Abstract
Soliva sessilis is a troublesome annual weed species in New Zealand turfgrass. This weed has been controlled selectively in New Zealand turfgrass for many years using pyridine herbicides such as clopyralid. However, in some golf courses, the continuous application of pyridine herbicides has resulted in the selection of S. sessilis populations that are resistant to these herbicides. This study focuses on a clopyralid-resistant population of S. sessilis collected from a golf course with a long history of clopyralid applications. The resistant phenotype of S. sessilis was highly resistant to clopyralid (over 225-fold). It was also cross-resistant to dicamba, MCPA and picloram but not mecoprop. The level of resistance to dicamba was high (7-14-fold) but much lower (2-3-fold) for both MCPA and picloram. The phenotype was morphologically distinct from its susceptible counterpart. Individuals of the clopyralid-resistant phenotype had fewer lobes on their leaves and were slightly larger compared to the susceptible phenotype. Resistant individuals also had a larger leaf area and greater root dry weight than the susceptible plants. An evaluation of internal transcribed spacer (ITS) regions confirmed that clopyralid-resistant phenotypes are conspecific with S. sessilis. In summary, the cross-resistance to several auxinic herbicides in this S. sessilis phenotype greatly reduces chemical options for controlling it; thus, other integrated management practices may be needed such as using turfgrass competition to reduce weed germination. However, the morphological differences between resistant and susceptible plants make it easy to see, which will help with its management.
Introduction
Weeds are unwanted plant species that can be troublesome in agricultural and non-agricultural situations [1]. Since the first herbicide was commercialized, chemical weed control has been the preferred method for managing weed populations [2]. The popularity of herbicides for weed control has not been without consequences and as predicted in the early days of the commercialization of herbicides, the occurrence of herbicide resistance in weed populations was inevitable [3]. To date there are over 500 unique cases of herbicide-resistant weed species globally [4]. In New Zealand, currently there are 25 confirmed cases of herbicide-resistant weeds [5], two of which were reported in turfgrass [6, 7].
Soliva sessilis, a member of the Asteraceae family, is a low growing winter annual weed species [8]. S. sessilis is originally from South America [9], and some of its common names include lawn burweed, field burrweed, lawnweed, bindii, bindy-eye, carpet burweed and Onehunga weed. In New Zealand, S. sessilis is primarily a troublesome weed species in turfgrass [5]. The germination of S. sessilis seeds occurs in late summer or early autumn when the soil becomes moist, and turfgrass has not recovered from summer dieback. Once the S. sessilis plants are established, they grow throughout winter [9]. There is little known about the reproductive biology of S. sessilis, but it has been noted that S. sessilis produces seeds in spring and, in summer when the mature plants of S. sessilis die back, the seeds are shed on the soil surface [9]. Each seed has a sharp spine that can penetrate the skin of bare feet, thus making this species a nuisance in turfgrass areas, especially near playgrounds and in home lawns [10]. Also, S. sessilis can be a vigorous competitor in short turfgrass, and once the plants die back, they leave patches of bare soil that can be used as a niche for other weed species establishment [11].
Weed management practices for turfgrass weed species such as S. sessilis involve mechanical, cultural and chemical options [12]. Hand pulling of established plants was found effective since S. sessilis plants are shallow-rooted and can easily be pulled out [13]. However, this approach is labour-intensive and is not cost-effective in large areas of turfgrass. Mowing does not affect S. sessilis due to the prostrate growth habit of S. sessilis. Flaming as a cultural practice was found effective in reducing S. sessilis populations [13]; however, the best results can only be achieved by using high intensity fires [14], which are damaging to turfgrass. Keeping turfgrass dense during autumn when the seeds normally germinate will prevent a new cohort from establishing, but this weed causes problems in turfgrass that has died back due to summer dryness [10]. Compared to other weed control practices, chemical options are more desirable as they provide more efficient and selective control of S. sessilis, hence they have been the most common practice for S. sessilis control in turfgrass in New Zealand [5].
Selective control of S. sessilis in New Zealand occasionally makes use of contact herbicides such as bentazone or ioxynil for seedlings, but generally involves synthetic auxin herbicides such as clopyralid, triclopyr, picloram, dicamba and MCPA for older plants [15]. Synthetic auxin herbicides mimic the natural auxin indole-3-acetic acid (IAA), hence they can bind to the same target receptor as IAA [16–18]. Clopyralid has been widely used to control S. sessilis in turfgrass in New Zealand because of its effectiveness and selectivity in fine turfgrass [5, 6]. However, in the early 2000s, a population of S. sessilis was found resistant to clopyralid in a New Zealand golf course which had a long history of pyridine herbicide applications, especially clopyralid and triclopyr [6]. Worldwide, there are currently over 40 weed species confirmed as having evolved resistance to synthetic auxin herbicides [4]. We understand this is the only reported case in the world of clopyralid-resistant S. sessilis.
The initial report of this case showed only that there were significant differences between this phenotype and susceptible S. sessilis to recommended rates of clopyralid, triclopyr, and mixtures of picloram with triclopyr and with 2,4-D [6]. In work reported here, we evaluate the magnitude of resistance to clopyralid in this phenotype, and also investigate the magnitude of resistance within some of the other synthetic auxin herbicides used in turfgrass. Also, we provide further details about the differences in growth traits between clopyralid-resistant and clopyralid-susceptible phenotypes of S. sessilis, and check whether this phenotype is still genetically the same species given the differences in morphology.
Materials and methods
Plant material
The response of a clopyralid-resistant population of S. sessilis was compared to a susceptible one. The clopyralid-resistant population (OR) was the population mentioned above which also showed some resistance to triclopyr and picloram, and was originally from a golf course at Helensville (36°38’27.9"S 174°30’43.0"E) near Auckland [6]. Seeds were collected from survivors of plants that had been treated with clopyralid and were kept at 5°C. To multiply these seeds, 20 plants were grown from seeds that had been collected after the preliminary experiment. For this, five seeds were placed on the surface of potting mix (20% Pacific Pumice (7 mm), 30% fibre, 50% bark) containing slow release fertilizer (Woodace, PA, USA) within polythene bags, and the seeds were covered with a 1cm layer of potting mix. The pots were placed in a heated glasshouse at average daily max/min temperatures of 22.2/20.5°C, and an average relative humidity (RH) of 58%. Seedlings were then thinned to one per pot at 1 week after emergence, and the plants were left to establish before they were shifted to a shadehouse in late autumn. The plants were kept in the shadehouse under natural light throughout winter. The average maximum and minimum temperatures during winter in the unheated glasshouse were 10.5°C to 6.4°C respectively. A susceptible population (OS) was collected from a site close to the Helensville Golf Club (36°38’18.6"S 174°30’23.2"E), but previous applications of any herbicides including clopyralid at this site were unlikely. The plants from population OS were also kept in the same shadehouse as population OR. Sine the resistant and susceptible plants flowered at different times, the cross-pollination between them was unlikely. The seeds from each population were collected at maturity in early summer and stored at 5°C until the beginning of this research.
Response to clopyralid
The response of population OR to clopyralid (Versatill, 300 g ae L-1 as amine salt) was compared to population OS using a dose-response experiment. Plants of each population were established from seeds. For this, 30 seeds were placed in each planter bag (PB 5, 120mm x 120mm x 200mm, 3 L) filled with potting mix and fertilizer as described above. The pots were kept in a heated glasshouse under natural light at average daily max/min temperatures of 23.4/19.8°C, and an average RH of 52%. At one week after emergence, the seedlings were thinned to 15 seedlings in each pot, and the plants were left to establish before they were sprayed at the 4–5 leaf stage with clopyralid. Clopyralid was applied at 0, 37.5, 75, 150, 300 (recommended rate), 600, 1200, 2400 and 4800 g ae ha-1 in an initial dose-response experiment. The doses were chosen to cover the whole range of responses from no effect to complete death of plants [19]. The plants were treated with clopyralid using a laboratory track sprayer which delivered 230 L ha−1 of spray solution at 200 kPa. The treated plants were then returned to the same glasshouse and kept for 4 weeks before evaluating the response of plants to herbicide treatments. The average daily maximum and minimum temperatures during the 4 weeks following treatment were 23.1 and 20.1°C respectively, and the average RH was 59%. To evaluate the response of plants to clopyralid, the number of plants that survived the application was recorded to calculate the percentage of surviving plants for each rate. This experiment used a randomized design with four replicates (i.e. four pots) and then was repeated using the same method outlined above, but in the second dose-response experiment, plants were treated with 0, 37.5, 75, 150, 300, 600, 1200, 2400 and 4800, 9600 and 19200 g ae ha-1 of clopyralid. Higher doses were added in the second experiment because the highest rate of clopyralid used in the first dose-response caused no mortality in the resistant phenotype.
Cross-resistance to other synthetic auxin herbicides
The pattern of cross-resistance to MCPA (MCPA 750, 750 g ae L-1 as the dimethylamine salt), picloram (Spike, 200 g ae L-1 as amine salt), dicamba (Kamba 500, 500 g ae L-1 as dimethylamine salt) and mecoprop-p (Duplosan KV, 600 g ae L-1 as potassium salt of the optically active isomer) was evaluated for population OR and the response was compared to population OS using the same dose-response experiment method as outlined above. Plants were established and grown as described above. The rates used for each herbicide are summarized in Table 1. This experiment used a randomized design with four replicates (i.e. four pots) and was conducted twice.
Table 1. Herbicides that were applied to clopyralid-resistant (OR) and clopyralid susceptible (OS) populations in dose-response experiments.
| Herbicide | Rate (g ae ha-1) |
|---|---|
| Picloram | 0, 25, 50, 100, 200, 400, 800, 1600 and 3200 |
| MCPA | 0, 93.75, 187.5, 375, 750, 1500, 3000, 6000 and12000 |
| Dicamba | 0, 100, 200, 400, 800, 1600, 3200 and 6400 |
| Mecoprop-p | 0, 150, 300, 600, 1200, 2400, 4800 and 9600 |
Growth characteristics
Preliminary experiments reported that certain growth characteristics of clopyralid-resistant S. sessilis were different to susceptible plants [6]. Here, we quantified and compared the growth characteristics of the clopyralid-resistant with clopyralid-susceptible phenotypes of S. sessilis. Plants of each population were established from seeds using the method outlined above. At emergence, the seedlings were transplanted into pots (PB3, 100mm x 100mm x 200mm, 1.7 L) containing the same potting mix and slow-release fertilizer as those for the dose-response experiments with each pot containing only one seedling. The pots were kept under the same conditions as outlined for the dose-response experiments. At 40 days after emergence, the distance between the tips of the longest leaves either side of the rosette (rosette width) was measured in one direction to estimate the diameter of the plant rosette. A second measurement was then made perpendicular to the first measurement, and the two measurements were averaged for each plant. The plants were photographed before removing the plants (shoot plus root) from each pot. The photographs were used to study the morphological differences in leaflet lobes between resistant and susceptible phenotypes. The harvested plants were divided into root and shoot, and the root was washed with tap water. The leaf area of each harvested plant was measured using a digital leaf area meter (LiCor model-3100; LiCor, Lincoln, USA). The harvested shoot and root materials were oven-dried separately at 80°C for 48 h then weighed. This experiment consisted of eight replicates and was conducted twice.
Evaluation of internal transcribed spacer regions
Internal transcribed spacer (ITS) regions are DNA markers that can be used to identify plant species [20]. The ITS of the clopyralid-resistant phenotype was evaluated and compared to those published for S. sessilis previously [21]. For this, initially, the genomic DNA was extracted from the leaves taken from clopyralid-resistant S. sessilis using a method described previously [22]. To amplify the ITS regions (ITS1 and ITS2), previously published primers [23] with some modifications were used. The forward (ITS-18SF: 5`-GAACCTTATCGTTTAGAGGAAGGAG-3`) and reverse (ITS-26R: 51`-AAGCCGCCCGATTTTCAAGC-3`) primers cover 840 bp portion of the S. sessilis ribosomal RNA gene (KX064030.1) flanking both ITS1 and ITS2 regions. Polymerase chain reaction (PCR) was used to amplify the ITS regions. The PCR reaction contained 12.5 μl of Q5® high-fidelity 2X master mix (NEB, UK), 20 ng of DNA template, 0.4 μM of each forward and reverse primer and nuclease-free water to bring the volume of reaction to 25 μl. The PCR thermocycling program included initial denaturation at 98°C (one cycle of 30 s), denaturation at 98°C (35 cycles of 10 s), 35 cycles of 30 s annealing at 55°C, 35 cycles of 15 s extension at 72°C, followed by one cycle final extension at 72°C (2 min). The PCR products were then loaded on a 1x LB (lithium borate) 1% agarose gel (0.5 μg ml-1 ethidium bromide) before they were run at 5 V cm-1 for 0.5 h and visualized under UV illumination using a Gel Doc XR 2000 system (Bio-Rad Laboratories). The PCR products were then sequenced by the Massey Genome Service using the same forward and reverse primers outlined above and the DNA sequenced data were analyzed, assembled and compared using an online sequence alignment tool, Emboss Needle (https://www.ebi.ac.uk) [24]. The ITS region sequence from this study was compared to the ITS region sequences of S. sessilis (AM774471.1), S. anthemifolia (AY947414.1), S. mutisii (HE860705.1), and S. stolonifera (AJ864601.1) available in the data set using the basic local search alignment tool (Blast) (https://blast.ncbi.nlm.nih.gov/Blast.cgi), and the sequences with highest blast scores were considered best hit sequences. As ITS2 regions contain enough variability to distinguish closely related species [20], the secondary structures of ITS2 region was predicted and assessed on the ITS2 database web server (http://its2.bioapps.biozentrum.uni-wuerzburg.de) [25]. In addition, the Kimura‐2‐parameter (K2P) model was used to calculate genetic distance within interspecies using MEGA X software [26].
Statistical analyses
The survival data from dose-response experiments were fitted to a three-parameter log-logistic model (Eq 1) after they were checked for normality (Shapiro–Wilk test) and homogeneity of variance (Levene’s tests).
| (1) |
where Y is plant survival, d is the upper limit, x is the herbicide rate, LD50 is the herbicide rate corresponding to 50% reduction in plant survival, and b is the slope around LD50. The dose-response data were analyzed using the drc package in R v. 3.1.2. [27], and the LD50 estimates of resistant and susceptible populations for each herbicide were compared using the ‘compParm’ statement in the drc package [27]. The data from both dose-response experiments were analyzed separately due to the variability in response to herbicides between two runs. The data from the growth characteristic experiments were pooled as there was no significant difference between the two runs (p > 0.05). The differences in growth characteristics between populations OR and OS were statistically analyzed and compared using a Student`s t-test at a 5% probability.
Results
Clopyralid dose-response experiments
The results from the first clopyralid dose-response experiment revealed a high level of resistance to clopyralid for OR population compared to the susceptible population (OS). While all OS plants treated at 300 g ae clopyralid ha-1 were completely dead at 4 weeks after application, even 4800 g ae clopyralid ha-1 did not cause any mortality in the plants of OR population (Fig 1A, Table 2). In the second clopyralid dose-response experiment, the range of clopyralid rates was further extended to generate a better dose-response curve for estimating the level of clopyralid resistance in population OR. All the plants of OS population were completely controlled at 150 g ae clopyralid ha-1; however, there was only 18% mortality recorded for the plants of population OR treated at 19200 g ae clopyralid ha-1 (Fig 1B, Table 2). Based on these results, it appeared that population OR was highly resistant to clopyralid with a level of resistance over 225-fold.
Fig 1.
Fitted clopyralid dose-response curves for two S. sessilis populations, the resistant population OR and the susceptible population OS in (a) the first and (b) second dose-response experiments. The percentage of survival of treated plants was used to produce the fitted curves. Vertical bars represent ± standard error of the mean.
Table 2. Parameters (see footnote) estimated from the four-parameter log-logistic model analysis of clopyralid dose-response experiments for clopyralid-resistant (OR) and susceptible (OS) populations evaluated at 4 weeks after treatment.
| First dose-response experiment | ||||
| Population | d (±SE) | b (±SE) | LD50 (±SE) | LD50 RF |
| OR | 100 (0.6) | 1.4 (N/A) | >4800 (N/A) | >33.1 |
| OS | 100 (1.0) | 5.4 (0.8) | 145.0 (2.0) | |
| P-value | NA | |||
| Second dose-response experiment | ||||
| Population | d (±SE) | b (±SE) | LD50 (±SE) | R/S LD50 |
| OR | 100.5 (1.1) | 1.6 (0.4) | >19200 (N/A) | >225.9 |
| OS | 99.0 (2.9) | 3.2 (0.4) | 83.9 (3.8) | |
| P-value | N/A | |||
d = the upper limit, b = the slope around the LD50, LD50 = the rate of herbicide (g ae ha-1) required to cause 50% mortality, SE = standard error LD50 RF = resistant/susceptible factor based on LD50 ratios. N/A = not applicable.
Cross-resistance dose-response experiments
Results from dose-response experiments for other synthetic auxin herbicides showed that population OR was cross-resistant to picloram, MCPA and dicamba, but with different levels of resistance to each herbicide (Fig 2, Table 3).
Fig 2.
Fitted dose-response curves for two S. sessilis populations, the resistant population OR and the susceptible population OS in (a) first and (b) second picloram dose-response experiments, (c) first and (d) second MCPA dose-response experiments, e) first and (f) second dicamba dose-response experiments, and (g) first and (h) second mecoprop dose-response experiments. The percentage of survival of treated plants was used to produce the fitted curves. Vertical bars represent ± standard error of the mean.
Table 3. Parameters (see footnote) estimated from the four-parameter log-logistic model analysis of picloram, MCPA, dicamba and mecoprop dose-response experiments for clopyralid-resistant (OR) and susceptible (OS) populations evaluated at 4 weeks after treatment.
| First dose-response experiment | |||||
| Population | d (±SE) | b (±SE) | LD50 (±SE) | LD50 RF | |
| Picloram | OR | 102.9 (2.6) | 2.1 (0.2) | 146.1 (10.1)a | 2.6 |
| OS | 102.3 (2.9) | 3.7 (0.5) | 55.2 (2.4)b | ||
| P-value | 0.006 | ||||
| MCPA | OR | 100.7 (2.6) | 2.7 (0.4) | 1048.7 (64.8)a | 2.9 |
| OS | 100.1 (4.5) | 1.7 (0.2) | 363.9 (37.1)b | ||
| P-value | 0.0008 | ||||
| Dicamba | OR | 99.8 (2.6) | 2.5 (0.5) | 2072.6 (232.0)a | 13.8 |
| OS | 99.2 (5.0) | 2.7 (0.4) | 149.7 (12.9)b | ||
| P-value | 0.0001 | ||||
| Mecoprop-p | OR | 99.9 (2.6) | 2.2 (0.3) | 3077.8 (238.0)b | 0.6 |
| OS | 98.0 (3.4) | 1.1 (0.2) | 5459.3 (708.6)a | ||
| P-value | 0.0001 | ||||
| Second dose-response experiment | |||||
| Population | d (±SE) | b (±SE) | LD50 (±SE) | LD50 RF | |
| Picloram | OR | 101.5 (2.0) | 2.7 (0.4) | 119.8 (4.3)a | 2.4 |
| OS | 97.0 (2.8) | 3.0 (0.4) | 50.8 (2.1)b | ||
| P-value | 0.0001 | ||||
| MCPA | OR | 99.8 (1.8) | 3.9 (0.5) | 460.7 (15.3a) | 2.1 |
| OS | 101.6 (2.5) | 2.8 (0.3) | 227.8 (9.7)b | ||
| P-value | 0.002 | ||||
| Dicamba | OR | 100.2 (1.2) | 3.7 (0.4) | 1572.9 (42.5)a | 9.5 |
| OS | 99.4 (2.5) | 2.6 (0.2) | 165.6 (6.9)b | ||
| P-value | 0.0001 | ||||
| Mecoprop-P | OR | 101.7 (3.4) | 2.2 (0.2) | 545.5 (38.1)b | 0.8 |
| OS | 100.5 (3.4) | 2.1 (0.3) | 658.1 (49.3)a | ||
| P-value | 0.04 | ||||
d = the upper limit, b = the slope around the LD50, LD50 = the rate of herbicide (g ae ha-1) required to cause 50% mortality, SE = standard error LD50 RF = resistant/susceptible factor based on LD50 ratios. Different letters within one herbicide treatment indicate significant differences between the two populations, according to t-tests (P < 0.05).
All plants of population OS treated at 100 g ae picloram ha-1 were completely controlled in both dose-response experiments, whereas 100% mortality was only recorded for population OR at 800 and 400 g ae picloram in the first and second dose-response experiments, respectively. Based on the values for 50% reduction in survival of individuals (LD50 values), population OR was estimated to be 2.6- and 2.4-fold more resistant to picloram relative to population OS (Fig 2A and 2B).
Population OR also showed a low level of resistance to MCPA (Table 3). Based on the LD50 values, population OR was found to be 2.9 and 2.1 times less sensitive to MCPA compared to population OS, in the first and second dose-response experiments, respectively (Table 3). MCPA application rates of 750 and 375 ae ha-1 resulted in 100% mortality in all plants of population OS in the first and second dose-response experiments respectively, while greater rates of MCPA were needed to provide complete control of the individuals of population OR in both experiments (Fig 2C and 2D).
Population OR displayed a high level of resistance to dicamba in both dose-response experiments (Table 3). In both dose-response experiments, the individuals of population OS were completely dead at 800 g ae dicamba ha-1, while at this dicamba rate, only 10% mortality was recorded for population OR (Fig 2E and 2F). The LD50 values for population OR when treated with dicamba were found to be significantly greater than those of population OS in both dose-response experiments (Table 3). Population OR was 13.7-fold more resistant to dicamba than population OS, based on the LD50 R/S ratio in the first run, and a 9.5-fold difference was recorded in the second run (Table 3).
Population OR was found to be more sensitive than population OS when treated with mecoprop-P (Fig 2G and 2H). Comparison of the LD50 values showed significant differences between the populations, indicating a small negative cross-resistance to mecoprop in population OR (Table 3).
Determination of differences in growth traits
To quantify the differences in growth characteristics between clopyralid-resistant and clopyralid-susceptible individuals, several traits were evaluated. At the cotyledon stage, there were no noticeable differences between the two populations (Fig 3A). However, differences in growth traits between the individuals of the two populations were evident with the appearance of true leaves. The leaflet shape differed between the two populations, with individuals of population OS having more lobes on each leaf than OR plants (Fig 3B and 3C).
Fig 3.
Variation in leaf morphology at (a) seedling (b) the 2–3 leaf and (c) the 5–6 leaf stage between clopyralid-resistant and clopyralid-susceptible plants.
When plants were compared at 40 days after emergence, the individuals of population OR were significantly larger as determined by their rosette width, leaf area and shoot dry weight (Table 4). There were also differences between both populations in their root size as the individuals of OR population had a larger root dry weight after 40 days of growth. Therefore, the total dry weight of population OR plants was also greater after 40 days than the OS plants. However, there were no significant differences in shoot/root ratios between the populations (Table 4).
Table 4. Growth analysis of clopyralid-resistant (OR) and clopyralid -susceptible (OS) plants at 40 days after emergence.
| Population | RosetteWidth (cm) | Leaf area (cm-2) | Shoot dry weight (g) | Root dry weight (g) | Total dry weight (g) | Shoot/root ratio |
|---|---|---|---|---|---|---|
| OR | 11.2a | 24.2a | 0.144a | 0.0283a | 0.172a | 5.3 |
| OS | 8.5b | 14.7b | 0.103b | 0.0209b | 0.124b | 4.9 |
| P-value | 0.0001 | 0.0001 | 0.0001 | 0.005 | 0.0001 | 0.322 |
Mean values followed by different letters are significantly different between the two populations, according to t-tests (P < 0.05).
Comparison of sequence variation in ITS regions
The ITS primers used in this research successfully amplified the ITS regions. The results from the ITS1 region sequence alignment showed that the ITS1 region of population OR had 100% sequence homology to that of S. sessilis while it only had 94.5% sequence homology to the ITS1 region of Soliva anthemifolia and Soliva mutisii (Fig 4). Similarly, the ITS2 region sequence of population OR showed the greatest level of homology (99.60%) with the ITS2 region sequence of S. sessilis (Fig 5). The ITS2 region sequence of population OR had 95.50, 94.0 and 90.80% homology with that of S. anthemifolia, S. mutisii and Soliva stolonifera, respectively (Fig 5). Overall, these results showed that individuals of population OR had identical ITS region sequences with S. sessilis and the ITS2 region appeared to provide higher identification efficiency compared to the ITS1 region.
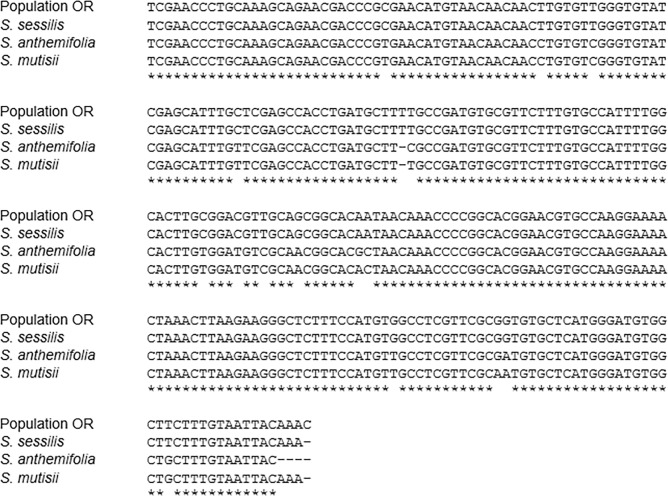
Fig 4. Sequence alignment of the internal transcribed spacer 1 (ITS1) regions of clopyralid-resistant population (OR), S. sessilis, S. anthemifolia, and S. mutisii.
Hyphens (-) denote alignment gaps and asterisks donates residues conserved in all sequences.
Fig 5. Sequence alignment of the internal transcribed spacer 2 (ITS2) regions of clopyralid-resistant population (OR), S. sessilis, S. anthemifolia, S. mutisii and S. stolonifera.
Hyphens (-) denote alignment gaps and asterisks donate residues conserved in all sequences.
Differences in the ITS2 region sequence properties are shown in Table 5. The results showed that the individuals of population OR had the same guanine-cytosine (GC) content as S. sessilis and the interspecific genetic distance between S. sessilis and population OR was found to be very small (1 × 10−10), indicating that genetic information in ITS2 region sequences between these two are very close. The GC content in the other Soliva species was found to be lower than that of the individuals of population OR, with S. stolonifera having the lowest GC content. In addition, compared to S. sessilis, greater values were recorded for interspecific distance in the ITS2 region sequences of S. anthemifolia, S. mutisii and S. stolonifera, indicating that there was a great interspecies genetic distance between all three species and individuals of population OR. The ITS2 region secondary structures of these four Soliva species and the individuals of population OR are illustrated in Fig 6. Individuals of population OR had the same ITS2 region structure as that of S. sessilis (Fig 6A and 6B). However, there were several structural differences between population OR and other Soliva species in the ITS2 region secondary structure. For instance, there was a large bulge in the Helix II of individuals of population OR while S. anthemifolia, S. mutisii and S. stolonifera had a smaller “bulge” in the same region of Helix II (Fig 6A, and 6C–6E). In addition, there were differences in the number and position of the loops on Helices I and III between the ITS2 secondary structure of population OR and those of S. anthemifolia, S. mutisii and S. stolonifera. Taken together, these results revealed that based on the genetic information in ITS2 region, the individuals of population OR were conspecific with S. sessilis.
Table 5. Internal transcribed spacer 2 (ITS2) region properties of different Soliva species and clopyralid-resistant S. sessilis population (OR).
| Species | Base number (bp) | GC%# | Genetic distance* |
|---|---|---|---|
| Population OR | 243 | 58.0 | - |
| S. sessilis | 243 | 58.0 | 10×e-10 |
| S. anthemifolia | 229 | 55.5 | 0.064 |
| S. mutisii | 220 | 54.5 | 0.067 |
| S. stolonifera | 222 | 51.8 | 0.13 |
# the percentage of guanine-cytosine content.
* Kimura‐2‐parameter (K2P) model was used to calculate genetic distance.
Fig 6.
The predicted internal transcribed spacer 2 (ITS2) secondary structure of (a) clopyralid-resistant population (OR), (b) S. sessilis, (c), S. anthemifolia, (d) S. mutisii and (e) S. stolonifera. The four helices are labelled I–IV. The secondary structures were predicted and assessed using the ITS2 database web server (http://its2.bioapps.biozentrum.uni-wuerzburg.de).
Discussion
Globally, there are over 40 cases of resistance to synthetic auxin herbicide in weed species (both monocotyledon and dicotyledon) [4]. In New Zealand, there are five cases of resistance to synthetic auxin herbicides [28–30], of which S. sessilis is the only synthetic auxin herbicide-resistant species reported in turfgrass [5]. Clopyralid-resistant S. sessilis was initially reported in the early 2000s [5]; however, the level of resistance to clopyralid and the pattern of cross-resistance to other synthetic auxin herbicides were unknown in this resistant population. In this research, we recorded a very high level of resistance to clopyralid in the resistant phenotype. To the best of our knowledge, such a high level of resistance (> 225-fold) has not been reported for any of the weed species resistant to synthetic auxin herbicides. For instance, resistant phenotypes of Bassia scoparia and Chenopodium album were found to be 4.6-fold and 19-fold more resistant to dicamba respectively compared to their susceptible counterparts [28, 31]. Determining the level of resistance can hint at the potential mechanism of resistance [32]. The level of resistance to herbicides is also a function of other factors such as the type of mutation, the zygosity status of individuals for a specific mutation, and the number of mechanisms associated with resistance to a herbicide, with individuals that have accumulated multiple mechanisms of resistance displaying greater levels of herbicide resistance [33–36]. Taken together, the high level of clopyralid resistance in S. sessilis recorded in this research may suggest a different mechanism of resistance compared to that of other cases of synthetic auxin herbicide resistance, although the presence of multiple mechanisms of resistance cannot be ruled out.
Evaluating the pattern of cross resistance can inform us of alternative chemical options for managing herbicide resistance [37]. The pattern of cross-resistance can vary based on the herbicidal modes of action [37], and the type of the mutations associated with the mechanism of resistance [35, 38, 39]. The results of this research showed that clopyralid-resistant S. sessilis was cross-resistant to dicamba, picloram and MCPA but not mecoprop. In addition, the clopyralid-resistant phenotype had a high level of resistance only to dicamba, while the level of resistance to MCPA and picloram was relatively low. Both clopyralid and picloram belong to the pyridine carboxylic acid class of synthetic auxin herbicides, while dicamba and MCPA belong to the benzoic acid and phenoxy acid classes, respectively.
The pattern and level of cross-resistance to different classes of synthetic auxin herbicides have been shown to vary for other resistant weed species [37]. For instance, picloram-resistant Centaurea solstitialis was found to be highly cross-resistant to clopyralid while it showed a low level of cross-resistance to dicamba and no cross-resistance to 2,4-D [40]. Dicamba-resistant C. album was highly cross-resistant to picloram and aminopyralid (pyridine carboxylic acids), but it was not cross-resistant to either 2,4-D or mecoprop (phenoxy acids) [41]. The varied patterns of cross-resistance to synthetic auxin herbicides in a dicamba-resistant phenotype of B. scoparia was associated with a single mutation within a highly conserved region of an AUX/indole-3-acetic acid (IAA) protein, IAA16 [42]. The dicamba-resistant phenotypes were cross-resistant to 2,4-D, picloram, fluroxypyr (pyridine carboxylic acid) and quinclorac (quinoline carboxylic acid) [42]. Varying patterns of cross-resistance recorded in this research and others can be attributed to different mechanisms or specific mutations associated with resistance to synthetic auxin herbicides. For example, a specific point mutation may only confer resistance to a class of herbicides or a small number of chemicals within a herbicide group [37].
Although these results suggest no cross resistance to mecoprop in the resistant S.sessilis plants, this herbicide has generally been considered to be poor at controlling S. sessilis [43]. Herbicides registered for use in New Zealand to control this weed in turfgrass that contain mecoprop are either mixtures with ioxynil and bromoxynil, or mixtures with MCPA and dicamba [15]. Data in Fig 2 shows that good control was only achieved at rates exceeding 1000 g ae ha-1 of mecoprop, the optically active isomer, which is equivalent to 2000 g ae ha-1 of the normal mecoprop present in many turfgrass herbicides used in New Zealand. The highest recommended rate of the mixture with MCPA and dicamba is needed to reach a rate of 2000 g ae ha-1 of mecoprop, and the cross-resistance to dicamba means there will be little assistance from this component. Mecoprop is not used alone as a turfgrass herbicide as it does not control a particularly wide range of weed species [15]. Most other turfgrass herbicides in New Zealand make use of clopyralid, triclopyr and picloram to control S. sessilis, all of which are not suitable for the resistant phenotype. Earlier research showed that the resistant phenotype can be controlled by the mecoprop + ioxynil + bromoxynil formulation available in New Zealand and also bentazone [6]. Both herbicides need to be applied while the seedlings are young though to get good control [15], so are less versatile than herbicides such as clopyralid that would normally be used in spring on older plants. It will probably be necessary for turfgrass managers with this resistant winter annual weed to depend more on keeping the turfgrass competitive during autumn to avoid germination rather than relying just on herbicide applications in spring as currently occurs.
In this study, variations in growth traits between clopyralid-resistant and clopyralid-susceptible phenotypes of S. sessilis were recorded. The results show that the clopyralid-resistant plants had fewer lobes on their leaves, but they were larger compared to their susceptible counterparts. Such distinct growth characteristics can be used by turfgrass managers for identifying the clopyralid-resistant S. sessilis. Variations in growth traits have been observed for Arabidopsis lines with mutations within their auxin receptor proteins [44]. Phenotypic variations have also been recorded for other synthetic auxin herbicide- resistant weeds. For instance, dicamba-resistant C. album phenotypes were found to be shorter, more branched and their leaves were less jagged compared to the wild-type [45]. Picloram-resistant phenotypes of Sinapis arvensis were found to have a serrated leaf margin while their susceptible counterparts had a smooth leaf margins [46]. The dicamba-resistant phenotype of B. scoparia was found to be shorter and had more ovate leaf blades compared to the susceptible ones [42]. The growth characteristics observed in the dicamba-resistant phenotype of B. scoparia were attributed to a single mutation within IAA16 gene [42]. The mechanism associated with resistance to dicamba and picloram in C. album [47] and S. arvensis [48] phenotypes outlined above has not been completely elucidated but non-target site mechanisms (herbicide enhanced metabolism and reduced herbicide absorption/translocation) were not associated with the resistance to dicamba [37] and picloram [48] in either species. Therefore, it is likely that a mutation in an auxin receptor protein [17] is associated with the mechanisms of resistance and the growth traits manifested by each of these resistant phenotypes. For instance, it is known that mutations in the Auxin/indole-3-acetic acid (Aux/IAA) transcription factors, IAA9, axr5-1/IAA1, shy2/IAA3, axr2/IAA7, IAA16 and IAA28 result in abnormalities in leaf shape and development [49].
ITS regions have been used as barcodes in plant taxonomy to identify plant species [50]. In order to confirm if the individuals of population OR were correctly identified as S. sessilis, we amplified the ITS region sequence of the individuals of population OR and the resultant sequence was compared to those of other Soliva species available in the National Center for Biotechnology Information (NCBI) database. The results showed that individuals of population OR shared the same ITS sequence as that of S. sessilis. Also, the results from interspecies genetic distances and the ITS2 secondary structure provided further evidence that the individuals of population OR are conspecific with S. sessilis.
Conclusion
In conclusion, the results from this research confirm a very high level of resistance to clopyralid in S. sessilis. An assessment of the extent and level of cross-resistance to other synthetic auxin herbicides recommended for weed management in turfgrass showed that only mecoprop had no cross-resistance to this clopyralid-resistant phenotype. The greatly reduced number of lobes on each leaf associated with clopyralid-resistance can be used by turfgrass managers to detect the resistant plants and manage them accordingly. Future studies will involve evaluating the extent of the problem in turfgrass areas in New Zealand, understanding the mode of inheritance and investigating the molecular basis of resistance to clopyralid in S. sessilis.
Data Availability
All relevant data are within the paper.
Funding Statement
Financial support for the authors was provided by the Endeavour fund (C10X1806, Managing Herbicide Resistance) from the New Zealand Ministry for Business Innovation and Employment. The funder did not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Oerke EC. Crop losses to pests. J Agric Sci. 2005;144(1):31–43. Epub 12/09. doi: 10.1017/S0021859605005708 [DOI] [Google Scholar]
- 2.Harrington KC, Ghanizadeh H. Herbicide application using wiper applicators—A review. Crop Prot. 2017;102:56–62. 10.1016/j.cropro.2017.08.009. [DOI] [Google Scholar]
- 3.Ghanizadeh H, Harrington KC. Perspectives on non-target site mechanisms of herbicide resistance in weedy plant species using evolutionary physiology. AoB Plants. 2017;9(5):plx035. doi: 10.1093/aobpla/plx035 PMC5585855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heap I. The international survey of herbicide resistant weeds [online]. http://www.weedscience.com [Accessed 01.11.2020]. 2020. [Google Scholar]
- 5.Ghanizadeh H, Harrington KC. Herbicide resistant weeds in New Zealand: state of knowledge. New Zeal J Agr Res. 2019:(in press). doi: 10.1080/00288233.2019.1705863 [DOI] [Google Scholar]
- 6.Harrington KC, Ward AJ, Wells DM. Herbicide resistance in black nightshade and Onehunga weed. New Zeal Plant Prot. 2001;54:152–6. CABI:20023132603. [Google Scholar]
- 7.Ghanizadeh H, Mesarich CH, Harrington KC. Molecular characteristics of the first case of haloxyfop-resistant Poa annua. Sci Rep. 2020;10:4231. doi: 10.1038/s41598-020-61104-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popay I, Champion P, James T. An illustrated guide to common weeds of New Zealand. New Zealand: New Zealand Plant Protection Society; 2010. [Google Scholar]
- 9.Maxwell CD, Jacob N, Bollard S, Lovell P. Factors affecting establishment and survival of Soliva (Onehunga weed) at Auckland, New Zealand. New Zeal J Bot. 1986;24(1):79–87. doi: 10.1080/0028825X.1986.10409722 [DOI] [Google Scholar]
- 10.Lovell PH, Maxwell CD, Jacob N. Variation in cypsela morphology in Soliva valdiviana and S. pterosperma (Anthemideae, Asteraceae) in a local population at Auckland, New Zealand. New Zeal J Bot. 1986;24(4):657–64. doi: 10.1080/0028825X.1986.10409949 [DOI] [Google Scholar]
- 11.Johnson CO. Germination, establishment, and spread of Soliva valdiviana (Compositae). New Zeal J Bot. 1980;18(4):487–93. doi: 10.1080/0028825X.1980.10425171 [DOI] [Google Scholar]
- 12.Brosnan JT, Elmore MT, Bagavathiannan MV. Herbicide-resistant weeds in turfgrass: current status and emerging threats. Weed Technol. 2020;34(3):424–30. Epub 2020/06/29. doi: 10.1017/wet.2020.29 [DOI] [Google Scholar]
- 13.Polster DF. Eradicating carpet burweed (Soliva sessilis) in Canada. Clements DR, Darbyshire SJ, editors. Sainte-Anne-de Bellevue, Canada: Canadian Weed Science Society; 2007. 71–81 p. [Google Scholar]
- 14.Dennehy C, Alverson ER, Anderson HE, Clements DR, Gilbert R, Kaye TN. Management strategies for invasive plants in pacific Northwest Prairies, Savannas, and oak woodlands. Northwest Sci. 2011;85(2):329–51, 23. [Google Scholar]
- 15.Young S. New Zealand Novachem Agrichemical Manual 2020: Agrimedia. Pp.864., New Zealand.; 2020.
- 16.Grossmann K. Mode of action of auxin herbicides: a new ending to a long, drawn out story. Trends Plant Sci. 2000;5(12):506–8. doi: 10.1016/s1360-1385(00)01791-x CABI:20013001757. [DOI] [PubMed] [Google Scholar]
- 17.Christoffoleti PJ, Figueiredo MRAd, Peres LEP, Nissen S, Gaines TA. Auxinic herbicides, mechanisms of action, and weed resistance: a look into recent plant science advances. Scientia Agricola. 2015;72(4):356–62. CABI:20153225726. [Google Scholar]
- 18.Grossmann K. Quinclorac belongs to a new class of highly selective auxin herbicides. Weed Sci. 1998;46(6):707–16. Epub 2017/06/12. doi: 10.1017/S004317450008975X [DOI] [Google Scholar]
- 19.Seefeldt SS, Jensen JE, Fuerst EP. Log-logistic analysis of herbicide dose-response relationships. Weed Technol. 1995;9(2):218–27. CABI:19952310789. [Google Scholar]
- 20.Yao H, Song J, Liu C, Luo K, Han J, Li Y, et al. Use of ITS2 region as the universal DNA barcode for plants and animals. PLOS ONE. 2010;5(10):e13102. doi: 10.1371/journal.pone.0013102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Himmelreich S, Källersjö M, Eldenäs P, Oberprieler C. Phylogeny of southern hemisphere Compositae-Anthemideae based on nrDNA ITS and cpDNA ndhF sequence information. Plant Syst Evol. 2008;272(1):131–53. doi: 10.1007/s00606-007-0634-y [DOI] [Google Scholar]
- 22.Ghanizadeh H, Harrington KC, Mesarich CH. The target site mutation Ile-2041-Asn is associated with resistance to ACCase-inhibiting herbicides in Lolium multiflorum. New Zeal J Agr Res. 2019;63(3):416–29. doi: 10.1080/00288233.2019.1620296 [DOI] [Google Scholar]
- 23.Rydin C, Pedersen KR, Friis EM. On the evolutionary history of Ephedra: Cretaceous fossils and extant molecules. Proc Natl Acad Sci USA. 2004;101(47):16571–6. doi: 10.1073/pnas.0407588101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019;47(W1):W636–W41. doi: 10.1093/nar/gkz268 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz J, Müller T, Achtziger M, Seibel PN, Dandekar T, Wolf M. The internal transcribed spacer 2 database—a web server for (not only) low level phylogenetic analyses. Nucleic Acids Res. 2006;34:704–7. Epub 2006/07/18. doi: 10.1093/nar/gkl129 ; PubMed Central PMCID: PMC1538906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–9. Epub 2018/05/04. doi: 10.1093/molbev/msy096 ; PubMed Central PMCID: PMC5967553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritz C, Baty F, Streibig JC, Gerhard D. Dose-response analysis using R. PLOS ONE. 2016;10(12):e0146021. doi: 10.1371/journal.pone.0146021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghanizadeh H, Harrington KC, James TK, Woolley DJ. A quick test using seeds for detecting dicamba resistance in fathen (Chenopodium album). Aus J Crop Sci. 2015;9(4):337–43. CABI:20153190168. [Google Scholar]
- 29.Ghanizadeh H, Harrington KC. Ecological evidence for the fitness trade-off in triazine resistant Chenopodium Album L.: Can we exploit the cost of resistance? Agronomy. 2019;9:253. 10.3390/agronomy9090523. [DOI] [Google Scholar]
- 30.Ghanizadeh H, Harrington KC. Weed management in New Zealand pastures. Agronomy. 2019;9(8):448. doi: 10.3390/agronomy9080448 [DOI] [Google Scholar]
- 31.Cranston HJ, Kern AJ, Hackett JL, Miller EK, Maxwell BD, Dyer WE. Dicamba resistance in kochia. Weed Sci. 2001;49(2):164–70. doi: 10.1614/0043-1745(2001)049[0164:DRIK]2.0.CO;2 CABI:20013069361. [DOI] [Google Scholar]
- 32.Burgos NR, Tranel PJ, Streibig JC, Davis VM, Shaner D, Norsworthy JK, et al. Review: confirmation of resistance to herbicides and evaluation of resistance levels. Weed Sci. 2013;61(1):4–20. CABI:20133044693. [Google Scholar]
- 33.Yu Q, Collavo A, Zheng M-Q, Owen M, Sattin M, Powles SB. Diversity of acetyl-coenzyme a carboxylase mutations in resistant Lolium populations: Evaluation using clethodim. Plant Physiol. 2007;145(2):547–58. doi: 10.1104/pp.107.105262 WOS:000249893600023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghanizadeh H, Buddenhagen CE, Harrington KC, James TK. The genetic inheritance of herbicide resistance in weeds. Critic Rev Plant Sci. 2019;38(4):295–312. doi: 10.1080/07352689.2019.1665769 [DOI] [Google Scholar]
- 35.Ghanizadeh H, Harrington KC. Non-target site mechanisms of resistance to herbicides. Critic Rev Plant Sci. 2017;36(1):24–34. 10.1080/07352689.2017.1316134. [DOI] [Google Scholar]
- 36.Ghanizadeh H, Harrington KC. Restricted glyphosate translocation in Lolium multiflorum is controlled by a single incomplete dominant nuclear gene. New Zeal J Crop Hort Sci. 2018;46(4):346–53. doi: 10.1080/01140671.2018.1449124 [DOI] [Google Scholar]
- 37.Beckie HJ, Tardif FJ. Herbicide cross resistance in weeds. Crop Prot. 2012;35:15–28. [Google Scholar]
- 38.Yu Q, Powles SB. Resistance to AHAS inhibitor herbicides: current understanding. Pest Manag Sci. 2014;70(9):1340–50. doi: 10.1002/ps.3710 [DOI] [PubMed] [Google Scholar]
- 39.Kaundun SS. Resistance to acetyl-CoA carboxylase-inhibiting herbicides. Pest Manag Sci. 2014;70(9):1405–17. doi: 10.1002/ps.3790 [DOI] [PubMed] [Google Scholar]
- 40.Fuerst EP, Sterling TM, Norman MA, Prather TS, Irzyk GP, Wu Y, et al. Physiological characterization of picloram resistance in yellow starthistle. Pest Biochem Physiol. 1996;56(2):149–61. CABI:19972302312. [Google Scholar]
- 41.Ghanizadeh H, Harrington KC. Cross-resistance to auxinic herbicides in dicamba-resistant Chenopodium album. New Zeal J Agr Res. 2017;60:45–53. doi: 10.1080/00288233.2016.1238397 [DOI] [Google Scholar]
- 42.LeClere S, Wu C, Westra P, Sammons RD. Cross-resistance to dicamba, 2,4-D, and fluroxypyr in Kochia scoparia is endowed by a mutation in an AUX/IAA gene. Proc Natl Acad Sci USA. 2018;115(13):2911–20. doi: 10.1073/pnas.1712372115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthews LJ. Weed control by chemical methods. Wellington; New Zealand: Government Printer; 1975. 710 pp p. [Google Scholar]
- 44.Prigge MJ, Platre M, Kadakia N, Zhang Y, Greenham K, Szutu W, et al. Genetic analysis of the Arabidopsis TIR1/AFB auxin receptors reveals both overlapping and specialized functions. Elife. 2020;9:e54740. doi: 10.7554/eLife.54740 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghanizadeh H, Harrington KC. Fitness costs associated with multiple resistance to dicamba and atrazine in Chenopodium album. Planta. 2019;249(3):787–97. doi: 10.1007/s00425-018-3040-5 [DOI] [PubMed] [Google Scholar]
- 46.Mithila J, McLean MD, Chen S, Christopher Hall J. Development of near-isogenic lines and identification of markers linked to auxinic herbicide resistance in wild mustard (Sinapis arvensis L.). Pest Manag Sci. 2012;68(4):548–56. doi: 10.1002/ps.2289 [DOI] [PubMed] [Google Scholar]
- 47.Ghanizadeh H, Harrington KC, James TK. A comparison of dicamba absorption, translocation and metabolism in Chenopodium album populations resistant and susceptible to dicamba. Crop Prot. 2018;110: 112–6. doi: 10.1016/j.cropro.2018.04.007 [DOI] [Google Scholar]
- 48.Peniuk MG, Romano ML, Hall JC. Physiological investigations into the resistance of a wild mustard (Sinapis arvensis L.) biotype to auxinic herbicides. Weed Res. 1993;33(6):431–40. doi: 10.1111/j.1365-3180.1993.tb01959.x CABI:19932340176. [DOI] [Google Scholar]
- 49.Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, et al. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell. 2005;17(10):2676–92. Epub 2005/08/26. doi: 10.1105/tpc.105.033415 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin Y, Li M, Cao Y, Gao Y, Zhang W. Molecular thresholds of ITS2 and their implications for molecular evolution and species identification in seed plants. Sci Rep. 2017;7(1):17316. doi: 10.1038/s41598-017-17695-2 [DOI] [PMC free article] [PubMed] [Google Scholar]