Abstract
Leiomyomas are the most common benign tumors of the uterus. On the opposite side, leiomyosarcomas are rare malignant uterine tumors that account for a significant proportion of uterine cancer deaths. Especially when large and degenerated, leiomyomas and leiomyoma variants can have overlapping imaging characteristics with those of leiomyosarcomas. Although not always possible, it is paramount to be able to differentiate between leiomyomas and leiomyosarcomas on imaging, as the therapeutic management can differ. This pictorial review aims to familiarize radiologists with imaging features of leiomyomas and various types of leiomyoma degeneration and variants, together with their pathology correlates.
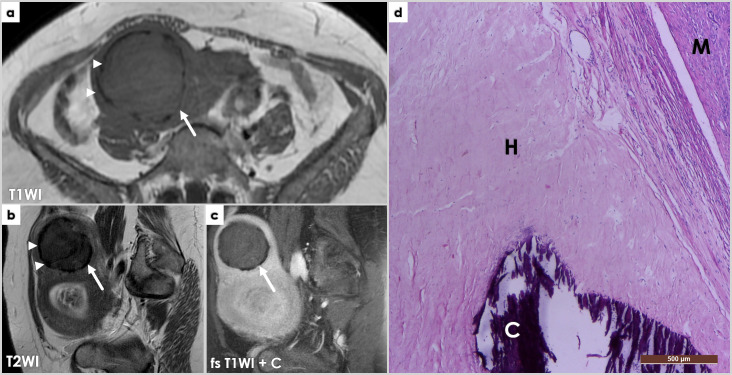
Introduction
Uterine leiomyomas are benign smooth muscle tumors of myometrial origin, representing by far the most common gynecologic neoplasm. Leiomyomas vary in size and can be solitary or multiple, with variable locations in relation to uterine wall-depth, broadly classified as intramural, submucosal, or subserosal. For a detailed, standardized nomenclature of leiomyomas, radiologists can use the FIGO classification,1 schematized in Figure 1. Rarely, a pedunculated leiomyoma can detach from the uterus and adhere to the broad ligament, salpinx or ovary, resulting in a so-called parasitic leiomyoma (Figure 2), completely separated from the uterus.
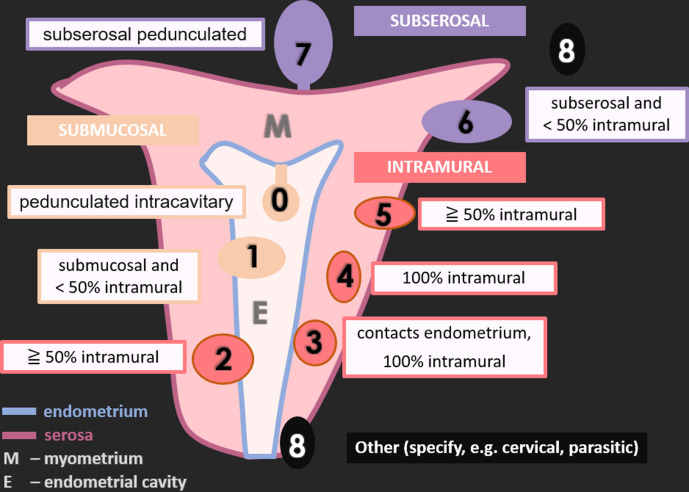
Figure 1.
Schematic representation of uterine leiomyomas according to FIGO classification.
FIGO 0: pedunculated intracavitary (submucosal); FIGO 1: submucosal and <50% intramural; FIGO 2: ≧ 50% intramural; FIGO 3: contacts endometrium, 100% intramural; FIGO 4: 100% intramural; FIGO 5: subserosal ≧ 50% intramural; FIGO 6: subserosal <50% intramural; FIGO 7: subserosal pedunculated; FIGO 8: other (specify e.g., cervical, parasitic).
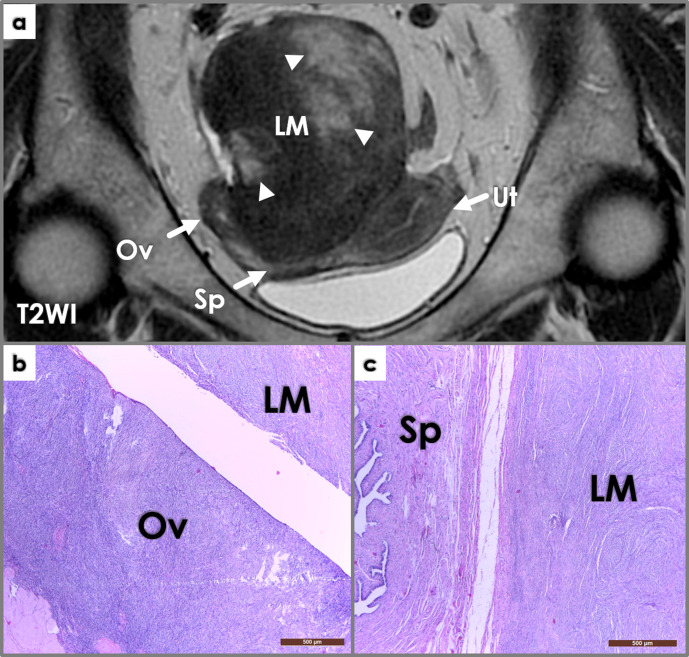
Figure 2.
Coronal T2WI image (a) shows a large intrapelvic mass (LM), in close contact to the right adnexa (Ov – ovary, Sp – salpinx) and posterior wall of the uterus (Ut). The mass is mostly solid (iso/hypointense signal intensity compared to muscle on T2WI), but also contains cystic areas (hyperintense signal intensity on T2WI, arrowheads). Macroscopic examination during surgery reveals close attachment of the mass to the right ovary and salpinx; the mass was connected to the ovary through a thin pedicle. Photomicrographs (b, c, H&E stain, 4x) show whorled interlacing fascicles of typical smooth muscle cells, with no atypia and no coagulative tumor necrosis – findings consistent with a parasitic leiomyoma (LM) histology – closely located near the right ovary (Ov, left image) and salpinx (Sp, right image).
Accounting for less than 1% of all uterine malignancies, leiomyosarcomas lie at the opposite ends of the pathologic spectrum of myometrial neoplasms. Leiomyosarcomas represent about 70% of uterine sarcomas and are responsible for a significant proportion of uterine cancer deaths.2
The decision of benign versus malignant can be rather difficult at times for the radiologist to distinguish – as leiomyomas and leiomyoma variants, especially when large, can show imaging features suspicious for malignancy.
This pictorial review aims to present imaging features correlated with histopathological findings of uterine smooth muscle tumors’ spectrum, from benign ordinary leiomyoma with its various forms of degeneration and variants, to smooth muscle tumor of uncertain malignant potential (STUMP) and malignant leiomyosarcoma. Atypical features that could mislead towards a diagnosis of malignancy will also be highlighted.
I.Imaging techniques
Imaging of uterine tumors facilitates treatment planning. However, there is no reliable up-to-date imaging method for certainty in differentiating smooth muscle benign versus malignant tumors.
A) Ultrasound (US)
US is the first-line imaging modality in symptomatic patients with leiomyomas. On US, they present as homogeneous, iso/hypoechoic, well-defined, round/oval intramyometrial masses. They may be heterogeneous due to internal degeneration. Calcifications can be seen peripherally as hyperechoic foci with acoustic shadowing, or centrally, with an internal speckled pattern. Lipoleiomyomas, when containing pure fat (due to adipose metaplasia), contain internal hyper-echogenic areas within (Figure 3a). Cystic degeneration, hemorrhage, or proteolytic liquefaction can present as hypo/anechoic areas or fluid–fluid layers, requiring additional imaging techniques for further characterization.3
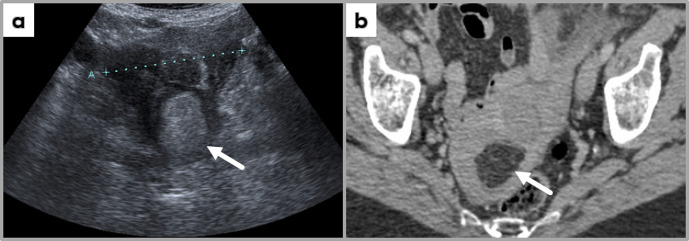
Figure 3.
Trans-abdominal ultrasound image (a) and contrast-enhanced axial CT image at the level of uterus, showing a uterine fundus mass hyperechogenic on ultrasound; the mass demonstrates fat-specific HU values, most likely representing a uterine lipoleiomyoma (incidental finding at CT examination performed for another clinical indication, followed by US assessment of the pelvis).
B) Computed Tomography (CT)
Except for incidentally diagnosing a pelvic mass as a probable leiomyoma, CT has no indication in assessing uterine masses. It might have some value in postuterine artery embolization treatment follow-up.1
On CT, leiomyomas are seen as iso/hypodense uterine masses, with various amount of calcification (usually peripheral). Necrosis or cystic degeneration (low-attenuation, Hounsfield units, HU range [0;10]), hemorrhage (high attenuation, HU range [45;70]), and fat-containing leiomyomas (fat attenuation, HU range [−120; −60] – Figure 3b) might be suggested based on the HU evaluation.
C) Magnetic Resonance Imaging (MRI)
MRI provides superior soft tissue characterization and is very useful in pre-treatment planning (precise location, number, and size). It also performs better than other imaging techniques in regards to differential diagnostic controversy, being able to depict features suggestive of malignancy in a uterine myometrial mass, which could change the treatment course.1,4
A non-degenerated leiomyoma has low T1WI/T2WI signal intensity (SI)4 and exhibits contrast enhancement following contrast administration (Figure 4a–c). The larger ones can show a high T2WI-SI rim (pseudo capsule), as a consequence of peritumoral edema due to varying degree of venous or lymphatic compression.5,6
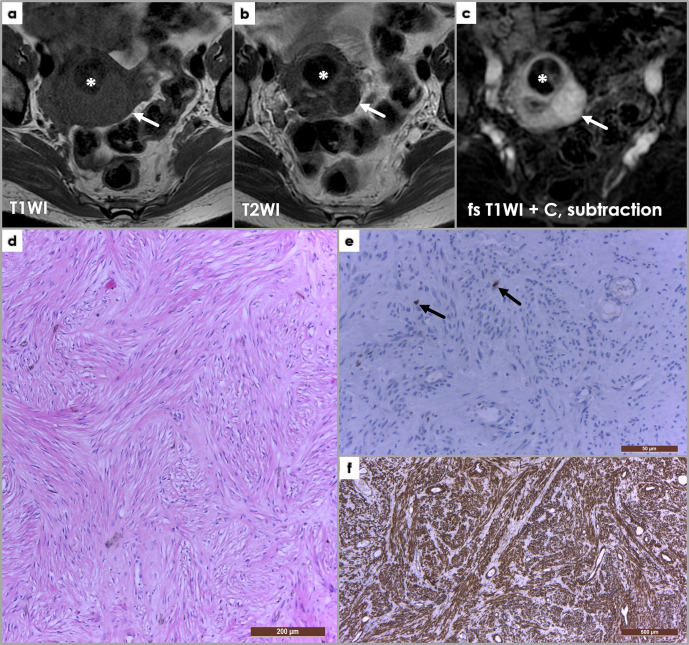
Figure 4.
Axial T1WI (a), T2WI (b) and subtraction fat-saturated post-contrast MRI images, depicting an ordinary non-degenerated leiomyoma (arrows) isointense signal intensity on T1WI (a), hypointense on T2WI (b) and enhancing post-contrast administration. Asterisk (*) depicts a hyalinized leiomyoma, which shows hypointense signal intensity on both T1WI and T2WI, and no or little enhancement on post-contrast sequences. (d) Photomicrograph of a uterine leiomyoma histology, presenting a whorled (fascicular) pattern of smooth muscle bundles – spindled cells with eosinophilic cytoplasm – separated by well-vascularized connective tissue (H&E staining, x100). Immunohistochemistry in leiomyomas: (e) Ki-67 staining (x400), (f) SMA (smooth muscle actin) stain (x40). Ki-67 is a marker of cell proliferation (nuclear stain, cytoplasmic staining is disregarded), and is negative in leiomyomas (Ki-67 positivity <1%) – only two nuclei positive for Ki-67 shown (arrows, (e); diffuse SMA positivity (f) of the spindle-shaped cells.
DWI and MR spectroscopy have been studied to try to accurately differentiate leiomyosarcomas from leiomyomas, but since significant overlap still hinder that, these methods are not entirely reliable.7
1. II. Radiology-pathology correlation ordinary leiomyomas
Non-degenerated leiomyomas are well-defined tumors, with a microscopic whorled pattern of smooth muscle fascicles separated by well-vascularized connective tissue (Figure 4d). Atypia and mitotic figures are absent (or <10/HPFs – high power fields) and no coagulative tumor cell necrosis (CTCN) is seen. Ki-67 is a marker of cell proliferation (nuclear stain, cytoplasmic staining is disregarded), and is negative in leiomyomas (Ki-67 positivity <1%) (Figure 4e). Smooth muscle origin is confirmed through positive SMA (smooth muscle actin) stain (Figure 4f).
2. Degenerated leiomyomas and variants
Degenerative changes in leiomyomas are frequent and not rarely coexist within the same tumor. They are usually a hallmark of vascular insufficiency, which often occurs because of increase in size.
A) Hyaline degeneration or hyaline necrosis is the most common type of degeneration, found in up to 60% of leiomyomas.
On MRI, hyaline-degenerated leiomyomas (Figure 5a–c) are usually similar to non-degenerated fibroids. Sometimes, hyaline necrosis can be depicted as thin, ill-defined high T2WI-SI bands/areas, scattered throughout the tumor. Hyalinization narrows the extracellular space, resulting in decreased enhancement when assessed on dynamic-contrast-enhanced MR scans.6,8
Figure 5.
Axial T1WI (a) and sagittal T2WI (b) and fat-saturated post-contrast T1WI (c) showing two large uterine masses, one located superiorly (arrows), at the uterine fundus, showing hypointense signal intensity on both T1- (a) and T2-WI (b), with a discontinuous, markedly hypointense peripheral rim (arrowheads, (a, b), and no contrast enhancement (c). Off note, the partially captured lower anterior uterine mass was also a leiomyoma with hyaline degeneration and hyaline necrosis, hence the high signal intensity T2WI areas (b) partially captured on these images. (d) Completely degenerated hyaline leiomyoma, with peripheral calcifications: microscopy image (H&E stain, (x40). The hyaline (H) manifests as homogeneously red pink substances replacing the preexisting bundles of long smooth muscle cells. Calcified nodules (C) are easily identified on H&E stain, by intense uniform basophilic deposits in a dense collagenous stroma (right lower image) – corresponding to the peripheral areas of low T1-/T2-WI signal intensity on MRI (previous image). M – normal myometrium.
On pathology, hyaline degeneration represents the deposition of collagen fibers within the extracellular space of poorly vascularized areas, appearing as homogenous eosinophilic bands in between viable and necrotic cells. Dystrophic calcifications are a feature of long-standing hyaline degenerated leiomyomas, exhibiting peripheral signal voids on MRI.9 (Figure 5d)
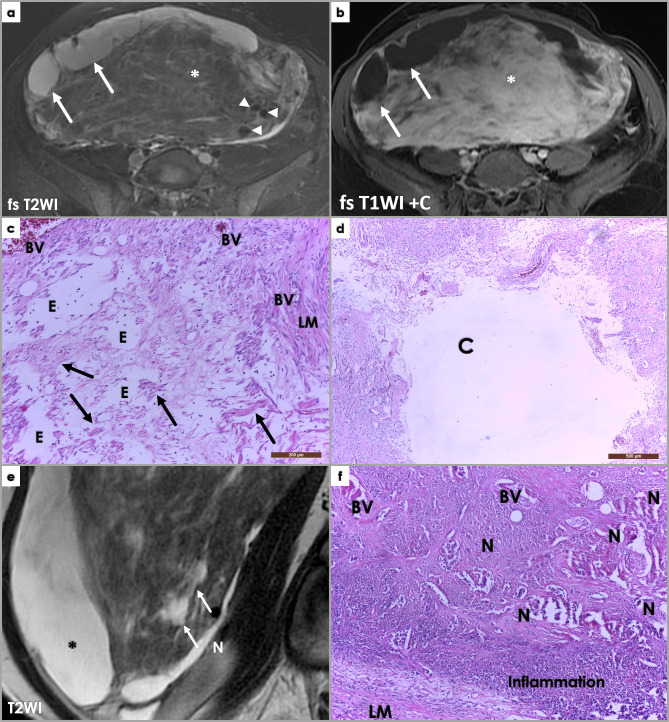
B) Cystic degeneration is characterized by the presence of cystic components of variable number and size within the leiomyomas, resulting from liquefactive, extreme hyaline necrosis. MR imaging depicts them as well-defined internal areas with homogeneous fluid-like SI (low T1WI/markedly high T2WI-SI) and no enhancement on following contrast administration.9 Differential diagnosis on imaging includes edema (also high T2WI-SI). (Figure 6a–d)
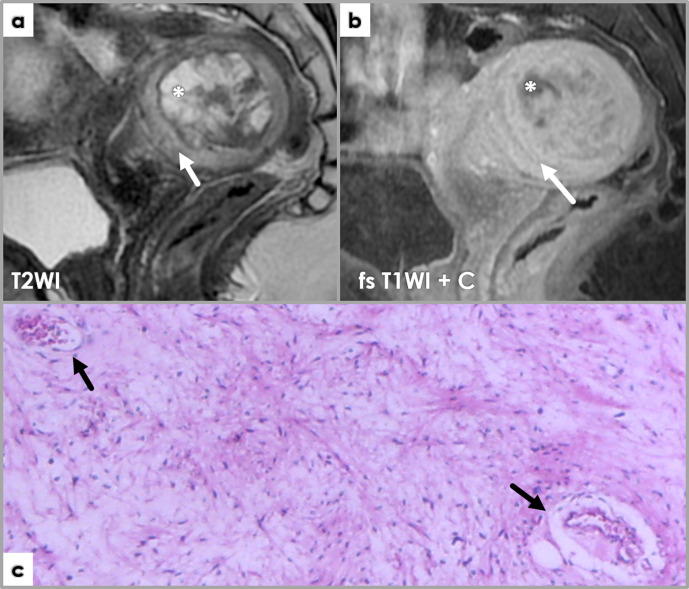
Figure 6.
Large subserosal uterine tumor proving to be a leiomyoma with cystic degeneration and ischemic necrosis at pathology examination. Axial fat-saturated (fs) T2WI (a) and post-contrast T1WI (b) MR images showing large heterogeneous intra-abdominal mass, with solid and cystic components. The cystic components (arrows) show hyperintense signal intensity on fs T2WI (a), and no contrast enhancement on post-contrast imaging (b). The solid component (*) shows mostly isointense to muscle signal intensity on both T1WI (not shown)and T2WI (a) Areas of high T2WI signal intensity scattered between the isointense leiomyoma portions reflect edema (asterisk, (a), and contrary to the cystic components, these area are avidly enhancing post Gadolinium administration (b). Note signal void on the T2 image, corresponding to large feeding vessels of the leiomyoma (arrowheads, (a). Photomicrograph showing edema (E) (H&E stain, 100x). Scattered spindle-shaped smooth muscle cells (arrow) on a background of extensive hypocellular areas consistent with an edematous fibroma. LM – leiomyoma histology. BV – blood vessels. Photomicrograph (d) of previously described uterine leiomyoma showing a cystic areas (C) (H&E stain, 40x). At gross pathology, a 3.5/4 cm necrotic area was described, and we tried to retrospectively link it to imaging features of necrosis (although imaging cannot reliably differentiate among different types of necrosis) – arrow, T2 hyperintense area, not so well defined as the cystic components (*) – differential for this would include edema. Photomicrograph (f) showing areas of extensive necrosis (H&E stain, 100x). The area was recently affected and completely necrotic; reactive inflammatory response visible in the periphery of the necrotic area (role: cellular debris disintegration). No cytologic atypia is noted in the residual leiomyoma tissue (LM).
Hyaline necrosis versus tumor necrosis (or CTCN). In contrast to hyaline necrosis, CTCN is characterized by abrupt transition between viable and necrotic cells, without interposing zones of collagen deposition, and is highly characteristic of leiomyosarcomas.10 However, early stages of hyaline necrosis are completely necrotic (as collagen deposition takes time), and show similar abrupt transition between viable and nonviable tumor cells as CTCN. Moreover, hyaline necrosis may coexist with CTCN. This makes the distinction difficult, thus, “early”, non-well-characterized necrosis should always be weighted in conjunction with the presence of nuclear atypia and mitotic activity before reaching a diagnosis. (Figure 6e–f)
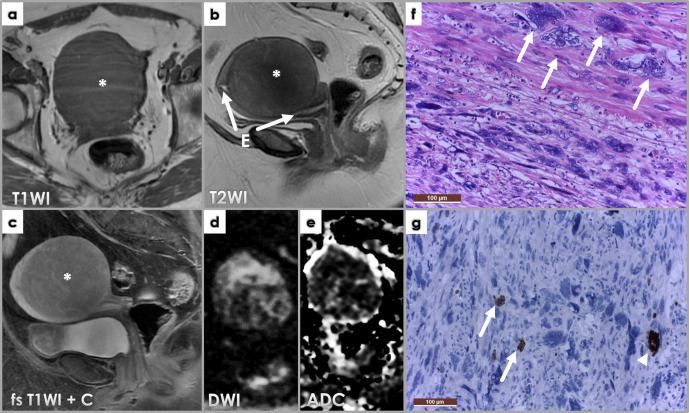
C) Hemorrhagic degeneration (red/cavernous degeneration) is a consequence of coagulative necrosis, due to acute obstruction of blood supply and tumor infarction. It is associated with pregnancy and oral contraceptive administration. Symptoms may include persistent acute abdominal pain, fever, and leukocytosis.9 Red degenerated leiomyomas typically have high T1WI-SI (with and without fat-suppression) due to the paramagnetic effect of methemoglobin and variable T2WI-SI, according to various stages of hemoglobin degradation through the physiological process of blood’s aging.11 Enhancement is absent, blood flow to the affected component being completely lost.5 (Figure 7)
Figure 7.
Axial fat-saturated T1WI (a) and T2WI (b), sagittal T2WI (c), axial DWI (d), ADCmap (e) and fat-saturated post-contrast T1WI (f) images showing a large intramural leiomyoma within the anterior uterine body. The mass is showing uniform high T1WI signal intensity and low T2WI, suggestive of red degeneration of the fibroid. Low DWI signal intensity and low ADC values depict the so-called T2-blackout effect – explained by extremely low T2WI signal intensity due to the paramagnetic effects of intracellular deoxyhemoglobin described with subacute hematomas. No enhancement is depicted on post-contrast imaging.
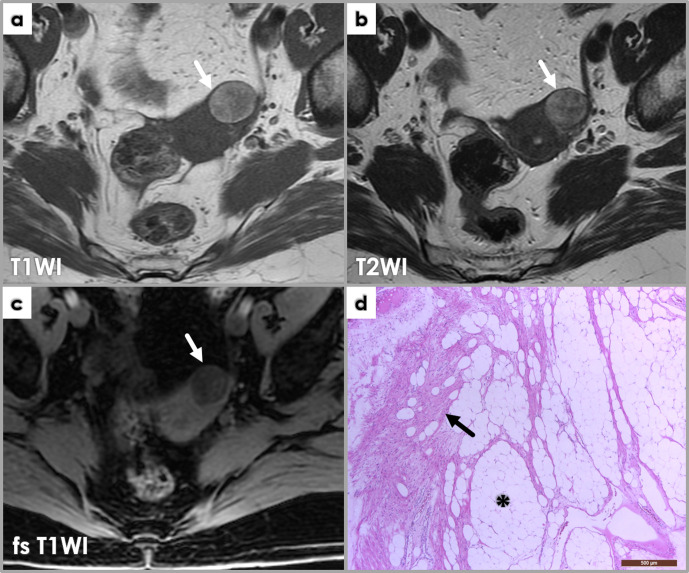
D) Fatty degeneration, seen in lipoleiomyomas, exhibits typical fat SI on MRI with high T1WI/T2WI-SI and loss of SI when using fat suppression techniques. It may arise in any part of the uterus, or in a pre-existing ordinary leiomyoma consequently to adipose metaplasia. A blend of ordinary leiomyoma cells and mature adipocytes is the hallmark of lipoleiomyomas. The adipocyte content varies; when abundant, the tumor will be yellowish on gross examination.12 (Figure 8)
Figure 8.
Axial MRI images (a, b, c) demonstrate a well-defined lesion (arrows) arising from the left uterine fundus which is of high T1WI (a) and high T2WI (b) signal intensities. There is loss of signal on the fat-saturated T1WI sequence (c) equal to that of subcutaneous fat, consistent with a subserosal lipoleiomyoma. Photomicrograph (d) of lipoleiomyoma corresponding to fatty areas described at imaging, displaying a mix of smooth muscle cells (arrow) and adipocytes (*).
E) Myxoid degeneration typically exhibits low/iso-T1WI and markedly increased heterogeneous T2WI-SI and can have a characteristic laminated enhancement pattern. This is explained by the stromal enhancement, but no contrast uptake within the interposing myxoid areas,6 consisting of extracellular mucin (mucopolysaccharides rich in hyaluronic acid). Benign smooth muscle cells in a myxoid background consisted of glycosaminoglycans are characteristic of myxoid leiomyomas.9 (Figure 9)
Figure 9.
Sagittal T2WI (a) and fat-saturated post-contrast T1WI (b) showing a degenerated myxoid (arrows) and cystic (*) leiomyoma. Myxoid peripheral areas (arrows) are T2WI hyperintense (a), and exhibit characteristic laminated contrast enhancement on fs T1WI + C (b). Pathology image (c) shows myxoid degeneration area within a leiomyoma: abundant pink-bluish myxoid connective tissues (containing large vessels, arrows) between islands of elongated spindle smooth muscle cells (Sm) with fibrillary acidophilic cytoplasm and elongated nuclei with fusiform or blunt ends. Atypia is absent or at most mild, and mitotic count should be no more than two mitoses/10 HPF. Scattered lymphocytes can be noted throughout.
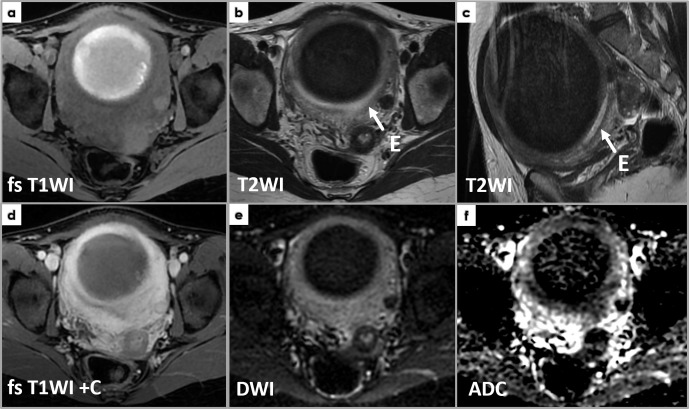
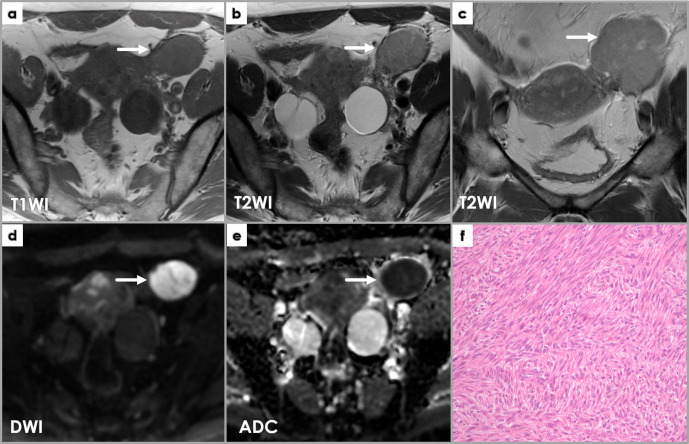
F) Cellular leiomyomas represent a leiomyoma variant that shows low T1WI/intermediate-to-high T2WI-SI and mild enhancement. Due to their hypercellularity, cellular leiomyomas exhibit markedly restricted diffusion (high DWI SI and low ADC values), potentially misleading toward a malignancy suspicion. Diagnosis is based on pathology as this variant shows hypercellularity in comparison with normal myometrium, and no atypia or CTCN are noted.12 (Figure 10)
Figure 10.
Axial T1WI (a) and T2WI (b), coronal T2WI (c), axial DWI (d) and ADCmap (e) show a well-defined mass (arrows) near the left of but completely separated from the uterine body, exhibiting intermediateT2WI signal intensity (a, b) and low T1WI signal intensity (c), as well as restricted diffusion (high DWI signal intensity and low ADC values). Intraoperatively, it was found to belong to the left round ligament of the uterus. Pathology microscopy (H&E stain) image (f) shows features of a cellular leiomyoma: hyper-cellularity with few mitoses (less than 4/10 HPF); small spindle cells with fusiform or round vesicular nuclei with no nuclear atypia or tumor cell necrosis.
G) Leiomyoma with bizarre nuclei (symplastic leiomyoma) and mitotically active leiomyomas present as solid-cystic tumors, with solid components showing low/isointense T1WI-SI, high T2WI-SI, and heterogeneous enhancement. Restricted diffusion can be present, with ADC values that sometimes overlap those of leiomyosarcomas. At pathology examination, leiomyomas with bizarre nuclei show large atypical multinucleated cells, moderate-to-severe atypia, but <10 mitoses/10HPF and no CTCN.10 (Figure 11)
Figure 11.
Axial T1WI (a), sagittal T2WI (b), sagittal fat-saturated post-contrast T1WI (c), axial DWI (d) and ADCmap (c) of an intramyometrial large mass (*), compressing the endometrium anteriorly (arrows on T2WI, E – endometrium, high T2WI signal), well delineated, showing low T1WI and T2WI intensity – isointense to normal myometrium, with diffuse and heterogeneous enhancement (e). Focal areas showing diffusion restriction (high DWI signal) with ADC values as low as 0.5 × 10−3 mm2/s. (f) Photomicrograph (H&E stain, 10x) showing a symplastic leiomyoma, containing bizarre multinucleated smooth muscle cells (arrows) with moderate-to-severe atypia, but less than 10 mitotic figures/10 HPF and no tumor cell necrosis. Photomicrograph (g) (Ki-67 staining, 10x) showing a Ki-67 index of 1% – only one nucleus positive for Ki-67 (arrowhead), cytoplasmic staining being disregarded (arrows).
3. Smooth-muscle tumors of uncertain malignant potential (STUMPs)
Uterine STUMPs represent smooth-muscle tumors with equivocal histopathological features, insufficient for neither leiomyoma nor leiomyosarcoma diagnosis. Although they rarely prove to have a malignant behavior (e.g., recurrence, metastases),6 as malignancy cannot be excluded, these patients receive long-term imaging follow-up after surgical removal.6,13 As STUMP is a histopathological exclusion diagnosis, reached by a tumor not meeting criteria for leiomyoma or leiomyosarcoma, the imaging features are not specific. Moreover, given their rarity and the fact that they are often grouped with malignant tumors when included in studies,6,14,15 it is almost impossible to associate imaging particularities with STUMPs based on existing literature. The MRI features vary from ordinary leiomyoma14 and typical degenerated leiomyoma appearances15 (as we also show in Figure 12), to resembling leiomyosarcomas.16
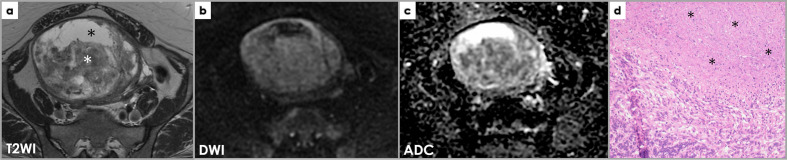
Figure 12.
Axial T2WI (a), DWI (b) and ADC map (c) images demonstrate a large heterogeneous subserosal uterine mass with areas of high signal intensities which are low on T1WI (not shown), consistent with fluid, indicating cystic change (black *). The solid component of the mass (white *) does not show restricted diffusion) and there are no other suspicious features for malignancy; it was interpreted by the radiologist as most likely a leiomyoma with cystic degeneration. However, the patient underwent hysterectomy due to the large size of the mass and the pathology was that of a STUMP. Photomicrograph (d) of a smooth muscle tumor with tumor cell necrosis (*), but no significant cytological atypia or mitotic activity. Despite the bland cytological features and absence of significant mitotic activity, the findings are in keeping with a smooth muscle tumor of uncertain malignant potential (STUMP), in view of the presence of tumor cell necrosis.
4. Leiomyosarcomas
It is critical to be able to differentiate between leiomyomas and leiomyosarcomas on imaging, as therapeutic management is different. A suspicion of leiomyosarcoma most often leads to hysterectomy, while symptomatic leiomyomas (even larger ones) can benefit from alternative, patient-individualized treatments such as hormonal therapy, MRI-guided, high-intensity focused US, uterine artery embolization (UAE), myomectomy or morcellation. The prerequisite for treatment that does not involve hysterectomy is establishing the benign nature of the targeted lesion. Table 1 summarizes MRI features of leiomyomas and subtypes versus leiomyosarcomas. Combining these different features could improve diagnostic accuracy of uterine smooth muscle tumors on imaging.4
Table 1.
MRI features of leiomyomas and subtypes versus leiomyosarcomas
| Ordinary LM | Degenerated LM | Highly cellular LM | LMS | |
|---|---|---|---|---|
| Border | Well defined; can show a low T2 pseudocapsule (peri-tumoral edema due to venous/lymphatic compression) |
Irregular, nodular or lobulated | ||
| Enhancement | Variable, generally homogeneous | Heterogeneous with non-enhancing areas (hyaline degeneration, cystic components, red degeneration) | Avid, generally homogeneous | Early, heterogeneous, with central poor enhancement (necrotic areas) |
| High T1WI SI (hemorrhage) | Absent (low T1WI SI) |
Present in red degeneration (infarction-type necrosis) |
Absent (low T1WI SI) | Present (areas of high T1WI SI) |
| T2WI SI | Low T2WI SI | High in myxoid/cystic degeneration and oedematous areas | Intermediate-to-high T2WI SI | Intermediate-to-high areas mixed with “T2 dark” areas |
| Endometrial interface | Can appear displaced or compressed, but preserved; no endometrial thickening | Disrupted, thickened endometrium | ||
| Restricted Diffusion | Absent | Present in red degeneration (hemorrhage can restrict diffusion) |
Present (high cellularity) |
Present (high cellularity of the solid component) |
| ADC values ± SD (10–3mm2/s) |
0.88 ± 0.27 to 1.40 ± 0.31 | 1.17 ± 0.17 to 1.7 ± 0.11 | 1.13 ± 0.18 to 1.43 ± 0.58 | 0.79 ± 0.21 (SD) to 1.17 ± 0.1 |
| Ordinary LM | Degenerated LM | Highly cellular LM | LMS | |
| Border | Well defined; can show a low T2 pseudocapsule (peri-tumoral edema due to venous/lymphatic compression) |
Irregular, nodular or lobulated | ||
| Enhancement | Variable, generally homogeneous | Heterogeneous with non-enhancing areas (hyaline degeneration, cystic components, red degeneration) | Avid, generally homogeneous | Early, heterogeneous, with central poor enhancement (necrotic areas) |
| High T1WI SI (hemorrhage) | Absent (low T1WI SI) |
Present in red degeneration (infarction-type necrosis) |
Absent (low T1WI SI) | Present (areas of high T1WI SI) |
SI, signal intensity.
In a study by Lakhman et al, the presence of 3 or more MRI imaging features such as irregular borders, hyperintense T1WI-SI (hemorrhage), “T2 dark” areas, central unenhanced areas), increases the sensitivity and specificity of leiomyosarcoma diagnosis up to 95–100%17 (Figure 13). Although several studies have tried to establish ADC value cut-offs for differentiating between different types of leiomyomas and leiomyosarcoma18–22 (values presented in Table 1), there is significant overlap between benign and malignant; ADC values should be interpreted with caution, in conjunction with other imaging features. In a recent study by Sahin et al,23 they show that the likelihood of a myometrial mass to represent leiomyosarcoma in a post-menopausal patient can be predicted with high accuracy at non-contrast MRI by endometrial interface disruption and endometrial thickening.
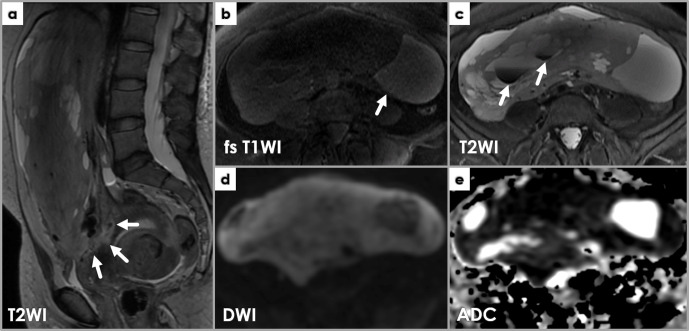
Figure 13.
MRI showing imaging features suspicious for leiomyosarcoma. Sagittal T2WI (a) showing a large, heterogeneous mass with irregular borders (arrows) approaching the endometrium. Axial T1WI (b) and T2WI (c) depict internal high T1WI SI areas (b, arrow) and low T2WI SI areas (c, arrows). Restricted diffusion is noted throughout the mass on DWI (d) and ADC (e) images.
From the pathologist point of view, a malignancy diagnosis in uterus smooth muscle tumors is relatively straightforward: leiomyosarcomas display marked nuclear atypia, high mitotic rate (≥10 mitoses/10 HPFs), and tumor cell necrosis (2 out of 3 needed to reach a leiomyosarcoma diagnosis).10 (Figure 14)
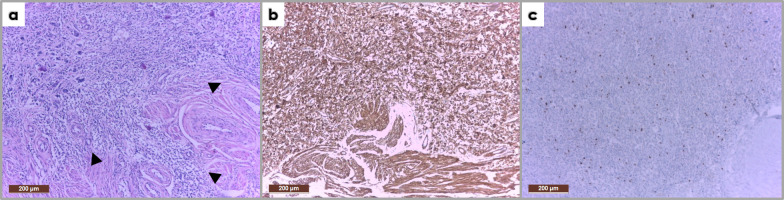
Figure 14.
Photomicrograph (H&E stain, 10x) of the mass described within Figure 13 showing marked pleomorphism with hypercellular areas; medium-sized cells, others with fusiform aspect, with large, rounded and oval nuclei (a). The tumor infiltrates and extends beyond the myometrium (arrowheads). Immunohistochemistry (IHC) shows positive SMA staining (b) and a high Ki-67 proliferation index (c). The described histological features together with the IHC profile correspond to a uterine leiomyosarcoma.
Summary
This pictorial review aims to familiarize radiologists with MRI appearances of different leiomyoma subtypes, correlated with their key histopathological features. MRI is the best imaging technique to distinguish benign leiomyomas versus leiomyosarcomas. MRI findings such as irregular margins, post-contrast MRI heterogeneity with non-enhanced central areas, hemorrhage (high T1WI signal intensity areas), T2 dark areas, disrupted endometrium and restricted diffusion can raise the suspicion for malignancy, although different leiomyoma subtypes can exhibit at least one of these imaging findings. When combined, these MRI features improve accuracy and specificity for preoperative diagnosis of STUMP/leiomyosarcoma.
Contributor Information
Vlad Bura, Email: vlad.t.bura@gmail.com.
Roxana Maria Pintican, Email: roxana.pintican@gmail.com.
Reka Emma David, Email: david.reka.emma@gmail.com.
Helen Clare Addley, Email: helenclare.addley@addenbrookes.nhs.uk.
Janette Smith, Email: janette.smith@addenbrookes.nhs.uk.
Mercedes Jimenez-Linan, Email: mercedes.jimenez-linan@addenbrookes.nhs.uk.
Janice Lee, Email: janice.lee2@addenbrookes.nhs.uk.
Susan Freeman, Email: sue.freeman@addenbrookes.nhs.uk.
Carmen Georgiu, Email: carmengeorgiu@hotmail.com.
REFERENCES
- 1.Kubik-Huch RA, Weston M, Nougaret S, Leonhardt H, Thomassin-Naggara I, Horta M, et al. European Society of urogenital radiology (ESUR) guidelines: MR imaging of leiomyomas. Eur Radiol 2018; 28: 3125–37. doi: 10.1007/s00330-017-5157-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juhasz-Böss I, Gabriel L, Bohle RM, Horn LC, Solomayer E-F, Breitbach G-P. Uterine leiomyosarcoma. Oncol Res Treat 2018; 41: 680–6. doi: 10.1159/000494299 [DOI] [PubMed] [Google Scholar]
- 3.Barral M, Placé V, Dautry R, Bendavid S, Cornelis F, Foucher R, et al. Magnetic resonance imaging features of uterine sarcoma and mimickers. Abdom Radiol 2017; 42: 1762–72. doi: 10.1007/s00261-017-1076-9 [DOI] [PubMed] [Google Scholar]
- 4.Smith J, Zawaideh JP, Sahin H, Freeman S, Bolton H, Addley HC. Differentiating uterine sarcoma from leiomyoma: BET1T2ER Check! Br J Radiol 2021; 0: 20201332. doi: 10.1259/bjr.20201332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murase E, Siegelman ES, Outwater EK, Perez-Jaffe LA, Tureck RW. Uterine leiomyomas: histopathologic features, MR imaging findings, differential diagnosis, and treatment. Radiographics 1999; 19: 1179–97. doi: 10.1148/radiographics.19.5.g99se131179 [DOI] [PubMed] [Google Scholar]
- 6.Arleo EK, Schwartz PE, Hui P, McCarthy S. Review of leiomyoma variants. AJR Am J Roentgenol 2015; 205: 912–21. doi: 10.2214/AJR.14.13946 [DOI] [PubMed] [Google Scholar]
- 7.DeMulder D, Ascher SM. Uterine leiomyosarcoma: can MRI differentiate leiomyosarcoma from benign leiomyoma before treatment? AJR Am J Roentgenol 2018; 211: 1405–15. doi: 10.2214/AJR.17.19234 [DOI] [PubMed] [Google Scholar]
- 8.Shimada K, Ohashi I, Kasahara I, Watanabe H, Ohta S, Miyasaka N, et al. Differentiation between completely hyalinized uterine leiomyomas and ordinary leiomyomas: three-phase dynamic magnetic resonance imaging (MRI) vs. diffusion-weighted MRI with very small b-factors. J Magn Reson Imaging 2004; 20: 97–104. doi: 10.1002/jmri.20063 [DOI] [PubMed] [Google Scholar]
- 9.Foti P V, Tilocca C, Sigona A, et al. Benign neoplasms of the uterus: MR imaging of leiomyomas with Radiologic-Pathologic correlation. WCRJ 2015; 2. [Google Scholar]
- 10.Oliva E. Practical issues in uterine pathology from banal to bewildering: the remarkable spectrum of smooth muscle neoplasia. Mod Pathol 2016; 29 Suppl 1(S1): S104–20. doi: 10.1038/modpathol.2015.139 [DOI] [PubMed] [Google Scholar]
- 11.Lall C, Bura V, Lee TK, Bhosale P, Faria SC, Choi J-I, et al. Diffusion-Weighted imaging in hemorrhagic abdominal and pelvic lesions: restricted diffusion can mimic malignancy. Abdom Radiol 2018; 43: 1772–84. doi: 10.1007/s00261-017-1366-2 [DOI] [PubMed] [Google Scholar]
- 12.Sugino N. Uterine Fibroids and Adenomyosis. Sugino N, ed. Singapore: Springer; 2018. [Google Scholar]
- 13.Rizzo A, Ricci AD, Saponara M, DE Leo A, Perrone AM, DE Iaco P, et al. Recurrent uterine smooth-muscle tumors of uncertain malignant potential (stump): state of the art. Anticancer Res 2020; 40: 1229–38. doi: 10.21873/anticanres.14064 [DOI] [PubMed] [Google Scholar]
- 14.Tanaka YO, Nishida M, Tsunoda H, Okamoto Y, Yoshikawa H. Smooth muscle tumors of uncertain malignant potential and leiomyosarcomas of the uterus: Mr findings. J Magn Reson Imaging 2004; 20: 998–1007. doi: 10.1002/jmri.20207 [DOI] [PubMed] [Google Scholar]
- 15.Cornfeld D, Israel G, Martel M, Weinreb J, Schwartz P, McCarthy S. MRI appearance of mesenchymal tumors of the uterus. Eur J Radiol 2010; 74: 241–9. doi: 10.1016/j.ejrad.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 16.Bolan C, Caserta MP. MR imaging of atypical fibroids. Abdom Radiol 2016; 41: 2332–49. doi: 10.1007/s00261-016-0935-0 [DOI] [PubMed] [Google Scholar]
- 17.Lakhman Y, Veeraraghavan H, Chaim J, Feier D, Goldman DA, Moskowitz CS, et al. Differentiation of uterine leiomyosarcoma from atypical leiomyoma: diagnostic accuracy of qualitative MR imaging features and feasibility of texture analysis. Eur Radiol 2017; 27: 2903–15. doi: 10.1007/s00330-016-4623-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato K, Yuasa N, Fujita M, Fukushima Y. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. Am J Obstet Gynecol 2014; 210: 368.e1–368.e8. doi: 10.1016/j.ajog.2013.12.028 [DOI] [PubMed] [Google Scholar]
- 19.Lin G, Yang L-Y, Huang Y-T, Ng K-K, Ng S-H, Ueng S-H, et al. Comparison of the diagnostic accuracy of contrast-enhanced MRI and diffusion-weighted MRI in the differentiation between uterine leiomyosarcoma / smooth muscle tumor with uncertain malignant potential and benign leiomyoma. J Magn Reson Imaging 2016; 43: 333–42. doi: 10.1002/jmri.24998 [DOI] [PubMed] [Google Scholar]
- 20.Namimoto T, Yamashita Y, Awai K, Nakaura T, Yanaga Y, Hirai T, et al. Combined use of T2-weighted and diffusion-weighted 3-T MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol 2009; 19: 2756–64. doi: 10.1007/s00330-009-1471-x [DOI] [PubMed] [Google Scholar]
- 21.Tamai K, Koyama T, Saga T, Morisawa N, Fujimoto K, Mikami Y, et al. The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol 2008; 18: 723–30. doi: 10.1007/s00330-007-0787-7 [DOI] [PubMed] [Google Scholar]
- 22.Sun S, Bonaffini PA, Nougaret S, Fournier L, Dohan A, Chong J, et al. How to differentiate uterine leiomyosarcoma from leiomyoma with imaging. Diagn Interv Imaging 2019; 100: 619–34. doi: 10.1016/j.diii.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 23.Sahin H, Smith J, Zawaideh JP, Shakur A, Carmisciano L, Caglic I, et al. Diagnostic interpretation of non-contrast qualitative MR imaging features for characterisation of uterine leiomyosarcoma. Br J Radiol 2021; 0: 20210115. doi: 10.1259/bjr.20210115 [DOI] [PMC free article] [PubMed] [Google Scholar]