Summary
Many organisms use genetic self-recognition systems to distinguish themselves from conspecifics. In the cnidarian, Hydractinia symbiolongicarpus, self-recognition is partially controlled by allorecognition 2 (Alr2). Alr2 encodes a highly polymorphic transmembrane protein that discriminates self from nonself by binding in trans to other Alr2 proteins with identical or similar sequences. Here, we focused on the N-terminal domain of Alr2, which can determine its binding specificity. We pair ancestral sequence reconstruction and experimental assays to show that amino acid substitutions can create sequences with novel binding specificities either directly (via one mutation) or via sequential mutations and intermediates with relaxed specificities. We also show that one side of the domain has experienced positive selection and likely forms the binding interface. Our results provide direct evidence that point mutations can generate Alr2 proteins with novel binding specificities. This provides a plausible mechanism for the generation and maintenance of functional variation in nature.
Subject areas: Molecular Genetics, Molecular Biology, Evolutionary Biology
Graphical abstract

Highlights
-
•
Three binding specificities evolved in a clade of five domain 1 sequences
-
•
One new specificity evolved via a single amino acid mutation
-
•
Another new specificity evolved through a dual-specificity intermediate
-
•
Sequence analyses suggest a possible binding interface
Molecular genetics; Molecular biology; Evolutionary biology
Introduction
The ability to discriminate self from same-species nonself (often referred to as allorecognition) has evolved in plants (Fujii et al., 2016), fungi (Paoletti, 2016; Gonçalves et al., 2020), slime molds (Kundert and Shaulsky, 2019), marine invertebrates (Nicotra, 2019), and bacteria (Gibbs and Greenberg, 2011; Pathak et al., 2013; Cao et al., 2019). In all cases, it is based on an organism's genotype at polymorphic loci. This polymorphism is thought to be maintained by a form of balancing selection called negative frequency-dependent selection (Wright, 1939; Kimura and Crow, 1964). Under negative frequency-dependent selection, alleles become more fit as they become less frequent. This is because rare alleles are unlikely to be shared by chance, making them better markers of self. New alleles, the rarest of all, spread in a population until their frequencies reach that of other alleles (Richman and Kohn, 2000). These dynamics can maintain tens to hundreds of self-recognition alleles in a population (Casselton and Olesnicky, 1998; Lawrence, 2000; Gloria-Soria et al., 2012; James, 2015; Nydam et al., 2017; Goncalves et al., 2019). How new, functional self-recognition alleles are generated and ultimately contribute to this extreme polymorphism remains a puzzle.
Hydractinia symbiolongicarpus is a colonial cnidarian that uses proteins that are their own ligand for allorecognition (Frank et al., 2020). Hydractinia colonies begin when a sexually produced larva settles on a hermit crab shell and metamorphoses into a polyp. The animal then expands across the shell by elongating stolons (extensions of its gastrovascular system) or mat (a plate of tissue that fills the space between stolons), from which new polyps grow to form a mature colony. As it grows, a colony's stolons and mat edges meet and fuse to create an anastomosing network of gastrovascular canals embedded in a continuous sheet of mat. The colony will also fuse to itself as it grows around the shell or recovers from injury. Because nearly half of all shells bear more than one colony (Yund et al., 1987), colonies also frequently encounter conspecifics. This usually elicits an aggressive rejection response in which the colonies fight by firing nematocysts (harpoon-like organelles) until one dies (Nicotra and Buss, 2005).
Previous experiments with inbred, laboratory strains of Hydractinia have demonstrated that colonies can distinguish self from nonself by their genotype at two linked genes called allorecognition 1 (Alr1) and allorecognition 2 (Alr2) (Cadavid et al., 2004; Powell et al., 2007, 2011). Animals that shared at least one allele at both loci fused, while those that shared no alleles at either Alr1 or Alr2 rejected. If colonies only shared alleles at one locus, they fused but then separated. Because only two alleles were present at each locus in these strains, it was impossible to determine how similar alleles need to be for colonies to fuse. In addition, subsequent experiments with wild-type colonies have strongly suggested at least one additional allorecognition locus exists in the genomic region encoding Alr1 and Alr2 (Powell et al., 2007, 2011; Nicotra et al., 2009; Rosa et al., 2010).
Alr1 and Alr2 both encode type I transmembrane proteins with tandem Ig-like domains in their extracellular regions (Nicotra et al., 2009; Rosa et al., 2010). Each Alr is capable of cell-to-cell (i.e., trans) homophilic binding (Karadge et al., 2015). Binding is restricted to isoforms with identical or very similar sequences (Karadge et al., 2015). These results, combined with the fact that Hydractinia must share Alr1 and Alr2 alleles to recognize each other as self, have led to the hypothesis that homophilic binding of Alr1 and Alr2 between colonies is part of the in vivo self-recognition mechanism.
Alr1 and Alr2 are also highly polymorphic. A study of Alr2 identified 183 distinct Alr2 amino acid sequences from a single population (Gloria-Soria et al., 2012). Alr1 is expected to be similarly diverse based on the extreme levels of sequence polymorphism observed in 20 sequenced alleles (Rosa et al., 2010). These observations suggest hundreds of distinct binding specificities could exist in nature.
Two features of Hydractinia's natural history likely contribute to the evolution of this extreme polymorphism. First, colonies must be able to compete for space while simultaneously retaining the ability to recognize and fuse to themselves. Thus, a new allele that binds only to itself is favored because it permits a colony to compete with every other Hydractinia in the population but still fuse with itself. Second, Hydractinia has a pluripotent stem cell lineage that can differentiate into germ cells at any point in the colony's life. Fusion allows these stem cells to migrate from one colony into the other, where they could dominate its gametic output. This phenomenon, called stem cell parasitism, has been observed anecdotally in Hydractinia (Künzel et al., 2010; Dubuc et al., 2020) and is thought to be a common trait in most colonial organisms (Buss, 1987; Stoner and Weissman, 1996; Stoner et al., 1999; Laird et al., 2005; Aanen et al., 2008). Thus, a new allele that restricts fusion to self would be favored because it would reduce the risk of stem cell parasitism.
It has been assumed that novel Alr1 and Alr2 alleles are generated by random mutations that are then subjected to negative frequency-dependent selection. This raises the question of whether point mutations, by themselves, can generate alleles with novel homophilic binding specificities and, furthermore, whether this type of mutation could, in part, explain the large number of binding specificities thought to exist in natural populations.
Here, we sought to determine how binding specificities evolve in the N-terminal domain of Alr2. This domain, referred to as “domain 1,” is the most polymorphic region of Alr2. Changes in domain 1 can prevent Alr2 proteins from binding and therefore might be able to generate alleles with new identities. To determine how this domain has evolved in nature, we identified a clade of five domain 1 sequences encoding isoforms that differed by six or fewer amino acids. We then used ancestral sequence reconstruction and in vitro binding assays to determine the evolutionary history of the clade. Our results demonstrate that the binding specificity of domain 1 can be altered by single amino acid changes, resulting in novel specificities or intermediates with broadened specificities. Finally, we show that one face of the predicted domain 1 structure appears to be under diversifying selection, which also allows us to hypothesize that Alr2 protein-protein interactions occur in a side-to-side manner.
Results
Point mutations in domain 1 can create new binding specificities
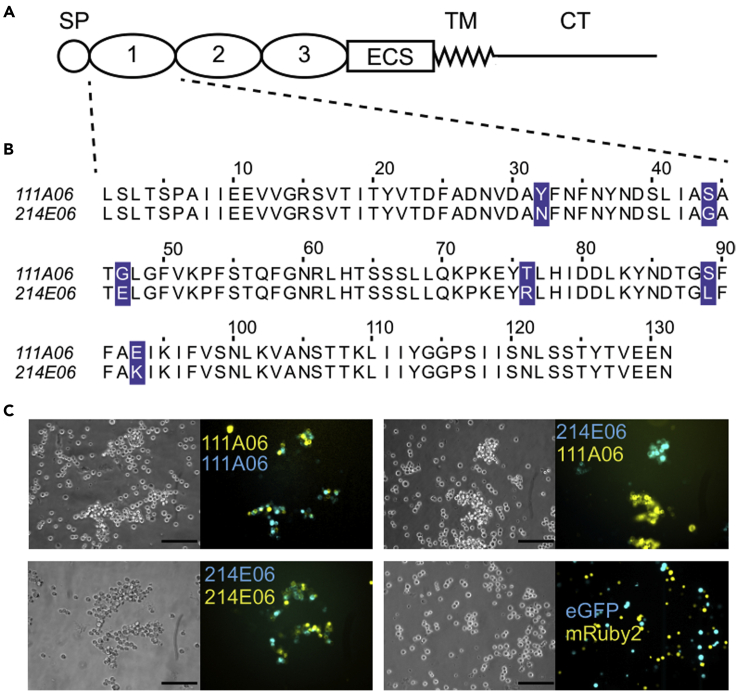
We searched a data set of full-length, naturally occurring Alr2 alleles (Nicotra et al., 2009; Gloria-Soria et al., 2012) and identified two (111A06 and 214 × 106) that encoded Alr2 allelic isoforms (hereafter, “isoforms”) with six amino acid differences in domain 1 and identical sequences across the rest of the extracellular region (Figures 1A and 1B). Using cell aggregation assays (Karadge et al., 2015), we found that each isoform bound to itself across opposing cell membranes but did not bind to the other (Figure 1C). We therefore sought to identify the amino acid differences that prevented them from binding to each other.
Figure 1.
Isoform-specific, homophilic binding of Alr2 isoforms
(A) Alr2 protein structure. SP = Signal peptide, ECS = Extracellular spacer, TM = Transmembrane domain, CT = Cytoplasmic tail.
(B) Multiple sequence alignment of 111A06 and 214E06 domain 1. Polymorphisms highlighted in purple.
(C) Cell aggregation assays of 111A06 and 214E06. Cells transfected with vectors encoding only fluorescent proteins (eGFP or mRuby2) do not form aggregates (bottom right). Scale bar = 100 μm.
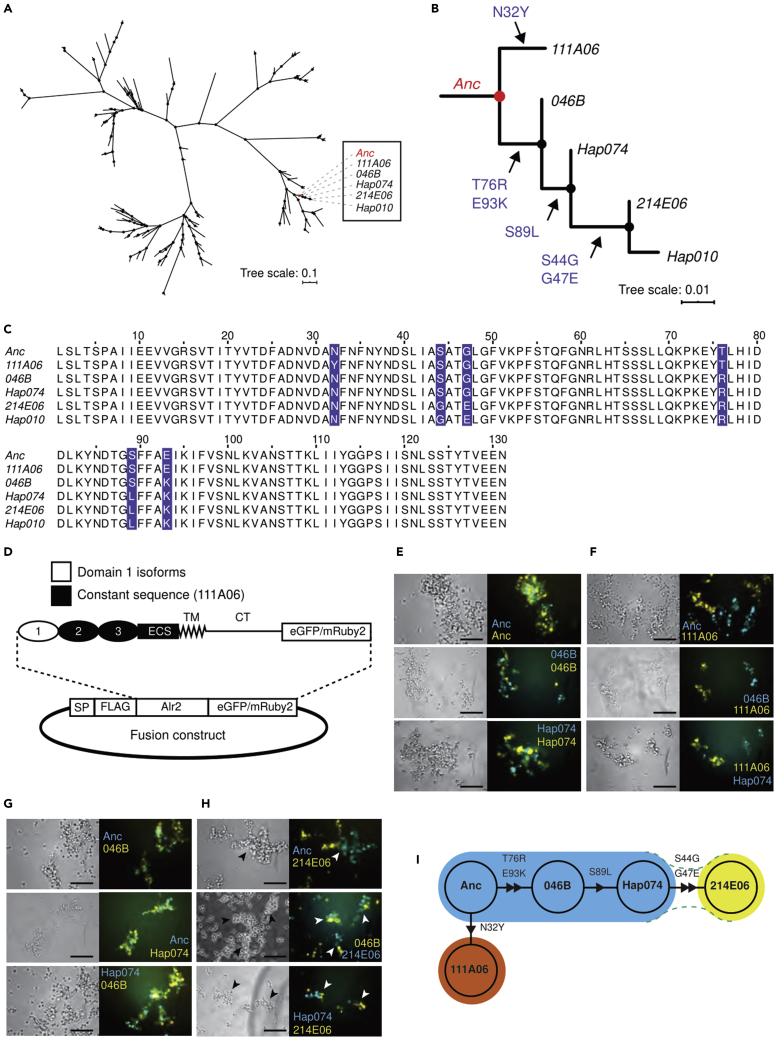
Each amino acid difference between 111A06 and 214E06 is the result of one point mutation. To reconstruct the evolutionary history of these mutations, we created a phylogeny of all known domain 1 coding sequences (Figure 2A). 111A06 and 214E06 were located in a clade with three additional sequences (Figure 2B). We then used ancestral sequence reconstruction to infer the sequence of each node. All but the ancestral node (Anc) were predicted to be identical to an extant sequence (Figure 2C). Because 214E06 and Hap010 differed only by a single synonymous mutation, we used 214E06 to represent their shared amino acid sequence.
Figure 2.
Evolution of novel binding specificities via point mutation
(A) Maximum-likelihood tree of 146 domain 1 coding sequences.
(B) Expansion of clade that includes 111A06 and 214E06. Allele names on branch. Amino acid changes indicated along branches.
(C) Multiple sequence alignment of clade. Variant residues highlighted.
(D) Plasmid map Alr2 fusion proteins. (E-H) Representative images of cell aggregation assays.
(E) Anc, 046B, and Hap074 against themselves.
(F) Anc, 046B, and Hap074 against 111A06.
(G) All pairwise combinations of Anc, 046B, and Hap074.
(H) Anc, 046B, and Hap074 versus 214E06. Arrowheads point to semi-mixed aggregates (See also Figure S1). Scale bar = 100 μm.
(I) Node network of isoforms colored by binding specificity. Triangles indicate the hypothesized direction of mutation from Anc. Green dotted lines indicate weaker heterophilic interactions.
To determine the binding specificity of the domain 1 isoforms encoded by these sequences, we expressed each as a fusion to domain 2 through the cytoplasmic tail of the 111A06 isoform, with a C-terminal fluorescent protein tag (Figure 2D). The resulting isoforms were tested against themselves and each other in cell aggregation assays (Figures 2E–2H). Each isoform, including the predicted ancestor, Anc, caused cells to form multicellular aggregates, indicating it was capable of homophilic binding (Figure 2E). In pairwise assays, 111A06 did not form mixed aggregates with any isoform, indicating it had a unique binding specificity within the clade (Figure 2F). In contrast, Anc, 046B, and Hap074 all formed mixed aggregates with each other, indicating a shared binding specificity (Figure 2G).
In assays that paired 214E06 with Anc, 046B, or Hap074, we observed single-color aggregates, some of which appeared to adhere to aggregates of a different color (Figure 2H, arrowheads). These semimixed aggregates were repeatable (Figure S1A) and qualitatively different from the mixed aggregates it formed when paired with itself and from the completely separate aggregates it formed with the other four isoforms. This ruled out a defect in 214E06 that prevented homophilic binding or caused it to bind to any isoform. Semimixed aggregates have been observed in studies of cell adhesion molecules that have strong homophilic affinities but weaker heterophilic affinities (Katsamba et al., 2009; Goodman et al., 2016). Because of this, we concluded that 214E06 binds more weakly to Anc, 046B, and Hap074 than to itself and that it therefore had a different binding profile from the other isoforms.
Our results are consistent with the following evolutionary history (Figure 2I). An ancestral sequence, Anc, underwent a single mutation, N32Y, which created a daughter sequence, 111A06, with a novel binding specificity. In a separate lineage, the Anc sequence underwent two mutations, T76R and E93K to create 046B, which retained the ability to bind to Anc. A third mutation, S89L, then created Hap074, which also remained able to bind Anc and 046B. Two more mutations, S44G and G47E, then created 214E06, which bound more weakly to the ancestral isoforms than to itself (Figure 2I, dotted lines). The result is a clade in which we can discern three binding specificities, one of which arose via a single-point mutation.
New homophilic specificities can evolve via less restricted intermediates
Within the phylogeny, two pairs of mutations occurred within single branches (Figure 2B), preventing us from determining which came first. To determine whether the missing single-step intermediates were functional (i.e., able to bind homophilically) or had a different binding specificity from their parent and daughter sequences, we recreated each one (Figure 3A) and tested it in cell aggregation assays. We found each intermediate could bind homophilically (Figure 3B), thus ruling out the possibility that there were nonfunctional intermediates in the clade.
Figure 3.
Domain 1 isoforms can evolve via intermediates with broadened specificity
(A) Expanded node network including hypothesized single-step mutants between Anc and 046B, Hap074 and 214E06. Scale bar = 100 μm and applies to all images.
(B–H) Representative images of cell aggregation assays.
(B) Mutants tested against themselves.
(C) Anc-T76R and Anc-E93K tested against Anc, 046B, Hap074.
(D) Anc-T76R versus 111A06 (left) and Anc-E93K versus 111A06 (right). Semi-mixed aggregates indicated with arrowheads (See also Figure S1A).
(E) Anc-T76R and Anc-E93K versus 214E06 (See also Figure S1C).
(F) Hap074-S44G and Hap074-G47E versus 111A06.
(G) Hap074-S44G and Hap074-G47E versus 214E06.
(H) Hap074-S44G and Hap074-G47E versus all remaining isoforms.
We next tested the specificity of each missing intermediate. The first pair, Anc-T76R and Anc-E93K, formed mixed aggregates with Anc, 046B, and Hap074 (Figure 3C). Assays pairing Anc-T76R with 111A06 resulted in single-color aggregates (Figure 3D), but those pairing Anc-E93K with 111A06 resulted in a few semimixed aggregates (Figure 3D, arrowheads, Figure S1B). Both mutants also formed semimixed aggregates when paired with 214E06 (Figures 3E and S1C). Thus, evolution from Anc to 046B is unlikely to have involved a significant change in binding specificity (Figure 3A).
In contrast, the specificity of the second pair of intermediates, Hap074-S44G and Hap074-G47E, was different from their parent and daughter sequences. These mutants failed to form mixed aggregates with 111A06 (Figure 3F) but did form mixed aggregates with 214E06 (Figure 3G) and all other ancestral sequences (Figure 3H). We did not observe semimixed aggregates in any assay. These results suggest the first mutation on the path from Hap074 to 214E06, either S44G or G47E, created a sequence that could still bind Hap074 (Figure 3A). The acquisition of the second mutation then generated a new allele, 214E06, which remained able to bind its parent sequence, but had a weaker affinity for Hap074. The evolution of new domain 1 sequences can therefore proceed through intermediates with broader specificities than their parental or daughter sequences.
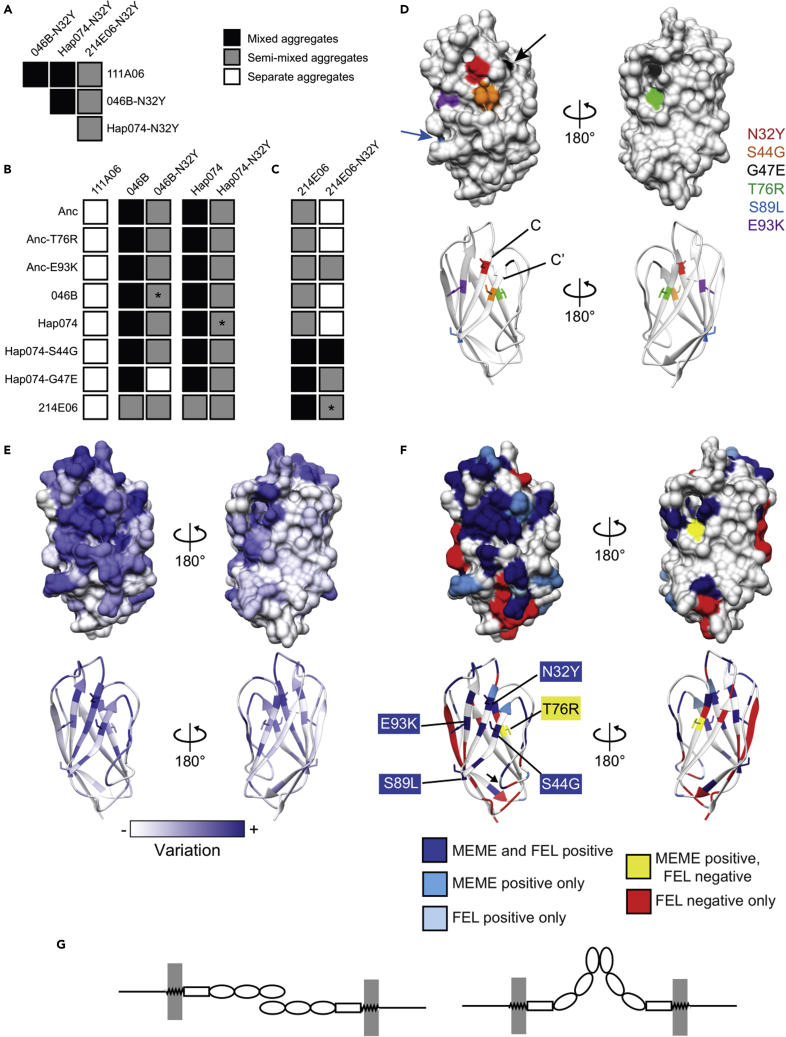
The N32Y mutation preserves homophilic binding and alters specificity
Isoform 111A06 evolved when position 32 mutated from Asn to Tyr in Anc. We therefore hypothesized the N32Y mutation might turn 046B or Hap074, which had the same specificity as Anc, into isoforms with the same specificity as 111A06. To test this, we generated 046B-N32Y and Hap074-N32Y. In assays with themselves, each formed mixed aggregates, indicating the mutation did not disrupt homophilic binding (Figure S1D). In pairwise assays with each other and 111A06, the mutants formed mixed aggregates, indicating they had gained the ability to bind 111A06 and each other (Figures 4A and S1G). In pairwise assays with their immediate ancestors, however, the mutants formed semimixed aggregates (Figure 4B, asterisks, and Figure S1E). This indicated each could still bind its ancestor, albeit more weakly than it did itself. Finally, we performed pairwise assays with the remaining isoforms in the clade. This showed the mutants had different specificities than 111A06, 046B, or Hap074 (Figures 4B and S2). In sum, the N32Y altered the specificities of 046B and Hap074 but did not generate daughter sequences with the same specificity as 111A06.
Figure 4.
Effects of N32Y mutation on binding specificity and structural analysis
(A) Results of assays between N32Y mutants and 111A06 (See also Figures S1G and S1H).
(B) Binding profiles of 111A06, 046B-N32Y, Hap074-N32Y, 046B, and Hap074. Asterisk denotes the result of an allele and its N32Y mutant.
(C) Binding profiles of 214E06 and 214E06-N32Y.
(D) Predicted structure of Anc domain 1. Six variant residues labeled.
(E) Sequence conservation mapped onto domain 1.
(F) Residues predicted to have experienced either diversifying or purifying selection mapped onto domain 1. Colors correspond to the predictions of MEME and/or FEL. Arrowhead indicates the one residue predicted to be under positive selection by FEL only.
(G) Hypothetical binding topologies Alr2.
We next tested whether the N32Y mutation would alter the specificity of 214E06, the remaining domain 1 isoform known to exist in nature. We generated 214E06-N32Y and found it formed mixed aggregates with itself (Figure S1D), indicating it was able to bind homophilically to itself. It also formed semimixed aggregates with 214E06 (Figure 4C, asterisk, and Figure S1F), indicating a reduced binding affinity for its immediate ancestor compared to itself. However, 214E06-N32Y only formed semimixed aggregates with 111A06, 046B-N32Y, and Hap074-N32Y (Figure 4A). Thus, simply sharing a Tyr at position 32 was insufficient for isoforms to bind each other as strongly as they did themselves. Pairwise assays with the remaining isoforms revealed 214E06-N32Y to have a different binding profile than 214E06, with the exception of the mixed aggregates formed with Hap074-S44G (Figures 4C and S3). The effect of the N32Y mutation thus depends on the sequence context in which it occurs.
Structural and evolutionary analyses suggest a potential binding interface
In this study, three mutations changed the binding specificity of domain 1 (N32Y, S44G, and G47E), and three others did not. To investigate how these mutations might affect the tertiary structure of domain 1—and thus its binding specificity—we used I-TASSER to predict their structures. All were predicted to fold like V-set Ig-domains, which was consistent with previous work (Nicotra et al., 2009). Five mutations mapped to one face of the predicted beta-sandwich, with the three specificity-altering mutations in close proximity to each other in beta-strands C and C’ (Figure 4D shows the structure of Anc for illustration). This suggested these strands are involved in homophilic binding between compatible domain 1 isoforms.
To gain further insight into the mechanism of homophilic binding, we compared the predicted structures of domains differing by a single amino acid (e.g., Anc vs 111A06). We noted many differences in the orientation of the mutated residues and their nearby amino acids. However, molecular dynamics simulations indicated these orientations were probably unstable (data not shown), so we did not analyze the models any further.
As an alternative approach to identify functionally important parts in domain 1, we reasoned that selection should increase sequence variation at or near the binding site. We therefore calculated the level of sequence variation at each site across all known domain 1 sequences, then mapped this metric onto the predicted structure of Anc. We found that most of the variable sites were also concentrated on the side of the domain that includes strands C and C′ and residues 32, 44, and 47 (Figure 4E).
One explanation for this increase in variation is that positive (diversifying) selection is acting on amino acid positions at the binding interface because this can generate new specificities.
Although current sequence-based methods do not allow one to test whether a single mutation on a single-branch experienced positive selection (Murrell et al., 2012; Spielman et al., 2019), we were able to test whether positive selection has acted on specific sites in domain 1 across the entire phylogeny of domain 1 sequences. To do this, we analyzed the alignment of all known domain 1 sequences with MEME (Murrell et al., 2012) and FEL (Pond and Frost, 2005). Thirty sites were predicted to have experienced positive selection and were concentrated on the side of the domain that includes strands C and C’ (Figure 4F, Table S1). Twenty sites were predicted to be under negative (purifying) selection and mapped to this side of the domain.
With respect to the six positions at which mutations occurred in our clade of interest, sites 32, 44, 89, and 93 were predicted to have experienced positive selection on at least one branch of the full phylogeny, but site 47 was not. Site 76 was predicted by MEME to be under positive selection, but by FEL to be under negative selection, a pattern consistent with a burst of diversifying selection against a background of purifying selection (Spielman et al., 2019). In all, these results are consistent with positive selection acting to increase sequence variation at sites on a probable binding face.
Taken together, these evolutionary signatures also suggest Alr2 proteins might bind via “side-to-side” interactions at their N-terminal domains. We speculate these interactions could occur in either an antiparallel or parallel topology (Figure 4G).
Discussion
Domain 1 is the most polymorphic region of Alr2 (Gloria-Soria et al., 2012). Here, we demonstrate that sequence differences in this domain can prevent Alr2 isoforms from binding to each other. Then, by reconstructing the history of a small domain 1 sequence family, we show that new sequences capable of discriminating between themselves and their ancestors can evolve via point mutation. This can occur with as little as one mutation or via sequential mutations leading through intermediates with relaxed specificities. The fact that so few mutations occurred within this family also increases our confidence in our sequence reconstructions. Because sequence differences in domain 1 are sufficient to alter Alr2 specificity, these mutations may have generated Alr2 alleles with novel identities. Moreover, because the sequences in this study were drawn from a single population, our results show that natural selection maintains ancestral sequences alongside one encoding new specificities. Thus, our results reveal a mechanism capable of generating, maintaining, and increasing the functional diversity of Alr2.
In this study, we failed to identify domain 1 sequences that could not bind homophilically. This is somewhat surprising because alleles incapable of homophilic binding might be expected to exist in nature. Colonies that are Alr2a/null (where a is an allele encoding a homophilic binding protein and null cannot bind homophilically) might be functionally equivalent to Alr2a/a colonies. This is possible because fusions between colonies sharing only one allele are identical to fusions between colonies that share two alleles. Colonies with null alleles might even have a fitness advantage because the probability that they will fuse with nonself is reduced from the sum of two allele frequencies to the frequency of a single allele. So, why have we not detected null alleles in this and a previous study (Karadge et al., 2015)? One possibility is that null alleles are rare, and we have not found one yet because we have only studied ∼5% of sequence variation at Alr2. A second possibility is that Alr2a/null animals are not, in fact, equivalent to Alr2a/a animals. This might be true if Alr2 has essential functions beyond self-recognition at the colony border. In fact, we suspect this is the case because Alr2 is constitutively expressed from embryonic development through adulthood and across all tissues in a colony (Nicotra et al., 2009). Alr2 might therefore be required to maintain adhesion between epithelial cell layers. If true, Alr2wt/null colonies might be unfit, and Alr2null/null animals might be inviable. This would also place an upper limit on the total frequency of null alleles in a population. A third possibility is that our assay is unable to detect null alleles. This would be the case if cells aggregate in our assay at a lower affinity than that required for colonies to recognize a tissue as self.
Assuming our assay does correlate with the in vivo function of Alr2, the observation that three sequences (Anc, 046B, and Hap074) encode the same binding specificity might also seem surprising, since their common specificity would make them less fit than 111A06 or 214E06. Alr2 allele frequencies might differ from expected equilibrium frequencies if the population has experienced changes in gene flow or recent bottlenecks. Similarly, if there are beneficial alleles at nonallorecognition genes tightly linked to Alr2, some Alr2 alleles might have higher than expected frequencies due to genetic hitchhiking. In addition, we note that the tree for this clade does not represent actual allele frequencies because we removed duplicate sequences prior to constructing the phylogeny. Indeed, in the original study (Gloria-Soria et al., 2012), which reported near-saturation sampling of a single population in Long Island Sound, USA, the 111A06 specificity was represented by three alleles, the Anc/046B/Hap074 specificity by five alleles, and the 214E06 specificity by three alleles. Although essentially anecdotal, this distribution is closer to the expectation of equal phenotype frequencies. These considerations suggest that future work elucidating the population genetics of Hydractinia, comprehensively assessing the full breadth of Alr2 binding specificity diversity, and annotating genomic regions linked to Alr1 and Alr2 will be fruitful.
Many positions in domain 1 appear to have experienced positive selection that was either episodic (i.e., limited to particular branches and detected by MEME) or pervasive (under pressure throughout the phylogeny and detected by FEL). As previously mentioned, our evolutionary analyses cannot not tell us whether the six specific mutations that occurred within the branches of our clade experienced positive selection. What we can say is that four mutations occurred at positions under positive selection somewhere in the phylogeny. Two of these mutations (at positions 32 and 44) altered binding specificity and two did not (89 and 93). One interpretation of this is that nonsynonymous mutations are favored at these positions because they can alter specificity in some sequence contexts, some of which are present in other branches of the tree. Alternatively, these latter mutations might actually alter specificity at a level that our assays could not detect. With respect to position 47, which was not found to be under positive selection but which did alter binding specificity, it is possible that positive selection was present but neither MEME nor FEL had power to detect positive selection because the branches were short. The same explanation could apply to position 76, although our results suggest that positive selection acted only briefly and against a background of strong negative selection. This would also be consistent with mutations at this position altering specificity elsewhere in the larger tree. Several sites in the hypothesized binding surface were also predicted be under negative selection. These sites could be highly conserved because altering them would render the domain incapable of homophilic binding at all. Further work to complement these analyses with functional assays will answer these questions.
Because our study identified residues that affect homophilic binding specificity, we attempted to use structural modeling to identify the biophysical basis of this specificity. Ultimately, we determined that the models produced by I-TASSER were not reliable enough for us to do so. Understanding the biophysical mechanism of this exquisite specificity must therefore await experimentally determined structures. We were, however, able to use sequence variation to generate a hypothesis for how the proteins interact. Across all Alr2 alleles, positions with the highest degree of variation, and those experiencing diversifying selection, were predicted to occur on one side of the Ig-like beta barrel. This suggests that the N-terminal domains of Alr2 bind in a side-to-side manner.
Although we focused here on domain 1, other regions might also determine binding specificity. Evidence for this comes from the fact that the entire extracellular region of Alr2 is polymorphic, and the prediction that residues in domains 2–3 and the ECS are predicted to have experienced diversifying selection (Nicotra et al., 2009; Gloria-Soria et al., 2012). Point mutations in these regions might also give rise to new alleles. Recombination might also generate novel binding specificities. Domains 1–3 and the ECS are each encoded by single exons. These exons frequently recombine between Alr2 alleles and get shuffled between Alr2 and several adjacent pseudogenes via gene conversion or unequal crossing over (Gloria-Soria et al., 2012). This could generate chimeric domain 1 sequences with novel specificities. It might also bring together new combinations of domains 1–3 or the ECS that would have different specificities than either of the nonrecombinant parental alleles.
In light of our results, Hydractinia would be a productive system in which to study “sequence space”—the theoretical universe of all possible peptides of a given length. Long-standing questions about how many functional variants of a protein exist in sequence space, how many of these actually appear in nature, and whether evolution is constrained in its ability to reach them remain unresolved (Weinreich et al., 2006; Povolotskaya and Kondrashov, 2010; Podgornaia and Laub, 2015). Because natural selection drives the continued evolution of new allorecognition alleles, allorecognition loci like Alr2 are essentially natural experiments exploring sequence space.
Limitations of the study
The main limitation of this study is the qualitative nature of our cell aggregation assays. Although such assays are commonly used to test binding in cell adhesion molecules (Kasinrerk et al., 1999; Katsamba et al., 2009; Schreiner and Weiner, 2010; Thu et al., 2014; Rubinstein et al., 2015; Goodman et al., 2016), it can be difficult to draw conclusions from them about quantitative binding affinities. This is particularly true here because we used transient transfections, which led to unavoidable variation in the expression of each Alr2 isoform between cell populations. This prevented us from using measures of aggregation speed or aggregate size to infer their binding strength. In other words, in this study, assays with just one allele reveal whether the encoded protein can bind to itself in trans but do not indicate its homophilic binding affinity. Similarly, assays in which two alleles are present only reveal whether homophilic or heterophilic interactions were favored. Therefore, it is possible that isoforms that did not bind each other in our assays would, in fact, bind heterophilically if homophilic interactions were prevented, as would likely be the case if they were expressed on the outward facing epithelia of opposing Hydractinia colonies. With this limitation in mind, we conservatively interpreted “semimixed” aggregates as indicating that two isoforms had heterophilic affinities that were relatively weaker than their homophilic affinities. We hypothesize this type of aggregate formed because the difference in affinities led to homophilic clusters that then associated heterophilically. This interpretation is in line with what is thought to happen when similar aggregates form with cadherins and other immunoglobulin superfamily cell adhesion proteins (Katsamba et al., 2009; Goodman et al., 2016). These caveats should be kept in mind when extrapolating our results to nature. Resolving this issue will require quantitative assays paired with transgenic experiments to ectopically express these alleles in living colonies and determine their phenotypic effect, an experimental approach now possible thanks to recent advances in Hydractinia functional genomics (Sanders et al., 2018).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: cell lines | ||
| HEK293T cells | ATCC | Cat# CRL-3216 |
| Recombinant DNA | ||
| pFLAG-CMV3-111A06-eGFP | This paper | pUP801 |
| pFLAG-CMV3-111A06-mRuby2 | This paper | pUP738 |
| pFLAG-CMV3-214E06-eGFP | This paper | pUP836 |
| pFLAG-CMV3-214E06-mRuby2 | This paper | pUP746 |
| pFLAG-CMV3-Anc-eGFP | This paper | pUP871 |
| pFLAG-CMV3-Anc-mRuby2 | This paper | pUP748 |
| pFLAG-CMV3-046B-eGFP | This paper | pUP872 |
| pFLAG-CMV3-046B-mRuby2 | This paper | pUP750 |
| pFLAG-CMV3-Hap074-eGFP | This paper | pUP894 |
| pFLAG-CMV3-Hap074-mRuby2 | This paper | pUP752 |
| pFLAG-CMV3-214E06-N32Y-eGFP | This paper | pUP838 |
| pFLAG-CMV3-214E06-N32Y-mRuby2 | This paper | pUP839 |
| pFLAG-CMV3-Hap074-G47E-eGFP | This paper | pUP875 |
| pFLAG-CMV3-Hap074-G47E-mRuby2 | This paper | pUP876 |
| pFLAG-CMV3-Hap074-S44G-eGFP | This paper | pUP878 |
| pFLAG-CMV3-Hap074-S44G-mRuby2 | This paper | pUP877 |
| pFLAG-CMV3-Anc-T76R-eGFP | This paper | pUP866 |
| pFLAG-CMV3-Anc-T76R-mRuby2 | This paper | pUP865 |
| pFLAG-CMV3-Anc-E93K-eGFP | This paper | pUP879 |
| pFLAG-CMV3-Anc-E93K-mRuby2 | This paper | pUP880 |
| pFLAG-CMV3-046B-N32Y-eGFP | This paper | pUP892 |
| pFLAG-CMV3-046B-N32Y-mRuby2 | This paper | pUP794 |
| pFLAG-CMV3-Hap074-N32Y-eGFP | This paper | pUP893 |
| pFLAG-CMV3-Hap074-N32Y-mRuby2 | This paper | pUP795 |
| Software and algorithms | ||
| I-TASSER v5.1 | Roy et al. (2010); Yang et al. (2015); Zhang (2008) | https://zhanglab.ccmb.med.umich.edu/I-TASSER/download/ |
| Multialign Viewer (Chimera v1.14) | USCF Chimera Meng et al., 2006; Pettersen et al. (2004) | NA |
| MEME (HyPhy 2.2.4) | Murrell et al., 2012 | http://hyphy.org/w/index.php/Download |
| FEL (HyPhy 2.2.4) | Pond and Frost (2005) | http://hyphy.org/w/index.php/Download |
| ImageJ v1.53a | Abràmoff et al., 2004; Schneider et al. (2012) | https://imagej.nih.gov/ij/ |
| Other | ||
| TransIT-293 | Mirus Bio | Cat#MIR 2700 |
| 35μm strainer mesh | Steller scientific | Cat#FSC-FLTCP |
| DNase I | Sigma | Cat#D4527-10KU |
| 24-well ultra-low attachment plate | Fisher Scientific | Cat#07-200-602 |
| Orbital rotator | IBI Scientific | Model# BBUAAUVIS |
| NEBuilder HiFi DNA Assembly | New England Biolabs | Cat#E2621S |
| Hap200 | Genbank | JX048906.1 |
| Hap199 | Genbank | JX048905.1 |
| Hap197 | Genbank | JX048904.1 |
| Hap196 | Genbank | JX048903.1 |
| Hap195 | Genbank | JX048902.1 |
| Hap194 | Genbank | JX048901.1 |
| Hap193 | Genbank | JX048900.1 |
| Hap192 | Genbank | JX048899.1 |
| Hap191 | Genbank | JX048898.1 |
| Hap190 | Genbank | JX048897.1 |
| Hap189 | Genbank | JX048896.1 |
| Hap188 | Genbank | JX048895.1 |
| Hap187 | Genbank | JX048894.1 |
| Hap186 | Genbank | JX048893.1 |
| Hap185 | Genbank | JX048892.1 |
| Hap184 | Genbank | JX048891.1 |
| Hap183 | Genbank | JX048890.1 |
| Hap182 | Genbank | JX048889.1 |
| Hap181 | Genbank | JX048888.1 |
| Hap180 | Genbank | JX048887.1 |
| Hap179 | Genbank | JX048886.1 |
| Hap178 | Genbank | JX048885.1 |
| Hap176 | Genbank | JX048884.1 |
| Hap175 | Genbank | JX048883.1 |
| Hap173 | Genbank | JX048881.1 |
| Hap172 | Genbank | JX048880.1 |
| Hap171 | Genbank | JX048879.1 |
| Hap169 | Genbank | JX048877.1 |
| Hap168 | Genbank | JX048876.1 |
| Hap167 | Genbank | JX048875.1 |
| Hap166 | Genbank | JX048874.1 |
| Hap165 | Genbank | JX048873.1 |
| Hap164 | Genbank | JX048872.1 |
| Hap163 | Genbank | JX048871.1 |
| Hap161 | Genbank | JX048869.1 |
| Hap159 | Genbank | JX048867.1 |
| Hap158 | Genbank | JX048866.1 |
| Hap157 | Genbank | JX048865.1 |
| Hap156 | Genbank | JX048864.1 |
| Hap155 | Genbank | JX048863.1 |
| Hap154 | Genbank | JX048862.1 |
| Hap153 | Genbank | JX048861.1 |
| Hap152 | Genbank | JX048860.1 |
| Hap151 | Genbank | JX048859.1 |
| Hap150 | Genbank | JX048858.1 |
| Hap149 | Genbank | JX048857.1 |
| Hap148 | Genbank | JX048856.1 |
| Hap147 | Genbank | JX048855.1 |
| Hap146 | Genbank | JX048854.1 |
| Hap145 | Genbank | JX048853.1 |
| Hap144 | Genbank | JX048852.1 |
| Hap143 | Genbank | JX048851.1 |
| Hap142 | Genbank | JX048850.1 |
| Hap141 | Genbank | JX048849.1 |
| Hap139 | Genbank | JX048847.1 |
| Hap138 | Genbank | JX048846.1 |
| Hap137 | Genbank | JX048845.1 |
| Hap136 | Genbank | JX048844.1 |
| Hap135 | Genbank | JX048843.1 |
| Hap134 | Genbank | JX048842.1 |
| Hap133 | Genbank | JX048841.1 |
| Hap132 | Genbank | JX048840.1 |
| Hap131 | Genbank | JX048839.1 |
| Hap130 | Genbank | JX048838.1 |
| Hap129 | Genbank | JX048837.1 |
| Hap128 | Genbank | JX048836.1 |
| Hap127 | Genbank | JX048835.1 |
| Hap126 | Genbank | JX048834.1 |
| Hap125 | Genbank | JX048833.1 |
| Hap124 | Genbank | JX048832.1 |
| Hap123 | Genbank | JX048831.1 |
| Hap122 | Genbank | JX048830.1 |
| Hap121 | Genbank | JX048829.1 |
| Hap120 | Genbank | JX048828.1 |
| Hap119 | Genbank | JX048827.1 |
| Hap118 | Genbank | JX048826.1 |
| Hap117 | Genbank | JX048825.1 |
| Hap116 | Genbank | JX048824.1 |
| Hap115 | Genbank | JX048823.1 |
| Hap114 | Genbank | JX048822.1 |
| Hap113 | Genbank | JX048821.1 |
| Hap112 | Genbank | JX048820.1 |
| Hap111 | Genbank | JX048819.1 |
| Hap110 | Genbank | JX048818.1 |
| Hap109 | Genbank | JX048817.1 |
| Hap108 | Genbank | JX048816.1 |
| Hap107 | Genbank | JX048815.1 |
| Hap106 | Genbank | JX048814.1 |
| Hap105 | Genbank | JX048813.1 |
| Hap104 | Genbank | JX048812.1 |
| Hap103 | Genbank | JX048811.1 |
| Hap102 | Genbank | JX048810.1 |
| Hap101 | Genbank | JX048809.1 |
| Hap100 | Genbank | JX048808.1 |
| Hap099 | Genbank | JX048807.1 |
| Hap098 | Genbank | JX048806.1 |
| Hap097 | Genbank | JX048805.1 |
| Hap096 | Genbank | JX048804.1 |
| Hap095 | Genbank | JX048803.1 |
| Hap094 | Genbank | JX048802.1 |
| Hap093 | Genbank | JX048801.1 |
| Hap092 | Genbank | JX048800.1 |
| Hap091 | Genbank | JX048799.1 |
| Hap090 | Genbank | JX048798.1 |
| Hap089 | Genbank | JX048797.1 |
| Hap088 | Genbank | JX048796.1 |
| Hap087 | Genbank | JX048795.1 |
| Hap086 | Genbank | JX048794.1 |
| Hap085 | Genbank | JX048793.1 |
| Hap084 | Genbank | JX048792.1 |
| Hap083 | Genbank | JX048791.1 |
| Hap082 | Genbank | JX048790.1 |
| Hap081 | Genbank | JX048789.1 |
| Hap080 | Genbank | JX048788.1 |
| Hap079 | Genbank | JX048787.1 |
| Hap078 | Genbank | JX048786.1 |
| Hap077 | Genbank | JX048785.1 |
| Hap076 | Genbank | JX048784.1 |
| Hap075 | Genbank | JX048783.1 |
| Hap074 | Genbank | JX048782.1 |
| Hap073 | Genbank | JX048781.1 |
| Hap072 | Genbank | JX048780.1 |
| Hap071 | Genbank | JX048779.1 |
| Hap070 | Genbank | JX048778.1 |
| Hap069 | Genbank | JX048777.1 |
| Hap068 | Genbank | JX048776.1 |
| Hap067 | Genbank | JX048775.1 |
| Hap066 | Genbank | JX048774.1 |
| Hap065 | Genbank | JX048773.1 |
| Hap064 | Genbank | JX048772.1 |
| Hap063 | Genbank | JX048771.1 |
| Hap062 | Genbank | JX048770.1 |
| Hap061 | Genbank | JX048769.1 |
| Hap060 | Genbank | JX048768.1 |
| Hap058 | Genbank | JX048766.1 |
| Hap057 | Genbank | JX048765.1 |
| Hap056 | Genbank | JX048764.1 |
| Hap054 | Genbank | JX048762.1 |
| Hap051 | Genbank | JX048759.1 |
| Hap048 | Genbank | JX048756.1 |
| Hap046 | Genbank | JX048754.1 |
| Hap045 | Genbank | JX048753.1 |
| Hap044 | Genbank | JX048752.1 |
| Hap043 | Genbank | JX048751.1 |
| Hap042 | Genbank | JX048750.1 |
| Hap041 | Genbank | JX048749.1 |
| Hap040 | Genbank | JX048748.1 |
| Hap039 | Genbank | JX048747.1 |
| Hap038 | Genbank | JX048746.1 |
| Hap037 | Genbank | JX048745.1 |
| Hap036 | Genbank | JX048744.1 |
| Hap035 | Genbank | JX048743.1 |
| Hap034 | Genbank | JX048742.1 |
| Hap033 | Genbank | JX048741.1 |
| Hap032 | Genbank | JX048740.1 |
| Hap031 | Genbank | JX048739.1 |
| Hap030 | Genbank | JX048738.1 |
| Hap029 | Genbank | JX048737.1 |
| Hap028 | Genbank | JX048736.1 |
| Hap027 | Genbank | JX048735.1 |
| Hap026 | Genbank | JX048734.1 |
| Hap025 | Genbank | JX048733.1 |
| Hap024 | Genbank | JX048732.1 |
| Hap022 | Genbank | JX048730.1 |
| Hap021 | Genbank | JX048729.1 |
| Hap020 | Genbank | JX048728.1 |
| Hap019 | Genbank | JX048727.1 |
| Hap018 | Genbank | JX048726.1 |
| Hap017 | Genbank | JX048725.1 |
| Hap016 | Genbank | JX048724.1 |
| Hap015 | Genbank | JX048723.1 |
| Hap014 | Genbank | JX048722.1 |
| Hap012 | Genbank | JX048720.1 |
| Hap010 | Genbank | JX048718.1 |
| Hap008 | Genbank | JX048716.1 |
| Hap007 | Genbank | JX048715.1 |
| Hap006 | Genbank | JX048714.1 |
| Hap005 | Genbank | JX048713.1 |
| Hap004 | Genbank | JX048712.1 |
| Hap003 | Genbank | JX048711.1 |
| Hap002 | Genbank | JX048710.1 |
| Hap001 | Genbank | JX048709.1 |
| Hap174 | Genbank | JX048882.1 |
| Hap162 | Genbank | JX048870.1 |
| Hap160 | Genbank | JX048868.1 |
| Hap050 | Genbank | JX048758.1 |
| Hap013 | Genbank | JX048721.1 |
| Hap009 | Genbank | JX048717.1 |
| LH09_466G04 | Genbank | JX049024.1 |
| LH09_466B06 | Genbank | JX049023.1 |
| LH09_465F03 | Genbank | JX049022.1 |
| LH09_465B09 | Genbank | JX049021.1 |
| LH09_459C06 | Genbank | JX049020.1 |
| LH09_459C03 | Genbank | JX049019.1 |
| LH09_454D03 | Genbank | JX049018.1 |
| LH09_452H02 | Genbank | JX049017.1 |
| LH09_449H03 | Genbank | JX049016.1 |
| LH09_449F06 | Genbank | JX049015.1 |
| LH09_447F08 | Genbank | JX049014.1 |
| LH09_447D09 | Genbank | JX049013.1 |
| LH09_443D04 | Genbank | JX049012.1 |
| LH09_443B07 | Genbank | JX049011.1 |
| LH09_436B04 | Genbank | JX049010.1 |
| LH09_435B06 | Genbank | JX049009.1 |
| LH09_435B05 | Genbank | JX049008.1 |
| LH09_431F06 | Genbank | JX049007.1 |
| LH09_431C08 | Genbank | JX049006.1 |
| LH09_429C03 | Genbank | JX049005.1 |
| LH09_429A03 | Genbank | JX049004.1 |
| LH09_425B08 | Genbank | JX049003.1 |
| LH09_425B07 | Genbank | JX049002.1 |
| LH09_417C08 | Genbank | JX049001.1 |
| LH09_417B05 | Genbank | JX049000.1 |
| LH09_406B01 | Genbank | JX048999.1 |
| LH09_396C10 | Genbank | JX048998.1 |
| LH09_396B06 | Genbank | JX048997.1 |
| LH09_396A08 | Genbank | JX048996.1 |
| LH09_395G03 | Genbank | JX048995.1 |
| LH09_386C08 | Genbank | JX048994.1 |
| LH09_384F06 | Genbank | JX048993.1 |
| LH09_384E03 | Genbank | JX048992.1 |
| LH09_380B02 | Genbank | JX048991.1 |
| LH09_380A03 | Genbank | JX048990.1 |
| LH09_274C02 | Genbank | JX048989.1 |
| LH09_271B05 | Genbank | JX048988.1 |
| LH09_270D09 | Genbank | JX048987.1 |
| LH09_270C02 | Genbank | JX048986.1 |
| LH09_268E09 | Genbank | JX048985.1 |
| LH09_268B05 | Genbank | JX048984.1 |
| LH09_265F08 | Genbank | JX048983.1 |
| LH09_265B10 | Genbank | JX048982.1 |
| LH09_261C01 | Genbank | JX048981.1 |
| LH09_249C04 | Genbank | JX048980.1 |
| LH09_249B04 | Genbank | JX048979.1 |
| LH09_248A06 | Genbank | JX048978.1 |
| LH09_244H05 | Genbank | JX048977.1 |
| LH09_244B05 | Genbank | JX048976.1 |
| LH09_230G03 | Genbank | JX048975.1 |
| LH09_230F04 | Genbank | JX048974.1 |
| LH09_230B04 | Genbank | JX048973.1 |
| LH09_214H10 | Genbank | JX048972.1 |
| LH09_214E06 | Genbank | JX048971.1 |
| LH09_212C05 | Genbank | JX048970.1 |
| LH09_212B03 | Genbank | JX048969.1 |
| LH09_205E03 | Genbank | JX048968.1 |
| LH09_205C02 | Genbank | JX048967.1 |
| LH09_202E04 | Genbank | JX048966.1 |
| LH09_162G06 | Genbank | JX048965.1 |
| LH09_162D02 | Genbank | JX048964.1 |
| LH09_158D11 | Genbank | JX048963.1 |
| LH09_158A08 | Genbank | JX048962.1 |
| LH09_158A03 | Genbank | JX048961.1 |
| LH09_145D12 | Genbank | JX048960.1 |
| LH09_116B02 | Genbank | JX048959.1 |
| LH09_111C09 | Genbank | JX048958.1 |
| LH09_111A06 | Genbank | JX048957.1 |
| LH09_110C01 | Genbank | JX048956.1 |
| LH09_110B01 | Genbank | JX048955.1 |
| LH09_085A06 | Genbank | JX048954.1 |
| LH09_084B07 | Genbank | JX048953.1 |
| LH09_083D05 | Genbank | JX048952.1 |
| LH09_083C10 | Genbank | JX048951.1 |
| LH09_082F03 | Genbank | JX048950.1 |
| LH09_082D07 | Genbank | JX048949.1 |
| LH09_078E08 | Genbank | JX048948.1 |
| LH09_068F07 | Genbank | JX048947.1 |
| LH09_068B01 | Genbank | JX048946.1 |
| LH09_064D04 | Genbank | JX048945.1 |
| LH09_064C05 | Genbank | JX048944.1 |
| LH09_061G09 | Genbank | JX048943.1 |
| LH09_061G06 | Genbank | JX048942.1 |
| LH09_059C03 | Genbank | JX048941.1 |
| LH09_058G02 | Genbank | JX048940.1 |
| LH09_058C05 | Genbank | JX048939.1 |
| LH09_055H01 | Genbank | JX048938.1 |
| LH09_055A07 | Genbank | JX048937.1 |
| LH09_054E03 | Genbank | JX048936.1 |
| LH09_054D05 | Genbank | JX048935.1 |
| LH09_052D03 | Genbank | JX048934.1 |
| LH09_052C02 | Genbank | JX048933.1 |
| LH09_051E03 | Genbank | JX048932.1 |
| LH09_051B03 | Genbank | JX048931.1 |
| LH09_048E02 | Genbank | JX048930.1 |
| LH09_044F06 | Genbank | JX048929.1 |
| LH09_044C10 | Genbank | JX048928.1 |
| LH09_042B02 | Genbank | JX048927.1 |
| LH09_042A05 | Genbank | JX048926.1 |
| LH09_039G03 | Genbank | JX048925.1 |
| LH09_037B08 | Genbank | JX048924.1 |
| LH09_037A08 | Genbank | JX048923.1 |
| LH09_035C05 | Genbank | JX048922.1 |
| LH09_034H09 | Genbank | JX048921.1 |
| LH09_032F03 | Genbank | JX048920.1 |
| LH09_032E02 | Genbank | JX048919.1 |
| LH09_024B01 | Genbank | JX048918.1 |
| LH09_023H02 | Genbank | JX048917.1 |
| LH09_023E08 | Genbank | JX048916.1 |
| LH09_019D10 | Genbank | JX048915.1 |
| LH09_018D08 | Genbank | JX048914.1 |
| LH09_018B08 | Genbank | JX048913.1 |
| LH09_005G06 | Genbank | JX048912.1 |
| LH09_005E05 | Genbank | JX048911.1 |
| LH09_004E06 | Genbank | JX048910.1 |
| LH09_004B06 | Genbank | JX048909.1 |
| LH09_001E02 | Genbank | JX048908.1 |
| 14_7F | Genbank | JX048907.1 |
| OQ-6Db | Genbank | HM013632.1 |
| OQ-6Da | Genbank | HM013631.1 |
| LH07:060b | Genbank | HM013630.1 |
| LH07:060a | Genbank | HM013629.1 |
| LH07:049b | Genbank | HM013628.1 |
| LH07:049a | Genbank | HM013627.1 |
| LH07:046b | Genbank | HM013626.1 |
| LH07:046a | Genbank | HM013625.1 |
| LH07:043a | Genbank | HM013624.1 |
| LH07:041b | Genbank | HM013623.1 |
| LH07:041a | Genbank | HM013622.1 |
| LH07:037b | Genbank | HM013621.1 |
| LH07:037a | Genbank | HM013620.1 |
| LH07:036b | Genbank | HM013619.1 |
| LH07:036a | Genbank | HM013618.1 |
| LH07:026b | Genbank | HM013617.1 |
| LH07:026a | Genbank | HM013616.1 |
| LH06:050b | Genbank | HM013613.1 |
| LH06:049b | Genbank | HM013611.1 |
| LH06:028b | Genbank | HM013609.1 |
| LH06:028a | Genbank | HM013608.1 |
| LH06:003b | Genbank | HM013607.1 |
| alr2-W60b | Genbank | FJ207419.1 |
| alr2-W60a | Genbank | FJ207418.1 |
| alr2-W49b | Genbank | FJ207417.1 |
| alr2-W49a | Genbank | FJ207416.1 |
| alr2-W41b | Genbank | FJ207415.1 |
| alr2-W41a | Genbank | FJ207414.1 |
| alr2-W36b | Genbank | FJ207413.1 |
| alr2-W36a | Genbank | FJ207412.1 |
| alr2-W14b | Genbank | FJ207411.1 |
| alr2-W14a | Genbank | FJ207410.1 |
| alr2-LH49b | Genbank | FJ207402.1 |
| alr2-LH49a | Genbank | FJ207401.1 |
| alr2-LH03i | Genbank | FJ207396.1 |
| alr2-LH03a | Genbank | FJ207395.1 |
| LH07:014b | Genbank | HM013615.1 |
| LH07:014a | Genbank | HM013614.1 |
| LH06:050a | Genbank | HM013612.1 |
| LH06:049a | Genbank | HM013610.1 |
| LH06:003a | Genbank | HM013606.1 |
| alr2-LH53b | Genbank | FJ617568.1 |
| alr2-LH53a | Genbank | FJ617567.1 |
| alr2-LH04b | Genbank | FJ617566.1 |
| alr2-LH04a | Genbank | FJ617565.1 |
| alr2-R | Genbank | FJ207409.1 |
| alr2-LH82b | Genbank | FJ207408.1 |
| alr2-LH82a | Genbank | FJ207407.1 |
| alr2-LH58b | Genbank | FJ207406.1 |
| alr2-LH58a | Genbank | FJ207405.1 |
| alr2-LH57b | Genbank | FJ207404.1 |
| alr2-LH57a | Genbank | FJ207403.1 |
| alr2-LH09b | Genbank | FJ207400.1 |
| alr2-LH09a | Genbank | FJ207399.1 |
| alr2-LH08b | Genbank | FJ207398.1 |
| alr2-LH08a | Genbank | FJ207397.1 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Matthew Nicotra (matthew.nicotra@pitt.edu).
Materials availability
This study did not generate new reagents. Plasmids generated in this study are available from the Lead Contact upon request.
Data and code availability
This paper analyzes existing, publicly available data. These accession numbers for the datasets are listed in the key resources table. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
HEK293T cells (ATCC Cat# CRL-3216) were cultured at 37°C with 5% CO2 in accordance with ATCC guidelines. Complete HEK culture medium was made using DMEM (Fisher Science, SH30081.01), 10% fetal bovine serum (Thermofisher Scientific, #16000044), 0.001% beta-mercaptoethanol (Fisher Scientific, 21-985-023), 100 U/mL penicillin and 100 mg/mL streptomycin (Sigma, P4333-100ML).
Method details
Alr2 sequence acquisition and processing
Alr2 alleles 111A06 and 214E06 were identified from previously published Alr2 sequences (Gloria-Soria et al., 2012). To obtain a dataset of Alr2 domain 1 sequences, we downloaded all 373 Hydractinia symbiolongicarpus Alr2 cDNA sequences from GenBank, aligned them with MAFFT (Katoh et al., 2005) as implemented in Jalview 2.10.5 (Waterhouse et al., 2009), then trimmed the alignment leaving only the region encoding domain 1. Duplicate sequences were then removed with ElimDupes (www.hiv.lanl.gov), to yield 146 distinct domain 1 cDNA sequences, encoding 137 distinct amino acid sequences.
Phylogenetic analysis and ancestral state reconstruction
The 146 domain 1 cDNA sequences were aligned with PRANK (Löytynoja, 2014), a codon-aware alignment program (File S1). The alignment was then used to construct a phylogenetic tree using maximum likelihood through IQ-TREE (http://iqtree.cibiv.univie.ac.at/) (Trifinopoulos et al., 2016) (File S2). From the web portal, the defaults settings were used with codon selected for the sequence type, standard/universal genetic code, ultrafast bootstrap analysis with a maximum of 1000 alignments, 0.99 minimum correlation coefficient, 1000 replicates of SH-aLRT branch test, 0.5 perturbation strength, and 100 set for the IQ-TREE stopping rule. Ancestral states were estimated using the phylogenetic tree generated from IQ-TREE and the ancestral reconstruction function within PRANK (File S3) (Dutheil and Boussau, 2008; Löytynoja, 2014). An unrooted tree was generated using iTOL v5.5.1 with one iteration of equal-daylight (Letunic and Bork, 2019).
Constructs for ectopic expression of Alr2 alleles
The plasmid backbone used for all constructs in this study was the pFLAG-CMV-3 (Sigma, E6783). Previously, it was determined that the N-terminal FLAG tag did not have an effect on the binding capability of Alr2 (Karadge et al., 2015). The Hydractinia Alr2 allele sequences were optimized for human expression using the Integrated DNA Technologies (IDT) Codon Optimization Tool (https://www.idtdna.com/CodonOpt). The full Alr2 sequence (domain 1 in the ectodomain through the cytoplasmic tail) for 111A06 and domain 1 sequences for Anc, 046B, Hap074, and 214 × 106 were ordered as gBlocks Gene Fragments from IDT. All other mutant domain sequences were ordered from Twist Bioscience as Gene Fragments. Coding sequences for fluorescent proteins were cloned from vectors encoding eGFP and mRuby2 (gift from Michael Davidson, Addgene plasmid #54614 (Lam et al., 2012)). Cloning was performed using the NEBuilder HiFi DNA Assembly (New England Biolabs, E2621S) with primers designed to amplify the vector and insert sequences with ≥20 bp overlap. The FLAG-111A06-eGFP/mRuby2 plasmids (pUP801, pUP746) were cloned first and then used as the template for cloning in the other domain 1 isoforms. Within the construct, linker sequences were used before (Leu-Ala-Ala-Ala) and after (Gly-Pro-Pro-Val-Glu-Lys) the Alr2 allele.
Expression of Alr2 alleles in mammalian cells
To prepare plasmids for transfection, plasmids were transformed into chemically competent bacteria and isolated from cultures using the GeneJET Plasmid Midi-prep Kit (Thermofisher Scientific, K0481) or the PureLink HiPure Plasmid Maxiprep Kit (Thermofisher Scientific, K2100006). Plasmids were transiently transfected into HEK293T cells using TransIT-293 (Mirus Bio, MIR 2700) according to the manufacturer's instructions. To summarize, on day 1, HEK293T cells were plated in a 12-well plate (Fisher Scientific, #353043) at a density of 3x105/well in 1 mL of complete HEK medium to achieve approximately 60-70% confluency on Day 2. On Day 2, the transfection mixture was prepared in a total volume of 100 μL using 1 ug (X μl) of plasmid DNA (plasmid concentrations between 300ng and 1000ng/μl), diluted with optiMEM (Gibco, #31985-070) (97-X μl), and 3 μL of TransIT-293 reagent. While incubating the DNA:lipid complexes, the cells were washed using 500 μL of DPBS (Fisher Scientific, BW17-512F), incubated with 1 mL transfection medium (complete HEK medium without antibiotics), and replaced in the 5% CO2 incubator. Once the DNA:lipid complexes had incubated, the 100 μL mixture was added to the appropriate well, the plate gently shaken back and forth and then replaced in the incubator. On Day 4, cells were used in the aggregation assay.
Aggregation assay
Our aggregation protocol is adapted from previous work (Karadge et al., 2015). To summarize, previously transfected HEK293T cells were incubated with 0.25% Trypsin/0.1% EDTA solution (Corning, MT25053CI), washed in complete HEK culture medium, mechanically disrupted via pipette, and filtered through a 35μm strainer mesh (Steller scientific, FSC-FLTCP) to create a single cell suspension. For each aggregation assay, a total of 5x104 cells were resuspended in 500 μL aggregation assay medium (complete HEK medium, 70 U/ml DNase I [Sigma, D4527-10KU], and 2 mM EGTA [Goldbio, E−217-25]) and added to one well of a 24-well ultra-low attachment plate (Fisher Scientific, 07-200-602). When testing isoforms pairwise, 2.5x104 cells of each transfection were added to the same well and resuspended in a total of 500 μL. The plate was incubated for one hour at 37°C in 5% CO2 on an orbital rotator (IBI Scientific, Model# BBUAAUVIS) set at 90 rpm. Assays were visualized using an inverted fluorescence microscope (Nikon Eclipse TS100). Each pairwise assay was repeated at least three times. In cases when the assay results could not be viewed immediately, cell aggregates were fixed by adding 500 μL of 8% paraformaldehyde (Fisher, AA433689M) diluted in DPBS to each well and the results imaged within 5 h. All images and merged images were processed using ImageJ (Abràmoff et al., 2004; Schneider et al., 2012).
Sequence variability and visualization of domain 1
The structure for the Anc domain 1 isoform was predicted using I-TASSER v5.1 (Zhang, 2008; Roy et al., 2010; Yang et al., 2015) which resulted in a domain with a V-set like fold. To visualize the variable positions within domain 1, the aligned 137 protein sequences were uploaded to the Multialign Viewer in UCSF Chimera (Pettersen et al., 2004; Meng et al., 2006) and the conservation rendered onto the structure. Sites under positive selection were identified using MEME (Murrell et al., 2012) and FEL (Pond and Frost, 2005) as implemented in HyPhy 2.5.8 (Pond et al., 2019). Both algorithms were run using synonymous rate variation and significance threshold of p = 0.1, as recommended by the developers (Spielman et al., 2019).
Quantification and statistical analysis
Sites under positive and/or negative selection were identified using statistical tests as implemented in MEME and FEL, at significance thresholds of p = 0.1. No other quantification or statistical analyses were performed in this study.
Acknowledgments
Molecular graphics and analyses were performed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311. We thank Kristina Paris for assistance and discussion with running molecular dynamics for the models. M.N. was supported by NSF grant IOS-1557339. A.H. was supported by NIH T32 AI074491.
Author contributions
A.H. and T.C. performed the experiments. A.H. did the data analysis and structural visualizations. A.H. and M.N. designed the experiments and wrote the paper.
Declaration of interests
The authors declare no competing interests.
Published: July 23, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102811.
Supplemental information
References
- Aanen D.K., Debets A.J.M., Visser J.A.G.M. De, Hoekstra R.F. The social evolution of somatic fusion. Bioessays. 2008;30:1193–1203. doi: 10.1002/bies.20840. [DOI] [PubMed] [Google Scholar]
- Abràmoff M.D., Magalhães P.J., Ram S.J. Image processing with ImageJ. Biophotonics Int. 2004;7:36–42. [Google Scholar]
- Buss L.W. Princeton University Press; 1987. The Evolution of Individuality. [Google Scholar]
- Cadavid L.F., Powell A.E., Nicotra M.L., Moreno M., Buss L.W. An invertebrate histocompatibility complex. Genetics. 2004;167:357–365. doi: 10.1534/genetics.167.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P., Wei X., Awal P., Müller R. A highly polymorphic receptor governs many distinct self-recognition types within the Myxococcales order. MBio. 2019;10:1–15. doi: 10.1128/mBio.02751-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casselton L.A., Olesnicky N.S. Molecular genetics of mating recognition in Basidiomycete fungi. Microbiol. Mol. Biol. Rev. 1998;62:55–70. doi: 10.1128/mmbr.62.1.55-70.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc T.Q., Schnitzler C.E., Chrysostomou E., Mcmahon E.T., Gahan J.M., Buggie T., Gornik S.G., Hanley S., Barreira S.N., Gonzalez P. Transcription factor AP2 controls cnidarian germ cell induction. Science. 2020;367:757–762. doi: 10.1126/science.aay6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutheil J., Boussau B. 2008. Of Libraries and Programs 12; pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank U., Nicotra M.L., Schnitzler C.E. The colonial cnidarian Hydractinia. Evodevo. 2020;11:7–12. doi: 10.1186/s13227-020-00151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Kubo K., Takayama S. Non-self- and self-recognition models in plant self-incompatibility. Nat. Plants. 2016;2:1–9. doi: 10.1038/NPLANTS.2016.130. [DOI] [PubMed] [Google Scholar]
- Gibbs K.A., Greenberg E.P. Territoriality in proteus: advertisement and aggression. Chem. Rev. 2011;111:188–194. doi: 10.1021/cr100051v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloria-Soria A., Moreno M.A., Yund P.O., Lakkis F.G., Dellaporta S.L., Buss L.W. Evolutionary genetics of the hydroid allodeterminant alr2. Mol. Biol. Evol. 2012;29:3921–3932. doi: 10.1093/molbev/mss197. [DOI] [PubMed] [Google Scholar]
- Gonçalves A.P., Heller J., Rico-ramírez A.M., Daskalov A., Rosenfield G., Glass N.L. conflict, competition, and cooperation regulate social interactions in Filamentous fungi. Annu. Rev. Microbiol. 2020;74:693–712. doi: 10.1146/annurev-micro-012420-080905. [DOI] [PubMed] [Google Scholar]
- Goncalves A.P., Heller J., Span E.A., Rosenfiled G., Do H.P., Palma-Guerrero J., Requena N., Marletta M.A., Glass N.L. Allorecognition upon fungal cell-cell contact determines social cooperation and impacts the acquisition of multicellularity article allorecognition upon fungal cell-cell contact determines social cooperation. Curr. Biol. 2019;29:3006–3017. doi: 10.1016/j.cub.2019.07.060. [DOI] [PubMed] [Google Scholar]
- Goodman K.M., Yamagata M., Jin X., Mannepalli S., Katsamba P.S., Ahlsen G., Sergeeva A.P., Honig B., Sanes J.R., Shapiro L. Molecular basis of sidekick-mediated cell-cell adhesion and specificity. Elife. 2016;5:1–21. doi: 10.7554/eLife.19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James T.Y. Why mushrooms have evolved to be so promiscuous: insights from evolutionary and ecological patterns. Fungal Biol. Rev. 2015;29:167–178. doi: 10.1016/j.fbr.2015.10.002. [DOI] [Google Scholar]
- Karadge U.B., Gosto M., Nicotra M.L. Allorecognition proteins in an invertebrate exhibit homophilic interactions. Curr. Biol. 2015;25:2845–2850. doi: 10.1016/j.cub.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinrerk W., Tokrasinwit N., Phunpae P. CD147 monoclonal antibodies induce homotypic cell aggregation of monocytic cell line U937 via LFA-1/ICAM-1 pathway. Immunology. 1999;96:184–192. doi: 10.1046/j.1365-2567.1999.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Kuma K., Toh H., Miyata T. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsamba P., Carroll K., Ahlsen G., Bahna F., Vendome J., Posy S., Rajebhosale M., Price S., Jessell T.M., Ben-Shaul A. Linking molecular affinity and cellular specificity in cadherin-mediated adhesion. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11594–11599. doi: 10.1073/pnas.0905349106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Crow J.F. The number of alleles that can be maintained in a finite population. Genetics. 1964;49:725–738. doi: 10.1093/genetics/49.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundert P., Shaulsky G. Cellular allorecognition and its roles in Dictyostelium development and social evolution. Int. J. Dev. Biol. 2019;393:383–393. doi: 10.1387/ijdb.190239gs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzel T., Heiermann R., Frank U., Müller W., Tilmann W., Bause M., Nonn A., Helling M., Schwarz R.S., Plickert G. Migration and differentiation potential of stem cells in the cnidarian Hydractinia analysed in eGFP-transgenic animals and chimeras. Dev. Biol. 2010;348:120–129. doi: 10.1016/j.ydbio.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Laird D.J., De Tomaso A.W., Weissman I.L. Stem cells are units of natural selection in a colonial ascidian. Cell. 2005;123:1351–1360. doi: 10.1016/j.cell.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Lam A.J., St-pierre F., Gong Y., Marshall J.D., Cranfill P.J., Baird M.A., Mckeown M.R., Wiedenmann J., Davidson M.W., Schnitzer M.J. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods. 2012;9:1005–1012. doi: 10.1038/NMETH.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M.J. Population genetics of the homomorphic self-incompatibility polymorphisms in flowering plants. Ann. Bot. 2000;85:221–226. [Google Scholar]
- Letunic I., Bork P. Interactive Tree of Life (iTOL) v4: recent updates and. Nucleic Acids Res. 2019;47:256–259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löytynoja A. Phylogeny-aware alignment with PRANK. Methods Mol. Biol. 2014;1079:155—170. doi: 10.1007/978-1-62703-646-7_10. [DOI] [PubMed] [Google Scholar]
- Meng E.C., Pettersen E.F., Couch G.S., Huang C.C., Ferrin T.E. Tools for integrated sequence-structure analysis with UCSF Chimera. BMC Bioinformatics. 2006;7:1–10. doi: 10.1186/1471-2105-7-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell B., Wertheim J.O., Moola S., Weighill T., Scheffler K., Pond S.L.K. Detecting Individual sites subject to episodic diversifying selection. PLoS Genet. 2012;8:1–10. doi: 10.1371/journal.pgen.1002764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra M.L. Invertebrate allorecognition. Curr. Biol. 2019;29:R463–R467. doi: 10.1016/j.cub.2019.03.039. [DOI] [PubMed] [Google Scholar]
- Nicotra M.L., Buss L.W. A test for larval kin aggregations. Biol. Bull. 2005;208:157–158. doi: 10.2307/3593147. [DOI] [PubMed] [Google Scholar]
- Nicotra M.L., Powell A.E., Rosengarten R.D., Moreno M., Grimwood J., Lakkis F.G., Dellaporta S.L., Buss L.W. A hypervariable invertebrate allodeterminant. Curr. Biol. 2009;19:583–589. doi: 10.1016/j.cub.2009.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nydam M.L., Stephenson E.E., Waldman C.E., Tomaso A.W. De. Balancing selection on allorecognition genes in the colonial ascidian Botryllus schlosseri. Dev. Comp. Immunol. 2017;69:60–74. doi: 10.1016/j.dci.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Paoletti M. Vegetative incompatibility in fungi: from recognition to cell death, whatever does the trick. Fungal Biol. Rev. 2016;30:152–162. doi: 10.1016/j.fbr.2016.08.002. [DOI] [Google Scholar]
- Pathak D.T., Wei X., Dey A., Wall D. Molecular recognition by a polymorphic cell surface receptor governs cooperative behaviors in bacteria. PLoS Genet. 2013;9:1–12. doi: 10.1371/journal.pgen.1003891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera — a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Podgornaia A.I., Laub M.T. Pervasive degeneracy and epistasis in a protein-protein interface. Science. 2015;347:673–677. doi: 10.1126/science.1257360. [DOI] [PubMed] [Google Scholar]
- Pond S.L.K., Frost S.D.W. Not so different after all: a comparison of methods for detecting amino acid sites under selection. Mol. Biol. Evol. 2005;22:1208–1222. doi: 10.1093/molbev/msi105. [DOI] [PubMed] [Google Scholar]
- Pond S.L.K., Poon A.F.Y., Velazquez R., Weaver S., Hepler N.L., Murrell B., Shank S.D., Magalis B.R., Bouvier D., Nekrutenko A. HyPhy 2.5 — a Customizable platform for evolutionary hypothesis testing using Phylogenies. Mol. Biol. Evol. 2019;37:295–299. doi: 10.1093/molbev/msz197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povolotskaya I.S., Kondrashov F.A. Sequence space and the ongoing expansion of the protein universe. Nature. 2010;465:922–926. doi: 10.1038/nature09105. [DOI] [PubMed] [Google Scholar]
- Powell A.E., Moreno M., Gloria-soria A., Lakkis F.G., Dellaporta S.L., Buss L.W. Genetic background and allorecognition phenotype in Hydractinia symbiolongicarpus. G. 2011;1:499–503. doi: 10.1534/g3.111.001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell A.E., Nicotra M.L., Moreno M.A., Lakkis F.G., Dellaporta S.L., Buss L.W. Differential effect of allorecognition loci on phenotype in Hydractinia symbiolongicarpus (Cnidaria: Hydrozoa) Genetics. 2007;177:2101–2107. doi: 10.1534/genetics.107.075689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman A.D., Kohn J.R. Evolutionary genetics of self-incompatibility in the Solanaceae. Plant Mol. Biol. 2000;42:169–179. [PubMed] [Google Scholar]
- Rosa S.F.P., Powell A.E., Rosengarten R.D., Nicotra M.L., Moreno M.A., Grimwood J., Lakkis F.G., Dellaporta S.L., Buss L.W. Hydractinia allodeterminant alr1 resides in an immunoglobulin superfamily-like gene complex. Curr. Biol. 2010;20:1122–1127. doi: 10.1016/j.cub.2010.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Kucukural A., Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein R., Thu C.A., Goodman K.M., Wolcott H.N., Bahna F., Mannepalli S., Ahlsen G., Chevee M., Halim A., Clausen H. Molecular logic of neuronal self-recognition through protocadherin domain interactions. Cell. 2015;163:629–642. doi: 10.1016/j.cell.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S.M., Ma Z., Hughes J.M., Riscoe B.M., Gibson G.A., Watson A.M., Flici H., Frank U., Schnitzler C.E., Baxevanis A.D., Nicotra M.L. CRISPR/Cas9-mediated gene knockin in the hydroid Hydractinia symbiolongicarpus. BMC Genomics. 2018;19:1–17. doi: 10.1186/s12864-018-5032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner D., Weiner J.a. Combinatorial homophilic interaction between gamma-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14893–14898. doi: 10.1073/pnas.1004526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman S.J., Weaver S., Shank S.D., Magalis B.R., Li M., Pond S.L.K. Evolutionary Genomics. Methods in Molecular Biology. 2019. Evolution of viral genomes: interplay between selection, recombination, and other forces; pp. 427–468. [DOI] [PubMed] [Google Scholar]
- Stoner D.S., Rinkevich B., Weissman I.L. Heritable germ and somatic cell lineage competitions in chimeric colonial protochordates. Proc. 1999;96:9148–9153. doi: 10.1073/pnas.96.16.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner D.S., Weissman I.L. Somatic and germ cell parasitism in a colonial ascidian: possible role for a highly polymorphic allorecognition system. Proc. Natl. Acad. Sci. U. S. A. 1996;93:15254–15259. doi: 10.1073/pnas.93.26.15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thu C.A., Chen W.V., Rubinstein R., Chevee M., Wolcott H.N., Felsovalyi K.O., Tapia J.C., Shapiro L., Honig B., Maniatis T. Single-cell identity generated by combinatorial homophilic interactions between alpha, beta,and gamma protocadherins. Cell. 2014;158:1045–1059. doi: 10.1016/j.cell.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifinopoulos J., Nguyen L., Haeseler A.V., Minh B.Q. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44:232–235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A.M., Procter J.B., Martin D.M.A., Clamp M., Barton G.J. Jalview Version 2 — a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D.M., Delaney N.F., DePristo M.A., Hartl D.L. Darwinian evolution can follow only very few mutational paths to fitter proteins. Science. 2006;312:111–114. doi: 10.1126/science.1123539. [DOI] [PubMed] [Google Scholar]
- Wright S. The distribution of self-sterility alleles in populations. Genetics. 1939;24:538–552. doi: 10.1093/genetics/24.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Yan R., Roy A., Xu D., Poisson J., Zhang Y. The I-TASSER suite: protein structure and function prediction. Nat. Publ. Gr. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yund P., Cunningham C.W., Buss L.W. Recruitment and postrecruitment interactions in a colonial hydroid. Ecology. 1987;68:971–982. doi: 10.2307/1938368. [DOI] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:1–18. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This paper analyzes existing, publicly available data. These accession numbers for the datasets are listed in the key resources table. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.