Abstract
Textile dyes from wastewater effluent are highly toxic to both living species and aqueous environments. An environmentally friendly method to remove hazardous dyes from wastewater in the textile industry has been a challenge. Chitosan (CS) and activated carbon (AC) are widely used as adsorbents for dye removal. However, the poor porosity and unsatisfactory stability of CS and the unfriendly cost of AC limited their applications to be used alone as a single adsorbent. Here, we report a novel method to prepare a CS/AC membrane using PEG10000 as a porogen and sodium tripolyphosphate (TPP) as a cross-linking agent. The adsorption efficiency and reusability of the PEG/TPP-modified CS/AC membrane to remove RhB were investigated based on dynamic and static adsorption models. The results reveal that the adsorption performance of CS/AC membranes was significantly improved after the PEG/TPP modification based on the abundance macroporous structure. The modified CS/AC membrane with a 30% AC doping ratio exhibited an excellent adsorption efficiency of 91.29 and 73.91% in the dynamic and static adsorption processes, respectively. These results provide new insights into designing membranes to remove dyes from wastewater efficiently.
1. Introduction
With the development of the textile and dyestuff industries, the wastewater effluent without adequate treatment can cause significant environmental problems.1,2 The wastewater containing harmful residual dyes and other auxiliary chemical additives causes irreversible damage to both living species and the aqueous environment.3,4 For instance, the aquatic environment becomes unbalanced due to excessive proliferation of algae and suffocation of aquatic organisms under the influence of the dyeing effluent in a watercourse.5,6 Therefore, efficient treatments to the residual pollutants in the wastewater, especially textile dyeing, must be performed before discharge. Rhodamine B (RhB) is a widely used organic colorant in textile and dyestuff industries.7 However, it is highly toxic with potential carcinogenic and mutagenic properties caused by benzidine and other aromatic compounds.8 Furthermore, RhB interferes with the natural purification process for aquatic life through impeding light penetration and photosynthesis.9 Therefore, it is necessary to make systematic efforts to avoid the destructive impact of RhB in effluent treatment.
With this in mind, municipal feasible effluent treatment systems are widely used for effluent purification and reclamation, including physicochemical flocculation,10 precipitation,11 ozonation,12 electrocoagulation,13 adsorption,14,15 and other comprehensive technologies, varying in economical cost, operation, and effectiveness. Most of them have limitations such as low selectivity, high energy consumption, low efficiency, and many toxic chemicals involved.16 Among them, adsorption, in which adsorbents are applied for adsorption contaminants, provides various advantages for decolorizing textile dyeing effluent, such as low operation cost, excellent purification, and low energy consumption.17
Several adsorbents have been developed in dyeing effluent treatment, such as activated sludge,18 alum,19 coal ash,20 silicate,21 acetylacetone,22 graphene-based nanomaterials,23−25 and silica gel.26 However, these adsorbents bring about low effectiveness with incomplete color removal and are not entirely eco-friendly to the environment. In this respect, the natural polysaccharide chitosan (CS) is highly regarded due to its unique chelation and adsorption performance, excellent biodegradability, nontoxicity, and commercial values.27 CS, a deacetylated product of chitin, contains amine and hydroxyl functional groups for excellent adsorption performance.28,29 In the effluent treatment process, supramolecular conjugates are formed on the surface of CS under hydrogen-bonding, electrostatic attraction, and van der Waals forces between CS and RhB, allowing excellent chemical adsorption in color removal.28 Moreover, the acylation reaction takes place between CS and RhB to form compounds in effluent treatment.30 However, insufficient mechanical and chemical stability hinders further development of CS.31 Cross-linking and doping help to improve the chemical stability and mechanical strength of CS.32 Various adsorbents, such as activated carbon (AC),33,34 magnetic materials,35 and graphene oxide,36 have been used as CS adulterants to improve their applicability with satisfactory mechanical properties. Among them, AC as a common adsorbent has attracted significant attention due to its excellent porous structure and large specific surface area.37−41 Due to bad degradation, high cost, and tough separation from the water body, AC is restricted into small-scale effluent treatment.33 Fortunately, the combination of CS/AC doping version paves the way to achieve better adsorption performance through complementary advantages from both of them.
The porosity of adsorptions poses a significant impact on their adsorption performance. Although different forms of CS (beads and film) are prepared for effluent treatment, there are generally nonporous structures on their surface. To achieve the high adsorption capability of CS, it is necessary to possess a porous structure.42 As far as we know, adding a cross-linking agent, sodium tripolyphosphate (TPP) solution, to CS can enhance the chemical stability and form a microstructure improvement in its adsorption performance. Several works have been reported on water pollutant adsorption using CS/carbonaceous material composites, but little research was based on the porous structure of CS/AC composite membranes used for dye removal. In this study, porous CS/AC (P-CS/AC) membranes with different AC doping ratios were prepared using the porogen PEG and the cross-linking agent TPP. We explored the influence of the AC doping ratios on the adsorption performance of the CS/AC membrane. Besides, we compared the adsorption performance and reusability of P-CS/AC membranes and nonporous CS/AC (N-CS/AC) membranes based on dynamic and static adsorption models.
2. Results and Discussion
2.1. Morphology of a Porous Chitosan Membrane
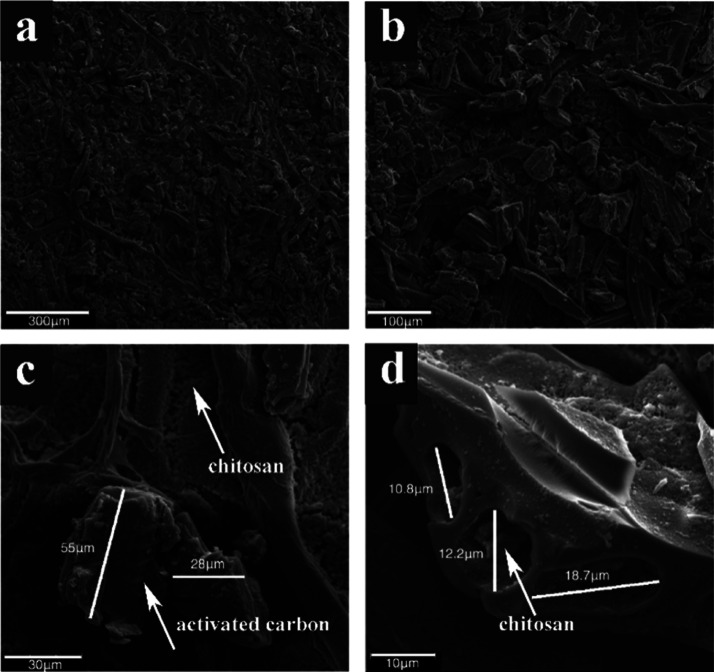
The surface morphology and porous structure of a TPP cross-linking P-CS membrane are shown in Figure 1. It was shown that CS infiltrated in cellulose filter paper and occupied most of the gap between filter paper fibers forming a uniform P-CS membrane (Figure 1a). The boundary between the inner filter paper and the outer CS coating could be observed in the cross-sectional image of the P-CS membrane (Figures 1b and S1), and the thickness of the CS coating was about 100 nm. Furthermore, the macroporous structure of the P-CS membrane could be observed in Figure 1c,d. The macroporous structure of the P-CS membrane was attributed to the dissolution of the water-soluble porogen PEG, and the macroporous structure was left in the P-CS membrane after that. Also, TPP created a porous structure during the cross-linking process. The cooperation of the porogen PEG and the cross-linking agent TPP formed an excellent three-dimensional network porous structure in the P-CS membranes.
Figure 1.
Scanning electron microscopy images of the TPP cross-linking P-CS membrane. (a) Surface macromorphology, magnification = 500, (b) cross-sectional, magnification = 500, and surface microtopography, (c) magnification = 5000 and (d) magnification = 10,000.
The porosity as an essential factor of adsorption performance may be affected by the drying process. We explored the influence of different drying processes on porosity. Figure S2 shows that three different drying conditions led to three-dimensional network porous structure shapes with size ranges from 0.2 to 3 μm. Compared with CS coating weight and mean porosity (Table S1), there was not much difference between different drying conditions. The porous structure had been formed during the modified process of adding a porogen and cross-linking agent, and the subsequent drying process had little effect on the pore structure. Considering the operation and economic factors, air-drying was the best choice for preparing the P-CS membrane.
2.2. Characterization of Porous Chitosan/Activated Carbon Membranes
AC possesses a plentiful porous structure and a high specific surface area that could be doped into the P-CS membrane to improve its mechanical stability and adsorption performance. TPP cross-linking P-CS/AC membranes with different AC doping ratios (10, 20, 30, and 40%) were prepared in our work. CS acted as an adsorbent and a binder to create good adhesion between the matrix and AC. The morphology of P-CS/AC membranes is exhibited in Figure 2. AC was evenly distributed on the surface of the composite membrane with the inlaid form. CS adhered or filled the surface or the inner space of AC. Porous AC, together with the porogen PEG and the cross-linking agent TPP, formed a macroporous structure of P-CS/AC membranes.
Figure 2.
Scanning electron micrographs of TPP cross-linking P-CS/AC membranes with different AC doping ratios, (a) 40, (b) 30, (c) 20, and (d) 10%.
2.3. Dynamic Adsorption Performance of Porous Chitosan/Activated Carbon Membranes
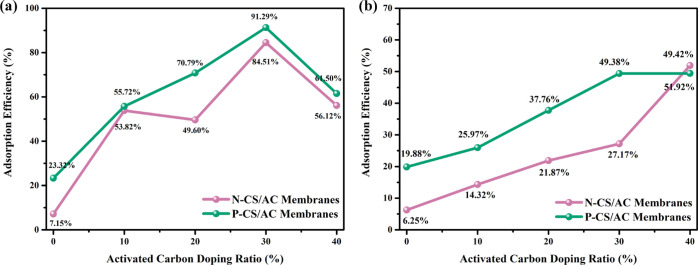
The adsorption performance of N-CS/AC and P-CS/AC membranes with different AC doping ratios is exhibited in Figure 3. In most circumstances, the adsorption efficiency of P-CS/AC membranes was much higher than that of N-CS/AC membranes, which demonstrated that the addition of AC improved the adsorption performance of CS membranes. It was beneficial to the adsorption of pollutants based on a macroporous structure with a large adsorption contact area.
Figure 3.
Adsorption efficiency of (a) first used and (b) reused N-CS/AC membranes and P-CS/AC membranes with different AC doping ratios.
Different amounts of AC had different effects on adsorption performance, as shown in Figure 3a. In P-CS/AC membranes, the adsorption efficiency increased with the increase in AC doping while the AC doping ratio was less than 30%, and P-CS/AC membranes exhibited the maximum adsorption efficiency of 91.29% at a 30% AC doping ratio. CS with a large number of amino groups formed compounds with RhB molecules under the acylation effect. Meanwhile, supramolecular polymers also formed on the surface of CS through hydrogen-bonding and electrostatic attraction.30 Thus, CS played an important role in the chemisorption of RhB. AC played an important role in physical adsorption on RhB with its natural three-dimensional network macroporous structure.43−45 Under the combination of physical and chemical adsorption, P-CS/AC membranes produced an excellent adsorption efficiency. However, the adsorption performance of the P-CS/AC membranes decreased with the excessive addition of AC because excessive doping AC covered the P-CS surface. The chemical adsorption performance of CS was hindered, and thus, the synergistic adsorption performance was destroyed after excessive doping AC. The balance between the amount of AC and CS was also shown in N-CS/AC membranes.
To make effluent treatment more economical, CS/AC membranes were resued as secondary regeneration membranes to purified RhB solution. Although the adsorption efficiency of P-CS/AC and N-CS/AC membranes in the secondary adsorption process was lower than the first used, the P-CS/AC membranes exhibited much higher adsorption efficiency than the N-CS/AC membranes (Figure 3b), which demonstrated that CS/AC membranes exhibited better stability after modification. The decrease in adsorption efficiency of CS/AC membranes in the regeneration process related to the decline of the content of nitrogen in CS, which acted as the activated sites of the adsorbent.46,47 The loss of incompletely attached AC of the CS membranes in the regeneration process also led to decreased adsorption efficiency. In order to minimize the loss of AC, in-depth exploration of the concentration of CS and drying modes. Also, cross-linking agents such as glutaraldehyde could also improve the adhesion between AC and CS.48 P-CS/AC-30% and P-CS/AC-40% membranes showed better adsorption performance in the reused process. Thus, P-CS/AC membranes exhibited excellent adsorption performance, reusability, and environmental friendliness.
2.4. Static Adsorption Performance of Porous Chitosan/Activated Carbon Membranes
To explore the static adsorption performance of CS/AC membranes, we constructed static adsorption models. Ten small pieces (diameter = 6 mm) were taken from any position of N-CS/AC and P-CS/AC membranes with different AC doping ratios. The pieces were added into 5 mL of 10 mg/mL RhB solution and stirred under 150 rpm for 1 h, and the absorbance of stock solutions was tested. For P-CS/AC membranes, the adsorption efficiency increased as the doping ratio of AC increased, but when the AC doping ratios were higher than 30%, the adsorption efficiency increased slowly (Figure 4). This phenomenon was similar to the dynamic adsorption model, which may be due to the excessive AC damage to the pore structure of P-CS/AC membranes. In the static adsorption model, the adsorption efficiency of P-CS/AC membranes was slightly higher than that of N-CS/AC membranes.
Figure 4.
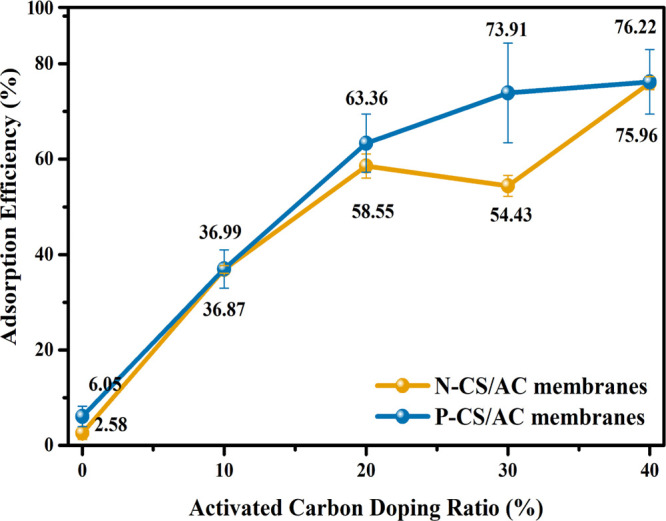
Adsorption efficiency of N-CS/AC membranes and P-CS/AC membranes in a static adsorption experiment.
The adsorption quantity of CS/AC membranes is listed in Figure 5. When the AC doping ratio was 0%, the adsorption quantity of the P-CS membrane was significantly higher than that of untreated N-CS membranes. This indicated that the pore-forming treatment with the porogen agent PEG and the cross-linking agent TPP could improve the adsorption performance of CS membranes. With the addition of AC, the adsorption quantity of CS/AC membranes improved. However, the adsorption quantity of P-CS/AC membranes is slightly higher than that of N-CS/AC membranes. This showed that the pore structure of AC played a major role in static adsorption.
Figure 5.

Adsorption quantity of N-CS/AC membranes and P-CS/AC membranes with different AC doping ratios.
Since the adsorption performance of AC at 30 and 40% was better than others, we explored their reused adsorption efficiency, as shown in Figure 6. The adsorption efficiency of reused CS/AC membranes was lower than that of the original membranes. However, the adsorption efficiency of P-CS/AC membranes was still higher than that of N-CS/AC membranes, which indicated that PEG/TPP treatment could improve the adsorption stability of CS/AC membranes.
Figure 6.

Adsorption efficiency of first used and reused CS/AC membranes with 30 and 40% AC doping ratios (R1: first used membranes and R2: reused membranes).
3. Conclusions
In this study, different doping ratios of AC were added to improve the adsorption performance of CS membranes, and the CS/AC membranes were modified by adding the porogen PEG and the cross-linking agent TPP. The dynamic and static adsorption models were prepared to compare the adsorption performance of P-CS/AC membranes and N-CS/AC membranes with different AC doping ratios. In the dynamic adsorption model, the P-CS/AC membrane with porous structures showed better adsorption efficiency, and a 30% AC doping ratio achieved the best adsorption performance. In the static adsorption model, AC played a major role in improving the adsorption performance of the CS/AC membrane, and PEG/TPP modification could improve its adsorption performance stability. Besides, the PEG/TPP modification treatment could improve the adsorption performance and reused stability of the CS/AC membranes, and a 30% AC doping ratio acted as a reference for doping amount. This work plays a vital role in designing the CS/AC membrane economically.
4. Experimental Section
4.1. Materials
Chitosan (CS, Shanghai, China), polyethylene glycol (PEG10000, Shanghai, China), sodium tripolyphosphate (TPP, Shanghai, China), and cellulose filter paper (pore size is 70 mm, Shanghai, China) were used as received. Acetic acid, sodium hydroxide, glycerin, and activated carbon powder used were of analytical grades. Rhodamine B used was of biotechnology grade. Ultrapure water was used in all experiments.
4.2. Preparation of a Porous Chitosan Membrane
First, 0.1 g of CS was dissolved in 10 mL of 1% v/v acetic acid aqueous solution under 200 rpm at 60 °C for 5 h. The mixture was then filtered through a 1000 mesh nylon microporous membrane to obtain pure CS solution. After that, 0.5 g of PEG as a porogen was added to the solution under stirring at 200 rpm for 1 h at room temperature, followed by sonication to remove air bubbles. To obtain a uniform P-CS membrane, 5 mL of mixed solution was slowly poured onto the cellulose filter membrane and evaporated at room temperature for about 12 h. The membrane was then immersed into 50 mL of 5% w/v NaOH solution for 12 h and washed with ultrapure water until neutral.
To improve the stability of the P-CS membrane, TPP as a cross-linking agent was introduced. First, 50 mL of 10% w/v TPP solution was prepared and adjusted to pH = 4 using HCl aqueous solution. Then, the CS membrane was immersed in the TPP solution for about 2 h and washed with ultrapure water until neutral. To prevent shrinkage of P-CS membranes, the membranes were treated with 50 mL of glycerol solution (20% v/v) for 1 h. Three drying methods (air-drying at room temperature, oven-drying at 40 °C, and freeze-drying) were introduced to dry the membrane, respectively.
4.3. Preparation of a Porous and Nonporous Chitosan/Activated Carbon Membrane
As for P-CS/AC membranes, 0.1 g of CS was dissolved in 10 mL of 1% v/v acetic acid aqueous solution and filtered to obtain pure CS solution. A total of 0.5 g of PEG was added to CS solution at 200 rpm for 1 h and sonicated to remove air bubbles. Then, different amounts of prerefined AC (10, 20, 30, and 40 mg) were suspended into 10 mL of CS solution, respectively. The suspensions were vigorously stirred for 10 min. Then, 5 mL of mixture solutions were slowly poured onto cellulose filter membranes and evaporated at room temperature for 12 h. The membranes were immersed into 50 mL of 5% w/v NaOH solution for 12 h and washed with ultrapure water until neutral. After that, the membranes were immersed in 50 mL of TPP solution (10% w/v, pH = 4) for 2 h and washed with ultrapure water until neutral. The membranes were treated with 50 mL of glycerol solution (20% v/v) for 1 h. Finally, the membranes were treated with air-drying for 24 h.
The preparation of N-CS/AC membranes was similar to that of the P-CS/AC membranes, except for PEG and TPP treatments. The N-CS/AC membranes without PEG/TPP treatment were obtained in the end.
where mac (mg) and mcs (mg) are the mass of AC and CS, respectively.
4.4. Dynamic Adsorption Studies
The adsorption performance of P-CS/AC membranes and N-CS/AC membranes with different AC doping ratios was investigated. A total of 10 mL of 10 mg/L RhB solution was filtered through the CS/AC membranes. Then, the filtered RhB solutions were collected for further analysis. The concentration of filtered RhB solutions was examined using a UV–vis spectrophotometer with an excitation wavelength at λ = 554 nm. The adsorption efficiency of membranes toward RhB was calculated as follows
where C0 and Ce are the stock and filtered concentrations of RhB, respectively.
For reused adsorption performance, the used membranes were soaked into ethanol solution for 1 h to remove adsorbed RhB and dried for secondary use.
4.5. Static Adsorption Studies
The static adsorption studies were performed with a series of adsorption experiments at 20 °C. The small circle pieces (diameter = 6 mm) were cut from random positions of the prepared CS/AC membranes with a punch. Then, 10 small pieces of each CS/AC membrane were placed into 5 mL of 10 mg/mL RhB solutions (pH = 8.4) and stirred at 150 rpm for 1 h. The final RhB solutions were collected for further test.
The adsorption quantity was calculated as follows
where V is the volume of stock RhB solution, C0 and Ce are the stock and filtered concentrations of RhB, respectively, and m is the quality of the CS/AC layer or the membrane.
The operation of reused adsorption studies was similar to the dynamic adsorption.
Acknowledgments
The authors would like to acknowledge the support from the National Natural Science Foundation of China (grant no. 51671114 and U1806219), the Natural Science Foundation of Shandong Province (ZR2019BEM024), the Shenzhen Fundamental Research Program (JCYJ20190807092803583), the Natural Science Foundation of Jiangsu Province (grant no. BK20190205), the Guangdong Basic and Applied Basic Research Foundation (grant no. 2019A1515110846), and the Fundamental Research Funds of Shandong University (grant no. 2018CJ047). The Special Funding also supports this work in the Project of the Qilu Young Scholar Program of Shandong University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01444.
SEM image of the cross-sectional of CS membrane and SEM images and characteristics of TPP cross-linked CS membranes in different drying processes (PDF)
Author Contributions
# J.Y. and Y.H. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Hu E.; Shang S.; Chiu A. K. Removal of Reactive Dyes in Textile Effluents by Catalytic Ozonation Pursuing on-Site Effluent Recycling. Molecules 2019, 24, 2755. 10.3390/molecules24152755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithya R.; Thirunavukkarasu A.; Sathya A. B.; Sivashankar R. Magnetic materials and magnetic separation of dyes from aqueous solutions: a review. Environ. Chem. Lett. 2021, 19, 1275. 10.1007/s10311-020-01149-9. [DOI] [Google Scholar]
- Banat I. M.; Nigam P.; Singh D.; Marchant R. Microbial decolorization of textile-dyecontaining effluents: A review. Bioresour. Technol. 1996, 58, 217–227. 10.1016/s0960-8524(96)00113-7. [DOI] [Google Scholar]
- Moradihamedani P. Recent advances in dye removal from wastewater by membrane technology: a review. Polym. Bull. 2021, 10.1007/s00289-021-03603-2. [DOI] [Google Scholar]
- Figueiredo S. A.; Boaventura R. A.; Loureiro J. M. Color removal with natural adsorbents: modeling, simulation and experimental. Sep. Purif. Technol. 2000, 20, 129–141. 10.1016/s1383-5866(00)00068-x. [DOI] [Google Scholar]
- Husain Q. Peroxidase mediated decolorization and remediation of wastewater containing industrial dyes: a review. Rev. Environ. Sci. Biotechnol. 2010, 9, 117–140. 10.1007/s11157-009-9184-9. [DOI] [Google Scholar]
- Nayeri D.; Mousavi S. A. Dye removal from water and wastewater by nanosized metal oxides - modified activated carbon: a review on recent researches. J. Environ. Health Sci. Eng. 2020, 18, 1671–1689. 10.1007/s40201-020-00566-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lops C.; Ancona A.; Di Cesare K.; Dumontel B.; Garino N.; Canavese G.; Hérnandez S.; Cauda V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal., B 2019, 243, 629–640. 10.1016/j.apcatb.2018.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merouani S.; Hamdaoui O.; Saoudi F.; Chiha M.; Pétrier C. Influence of bicarbonate and carbonate ions on sonochemical degradation of Rhodamine B in aqueous phase. J. Hazard. Mater. 2010, 175, 593–599. 10.1016/j.jhazmat.2009.10.046. [DOI] [PubMed] [Google Scholar]
- Pathe P. P.; Biswas A. K.; Rao N. N.; Kaul S. N. Physico-chemical treatment of wastewater from clusters of small scale cotton textile units. Environ. Technol. 2005, 26, 313–328. 10.1080/09593332608618562. [DOI] [PubMed] [Google Scholar]
- Cheng K. Y.; Kaksonen A. H.; Douglas G. B. Sequential in situ hydrotalcite precipitation and biological denitrification for the treatment of high-nitrate industrial effluent. Bioresour. Technol. 2014, 172, 373–381. 10.1016/j.biortech.2014.09.050. [DOI] [PubMed] [Google Scholar]
- Hu E.; Shang S.; Tao X.-m.; Jiang S.; Chiu K.-l. Regeneration and reuse of highly polluting textile dyeing effluents through catalytic ozonation with carbon aerogel catalysts. J. Clean. Prod. 2016, 137, 1055–1065. 10.1016/j.jclepro.2016.07.194. [DOI] [Google Scholar]
- Palácio S. M.; Espinoza-Quiñones F. R.; Módenes A. N.; Oliveira C. C.; Borba F. H.; Silva F. G. Jr Toxicity assessment from electro-coagulation treated-textile dye wastewaters by bioassays. J. Hazard. Mater. 2009, 172, 330–337. 10.1016/j.jhazmat.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Sun Z.; Yang J.; Qi Y.; Wang F.; Hong W.; Li H.; Jiang Y. Facile preparation of hydroxyl-rich mesoporous magnesium silicate with excellent adsorption performance. Surf. Interfaces 2020, 20, 100519. 10.1016/j.surfin.2020.100519. [DOI] [Google Scholar]
- Chen H.; Zhou Y.; Wang J.; Lu J.; Zhou Y. Polydopamine modified cyclodextrin polymer as efficient adsorbent for removing cationic dyes and Cu2+. J. Hazard. Mater. 2020, 389, 121897. 10.1016/j.jhazmat.2019.121897. [DOI] [PubMed] [Google Scholar]
- Yan M.; Huang W.; Li Z. Chitosan cross-linked graphene oxide/lignosulfonate composite aerogel for enhanced adsorption of methylene blue in water. Int. J. Biol. Macromol. 2019, 136, 927–935. 10.1016/j.ijbiomac.2019.06.144. [DOI] [PubMed] [Google Scholar]
- Burakov A. E.; Galunin E. V.; Burakova I. V.; Kucherova A. E.; Agarwal S.; Tkachev A. G.; Gupta V. K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. 10.1016/j.ecoenv.2017.11.034. [DOI] [PubMed] [Google Scholar]
- Pala A.; Tokat E. Color removal from cotton textile industry wastewater in an activated sludge system with various additives. Water Res. 2002, 36, 2920–2925. 10.1016/s0043-1354(01)00529-2. [DOI] [PubMed] [Google Scholar]
- Dwyer J.; Griffiths P.; Lant P. Simultaneous colour and DON removal from sewage treatment plant effluent: alum coagulation of melanoidin. Water Res. 2009, 43, 553–61. 10.1016/j.watres.2008.10.053. [DOI] [PubMed] [Google Scholar]
- Butt M. T.; Imtiaz N.; Ahmed S.; Arif F.; Khan S. R. Colour Removal from Textile Dyeing Wastewater Using Different Adsorbents. Pak. J. Sci. Ind. Res. 2010, 53, 81–84. [Google Scholar]
- Sun Z.; Duan X.; Srinivasakannan C.; Liang J. Preparation of magnesium silicate/carbon composite for adsorption of rhodamine B. RSC Adv. 2018, 8, 7873–7882. 10.1039/c7ra12848g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F.; Sheng B.; Wang Z.; Xue Y.; Liu J.; Ma T.; Bush R.; Kušić H.; Zhou Y. Performance of UV/acetylacetone process for saline dye wastewater treatment: Kinetics and mechanism. J. Hazard. Mater. 2021, 406, 124774. 10.1016/j.jhazmat.2020.124774. [DOI] [PubMed] [Google Scholar]
- Faysal Hossain M.; Akther N.; Zhou Y. Recent advancements in graphene adsorbents for wastewater treatment: Current status and challenges. Chin. Chem. Lett. 2020, 31, 2525–2538. 10.1016/j.cclet.2020.05.011. [DOI] [Google Scholar]
- Lei X.; You M.; Pan F.; Liu M.; Yang P.; Xia D.; Li Q.; Wang Y.; Fu J. CuFe2O4@GO nanocomposite as an effective and recoverable catalyst of peroxymonosulfate activation for degradation of aqueous dye pollutants. Chin. Chem. Lett. 2019, 30, 2216–2220. 10.1016/j.cclet.2019.05.039. [DOI] [Google Scholar]
- Ma Y.; Zhi L. Functionalized Graphene Materials: Definition, Classification, and Preparation Strategies. Acta Phys. Chim. Sin. 2021, 37, 2101004. 10.3866/pku.whxb202101004. [DOI] [Google Scholar]
- Desai M.; Mehta M. Tertiary Treatment for Textile Waste Water-A Review. Int. J. Eng. Sci. Res. Technol. 2014, 5, 1579–1585. [Google Scholar]
- Sarode S.; Upadhyay P.; Khosa M. A.; Mak T.; Shakir A.; Song S.; Ullah A. Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. 10.1016/j.ijbiomac.2018.10.089. [DOI] [PubMed] [Google Scholar]
- Blackburn R. S. Natural Polysaccharides and Their Interactions with Dye Molecules: Applications in Effluent Treatment†. Environ. Sci. Technol. 2004, 38, 4905–4909. 10.1021/es049972n. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Lu J.; Zhou Y.; Liu Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. 10.1016/j.envpol.2019.05.072. [DOI] [PubMed] [Google Scholar]
- Inyinbor A. A.; Adekola F. A.; Olatunji G. A. Liquid phase adsorptions of Rhodamine B dye onto raw and chitosan supported mesoporous adsorbents: isotherms and kinetics studies. Appl. Water Sci. 2017, 7, 2297–2307. 10.1007/s13201-016-0405-4. [DOI] [Google Scholar]
- Elsabee M. Z.; Morsi R. E.; Al-Sabagh A. M. Surface active properties of chitosan and its derivatives. Colloids Surf., B 2009, 74, 1–16. 10.1016/j.colsurfb.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Auta M.; Hameed B. H. Coalesced chitosan activated carbon composite for batch and fixed-bed adsorption of cationic and anionic dyes. Colloids Surf., B 2013, 105, 199–206. 10.1016/j.colsurfb.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Sharififard H.; Shahraki Z. H.; Rezvanpanah E.; Rad S. H. A novel natural chitosan/activated carbon/iron bio-nanocomposite: Sonochemical synthesis, characterization, and application for cadmium removal in batch and continuous adsorption process. Bioresour. Technol. 2018, 270, 562–569. 10.1016/j.biortech.2018.09.094. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Wei J.; Duyar M. S.; Ordomsky V. V.; Khodakov A. Y.; Liu J. Carbon-based catalysts for Fischer-Tropsch synthesis. Chem. Soc. Rev. 2021, 50, 2337–2366. 10.1039/d0cs00905a. [DOI] [PubMed] [Google Scholar]
- Liu H.; Ren X.; Chen L. Synthesis and characterization of magnetic metal-organic framework for the adsorptive removal of Rhodamine B from aqueous solution. J. Ind. Eng. Chem. 2016, 34, 278–285. 10.1016/j.jiec.2015.11.020. [DOI] [Google Scholar]
- Shao L.; Chang X.; Zhang Y.; Huang Y.; Yao Y.; Guo Z. Graphene oxide cross-linked chitosan nanocomposite membrane. Appl. Surf. Sci. 2013, 280, 989–992. 10.1016/j.apsusc.2013.04.112. [DOI] [Google Scholar]
- Krahnstöver T.; Plattner J.; Wintgens T. Quantitative detection of powdered activated carbon in wastewater treatment plant effluent by thermogravimetric analysis (TGA). Water Res. 2016, 101, 510–518. 10.1016/j.watres.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Gad H. M.; El-Sayed A. A. Activated carbon from agricultural by-products for the removal of Rhodamine-B from aqueous solution. J. Hazard. Mater. 2009, 168, 1070–1081. 10.1016/j.jhazmat.2009.02.155. [DOI] [PubMed] [Google Scholar]
- da Silva Lacerda V.; López-Sotelo J. B.; Correa-Guimarães A.; Hernández-Navarro S.; Sánchez-Báscones M.; Navas-Gracia L. M.; Martín-Ramos P.; Martín-Gil J. Rhodamine B removal with activated carbons obtained from lignocellulosic waste. J. Environ. Manage. 2015, 155, 67–76. 10.1016/j.jenvman.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Liu X.; Lai D.; Wang Y. Performance of Pb(II) removal by an activated carbon supported nanoscale zero-valent iron composite at ultralow iron content. J. Hazard. Mater. 2019, 361, 37–8. 10.1016/j.jhazmat.2018.08.082. [DOI] [PubMed] [Google Scholar]
- Tian H.; Liang J.; Liu J. Nanoengineering Carbon Spheres as Nanoreactors for Sustainable Energy Applications. Adv. Mater. 2019, 31, 1903886. 10.1002/adma.201903886. [DOI] [PubMed] [Google Scholar]
- Yang L.; Hsiao W. W.; Chen P. Chitosan–cellulose composite membrane for affinity purification of biopolymers and immunoadsorption. J. Membr. Sci. 2002, 197, 185–197. 10.1016/s0376-7388(01)00632-9. [DOI] [Google Scholar]
- Wang J.; Cheng G.; Lu J.; Chen H.; Zhou Y. PDA-cross-linked beta-cyclodextrin: a novel adsorbent for the removal of BPA and cationic dyes. Water Sci. Technol. 2020, 81, 2337–2350. 10.2166/wst.2020.286. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Li Y.; Chen H.; Lu J.; Yu G.; Möslang M.; Zhou Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J. Hazard. Mater. 2020, 382, 121040. 10.1016/j.jhazmat.2019.121040. [DOI] [PubMed] [Google Scholar]
- Duan C.; Ma T.; Wang J.; Zhou Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process. Eng. 2020, 37, 101339. 10.1016/j.jwpe.2020.101339. [DOI] [Google Scholar]
- Rahmi; Lelifajri; Nurfatimah R. Preparation of polyethylene glycol diglycidyl ether (PEDGE) crosslinked chitosan/activated carbon composite film for Cd2+ removal. Carbohydr. Polym. 2018, 199, 499–505. 10.1016/j.carbpol.2018.07.051. [DOI] [PubMed] [Google Scholar]
- Salehi E.; Madaeni S. S.; Rajabi L.; Derakhshan A. A.; Daraei S.; Vatanpour V. Static and dynamic adsorption of copper ions on chitosan/polyvinyl alcohol thin adsorptive membranes: Combined effect of polyethylene glycol and aminated multi-walled carbon nanotubes. Chem. Eng. J. 2013, 215–216, 791–801. 10.1016/j.cej.2012.11.071. [DOI] [Google Scholar]
- Galan J.; Trilleras J.; Zapata P. A.; Arana V. A.; Grande-Tovar C. D. Optimization of Chitosan Glutaraldehyde-Crosslinked Beads for Reactive Blue 4 Anionic Dye Removal Using a Surface Response Methodology. Life 2021, 11, 85. 10.3390/life11020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.