Abstract
Emtricitabine/tenofovir disoproxil fumarate [FTC-TDF] is a daily oral medication taken by HIV-negative individuals for pre-exposure prophylaxis (PrEP) to prevent human immunodeficiency virus (HIV) infection. A higher incidence of sexually transmitted infections (STIs) among PrEP users has been reported compared to STI incidence before PrEP use. Asymptomatic incident STI rates were investigated among 78 patients presenting for PrEP in Honolulu, Hawai‘i, from April 2018 to May 2019. Testing for oropharyngeal gonorrhea, urethral gonorrhea and chlamydia, rectal gonorrhea and chlamydia, and syphilis was performed. Incident STI percentages were calculated at each follow-up visit. Ninety-seven percent of patients were men who have sex with men (MSM). Forty-seven percent of patients had follow-up data 6 months after initiation and 28% after 1 year. Thirty-two percent of patients self-reported an STI before initiating PrEP. More than half reported anonymous partners. There were 35 positive STI tests during the study period, and 25% of patients had one or more positive tests during this time. At initiation, 17% of patients were found to have an STI, followed by 16% at 3 months, 14% at 6 months, 8% at 9 months, and 5% at 12 months. At all visits, chlamydia was the most common STI detected; at 6 months, 18% of all rectal tests were positive for chlamydia. There were inconsistent condom use and high STI rates from screening during PrEP initiation and follow-up, offering an opportunity to identify asymptomatic STIs in this population. This study is the first report in Hawai‘i of STI rates among PrEP users.
Keywords: Pre-Exposure Prophylaxis, Sexually transmitted infections, HIV prevention
Introduction
Emtricitabine/tenofovir disoproxil fumarate [FTC-TDF], a daily oral medication taken by HIV-negative individuals as pre-exposure prophylaxis (PrEP) to prevent human immunodeficiency virus (HIV), was approved in 2012 by the Food and Drug Administration.1,2. The Centers for Disease Control and Prevention (CDC) recommends PrEP for patients with “substantial risk for acquiring HIV infection,” including men who have sex with men (MSM), heterosexual men and women, and injection drug users.1 For MSM, substantial risk includes those with an HIV-positive sexual partner, recent bacterial sexually transmitted infection (STI), a high number of sex partners, inconsistent or no condom use, or commercial sex work.
FTC-TDF is an effective tool for HIV prevention, as shown in randomized control trials, reducing HIV acquisition in MSM by as much as 92% among subjects with a detectable drug level.3 An open-label extension of this initial study found a 97% relative reduction of HIV incidence when PrEP was taken on demand.4 Studies have also demonstrated that rapid high coverage roll-out of PrEP among MSM reduced HIV incidence in the cohort prescribed PrEP and statewide in New South Wales, Australia.5 New HIV infections decreased significantly in New South Wales from 295 in the 12 months before the roll-out to 221 in the 12 months after the roll-out (relative risk reduction, 25.1%; 95% confidence interval [CI], 10.5–37.4), with only 2 new HIV infections in the nearly 3700 study participants.5
Despite this efficacy, studies have indicated that PrEP may be correlated with an increased STI incidence.6–8 In the general US population, the CDC reports an increase in 2018 STI rates compared to 2017, in primary and secondary syphilis (11 per 100 000 people, an increase of 14%), chlamydia (540 per 100 000 people, an increase of 3%), and gonorrhea (179 per 100 000 people, an increase of 5%).9 MSM are disproportionally affected by STIs, accounting for nearly 54% of new primary and secondary syphilis cases in 2018.9 Further, gonorrhea diagnoses doubled among MSM over the previous 5 years (from 186 943 to 341 401 cases).9 A higher incidence of STIs among MSM PrEP users continues to be reported when compared to STI incidence before PrEP use.6,7 It is unclear if these increases are due to a rise in STI testing,10 an increase in partner numbers,6 or an increase in condomless anal sex acts.11 To date, only one study, Pre-Exposure Option for Reducing HIV in the UK (PROUD), an open-label randomized clinical trial comparing immediate to deferred daily Truvada for HIV-negative gay men, was explicitly designed to detect changes in sexual risk behavior by comparing MSM in the United Kingdom who knew they were on PrEP (randomized to immediate start) to those who knew they were not on PrEP (randomized to a 1-year delay).12 Although there were more condomless sex acts among the participants actively taking PrEP compared to those not on PrEP, there was no increase in STIs among the PrEP group.12,13
STI monitoring is also an essential component of the fight against antibiotic-resistant gonorrhea.14,15 Honolulu, Hawai‘i is a sentinel site for gonorrhea surveillance for the Strengthening the United States Response to Resistant Gonorrhea (SURRG) program, which began in 2016.16,17 SURRG encourages surveillance and capacity building for culture-based gonorrhea surveillance and response. Given that undiagnosed STIs can lead to more severe health problems, including infertility, ectopic pregnancy, and increased HIV risk, STI screening among PrEP users continues to be an important component of PrEP visits and HIV prevention.18
This paper describes incident STI rates among asymptomatic patients presenting for PrEP visits at an HIV prevention and treatment clinic for patients in Hawai‘i.
Methods
From April 2018 to May 2019, a chart review was performed of all patients presenting to the clinic for PrEP. Clinic results were collected into a secured de-identified database. As per protocol from the University of Hawai‘i Office of Research Compliance Human Studies Program Worksheet 301 sections I and II, this study did not meet the federal definition of research, requiring no further application.
This chart review included patients presenting for their initial PrEP visit and those presenting for subsequent follow-up visits. If a patient was on PrEP at another location, the intake process was the same as a patient new to PrEP, and the first visit at this clinic was still counted as an initial PrEP visit. If the patient started PrEP out of state, they were not included in this study. Some patients presented within the study period for a follow-up PrEP visit only, comprising the initial visit and some subsequent regular 3-month follow-up visits may have occurred before the study period. Relevant STI data was abstracted from those previous visits. Only patients who initiated PrEP within 12 months of the study period were included in the review.
Information about alcohol use, drug use, partner preference, and history of previous STIs was obtained from the initial PrEP visit. “Some” alcohol use was defined at the time of PrEP initiation as drinking alcohol weekly or monthly. Illicit drug use was defined as any type of drug use at the time of PrEP initiation. At this initial visit, patients were asked whether they used condoms with sexual partners in the previous 90 days. If yes, they were then asked to estimate the percentage of partners with whom they used condoms. Age was determined as the age during the earliest visit associated with the study.
Laboratory work was required before PrEP initiation and included HIV antigen and antibody testing, Testing for hepatitis C antibody, hepatitis B surface antigen, hepatitis B surface antibody, and hepatitis B core antibody, syphilis testing with rapid plasma reagin (RPR), and urine-based urethral gonorrhea and chlamydia testing with nucleic acid amplification test (NAAT). HIV, RPR, and urine-based urethral testing were ordered before each follow-up visit. Testing occurred at a private laboratory or the Hawai‘i State Department of Health (DOH). At the initial PrEP visit and each subsequent visit, patients were offered oropharyngeal swabbing to test for gonorrhea, a self-collection kit to obtain a rectal swab for gonorrhea and chlamydia testing, and urine-based urethral testing if it was not done before the visit. Screening is not offered for oropharyngeal chlamydia. Swabbing was done on-site in the clinic. Oropharyngeal and rectal swab specimens were sent to the DOH for NAAT, and results were mailed to the clinic. Patients were notified of their results. Patients with positive results were recalled to the clinic for treatment. For patients with a positive gonorrhea result, a culture specimen was first obtained for antibiotic susceptibility testing (AST), followed by treatment.
The presence of an STI was based on documented laboratory results showing positive oropharyngeal or rectal gonorrhea, positive urine NAAT results for urethral gonorrhea or chlamydia, positive rectal NAAT results for chlamydia, or reactive RPR. All reactive RPRs were confirmed with a treponemal-specific antibody test. Incident STI percentages were calculated based on a denominator of the number of patients tested for that specific STI at each relevant visit.
Screening was also done in the case of patients presenting with an STI contact or symptoms. Gonococcal cultures for AST were obtained before treatment. Treatment was given empirically to symptomatic patients and contacts. However, STIs diagnosed from symptomatic patients and contacts were not included in this study, as this study was investigating the identification of asymptomatic individuals who would otherwise have not undergone treatment. Chart review was done after each visit, and STI testing and results were recorded as they were received.
Results
From April 2018 to May 2019, a total of 87 individuals on or seeking PrEP presented for a clinic visit. One patient presenting for PrEP was on it previously in another state and was not included in the study. Eight patients presented for follow-up visits but initiated PrEP more than 1 year prior and thus were not included in the study. Of these 78 patients included in the review, a total of 55 patients (71%) initiated PrEP during the study period. Twenty-three patients initiated PrEP within 1 year prior (29%) and were seen for a follow-up visit during the study period. Patient demographics are summarized in Table 1. The median age was 33 years. The majority of the patients were male (96%), and of those, 93% had only male partners. There were no self-identified transgender patients. Sixty-two percent of patients reported having anonymous partners (4 patients did not report any answer). Thirty-two percent of patients reported having a history of any STI before PrEP initiation. Seventy-six percent of patients reported they used condoms in the 90 days before PrEP initiation (2 of 78 patients did not report condom use status). Among 58 condom users, 36 patients reported an actual percentage of time that they used condoms in the 90 days prior, which was on average 65% (not shown in table). Eighty-two percent reported some alcohol use. Forty-seven percent reported illicit drug use. The most common drug used was marijuana at 33%, followed by “poppers” (amyl nitrite), at 12%.
Table 1.
Demographics and Behavioral Variables of Patients at Pre-Exposure Prophylaxis Initiation (N=78)
| Demographic | |
|---|---|
| Initiated PrEP during the study period*, n (%) | 55 (71) |
| Initiated PrEP 1 year before study period*, n (%) | 23 (29) |
| Median age, years | 33 |
| Sex, n (%) | |
| Men | 75 (96) |
| Women | 3 (4) |
| Among 75 men, sex partners, n (%) | |
| Men only | 70 (93) |
| Men and Women | 4 (6) |
| Transgender | 1 (1) |
| Among 3 women, sex partners, n (%) | |
| Men only | 3 (100) |
| Anonymous partners, n (%) | 46 (62) |
| Missing | 4 |
| STI reported before PrEP, n (%) | 25 (32) |
| Reported condom use before PrEP initiation, n (%) | 58 (76) |
| Missing | 2 |
| Weekly or monthly alcohol use, n (%) | 64 (82) |
| Drug use, n (%)† | 37 (47) |
| Marijuana | 26 (33) |
| Poppers | 9 (12) |
| Ecstasy | 5 (6) |
| Other | 4 (5) |
Abbreviation: PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection.
Study period during April 1, 2018, and May 31, 2019
Some patients reported multiple drug use
Figure 1 illustrates the number and percentage of gonorrhea infections among those tested by the site for each visit. Figure 2 shows the number and percentage of chlamydia infections and the number of new syphilis infections among those tested by the site for each visit. For each figure, the number of cases and the total number of tested patients are also shown by the site for each visit.
Figure 1.
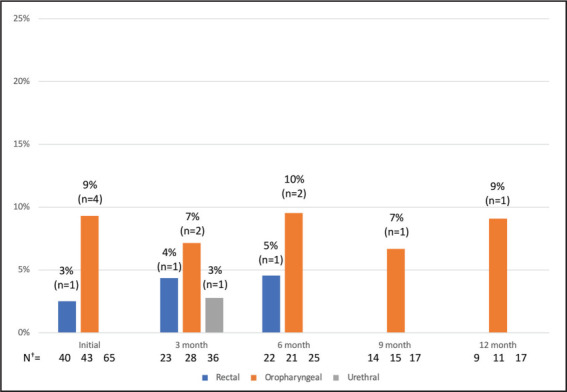
Number and Percentage of Gonorrhea Infections by Site at Initiation and Follow-Up*
*The numbers above each bar are the percentage and number of infections detected.
†N=Total number of patients tested for rectal, oropharyngeal, and urethral gonorrhea, respectively.
Figure 2.
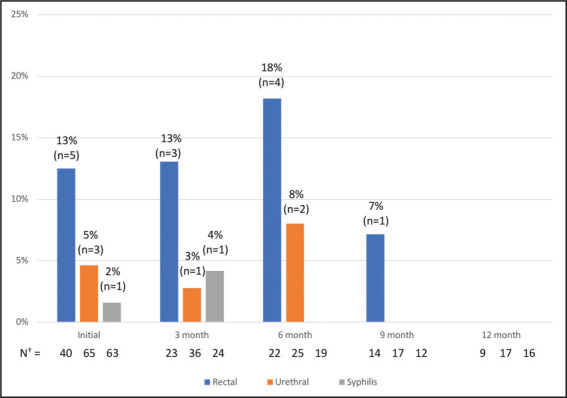
Number and Percentage of Chlamydia Infections by Site and Syphilis Infections at Initiation and Follow-Up*
*The numbers above each bar are the percentage and number of infections detected
†N=Total number of patients tested for rectal chlamydia, urethral chlamydia, and syphilis, respectively.
At the initial PrEP visit, 78 patients were tested for STIs, and a total of 14 positive tests were detected (Figures 1 and 2). The number of patients tested for specific STIs differed, given that some patients declined specific tests based on their perceived risk, other tests were not ordered, and some patients were tested for certain STIs elsewhere. At the initial PrEP visit, there were 40 completed rectal swabs, 43 oropharyngeal swabs, 65 urine-based tests, and 63 syphilis tests. Seventeen percent of all patients (n=13) presenting for PrEP screened positive for an STI at PrEP initiation. The most common STI detected at PrEP initiation was rectal chlamydia (13% of all patients tested with a rectal swab). Nine percent of all patients tested with an oropharyngeal swab were positive for oropharyngeal gonorrhea. Rectal gonorrhea was detected in 3% of all patients tested with a rectal swab. Urethral chlamydia was detected in 5% of all patients tested, and newly diagnosed syphilis was detected in 2% of all patients tested—one of the 14 patients presented with two positive STI tests.
For the 3-month follow-up visit, 50 patients had STI data. There were 23 completed rectal swabs, 28 oropharyngeal swabs, 36 urine-based tests for urethral gonorrhea and chlamydia, and 24 syphilis tests. A total of 9 positive STI tests were detected (Figures 1 and 2). Sixteen percent of all patients (n=8) at the 3-month follow-up screened positive for an STI. The most common STI detected was rectal chlamydia (13%), followed by oropharyngeal gonorrhea (7%), rectal gonorrhea (4%), syphilis (4%), urethral gonorrhea (3%), and urethral chlamydia (3%). One patient was noted to have two positive STI tests; this individual was previously tested for and treated for two positive STI tests at PrEP initiation.
For the 6-month follow-up visit, 37 patients had STI data. There were 22 completed rectal swabs, 21 oropharyngeal swabs, 25 urine-based tests, and 19 syphilis tests. A total of 9 positive STI tests were detected (Figures 1 and 2). Fourteen percent of all patients (n=5) with 6-month follow-up data had an STI at this visit. One patient presented with 2 positive STI tests, and 1 patient presented with 4 positive STI tests. Two out of the 6 patients had previous positive STI tests detected in the clinic during the study period. The most common STI was rectal chlamydia (18%), followed by oropharyngeal gonorrhea (10%), urethral chlamydia (8%), and rectal gonorrhea (5%). There were no urethral gonorrhea cases and no new syphilis cases.
For the 9-month follow-up visit, 25 patients had STI data. There were 14 completed rectal swabs, 15 oropharyngeal swabs, 17 urine-based tests, and 12 syphilis tests. A total of 2 positive STI tests were detected (Figures 1 and 2). Eight percent of all patients (n=2) with 9-month follow-up data had an STI at this visit. There was 1 positive oropharyngeal gonorrhea test (7%) and 1 positive rectal chlamydia test (7%). Both patients had 1 positive STI test only. Both patients who tested positive at 9 months had previous positive STIs detected in the clinic during the study period.
At 12 months of follow-up, 22 patients had STI data. There were 9 completed rectal swabs, 11 oropharyngeal swabs, 17 urine-based tests, and 16 syphilis tests. One STI was detected (Figures 1 and 2). Five percent of all individuals (n=1) with 12-month follow-up data had an STI at this visit. There was 1 positive oropharyngeal gonorrhea test (9%) (Figures 1 and 2). The patient who tested positive for an STI at 12 months had previous positive STIs detected in the clinic during the study period.
Among the 78 patients during this study period, there were a total of 35 positive test results. Three percent of infections were syphilis (n=2), 13% were oral gonorrhea (n=10), 17% were rectal chlamydia (n=13), 4% were rectal gonorrhea (n=3), 8% were urethral chlamydia (n=6) and 1% urethral gonorrhea (n=1). These 35 positive tests occurred in 20 patients during this study period, representing 26% of the study population. During the study period, 15% percent of patients had 1 positive test (n=12), 5% had 2 positive tests (n=4), 3% had 3 positive tests (n=2), 1% had 4 positive tests (n=1), and 1% had 5 positive tests (n=1).
Discussion
This study found consistent positive STI tests among asymptomatic MSM on PrEP at initiation and at each of the follow-up visits. To date, this is the first report in Hawai‘i of STI rates among PrEP users. Seventy-eight unique patients were identified who presented to the clinic for PrEP from April 2018 to May 2019, nearly half of whom had follow-up data 6 months after they initiated PrEP. A third of them had follow-up data after 1 year. At all of the visits in this study, asymptomatic STI cases were screened for and detected in this at-risk population. One-third of the study population self-reported an STI before initiating PrEP. This statistic is lower than reports from the PROUD study in England, where 64% of the population reported an STI in the prior 12 months.12 A quarter of the patients in the present study tested positive for 1 or more STIs, which was also slightly lower than that noted in the literature.12,19 A 2016 study by Liu et al of 557 MSM PrEP participants in Miami, DC, and San Francisco found after 48 weeks of follow up, 51% of participants tested positive for 1 or more STIs during quarterly testing (syphilis, rectal gonorrhea or chlamydia, urethral gonorrhea or chlamydia, or oropharyngeal gonorrhea), with 26% of participants testing positive at baseline.19 The PROUD study detected similar rates as Liu, finding 152 of 265 (57%) participants in the immediate PrEP arm tested positive for 1 or more STIs (243 person-years of follow-up) and 124 of 247 participants (50%) in the delayed PrEP arm tested positive for one or more STIs (222 person-years of follow-up) during routine screening every 3 months.12 Lower STI rates were demonstrated in Hawai‘i by comparison; however, in the Liu and PROUD studies, each participant was screened regularly as part of the study protocol, while not all of the Hawai‘i participants presenting for 3-month follow-up visits received complete STI testing.
The CDC recommends STI testing every 3 to 6 months; this study demonstrates that testing every 3 months successfully detects asymptomatic infections in this MSM population in Hawai‘i.1 Seventeen percent of this study population tested positive at PrEP initiation; at most of these follow-up visits, chlamydia was the most common STI. This data is consistent with prior PrEP/STI studies showing high rates of chlamydia among MSM PrEP users, though gonorrhea is also noted in several large studies.20,21 A study by Traeger et al. in Victoria, Australia found incidences of 45.0 and 39.0 per 100 person-years for chlamydia and gonorrhea, respectively, and similar to this study in Hawai‘i, there was a subgroup of patients who experienced reinfections.7 This underscores that reinfections are common and that repeat testing every 3 months is effective; at each follow-up visit, positive STI tests were detected among those patients who had positive STI tests at previous visits. At the 9-month and 12-month visit, all positive STI tests were detected in patients with previous positive tests during the study period. The 3-month follow-up interval also provides an opportunity for consistent counseling and education about condom use and risky behavior to prevent further STIs and prevent transmission to their sexual networks and community at the population level.22 Hsu et al studied characteristics of individuals presenting with repeat STIs within the Massachusetts STI surveillance system; interestingly, data from this state system showed that these patients presented at multiple clinic locations, suggesting that providers might not know the extent of repeat infections and patients’ heightened risk.23 This Honolulu clinic is a large provider of specialized PrEP, HIV, and STI services; therefore, it is in a unique position to provide counseling and STI services to these high-risk patients.
Finally, frequent testing in this MSM population is important for the monitoring of multidrug-resistant gonococci (MDRGC) and extensively-drug resistant gonococci (XDR-GC) in Hawai‘i. The islands sit geographically near Australia, Japan, Thailand, and China, where resistant strains may reside and be introduced through travel.24–26
This study also highlights that patients in Hawai‘i engage in anonymous sexual activity, although this study does not have information about whether this is linked to condom use. While patients in this study reported whether condoms were used in the preceding 90 days before PrEP initiation, data were not consistently collected about sexual practices before starting PrEP, such as the use of condoms with anonymous partners or the use of condoms per sex act. A major limitation of the study is that it could not determine if anonymous sexual activity or condomless anal sex changed following PrEP. In particular, documentation of condom use included varied responses and did not detail whether condom use decreased after the implementation of PrEP. Further, recall of condom use might be varied or incorrect, especially with multiple partners or multiple sex acts per partner. Prior studies have shown that PrEP may increase risky behavior.4,12 However, Liu et al showed decreasing condomless receptive anal intercourse in Miami and DC, but not San Francisco,19 highlighting the need for further study in Hawai‘i. The clinic has recently begun to document the number of partners since the last visit. Of these, the number of anonymous partners, the types of sexual acts, and the numbers of partners with whom condoms were used for anal or vaginal sex. This finding represents an area for further research in Hawai‘i.
Another major limitation was that not all patients completed multi-site testing for each STI at each follow-up visit due to patient refusal to test for certain STIs or physician error in screening. This limitation could have resulted in missing asymptomatic infections, which would have otherwise been found on routine screening, thus underestimating these results due to missing data. Further, lower STI rates compared to other studies might be due to a smaller Hawai‘i population, only one clinic location, and selection bias. Also, the only information regarding STIs before PrEP initiation was based on self-report, and self-reported STIs are notably inaccurate and underestimate the true occurrence.27 Thus, this study was unable to determine whether there was an increase, decrease, or stable occurrence of STI incidence after PrEP initiation in this population. Additional weaknesses of this study include a small total number of participants and small follow-up numbers. No statistical analyses of associations between patients presenting with STI and possible risk factors were performed. This weakness represents an area for further research.
In conclusion, among 78 patients presenting for PrEP during approximately 1 year, routine screening for STIs produced results consistent with other studies.
Acknowledgments
The authors thank the clinical and laboratory staff and the study participants.
Abbreviations and Acronyms
- AIDS
acquired immunodeficiency syndrome
- AST
antibiotic susceptibility testing
- CDC
Centers for Disease Control and Prevention
- DC
District of Columbia
- DOH
Department of Health
- FTC-TDF
Emtricitabine/tenofovir disoproxil fumarate
- HIV
human immunodeficiency virus
- MDR-GC
multidrug-resistant gonococci
- MSM
men who have sex with men
- NAAT
nucleic acid amplification test
- PrEP
pre-exposure prophylaxis
- PROUD
Pre-Exposure Option for Reducing HIV in the UK
- RPR
rapid plasma reagin
- STI
sexually transmitted infection
- SURRG
Strengthening the United States Response to Resistant Gonorrhea
- XDR-GC
extensively-drug resistant gonococci
Contributor Information
Kallan S. Ross, Hawai‘i Island Family Medicine, Hilo Medical Center, Hilo, HI (KSR).
Abigail C. Santos, Combined Rush University Medical Center and Cook County Hospital, Chicago, IL (ACS).
Timothy J. McCormick, Harm Reduction Services Branch, Communicable Disease and Public Health Nursing Division, Hawai‘i State Department of Health (TJM).
Conflict of Interest
None of the authors identify any conflict of interest.
References
- 1.Centers for Disease Control and Prevention US Public Health Service Preexposure Prophylaxis for the Prevention of HIV Infection in the United States - 2017 Update A Clinical Practice Guideline. 2018. Mar, Available from: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed October 24, 2019.
- 2.Food and Drug Administration Truvada for PrEP Fact Sheet: Ensuring Safe and Proper Use. 2012. Available from: https://www.fda.gov/media/83586/download. Accessed March 13, 2021.
- 3.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363((27)):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina JM, Charreau I, Spire B, et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV. 2017;4((9)):e402–e410. doi: 10.1016/S2352-3018(17)30089-9. [DOI] [PubMed] [Google Scholar]
- 5.Grulich AE, Guy R, Amin J, et al. Population-level effectiveness of rapid, targeted, high-coverage roll-out of HIV pre-exposure prophylaxis in men who have sex with men: the EPIC-NSW prospective cohort study. Lancet HIV. 2018;5((11)):e629–e637. doi: 10.1016/S2352-3018(18)30215-7. [DOI] [PubMed] [Google Scholar]
- 6.Traeger MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis. 2018;67((5)):676–686. doi: 10.1093/cid/ciy182. [DOI] [PubMed] [Google Scholar]
- 7.Traeger MW, Cornelisse VJ, Asselin J, et al. Association of HIV preexposure prophylaxis with incidence of sexually transmitted infections among individuals at high risk of HIV infection. JAMA. 2019;321((14)):1380–1390. doi: 10.1001/jama.2019.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher C, Wu L, Chandran A, et al. Sexually transmitted infection Screening among gay, bisexual and other men who have sex with men prescribed Pre-exposure Prophylaxis in Baltimore City, Maryland. Clin Infect Dis. 2020;71((10)):2637–2644. doi: 10.1093/cid/ciz1145. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control Sexually Transmitted Disease Surveillance, 2018. 2018. Available from: https://www.cdc.gov/std/stats18/default.htm. Accessed October 24, 2019.
- 10.Golub SA, Pena S, Boonrai K, Douglas N, Hunt M, Radik A. Conference on Retroviruses and Opportunistic Infections. Boston, MA: 2016. STI Data From Community-Based PrEP Implementation Suggest Changes to CDC Guidelines. Available from: https://www.croiconference.org/abstract/sti-data-community-based-prep-implementation-suggest-changes-cdc-guidelines-0/. Accessed October 24, 2019. [Google Scholar]
- 11.Werner RN, Gaskins M, Nast A, Dressler C. Incidence of sexually transmitted infections in men who have sex with men and who are at substantial risk of HIV infection - A meta-analysis of data from trials and observational studies of HIV pre-exposure prophylaxis. PLoS One. 2018;13((12)):e0208107. doi: 10.1371/journal.pone.0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387((10013)):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolling DI, Desai M, McOwan A, et al. An analysis of baseline data from the PROUD study: an open-label randomised trial of pre-exposure prophylaxis. Trials. 2016;17:163. doi: 10.1186/s13063-016-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13((12)):1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 15.Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N Engl J Med. 2012;366((6)):485–487. doi: 10.1056/NEJMp1112456. [DOI] [PubMed] [Google Scholar]
- 16.Papp JR, Abrams AJ, Nash E, et al. Azithromycin resistance and decreased ceftriaxone susceptibility in Neisseria gonorrhoeae, Hawaii, USA. Emerg Infect Dis. 2017;23((5)):830–832. doi: 10.3201/eid2305.170088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pham CD, Sharpe S, Schlanger K, et al. Emergence of Neisseria gonorrhoeae strains harboring a novel combination of azithromycin-attenuating mutations. Antimicrob Agents Chemother. 2019;63((4)):1–8. doi: 10.1128/AAC.02313-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenness SM, Weiss KM, Goodreau SM, et al. Incidence of gonorrhea and chlamydia following human immunodeficiency virus preexposure prophylaxis among men who have sex with men: a modeling study. Clin Infect Dis. 2017;65((5)):712–718. doi: 10.1093/cid/cix439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2016;176((1)):75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lal L, Audsley J, Murphy DA, et al. Medication adherence, condom use and sexually transmitted infections in Australian preexposure prophylaxis users. AIDS. 2017;31((12)):1709–1714. doi: 10.1097/QAD.0000000000001519. [DOI] [PubMed] [Google Scholar]
- 21.Hoornenborg E, Coyer L, Achterbergh RCA, et al. Sexual behaviour and incidence of HIV and sexually transmitted infections among men who have sex with men using daily and event-driven pre-exposure prophylaxis in AMPrEP: 2 year results from a demonstration study. Lancet HIV. 2019;6((7)):e447–e455. doi: 10.1016/S2352-3018(19)30136-5. [DOI] [PubMed] [Google Scholar]
- 22.Doherty IA, Padian NS, Marlow C, Aral SO. Determinants and Consequences of Sexual Networks as They Affect the Spread of Sexually Transmitted Infections. J Infect Dis. 2005;191((Supplement_1)):S42–S54. doi: 10.1086/425277. [DOI] [PubMed] [Google Scholar]
- 23.Hsu KK, Molotnikov LE, Roosevelt KA, et al. Characteristics of cases with repeated sexually transmitted infections, Massachusetts, 2014-2016. Clin Infect Dis. 2018;67((1)):99–104. doi: 10.1093/cid/ciy029. [DOI] [PubMed] [Google Scholar]
- 24.Lahra MM, Martin I, Demczuk W, et al. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis. 2018;24((4)) doi: 10.3201/eid2404.171873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Australian Government, Department of Health Multidrug Resistant Gonorrhea. Available from: https://www1.health.gov.au/internet/main/publishing.nsf/Content/ohp-gonorrhoea.htm. Accessed May 15, 2020.
- 26.European Centre for Disease Prevention and Control . Extensively drug-resistant (XDR) Neisseria gonorrhoeae in the United Kingdom and Australia. Stockholm: European Centre for Disease Prevention and Control; 2018. pp. 1–11. [Google Scholar]
- 27.Cunningham NJ, Beymer MR, Javanbakht M, Shover CL, Bolan RK. Concordance between self-reported STI history and biomedical results among men who have sex with men in Los Angeles, California. Sex Transm Infect. 2017;93((7)):514–519. doi: 10.1136/sextrans-2016-052933. [DOI] [PMC free article] [PubMed] [Google Scholar]


