Abstract
N-Aryl-3,4-dihydroisoquinoline carbothioamide analogues 1–22 were synthesized by a simple one-step reaction protocol and subjected to in vitro urease inhibition studies for the first time. All compounds 1–22 were found active and showed significant to moderate urease inhibitory potential. Specifically, analogues 1, 2, 4, and 7 were identified to be more potent (IC50 = 11.2 ± 0.81–20.4 ± 0.22 μM) than the standard thiourea (IC50 = 21.7 ± 0.34 μM). The structure–activity relationship showed that compounds bearing electron-donating groups showed superior activity. Molecular docking study on the most active derivatives revealed a good protein–ligand interaction profile against the corresponding target with key interactions, including hydrogen bonding, hydrophobic, and π-anion interactions.
Introduction
Urea is the chief nitrogenous left-over of biological systems, which is quickly metabolized by the action of microorganisms.1−3 Urease enzyme (EC 3.5.1.5) is present in bacteria, fungi, and plants. It plays a pivotal role in the nitrogen cycle, as it supplies nitrogen for the growth of microorganisms by catalyzing the hydrolysis of urea into ammonia and carbon dioxide at the rate approximately 1014 faster than a noncatalyzed reaction.4−6 Urease is important for hydrolysis of urea; nevertheless, its over expression leads to various problems in living organisms and causes environmental and economic damages.7 In humans and other animals, urease hyperactivity leads to various pathological problems, such as kidney stones, peptic ulcers, infection-induced reactive arthritis, and pyelonephritis.8 Urease is indispensable for colonization of human gastric mucosa by Helicobacter pylori. The ammonia produced from the hydrolysis of urea is toxic for various gastric cell lines, and it gives a suitable breeding ground for H. pylori leading to gastric ulcerations and cancers. It has also been reported that urease facilitates infectious stone formation in the kidneys, caused by Yersinia enterocolitica and Proteus mirabilis.9,10 In the agricultural sector, excessive levels of soil urease can rapidly degrade the fertilizer urea and result in phytopathic effects. In addition, loss of volatilized ammonia leads to poor crop yields.11−13 Therefore, the discovery of efficient and secured urease inhibitors is an important area in pharmaceutical and plant science due to the contribution of urease in a large number of pathological conditions and for agriculture applications.14,15
Heterocycles are also the central nucleus of numerous biologically active compounds and marketed drugs. Tetrahydroquinoline is a nitrogen-containing bicyclic compound, having a benzene ring fused with piperidine, and belongs to an important class of natural alkaloids. It is also found in mammalian alkaloids, e.g., salsolinecarboxylic acid, ecteinascidine family, and cactus alkaloids.16−18 Tetrahydroisoquinoline was prepared by the Pictet–Spengler reaction, hydrogenation of isoquinolines, Bischler–Napieralski cyclization of β-arylethylamides, and Pomeranz–Fritsch reaction.19−22 Tetrahydroisoquinolines are mostly used as intermediates for the manufacturing of pharmaceutical drugs with high therapeutic potencies.23 This partially aromatic alkaloid has attracted significant attention due to the indispensable biological activities, such as antitumor, antibacterial, and antihuman immunodeficiency virus activities. It plays an important role in the research to find a treatment for Parkinson’s disease.24−26 Quinazolinone derivatives bearing a coumarin nucleus exhibited potent lipase and α-glucosidase inhibitory activities.27
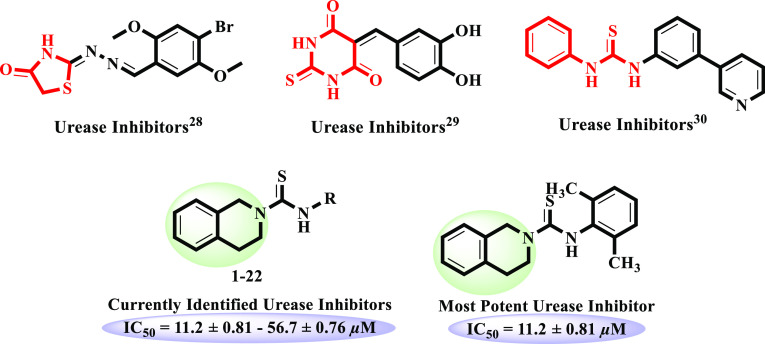
Our research group has reported a range of nitrogen-containing heterocycles, including thiazolidinone, thioxodihydropyrimidindione, and thiourea derivatives, for their potential urease inhibitory activities.28−30 Quinazolinone moieties linked with a range of heterocycles, such as coumarin, furan, triazole, and thiadiazole, as well as with hydrazone and thiosemicarbazide functionalities were reported as active urease inhibitors.31−33 It is noteworthy that tetrahydroisoquinoline has structural resemblance to a quinazoline ring. Furthermore, tetrahydroisoquinoline is not yet reported for urease inhibitory activity; therefore, we intended to explore this class for the said activity. Additionally, it is important to note that the thiourea moiety is also present in all synthetic analogues. Hence, the current research is based on the synthesis of N-aryl-3,4-dihydroisoquinoline-based carbothioamide analogues, in vitro urease inhibitory activity, and molecular docking studies (Figure 1).
Figure 1.
Rationale of the present research work.
Results and Discussion
N-Aryl-3,4-dihydroisoquinoline carbothioamide analogues 1–22 were synthesized by treating isothiocyanate derivatives under basic conditions (K2CO3). The reaction was carried out in acetone by stirring at room temperature for 5–30 min (Scheme 1). Reaction completion was periodically observed by thin layer chromatography (TLC) (hexane/ethyl acetate 8:2). The solvent was evaporated under reduced pressure after completion of reaction. Work-up and crystallization afforded the pure products. Structural elucidation of all synthetic compounds 1–22 was done by distinct spectroscopic techniques such as FAB (+ve), FAB (−ve), EI-MS, and 1H-NMR.Structures of compounds 13 and 22 are new; however, HR-MS-FAB (+ve) and 13C-NMR were performed on new derivatives.
Scheme 1. Synthesis of N-Aryl-3,4-Dihydroisoquinoline Carbothioamide Analogues 1–22.
Urease Inhibitory Activity In Vitro
N-Aryl-3,4-dihydroisoquinoline carbothioamide analogues 1–22 were subjected for the first time to possible urease inhibitory activity. It is interesting to mention that the whole library of compounds was found to be active against urease enzyme with IC50 ranges between 11.2 ± 0.81 and 56.7 ± 0.76 μM. Interestingly, compounds 1 (IC50 = 20.4 ± 0.22 μM), 2 (IC50 = 11.2 ± 0.81 μM), 4 (IC50 = 15.5 ± 0.49 μM), and 7 (IC50 = 18.5 ± 0.65 μM) showed more potent urease inhibitory activity as compared to the standard thiourea (IC50 = 21.7 ± 0.34 μM). However, rest of the derivatives showed good to moderate inhibitory potential (Table 1).
Table 1. In Vitro Urease Inhibitory Activity Results of N-Aryl-3,4-Dihydroisoquinoline Carbothioamide Analogues 1–22.

SEM (standard error mean)
Thiourea (standard inhibitor for urease enzyme)
Structure–Activity Relationship (SAR)
For the sake of simplification of the SAR, it is discussed on the basis of variation in the nature and position of substitutes on the aryl ring.
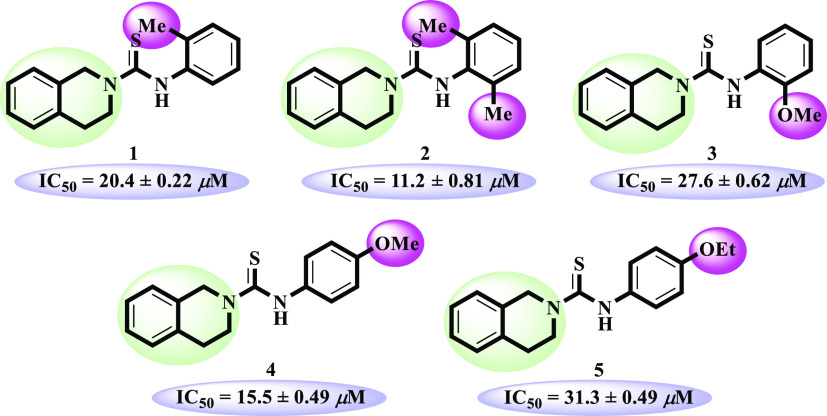
Electron-donating alkyl and alkoxy bearing analogues 1–5 were identified as excellent urease inhibitors. Among them, o-dimethyl-substituted compound 2 (IC50 = 11.2 ± 0.81 μM) was found to be the most potent inhibitor against urease enzyme, even more active than the standard thiourea (IC50 = 21.7 ± 0.34 μM). Lacking of one methyl as in the case of compound 1 (IC50 = 20.4 ± 0.22 μM) showed slightly decreased activity compared to compound 2. Among the methoxy-substituted compounds, p-methoxy-substituted compound 4 (IC50 = 15.5 ± 0.49 μM) exhibited excellent inhibitory activity, but changing the position of methoxy from para to ortho in compound 3 (IC50 = 27.6 ± 0.62 μM) decreased the inhibitory potential of the analogue. p-Ethoxy-substituted compound 5 (IC50 = 31.3 ± 0.49 μM) demonstrated enzyme inhibition comparable to the standard (Figure 2).
Figure 2.
Structure–activity relationship of compounds 1–5.
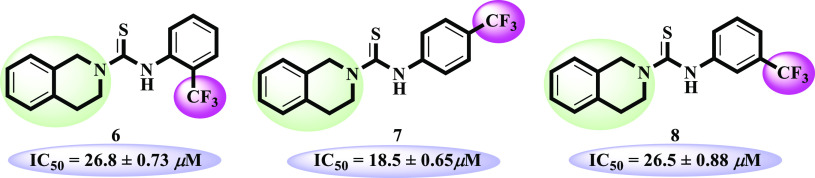
p-Trifluoro-substituted compound 7 (IC50 = 18.5 ± 0.65 μM) was the third most active compound of series. However, positional isomers 6 (IC50 = 26.8 ± 0.73 μM) and 8 (IC50 = 26.8 ± 0.88 μM) showed less inhibitory potential relative to compound 7. This activity comparison confirmed that trifluoro substitution at the para position uplifts the inhibitory strength to a good extent (Figure 3).
Figure 3.
Structure–activity relationship of compounds 6–8.
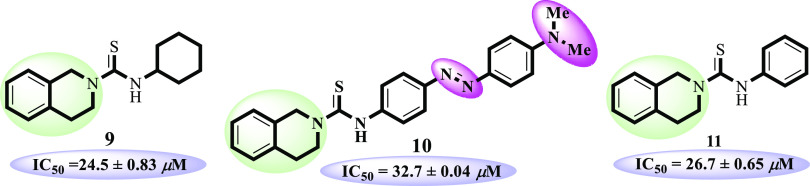
Compound 9 (IC50 = 24.5 ± 0.83 μM) is exceptionally substituted with an aliphatic cyclohexyl ring and interestingly found to be more active than 10 (IC50 = 32.7 ± 0.04 μM) and 11 (IC50 = 26.7 ± 0.65 μM). Compound 9 also showed potential comparable to standard thiourea (IC50 = 21.7 ± 0.34 μM). Replacement of cyclohexyl with a benzyl ring in analogue 11 and p-dimethylamino)phenyl)diazenyl substitution in 10 leads to decline in the inhibitory potential (Figure 4).
Figure 4.
Structure–activity relationship of compounds 9–11.
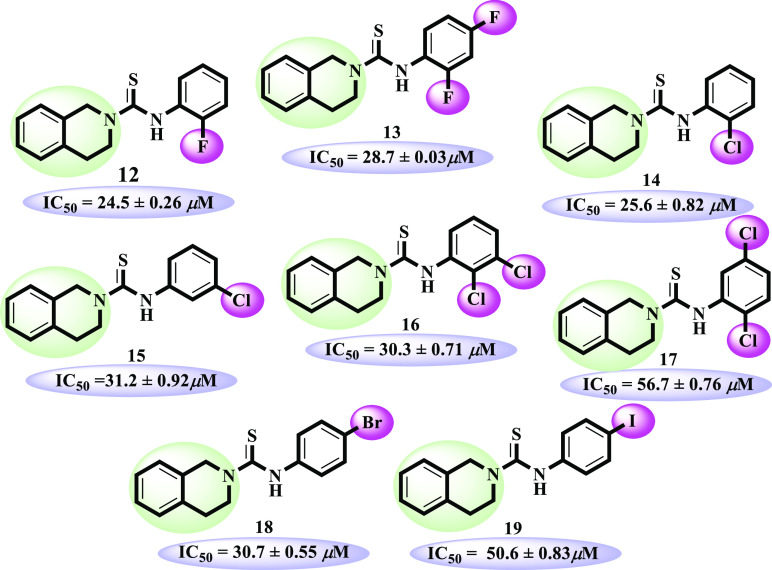
Compounds 12–19 possessing halogen substitutions (F, Cl, Br, and I). Except compounds 17 and 19, all derivatives were identified as good urease inhibitors. o-Fluoro-substituted analogue 12 (IC50 = 24.5 ± 0.26 μM) showed excellent inhibitory activity in the halogen-bearing group. Addition of another fluoro at the para position as in analogue 13 (IC50 = 28.7 ± 0.03 μM) leads to slightly decreased potential. o-Chloro-substituted analogue 14 (IC50 = 25.6 ± 0.82 μM) demonstrated almost similar activity to o-fluoro-substituted analogue 12. Moving the chloro group from the ortho to meta position as in compound 15 (IC50 = 31.2 ± 0.92 μM) showed a decreased inhibitory activity. However, o, m-dichloro-substituted derivative 16 (IC50 = 30.3 ± 0.71 μM) showed close inhibitory potential to that of m-chloro-substituted analogue 15. Surprisingly, the dichloro-substituted positional isomer of compound 16, that is, 17 (IC50 = 56.7 ± 0.76 μM), showed much less inhibitory potential, which demonstrates that dichloro groups do not really work like the monochloro group in urease inhibition. Furthermore, p-bromo-containing compound 18 (IC50 = 30.7 ± 0.55 μM) also showed inhibitory potential comparable to standard. Another p-iodo-containing compound 19 (IC50 = 50.6 ± 0.83 μM) showed much lesser inhibitory potential than mono F-, Cl-, and Br-bearing analogues. The moderate inhibitory activities of compounds 17 and 19 might be due to not attaining the conformations to well-fit in the active site of enzyme (Figure 5).
Figure 5.
Structure–activity relationship of compounds 12–19
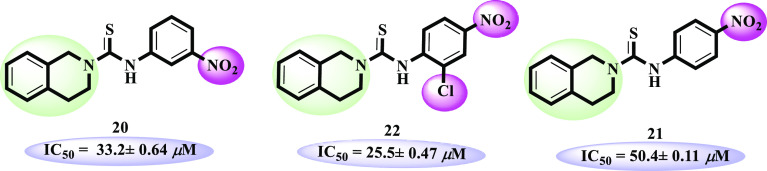
Among compounds 20–22, o-chloro-p-nitro-bearing compound 22 (IC50 = 25.5 ± 3.4 μM) was found to be significantly active. Compound 21 (IC50 = 50.4 ± 0.11 μM) lacking o-chloro showed moderate inhibitory potential compared to 22. However, its positional isomer 20 (IC50 = 33.2 ± 0.64 μM) having m-nitro substitution revealed a better inhibitory activity (Figure 6).
Figure 6.
Structure–activity relationship of compounds 11–13
The SAR showed that electron-donating groups such as OMe and Me play an important role in significant activity of molecules. However, other groups also demonstrated the significant activities when placed in certain numbers and positions.
Docking Study
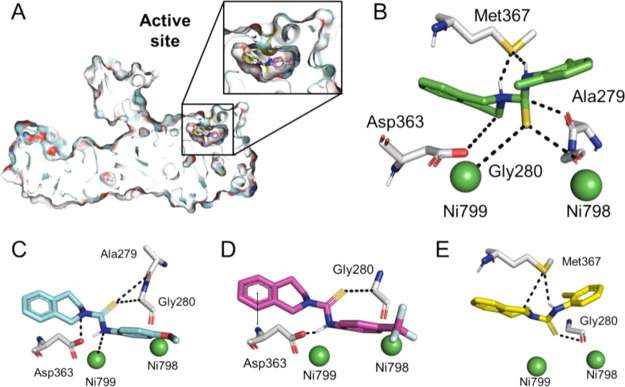
Molecular docking study was performed in order to elucidate in vitro studies of all synthesized compounds 1–22. Subsequently, all compounds showed fit-well binding mode with different affinities and correlate well with the in vitro studies (Figure 7A). All compounds have similar and related chemistry with slight modification at different positions. These different functionalities have found a wide difference in the interaction of these compounds to the active site. Among all, total eight compounds showed a good docking score and ligand interaction with active site residues. Compound 2 (Figure 7B) was found to be most effective, likewise the in vitro study. This compound showed maximum interaction with the active site of protein. The detail of interaction has been given in Table 2. Although this compound is quite like compound 1, the only difference is that compound 1 has one methyl group at the -para position; however, compound 2 has two methyl groups at -ortho and -para positions. The difference in enzymatic activity might be due to electron-donating moieties, which therefore more aggressively interact with the active site of enzyme. In other words, the chances of resonance are also more in compound 2 as both methyl groups are in the same plane and so can provide stability to the corresponding reacting species, which may be one of the reasons that the IC50 value of compound 2 is IC50 = 11.2 ± 0.81 μM and that of compound 1 is IC50 = 20.4 ± 0.22 μM as shown in Table 2 and Figure 7E. It has also been found that not only direct attachment of the electron-donating group becomes the cause of good activity, but their attachment to the electron-withdrawing group is also important, which in turn attaches to the ring of the compound. The better docking score, activity, and interaction of compound 4 (Figure 7C) were mainly due to this reason. In compound 4, although oxygen has been directly attached to the ring at one side and to methyl at the other, now instead oxygen withdraws electrons from the ring, and the methyl donates electrons, and hence, the ring remains electron rich.
Figure 7.
Binding mode of the synthesized compounds. (A) Surface representation of the enzyme with embedded ligands; (B) Binding mode of compound 2; (C) for compound 4; and (D) for compound 7; and (E) for compound 1. The double sided arrow indicates pi-H bonds.
Table 2. Protein–Ligand Interactions of all Compoundsa.
| interaction details | |||||||
|---|---|---|---|---|---|---|---|
| compounds | ligand atoms | receptor atom | interaction | distance | energy Kcal/mol | residue | docking score |
| 1 | S 13 | O | H-donor | 3.09 | 1.6 | GLY 280(C) | –7.55 |
| C 7 | SD | H-donor | 3.67 | –1.5 | MET 367(C) | ||
| 2 | 6-ring | CB | pi-H | 3.72 | –0.6 | ASP 363(C) | –8.36 |
| N 12 | SD | H-donor | 3.21 | –2.5 | MET 367(C) | ||
| C 11 | O | H-donor | 3.16 | –1.4 | ALA 279(C) | ||
| N 8 | SD | H-donor | 3.15 | –6.9 | MET 367(C) | ||
| C 7 | OD2 | H-donor | 3.28 | –1.3 | ASP 363(C) | ||
| 3 | N 12 | O | H-donor | 2.72 | –0.9 | ALA 279(C) | –6.38 |
| C 7 | SD | H-donor | 3.30 | –0.7 | MET 367(C) | ||
| 4 | S 13 | O | H-donor | 3.84 | –1.8 | ALA 279(C) | –8.19 |
| N 8 | OD2 | H-donor | 2.96 | –7.0 | ASP 363(C) | ||
| 5 | 6-ring | CB | pi-H | 4.54 | –0.5 | ASP 363(C) | –6.84 |
| S 13 | CA | H-acceptor | 3.63 | –0.8 | GLY 280(C) | ||
| 6 | F 21 | N | H-acceptor | 2.98 | –0.5 | ALA 366(C) | –6.26 |
| C 7 | SD | H-donor | 3.15 | –0.5 | MET 367(C) | ||
| 7 | S 13 | SD | H-acceptor | 3.35 | –0.9 | GLY 280(C) | –6.51 |
| N 2 | OD2 | H-donor | 2.0 | –6.5 | ASP 363(C) | ||
| 6-ring | NE2 | Pi-H | 3.65 | –0.5 | ASP 363(C) | ||
| 8 | F 21 | N | H-acceptor | 2.83 | –0.7 | ALA 366(C) | –6.63 |
| 9 | 6-ring | CB | pi-H | 3.83 | –0.5 | ASP 363(C) | –6.74 |
| S 13 | CG2 | H-acceptor | 4.47 | –0.5 | GLY 280 (C) | ||
| S 13 | CA | H-acceptor | 3.17 | –0.9 | THR 301(C) | ||
| C 9 | SD | H-donor | 3.45 | –0.6 | MET 367(C) | ||
| 10 | S 13 | CA | H-acceptor | 4.08 | –0.8 | HIS 315(C) | –5.21 |
| C 19 | OG1 | H-donor | 2.67 | –0.5 | THR 301(C) | ||
| N 12 | NE2 | H-donor | 3.08 | –1.6 | HIS 315(C) | ||
| 11 | 6-ring | 5-ring | pi-Pi | 3.28 | –0.0 | HIS 323(C) | –7.04 |
| N 12 | O | H-donor | 2.89 | –3.3 | ALA 279(C) | ||
| 12 | 6-ring | CB | pi-H | 3.82 | –0.8 | ASP 363(C) | –6.77 |
| N 12 | SD | H-donor | 3.39 | –1.3 | MET 367(C) | ||
| C 7 | SD | H-donor | 3.36 | –0.6 | MET 367(C) | ||
| 13 | 6-ring | CB | pi-H | 3.77 | –0.5 | ASP 363(C) | –6.08 |
| 6-ring | NH2 | pi-cation | 3.69 | –0.5 | ARG 339(C) | ||
| 14 | 6-ring | CB | pi-H | 3.97 | –0.5 | ASP 363(C) | –6.38 |
| CL 20 | O | H-donor | 3.55 | –1.1 | LYS 169(C) | ||
| 15 | 6-ring | CB | pi-H | 4.39 | –0.6 | ASP 363(C) | –6.39 |
| 16 | 6-ring | 5-ring | pi-pi | 3.28 | –0.0 | HIS 323(C) | –7.31 |
| 17 | S 13 | CA | H-acceptor | 3.17 | –1.0 | GLY 280(C) | –6.59 |
| N 8 | SD | H-donor | 4.00 | –0.5 | MET 367(C) | ||
| 18 | 6-ring | CB | pi-H | 3.78 | –0.8 | ASP 363(C) | –7.01 |
| C 9 | SD | H-donor | 3.48 | –0.6 | MET 367(C) | ||
| 19 | N 12 | SD | H-donor | 4.23 | –0.6 | MET 367(C) | –6.70 |
| C 9 | SD | H-donor | 3.61 | –0.6 | MET 367(C) | ||
| 20 | 6-ring | CB | pi-H | 3.53 | –0.5 | ASP 363(C) | –6.65 |
| O 21 | N | H-acceptor | 2.62 | –2.0 | ALA 366(C) | ||
| 21 | 6-ring | 5-ring | Pi-pi | 3.49 | –0.0 | HIS 323(C) | –7.22 |
| O 22 | N | H-acceptor | 2.81 | –1.9 | ALA 366(C) | ||
| 22 | 6-ring | CB | pi-H | 3.58 | –0.6 | ASP 363(C) | –7.88 |
| S 13 | CA | H-acceptor | 4.47 | –1.0 | GLY 280(C) | ||
H-donor (hydrogen bond donor), H-acceptor (hydrogen bond acceptor), pi-cation, pi-electron, and cation interaction
From docking analysis, it has also been found that not only the electron-donating group enhances the activity of the inhibitor, but its position is also important. The presence of a functional group is more effective at para positions; therefore, compound 7 (Figure 7D) has very good interactions and activity as compared to 6. The only difference is, in compound 7, CF3 is attached at the para position, while the same functionality is present at the ortho position in compound 6. However, CF3 is an electron-withdrawing group, but still compound 7 has a good IC50 value, that is, 18.5 ± 0.65 μM, and very better binding interaction as shown in Table 2. Nonetheless, compound 6 has an IC50 value of 26.5 ± 0.88 μM with poor or no interaction and a high docking score. Other compounds 9, 12, 14, and 22 have comparable activity with thiourea and better capabilities of inhibition. Compound 9 has a simple cyclohexane ring as compared to an aromatic ring. As the cyclohexane ring does not have resonance and no affinity for electrons, the electron cloud remains at the adjacent more electronegative sulfur, which established interaction with the binding site.
It has been found from docking study that those compounds are found to be more active, which have an electron-donating group at −ortho/–para positions. Similarly, electron-withdrawing groups if present at the –ortho position may not be so bad for reducing the interaction of the compound with active site residues. If the electron-withdrawing group is present at –para or specially at –meta positions, the activity may have been ceased.
Conclusions
A library of N-aryl-3,4-dihydroisoquinoline carbothioamide 1–22 was synthesized and evaluated for urease inhibitory activity in vitro. Compounds 1, 2, 4, and 7 showed more potent inhibitory potential than the standard thiourea. Screening results and the SAR suggested that the electron-donating groups and their positions played an important role in the inhibitory potential. Docking results of the most active compounds 1, 2, 4, and 7 showed a good protein–ligand interaction profile against the corresponding target. This ongoing study has identified a library of lead molecules as urease inhibitors that would be beneficial in future for the designing and development of drugs for the treatment of ulcer and its complications.
Experimental Section
Materials and Methods
All reagents were purchased from Merck (Germany) and Sigma Aldrich (USA). The NMR spectra were recorded on a Bruker AM spectrometer (400 MHz). Mass spectra (EI-MS, HR-EI-MS, and FAB) were recorded on MAT 312, MAT 113D, and JEOL JMS-600H mass spectrometers. Melting points were determined by using a B̈chi Melting Point-560 apparatus. TLC was carried out using precoated silica gel-254 Merck, Germany. Spots were visualized under UV light at 366 and 254 nm. Chemical shift values were recorded in ppm with respective to DMSO-d6 as the reference, and coupling constants are presented in Hz.
General Procedure for the Synthesis of Compounds 1–22
1,2,3,4-Tetrahydroisoquinoline (1 mmol) and aryl isothiocyanate derivatives (1 mmol) were taken in acetone (10 mL) into a 100 mL round-bottomed flask. Then, potassium carbonate, K2CO3, (1 mmol) was added in the reaction mixture and stirred at room temperature for 5–30 min. Product formation was monitored through TLC analysis (hexane/ethyl acetate 8:2). As the reaction proceeded, precipitates appeared in the reaction mixture. After completion of reaction, the solvent was evaporated under reduced pressure, and the residue was washed thoroughly with water to afford crude products, which were crystallized from ethanol. Compounds 1–22 were characterized by different spectroscopic techniques, including 1H-, 13C-NMR, FAB, EI-MS, HRFAB-MS, and HREI-MS.
N-(2-Methylphenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (1) CAS # 436109–97-6
Yield: 93%; M.P.: 154–156 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.16 (s, 1H, NH), 7.12 (ovp., 8H, H-6, H-7, H-8, H-9, H-3′, H-4′, H-5′, H-6′), 5.05 (s, 2H, CH2–1), 4.05 (t, J3,4= 11.6 Hz, 2H, CH2–3), 2.98 (t, J4,3 = 11.6 Hz, 2H, CH2–4), 2.15 (s, 3H, CH3); EI-MS: m/z (% rel. abund.) 282 [M+, 68], 267 (18), 176 (20), 149 (100), 132 (94), 104 (61), 91 (40).
N-(2,6-Dimethylphenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (2) CAS # 216774-22-0
Yield: 93%; M.P.: 246–248 °C; 1H-NMR (400 MHz, DMSO-d6), δH 8.91 (s, 1H, NH), 7.23 (ovp., 4H, H-6, H-7, H-8, H-9), 7.05 (s, 3H, H-2′, H-3′, H-4′), 5.08 (s, 2H, CH2–1), 4.07 (t, J3,4= 12.0 Hz, 2H, CH2–3), 2.95 (t, J4,3 = 12.0 Hz, 2H, CH2–4), 2.11 (s, 6H, CH3–2′, CH3–6′); EI-MS: m/z (% rel. abund.) 296 [M+, 61], 281 (52), 191 (1), 176 (12), 163 (100), 132 (100), 104 (49).
N-(2-Methoxyphenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (3) CAS # 406925-60-8
Yield: 96%; M.P.: 137–139 °C; 1H-NMR (400 MHz, DMSO-d6), δH 8.78 (s, 1H, NH), 7.24 (ovp., 6H, H-6, H-7, H-8, H-9, H-3′, H-5′), 7.02 (d, J6’,5′ = 7.6 Hz, 1H, H-6′), 6.91 (td, J4’(5′,3′) = 8.4 Hz, J4’,6′ = 1.2 Hz, 1H, H-4′), 5.03 (s, 2H, CH2–1), 4.04 (t, J3,4= 12.0 Hz, 2H, CH2–3), 3.74 (s, 3H, −OCH3), 2.93 (t, J4,3 = 11.6 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 298 [M+, 31], 267 (57), 176 (10), 165 (100), 132(91), 122(62), 104 (52).
N-(4-Methoxyphenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (4) CAS # 502868-57-7
Yield: 92%; M.P.: 150–152 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.16 (s, 1H, NH), 7.12 (ovp., 6H, H-6, H-7, H-8, H-9, H-1′, H-6′), 6.87 (d, J3’,2′ = J6’,5′ = 8.8 Hz, 2H, H-3′, H-5′), 5.02 (s, 2H, CH2–1), 4.05 (t, J3,4= 12.0 Hz, 2H, CH2–3), 3.73 (s, 2H, −OCH3), 2.93 (t, J4,3 = 11.6 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 298 [M+, 8], 176 (6), 165 (100), 132 (82), 122 (44), 104 (68).
N-(4-Ethoxyphenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (5) CAS # 897342-81-3
Yield: 91%; M.P.: 191–193 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.15 (s, 1H, NH), 7.21 (ovp., 2H, H-2′, H-6′), 7.20 (ovp., 4H, H-6, H-7, H-8, H-9), 6.85 (d, J3’,2′ = J5’,6′ = 8.8 Hz, 2H, H-3′, H-5′), 5.01 (s, 2H, CH2–1), 4.05 (q, JCH2,CH3 = 12.0 Hz, 2H, -OCH2), 3.98 (t, J3,4= 11.6 Hz, 2H, CH2–3), 2.92 (t, J4,3 = 11.6 Hz, 2H, CH2–4), 1.32 (t, JCH3,CH2 = 14.0 Hz, 3H, −CH3); EI-MS: m/z (% rel. abund.) 312 [M+, 4], 179 (87), 151 (100), 132 (73), 104 (56).
N-(2-(Trifluoromethyl)phenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (6) CAS # 575493-14-0
Yield: 93%; M.P.: 157–159 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.22 (s, 1H, NH), 7.70 (d, J3’,4′ = 8.0 Hz, 1H, H-3′), 7.66 (t, J4’(3′,5′) = 7.8 Hz, 1H, H-4), 7.47 (t, J5’(4′,6′) = 7.4 Hz, 1H, H-5), 7.40 (d, J6’,5′ = 8.0 Hz, 1H, H-6), 7.22 (ovp., 4H, H-6, H-7, H-8, H-9), 5.06 (s, 2H, CH2–1), 4.06 (t, J3,4= 11.6 Hz, 2H, CH2–3), 2.94 (t, J4,3 = 11.6 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 336 [M+, 100], 267 (30), 203 (62), 176 (14), 145 (22), 132 (94), 104 (32).
N-(4-(Trifluoromethyl)phenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (7) CAS # 892722-67-7
Yield: 95%; M.P.: 161–163 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.55 (s, 1H, NH), 7.64 (d, J3’,2′ = J5’,6′ = 8.8 Hz, 2H, H-3′, H-5′), 7.57 (d, J2’,3′ = J6’,5′ = 8.4 Hz, 2H, H-2′, H-6′), 7.23 (ovp., 4H, H-6, H-7, H-8, H-9), 5.04 (s, 2H, CH2–1), 4.08 (t, J3,4= 12.0 Hz, 2H, CH2–3), 2.96 (t, J4,3 = 11.6 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 336 [M+, 36], 203 (100), 176 (10), 145 (69), 132 (80), 104 (65).
N-(3-(Trifluoromethyl)phenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (8) CAS #216774-42-4
Yield: 98%; M.P.: 197–199 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.50 (s, 1H, NH), 7.71 (s, 1H, H-2′), 7.68 (d, J6’,5′ = 8.4 Hz, 1H, H-6′), 7.52 (t, J3’(2′,4′) = 7.8 Hz, 1H, H-3′), 7.44 (d, J4’,3′ = 7.6 Hz, 1H, H-4′), 7.23 (ovp., 4H, H-6, H-7, H-8, H-9), 5.05 (s, 2H, CH2–1), 4.08 (t, J3,4= 12.0 Hz, 2H, CH2–3), 2.97 (t, J4,3 = 11.6 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 336 [M+,100], 317 (6), 203 (96), 176 (18), 145 (43), 132 (84), 104 (39).
N-Cyclohexyl-3,4-dihydroisoquinoline-2(1H)-carbothioamide (9)34
Yield: 89%; M.P.: 226–228 °C; 1H-NMR (400 MHz, DMSO-d6), δH 7.20 (ovp., 5H, NH, H-6, H-7, H-8, H-9), 4.88 (s, 2H, CH2–1), 4.25 (m, 1H, H-1′), 3.92 (t, J3,4= 12.0 Hz, 2H, CH2–3), 1.88 (t, J4,3 = 12.0 Hz, 2H, CH2–4), 1.71 (m, 4H, CH2–2′, CH2–6′), 1.33 (ovp., 6H, CH2–3′, CH2–4′, CH2–5′); EI-MS: m/z (% rel. abund.), 274 [M+, 53], 191 (6), 141 (33), 132 (100), 104 (54).
(E)-N-(4-((4-(Dimethylamino)phenyl)diazenyl)phenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (10)
Yield: 99%; M.P.: 206–208 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.49 (s, 1H, NH), 7.77 (d, J3’,2′/5′,6′ = 8.8 Hz, 2H, H-3′, H-5′), 7.50 (d, J2’,3′/6′,5′ = 8.8 Hz, 2H, H-2′, H-6′), 7.23 (d, J2”,3″/6″5” = 8.8 Hz, 2H, H-2″, H-6″), 7.22 (ovp., 4H, H-6, H-7, H-8, H-9), 6.83 (d, J3”,2″/5″,6″ = 9.2 Hz, 2H, H-3″, H-5″), 5.04 (s, 2H, CH2–1), 4.09 (t, J3,4= 12.0 Hz, 2H, CH2–3), 3.04 (s, 6H, N(CH3)2), 2.97 (t, J4,3 = 11.6 Hz, 2H, CH2–4); 13C-NMR (DMSO-d6, 125 MHz): δC 180.5, 152.2, 148.6, 142.6, 142.6135.0, 133.4, 128.1, 126.7, 126.2, 126.2, 125.0, 125.0, 124.4, 124.4, 121.6, 121.6, 111.6, 111.6, 50.1, 46.1, 39.7, 39.7, 28.1; FAB (Pos.) MS m/z = 416 [M-H]+1; HRFAB (Pos.) MS, m/z Found 416.1902 cal. 416.1909.
N-Phenyl-3,4-dihydroisoquinoline-2(1H)-carbothioamide (11).34
Yield: 93%; M.P.: 143–145 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.29 (s, 1H, NH), 7.23 (ovp., 9H, H-6, H-7, H-8, H-9, H-2′, H-3′, H-4′, H-5′, H-6′), 5.02 (s, 2H, CH2–1), 4.06 (t, J3,4= 12.0 Hz, 2H, CH2–3), 2.95 (t, J4,3 = 11.6 Hz, 2H, CH2–4); FAB (Neg.) MS m/z = 267 [M-H]−1.
N-(2-Fluorophenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (12) CAS # 455299-84-0
Yield: 97%; M.P.: 109–111 °C; 1H-NMR (400 MHz, DMSO-d6), δH 8.91 (s, 1H, NH), 7.23 (ovp., 8H, H-6, H-7, H-8, H-9, H-3′, H-4′, H-5′, H-6′), 5.05 (s, 2H, CH2–1), 4.06 (t, J3,4= 12.0 Hz, 2H, CH2–3), 2.95 (t, J4,3 = 12.0 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 286 [M+, 100], 266 (11), 176 (28), 132 (94), 104 (30), 95 (15).
N-(2,4-Difluorophenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide(13) CAS # 454428-71-8
Yield: 87%; M.P.: 207–209 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.12 (s, 1H, NH), 7.31 (ovp., 6H, H-6, H-7, H-8, H-9, H-6′, H-5′), 7.06 (m, 1H, H-3′), 5.04 (s, 2H, CH2–1), 4.05 (t, J3,4= 11.6 Hz, 2H, CH2–3), 2.95 (t, J4,3 = 12.0 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 304 [M+, 82], 284 (4), 176 (23), 171 (100), 132 (73), 104 (39).
N-(2-Chlorophenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (14) CAS # 216774-62-8
Yield: 86%; M.P.: 188–190 °C; 1H-NMR (400 MHz, DMSO-d6) δH 9.23 (s, 1H, NH), 7.48 (d, J6’,5′ = 8.8 Hz 1H, H-6′), 7.31 (d, J9,8 = J6,7 = 4.6 Hz, 2H, H-9, H-6), 7.27 (ovp., 5H, H-3, H-7, H-8, H-4′, H-5′), 5.06 (s, 2H, CH2–1), 4.06 (t, J3,4= 12.0 Hz, 2H, CH2–3), 2.95 (t, J4,3 = 8.0 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 302 [M+, 1], 304 (M + 2, 0.3), 267 (56), 169 (100), 132 (78), 104 (57).
N-(3-Chlorophenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (15) CAS # 216774-88-8
Yield: 91%; M.P 190–192 °C; 1H-NMR (400 MHz, DMSO-d6) δH 9.38 (s, 1H, NH), 7.44 (s, 1H, H-2′), 7.31 (ovp., 2H, H-4′, H-6′), 7.22 (ovp., 4H, H-6, H-7, H-8, H-9), 7.16 (m, 1H, H-5′), 5.02 (s, 2H, CH2–1), 4.06 (t, J3,4= 11.6 Hz, 2H, CH2–3), 2.95 (t, J4,3 = 11.6 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 302 [M+, 10], 304 (M + 2, 4), 269 (3), 169 (100), 132 (77), 104 (55).
N-(2,3-Dichlorophenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (16) CAS # 894156-87-7
Yield: 90%; M.P.: 186–188 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.30 (s, 1H, NH), 7.26 (ovp., 7H, H-6, H-7, H-8, H-9, H-4′, H-5′, H-6′), 5.04 (s, 2H, CH2–1), 4.06 (t, J3,4= 8.7 Hz, 2H, CH2–3), 2.95 (t, J4,3 = 8.7 Hz, 2H, CH2–4); FAB (Neg.) MS m/z = 335 [M–H]−1.
N-(2,5-Dichlorophenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (17) CAS # 897511-80-7
Yield: 90%; M.P.: 193–195 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.14 (s, 1H, NH), 7.51 (d, J3’,4′ = 8.4 Hz, 1H, H-3′), 7.42 (d, J6’,4′ = 2.4 Hz, 1H, H-6′), 7.35 (dd, J4’,6′ = 2.4 Hz, J4’,3′ = 6.0 Hz, 1H, H-4′), 7.23 (ovp., 4H, H-6, H-7, H-8, H-9), 5.06 (s, 2H, CH2–1), 4.06 (t, J3,4= 11.6 Hz, 2H, CH2–3), 2.96 (t, J4,3 = 12.0 Hz, 2H, CH2–4); FAB (Neg.) MS m/z = 335 [M–H]−1.
N-(4-Bromophenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (18) CAS # 894230-32-1
Yield: 89%; M.P.: 168–170 °C; 1H-NMR: (400 MHz, DMSO-d6), δH 9.49 (s, 1H, NH), 7.56 (ovp., 8H, H-6, H-7, H-8, H-9, H-4′, H-5′, H-6′), 5.02 (s, 2H, CH2–1), 4.06 (t, J3,4= 12.0 Hz, 2H, CH2–3), 2.94 (t, J4,3 = 11.6 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 348 [M+, 56], 350 [M + 2, 55], 215 (99), 176 (23), 155 (18), 132 (100), 104 (47) 78 (10).
N-(4-Iodophenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (19) CAS # 2208789-27-7
Yield: 98%; M.P.: 170–172 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.33 (s, 1H, NH), 7.72 (t, J8 (7,9) = 3.6 Hz, 1H, H-8), 7.46 (m, 2H, H-3′, H-5′), 7.22 (ovp., 4H, H-6, H-9, H-2′, H-6′), 7.11 (t, J7 (6,8) = 8.0 Hz, 1H, H-7), 5.02 (s, 2H, CH2–1), 4.12 (t, J3,4= 12.0 Hz, 2H, CH2–3), 2.95 (t, J4,3 = 11.6 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 394 [M+, 42], 261 (100), 203 (2), 176 (4), 132 (72), 104 (46).
N-(3-Nitrophenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (20) CAS # 708228-72-2
Yield: 95%; M.P.: 193–195 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.65 (s, 1H, NH), 8.30 (t, J2’(4′,6′) = 4.0 Hz, 1H, H-2′), 7.95 (dd, J4’,2′ = 4.0 Hz, J4’,5′ = 8.0 Hz, 1H, H-4′), 7.85 (dd, J6’,2′ = 4.0 Hz, J6’,5′ = 8.0 Hz, 1H, H-6′), 7.59 (t, J5’(4′,6′) = 8.0 Hz, 1H, H-5′), 7.24 (ovp., 4H, H-6, H-7, H-8, H-9), 5.06 (s, 2H, CH2–1), 4.10 (t, J3,4= 12.0 Hz, 2H, CH2–3), 2.98 (t, J4,3 = 12.0 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 313 [M+, 3], 180 (100), 132 (88), 104 (82).
N-(4-Nitrophenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (21) CAS # 583820-27-3
Yield: 97%; M.P.: 187–189 °C; 1H-NMR (400 MHz, DMSO-d6) δH 9.80 (s, 1H, NH), 8.15 (d, J3’,2′= J5’,6′ = 6.0 Hz, 2H, H-3′, H-5′), 7.61 (d, J2’,3′ = J6’,5′ = 6.0 Hz, 2H, H-2′, H-6′), 7.22 (ovp., 4H, H-6, H-7, H-8, H-9), 5.03 (s, 2H, CH2–1), 4.09 (t, J3,4= 12.0 Hz, 2H, CH2–3), 2.97 (t, J4,3 = 12.0 Hz, 2H, CH2–4); EI-MS: m/z (% rel. abund.) 313 [M+, 2], 180 (79), 132 (100), 122 (28), 104 (95).
N-(2-Chloro-4-nitrophenyl)-3,4-dihydroisoquinoline-2(1H)-carbothioamide (22)
Yield: 83%; M.P.: 208–210 °C; 1H-NMR (400 MHz, DMSO-d6), δH 9.89 (s, 1H, NH), 8.32 (d, J3’,5′ = 2.4 Hz, 1H, H-3′), 8.18 (dd, J5’,3′ = 2.4 Hz, J5’,6′ = 6.0 Hz, 1H, H-5′), 7.64 (d, J6’,5′ = 8.8 Hz, 1H, H-6′), 7.25 (ovp., 4H, H-6, H-7, H-8, H-9), 5.07 (s, 2H, CH2–1), 4.09 (t, J3,4= 11.6 Hz, 2H, CH2–3), 2.98 (t, J4,3 = 11.6 Hz, 2H, CH2–4); FAB (Neg), MS m/z = 346 [M–H]−1.
Urease Inhibitory Assay
Urease inhibitory activity was performed by following the method of Weatherburn (1967). The test compound (250 μM, 5 μL) was incubated with urease solution (1 U/well) (25 μL) for 15 min at 30 °C. After this, urea (substrate) (100 mM, 55 μL) was added, and then, the plate was incubated for 10 min at 30 °C. Then, 70 μL of alkali reagents (0.5% w/v sodium hydroxide and 0.1% sodium hypochlorite, and 45 μL of phenol (1% w/v phenol and 0.005% w/v sodium nitroprusside)) was added. The plate was again incubated for 50 min at 30 °C. Urease activity was calculated with the rate of production of ammonia, and alteration in absorbance was examined at 630 nm with an ELISA plate reader (Spectra Max M2, Molecular Devices, CA, USA). Thiourea was used as a standard control.6
Molecular Docking Study
The Molecular Operating Environment (MOE) software package was used to perform molecular docking study in order to explore the binding mode of the synthesized compounds within the active site of urease enzyme. First, the structure coordinates of synthesized compounds were generated using a molecular builder tool implemented in the MOE software package. Next, all the compounds were subjected for protonation and energy minimization using the default parameters of the MOE, that is, gradient: 0.05, Force Field: MMFF94X. The structure coordinates of the target protein were retrieved from the protein databank using PDB code 4UBP. All the water molecules were removed; later on, 3D protonation was carried using the MOE software package. The energy minimized was conducted in order to get a stable conformation of the protein using the default parameters of the MOE software package. The default parameters for docking studies were used, that is, Placement: Triangle Matcher, rescoring 1: London dG, Refinement: Forcefield, Rescoring 2: GBVI/WSA. For each ligand, a total of 10 conformations were allowed, and the top-ranked conformations based on the docking score were selected for additional analysis.
Acknowledgments
The authors are thankful to the Pakistan Academy of Sciences for providing financial support to Project No. (5-9/PAS/440).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01182.
The authors declare no competing financial interest.
Supplementary Material
References
- Qin Y.; Cabral J. M. Kinetic studies of the urease-catalyzed hydrolysis of urea in a buffer-free system. Biotechnol. Appl. Biochem. 1994, 49, 217–240. 10.1007/BF02783059. [DOI] [PubMed] [Google Scholar]
- Lodhi M. A.; Shams S.; Choudhary M. I.; Lodhi A.; Ul-Haq Z.; Jalil S.; Nawaz S. A.; Khan K. M.; Iqbal S.; Rahman A. U. Structural basis of binding and rationale for the potent urease inhibitory activity of biscoumarins. Bio. Med. Res. int. 2014, 1–12. 10.1155/2014/935039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervez H.; Iqbal M. S.; Tahir M. Y.; Nasim F. U. H.; Choudhary M. I.; Khan K. M. In vitro cytotoxic, antibacterial, antifungal and urease inhibitory activities of some N4-substituted isatin-3-thiosemicarbazones. J. Enzymem Inhib. Med. Chem. 2008, 23, 848–854. 10.1080/14756360701746179. [DOI] [PubMed] [Google Scholar]
- Arshad T.; Khan K. M.; Rasool N.; Salar U.; Hussain S.; Asghar H.; Ashraf M.; Wadood A.; Riaz M.; Perveen S.; Taha M.; Ismail N. H. 5-Bromo-2-aryl benzimidazole derivatives as non-cytotoxic potential dual inhibitors of α-glucosidase and urease enzymes. Bioorg. Chem. 2017, 72, 21–31. 10.1016/j.bioorg.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Ara R.; Ashiq U.; Mahroof-Tahir M.; Maqsood Z. T.; Khan K. M.; Lodhi M. A.; Choudhary M. I. Chemistry, urease inhibition, and phytotoxic studies of binuclear vanadium (IV) complexes. Chem. Biodiversity 2007, 4, 58–71. 10.1002/cbdv.200790007. [DOI] [PubMed] [Google Scholar]
- Naz F.; Latif M.; Salar U.; Khan K. M.; Al-Rashida M.; Ali I.; Ali B.; Taha M.; Perveen S. 4-Oxycoumarinyl linked acetohydrazide Schiff bases as potent urease inhibitors. Bioorg. Chem. 2020, 105, 104365 10.1016/j.bioorg.2020.104365. [DOI] [PubMed] [Google Scholar]
- Bremner J. M. Recent research on problems in the use of urea as a nitrogen fertilizer. Nitro. Eco.Trop. Soils 1995, 321–329. 10.1007/978-94-009-1706-4_30. [DOI] [Google Scholar]
- Ashraf M.; Hassan R.; Ahmadand I.; Khan M. Synthesis of N-Substituted Derivatives of N-(4-(N-(5-Chloro-2 methoxyphenyl) sulfamoyl) phenyl) acetamide with Potential Antiurease Activity. J. Chem. Soc. Pak. 2013, 35, 15–16. [Google Scholar]
- Mobley H. L.; Island M. D.; Hausinger R. P. Molecular biology of microbial ureases. Microbiol. Rev. 1995, 59, 451–480. 10.1128/MR.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayerdorffer E.; Ottenjann R. The role of antibiotics in Campylobacter pylori associated peptic ulcer disease. Scand. J. Gastroenterol. 1988, 23, 93–100. 10.3109/00365528809091721. [DOI] [PubMed] [Google Scholar]
- Arif Lodhi M.; Wadood A.; Iqbal S.; Khan K. M.; Iqbal Choudhary M. Three-dimensional quantitative structure–activity relationship (CoMSIA) analysis of bis-coumerine analogues as urease inhibitors. Med. Chem. Res. 2013, 22, 498–504. 10.1007/s00044-012-9999-8. [DOI] [Google Scholar]
- Gioacchini P.; Nastri A.; Marzadori C.; Giovannini C.; Antisari L. V.; Gessa C. Influence of urease and nitrification inhibitors on N losses from soils fertilized with urea. Biol. Fertil. Soils 2002, 36, 129–135. 10.1007/s00374-002-0521-1. [DOI] [Google Scholar]
- Taha M.; Ismail N. H.; Imran S.; Wadood A.; Rahim F.; Khan K. M.; Riaz M. Hybrid benzothiazole analogs as antiurease agent: Synthesis and molecular docking studies. Bioorg. Chem 2016, 66, 80–87. 10.1016/j.bioorg.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Khan A.; Hashim J.; Arshad N.; Khan I.; Siddiqui N.; Wadood A.; Ali M.; Arshad F.; Khan K. M.; Choudhary M. I. Dihydropyrimidine based hydrazine dihydrochloride derivatives as potent urease inhibitors. Bioorg. Chem. 2016, 64, 85–96. 10.1016/j.bioorg.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Ismail T. M.; Baharudin N. H.; Lalani M. S.; Mehboob S.; Khan K. M.; Yousuf S.; Siddiqui S.; Rahim F.; Choudhary M. I. Synthesis crystal structure of 2-methoxybenzoylhydrazones and evaluation of their α-glucosidase and urease inhibition potential. Med. Chem. Res. 2015, 24, 1310–1324. 10.1007/s00044-014-1213-8. [DOI] [Google Scholar]
- Tiwari R. K.; Singh D.; Singh J.; Chhillar A. K.; Chandra R.; Verma A. K. Synthesis, antibacterial activity and QSAR studies of 1,2-disubstituted-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolines. Eur. J. Med. Chem. 2006, 41, 40–49. 10.1016/j.ejmech.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Song P.; Ma F.; Wang F.; Wang X.; Patil R.; Ramagiri S.; Orr W. E.; Miller D. D.; Geisert E.; Yates C. R. Plasma and cerebrospinal fluid pharmacokinetics of the novel tetrahydroisoquinoline EDL-155 in rats. Cancer. Chemother. Pharmacol. 2008, 61, 1037. 10.1007/s00280-007-0563-z. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Xun K.; Wang Y.; Chen X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti-Cancer Drugs 2009, 20, 757–769. 10.1097/CAD.0b013e328330d95b. [DOI] [PubMed] [Google Scholar]
- Limuro A.; Yamaji K.; Kandula S.; Nagano T.; Kita Y.; Mashima K. Asymmetric hydrogenation of isoquinolinium salts catalyzed by chiral iridium complexes: Direct synthesis for optically active 1,2,3,4-tetrahydroisoquinolines. Angew. Chem., Int. Ed. 2013, 52, 2046–2050. 10.1002/anie.201207748. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Xiao Q.; Yin D. Synthesis of tetrahydroisoquinolines through TiCl4-mediated cyclization and Et3SiH reduction. Chin. Chem. Lett. 2020, 31, 729–732. 10.1016/j.cclet.2019.09.023. [DOI] [Google Scholar]
- Judeh Z. M.; Ching Bu C. B.; McCluskey A. J. The first Bischler–Napieralski cyclization in a room temperature ionic liquid. Tetrahedron Lett. 2002, 43, 5089–5091. 10.1016/S0040-4039(02)00998-X. [DOI] [Google Scholar]
- Banerjee S.; Liu F.; Sanchez D. M.; Martínez T. J.; Zare R. N. Pomeranz–Fritsch synthesis of isoquinoline: gas-phase collisional activation opens additional reaction pathways. J. Am. Chem. Soc. 2017, 139, 14352–14355. 10.1021/jacs.7b06813. [DOI] [PubMed] [Google Scholar]
- Groll B.; Schaaf P.; Mihovilovic M. D.; Schnurch M. Cu(I)-catalyzed one-pot decarboxylation-alkynylation reactions on 1,2,3,4-tetrahydroisoquinolines and one-pot synthesis of triazolyl-1,2,3,4-tetrahydroisoquinolines. J. Mol. Catal. A: Chem. 2017, 426, 398–406. 10.1016/j.molcata.2016.07.013. [DOI] [Google Scholar]
- Hatano H.; Takekawa F.; Hashimoto K.; Ishihara M.; Kawase M.; Qing C.; Tao W.; Sakagami H. Tumor-specific cytotoxic activity of 1,2,3,4-tetrahydroisoquinoline derivatives against human oral squamous cell carcinoma cell lines. Anticancer Res. 2009, 29, 3079–3086. [PubMed] [Google Scholar]
- Ngemenya M. N.; Hanna J. N.; Komtchou J. A.; Efange S. M. N. In vitro screening of 1-aryl-6-hydroxy-1,2,3,4-tetrahydroisoquinolines. Structure related activity against pathogenic bacteria. Asian Pac. J. Trop. Biomed. 2015, 5, 472–477. 10.1016/j.apjtb.2015.03.005. [DOI] [Google Scholar]
- Pingaew R.; Mandi P.; Nantasenamat C.; Prachayasittikul S.; Ruchirawat S.; Prachayasittikul V. Design, synthesis and molecular docking studies of novel N-benzenesulfonyl-1,2,3,4-tetrahydroisoquinoline-based triazoles with potential anticancer activity. Eur. J. Med. Chem. 2014, 81, 192–203. 10.1016/j.ejmech.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Menteşe E.; Karaali N.; Akyüz G.; Yılmaz F.; Ülker S.; Kahveci B. Synthesis and evaluation of α-glucosidase and pancreatic lipase inhibition by quinazolinone-coumarin hybrids. Chem. Heterocycl. Comp. 2016, 52, 1017–1024. 10.1007/s10593-017-2002-3. [DOI] [Google Scholar]
- Rahim F.; Zaman K.; Ullah H.; Taha M.; Wadood A.; Javed M. T.; Uddin I. Synthesis of 4-thiazolidinone analogs as potent in vitro anti-urease agents. Bioorg. Chem. 2015, 63, 123–131. 10.1016/j.bioorg.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Khan K. M.; Ali M.; Wadood A.; Khan M.; Lodhi M. A.; Perveen S.; Voelter W. Molecular modeling-based antioxidant arylidene barbiturates as urease inhibitors. J. Mol. Graphics Modell. 2011, 30, 153–156. 10.1016/j.jmgm.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Khan K. M.; Naz F.; Taha M.; Khan A.; Perveen S.; Choudhary M.; Voelter W. Synthesis and in vitro urease inhibitory activity of N, N′-disubstituted thioureas. Eur. J. Med. Chem. 2014, 74, 314–323. 10.1016/j.ejmech.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Menteşe E.; Akyüz G.; Yılmaz F.; Baltaş N.; Emirik M. Synthesis of some novel quinazolin-4 (3H)-one hybrid molecules as potent urease inhibitors. Arch. Pharm. 2018, 351, 1800182 10.1002/ardp.201800182. [DOI] [PubMed] [Google Scholar]
- Akyüz G.; Beriş F. Ş.; Kahveci B.; Menteşe E. Synthesis of novel 2, 3-disubstituted quinazolin-4 (3H)-one derivatives containing hydrazone skeleton as potent urease inhibitors and their antimicrobial activities. J. Heterocycl. Chem. 2019, 56, 3065–3072. 10.1002/jhet.3703. [DOI] [Google Scholar]
- Menteşe E.; Akyüz G.; Emirik M.; Baltaş N. Synthesis, in vitro urease inhibition and molecular docking studies of some novel quinazolin-4 (3H)-one derivatives containing triazole, thiadiazole and thiosemicarbazide functionalities. Bioorg. Chem. 2019, 83, 289–296. 10.1016/j.bioorg.2018.10.031. [DOI] [PubMed] [Google Scholar]
- Nguyen T. B.; Ermolenko L.; Al-Mourabit A. Three-component reaction between isocyanides, aliphatic amines and elemental sulfur: Preparation of thioureas under mild conditions with complete atom economy. Synthesis 2014, 46, 3172–3179. 10.1055/s-0034-1379327. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.