Abstract
Hepatic splenosis, a rare entity, is the ectopic implantation of splenic tissue into the hepatic parenchyma, most often incidentally seen in patients with a history of splenic trauma and splenectomy. We present a unique case of hepatic splenosis in a patient with hemosiderosis and splenectomy following the incidental finding of hepatic masses on pretransplant imaging. Final diagnosis was made based on cross-sectional imaging characteristics matching that of the left upper quadrant splenules alone. We discuss common characteristics of hepatic splenosis on multiple modalities, the effect of iron deposition on the imaging characteristics of hepatic and splenic tissue and how that impacts the differential and diagnosis. This case highlights the unique imaging characteristics hepatic splenosis can have particularly in the setting of hemosiderosis. Hepatic splenosis imaging diagnosis has a significant advantage over tissue diagnosis in terms of decreased risk, time and cost.
Keywords: Hepatic splenosis, Splenosis, Hemosiderosis, Siderosis, Iron overload, Iron deposition, Splenectomy, Hepatic masses
Abbreviations: ESRD, End Stage Renal Disease; CT, Computed Tomography; US, Ultrasound; MRI, Magnetic Resonance Imaging; HS, Hepatic Splenosis; Tc-99m-DRBC, Tc-99m labelled heat-denatured red blood cells
Introduction
Splenosis, originally defined by Buchbixder and Lipkoff, is the autotransplantation of functioning splenic tissue virtually anywhere in the body [1]. At up to 67%, splenosis is an overall common diagnosis in patients with a history of splenectomy or splenic trauma [1,2,3]. However, the overall incidence is uncertain, and likely higher, as the finding is often incidental [4]. Hepatic splenosis, on the other hand, is a rare finding and is often mistaken for a malignant hepatic mass based on its imaging features, such as rapid arterial enhancement, washout appearance, or elevated signal intensity on high b-value diffusion weighted imaging [1,5]. Confident diagnosis of this benign entity is beneficial to avoid unneeded invasive procedures and patient anxiety.
Here-in, we describe a case of hepatic splenosis in a patient with significant superimposed hemosiderosis in the setting of End Stage Renal Disease (ESRD), identified based on imaging characteristics alone. Hemosiderosis occurs in the setting of iron overload which leads to organ iron deposition. It is commonly appreciated on imaging of hemodialysis patients due to iron supplementation [6]. Iron overload significantly alters organ parenchymal characteristics, which can impact abdominopelvic differentials in diagnostic imaging. Magnetic Resonance Imaging (MRI), the gold standard for imaging diagnosis, can even be utilized to quantify the extent of iron accumulated [7].
Case report
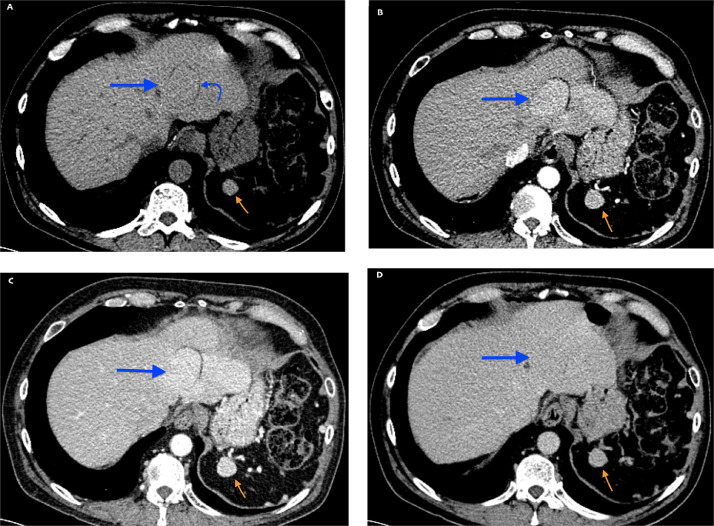
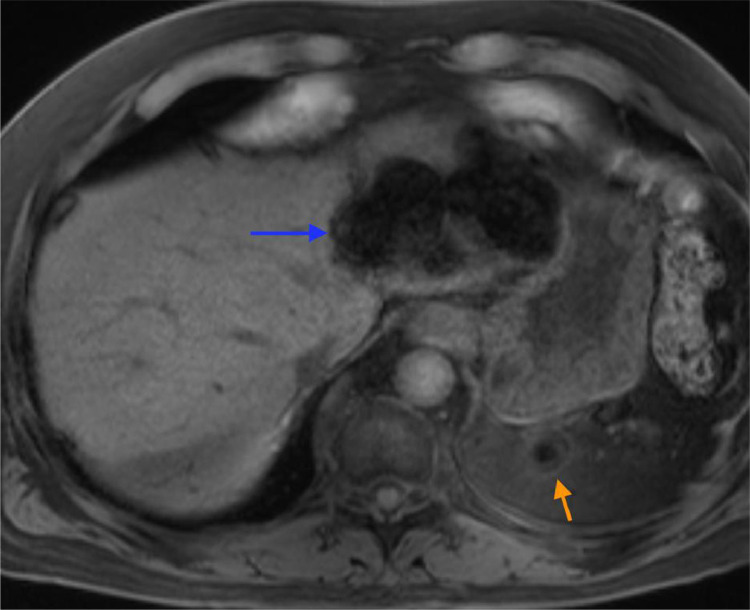
A 68-year-old Hispanic male with End Stage Renal Disease (ESRD), Type 2 Diabetes Mellitus, hypertension, and a remote history of splenectomy 50 years prior was found to have an incidental hepatic mass on a routine evaluation prior to receiving a renal transplant. His initial workup included an abdominal and pelvic Computed Tomography (CT) without contrast, Ultrasound (US) and laboratory studies. The CT was not initially found to reveal a hepatic abnormality. The abdominal US demonstrated a 3.5 cm isoechoic well circumscribed mass with hyperechoic rim in the left hepatic lobe [Fig. 7]. Further workup with a multiphase (noncontrast, arterial, venous, delayed) CT demonstrated evidence of a prior splenectomy, with two left upper quadrant splenules (residual or regenerated splenic tissue), a perigastric nodule consistent with another splenule, as well as larger, well-circumscribed lobulated lesions within the left hepatic lobe, enhancing greater than adjacent liver, including one with the appearance of a peripheral thin rim of fat attenuation Fig. 1, Fig. 2[. Differential diagnosis for this lesion remained broad with both malignant and benign etiologies thought possible. Initial laboratory studies for liver disease revealed normal hepatic function and a negative hepatitis panel. Because gadolinium contrast was being avoided due to severe renal failure, a Magnetic Resonance Imaging (MRI) without contrast was performed. On MRI T1 weighted images, the background hepatic parenchyma was overall increased T1-intensity as compared to paraspinous muscles [Fig. 4], additionally T2 weighted imaging demonstrated marked hepatic hypointenisty [Fig. 3]. Dual-sequence (gradient in and out of phase) images demonstrated a mean hepatic parenchyma ROI intensity value of 356 +/- 17 (2 standard deviations) on in phase imaging, while out of phase imaging revealed mean hepatic parenchyma ROI of 436 +/- 17; which (with longer TE in phase showing higher signal) is consistent with iron overload, given > 34% change in signal [Fig. 5]. Within the left hepatic lobe, there were two mass-like conglomerates corresponding to masses on CT, which demonstrated significant T1 hypointensity [Fig. 4], T2 isointensity [Fig. 3] and no restricted diffusion evident on high b-value DWI images [Fig. 6]. The conglomerate characteristics on both MRI and CT matched that of the left upper quadrant splenules on each sequence or phase. The diagnosis of hepatic splenosis was made, and thus the patient's renal transplant was not delayed.
Fig. 7.
Findings: Figure A: Real time sonographic images demonstrate isoechoic mass at the left hepatic lobe measured at 3.5 × 3.4 × 2.9 cm (straight blue arrows). Figure B: Sonographic image demonstrates surrounding hyperechoic rim (curved blue arrow) and posterior enhancement. Technique: Real time ultrasound of the liver using a curvilinear probe. (Color version of the figure is available online).
Fig. 1.
Findings: Axial CT images demonstrate a hepatic mass with surrounding fat border at the left hepatic lobe (blue arrow) as well as left upper quadrant splenule (orange arrow) with matching CT enhancement in each phase. Technique: Axial contrast enhanced CT abdomen and pelvis in noncontrast (A), arterial (B), venous (C), and delayed (D) phases. 120 KV, 105 mAs, 3 mm slice thickness, 150 ml Isovue IV contrast. (Color version of the figure is available online).
Fig. 2.
Findings: Coronal CT demonstrates two left hepatic lobe hepatic splenules with avid contrast enhancement in arterial phase imaging (B) with decreased enhancement on venous (A). The most superior splenule (blue arrows) demonstrates surrounding fat capsule, while the inferior demonstrates hepatic margin irregularity (white arrows). Technique: Coronal contrast enhanced CT abdomen and pelvis with multiple reformats. 120 KV, 264 mAs, 3 mm slice thickness, 150 ml Isovue IV contrast. (A) Venous phase imaging (B) Arterial phase imaging. (Color version of the figure is available online).
Fig. 4.
Findings: Axial T1 imaging with fat saturation demonstrates hyperintense T1 background hepatic parenchyma intensity as compared to the paraspinous muscles. Within the left hepatic lobe there are at least two splenules demonstrating T1 hypointensity. The left upper quadrant splenule (orange arrow) demonstrates marked T1 hypointensity, matching the signal intensity of the hepatic splenules. Technique: Axial T1 weighted image, noncontrast MRI (3T, TE =1.89, TR = 3.9, 4 mm slice thickness). (Color version of the figure is available online).
Fig. 3.
Findings: Axial (A) and Coronal (B) T2 weighted images demonstrate a markedly hypointense background hepatic parenchyma. Within the left hepatic lobe there is a hepatic splenule (blue arrow) with surrounding fatty capsule (curved blue arrow) which demonstrates marked T2 hypointensity (blue arrows) which matches the T2 signal intensity of the left upper quadrant splenule (orange arrow). Technique: (A) Axial T2-weighted, noncontrast MRI (3T, TR = 1600, TE = 86, 4 mm slice thickness) (B) Coronal T2-weighted, noncontrast MRI (3T, TR = 1500, TE = 92, 4 mm slice thickness). (Color version of the figure is available online).
Fig. 5.
Findings: Dual phase imaging with In (A) and Out (B) of phase imaging demonstrates hepatic parenchyma signal dropout on In phase imaging, compatible with hemosiderosis. Technique: (A) In phase image, noncontrast MRI (3T, TR = 200, TE = 2.46, 4 mm slice thickness). (B) Out of phase image, noncontrast MRI (3T, TR = 200, TE = 1.23, 4 mm slice thickness). (Color version of the figure is available online).
Fig. 6.
Findings: High b-value weighted imaging demonstrates no change in diffusion restriction within the hepatic splenule. Technique: B weighted, noncontrast MRI (3T, TR = 7800, TE = 58, 4 mm slice thickness). (Color version of the figure is available online).
Discussion
Hepatic splenosis, a benign finding, must be on the differential for any patient with a hepatic mass and a history of splenectomy or splenic trauma. Showering of splenic tissue into or near the hepatic parenchyma is thought to occur following a traumatic event to the spleen. One theory states that the splenic tissue implants on or around the hepatic capsule physically and then subsequently invaginates into the hepatic parenchyma. Another theory suggests that the splenic tissue embolizes via the portal system into the liver and subsequently grows [2,3,8,9]. The presence of this functional splenic tissue is often supported by the patient's lack of siderocytes, Howell-Jolly bodies, Heinz bodies, and pitted red blood cells on laboratory samples [4,5]. Perihepatic, intrahepatic and hepatic splenosis are often used interchangeably in the literature; for the purposes of this discussion, hepatic splenosis (HS) will be utilized.
Diagnosis of HS can present a dilemma, particularly when patients have other comorbidities which may increase the likelihood of a malignant etiology, such as cirrhosis, or when comorbidities alter imaging characteristics, such as in End Stage Renal Disease (ESRD). The diagnostic gold standard for splenosis is pathologic diagnosis via biopsy [1]. However, if possible, this should be avoided, as this is an invasive procedure accompanied by complication risks (i.e. bleeding, infection), patient inconvenience, and use of health system resources (including time of procedural physician and pathologist). The reference standard imaging modality is scintigraphy with Tc-99m labelled heat-denatured red blood cells (Tc-99m-DRBC) [5], [10], however increasingly splenosis is confidently diagnosed with multiphasic cross-sectional imaging, particularly MRI, with multiple published reports outlining imaging features of both general and hepatic splenosis [[1], [2], [3], [4], [5], [8], [9], [10], [11],].
Our case does follow some, but not all of the typical imaging findings seen with HS. HS are often found in the subcapsular region, either solitary or multiple, often with a surrounding capsule [9,4]. Ultrasound reveals well-circumscribed hypoechoic or isoechoic mass, often with a hyperechoic rim or posterior enhancement, as seen in our patient [1,2]. On noncontrast images, CT often demonstrates HS tissue as hypodense to the hepatic parenchyma [12]. On MRI, HS typically demonstrates homogeneous hypointensity on T1 weighted images and homogeneous hyperintensity on T2 weighted images [2], [5], [12] . On either modality with multiphase imaging, Ananthan et al. describes the HS enhancement as hyperintense to hepatic parenchyma with somewhat striated enhancement on arterial phase imaging and iso, hypo or hyperintense enhancement on venous phase imaging [12]. This is in contrast to our patients imaging findings which revealed marked hypointensity on T1 and T2 imaging and dimished intensity on high b-value images, caused by extensive hepatic and splenic iron deposition [Table 1].
Table 1.
Cross-sectional imaging in hepatic splenosis verses hepatic splenosis with hemosiderosis
| Hepatic Splenosis (typical) | Hepatic Splenosis (hemosiderosis) | |
|---|---|---|
| Noncontrast CT | Hypodense [12] | Isodense |
| Arterial Phase CT | Hyperdense [5], [12] | Hyperdense |
| Portal Venous Phase CT | Iso-, hypo-, hyper- dense [5], [10] | Hypodense |
| T1 Weighted MRI | Homogeneous hypointense [2], [5], [12] | Homogeneous hypointense |
| T2 Weighted MRI | Homogeneous hyperintense [2], [5], [12] | Homogeneous hypointense |
| Arterial Phase MRI | Hyperenhancement [5], [12] | Hyperenhancement |
| Portal venous Phase MRI | Iso-, hypo-, hyper- enhancement [5], [10] | Iso-, hypo-, hyper- enhancement |
| B value Images MRI | High Signal [1], [2], [10] | Low Signal |
Multiphase CT and multiphase MRI findings seen with typical hepatic splenosis (HS) compared with findings seen in our patient with hepatic splenosis with superimposed hemosiderosis.
Hemosiderosis has very specific findings on MRI which is both sensitive and specific for organ iron deposits [7]. Normally, iron is stored within hepatocytes as well as the reticuloendothelial system [7]. Buildup of iron in the reticuloendothelial system without causing organ damage is defined as hemosiderosis [13]. There are many causes of iron accumulation within the body due to either increased breakdown of iron containing cells or increased ingestion, absorption or infusion of iron, the latter of which is often necessary in ESRD patients on hemodialysis. Diagnostic gold standard is biopsy, however again, since invasive, biopsy can be safely avoided when imaging with MRI is noninvasive [6,7]. The superparamagnetic features of iron create unique signal characteristics on MRI which include increased T1 signal and decreased T2 signal. Dual-sequence imaging is utilized in the diagnosis of iron overload, where generally longer TE in-phase imaging will demonstrate decreased signal intensity (greater T2* effects) compared to shorter TE out of phase imaging [7,16]. Interestingly, high b-value diffusion weighted images, often utilized to aid in hepatic mass detection as well as splenule detection, are falsely decreased with iron deposition, as seen in our patient [1,14,15]. CT is less sensitive for siderosis and US has no role in siderosis detection [7]. Iron deposition significantly alters hepatic and splenic imaging characteristics, creating a unique diagnostic picture.
Our patient's imaging findings were not completely characteristic for HS, however when associating the findings of siderosis along with matching the splenic tissue imaging characteristics, lack of patient risk factors for hepatic malignancy and history of splenectomy, we were able to arrive at the diagnosis of HS based on history and imaging features alone.
We advise inclusion of hepatic splenosis to the differential in any patient with a hepatic mass and history of splenic trauma and/or splenectomy. Careful assessment of imaging characteristics, particularly matching concomitant left upper quadrant characteristic splenules will aid confident imaging diagnosis.
Teaching point
Hepatic splenosis is a benign finding which must be in the differential diagnosis for any patient with a history of splenic trauma or splenectomy. Hepatic splenosis can be diagnosed with cross-sectional imaging alone, utilizing the known characteristics of splenic tissue, and aided by concomitant splenic nodules characteristically in left upper quadrant, even if siderosis alters typical imaging features.
Patient Consent
The patient described in this case report gave writtenvoluntary informed consent for publication of this case report in April of 2020.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Vernuccio F., Dimarco M., Porrello G., Cannella R., Cusmà S., Midiri M., Brancatelli G. Abdominal splenosis and its differential diagnoses: What the radiologist needs to know. Curr Probl Diagn Radiol. Mar-Apr 2021;50(2):229–235. doi: 10.1067/j.cpradiol.2020.04.012. Epub 2020 May 19. PMID: 32540140. [DOI] [PubMed] [Google Scholar]
- 2.Tandon Y.K., Coppa C.P., Purysko A.S. Splenosis: a great mimicker of neoplastic disease. Abdom Radiol (NY) Nov 2018;43(11):3054–3059. doi: 10.1007/s00261-018-1601-5. PMID: 29651643. [DOI] [PubMed] [Google Scholar]
- 3.Vergara D., Ginolfi F., Moscati S., Giordano B., Ferrara N., Panico C., Imbriaco M. Multiple intra-hepatic and abdominal splenosis: an easy call if you know about it. Acta Radiol Open. 2018 May 11;7(5) doi: 10.1177/2058460118772324. PMID: 29780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mescoli C., Castoro C., Sergio A., Ruol A., Farinati F., Rugge M. Hepatic spleen nodules (HSN) Scand J Gastroenterol. May 2010;45(5):628–632. doi: 10.3109/00365521003587812. PMID: 20408775. [DOI] [PubMed] [Google Scholar]
- 5.Toh W.S., Chan K.S., Ding C.S.L., Tan C.H., Shelat V.G. Intrahepatic splenosis: a world review. Clin Exp Hepatol. Sep 2020;6(3):185–198. doi: 10.5114/ceh.2020.99509. Epub 2020 Sep 30. PMID: 33145425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rostoker G., Vaziri N.D., Fishbane S. Iatrogenic Iron Overload in Dialysis Patients at the Beginning of the 21st Century. Drugs. May 2016;76(7):741–757. doi: 10.1007/s40265-016-0569-0. PMID: 27091216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Queiroz-Andrade M., Blasbalg R., Ortega C.D., Rodstein M.A., Baroni R.H., Rocha M.S., Cerri G.G. MR imaging findings of iron overload. Radiographics. Oct 2009;29(6):1575–1589. doi: 10.1148/rg.296095511. PMID: 19959509. [DOI] [PubMed] [Google Scholar]
- 8.Teles G.N.S., Monteiro P.E.Z., Raphe R. Intrahepatic splenosis mimicking hepatic neoplasia. International Journal of Surgery Case Reports. 2018;44:47–50. doi: 10.1016/j.ijscr.2018.02.021. Epub 2018 Feb 17. PMID: 29475171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lake S.T., Johnson P.T., Kawamoto S., Hruban R.H., Fishman E.K. CT of splenosis: patterns and pitfalls. AJR Am J Roentgenol. Dec 2012;199(6):W686–W693. doi: 10.2214/AJR.11.7896. PMID: 23169741. [DOI] [PubMed] [Google Scholar]
- 10.Xuan Z., Chen J., Song P., Du Y., Wang L., Wan D., Zheng S. Management of intrahepatic splenosis: a case report and review of the literature. World J Surg Oncol. 28 Jun 2018;16(1):119. doi: 10.1186/s12957-018-1419-1. PMID: 29954390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin W.C., Lee R.C., Chiang J.H., Wei C.J., Chu L.S., Liu R.S., Chang C.Y. MR features of abdominal splenosis. American Journal of Roentgenology. Feb 2003;180(2):493–496. doi: 10.2214/ajr.180.2.1800493. PMID: 12540458. [DOI] [PubMed] [Google Scholar]
- 12.Ananthan K., Yusuf G.T., Kumar M. Intrahepatic and intra-abdominal splenosis: A case report and review of literature. World Journal of Hepatology. 27 Dec 2019;11(12):773–779. doi: 10.4254/wjh.v11.i12.773. PMID: 31966909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonkovsky H.L., Rubin R.B., Cable E.E., Davidoff A., Rijcken T.H., Stark D.D. Hepatic iron concentration: noninvasive estimation by means of MR imaging techniques. Radiology. Jul 1999;212(1):227–234. doi: 10.1148/radiology.212.1.r99jl35227. PMID: 10405746. [DOI] [PubMed] [Google Scholar]
- 14.Lewis S., Dyvorne H., Cui Y., Taouli B. Diffusion-weighted imaging of the liver: techniques and applications. Magn Reson Imaging Clin N Am. Aug 2014;22(3):373–395. doi: 10.1016/j.mric.2014.04.009. PMID: 25086935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandarana H., Do R.K., Mussi T.C., Jensen J.H., Hajdu C.H., Babb J.S., Taouli B. The effect of liver iron deposition on hepatic apparent diffusion coefficient values in cirrhosis. AJR Am J Roentgenol. Oct 2012;199(4):803–808. doi: 10.2214/AJR.11.7541. PMID: 22997371. [DOI] [PubMed] [Google Scholar]
- 16.Merkle E.M., Nelson R.C. Dual gradient-echo in-phase and opposed-phase hepatic MR imaging: a useful tool for evaluating more than fatty infiltration or fatty sparing. Radiographics. Sep 2006;26(5):1409–1418. doi: 10.1148/rg.265055711. [DOI] [PubMed] [Google Scholar]