Abstract
OBJECTIVES:
Extracorporeal membrane oxygenator support is a powerful clinical tool that is currently enjoying a resurgence in popularity. Wider use of extracorporeal membrane oxygenator support is limited by its significant risk profile and extreme consumption of resources. This study examines the role of markers of liver dysfunction in predicting outcomes of adult patients requiring extracorporeal membrane oxygenator support.
DESIGN:
Retrospective review.
SETTING:
Large extracorporeal membrane oxygenator center, Chicago, IL.
PATIENTS:
This study reports a single institution experience examining all adult patients for whom extracorporeal membrane oxygenator support was used over an 8-year period. Data were collected regarding patient demographics, details of extracorporeal membrane oxygenator support provided, laboratory data, and outcomes. Trends in liver function were examined for their ability to predict survival.
INTERVENTION:
Extracorporeal membrane oxygenator support, critical care.
MEASUREMENTS AND MAIN RESULTS:
Mean age was 50 years (range, 19–82 yr). There were 86 male patients (56.6%) and 66 female patients (43.4%). Indications for initiation of extracorporeal membrane oxygenator support included cardiac 76 patients (50.0%), respiratory 48 patients (31.6%), extracorporeal cardiopulmonary resuscitation 21 patients (13.3%), and combined cardiac/respiratory seven patients (4.6%). Mean duration of extracorporeal membrane oxygenator support was 17 days (range 1–223 d) or median 8 days (interquartile range, 4–17 d). Overall, in-hospital mortality was 56% (86/152). Forty-five percent of adult patients (68/152) surpassed at least one of the following established liver dysfunction thresholds: total bilirubin greater than 15 mg/dL, aspartate aminotransferase greater than 20× upper limit of normal, and alanine aminotransferase greater than 20× upper limit of normal. The multivariable logistic analysis yielded three significant findings associated with in-hospital mortality: highest total bilirubin greater than 15 (adjusted odds ratio = 4.40; 95% CI, 1.19–21.87; p = 0.04), age (adjusted odds ratio = 1.03; 95% CI, 1.00–1.05; p = 0.04), and highest lactate (adjusted odds ratio = 1.15; 95% CI, 1.06–1.26; p = 0.002).
CONCLUSIONS:
Increases in age, highest total bilirubin, and lactate all correlated with in-hospital mortality in multivariable analysis of patients requiring extracorporeal membrane oxygenator support.
Keywords: extracorporeal membrane oxygenator, liver dysfunction, mortality, outcomes
Extracorporeal membrane oxygenator (ECMO) support is a powerful clinical tool that is currently enjoying a resurgence in popularity. Its lure is an ability to rescue the sickest of patients, though, only in a proportion of cases. Wider use of ECMO is limited by its significant risk profile (1, 2) and extreme consumption of resources (3–6).
As the use of ECMO grows in popularity, clinically predictive variables will be necessary in order for patient selection to progress in a meaningful manner. There is a paucity of data in this area currently (7,8). Standard indicators such as liver function tests, known for their role in prognostication across many areas of medicine (9, 10), remain undefined in the ECMO population (11).
Prognostication with liver function tests in real time may be difficult, since there is a significant lag phase between liver injury and laboratory manifestations (12–14). The few existing studies in the literature that do examine liver function prognostication in ECMO currently report conflicting findings (15–19). This is an important piece of information in critically ill patients, since the failing liver is an organ that is not easily supported mechanically or with drugs.
In this study, we examine the association between markers of liver dysfunction and outcomes of adult patients requiring ECMO support. This may help guide/inform care of patients with liver dysfunction who are supported by ECMO.
METHODS
All investigations were approved by the Rush University Medical Center Institutional Review Board and had been conducted in compliance with its prescribed guidelines.
This study reports a single institution experience examining all adult patients for whom ECMO support was used. All consecutive patients over an 8-year period (2011–2019) were included in the study (if at any point supported by ECMO during hospital admission) with no patients excluded. Data were collected regarding patient demographics, details of ECMO support provided, laboratory data, and outcomes. Trends in liver function were examined for their ability to predict survival.
Rush University Medical Center is a quaternary hospital facility with established pediatric and adult ECMO programs. It is an Extracorporeal Life Support Organization (ELSO) designated “ECMO Center of Excellence.”
All ECMO care was provided in a dedicated cardiac surgery ICU. The ECMO service-line is headed and operated by the Division of Cardiac Surgery and implemented through an inclusive, multidisciplinary approach. This evolved over time, after introduction of hollow fiber oxygenators (Quadrox; Maquet, Rastatt, Germany) and expansion of adult ECMO in 2011. Two full-time, dedicated ECMO coordinators ensured uniformity in protocol, specialist training, and overall implementation of clinical care. Intersurgeon practice differences were minimized through a deliberate effort to standardize care and implement a coordinated multidisciplinary plan, daily. Full-time cardiac surgery ICU nurses, additionally trained as ECMO specialists, provided direct bedside ECMO circuit monitoring at all times. A bedside ECMO specialist was present in addition to each patient’s primary cardiac ICU nurse and in conjunction with the availability of cardiac perfusion for backup and consultation. All cannulation, circuit changes, and other procedures as appropriate were performed with perfusionists at the bedside.
Prior to November 15, 2015, Centrimag (Thoratec Corporation, Pleasanton, CA) centrifugal pumps were used and the Revolution (Sorin, Milan, Italy) or CardioHelp (Maquet, Rastatt, Germany) after November 15, 2015. Additional circuit components included the Quadrox oxygenator (Maquet), Carmeda-coated tubing (Medtronic, Minneapolis, MN), Biomedicus arterial/venous cannulae (Medtronic), and BioTrend (Medtronic) venous saturation/hematocrit monitoring system. IV heparin (5,000 units) was administered 5 minutes before cannulation.
Uniform data on all ECMO patients were prospectively collected and reported through the ELSO registry by two full-time ECMO coordinators. Additional data were collected to supplement information gathered for the ELSO registry. Data collected included demographic characteristics, technical ECMO data, laboratory data, and outcomes data.
Relevant data related to liver function was collected for aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin, albumin, international normalized ratio (INR), and lactate. For each variable, two values were collected: 1) the value immediately preceding cannulation and 2) the highest (lowest for albumin) observed value throughout the course of ECMO support. In addition to standard collection of liver function data as continuous variables, several binary metrics were used. Total bilirubin greater than 15 (mg/dL) was considered elevated as per established ELSO standards (20). AST, as well as ALT, was additionally evaluated when 20× greater in value than the upper limit of normal. This is consistent with established, defined criteria of shock liver and ischemic hepatitis (14, 21, 22). INR greater than 1.5 was used to signify an elevated value and separately evaluated (23).
For descriptive data, continuous variables were expressed as the mean ± sd or median and interquartile range (IQR), depending on whether the data fit a normal distribution; categorical variables were expressed as numbers and percentages. Univariable logistic regression was employed to determine the associations between liver function variables and in-hospital mortality. Multivariable logistic regression modeling was performed to identify liver function variables that were independently associated with in-hospital mortality. The C-statistic and Hosmer-Lemeshow goodness-of-fit tests were used to assess the logistic regression model’s discrimination and calibration. All statistical tests were two tailed, and a p less than 0.05 was considered statistically significant. All statistical analyses were performed using R statistical software (Version 4.0.3, R Foundation, Vienna, Austria).
RESULTS
A total of 152 patients received ECMO support during the 9-year study period. The number of patients annually receiving ECMO support increased relatively rapidly once the program became established.
Selected patient characteristics are summarized in Table 1. Mean age was 50 years (range, 19–82 yr). There were 86 male patients (56.6%) and 66 female patients (43.4%). Indications for initiation of ECMO support included cardiac 76 patients (50.0%), respiratory 48 patients (31.6%), extracorporeal cardiopulmonary resuscitation 21 patients (13.3%), and combined cardiac/respiratory seven patients (4.6%). Mean duration of ECMO support was 17 days (range, 1–223 d) or median 8 days (IQR, 4–17 d). Overall, in-hospital mortality was 56% (86/152). Bleeding as a complication was observed in the following distribution: cannulation site 32 patients (21.1%), gastrointestinal 23 patients (15.1%), surgical site 18 patients (11.8), disseminated intravascular coagulation five patients (3.3%), and brain four patients (2.6%).
TABLE 1.
Selected Baseline Characteristics
| Characteristics | Mean ± sd, Median (IQR), or n (%) |
|---|---|
| Total number of patients | 152 (100.0) |
| Age, yr | 50 ± 16 |
| Male | 86 (56.6) |
| Indications | |
| Extracorporeal cardiopulmonary resuscitation | 21 (13.8) |
| Respiratory | 48 (31.6) |
| Cardiac | 76 (50.0) |
| Respiratory/cardiac | 7 (4.6) |
| Extracorporeal membrane oxygenator days | 8 (4–17) |
| Bleeding | |
| Cannulation site | 32 (21.1) |
| Gastrointestinal | 23 (15.1) |
| Surgical site | 18 (11.8) |
| Disseminated intravascular coagulation | 5 (3.3) |
| Brain | 4 (2.6) |
| In-hospital mortality | 86 (56.6) |
IQR = interquartile range.
Using mean ± sd or median (IQR) depends on whether the data distribution is normal or not; Skewed data were presented in median (IQR) to reduce the effect of extreme values.
Specific liver function data for albumin, total bilirubin, ALT, AST, ALP, lactate, and INR are described below and summarized in Table 2. Albumin (g/dL) preceding initiation of ECMO, mean of 2.1, sd ± 0.7; lowest albumin (g/dL), mean of 1.6, sd ± 0.4; initiation total bilirubin (mg/dL), median of 1, IQR 0.7–2; highest total bilirubin (mg/dL), median of 4, IQR 2.3–10; patients with highest total bilirubin greater than 15 (mg/dL), n equals to 26 (17.1%); initiation ALT (U/L), median of 51, IQR: 21–174; highest ALT (U/L), median of 185, IQR 63.8–1,053; patients with highest ALT greater than 20 times upper end of normal, n equals to 44 (28.9%); initiation AST (U/L), median = 100, IQR 44.5–431; highest AST (U/L), median of 420, IQR 159.2–2,654; highest AST greater than 20 times upper end of normal, n equals to 59 (38.8%); initiation ALP (U/L), median of 77, IQR 49–114; highest ALP (U/L), median of 140, IQR 89–230; initiation lactate (mmol/L), 7.1 ± 5.7; highest lactate (mmol/L), 9.7 ± 6.5; initiation INR, median of 1, IQR 1.1–2; highest INR, median of 2, IQR 1.4–3; and number of patients with highest INR greater than 1.5, n equals to 106 (69.7%).
TABLE 2.
Liver Function Variables
| Variables | Mean ± sd, Median (IQR)or n (%) |
|---|---|
| Initiation albumin | 2.1 ± 0.7 |
| Lowest albumin | 1.6 ± 0.4 |
| Initiation total bilirubin | 1 (0.7–2) |
| Highest total bilirubin | 4 (2.3–10) |
| Highest total bilirubin > 15 | 26 (17.1) |
| Initiation ALT | 51 (21–174) |
| Highest ALT | 185 (63.8–1,053) |
| Highest ALT > 20 times upper end of normal | 44 (28.9) |
| Initiation AST | 100 (44.5–431) |
| Highest AST | 420 (159.2–2,654) |
| Highest AST > 20 times upper end of normal | 59 (38.8) |
| Initiation ALP | 77 (49–114) |
| Highest ALP | 140 (89–230) |
| Initiation lactate | 7.1 ± 5.7 |
| Highest lactate | 9.7 ± 6.5 |
| Initiation INR | 1 (1.1–2) |
| Highest INR | 2 (1.4–3) |
| Highest INR > 1.5 | 106 (69.7) |
ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, INR = international normalized ratio, IQR = interquartile range.
Forty-five percent of adult patients (68/152) surpassed at least one of the following established liver dysfunction thresholds: total bilirubin greater than 15 mg/dL, AST greater than 20× upper limit of normal, and ALT greater than 20× upper limit of normal.
In the initial univariable model of data analysis, a significant correlation with hospital mortality was identified in the following: age, highest total bilirubin greater than 15, highest ALT greater than 20× upper normal, highest AST greater than 20× upper normal, highest INR greater than 1.5, lowest albumin, and highest lactate. Additional details are presented in Table 3.
TABLE 3.
Univariable and Multivariable Logistic Regression for In-Hospital Mortality
| Variables | Unadjusted OR (95% CI) (Univariable Regression Analysis Results) | Adjusted OR (95% CI) (Multivariable Regression Analysis Results) |
|---|---|---|
| Age | 1.03 (1.00–1.05; p = 0.01a) | 1.03 (1.00–1.05; p = 0.04a) |
| Male | 0.96 (0.50–1.84; p = 0.91) | 1.19 (0.52–2.78; p = 0.68) |
| Highest total bilirubin > 15 | 7.67 (2.51–33.48; p < 0.01a) | 4.40 (1.19–21.87; p = 0.04a) |
| Highest alanine aminotransferase > 20 times upper end of normal | 5.22 (2.32–13.03; p < 0.01a) | 1.52 (0.25–7.91; p = 0.62) |
| Highest aspartate aminotransferase > 20 times upper end of normal | 6.32 (3.00–14.25; p < 0.01a) | 2.60 (0.61–13.82; p = 0.22) |
| Highest international normalized ratio > 1.5 | 3.65 (1.78–7.69; p < 0.01a) | 0.83 (0.31–2.13; p = 0.69) |
| Lowest albumin | 0.42 (0.19–0.89; p = 0.03a) | 0.44 (0.15–1.16; p = 0.11) |
| Highest alkaline phosphatase | 1.00 (1.00–1.00; p = 0.81) | 1.00 (1.00–1.00; p = 0.09) |
| Highest lactate | 1.22 (1.13–1.32; p < 0.01a) | 1.15 (1.06–1.26; p = 0.002a) |
OR = odds ratio.
aIndicates statistical significance.
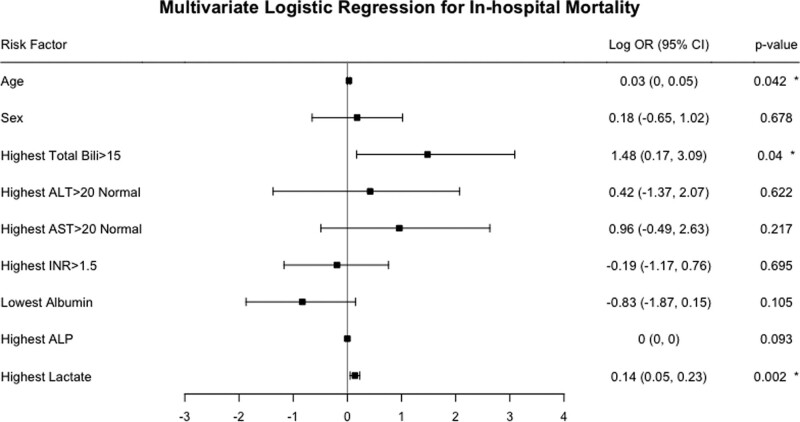
The final multivariable analysis yielded three variables that independently associated with mortality. Highest total bilirubin greater than 15 was associated with a 4.40-fold increase in the odds of in-hospital death after adjusting for other factors (adjusted odds ratio [OR] = 4.40; 95% CI, 1.19–21.87; p = 0.04). The association between age and in-hospital mortality was also statistically significant when adjusting for other factors: a 1-year increase in age was independently associated with a 3% odds increase (adjusted OR = 1.03; 95% CI, 1.00–1.05; p = 0.04) of in-hospital death. In addition, each one-unit increase in the highest lactate was associated with a 15% odds increase (adjusted OR = 1.15; 95% CI, 1.06–1.26; p = 0.002) of in-hospital death (Table 3 and Fig. 1). The final model demonstrated good calibration and discrimination (Hosmer-Lemeshow goodness-of-fit test: p = 0.22; C-statistic = 0.84).
Figure 1.
Multivariable logistic regression model for in-hospital mortality. Odds ratios (ORs) have been log transformed. *Indicates statistical significance. ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, INR = international normalized ratio.
DISCUSSION
Liver function is a proven prognostic indicator across a wide range of clinical applications, surgical and medical. As such, it merits contemporary evaluation in the rapidly expanding arena of ECMO support for critically ill patients. Several recent studies, similar in size and scope to our own, also attempt to characterize a prognostic role for liver function in guiding ECMO care for patients (15–18). To date, findings regarding this topic remain mixed with no clear, unifying trend identified among reports in the literature. It is also important to keep in mind that at the time of initiation of ECMO, liver function may be very difficult to quantify, objectively. Based on current testing methods, the first set of liver function tests at the time of ECMO initiation are often normal.
In examining our own data, we found that the results among the initial univariable analyses were much as we had anticipated. Age, highest total bilirubin, highest ALT greater than 20× upper normal, highest AST greater than 20× upper normal, highest INR greater than 1.5, lowest albumin, and highest lactate all correlated with increased mortality. It is notable that highest ALP demonstrated essentially no correlation. With the exception of albumin, all correlations were also very highly statistically significant.
In our final, multivariable model, most of these same factors failed to achieve statistical significance except for age, highest total bilirubin greater than 15, and lactate level. This may be related to ALT and AST levels being related to acute liver injury only. Increased mortality associated with age is an expected finding, and its presence in statistically significant form suggests that the study is not entirely underpowered—which is a concern with current ECMO studies limited by patient numbers.
Our finding of highest total bilirubin greater than 15 mg/dL predicted mortality with a corresponding OR of 4.4. The prognostic utility of total bilirubin has been adequately described and consistently supported in the pediatric ECMO literature at this threshold (> 15 mg/dL), and it is an established reporting variable in the ELSO database (20).
Highest lactate was the only other statistically significant liver-related predictor in our final multivariable model. Each 1 mmol/L increase in highest lactate was associated with a 15% probability increase of in-hospital death after adjusting for other factors.
Our findings corroborate some, but not all, reports found in the literature. It is worth examining liver function data from our study in conjunction with recent, similar studies from the literature (15).
Masha et al (16) conducted a retrospective institutional database query. They found that elevations in either bilirubin or lactate correlated with increased mortality in multivariable analysis. AST, ALT, and ALP failed to predict mortality in multivariable modeling. Of the markers evaluated, ALP demonstrated the least correlation with mortality, failing to achieve statistical significance even in initial univariable analysis. The authors observed that no patient survived with a peak serum bilirubin greater than 30 mg/dL (n = 21). Based on this observation, they suggested serum bilirubin greater than 30 mg/dL to be “a clear and significant indicator of death.” Similarly, they observed that even as a univariable factor, a 90% mortality threshold was observed using a serum bilirubin cutoff value of greater than 11 mg/dL. Elevations in bilirubin occur a few days after liver injury and cannot be used in the acute and early phase of ECMO support as a prognostic indicator.
Although not specifically part of our original study design, we briefly reexamined our data for comparison with the study by Masha et al (16). Within our own data, we noted 13 patients (8.5%) with a recorded total bilirubin greater than 30 mg/dL. Of these 13, three patients (23.1%) survived. Of the three surviving patients, two represented venovenous ECMO and one venoarterial ECMO. When using the study by Masha et al (16) “greater than 11 mg/dL” threshold, we identified 32 such patients (21.1%) within our study. Of the 32, 28 (87.5%) were never discharged from the hospital. This is comparable with the 90% mortality threshold observed by Masha et al (16).
Findings reported by Roth et al (17) differ from the findings reported by Masha et al (16). Roth et al (17) observed that ALP and total bilirubin were strong predictors of 30-day mortality in ECMO patients. Furthermore, ALP and total bilirubin remained predictive of long-term mortality as well. However, neither AST nor ALT was found to predict mortality. Low albumin levels were associated with long-term mortality, but not short term.
Ortiz et al (18) observed that an elevation of liver enzymes occurred in 65% of patients, roughly half of which (47%) had criteria of hypoxic hepatitis (20× normal). Two thirds of study patients demonstrated normalization of elevated enzymes within 5 days of ECMO initiation. None of these findings, including AST, ALT, 20× normal AST, and 20× normal ALT, were statistically significant in predicting outcomes. Ultimately, though, meaningful interpretation of the role of liver enzymes was likely difficult due to limited study power. Liver enzymes may also be difficult to interpret acutely, due to the delayed rise following liver injury which runs from 24 to 36 hours.
Although not a direct analysis of specific serum liver function laboratory values, Chou et al (24) examined outcomes in ECMO patients with liver cirrhosis. These patients were drawn from the entire national database of Taiwan, with numbers being sizeable, producing 233 cirrhotic patients supported by ECMO. Not surprisingly, cirrhosis was associated with a higher risk of in-hospital mortality, 76.4% versus 60.7% in the noncirrhotic. Age was also a statistically significant predictor of mortality. However, all-cause mortality rates did not significantly differ across different modified Child-Pugh scores. Clearly, the mechanism of liver dysfunction is very important in delineating which patients might benefit and identifying those that would not.
Reports in the literature, as well as our data presented in this study, are all likely limited in power to meaningfully separate differences among multiple, similar metrics of liver dysfunction. Looking at our own data, we saw an extremely strong trend among univariable data whereby liver function values uniformly predicted mortality. Most p values were less than 0.0001. We expected to observe strong correlation in the univariable data, though, not to such a high degree. We were surprised, however, to observe that our final multivariable model entirely eliminated a majority of the liver metrics as nonpredictive. Undoubtedly, greater patient numbers will be necessary to adequately power future studies. Though, as we have seen with the studies discussed above, as well as our own, even modestly powered studies can identify relevant associations. Once much larger patient numbers do become available, it will be interesting to better understand the role of statistical collinearity in a “family” of such similar variables as serum measurements of liver injury. Likely recognizing this issue, some studies have also examined ratios of liver injury markers (17, 25–28).
The degree of reversibility of liver dysfunction is key. This is important to understand which types of acute liver decompensation might be reversed with circulatory support using ECMO.
Our study attempts to contribute to the growing body of data evaluating liver function and prognostication in ECMO patients. With each passing year, worldwide trends indicate that ECMO is achieving greater acceptance and corresponding increases in its clinical utilization. It is anticipated that with this trend in ECMO, numbers will continue to grow, making available more and improved data to define how liver injury markers can guide clinical care.
Effective stewardship of ECMO as a costly resource will require that proper patient selection is guided by a correct understanding of risk. At the most fundamental level, it is anticipated that future studies will identify a threshold level (or pattern) of liver injury to establish contraindications to: 1) initiating ECMO support and 2) prolonging existing ECMO support. Clinicians are already accustomed to the paradigm of avoiding futility once profound disease progression has been demonstrated. Yet, because ECMO patients (or candidates) can quite rapidly escalate from moderate to exceedingly high-risk, the ability to glean additional information from liver injury markers would be helpful. Aberrations in liver function could potentially be well suited to signal imminent clinical deterioration, both rapidly and early. Patients correctly identified to be on the threshold of “just sick enough” to benefit from ECMO support might have their odds of survival optimized if ECMO is initiated at the earliest appropriate time. The potential benefits of such a strategy—sometimes ruling-in patients—is worth exploring. Finally, other applications of liver injury markers could include establishing indicators of when and how artificial liver support systems are to be initiated as an adjunct to ECMO in a fashion similar to dialysis (29, 30).
This study has several limitations. It is a single-center study and retrospective in nature. The liver function tests at the time of ECMO initiation can be misleading and normal. Our study does not claim to address that. This study is an attempt to provide pointers to aid decision-making in ECMO patients with liver dysfunction. Also, relatively low patient numbers in this experience may limit statistical inferences.
CONCLUSIONS
Increases in age, highest total bilirubin, and lactate all correlated with in-hospital mortality in multivariable analysis of adult patients requiring ECMO support. Clear patterns of prognostication have not yet been established within the currently available literature. It is expected that liver function studies will play a significant future role in patient selection criteria regarding: 1) appropriate initiation of ECMO, 2) as a criterion for termination of ECMO, and 3) possibly as a trigger for use of liver support devices.
Footnotes
The authors have disclosed that they do not have any conflicts of interest.
REFERENCES
- 1.Hu RTC, Broad JD, Osawa EA, et al. 30-day outcomes post veno-arterial extra corporeal membrane oxygenation (VA-ECMO) after cardiac surgery and predictors of survival. Heart Lung Circ 2020; 29:1217–1225 [DOI] [PubMed] [Google Scholar]
- 2.Schmidt M, Bréchot N, Combes A. Ten situations in which ECMO is unlikely to be successful. Intensive Care Med 2016; 42:750–752 [DOI] [PubMed] [Google Scholar]
- 3.Pellegrino V, Hockings LE, Davies A. Veno-arterial extracorporeal membrane oxygenation for adult cardiovascular failure. Curr Opin Crit Care 2014; 20:484–492 [DOI] [PubMed] [Google Scholar]
- 4.Crow S, Fischer AC, Schears RM. Extracorporeal life support: Utilization, cost, controversy, and ethics of trying to save lives. Semin Cardiothorac Vasc Anesth 2009; 13:183–191 [DOI] [PubMed] [Google Scholar]
- 5.Mishra V, Svennevig JL, Bugge JF, et al. Cost of extracorporeal membrane oxygenation: Evidence from the Rikshospitalet University Hospital, Oslo, Norway. Eur J Cardiothorac Surg 2010; 37:339–342 [DOI] [PubMed] [Google Scholar]
- 6.Thalanany MM, Mugford M, Hibbert C, et al. ; CESAR Trial Group. Methods of data collection and analysis for the economic evaluation alongside a national, multi-centre trial in the UK: Conventional ventilation or ECMO for severe adult respiratory failure (CESAR). BMC Health Serv Res 2008; 8:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Formica F, Avalli L, Colagrande L, et al. Extracorporeal membrane oxygenation to support adult patients with cardiac failure: Predictive factors of 30-day mortality. Interact Cardiovasc Thorac Surg 2010; 10:721–726 [DOI] [PubMed] [Google Scholar]
- 8.Wagner K, Risnes I, Abdelnoor M, et al. Is it possible to predict outcome in pulmonary ECMO? Analysis of pre-operative risk factors. Perfusion 2008; 23:95–99 [DOI] [PubMed] [Google Scholar]
- 9.Fuhrmann V, Kneidinger N, Herkner H, et al. Impact of hypoxic hepatitis on mortality in the intensive care unit. Intensive Care Med 2011; 37:1302–1310 [DOI] [PubMed] [Google Scholar]
- 10.O’Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989; 97:439–445 [DOI] [PubMed] [Google Scholar]
- 11.Nagy Á, Holndonner-Kirst E, Eke C, et al. Model for end-stage liver disease scores in veno-arterial extracorporeal membrane oxygenation. Int J Artif Organs 2020; 43:684–691 [DOI] [PubMed] [Google Scholar]
- 12.Yu LH, Yu WL, Zhao T, et al. Post-operative delayed elevation of ALT correlates with early death in patients with HBV-related hepatocellular carcinoma and post-hepatectomy liver failure. HPB (Oxford) 2018; 20:321–326 [DOI] [PubMed] [Google Scholar]
- 13.Mazzeffi M, Kon Z, Sanchez P, et al. Impact of acute liver failure on mortality during adult ECLS. Intensive Care Med 2016; 42:299–300 [DOI] [PubMed] [Google Scholar]
- 14.Henrion J, Schapira M, Luwaert R, et al. Hypoxic hepatitis: Clinical and hemodynamic study in 142 consecutive cases. Medicine (Baltimore) 2003; 82:392–406 [DOI] [PubMed] [Google Scholar]
- 15.Kaestner F, Rapp D, Trudzinski FC, et al. High serum bilirubin levels, NT-pro-BNP, and lactate predict mortality in long-term, severely ill respiratory ECMO patients. ASAIO J 2018; 64:232–237 [DOI] [PubMed] [Google Scholar]
- 16.Masha L, Peerbhai S, Boone D, et al. Yellow means caution: Correlations between liver injury and mortality with the use of VA-ECMO. ASAIO J 2019; 65:812–818 [DOI] [PubMed] [Google Scholar]
- 17.Roth C, Schrutka L, Binder C, et al. Liver function predicts survival in patients undergoing extracorporeal membrane oxygenation following cardiovascular surgery. Crit Care 2016; 20:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ortiz AB, LaManna I, Antonucci E, et al. Altered liver function in patients undergoing veno-arterial extracorporeal membrane oxygenation therapy. Minerva Anesthesiol 2017; 83:255–265 [DOI] [PubMed] [Google Scholar]
- 19.Lyu L, Yao J, Gao G, et al. Incidence, risk factors, and outcomes of hyperbilirubinemia in adult cardiac patients supported by veno-arterial ECMO. Artif Organs 2018; 42:148–154 [DOI] [PubMed] [Google Scholar]
- 20.Brogan TV, Lequier L, Lorusso R, et al. : Extracorporeal Life Support: The ELSO Red Book. Fifth Edition. Ann Arbor, MI, Extracorporeal Life Support Organization, 2017 [Google Scholar]
- 21.Rawson JS, Achord JL. Shock liver. South Med J 1985; 78:1421–1425 [DOI] [PubMed] [Google Scholar]
- 22.Ciobanu AO, Gherasim L. Ischemic hepatitis - intercorrelated pathology. Maedica (Bucur) 2018; 13:5–11 [PMC free article] [PubMed] [Google Scholar]
- 23.McDowell Torres D, Stevens RD, Gurakar A. Acute liver failure: A management challenge for the practicing gastroenterologist. Gastroenterol Hepatol (N Y) 2010; 6:444–450 [PMC free article] [PubMed] [Google Scholar]
- 24.Chou AH, Wu VC, Chen DY, et al. Outcome of extracorporeal membrane oxygenation support in patients with liver cirrhosis: A nationwide population-based cohort study. Eur J Cardiothorac Surg 2020; 58:519–527 [DOI] [PubMed] [Google Scholar]
- 25.Nyblom H, Björnsson E, Simrén M, et al. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int 2006; 26:840–845 [DOI] [PubMed] [Google Scholar]
- 26.Giannini E, Botta F, Fasoli A, et al. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci 1999; 44:1249–1253 [DOI] [PubMed] [Google Scholar]
- 27.Sheth SG, Flamm SL, Gordon FD, et al. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1998; 93:44–48 [DOI] [PubMed] [Google Scholar]
- 28.Nyblom H, Berggren U, Balldin J, et al. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol 2004; 39:336–339 [DOI] [PubMed] [Google Scholar]
- 29.García Martínez JJ, Bendjelid K. Artificial liver support systems: What is new over the last decade? Ann Intensive Care 2018; 8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabata S, Cavarocchi NC, Hirose H. Successful management of severe liver failure on venoarterial extracorporeal membrane oxygenation using molecular adsorbent recirculating systeme. J Heart Lung Transplant 2012; 31:1322–1323 [DOI] [PubMed] [Google Scholar]