Abstract
Transcatheter edge-to-edge repair has revolutionized the management of mitral regurgitation in the high surgical-risk population. Iatrogenic atrial septal defects (iASDs) are an obligatory consequence of the procedure. The long-term sequelae of persistent iASDs are unknown but are believed to be dependent on their size, directionality of flow, and underlying hemodynamics. We discuss an uncommon scenario of a post–transcatheter edge-to-edge repair iASD that required immediate closure. (Level of Difficulty: Intermediate.)
Key Words: cardiomyopathy, chronic heart failure, echocardiography, right ventricle, systolic heart failure, three-dimensional imaging, valve repair
Abbreviations and Acronyms: GDMT, guideline-directed medical therapy; iASD, iatrogenic atrial septal defect; LV, left ventricular; MR, mitral regurgitation; TEE, transesophageal echocardiography; TEER, transcatheter edge-to-edge repair
Graphical abstract

History Of Presentation
A 70-year-old man was referred to the structural heart team for decompensated heart failure and severe mitral regurgitation (MR) despite maximally tolerated guideline-directed medical therapy (GDMT). On examination, he was dyspneic and normotensive with an irregularly irregular pulse. Jugular venous pressure was 15 cm H2O, lung rales were evident, and there was a grade III/VI apical holosystolic murmur. Pro–B-type natriuretic peptide level was 4,880 pg/ml (normal <300 pg/ml).
Learning Objectives
-
•
To highlight the high prevalence of mitral regurgitation among adults >55 years of age in the United States.
-
•
To appreciate that TEER has benefit in addition to GDMT in secondary MR and therefore may become more commonly performed.
-
•
Because TEER is associated with iASD, a better understanding of its consequences is needed to best define those who may benefit from septal closure.
Medical History
The patient had a remote history of single-vessel coronary artery disease, aortic stenosis, and atrial fibrillation treated with bypass grafting of the diagonal artery, bioprosthetic aortic valve replacement, maze procedure, and left atrial appendage resection. He also had lung cancer, which was treated with a right upper lobectomy, and was morbidly obese with sleep apnea. The patient had been treated for heart failure with reduced ejection fraction and MR.
Differential Diagnosis
The differential diagnosis included primary MR or secondary MR or bioprosthetic aortic valve degeneration.
Investigations
Transthoracic echocardiography revealed a left ventricular (LV) ejection fraction of 50%, moderate to severe MR, LV end-systolic diameter of 4.1 cm, and normal aortic bioprosthetic valve function. Moderate to severe tricuspid regurgitation (TR), dilated right ventricle, and estimated right ventricular systolic pressure of 44 mm Hg were also noted. After intravenous diuresis, repeat imaging with transesophageal echocardiography (TEE) showed no change in MR severity (Video 1), A2-P2 malcoaptation, and moderate TR (Video 2). Right heart catheterization revealed pulmonary capillary wedge pressure of 36 mm Hg with V waves of 58 mm Hg. Coronary angiography revealed a patent left internal mammary artery to an occluded diagonal artery.
Management
The patient underwent mitral transcatheter edge-to-edge repair (TEER) with the MitraClip System (Abbott Laboratories, Abbott Park, Illinois) (Videos 1, 2 and 3). Electrocautery-facilitated transseptal puncture with a Brockenbrough needle (Medtronic, Minneapolis, Minnesota) was performed. The 22-F MitraClip delivery system was placed in the left atrium, and 2 MitraClip NTW devices and 1 NT device were placed, resulting in reduction in MR to 1 to 2+. The patient became hypoxic and did not improve with increased oxygen delivery. Bidirectional color flow Doppler across the atrial septum was noted at procedure completion (Videos 4, 5 and 6, Figure 1). Iatrogenic atrial septal defect (iASD) size was estimated to be 10 mm using two-dimensional and three-dimensional TEE. A 12-mm Amplatzer septal occluder device (Abbott Laboratories, Abbott Park, Illinois) was deployed across the iASD with no residual flow on TEE.
Figure 1.
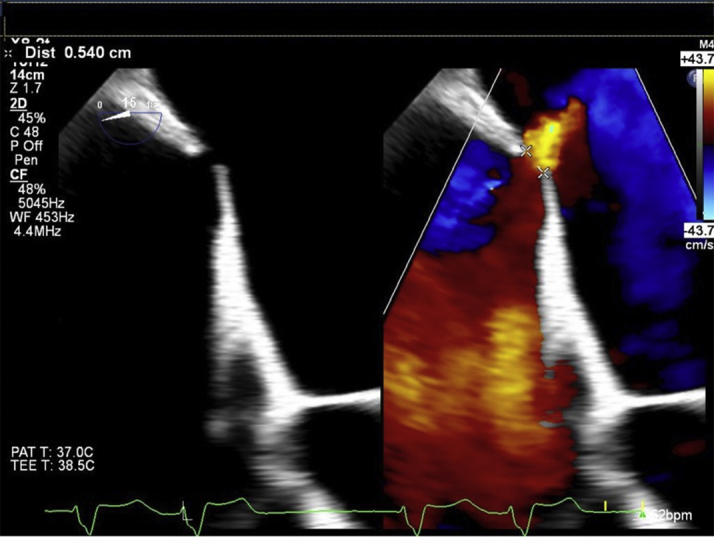
The Iatrogenic Atrial Septal Defect Was Measured at 0.5 cm in Diameter
Discussion
MR is the most common cause of moderate to severe valvular heart disease in the United States among adults >55 years of age. MR can be divided into 2 broad categories: primary (due to disease of the valve or chordae) and secondary (due to LV or left atrial pathology). In more than one-half of patients, MR is secondary, with most the result of LV remodeling (1). Unpropitious consequences of LV remodeling include annular dilatation and leaflet malcoaptation, impaired mitral valve closing forces, displacement of papillary muscles, and tethering of the leaflet; all may contribute to MR.
Transthoracic echocardiography remains the first-line imaging modality to characterize and quantify MR. TEE, and sometimes cardiac magnetic resonance imaging, are often used to further elucidate the etiology of MR. It should be noted that sedation administered during TEE may alter LV loading conditions and reduce MR severity. If a discrepancy exists between clinical findings and noninvasive testing assessment, exercise stress echocardiography is useful. The American College of Cardiology/American Heart Association guidelines for the management of patients with valvular heart disease define severe MR as having an effective regurgitant orifice area of at least 0.4 cm2 and a regurgitant volume of ≥60 ml, irrespective of the etiology (2). Of note, a consolidative approach should be used, and a single parameter in isolation should not be used to define severity.
The American Society of Echocardiography guidelines take into consideration the inherent inaccuracies of geometric assumptions and point out that secondary MR may be severe even with an effective regurgitant orifice area of ≥0.3 cm2 given the technical ascertainment challenges (3). For patients with secondary MR related to a dilated left ventricle and heart failure with reduced ejection fraction, medical optimization with GDMT is paramount. Surgical or transcatheter-based interventions are reserved for patients who have moderate to severe symptomatic MR despite maximally tolerated GDMT. The COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) trial for secondary MR with symptomatic heart failure found a significant decrease in hospitalizations for heart failure or death from any cause at 2 years for patients treated with GDMT plus TEER compared with those treated with GDMT alone (4). The MitraClip device was approved by the U.S. Food and Drug Administration in March 2019 for functional MR under the criteria of symptomatic heart failure with an ejection fraction of 20% to 50% on GDMT with persistent moderate to severe MR, an LV end-systolic dimension <7.0 mm, and a pulmonary artery systolic pressure <70 mm Hg. The American College of Cardiology/American Heart Association guidelines for the management of patients with valvular heart disease were last updated before the publication of the COAPT trial results and do not make recommendations for TEER in functional MR (2). The guidelines are currently under revision.
TEER requires left atrial access through a transseptal puncture using a 24-F guide catheter that tapers to 22-F at its distal tip and results in a residual iASD. Persistent iASDs 1 month after MitraClip deployment range between 43% and 82% (5). In addition to large catheter size, factors associated with persistent iASDs after the MitraClip procedure include longer procedure duration, extensive sheath movement, elevated left atrial pressure, and LV hypertrophy (6). Laceration of the atrial septum may also be caused by catheter manipulation. Certain studies have shown improved hemodynamics with post-MitraClip iASDs. Because iASD may have salutary effects on left heart failure hemodynamics, a large randomized multicenter clinical trial in patients with heart failure and preserved ejection fraction is underway to study the benefits of iASD in these patients (7). Other data, however, suggest an association between iASD and right-sided volume overload, heart failure hospitalizations, and death (8). There are limited data on iASD closure. Although our patient did not have persistent iASD, the recently published MITHRAS (A Randomized Trial of ASD Closure After IMVr) study prospectively randomized patients with persistent iASDs at 1-month post-procedure with a Qp:Qs ≥1.3 (9). The investigators found no difference in the primary endpoint of change in 6 min walking distance. Unlike those in the trial, our patient had known elevated right-sided pressures pre-procedure and a significant bidirectional shunt immediately post-procedure. Morikawa et al. (10) described elevated right atrial pressures with severe TR and pulmonary hypertension in the setting of significant reduction in left atrial pressure post-MitraClip deployment as contributing factors to development of right-to-left shunting. These factors were also seen in our patient and likely contributed to his bidirectional shunt.
The decision of whether and when to close the iASD is a challenging one. In our case, the decision to intervene immediately was based on the patient’s hypoxia post-transseptal puncture, multiple comorbidities, including poor pulmonary function, significant TR, right-to-left shunting, and pre-existing right heart dysfunction, which could be further compromised by worsening volume overload of the left-to-right shunting. Immediate closure was therefore considered superior to a watchful waiting approach.
Follow-Up
At his 30-day post-procedure follow up, the patient reported resolution of orthopnea and improved functional status. Repeat TEE showed trivial MR. Mean gradient across the mitral valve was 4 mm Hg at a heart rate of 48 beats/min (Videos 7, 8 and 9).
Conclusions
TEER has revolutionized nonsurgical interventions for secondary MR. With expanding indications for the MitraClip device, the consequences of iASD resulting from the procedure may become more manifest in clinical practice and the decision of whether and when to close the iASD more important. To date, data on iASD management from prospective trials are sparse, and no guideline recommendations exist. In the interim, immediate closure of iASDs is rarely indicated and should be considered only when the hemodynamic and clinical picture suggests an inability to tolerate shunting at the atrial level. Patients with elevated right-sided pressures portend a poor prognosis and should be identified before the procedure to anticipate adverse clinical consequences from the iASD.
FUNDING SUPPORT AND Author Disclosures
Dr. Ricciardi has received consulting and speaker fees from Abbott. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Mid-Esophageal Long-Axis Transesophageal Echocardiography View Demonstrating Malcoaptation of the A2 and P2 Leaflets, Between Which the Jet Originates
Three-Dimensional Reconstruction of the “Surgeon’s View” Demonstrating the Central Origin of the Regurgitant Jet Between the A2 and P2 Scallops
Transesophageal Echocardiography Mid-Esophageal Mitral Commissural View
Mid-esophageal 4-Chamber View Zoomed in to Demonstrate Bidirectional Flow Across the Iatrogenic Atrial Septal Defect on Color Flow Doppler and Significant Reduction in Mitral Regurgitation After Clip Placement
The Color Doppler Box Is Shifted Over to Demonstrate Significant Tricuspid Regurgitation. Bidirectional flow across the iatrogenic atrial septal defect is seen again.
Three-Dimensional Reconstruction of the Iatrogenic Atrial Septal Defect With Color Flow Doppler Across It
Mid-Esophageal Mitral Commissural View with 3 Well-Positioned Clips and Marked Improvement of Mitral Regurgitation as Seen on Color Flow Doppler
TrueVue Three-Dimensional Reconstruction of the “Surgeon’s View” of the Left Atrium Demonstrating an “En Face” View of the 3 Clips
Three-Dimensional Reconstruction of the Amplatzer Septal Occluder Device Enclosing the Iatrogenic Atrial Septal Defect
References
- 1.Dziadzko V., Dziadzko M., Medina-Inojosa J.R. Causes and mechanisms of isolated mitral regurgitation in the community: clinical context and outcome. Eur Heart J. 2019;40:2194–2202. doi: 10.1093/eurheartj/ehz314. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura R.A., Otto C.M., Bonow R.O. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 3.Zoghbi W.A., Adams D., Bonow R.O. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Stone G.W., Lindenfeld J., Abraham W.T. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 5.Hart E.A., Zwart K., Teske A.J. Haemodynamic and functional consequences of the iatrogenic atrial septal defect following MitraClip therapy. Neth Heart J. 2017;25:137–142. doi: 10.1007/s12471-016-0928-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadado A.J., Islam A. Iatrogenic atrial septal defect following the MitraClip procedure: a state-of-the-art review. Catheter Cardiovasc Interv. 2020 Jul 25 doi: 10.1002/ccd.29149. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Feldman T., Mauri L., Kahwash R. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]) Circulation. 2018;137:364–375. doi: 10.1161/CIRCULATIONAHA.117.032094. [DOI] [PubMed] [Google Scholar]
- 8.Schueler R., Öztürk C., Wedekind J.A. Persistence of iatrogenic atrial septal defect after interventional mitral valve repair with the MitraClip system: a note of caution. J Am Coll Cardiol Intv. 2015;8:450–459. doi: 10.1016/j.jcin.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Lurz P., Unterhuber M., Rommel K.P. Closure of iatrogenic atrial septal defect following transcatheter mitral valve repair: the randomized MITHRAS trial. Circulation. 2021;143:292–294. doi: 10.1161/CIRCULATIONAHA.120.051989. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa T., Miyasaka M., Flint N. Right-to-left shunt through iatrogenic atrial septal defect after MitraClip procedure. J Am Coll Cardiol Intv. 2020;13:1544. doi: 10.1016/j.jcin.2020.03.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mid-Esophageal Long-Axis Transesophageal Echocardiography View Demonstrating Malcoaptation of the A2 and P2 Leaflets, Between Which the Jet Originates
Three-Dimensional Reconstruction of the “Surgeon’s View” Demonstrating the Central Origin of the Regurgitant Jet Between the A2 and P2 Scallops
Transesophageal Echocardiography Mid-Esophageal Mitral Commissural View
Mid-esophageal 4-Chamber View Zoomed in to Demonstrate Bidirectional Flow Across the Iatrogenic Atrial Septal Defect on Color Flow Doppler and Significant Reduction in Mitral Regurgitation After Clip Placement
The Color Doppler Box Is Shifted Over to Demonstrate Significant Tricuspid Regurgitation. Bidirectional flow across the iatrogenic atrial septal defect is seen again.
Three-Dimensional Reconstruction of the Iatrogenic Atrial Septal Defect With Color Flow Doppler Across It
Mid-Esophageal Mitral Commissural View with 3 Well-Positioned Clips and Marked Improvement of Mitral Regurgitation as Seen on Color Flow Doppler
TrueVue Three-Dimensional Reconstruction of the “Surgeon’s View” of the Left Atrium Demonstrating an “En Face” View of the 3 Clips
Three-Dimensional Reconstruction of the Amplatzer Septal Occluder Device Enclosing the Iatrogenic Atrial Septal Defect


