, an increasing number of novel PTMs are being recognized. Increasing evidence supported that PTM does not exist as a separate part from other kinds of PTMs.30−34 Different PTMs can be regulated by the same enzyme. It is reported that crotonylation and 2-hydroxyisobutyrylation are mediated by p300, a member of lysine acetyltransferases.14,20 Therefore, our study explored the role of co-occurring KCr and Khib modification sites by MS in the ESRD state.
In our study, the molecular function in the GO term of 218 DMPs of dual KCr and Khib was highly enriched in the unfolded protein binding and protein binding involved in protein folding. KEGG analysis enriches in the pathway of protein processing in the ER. In the ER, a newly synthesized protein undergoes a range of modifications and proper folding by assistance from molecular chaperones and folding enzymes and then releases from the ER subsequently35 or targets for degradation if misfolding. The environment of hypoxia, inflammation, and oxidative stress are the characteristics of ESRD, which could not be relieved with maintenance HD. Environmental insults may lead to protein misfolding in ES, and the accumulation of misfolded or unfolded proteins (exceeding the chaperones’ folding capacity) is called ER stress. An adaptive signaling pathway called the unfolded protein response, whose branches include PERK, ATF-6, and inositol-requiring enzyme 1 (IRE-1) is triggered to restore ER homeostasis.36 Under unstressed conditions, these specialized ER-anchored sensor proteins bound with the ER chaperone Bip maintained in an inactive state.37 It is known that the ER stress is a component of chronic kidney disease and renal fibrosis.38−40 However, the pathogenic mechanisms remain unidentified. Bip can be seen as an ER stress marker.36 Bip is a master regulator in the ER and is closely related to chronic diseases, such as cardiovascular disease, neurodegenerative disease, and cancer.41 Under various stress conditions, PTM in Bip plays an important role in the foldase activity and intracellular location of Bip molecular.42,43 PTM reveals the potential utility in regulating the activity of Bip.44 When the unfolded protein diminishes, ADP ribosylation evolves to inactivate Bip for a rapid reaction for incoming unfolded proteins.44 Upon oxidative stress, glutathionylation and sulfenylation in Bip molecular can abolish its ATPase activity and enhance the ability to prevent protein aggregation.45,46 Other different PTMs among the BiP have been identified, including arginylation,47 phosphorylation,48 AMPylation,49 aldehyde adducts,50 and so forth. The relationship between Bip PTMs remains uncertain. Our study detected 9 KCr site modifications and 18 Khib site modifications on amino acids of BiP molecular. In addition, eight sites (K96, K154, K163, K352, K446, K523, K553, and K579) in Bip were identified as having both KCr and Khib modifications. Previous study showed that Bip sumoylation happened in K352.51 Between ESRD patients and normal controls, five differential KCr modification sites (K118, K446, K523, K553, and K579) and three differential Khib modification sites (K185, K213, and K164) were detected. Besides Bip, other proteins (GRP94, CNX, CRT, PDIs, GlcII, ERP57, Bap31, Hsp70, and Hsp90) further identified as downstream molecules of Bip are shown to be having both KCr and Khib modifications, indicating that many ER proteins may utilize the two PTMs to participate in ER processes. GRP94 as well as Bip are involved in the general folding biological process. Calnexin and calreticulin promote the efficient folding of glycoproteins.35 BAP31 has been reported to be involved in ER homeostasis and initiates cell death when a terminal misfold happens in the ER.52 PDIs whose activity can be regulated by S-glutathionylation can catalyze disulfide bond formation between protein cysteine residues in the ER. HSC70 and HSP90, as molecular chaperones, act together to regulate the folding of non-native nascent proteins in the ER and to further prevent protein misfolding and aggregation.53 Our study detected extensive co-occurring modification of KCr and Khib on the same site in several protein molecules in the KEGG pathway of protein processing in the ER. Thus, we put forward the following hypothesis: KCr and Khib modifications may involve regulating the ability of the processing protein of the ER of ESRD patients, especially through the key molecule Bip, to mediate the ER stress of ESRD patients.
The tissue/organ of individuals with ESRD may have an abnormality in energy metabolism and hypoxia because of malnutrition and anemia, which may induce ER stress.37,54,55 Patients with uremia often have complications of disordered glucose and lipid metabolism.56,57 Maintenance HD in ESRD has been considered as a catabolic event.58 Glycolysis is activated in this process, although the underlying mechanism is unclear.57 In our study, nine DMPs having both KCr and Khib modifications were related to the glycolysis/gluconeogenesis pathway, containing two key regulatory enzymes (hexokinase-1 coding by HK1 and pyruvate kinase coding by PKM) in the glycolysis part. The subcellular localization revealed that those nine proteins are located in the cytoplasm. Both KCr and Khib modifications co-occurring in the same site happened in two key enzymes, including HK1 (K315) and PKM (K62, K135, K261, K270, and K498). The two most abundant dual modification proteins were ENO1 and PGK1 with 15 sites and 8 sites, respectively. It is reported that P300 possesses the ability to regulate crotonylation and 2-hydroxyisobutyrylation modifications apart from acetylation.14,20 Lysine 2-hydroxyisobutyrylation and crotonylation comodify many proteins involved in glycolysis and host invasion of protozoon.13,32 In our study, lysine residue K228 in ENO1 comodified with Khib and KCr was on its surface, making it accessible for p300-mediating dynamic modifications to further decrease the glucose depletion-induced cell death by regulating glycolysis.14 Khib modification in K228 was a significant differential expression between ESRD patients and normal controls, while KCr in the site revealed no difference. It should be noted that KCr and Khib modifications in lysine K221 nearby K228 revealed a significant difference. The energy supply of ATP may affect protein folding in the ER by affecting the chaperone activity. The abnormal glycolysis/gluconeogenesis process, together with the ER stress, may induce cell apoptosis. ESRD is associated with a global decrease in the absolute total number of lymphocytes59 and higher apoptosis occurrence of T naive cells,60 which are the chief component of PBMCs. Abnormal glycolysis in human PBMCs may block Th17 differentiation and affect the survival of memory T cells.61 Thus, we propose to put forward the hypothesis that KCr and Khib comodifications may influence the number of immunocytes and further induce immune senescence in ESRD patients through the glycolysis/gluconeogenesis pathway and protein processing in the ER process, which may be a potential therapeutic direction in the future.
Our research still has some limitations. First, the proteomic dates were the average values of the pooled samples of ESRD patients and healthy controls. The individual information may be neglected in LC–MS/MS measurements. Second, the proposed hypothesis needs further experimental verification, which is lacking in this study. Third, we provided an overall co-occurring phenomenon of Khib and KCr modifications in the ESRD state. However, the differentially modified KCr or Khib may be up- or downregulated between ESRD and healthy state or may be regulated by the other modification (Khib or KCr) co-occurring in the same protein at even the same site which could not be distinguished. Overall, this work provides a new direction on the possible implication of KCr and Khib in the immunologic dysfunction of patients with ESRD.
Collectively, we present the first comprehensive of overlap DMPs of KCr and Khib modifications in PBMCs between ESRD patients and normal controls. The overlap DMPs of dual KCr and Khib were enriched in the pathway of protein processing in the ER and glycolysis/gluconeogenesis pathways. In addition, we first probed into the site-specific profile of co-occurring of KCr and Khib modifications with proteomic technology, which may provide new insight into the pathogenesis of ESRD through regulation between KCr and Khib modifications and even serve as a precious resource for a wide range of scientific research.
4. Materials and Methods
4.1. Sample Preparation
Twenty patients from Shenzhen People’s Hospital were diagnosed with ESRD and received HD treatment three times a week. All patients’ durations of dialysis were more than 6 months. Meanwhile, none of them had apparent HD complications, including dialysis disequilibrium syndrome, hemolysis, allergic reactions, intradialytic hypotension, intradialytic hypertension, and arrhythmia. The 20 patients’ peripheral blood samples were collected before the first HD for one week. Twenty healthy controls involved came from the Physical Center of Shenzhen People’s Hospital and could exclude chronic diseases (diabetes, hypertension, cancer, etc.). Every participant signed an informed consent form. The study was approved by the Clinical Research Ethics Committee of the Shenzhen People’s Hospital. The clinical parameters between ESRD patients and healthy controls are shown in Supporting Information Table S6. The peripheral blood of all participants was collected. PBMCs were isolated within 4 h and stored at −80 °C immediately.
4.2. Protein Extraction
The sample was ground into cell powder by liquid nitrogen. Four volumes of lysis buffer [8 M urea, 1% Triton X-100, 3 μM Trostatin A, 50 mM Nicotinamide, and 2 mM ethylenediaminetetraacetic acid (EDTA)] was added to the cell powder followed by sonication thrice using a high-intensity ultrasonic processor (Scientz). Cell debris was removed and filtered by centrifugation at 12,000g and 4 °C for 10 min. A bicinchoninic acid kit (Sigma Chemical Co., St. Louis, MO) was used to assay the protein concentration according to the manufacturer’s instructions. 200 μg of proteins from each sample was extracted followed by combining to a pool of 1.8 mg of ESRD patient samples and a pool of 1.8 mg of healthy control samples.
4.3. Trypsin Digestion and TMT
Before trypsinization, 100 mM triethylammonium bicarbonate was added to the protein sample to a final urea concentration of less than 2 M. Trypsin was added at 1:50 (pancreatin/protein) for the first digestion overnight and then 1:100 (pancreatin/protein) for a second 4 h digestion.
After trypsin digestion, the peptide was formed by desalination with a Strata X C18 SPE column (Phenomenex, Torrance, CA) and then vacuumed dry. The peptides were reconstituted and processed with a TMT label according to the manufacturer’s protocols.
4.4. Affinity Enrichment
Before Khib enrichment, anti-Khib beads (PTM Bio) were washed twice with PBS. Tryptic peptides were dissolved in IP solution (100 mM NaCl, 1 mM EDTA, 50 mM Tris–HCl, 0.5% NP-40, pH 8.0) and incubated with prewashed antibody beads at 4 °C overnight with gentle shaking. After the incubation, the beads were washed four times with NETN buffer and twice with ddH2O. Then, the bound peptides were removed from the beads with 0.1% trifluoroacetic acid for LC–MS/MS analysis. The electrospray voltage applied was set to 2.0 kV.
4.5. LC–MS/MS Analysis
The tryptic peptides were first redissolved in solvent A which contains 0.1% formic acid and straightly loaded onto a home-made reversed-phase analytical column with a size of 15 cm length and 75 mm inner diameter. The gradient contained a rise from 6 to 23% of solvent B (0.1% formic acid in 98% acetonitrile) for 26 min, 23 to 35% for 8 min, and increasing to 80% for 3 min and holding at 80% for the last 3 min. All of the above steps were at a constant flow rate of 400 nL/min on an EASYnLC 1000 UPLC system. Then, the peptides were submitted to a nanospray ionization source, followed by MS/MS in Q ExactiveTM Plus (Thermo) coupled online to a UPLC apparatus. The m/z scan range of the full scan was 350 to 1800. Intact peptides were detected with an Orbitrap at a resolution of 70,000. The peptides were selected for MS/MS by setting the normalized collision energy at 28, and the fragments were detected in the Orbitrap at a resolution of 17,500. A data-dependent procedure alternated between 1 MS scan and 20 MS/MS scans with a standard of 15.0 s dynamic exclusion duration. The automatic gain control parameter was set at 5 × 104, and the fixed first mass was set as 100 m/z.
4.6. Database Search
Maxquant search engine (v.1.5.2.8) was used for data analysis.62 Human database concatenated with a reverse decoy database was utilized for tandem MS data searching. Trypsin/P was set as the cleavage enzyme. Missing cleavages with ≤4 were allowed. The mass error was set to 20 ppm for the precursor in the first search and 5 ppm in the main search. The standard was set to 0.02 Da for fragments. Carbamidomethylation was set as fixed modification on cysteine, and crotonylation and oxidation on methionine and 2-hydroxyisobutyrylation on Lys were set as variable modifications. The false discovery rate was set at <1%, and the minimum score for modified peptides was adjusted to >40.
4.7. Statistical Analyses on Khib and Kcr Overlap Proteins
4.7.1. GO and KEGG
Lysine crotonylation dataset26 in the previous study from our laboratory was further utilized. We used a quantitative KCr/Khib-proteomic method to examine the KCr/Khib modification degree between ESRD and normal control, excluding the impact of the corresponding protein expression.63,64 Protein modifications (KCr/Khib) with a fold change ≥1.20 or ≤1/1.2 between ESRD patients and healthy controls were considered as DMPs. Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/) was applied to calculate the expression of overlapped DMPs with KCr/Khib. WOLF PSORT was utilized to predict the subcellular localization. GO enrichment analysis was performed by using Bingo in Cystoscape (v3.8.2).65 KEGG pathway enrichment was carried out according to ClueGO in Cystoscape.66 A corrected p-value < 0.05 was considered to be statistically significant of all bioinformatics analysis.
4.7.2. PPI Methods and Cluster Analysis with Molecular Complex Detection
STRING was utilized in the construction of the PPI network of the overlap DMPs of dual KCr and Khib. Then, two PPI networks of dual modified proteins involved in protein processing in ER and glycolysis/gluconeogenesis pathways were constructed, and the top three hub genes were identified by CytoHubba. Cluster analysis in the PPI network was processed in the MCODE (version 1.5.1) with configuration standard as follows: degree cutoff = 2, MCODE scores > 5, node score cutoff = 0.2, K-core = 2, and max. depth = 100. Cystoscape (version 3.8.2) was used to visualize the interaction network.
Acknowledgments
This work was supported by the Key Renal Laboratory of Shenzhen (grant number ZDSYS2015430161623417), the Guangxi Key Laboratory of Metabolic Diseases Research (grant number 20-065-76), the Science and Technology Plan of Shenzhen (grant number JCYJ20190807153405508), the Guangdong Provincial High-level Clinical Key Specialties (grant number SZGSP001), the Natural Science Foundation of Guangxi (grant number 2017GXNSFAA198185), the Science and Technology Plan of Shenzhen (grant number JCYJ201803 06140810282), the Key Research and Development Program of Guangdong Province (grant number 2019B020229001), and 2019 Dongguan Social Science and Technology Development (key) project (grant number 201950715002195).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01161.
Crotonylation and 2-hydroxyisobutyrylation sites on 78 kDa glucose-regulated protein/Bip of PBMC identified in this study; crotonylation and 2-hydroxyisobutyrylation sites on HK1 of PBMC identified in this study; crotonylation and 2-hydroxyisobutyrylation sites on PKM of PBMC identified in this study; crotonylation and 2-hydroxyisobutyrylation sites on ENO1 of PBMC identified in this study; crotonylation and 2-hydroxyisobutyrylation sites on PGK1 of PBMC identified in this study; and clinical results of ESRD patients and healthy controls (PDF)
Author Contributions
H.L. contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Costa E.; Rocha S.; Rocha-Pereira P.; Nascimento H.; Castro E.; Miranda V.; do Sameiro Faria M.; Loureiro A.; Quintanilha A.; Belo L. i. s.; Santos-Silva A. Neutrophil activation and resistance to recombinant human erythropoietin therapy in hemodialysis patients. Am. J. Nephrol. 2008, 28, 935–940. 10.1159/000142147. [DOI] [PubMed] [Google Scholar]
- Betjes M. G. H. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- Vaziri N. D.; Pahl M. V.; Crum A.; Norris K. Effect of uremia on structure and function of immune system. J. Renal Nutr. 2012, 22, 149–156. 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.; Pan J.; Shah P.; Ao M.; Thomas S. N.; Liu Y.; Chen L.; Schnaubelt M.; Clark D. J.; Rodriguez H.; Boja E. S.; Hiltke T.; Kinsinger C. R.; Rodland K. D.; Li Q. K.; Qian J.; Zhang Z.; Chan D. W.; Zhang H.; Pandey A.; Paulovich A.; Hoofnagle A.; Zhang B.; Mani D. R.; Liebler D. C.; Ransohoff D. F.; Fenyo D.; Tabb D. L.; Levine D. A.; Kuhn E.; White F. M.; Whiteley G. A.; Zhu H.; Shih I.-M.; Bavarva J.; McDermott J. E.; Whiteaker J.; Ketchum K. A.; Clauser K. R.; Ruggles K.; Elburn K.; Ding L.; Hannick L.; Zimmerman L. J.; Watson M.; Thiagarajan M.; Ellis M. J. C.; Oberti M.; Mesri M.; Sanders M. E.; Borucki M.; Gillette M. A.; Snyder M.; Edwards N. J.; Vatanian N.; Rudnick P. A.; McGarvey P. B.; Mertins P.; Townsend R. R.; Thangudu R. R.; Smith R. D.; Rivers R. C.; Slebos R. J. C.; Payne S. H.; Davies S. R.; Cai S.; Stein S. E.; Carr S. A.; Skates S. J.; Madhavan S.; Liu T.; Chen X.; Zhao Y.; Wang Y.; Shi Z. Integrated Proteomic and Glycoproteomic Characterization of Human High-Grade Serous Ovarian Carcinoma. Cell Rep. 2020, 33, 108276. 10.1016/j.celrep.2020.108276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previs M. J.; VanBuren P.; Begin K. J.; Vigoreaux J. O.; LeWinter M. M.; Matthews D. E. Quantification of protein phosphorylation by liquid chromatography-mass spectrometry. Anal. Chem. 2008, 80, 5864–5872. 10.1021/ac800337v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Andres O.; Sanchez-Niño M. D.; Cannata-Ortiz P.; Ruiz-Ortega M.; Egido J.; Ortiz A.; Sanz A. B. Histone lysine crotonylation during acute kidney injury in mice. Dis. Models Mech. 2016, 9, 633–645. 10.1242/dmm.024455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez P.; Parca L.; Diella F.; Mende D. R.; Kumar R.; Helmer-Citterich M.; Gavin A. C.; van Noort V.; Bork P. Deciphering a global network of functionally associated post-translational modifications. Mol. Syst. Biol. 2012, 8, 599. 10.1038/msb.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T.; Xia X.; Liu J.; Wang G.; Guo Y.; Guo X.; Wang X.; Sha J. Beyond single modification: Reanalysis of the acetylproteome of human sperm reveals widespread multiple modifications. J. Proteomics 2015, 126, 296–302. 10.1016/j.jprot.2015.05.022. [DOI] [PubMed] [Google Scholar]
- Samanta L.; Swain N.; Ayaz A.; Venugopal V.; Agarwal A. Post-Translational Modifications in sperm Proteome: The Chemistry of Proteome diversifications in the Pathophysiology of male factor infertility. Biochim. Biophys. Acta 2016, 1860, 1450–1465. 10.1016/j.bbagen.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Vu L. D.; Gevaert K.; De Smet I. Protein Language: Post-Translational Modifications Talking to Each Other. Trends Plant Sci. 2018, 23, 1068–1080. 10.1016/j.tplants.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Prabakaran S.; Lippens G.; Steen H.; Gunawardena J. Post-translational modification: nature’s escape from genetic imprisonment and the basis for dynamic information encoding. Wiley Interdiscip. Rev.: Syst. Biol. Med. 2012, 4, 565–583. 10.1002/wsbm.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.; Luo Z.; Qi S.; Huang J.; Xu P.; Wang X.; Gao L.; Li F.; Wang J.; Zhao W.; Gu W.; Chen Z.; Dai L.; Dai J.; Zhao Y. Landscape of the regulatory elements for lysine 2-hydroxyisobutyrylation pathway. Cell Res. 2018, 28, 111–125. 10.1038/cr.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.; Luo Z.; Ying W.; Cao Q.; Huang H.; Dong J.; Wu Q.; Zhao Y.; Qian X.; Dai J. 2-Hydroxyisobutyrylation on histone H4K8 is regulated by glucose homeostasis in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, 8782–8787. 10.1073/pnas.1700796114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.; Tang S.; Ji M.; Tang Z.; Shimada M.; Liu X.; Qi S.; Locasale J. W.; Roeder R. G.; Zhao Y.; Li X. p300-Mediated Lysine 2-Hydroxyisobutyrylation Regulates Glycolysis. Mol. Cell 2018, 70, 663–678.e6. 10.1016/j.molcel.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Andres O.; Suarez-Alvarez B.; Sánchez-Ramos C.; Monsalve M.; Sanchez-Niño M. D.; Ruiz-Ortega M.; Egido J.; Ortiz A.; Sanz A. B. The inflammatory cytokine TWEAK decreases PGC-1α expression and mitochondrial function in acute kidney injury. Kidney Int. 2016, 89, 399–410. 10.1038/ki.2015.332. [DOI] [PubMed] [Google Scholar]
- Dai L.; Peng C.; Montellier E.; Lu Z.; Chen Y.; Ishii H.; Debernardi A.; Buchou T.; Rousseaux S.; Jin F.; Sabari B. R.; Deng Z.; Allis C. D.; Ren B.; Khochbin S.; Zhao Y. Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. Nat. Chem. Biol. 2014, 10, 365–370. 10.1038/nchembio.1497. [DOI] [PubMed] [Google Scholar]
- Tan M.; Luo H.; Lee S.; Jin F.; Yang J. S.; Montellier E.; Buchou T.; Cheng Z.; Rousseaux S.; Rajagopal N.; Lu Z.; Ye Z.; Zhu Q.; Wysocka J.; Ye Y.; Khochbin S.; Ren B.; Zhao Y. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 2011, 146, 1016–1028. 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabari B. R.; Zhang D.; Allis C. D.; Zhao Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 2017, 18, 90–101. 10.1038/nrm.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabari B. R.; Tang Z.; Huang H.; Yong-Gonzalez V.; Molina H.; Kong H. E.; Dai L.; Shimada M.; Cross J. R.; Zhao Y.; Roeder R. G.; Allis C. D. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol. Cell 2015, 58, 203–215. 10.1016/j.molcel.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabari B. R.; Tang Z.; Huang H.; Yong-Gonzalez V.; Molina H.; Kong H. E.; Dai L.; Shimada M.; Cross J. R.; Zhao Y.; Roeder R. G.; Allis C. D. Intracellular Crotonyl-CoA Stimulates Transcription through p300-Catalyzed Histone Crotonylation. Mol. Cell 2018, 69, 533. 10.1016/j.molcel.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarska Z.; Ortega E.; Goudarzi A.; Huang H.; Kim S.; Márquez J. A.; Zhao Y.; Khochbin S.; Panne D. Structure of p300 in complex with acyl-CoA variants. Nat. Chem. Biol. 2017, 13, 21–29. 10.1038/nchembio.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. D. W.; Chandru A.; Watson P. J.; Song Y.; Blades M.; Robertson N. S.; Jamieson A. G.; Schwabe J. W. R.; Cowley S. M. Histone deacetylase (HDAC) 1 and 2 complexes regulate both histone acetylation and crotonylation in vivo. Sci. Rep. 2018, 8, 14690. 10.1038/s41598-018-32927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows R.; Denizot J.; Stellato C.; Cuomo A.; Jain P.; Stoyanova E.; Balázsi S.; Hajnády Z.; Liebert A.; Kazakevych J.; Blackburn H.; Corrêa R. O.; Fachi J. L.; Sato F. T.; Ribeiro W. R.; Ferreira C. M.; Perée H.; Spagnuolo M.; Mattiuz R.; Matolcsi C.; Guedes J.; Clark J.; Veldhoen M.; Bonaldi T.; Vinolo M. A. R.; Varga-Weisz P. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 2018, 9, 105. 10.1038/s41467-017-02651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.; Ke L.; Wang C.; Fan P.; Wu Z.; Xu X. Global Analysis of Lysine 2-Hydroxyisobutyrylome upon SAHA Treatment and Its Relationship with Acetylation and Crotonylation. J. Proteome Res. 2018, 17, 3176–3183. 10.1021/acs.jproteome.8b00289. [DOI] [PubMed] [Google Scholar]
- Martinez-Moreno J. M.; Fontecha-Barriuso M.; Martín-Sánchez D.; Sánchez-Niño M. D.; Ruiz-Ortega M.; Sanz A. B.; Ortiz A. The Contribution of Histone Crotonylation to Tissue Health and Disease: Focus on Kidney Health. Front. Pharmacol. 2020, 11, 393. 10.3389/fphar.2020.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Tang D.; Xu Y.; Zou Y.; Sui W.; Dai Y.; Diao H. Comprehensive analysis of lysine crotonylation in proteome of maintenance hemodialysis patients. Med 2018, 97, e12035 10.1097/md.0000000000012035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelli S.; Corbo M.; Iannuzzi F.; Negri L.; Blandini F.; Nistico R.; Feligioni M. The Involvement of Post-Translational Modifications in Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 313–335. 10.2174/1567205014666170505095109. [DOI] [PubMed] [Google Scholar]
- Gajjala P. R.; Fliser D.; Speer T.; Jankowski V.; Jankowski J. Emerging role of post-translational modifications in chronic kidney disease and cardiovascular disease. Nephrol., Dial., Transplant. 2015, 30, 1814–1824. 10.1093/ndt/gfv048. [DOI] [PubMed] [Google Scholar]
- Lobel L.; Cao Y. G.; Fenn K.; Glickman J. N.; Garrett W. S. Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Sci 2020, 369, 1518–1524. 10.1126/science.abb3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes M.; Hall B.; Foltz L.; Levy T.; Rikova K.; Gaiser J.; Cook W.; Smirnova E.; Wheeler T.; Clark N. R.; Lachmann A.; Zhang B.; Hornbeck P.; Ma’ayan A.; Comb M. Integration of protein phosphorylation, acetylation, and methylation data sets to outline lung cancer signaling networks. Sci. Signaling 2018, 11, eaaq1087 10.1126/scisignal.aaq1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C. E.; English D. M.; Cowley S. M. Acetylation & Co: an expanding repertoire of histone acylations regulates chromatin and transcription. Essays Biochem. 2019, 63, 97–107. 10.1042/ebc20180061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D.; Jiang N.; Zhang Y.; Wang D.; Sang X.; Feng Y.; Chen R.; Wang X.; Yang N.; Chen Q. Global Lysine Crotonylation and 2-Hydroxyisobutyrylation in Phenotypically Different Toxoplasma gondii Parasites. Mol. Cell. Proteomics 2019, 18, 2207–2224. 10.1074/mcp.ra119.001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Zhou X.; Zhai Z.; Li T. Co-occurring protein phosphorylation are functionally associated. PLoS Comput. Biol. 2017, 13, e1005502 10.1371/journal.pcbi.1005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks I. A.; Lyon D.; Young C.; Jensen L. J.; Vertegaal A. C. O.; Nielsen M. L. Site-specific mapping of the human SUMO proteome reveals co-modification with phosphorylation. Nat. Struct. Mol. Biol. 2017, 24, 325–336. 10.1038/nsmb.3366. [DOI] [PubMed] [Google Scholar]
- Braakman I.; Hebert D. N. Protein folding in the endoplasmic reticulum. Cold Spring Harbor Perspect. Biol. 2013, 5, a013201. 10.1101/cshperspect.a013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C.; Chevet E.; Oakes S. A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015, 17, 829–838. 10.1038/ncb3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N.; Talwar P.; Parimisetty A.; Lefebvre d’Hellencourt C.; Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. 2014, 8, 213. 10.3389/fncel.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky A. V. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat. Rev. Nephrol. 2017, 13, 681–696. 10.1038/nrneph.2017.129. [DOI] [PubMed] [Google Scholar]
- Yuan Q.; Xu T.; Chen Y.; Qu W.; Sun D.; Liu X.; Sun L. MiR-185-5p ameliorates endoplasmic reticulum stress and renal fibrosis by downregulation of ATF6. Lab. Invest. 2020, 100, 1436–1446. 10.1038/s41374-020-0447-y. [DOI] [PubMed] [Google Scholar]
- Wang Z.; do Carmo J. M.; Aberdein N.; Zhou X.; Williams J. M.; da Silva A. A.; Hall J. E. Synergistic Interaction of Hypertension and Diabetes in Promoting Kidney Injury and the Role of Endoplasmic Reticulum Stress. Hypertension 2017, 69, 879–891. 10.1161/hypertensionaha.116.08560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Lee J.; Liem D.; Ping P. HSPA5 Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene 2017, 618, 14–23. 10.1016/j.gene.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S. M.; Choi H. R.; Sung K. W.; Lee Y. J.; Kim S. T.; Kim D.; Mun S. R.; Hwang J.; Cha-Molstad H.; Ciechanover A.; Kim B. Y.; Kwon Y. T. The endoplasmic reticulum-residing chaperone BiP is short-lived and metabolized through N-terminal arginylation. Sci. Signaling 2018, 11, eaan0630 10.1126/scisignal.aan0630. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Ye Z.-w.; Chen W.; Culpepper J.; Jiang H.; Ball L. E.; Mehrotra S.; Blumental-Perry A.; Tew K. D.; Townsend D. M. Altered redox regulation and S-glutathionylation of BiP contribute to bortezomib resistance in multiple myeloma. Free Radical Biol. Med. 2020, 160, 755–767. 10.1016/j.freeradbiomed.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. E.; Petrova K.; Tomba G.; Vendruscolo M.; Ron D. ADP ribosylation adapts an ER chaperone response to short-term fluctuations in unfolded protein load. J. Cell Biol. 2012, 198, 371–385. 10.1083/jcb.201202005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Pareja K. A.; Kaiser C. A.; Sevier C. S. Redox signaling via the molecular chaperone BiP protects cells against endoplasmic reticulum-derived oxidative stress. eLife 2014, 3, e03496 10.7554/eLife.03496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Sevier C. S. Formation and Reversibility of BiP Protein Cysteine Oxidation Facilitate Cell Survival during and post Oxidative Stress. J. Biol. Chem. 2016, 291, 7541–7557. 10.1074/jbc.m115.694810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha-Molstad H.; Sung K. S.; Hwang J.; Kim K. A.; Yu J. E.; Yoo Y. D.; Jang J. M.; Han D. H.; Molstad M.; Kim J. G.; Lee Y. J.; Zakrzewska A.; Kim S.-H.; Kim S. T.; Kim S. Y.; Lee H. G.; Soung N. K.; Ahn J. S.; Ciechanover A.; Kim B. Y.; Kwon Y. T. Amino-terminal arginylation targets endoplasmic reticulum chaperone BiP for autophagy through p62 binding. Nat. Cell Biol. 2015, 17, 917–929. 10.1038/ncb3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot L. M.; Ting J.; Lee A. S. Identity of the immunoglobulin heavy-chain-binding protein with the 78,000-dalton glucose-regulated protein and the role of posttranslational modifications in its binding function. Mol. Cell. Biol. 1988, 8, 4250–4256. 10.1128/mcb.8.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A.; Chen A. J.; Nakayasu E. S.; Lazar C. S.; Zbornik E. A.; Worby C. A.; Koller A.; Mattoo S. A novel link between Fic (filamentation induced by cAMP)-mediated adenylylation/AMPylation and the unfolded protein response. J. Biol. Chem. 2015, 290, 8482–8499. 10.1074/jbc.m114.618348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan J. J.; Fritz K. S.; Backos D. S.; Shearn C. T.; Smathers R. L.; Jiang H.; MacLean K. N.; Reigan P. R.; Petersen D. R. Oxidative stress-mediated aldehyde adduction of GRP78 in a mouse model of alcoholic liver disease: functional independence of ATPase activity and chaperone function. Free Radical Biol. Med. 2014, 73, 411–420. 10.1016/j.freeradbiomed.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impens F.; Radoshevich L.; Cossart P.; Ribet D. Mapping of SUMO sites and analysis of SUMOylation changes induced by external stimuli. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 12432–12437. 10.1073/pnas.1413825111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K.; Han B.; Bai Y.; Ma X.-Y.; Ji Z.-N.; Xiong Y.; Miao S.-K.; Zhang Y.-Y.; Zhou L.-M. MiR-451a suppressing BAP31 can inhibit proliferation and increase apoptosis through inducing ER stress in colorectal cancer. Cell Death Dis. 2019, 10, 152. 10.1038/s41419-019-1403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K.; Haslbeck M.; Buchner J. The heat shock response: life on the verge of death. Mol. Cell. 2010, 40, 253–266. 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Li J.; Wang J. J.; Yu Q.; Wang M.; Zhang S. X. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009, 583, 1521–1527. 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D. E.; Chauhan V.; Koong A. C. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol. Cancer Res. 2005, 3, 597–605. 10.1158/1541-7786.mcr-05-0221. [DOI] [PubMed] [Google Scholar]
- Koppe L.; Nyam E.; Vivot K.; Manning Fox J. E.; Dai X.-Q.; Nguyen B. N.; Trudel D.; Attané C.; Moullé V. S.; MacDonald P. E.; Ghislain J.; Poitout V. Urea impairs β cell glycolysis and insulin secretion in chronic kidney disease. J. Clin. Invest. 2016, 126, 3598–3612. 10.1172/jci86181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee E. P.; Souza A.; Farrell L.; Pollak M. R.; Lewis G. D.; Steele D. J. R.; Thadhani R.; Clish C. B.; Greka A.; Gerszten R. E. Metabolite profiling identifies markers of uremia. J. Am. Soc. Nephrol. 2010, 21, 1041–2051. 10.1681/asn.2009111132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj D. S. C.; Welbourne T.; Dominic E. A.; Waters D.; Wolfe R.; Ferrando A. Glutamine kinetics and protein turnover in end-stage renal disease. Am. J. Physiol.: Endocrinol. Metab. 2005, 288, E37–E46. 10.1152/ajpendo.00240.2004. [DOI] [PubMed] [Google Scholar]
- Freitas G. R. R.; da Luz Fernandes M.; Agena F.; Jaluul O.; Silva S. C.; Lemos F. B. C.; Coelho V.; Elias D.-N.; Galante N. Z. Aging and End Stage Renal Disease Cause A Decrease in Absolute Circulating Lymphocyte Counts with A Shift to A Memory Profile and Diverge in Treg Population. Aging Dis. 2019, 10, 49–61. 10.14336/ad.2018.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang F.; Chen R.; Cao X.; Shen B.; Chen X.; Ding X.; Zou J. Premature aging of circulating T cells predicts all-cause mortality in hemodialysis patients. BMC Nephrol. 2020, 21, 271. 10.1186/s12882-020-01920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluxton D.; Petrasca A.; Moran B.; Fletcher J. M. Differential Regulation of Human Treg and Th17 Cells by Fatty Acid Synthesis and Glycolysis. Front. Immunol. 2019, 10, 115. 10.3389/fimmu.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyanova S.; Temu T.; Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. 10.1038/nprot.2016.136. [DOI] [PubMed] [Google Scholar]
- Hebert A. S.; Dittenhafer-Reed K. E.; Yu W.; Bailey D. J.; Selen E. S.; Boersma M. D.; Carson J. J.; Tonelli M.; Balloon A. J.; Higbee A. J.; Westphall M. S.; Pagliarini D. J.; Prolla T. A.; Assadi-Porter F.; Roy S.; Denu J. M.; Coon J. J. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell 2013, 49, 186–199. 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N.; Gao R.; Yang J.; Zhu Y.; Zhang Z.; Xu X.; Wang J.; Liu X.; Li Z.; Li Z.; Gong D.; Li J.; Bi J.; Kong C. Quantitative Global Proteome and Lysine Succinylome Analyses Reveal the Effects of Energy Metabolism in Renal Cell Carcinoma. Proteomics 2018, 18, e1800001 10.1002/pmic.201800001. [DOI] [PubMed] [Google Scholar]
- Shannon P.; Markiel A.; Ozier O.; Baliga N. S.; Wang J. T.; Ramage D.; Amin N.; Schwikowski B.; Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito R.; Smoot M. E.; Ono K.; Ruscheinski J.; Wang P.-L.; Lotia S.; Pico A. R.; Bader G. D.; Ideker T. A travel guide to Cytoscape plugins. Nat. Methods 2012, 9, 1069–1076. 10.1038/nmeth.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.