Abstract
Bacterial resistance to antimicrobial compounds is a growing concern in medical and public health circles. Overcoming the adaptable and duplicative resistance mechanisms of bacteria requires chemistry-based approaches. Engineered nanoparticles (NPs) now offer unique advantages toward this effort. However, most in situ infections (in humans) occur as attached biofilms enveloped in a protective surrounding matrix of extracellular polymers, where survival of microbial cells is enhanced. This presents special considerations in the design and deployment of antimicrobials. Here, we review recent efforts to combat resistant bacterial strains using NPs and, then, explore how NP surfaces may be specifically engineered to enhance the potency and delivery of antimicrobial compounds. Special NP-engineering challenges in the design of NPs must be overcome to penetrate the inherent protective barriers of the biofilm and to successfully deliver antimicrobials to bacterial cells. Future challenges are discussed in the development of new antibiotics and their mechanisms of action and targeted delivery via NPs.
Graphical Abstract

1. EMERGING ISSUE OF ANTIMICROBIAL RESISTANCE
1.1. Development of Antimicrobial Resistance.
Antimicrobials, such as antibiotics, are molecules produced naturally by bacteria for the putative function of inhibiting other bacteria. Antimicrobials also can be synthetically produced in the laboratory. Antimicrobials consume 30% of the total pharmaceutical budget in the US, but surprisingly only 1.6% of drug development allotments by major pharmaceutical companies are spent on antimicrobial development.1,2 The U.S. Centers for Disease Control (CDC) predicts that by the year 2050, antimicrobial resistant infections (ARIs) will result in over 10 million deaths annually, far exceeding deaths from all cancers and AIDS combined.3,4 The current economic burden created by ARIs for health-care costs and lost productivity is already estimated at $20 and $35 billion per year, respectively, and is projected to reach $100 trillion by 2050.5,6
ARIs are emerging as “one of the greatest global threats to human health”. The U.S. CDC reported that over 2 million people in the US are affected annually.4 Most recent estimates indicate over 162 000 antimicrobial-resistance deaths per year, with comparable numbers occurring in Europe.7 Over 1.3 million deaths occur worldwide due to tuberculosis alone. Infections due to methicillin-resistant Staphylococcus aureus (MRSA) contribute over 19 000 deaths annually and are responsible for 60–90% of hospital-acquired (i.e., nosocomial) infections.8 MRSA, as early as 2009, accounted for more deaths than HIV/AIDS and TB combined.9 The human and economic tolls of ARIs are already quite substantial and rising.
1.2. Antimicrobial Overuse and Development of Multidrug Resistant Bacterial Strains.
A decades-long reluctance to invest in the discovery and development of novel antibiotics has been coupled with the concurrent overuse of antimicrobials in agriculture and animal farming. For example, tens of millions of pounds per year are given to animals for nontherapeutic purposes, to largely enhance growth. In contrast, only 3 million pounds are used in humans.10 This has enabled resistance mechanisms to present-day antimicrobials to develop in food-related systems and clinical settings and be rapidly (e.g., days, weeks) transferred among pathogens within both hospital and community settings.11 The escalating crisis of ARIs has recently driven a greater awareness for the exploration and development of innovative antimicrobial therapies.11,12
The rapid emergence of ARIs and the arms race toward the development of novel bactericidal agents is one of the most urgent public health concerns in the United States. The development, screening, and testing of small-molecule antibiotics is costly and resource-intensive, thus limiting development of alternative treatments against resistant bacteria. As of December 2019, there were approximately 41 novel antimicrobials in clinical trials in the U.S. In general, the success rates of developing any new drug, including antimicrobials, are typically low, in which only approximately 20% of newly developed antimicrobials are approved as treatments. These primary limitations prompt an urgent need for the development of unique antimicrobials with precise targets. Limitations in current ARI treatments motivate a fundamental change in the approach for developing antimicrobials. The major disadvantages of conventional antimicrobial approaches involve the systemic toxicity and the inability of the antimicrobials to reach their desired effective concentration at the localized site of infection. Current drug therapies nonspecifically target the intended pathogen, commensal microbiome flora, and the host’s normal healthy cells.13,14 Therefore, the development of novel and other antimicrobial agents requires the identification of target pathway’s molecules, the quantity and concentration required, and specificity of the target. The bacteria structural and replication pathways are potential targets for the development of new antimicrobial agents.
Resistance genes for antimicrobials occur in many natural bacteria, as well as in clinical settings. ARI genes are often associated in clusters and therefore can be transferred among bacteria as groups of genes via transposons and other molecular mechanisms.15 This, coupled with their overuse, has resulted in the emergence of deadly multidrug resistant (MDR) superbugs, which are resistant to most or all available antibiotic therapies. Major MDR superbugs of concern are labeled with the acronym “ESKAPE” pathogens standing for Enterococcus faecium; Staphylococcus aureus; Klebsiella sp. Acginetobacter baumannii; Pseudomonas aeruginosa; and Enterobacter sp. These bacteria cause many US hospital infections and escape antimicrobial therapeutics.9 Resistance often originates from natural mechanisms that are already present in certain types of microbes and is spread through horizontal gene transfer (i.e., transformation, conjugation, transduction) to other bacteria when there are increased selective pressures.
The abuse and misuse of antibiotics together have contributed to the emergence of resistant bacteria threatening human health. Antimicrobial resistance has become one of the leading causes of death worldwide in recent years. Currently, strategies such as chemical modification of antibiotics, combinatorial therapy, photothermal agents, antimicrobial peptides, cationic polymers, and nanomaterials have been reported to be useful for conquering antimicrobial resistance.16
1.3. Nonspecific Effects of Antibiotics on the Beneficial Microbiome.
A relatively recent realization is that humans possess a complex array of over ten thousand different microbial species and strains, collectively called the human microbiome.17 This microbiome has coevolved with humans and is beneficial and even necessary for human health.18,19 A challenging issue, one that is most especially pronounced in western societies, is the overuse of antibiotics and their resulting effects of the gut microbiome. Humans are estimated to consist of approximately 37.2 trillion human cells but may possess over one hundred trillion bacterial cells. Given the variabilities in estimates and human size, the ratios of bacterial:human cells are between 3:1 and 1:1.20 This presents a diversity of potentially beneficial bacteria, which can be influenced in an adverse manner by antibiotic exposure and delivery. The nonspecific activities of most antibiotics often impart destructive consequences to the beneficial host flora in humans.
2. NANOPARTICLES FOR DELIVERY OF ANTIMICROBIALS
In the past decade, there have been great advances in nanomedicine that show promise for the treatment of bacterial infections. The inherent plasticity of microbial cells and their capabilities for the rapid evolution of resistance have driven the development of alternative approaches for the delivery of antimicrobials to pathogenic forms. nanoparticles (NPs), owing to their extremely small sizes relative to biological cells and often unique physical/chemical properties, have become efficient vehicles for drug delivery, especially for anticancer drugs, to cells (Figure 1). The continuous emergence of bacterial resistance and MDR strains have generated significant challenges resulting in an urgent need in the development of novel and effective antimicrobial agents. Among the most promising of these novel antimicrobial agents are metal NPs, which have shown strong antibacterial activity in an overwhelming number of studies. It is hypothesized that NPs with antibacterial activities have the potential to reduce or eliminate the evolution of resistant bacteria due to the nature of NPs being designed to target multiple pathways at once avoiding the development of resistant bacterial strains.
Figure 1.
Nanoparticle-based design strategies for intracellular delivery applications [Reprinted with permission from ref 27. Copyright 2011 Royal Society of Chemistry].
NPs can act as antimicrobial agents or the carriers for loading antimicrobial drugs to promote the bioavailability and their effectiveness.21 Antibacterial NPs without the need of drugs are developed using diverse materials, including metals, chitosan, and surfactants.22 The net-positive charge of cationic compounds can bind to the negatively charged membrane surface of bacterial cell walls, while the amphiphilic structure of some NPs prompts membrane damage.23 Another strategy involves the encapsulation of antibiotics into nanocarriers to enhance bacterial eradication and bioavailability. The delivery of drugs from nanosystems improves the efficacy, while reducing toxicity in comparison to conventional therapy. Given the high surface-to-volume ratio, the possibility of surface functionalization, and the capacity to load drug molecules, NPs contribute to the efficient antimicrobial activity with their high affinity to bacteria.14 The NPs can protect drugs from enzymatic attack and sustain the drug release to increase the half-life and bioavailability.24
Active targeting of NPs against bacteria can provide a suitable strategy to efficiently increase the therapeutic index. The nanocarriers can be functionalized with ligands to the bacterial surface to enhance the targeting to specific pathogens. The design of stimuli-responsive nanosystems is another concept approach for bacterial targeting through recognition of the bacterial microenvironment and the response in a dynamic process.25 NPs can be designed to respond to internal stimuli, including conditions varying in pH, concentrations of specific enzymes, or chemicals, which are associated with pathological conditions of infection and inflammation.26 The antibiotic targeting of bacteria can be also achieved by NP responses to external stimuli, such as magnetic, thermal, light, and ultrasound effects.
2.1. Cell Wall and Membrane Barriers of Bacteria.
Many studies have found that Gram-positive bacteria are more resistant (than Gram-negatives) to NP exposure and their NP-associated mechanisms of action. This has been attributed to the fundamental differences in the thickness and composition of cell walls between Gram-positive and Gram-negative bacteria. The bacterial cell wall is considered a single polymeric molecule that encloses the bacterium and is composed of glycan strands covalently linked to each other with an interconnecting peptide stem attached perpendicularly to alternating saccharides of the glycan strands.28 The alternating saccharides consist of an N-acetylglucosamine (GlcNAc, NAG) and N-acetylmuramic acid (MurNAc, NAM) connected by a β-1,4-glycosidic bond.28
Gram-negative bacteria, such as Escherichia coli, typically have a relatively thin cell wall, with 1–3 layers of peptidoglycan (~3–4 nm thick) and an additional outer membrane consisting of lipopolysaccharide (LPS; 1–3 nm thick).29 This structural arrangement is hypothesized to facilitate the entrance of ions released from NPs into the cell. Alternatively, the cell wall of Gram-positive bacteria, like S. aureus, is relatively thicker and consists of 10–20 layers of peptidoglycan and is 30 nm thick in Bacillus subtills, consisting of covalently attached teichoic and teichuronic acids.30,31 The proposed mechanism explaining the difference in susceptibility is in the direct interaction between NPs and the cell wall, which may be more detrimental for Gram-negative bacteria since they lack the thick peptidoglycan layer present in Gram-positive bacteria.
The surface charge and electronegativity of the cell wall also are hypothesized to play a significant role in the interactions between the bacterial cell walls and NPs or the ions released from these structures. It is known that both Gram-positive and Gram-negative bacteria have negatively charged cell walls; however, changes in the electronegativity of the cell wall can occur due to growth in different types of culture media.32,33 Additionally, the Gram-negative cell walls are surrounded by an outer membrane containing negatively charged LPS molecules. This imparts a higher affinity for the positive ions released by most NP constructs and leads to the increased uptake and overall accumulation of ions, which then causes intracellular damage. Several reactive oxygen species (ROS), including hydroxyl radicals, are negatively charged and therefore unable to easily penetrate the negatively charged cell membrane during NP exposure. The electrostatic charge plays an even more important role when charged capping agents are used during NP synthesis, further adding to the overall electrostatic attraction or repulsion.
2.2. Metallic Nanoparticles.
Transition metals are commonly used for antimicrobial NPs and are classified as metals with a density >5 g/cm3 and having valence electrons in two shells, rather than just the outer one. They form coordination bonds and perform a diversity of important biological functions, including hydroxylation, redox reactions, and electron transport.34 While many transition metals are essential to life in very small concentrations, they become toxic at higher concentrations. In metallic NPs, the metal is present in a neutral state and likely incapable of crossing the cell membrane. However, it has been demonstrated that metal NPs slowly release metal ions that are able to penetrate the cell membrane and disrupt the intracellular function and pathways.35 In general, trends in bactericidal activity often exhibit similarities to those observed in eukaryote cytotoxicity. Thus, most potent bactericidal agents are also toxic toward mammalian cells.36,37
Currently, the most effective antimicrobial metal oxide is ZnO, which exhibits efficacies comparable to Ag.38 Due to photocatalysis, ZnO-NPs are strongly bactericidal upon exposure with UV irradiation. Here, NP shape can influence efficacy. For example, ZnO nanorods were revealed to be better antimicrobials than nanospheres. Although, flower-shaped ZnO-NPs with exposed polar groups had even stronger efficacies.39 TiO2 NPs possess their antimicrobial activities through the production of OH• radicals. However, these same NPs exhibit minimal antibacterial properties in the absence of UV given their weak interactions with the negatively charged bacterial surface. Recent studies have shown that TiO2 NPs exhibited a high tendency to bind via van der Waals and receptor–ligand interactions to the OM of E. coli.40 A recent strategy involves metal doping of TiO2 to substantially increase antimicrobial activities and enable visible-light-induced photocatalytic activities.41,42
Au-NPs, in contrast, are both biocompatible and biologically inert. Internalization by eukaryote cells of Au-NPs is dependent on the size, shape, and charge of the NP. The fastest uptake was observed for 40–50 nm sizes and spherical shapes, when compared to nanorods with positively charged NPs, which penetrated more easily.43-45 Au-NPs, when functionalized with amino acid residues exhibiting a similar structure to antimicrobial peptides, facilitated strong electrostatic interactions between the cationic functionalization of the Au-NPs and the bacterial membrane. This interfered with membrane compactness and structure leading to its disruption and resulted in antibacterial activities against E. coli and S. aureus.46
Metal NPs and their associated antibacterial activities can be attributed to the formation of ROS and their affinity for interacting with thiol groups (R–SH) (Figure 2), especially the amino acid cysteine in proteins. This directly disrupts functioning of specific enzymes and/or breaks disulfide bridges required to maintain the integrity of protein folding and ultimately results in cell death. Metallic NPs have been extensively addressed elsewhere and will not be considered further here.47-54
Figure 2.
Schematic representation illustrating the antimicrobial mechanisms that have been demonstrated for various metal-based NPs [Reprinted with permission from ref 55. Copyright 2019 Shaikh et al.].
2.3. Noncytotoxic Nanoparticles for Delivery of Antimicrobials.
NPs can be engineered using a variety of materials for different applications. The size, shape, and surface charge of the NPs can be finely tuned by modulation of material types, contents, and preparation methods. Since most NP-based approaches utilize distinct mechanisms, structures, and pathways compared to those administered by conventional antibiotics, combined therapeutic regimens are favorable for tackling the rise of MDR bacteria bypassing their defense mechanisms. The multitarget action of NPs may overcome multidrug resistance by circumventing obstacles encountered by conventional antibiotics.14
Some commonly used nanosystems for antibiotic drugs include inorganic metal, polymeric, lipid-based, silica, micellar, and cell membrane-coated NPs.56 Superparamagnetic iron oxide NPs (SPIONs) have been widely investigated as potent bactericidal agents based on their magnetic hyperthermia properties.57 They have also been utilized as bacterial separation agents and bioimaging contrast agents for bacterial diagnoses.58 The hyperthermic properties lead to the increased bacterial membrane permeability and result in the killing of targeted bacteria.59 In order to potentiate their antibacterial activity, SPIONs can be functionalized with antibodies, antimicrobial peptides, and aptamers for targeting bacteria.60
Polymeric NPs can be fabricated with high biocompatibility and biodegradability. Chitosan-based NPs have been broadly used as drug delivery systems and display intrinsic antibacterial and antibiofilm activity due to their polycationic nature capable of disrupting bacterial membranes.61
The strategy of coating NPs with the lipids of natural cell membranes has gained much attention recently. Lipid-based NPs, including liposomes, nanoemulsions, and solid lipid NPs (SLNs) are widely utilized for the targeted delivery of antibacterial drugs. Lipid NPs easily fuse with the bacterial outer membrane, delivering the antimicrobial agent directly to the bacterial cells.62,63 As carriers for drug delivery, liposomes can prolong circulation time and accelerate cellular uptake, thus counteracting the selection of resistance. Liposomes can fuse with mammalian cells, tumor cells, and microbes, facilitating the transport of drugs across biological membranes.64
Due to their physiochemical stability, uniform porosity, large surface area, and biocompatibility, mesoporous silica NPs (MSNs) are widely used as drug delivery vehicles, biosensors, catalysts, and absorbents.65 Their tunable particle pore volume and surface chemistry can be modified so antibacterial agents can be neatly packaged inside the porous matrix and effectively shielded against enzymatic degradation and facilitate their passage through biomembranes.66
2.3.1. Surface Chemistry of Nanoparticles.
NPs design can be tailored to target specific cell types or pathways by tuning the nanomaterials parameters based on their size, shape, charge, hydrophobicity, rigidity, roughness, and surface functionalization.27,67 The physiochemical characteristics of NPs, including size, shape, and surface charge are critical for governing bacterial interactions and antibacterial activities and are summarized in Figure 1.
2.3.1.1. Size and Surface Charge.
NP size influences its interactions with bacterial cells. Typically, smaller-sized NPs exhibit a higher probability of interacting with a bacterial cell. The higher surface-to-mass ratio of smaller nanocomposites demonstrates increasing adaption and binding to bacterial surfaces.68 Additionally, smaller size NPs can more easily permeate into the bacterial membrane and enhance the overall antibacterial activity.69 In metallic NPs, smaller sizes increase particle dissolution rates into ions.70 Increased dissolution rates raise the potency of bacterial killing. NPs may aggregate into larger complexes before and after attachment on bacterial surfaces.71 NP aggregates of larger sizes interact with bacterial surfaces but in different manners when compared with dispersed NPs.72 However, aggregation decreased the overall reactivity with bacterial cells and resulted in lower antibacterial efficacies.73 It is generally recognized that smaller-sized NPs exhibit increased interactions and activities.
2.3.2. Increased Efficacies of Antimicrobials Delivered by NPs and Possible Mechanisms of Action.
A major goal of nanoengineering is to produce and utilize nontoxic NPs that avoid cross-reactivity with mammalian cells. The use of green chemistry to synthesize NPs may be advantageous over more common physical/chemical methods, owing to their ecofriendly, less toxic, and more economical syntheses.74,75 Applications of nontoxic NPs to act as carriers for antibiotics is especially relevant to the emerging global scale of antibiotic resistance. Nanocarriers can be designed for drug delivery to a specific site, organ, or cell type. Targeting capability can be achieved by coupling of antibodies, ligands, or other targeting moieties, such as sugars to the surface of the nanocarrier.67
An important aspect to consider when studying the interactions of nanomaterials in vivo and with cells is the dispersion of NPs in biological media and the potential agglomeration and stability over time. Agglomeration can impact and affect the corona composition and interactions with cells. NPs incubated with cells in the absence of serum or other biological media may lead to conclusions that are entirely irrelevant to their biological applications and physiochemical properties in vivo.76
2.4. Antimicrobial Peptides and Antibiotics Using Nanobased Strategies.
Approximately 60–70% of current antibiotics when delivered by conventional means are not effective against infections due to their inability to cross the bacterial cell surface and/or low retention times once in the cell. Many common antibiotics, such as the β-lactams and aminoglycosides, are hydrophilic in nature and unable to easily penetrate the bacterial cell wall.
Antimicrobial peptides (AMPs) have garnered interest as potential attractive alternatives to conventional antibiotics based on their unique physiochemical properties and broad or narrow spectrum activities against bacteria. Typically, AMPs are cationic amphipathic molecules that target and kill bacteria using two major mechanisms of actions. In the first, AMPs induce membrane disruption, which leads to lysis and cell death. In the second, AMPs are internalized into bacterial cells without membrane disruption and target key intracellular functions.77 AMPs including their mechanisms of action are further elaborated in a recent review.78
In a recent study, a class of antimicrobial polymers derived from bile acids were developed possessing facial amphiphilicity for enhanced interactions with the bacterial cell membrane.79 It was observed that the cholic acid–based cationic polymers exhibited higher effectivities against Gram-negative bacteria, particularly P. aeruginosa, compared to Gram-positive bacteria (e.g., S. aureus). The three cationic charges present on each cholic acid unit enable localization in the outer membrane via electrostatic interactions while the hydrophobic face of cholic acid inserts into the membrane.79
Some infections occur when bacteria reside within human cells, called intracellular pathogens. Most current antibacterial agents exhibit limited activity against intracellular bacteria. This is because antibiotic concentrations within the intracellular compartments of host cells, where pathogens are located, are typically less than the minimum inhibitory concentration (MIC) and contribute to the development of drug resistance of intracellular pathogens.80
Several types of NPs, including polymeric NPs, lipid-based NPs, and silica NPs, have been used for facile internalization into eukaryote host cells to eradicate intracellular micro-organisms.81 Such NPs can be designed having an increased affinity for eukaryote host cells (containing the intracellular pathogens) or having ligand conjugation on the NP surface for active targeting of cells. The absorption of drug molecules onto NP surfaces enables the delivery of relatively high concentrations of antimicrobials to a targeted site.82 Antimicrobials can either be passively sorbed, covalently bound to, or incorporated into a polymer matrix generating polymeric NPs with high biocompatibility and biodegradability.
3. ENTRY OF NANOPARTICLES INTO BACTERIA AND POTENTIAL FOR THE INTRACELLULAR DELIVERY OF DRUGS AND THERAPEUTICS
3.1. Cellular Barriers to Nanoparticles in Bacteria.
Entering NPs directly into a bacterium to control or kill the cell represents a new approach for antimicrobial drug delivery. The bacterial cell envelope consists of the cell membrane(s) and cell wall (CW) and represents a sophisticated and complex permeability barrier that can hinder the internalization/accumulation of antibiotics in bacterial cells. This results in selection toward acquiring bacterial resistance.83 Poor internalization could be potentially overcome if antibiotics were “carried” into bacterial cells.13
To reach a cytoplasmic target (e.g., such as proteins, nucleic acids) inside a Gram-negative bacterium, soluble antibiotics must cross three barriers: the outer membrane (OM), the cell wall (CW), and inner plasma membrane (PM).84 This tripartite barrier functions to protect the cell against exogeneous toxic compounds. The CW provides protection against osmotic pressures and mechanical damage, while maintaining the permeability of key molecules for bacterial metabolism and communication with the environment.13 Membrane transporters, e.g., porins or efflux pumps, function as primary penetration strategies regulating the internal accumulation of various hydrophilic molecules. Regulation of membrane permeability, involving the processes of influx and efflux, is modulated by the bacterial cell to regulate the intracellular concentration of various molecules and maintenance of a positive turgor pressure.85
3.2. Uptake by Cells and Synergistic Interactions of Small Molecules.
To be active against a bacterial pathogen, an antibiotic is required to reach a critical concentration threshold to inhibit the corresponding target. Diffusion of antibiotics through bacterial membranes must be facilitated and must overcome efflux pumps that remove antibiotics once in the periplasm, to attain a sufficient cytoplasmic concentration86,87 (Figure 3). Membranotropic compounds, such as polymyxin B and polymyxin E (colistin), perturb the integrity of bacterial membranes and enhance the bacterial susceptibility to various compounds. However, they can also act as opsonins for targeted killing by phagocytic cells. Polymyxin B nonapeptide (PMBN) lacks a fatty acid tail and plays a critical role by increasing the permeability of the bacterial plasma membrane (PM). This sensitizes bacterial cells to hydrophobic antibiotics while exhibiting minimal toxicity to eukaryotic cells.84
Figure 3.
Antimicrobial drug resistance mechanisms in bacteria. (1) Prevention of antimicrobial drug penetration into the bacterial cell by bacterial cell envelope. (2) Removal of antimicrobial drug from bacterial cell by nonspecific efflux pumps. (3) Acquisition of new genetic material from drug resistant bacterial strain. (4) Inactivation of antimicrobial drug by intracellular modification [Reprinted with permission from ref 70. Copyright 2018 Elsevier].
A proposed strategy now offered is to combine low (subinhibitory) concentrations of polymyxin with other clinical antibiotics to synergize their activities.84 As an example, the combination of meropenem and colistin demonstrated synergistic killing of Acinetobacter baumannii and P. aeruginosa.88 Similar results have been reported using a combination of colistin with a high dose of tigecycline, rifampin, and vancomycin.89-91 Interestingly, it has been demonstrated that PMBN (polymyxin B nonapeptide) and polymyxin B can act synergistically as a new family of antibacterial agents, peptide deformylase inhibitor against Gram-negative bacteria, such as E. coli, Enterobacter aerogenes, K., and P. aeruginosa.92 The increase in bacterial susceptibility was associated with an increase in entry of the new antibacterial agents using subinhibitory concentrations of polymyxin.92 Importantly, the use of polymyxin compounds in combination with another antibiotic may reduce the risk of resistant emergence.84
Interestingly, certain natural compounds can act as membrane permeabilizers. Eugenol, a terpenic compound and a major component of clove oil from Eugenia aromatica was reported to disrupt cytoplasmic membranes.93 When applied in combination with eugenol, ten antibiotics were shown to have minimum inhibitory concentrations (MICs) that were 5 to 1000-fold lower than when used alone, indicating a synergistic effect.94 The effect on bacterial membranes was also demonstrated through the observed enhancement of nitrocefin uptake associated with a sensitization of bacterial cells to lysis by lysozyme or detergents in the presence of eugenol.
An additional strategy to circumvent the membrane barrier is through destabilization of the LPS layer using chaotropic agents or detergents that consequently aid in the diffusion of hydrophilic compounds through the membrane lipid bilayer.95 EDTA has been shown to increase the susceptibility of bacteria producing metallo-β-lactamases toward β-lactams.96 Recently, squalamine, a natural aminosterol, has been characterized based on its antimicrobial activity and its ability to destabilize the OM of Gram-negative.97,98 More interestingly, squalamine in combination with other conventional antibiotics was demonstrated to be capable of restoring bacterial susceptibility to various antibiotic classes including β-lactams, fluoroquinolones, macrolides, phenicols, and cyclines in resistant isolates that lack the expression of porins.99 This class of amphiphilic derivatives has been suggested for direct use in the development of novel antibacterial agents, or alternatively to produce original adjuvants for rejuvenating activities of old conventional antibiotics on MDR bacteria.100,101 Finally, Tang and colleagues have developed amphiphilic antimicrobials that effectively slice open the outer membranes of Gram-negative bacterial to demonstrate their efficacy.79
3.3. Can Nanoparticle Design Facilitate Antimicrobial and Cellular Uptake into Bacterial Cells?
Different strategies have been proposed to overcome the bacterial envelope barrier (i.e., the OM, CW, and PM) and most involve altering the influx/efflux mechanisms of the cell, in order to restore the activity of antibiotics against resistant strains.85 NP and molecular transporters able to successfully interact with bacterial envelopes are unique approaches to carry oligonucleotides and poorly internalized antibiotics across the envelope.13 Also, a key advantage of NP carriers is potentially provided by a reduction of drug efflux from bacterial cells. This is due to the intracellular delivery of a high concentration of drug into cytoplasm that may overwhelm the efflux pumps.41,81 Consequently, the overall potential of NPs and molecular transporters to circumvent the bacterial envelop barrier depends upon the ability of the NP/transporters to efficiently interact with the different bacterial envelope structures.13
Key ideal features of drug delivery systems are biodegradability, biocompatibility, controlled drug transport, and targeted delivery to a tissue/organ.102 Drug absorption efficiency is directly proportional to the surface area of the absorbent and inversely proportional to NP particle size. Given their large surface-to-volume ratio and the diverse functionalization, NPs can be utilized as transporters for targeted drug delivery. Their use provides distinct advantages over alternative drug carrier systems due to a reduction in side effects observed for antibiotics and other antimicrobial agents. A study showed that superparamagnetic FeOH-NPs interact with microbial cells by directly penetrating the cell membrane and interfering with the transfer of transmembrane electrons.103
NPs that are surface loaded with antibiotics often exhibit enhanced killing efficiencies of bacteria, even those strains exhibiting resistance to the antibiotic.104 Recently, Decho and colleagues observed that when the beta lactam antibiotic penicillin G (PenG) was passively sorbed to NPs, their killing efficiencies dramatically increased against several types of penG-resistant bacteria, including MRSA strains.105 They postulated the “grenade hypothesis” suggesting that each NP contained a sufficiently large number of PenG molecules, and constituted a powerful package (i.e., a high concentration) delivered to a single bacterial cell, which overwhelmed the natural beta lactamase-based resistance mechanism.105
NPs themselves can lead to cell membrane damage caused by NP absorption, which sometimes is followed by penetration into the cell and cell death. Several studies have suggested that absorption on the CW followed by its disintegration was the primary mechanism of toxicity.49,106 Absorption of NPs results in CW depolarization, which alters the negatively charged CW and becomes more permeable. Using laser scanning confocal microscopy, the microbial cell membrane became visibly “blurred” upon exposure to AgNPs, indicative of CW degradation.107 The authors proposed that AgNPs followed a two-step mechanism of action. The first step involved disruption of the cell membranes and penetration of the AgNPs. In the second step, ROS are subsequently formed that inhibit the production of ATP and DNA replication. It was also noted that the production of ROS may be a primary step as well since its production counteracts the innate antioxidant defense mechanism and results in disruption of the cell wall.108
Developing novel antibacterial agents to combat the membrane barrier, antibiotic-efflux pump inhibitors (EPIs) and affect the multidrug efflux pumps of bacteria that have been a recent focus of investigation, with the goal of developing new agents having the capability to rejuvenate activities of traditional antibiotics against MDR bacteria.
4. PENETRATION OF BIOFILMS: THE MAJOR BARRIER OF IN SITU INFECTIONS
4.1. Why do Infections Preferentially Occur As Biofilms?
It has been realized for several decades that bacteria occur in two major forms or states.109 They occur as free-living suspended (i.e., planktonic) cells and as attached groups of cells called “biofilms”.110 Biofilms are multicellular, densely packed communities of bacteria that are surrounded by a self-secreted matrix of extracellular polymeric substances (EPSs).111 EPS is an operational term, which refers to a wide range of secreted polymeric molecules such as polysaccharides, proteins, DNA, and lipids.112-115
Biofilm formation plays a key role in the development of bacterial antibiotic resistance and in chronic and persistent infections.116-119 This is due to the many protective roles they afford to cells.120 While free-living bacterial cells are exposed to their immediate surrounding environment, biofilm cells embedded within an EPS matrix are provided many protective advantages. (1) The EPS matrix, secreted by microbial cells, is the primary emergent property of a biofilm; one that facilitates many subsequent advantages.110 The majority of biomass in a biofilm is contributed by EPSs, rather than cells.111,121 (2) Diffusion-slowing properties of EPSs. When EPSs form dense gels, there is a measurable reduction in the diffusivities of molecules and ions within a biofilm.122-124 This can slow (or prevent) antibiotics from reaching cells enclosed in the biofilm.125 (3) Localization of extracellular enzymes (e-enzymes). e-Enzymes are secreted by cells and localized within EPS. This affords cells the benefits of the enzymatic hydrolysis products. Certain e-enzymes, such as β-lactamases, hydrolyze lactam antibiotics (e.g., penicillin, ampicillin). Their concentrated extracellular presence can create a “minefield” for inbound diffusing antibiotics.126,127 Studies have also shown that when e-enzymes are localized within EPS, their denaturation over time is reduced.111 (4) Enhanced gene exchange. Plasmid DNA (i.e., containing various genetic capabilities) is released by cells into the EPS matrix. Localization in EPSs allows for more efficient (subsequent) uptake by cells (i.e., via transformation). Gene exchange also occurs more efficiently via conjugation since cells can be held in place by EPSs to allow transfer through pili. Both transformation and conjugation within biofilms are used to transfer important AR genes among cells.128,129 Additionally, the overall dynamic of biofilms represents a “breeding ground” for frequent resistant mutants.130,131 (5) Quorum sensing. Cell to cell chemical communication, called quorum sensing (QS), occurs most efficiently within biofilms. The diffusion-slowing properties of EPSs result in the sustained buildup of signals above threshold concentrations where gene expression changes are triggered and occur within groups of cells.132 QS allows the cells to act in a coordinated manner in their activities, such as production/secretion of e-enzymes and the upregulation of virulence genes in bacteria. (6) Persister cells. A relatively small portion of cells within a biofilm (i.e., and even within actively growing cultures) are physiologically inactive and called persister cells.133,134 Since most antibiotics target actively metabolizing cells, persisters are largely immune to antibiotic effects. If a biofilm community is effectively removed by antibiotics, persister cells remain viable (but inactive) and will become active again to repopulate once the antibiotic is no longer present. Many persistent and chronic infections are thought to occur via this mechanism. Persister cells are thought to be an inherent strategy for survival employed by bacteria against sudden stressors. Recently, a new class of antibiotics was discovered that are effective against MRSA and persister cells encompassing the dormant bacteria associated with troublesome chronic, recurring infections.135 In this study, two synthetic retinoids, CD437 and CD1530, were demonstrated to be able to kill both growing and persister cells of MRSA individually and in combination with the antibiotic gentamicin. The retinoids exhibited the capability to disrupt the bacterial membrane lipid bilayers, which was suggested to enhance the diffusion of gentamicin into the cells when coadministrated. Further investigation led to the development of an analog of CD437 that maintained the ability to efficiently kill persisters while reducing the toxicity observed to mammalian cells.135 (7) Amyloid proteins and structural stability. Amyloids are fibril-shaped proteins having a cross-β-sheet structure, where the β-sheets are stacked on top of each other.136They are well-known for their involvement in human pathological conditions such as Alzheimer’s, Parkinson’s, and Huntington’s diseases. For over a decade, however, it has been realized that bacteria also produce amyloids, which are multifunctional components of the EPS and therefore referred to as “functional amyloids (FA)”.137,138 Major FAs include CsgA (i.e. curli protein) found in a range of bacteria139 and FapC produced by members of the genus Pseudomonas. FA biogenesis is a tightly regulated process, and its release from the cell involves a series of related proteins, which transport the FA to the cell surface.140 Once outside the cell, the individual proteins undergo fibrillation, where imperfect repeat portions of the protein aggregate to form a cross-β-sheet structure.141,142 The interaction of FapC with membrane components such as the outer membrane lipopolysaccharide and a rhamnolipid (surfactant) enhance fibrillation.143 Bacterial amyloids often extend from the cell surface into the EPS and survival properties,144 acting as a form of structural rebar enhancing the physical architecture of biofilms. The genes for amyloids production and secreted formed appear to be present in a wide range of bacteria. Additionally, amyloids enhance the localization of quorum sensing signals and other small molecules within the biofilm matrix.145,146 Given the refractory nature of amyloids, they have likely evolved as a collective adaptation, which contributes to the overall resiliency of biofilms147 and likely serve other important roles as well. This presents amyloids as a target for potential antimicrobial drugs148 and for the development of antibiofilm technologies, as discussed below.
Finally, biofilm bacteria often produce superantigens to evade host immune systems. They are less restrained by the selective pressures associated with antibacterial agents then their respective planktonic bacterial cells.149 The combination of the protective synergistic effects of a biofilm constitute a formidable barrier to antibiotics. Their unique composition and structure shelter and protect the embedded microorganisms and facilitate their overall survival, whether in natural environments or in the human body. Biofilm bacteria display a 10- to 1000-fold higher resistance to antibiotic treatment than the planktonic form.16
4.2. Extracellular Bastion against Nanoparticle Delivery of Antibiotics?
While antibiotic delivery by NPs is a promising approach to overcome the biofilm barriers the ability of antimicrobials to penetrate and reach cells within a protective biofilm remains a prime limiting factor in controlling infections.63,129,150 Numerous studies have demonstrated that NPs can disrupt the membranes of individual bacterial cells.151-153 Also, NPs can be designed to contain a concentrated antibiotic package that can be released upon a single bacterial cell under the proper conditions. Therefore, the potential exists for NPs as an alternative approach to target bacterial biofilms depends upon them reaching cells.
Applications of metallic NPs in preventing and/or overcoming biofilm formation have included the use of AuNPs, AgNPs, Mg-NPs, ZnO-NPs, CuO-NPs, Fe3O4-NPs, and YF-NPs.151-158 In order to obtain greater prevention of biofilms, smaller size and a higher surface-to-mass ratio has been recommended. However, while size limitation effects on NP penetration of biofilms appears reasonable, they have been generalized based on few detailed data and insufficient characterization of the actual biofilms.159 In addition, NP shape also is considered to influence biofilm destruction. For example, NPs with rodlike shapes have appeared more effective than NPs with a spherical shape.152
Fusogenic NPs, NP targeting, and triggered drug release from nanocarriers are ideal strategies to maximize the exposure of the biofilm to drugs. Enzymes, such as deoxyribonuclease (DNase) and protease, have been loaded into NPs to hydrolyze the biofilm structure for enhanced penetration of antibacterial drugs or NPs.63 Magnetic NPs composed of iron oxide are also effective in the deep penetration into biofilms.160
4.3. Penetrating the Extracellular Matrix to Reach Pathogen Cells.
The EPS surrounding cells consists of a meshwork of (i.e., mostly charged) polymer molecules linked together in varying densities.161 However, they also contain much water, the majority of which (i.e., approximately 99%) is not bound to EPSs (Figure 4).162 For this reason, the water within the EPS is considered as “immobilized” water.162 The polymers may be densely packed to form gels, or more-loosely spread to form pliant moveable slime matrices.163 Lectin studies of EPSs in natural aggregates, coupled with confocal microscopy have shown the matrix to vary in composition over small (i.e., μm) spatial scales. Such small-scale variations in EPSs have been termed EPS microdomains.164-166
Figure 4.
Protective attributes of biofilms. Bacteria within biofilms secrete a wide range of extracellular polymeric substances, collectively called EPS, which secure attachment, slow the diffusion of antimicrobials such as antibiotics and NPs, and protect bacterial cells from external stressors. Within a biofilm, there is enhanced gene exchange (e.g., AR genes) and secretion of extracellular enzymes such as β-lactamases, that can degrade antibiotics. Together, these attributes enhance resistance of biofilm infections to antibiotics and other antimicrobial approaches.
4.3.1. Density.
Molecules, such as antibiotics, diffuse through water-filled pore spaces between adjacent polymers. Therefore, the relative sizes of the pore spaces become a limiting constraint in the diffusion-slowing properties of a biofilm.161 When considering the movement of (larger) nanosized particles, this relative spacing becomes especially important. Estimates of NP diffusion in biofilms have been made.167,168 Looser and less dense EPSs offer larger spacing between adjacent polymers and perhaps weaker polymer–polymer linkages, while more dense gels possess relatively smaller pore-spaces.169 One of the first insightful laboratory studies to address this directly manipulated the densities and relative pore spacing of artificial hydrogels of EPSs. This was found to directly influence the penetration and diffusive movement of NPs as determined using super-resolution fluorescence microscopy.170 More dense EPS gels reduce the movements of NPs compared with less dense EPSs, all else being constant. However, in situ biofilms exhibit much small-scale variability in the composition and density of EPSs making such direct measurements difficult. When linkages are abundant, the matrix is condensed to understand the porosity and exposed functional groups within the pores.
The effects of size, shape, and surface coatings of NPs on their penetration into biofilm matrices have not yet been thoroughly tested. To understand these effects more completely, it will require the characterization of different types of EPS matrices. EPS polymers are linked to each other through ion bridges and H-bonding.
Proteins and/or DNA are sometimes actively secreted by bacteria and comprise part of the EPS matrix. These components have been shown to provide structural stability and even rigidity to the biofilm.111 DNA often forms complexes with specific proteins and polysaccharides to act as a structural rebar to reinforce the EPS against expansion and contraction.171 Amyloid proteins may also be used in this manner since their β-sheet configurations make them less easily degraded.172,173
4.3.2. Size.
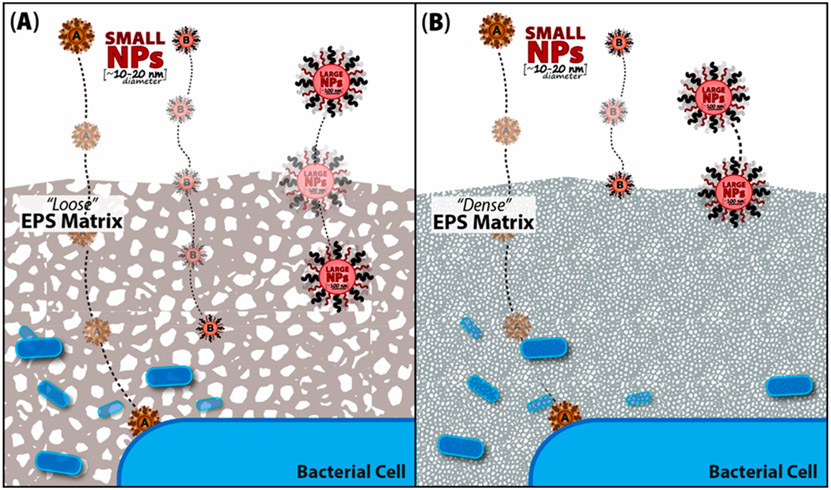
The size of NPs most certainly will limit their penetration into the EPS matrix (Figure 5). However, the surface properties or coatings of NPs have been shown to influence their migration into cells, biofilm matrices, and even human colonic mucus.76,135,174,175 Hydrophilic coatings on NPs such as polyethylene glycol (PEG) are being explored to penetrate different types of polymeric mucins. Functionalized gold NPs were found to be efficiently bound to natural estuarine biofilms.176 The EPSs act as an efficient sponge to concentrate NPs.177 This implies that certain NPs partially penetrate and are retained by the matrix. It additionally offers the potential for photodynamic activation. This uses infrared (IR) irradiation coupled with Au-NPs to treat and destroy superficial wound infections or other infections provided NPs can be specifically localized to the biofilm; a process that can also be potentially useful in removing biofilms from medical devices and implants.178 Designing NPs with relatively small sizes and coronas (i.e., coatings) that can facilitate penetration of EPSs represents the major hurdle in the successful treatment of infections.179,180
Figure 5.
Control of NP entry into biofilms. Small-scale changes in the relative density of EPS influences the penetration of NPs (red) into a biofilm. (A) Water-filled pore spaces (i.e., white spaces) in EPS (gray) are interconnected through which NPs diffuse into the matrix. It is hypothesized that (A) when EPS pore spacing is large NPs more easily penetrate the matrix. (B) However, when EPS are densely packed, pore spaces between adjacent polymers on average are small and limit their diffusivity. Therefore, only small diameter NPs (e.g., 10–30 nm dia), may penetrate to reach bacterial cells. The design of NPs with specific hydrated coatings (i.e., coronas) or surface properties may enhance their diffusion through EPS to reach infectious bacterial cells in a biofilm. NPs indicated “A” and “B” represent NPs with small diameters with different functional groups attached.
4.3.3. Surface Chemistry.
A large diversity of functionality has been imparted to the surfaces of NPs. Typically, the strategy of converting an inorganic surface to an organic surface with desired functionality relies on using a difunctional additive which contains a group which can bind or react with the inorganic surfaces and also has the desired organic functionality. For inorganic particles such as silica and related oxides, there are many commercially available silane coupling agents that have been developed over many years to perform this task and include large numbers of organic ligand chemistries.181 When the inorganic surface chemistry is less reactive with the silane coupling agents, commercially available titanate, zirconate, and aluminate coupling agents are available. There is extensive literature concerning the specific binding groups that are used for bioconjugation to many common NP surfaces including those for gold, silver, silica, carbons, quantum dots, magnetic NPs, and others.182-185
In addition to the attachment of small organic functionalities, the attachment of proteins, polymers, and other macromolecular species is very important and extends the range of surface properties that can be added to NPs. The grafting of polymers to NPs relies on four general strategies, physisorption, grafting-to, grafting-from, and grafting through, each having its own benefits and drawbacks. Physisorption is achieved through an attractive (noncovalent) physical association between a portion of a polymer chain and a surface. Grafting-to is similar in that the polymer chain must diffuse to a surface, but it is then attached through a covalent or stronger chemical linkage. These grafting strategies both have the advantage of attaching polymer chains directly to the surface, but physisorbed chains easily desorb from the surface. Only low graft densities can be achieved with these methods because surface-associated chains sterically limit additional polymer chains from diffusing to the surface. Higher graft densities can be achieved using a grafting-from approach where polymer chains are grown from a tethered initiating group. In the grafting-through approach, the polymerization is not initiated at the surface, but polymerizable functional groups on the surface are quickly incorporated into the growing polymer, resulting in a tethered chain (though not always at the chain end). For many preformed proteins and biological macromolecules, grafting-to is the dominant approach while grafting-from is often used for synthetic polymers due to the degree of control and broader range of graft densities that can be obtained.186,187
5. ONGOING APPROACHES USING NANOPARTICLE-BASED DELIVERY TO OVERCOME INFECTIONS
5.1. Can Old Antibiotics Be Reused with New Nanobased Delivery Approaches?
Most present-day antibiotics that have been introduced during the past 30 years are synthetic modifications of previously isolated natural forms.2 The use of antibiotic-loaded NPs for bacterial targeting and delivery is an ideal strategy given its numerous advantages over the conventional formulations, including improved stability, controlled antibiotic release, targeted capability, and increased bioavailability.188
Antibiotic-conjugated NPs exhibit higher antibacterial activities compared to free antibiotic or NPs alone. This suggests a synergistic effect between the NPs and antibiotics revealing different antibacterial mechanisms that are at play for these compounds.49,189,190 In a study using AgNPs in combination with several different antibiotics, penicillin G, amoxicillin, erythromycin, clindamycin, and vancomycin demonstrated enhanced efficacies against E. coli and S. aureus, respectively.189 This was performed in the absence of any direct intended associations of NPs with antibiotics. However, unintentional binding of the NP and antibiotic may have still occurred. Targeted delivery of cationic antibiotics, tetracycline and lincomycin, has been accomplished using bPEI-coated polyacrylic copolymer nanogels.191
5.2. Repurposing Bacterial Defenses into Offensive Attacks Using Nanobased Delivery of CRISPR-Cas Systems to Control Bacterial Metabolism.
Discovery of the clustered regularly interspaced short palindromic repeats (CRISPR) and their functional role in the prokaryotic adaptive immune system (CRISPR-associated system, Cas) has led to significant advances in genetic engineering. CRISPR-Cas is a site-specific gene editing tool, utilizing a naturally occurring RNA-guided endonuclease that functions to protect against viral infection in bacteria and archaea.192 More than 6000 research publications have appeared since applications began just a few years ago.
CRISPR-Cas systems have been extensively studied and modified for use in genetic modification by selective insertion, deletion, and/or mutation of targeted genes in most cellular organisms. A key difference in this system is the use of a short single-guided RNA sequences (sgRNA) as the specificity-determining factor in the formation of a dsDNA break at a targeted site in the genome compared to the other protein-based DNA-binding gene-editing techniques. The use of CRISPR/Cas9 avoids the requirement of protein engineering methods to develop site-specific nucleases against a specific DNA target sequence. While not discussed here, there are numerous published reviews providing an in-depth summary of the other biotechnological advances and uses of CRISPR-Cas genome engineering.192
Currently, applications of CRISPR-based antibacterials will provide several advances or modifications to the present methods available including the capability to target specific bacterial species within complex bacterial populations, delivering antibacterial agents to pathogenic bacteria, and delivery of therapeutic agents to infected host cells. Delivery of CRISPR-Cas9 antibacterial agents pose a unique challenge given in that the active protein–RNA complex (approximately 160-kDa) must be able to be transported across the bacterial membrane to be effective.193
To direct the CRISPR-Cas9 machinery to attack rather than provide a defense for bacteria, CRISPR guided RNAs must be designed to target either virulence factors and/or essential chromosomal genes specific to a particular bacterial species. These CRISPR guided RNAs will subsequently induce dsDNA breaks and typically result in lethality, especially if they are chromosomally targeted.
One approach that has been utilized by several groups already employs the species-level specificity of bacteriophages for CRISPR-Cas delivery. Using inert phages specific to the bacterial species of interest, the CRISPR-Cas9-targeting specific bacterial genes can be encoded in a phagemid enabling for packaging of CRISPR-Cas machinery into phage capsid.194 In 2014, a research study using phage-encoded CRISPR-Cas9 targeting antibiotic resistance in virulent strains of S. aureus concluded that the CRISPR-Cas9 antibacterials demonstrated species-specific pathogenic efficacy while sparing the non-targeted bacterial species This is an essential feature that is ideal for the development of novel, effective antibacterial agents, especially in the presence of the helpful human microbiome.195
By tuning the chemical properties of drug delivery of the CRISPR-Cas9, the targeting of healthy, uninfected cells can be prevented, allowing for the usage of lower dosage levels and thereby, reducing the overall cost of the treatment. Due to the structural diversity and wide range of sizes of phages, the traditional NP delivery strategies used are not currently reasonable nor practical. The use of different porous NPs to absorb phage-based treatments has been previously demonstrated; however, these methods are ineffective for large, nonsymmetrical phages, as the pore sizes are too small to effectively sorb the phages.196
One of the primary applications of the CRISPR-Cas9 system that has immense potential for use in metabolic engineering is by controlling the regulatory signaling pathways involved in the complex chemical communications for most cellular functions and synthesis pathways. The CRISPR-Cas9 gene-editing method has been used to facilitate the synthesis of valuable metabolic compounds, while concurrently reducing production of unwanted compounds and byproducts. This gene-editing system utilizes dCas9 to generate multiple tools that can block activities at a single site along complex multistep signaling pathways with no crosstalk. Since the “instructions” for the multiple steps in each signaling pathway are encoded in the genomic DNA, these processes are able to be controlled at each step—by either blocking or enabling transcription of the DNA at the promoter.197
5.3. Strategies in Disrupting Bacterial Cell–Cell Communication.
5.3.1. Nanoparticles as Signal Sponges.
To combat the increasing development of antibiotic resistance, one of the noncytotoxic approaches that has been proposed to control bacterial infections involves the use of NPs targeting the disruption of bacterial cell–cell chemical communication mechanisms and prevent the acquisition of resistance mechanisms toward antimicrobial therapies. The primary strategy that has been proposed for inhibiting biofilm formation involves targeting and interfering with quorum sensing (QS) molecules (Figure 6).198
Figure 6.
Targeting quorum sensing. Schematic of QS in bacteria as well as methods to block this signaling mechanism. AHL dependent QS within biofilms (left) can be blocked using competitive QS inhibition that outcompetes AHL for AHL receptors (middle) or quorum quenching enzymes that inactivate AHL signals (right) [Reprinted from ref 210. Copyright 2018 Beitelshees et al.].
QS communication involves the production, detection, and response to chemical environmental signaling molecules called autoinducers (AIs).199 AIs are secreted chemical signaling factors that are intracellularly synthesized and released into the extracellular environment, disperse through diffusion, and are recognized by adjacent cells and result in the alteration of gene expression accordingly dependent on population density. QS signals accumulate in the local environment as bacterial population density increases requiring threshold levels to be reached to produce a transcriptional response. The minimum behavioral unit, described as a quorum, corresponds to a particular bacterial cell density required to produce signals above threshold levels of target the signal and elicit a transcriptional response.
QS mechanisms are known to modulate a diversity of biological processes in bacteria involved in bioluminescence, sporulation, competence, antibiotic production, biofilm formation, and virulence factor secretion.200-202 In P. aeruginosa, the QS system is well-characterized and known to regulate the production of several compounds identified to play key roles in biofilm formation, including rhamnolipids, lectins (LecA/LecB), pyochelin, and pyoverdine siderophores.203-206 Different QS systems have been identified and are classified into several groups according to the type of autoinducer used, such as acyl-homoserine lactones (AHLs or AI-1), furanosyl borate diesters (AI-2s) and autoinducing peptides (AIPs), diffusible signal factors (DSFs), and diketopiperazines (DKPs).200
Based on the key roles that QS plays in bacterial virulence and pathogenesis, the disruption of bacterial communication systems is an emerging approach/strategy that has been described for the development of novel antimicrobial agents. This approach is referred to as “quorum quenching” (QQ) and includes any strategy targeted to interfere and/or disrupt microbial QS signaling (Figure 6). Inhibition can be done at several different steps in the signaling process: inhibition of autoinducer synthesis, degradation of autoinducer, and interception of its interaction(s) with the cognate receptor(s).207 Based upon the type of regulation elicited by QS signal(s), the agents used for targeting QS signaling should either inhibit or stimulate the QS-regulated gene expression, accordingly. As observed in the human pathogen Vibrio cholerae, QS signaling was demonstrated to repress biofilm formation and virulence factor production.208,209
NPs and related nanomaterials have been designed and utilized as QS inhibitors and NP-based carriers of compounds and/or molecules that possess QS quenching activity. In Vibrio fischeri, initial efforts using SiO2-NPs functionalized with β-cyclodextrin (β-CD) demonstrated an ability to bind AHLs and quench QS signaling, determined at the transcriptional level by the down-regulation of bacterial QS genes.211
Surface functionalized NPs with β-CD or N-acylated homoserine lactonase proteins (AiiA) can interfere with QS signaling molecules preventing them from reaching their designated target cognate receptor and thereby inhibiting the signal/receptor interaction.14 However, the hydrophobic cores of a β-CD are nonspecific in their binding of AHLs and/or other organic molecules. Future work should target incorporations of the binding domains for LuxR receptor proteins onto NPs to increase the specificity of AHL chelation by NPs. Bacterial QS systems function as major regulatory mechanisms of pathogenesis and are involved in the formation of biofilm structures. Using QS, bacterial populations are able to coordinate their gene expression, acquiring a competitive advantage to respond to environmental changes.200 Consequently, QS systems have promoted the formation of antibiotic tolerant communities. Therefore, bacterial biofilms pose a significant challenge to the efficacy of conventional antibiotics given that they serve as a recalcitrant form of bacterial growth that increases bacterial resistance to conventional antibiotics.14
Several studies demonstrated that bacteria are able to sense and store information regarding surface chemical exposure through the molecule cyclic adenosine monophosphate (cAMP). More recently, it was shown that the coupled cycling of cAMP levels and type IV pili (TFP) activities are coordinated to allow bacteria to adaptively adhere to surfaces. Interestingly, the same cAMP pattern was displayed by descendent cells across multiple generations allowing encompassed cells to persist in a coordinated, nonmotile state and remain as a biofilm.212
5.3.2. Signaling Analogs.
The identification and development of natural or synthetic chemical compounds and/or enzymes that facilitate quorum-sensing inhibition (QSI) has been an emerging approach to combat bacterial pathogenesis. Disruption of QS signaling mechanisms has been demonstrated by targeting the signaling molecules, signal biogenesis, or signal detection (Figure 6).213
Interfering with QS may provide a powerful, nonlethal strategy that does not select for resistance, and ultimately a tool for controlling virulence and antibiotic tolerance in QS pathogens. Such a tool can also be applied within antimicrobial chemotherapy to overcome bacterial infections. Currently, the methods that have been used to disrupt QS include: (1) antagonizing signal binding to LuxR-family receptor, (2) inhibition of signal production, (3) degrading signals, (4) trapping signals, and (5) suppression of synthase and receptor activities, stabilities, or productions (Figure 7).214
Figure 7.
Quorum quenching potential of nanomaterials. The agents interrupt QS signaling in different ways: (a) suppressing the production of autoinducers via reduction the activity of LuxI-type cognate receptor synthase and ATP binding cassettes, which produces AHLs and oligopeptides respectively; (b) degrading the autoinducers; (c) inhibiting the binding of autoinducers to the LuxR-type receptor protein; and (d) inhibiting biofilm formation [Reprinted with permission from ref 220. Copyright 2017 Taylor & Francis].
A diversity of compounds for QSI “signal analogs” have been identified and reported to inhibit bacterial communication processes.215-218 For example, analogs containing alterations in the acyl side chains of 3-oxo-C6-AHL for V. fischeri, 3-oxo-C12-AHL for P. aeruginosa, and 3-oxo-C8-AHL for Agrobacterium tumefaciens inhibited the binding of native AHLs.215,219 The cognate receptors were able to bind analogs at higher affinities compared to native AHL ligands, which resulted in the analogs inactivating AHL-mediated gene expression. The most extensively utilized QSI strategy that has been currently investigated involves the degradation and modification of QS signals in addition to the use of autoinducer antagonists. Smith and colleagues constructed a library of synthetic analogs to the P. aeruginosa las QS molecule, 3-oxo-C12-AHL, by substituting the homoserine lactone moiety with different alcohols, amines, and/or a 5- or 6-membered ring.216,217 Using a lasI promoter-fused gfp reporter strain for high-throughput screening of the compounds, three compounds were identified as antagonists. 3-Oxo-C12-(2-aminophenol) and 3-oxo-C12-(aminocyclopentanol) were demonstrated to inhibit the LasR activation attributed to the 3-oxo-C12-AHL–LasR interaction. Alternatively, 3-oxo-C12-(aminocyclohexanone) was observed to target not only LasR but also RhlR, which is an additional QS receptor in P. aeruginosa.
Similarly, a synthetic analog of C4-AHL, N-decanoyl cyclopentyl-amide (C10-CPA) was also reported to target both LasR and RhlR.221 C10-CPA inhibits lasB and rhlA gene activation by 3-oxo-C12-AHL and C4-AHL resulting in reductions in the elastase, pyocyanin, and rhamnolipid levels and biofilm formation. It was shown that changes in amide function coupled between the lactone ring and the fatty acid influence the AHL binding activity to receptor proteins, due to the amide forming hydrogen bonds with conserved tyrosine and aspartic acid in the AHL binding pocket.222 Compounds containing this modification have shown antagonistic behaviors with the V. fischeri LuxR receptor.
Selective and broad-spectrum antagonists effective across multiple species have been developed. C8-AHL, C10-AHL, 4-bromophenylpropionyl-AHL, and 4-iodophenylacetyl-AHL have been demonstrated to antagonize the AHL-binding to the TraR, LuxR, and LasR receptor proteins of A. tumefaciens, V. fischeri, and P. aeruginosa, respectively.223 It is still not well understood how the conformational change in the receptor occurs to distinguish between a “true ligand” (agonist) and “fake ligand” (antagonist). The crystal structures of the receptor–AHL binding form are available for a few species; however, it is imperative that we obtain both structures: an antagonist-binding form and a ligand-free form. Targeting the production or synthesis of AHL signals is an additional strategy for quorum sensing inhibition. Consequently, if no AHL signals are produced, no QS occurs, and therefore, bacterial communication is prevented from taking place. In an earlier study, several analogs of S-adenosyl-methionine (SAM), which is the second substrate for LuxI synthases, was demonstrated to inhibit the LuxI reaction.224 Recently a C8-AHL analog was identified to bind to AHL synthase resulting in the inhibition of its enzymatic activity.225 AHL synthesis inhibitors that are transition state analogs of MTAN (5′-methylthioadenosine/S-adenosylhomocysteine hydrolase) provide a strategy of inhibition that blocks not only AHL production but also the generation of AI-2 and pathogenicity.226 Coupling the recent results in QS disruptors with nanotargeted designs could provide fruitful approaches for future improvements in disrupting chemical communication in biofilms and ultimately in controlling infection-based pathogenicity itself.
5.3.3. Degradation of AHLs by Nanoparticle-Based Enzymes or Oxidants.
An alternative approach to the use of signal analogs involves the enzymatic breakdown of AHL signals (Figure 6). In natural bacteria, this occurs by two major classes of enzymes, called lactonases and acylases, both of which are produced by Gram-positive strains. The majority of work has focused on the lactonases, which hydrolyzes the lactone of an AHL. A lactonase enzyme from a Gram-positive Bacillus strain and is designated “AiiA”.227 Some lactonases can be specific in the AHLs that they target for hydrolysis. In Rhodococcus erythropolis, QsdA is a phophotriesterase (PTE) like protein capable of degrading AHLs with acyl chains of C6–C14 in length.228 AiiM protein in Microbacterium testaceum, an isolate from a potato leaf, was determined by an α/β hydrolase exhibiting a preference of C6 to C12-AHLs with 3-oxo substitutions compared to those without the substitution.229 A series of BpiB proteins (i.e., designated BpiB01, BpiB04, and BpiB07) isolated from soil-derived metagenomic libraries with traI-lacZ A. tumefaciens reporter strain, hydrolyzed the 3-oxo-C8-AHL. This also inhibited P. aeruginosa swarming motility and biofilm formation, which is regulated by C4-AHL and 3-oxo-C12-AHL.230 More recently, a new type of AHL lactonase isolated from the marine bacterium Pseudoalteromonas byunsanensis was described to encode a hybrid membrane protein containing GDSL hydrolase domain at the N-terminal of an RND (resistance-nodulation-cell-division)-type multidrug efflux transporter.231 The truncated form containing the GDSL hydrolase function was designated as QsdH and catalyzed hydrolyses of C4 to C12-AHLs (with or without 3-oxo substitution). The use of hydrolytic enzymes presents a signal specific tool to inhibit specific AHL signals, which can be potentially linked to NPs.
5.4. Degradation of Biofilms by Near-Infrared (NIR) Induced Oxidant Photoinactivation and Amyloid Inhibition.
As a promising strategy for antibacterial therapy, photodynamic therapy (PDT) has gained considerable attention recently. Photosensitizers (PSs) can be used to generate ROS upon visible light or infrared illumination in the presence of oxygen.178,232 More recently, considerable progress has been made on lanthanide-doped upconversion NPs (UCNPs) due to their unique properties of converting NIR light to ultraviolet or visible emission and activating PSs nearby for antibacterial applications. Sun and colleagues211 designed and synthesized a series of antimicrobial nanocomposite membranes containing novel UCNPs, which demonstrated potent antimicrobial activity upon activation by NIR against both Gram-positive S. aureus and Gram-negative E. coli. These nanocomposite membranes were revealed to provide potential practical applications in antibacterial wound dressing materials that are capable of sterilizing wound areas multiple times via external NIR irradiation.
As alluded to previously (section 4.1), amyloid proteins provide structural resiliency for the biofilm matrix. Therefore, the ability to decrease amyloid components or reduce fibrillation are being explored to reduce the structural efficacy of the biofilm itself. Recently, two well studied examples of plant-derived polyphenols are epigallocatechin gallate (EGCG) and penta-O-galloyl-β-d-glucose (PGG). EGCG was first shown to reduce amyloidogenic polypeptides.233 Subsequently, both EGCG and PGG have been shown to redirect the aggregation of FapC monomers oligomeric species, which ultimately results in a reduction in biofilm formation and susceptibility to antibiotic treatments.143,234 This demonstrates the biotechnologic potential for development of NP surfaces to act as carriers for delivery of such small molecules to bacterial cells, especially during the early stages of biofilm formation to reduce/prevent their resiliency to removal.
6. CONCLUSION
The applications of engineered NPs directly as antimicrobial agents or as carriers for the targeted delivery of antimicrobials offers much potential for removal of biofilm-related infections. However, fundamental issues must be understood and addressed before the resilient EPS matrix of biofilms can be successfully navigated. Significant hurdles remain in designing NPs to penetrate dense gel EPS matrices that often protect biofilms and in the controlled release of the antimicrobial agents. While smaller diameter (<20 nm) NPs with hydrophilic coatings may, at present, appear to have greater facility in penetrating EPS, the adaptable nature of bacteria and the biofilm itself will likely limit this as the only way to penetrate the biofilm. Optimizing delivery platforms and intelligent design of NPs will be essential for long-term efficacy. Finally, the precision, targeted delivery of antibiotics into specific biofilm infection sites is of paramount importance in the control of human infections but presents substantial challenge in NP development. At present, targeted activation may offer the most efficient solution. Further studies using controlled forms of hydrogels are needed to understand the natural variability present in biofilm matrices, and how these can be penetrated using NPs.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (RO1-149810) and the National Science Foundation, Biomaterials Program (1608151). Support for B.C.B. is also acknowledged from the SC SmartState Program.
ABBREVIATIONS
- AgNPs
silver nanoparticles
- AHL
acyl-homoserine lactone
- AiiA
N-acylated homoserine lactonase proteins
- AIPs
autoinducing peptides
- AIs
autoinducers
- AMPs
antimicrobial peptides
- AMR
antimicrobial resistance
- ARIs
antibiotic resistant infections
- β-CD
β-cyclodextrin
- CPP
cell penetrating peptide
- CRISPR
clustered regularly interspaced short palindromic repeats
- CW
cell wall
- DKPs
diketopeptides
- DSFs
diffusible soluble factors
- EPIs
efflux pump inhibitors
- EPSs
extracellular polymers
- LPS
lipopolysaccharide
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant Staphylococcus aureus
- NPs
nanoparticles
- OM
outer membrane
- PDT
photodynamic therapy
- PM
plasma membrane
- PMBN
polymyxin B nonapeptide
- PSs
photosensitizers
quorum quenching
- QS
quorum sensing
- QSI
quorum sensing inhibition
- ROS
reactive oxygen species
- sgRNA
single-guided RNA sequences
- SLNs
solid lipid nanoparticles
- UCNPs
upconversion nanoparticles
Footnotes
The authors declare no competing financial interest.
Contributor Information
Amjed Alabresm, Department of Environmental Health Sciences, University of South Carolina, Columbia, South Carolina 29208, United States; Department of Biological Development of Shatt Al-Arab & N. Arabian Gulf, Marine Science Centre, University of Basrah, Basrah, Iraq.
Savannah L. Chandler, Department of Chemistry and Biochemistry, University of South Carolina, Columbia, South Carolina 29208, United States.
Brian C. Benicewicz, Department of Chemistry and Biochemistry and USC NanoCenter, University of South Carolina, Columbia, South Carolina 29208, United States.
Alan W. Decho, Department of Environmental Health Sciences, University of South Carolina, Columbia, South Carolina 29208, United States
REFERENCES
- (1).Sipahi OR (2008) Economics of antibiotic resistance. Expert Rev. Anti-Infect. Ther 6 (4), 523–539. [DOI] [PubMed] [Google Scholar]
- (2).Fair RJ, and Tor Y (2014) Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem 6 (4), S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Shlaes DM (2010) Resistance. Antibiotics, 15–28. [Google Scholar]
- (4).Centers for Disease Control and Prevention (CDC). (2019) Antibiotic resistance threats in the United States; Atlanta. [Google Scholar]
- (5).Organization, W. H. (2015) Global Action Plan on Antimicrobial Resistance. Microbe Magazine 10, 354–355. [Google Scholar]
- (6).HHS. (2013) Environmental Justice Implementation Progress Report.
- (7).Burnham JP, Olsen MA, and Kollef MH (2019) Reestimating annual deaths due to multidrug-resistant organism infections. Infect. Control Hosp. Epidemiol 40 (1), 112–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Fischbach MA, and Walsh CT (2009) Antibiotics for emerging pathogens. Science 325 (5944), 1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, and Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis 48 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- (10).Gorbach SL (2001) Antimicrobial use in animal feed—time to stop. N. Engl. J. Med 345 (16), 1202–1203. [DOI] [PubMed] [Google Scholar]
- (11).Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Teillant A, and Laxminarayan R (2015) Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. U. S. A 112 (18), 5649–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Reardon S (2014) Phage therapy gets revitalized. Nature 510 (7503), 15–16. [DOI] [PubMed] [Google Scholar]
- (13).Santos RS, Figueiredo C, Azevedo NF, Braeckmans K, and De Smedt SC (2018) Nanomaterials and molecular transporters to overcome the bacterial envelope barrier: Towards advanced delivery of antibiotics. Adv. Drug Delivery Rev 136, 28–48. [DOI] [PubMed] [Google Scholar]
- (14).Zazo H, Colino CI, and Lanao JM (2016) Current applications of nanoparticles in infectious diseases. J. Controlled Release 224, 86–102. [DOI] [PubMed] [Google Scholar]
- (15).Schaack S, Gilbert C, and Feschotte C (2010) Promiscuous DNA: horizontal transfer of transposable elements and why it matters for eukaryotic evolution. Trends Ecol. Evol 25 (9), 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Gebreyohannes G, Nyerere A, Bii C, and Sbhatu DB (2019) Challenges of intervention, treatment, and antibiotic resistance of biofilm-forming microorganisms. Heliyon 5 (8), e02192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT, Creasy HH, Earl AM, FitzGerald MG, Fulton RS, et al. (2012) Structure, function and diversity of the healthy human microbiome. Nature 486 (7402), 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Sommer F, and Bäckhed F (2013) The gut microbiota—masters of host development and physiology. Nat. Rev. Microbiol 11 (4), 227–238. [DOI] [PubMed] [Google Scholar]
- (19).Stulberg E, Fravel D, Proctor LM, Murray DM, LoTempio J, Chrisey L, Garland J, Goodwin K, Graber J, Harris MC, et al. (2016) An assessment of US microbiome research. Nat. Microbiol 1 (1), 15015. [DOI] [PubMed] [Google Scholar]
- (20).Sender R, Fuchs S, and Milo R (2016) Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164 (3), 337–340. [DOI] [PubMed] [Google Scholar]
- (21).Baptista PV, McCusker MP, Carvalho A, Ferreira DA, Mohan NM, Martins M, and Fernandes AR (2018) Nanostrategies to fight multidrug resistant bacteria—“A Battle of the Titans. Front. Microbiol 9, 1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Jun B-H, Kim G, Noh MS, Kang H, Kim Y-K, Cho M-H, Jeong DH, and Lee Y-S (2011) Surface-enhanced Raman scattering-active nanostructures and strategies for bioassays. Nanomedicine 6 (8), 1463–1480. [DOI] [PubMed] [Google Scholar]
- (23).Chen C, Hu J, Zhang S, Zhou P, Zhao X, Xu H, Zhao X, Yaseen M, and Lu JR (2012) Molecular mechanisms of antibacterial and antitumor actions of designed surfactant-like peptides. Biomaterials 33 (2), 592–603. [DOI] [PubMed] [Google Scholar]
- (24).Walvekar P, Gannimani R, and Govender T (2019) Combination drug therapy via nanocarriers against infectious diseases. Eur. J. Pharm. Sci 127, 121–141. [DOI] [PubMed] [Google Scholar]
- (25).Canaparo R, Foglietta F, Giuntini F, Della Pepa C, Dosio F, and Serpe L (2019) Recent developments in antibacterial therapy: Focus on stimuli-responsive drug-delivery systems and therapeutic nanoparticles. Molecules 24 (10), 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Lee S, Stubelius A, Hamelmann N, Tran V, and Almutairi A (2018) Inflammation-responsive drug-conjugated dextran nanoparticles enhance anti-inflammatory drug efficacy. ACS Appl. Mater. Interfaces 10 (47), 40378–40387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Chou LY, Ming K, and Chan WC (2011) Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev 40 (1), 233–245. [DOI] [PubMed] [Google Scholar]
- (28).Dik DA, Fisher JF, and Mobashery S (2018) Cell-wall recycling of the Gram-negative bacteria and the nexus to antibiotic resistance. Chem. Rev 118 (12), 5952–5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Gan L, Chen S, and Jensen GJ (2008) Molecular organization of Gram-negative peptidoglycan. Proc. Natl. Acad. Sci. U. S. A 105 (48), 18953–18957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Silhavy TJ, Kahne D, and Walker S (2010) The bacterial cell envelope. Cold Spring Harbor Perspect. Biol 2 (5), a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Misra G, Rojas ER, Gopinathan A, and Huang KC (2013) Mechanical consequences of cell-wall turnover in the elongation of a Gram-positive bacterium. Biophys. J 104 (11), 2342–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Holme T, Lindberg A, Garegg P, and Onn T (1968) Chemical composition of cell-wall polysaccharide of rough mutants of Salmonella typhimurium. J. Gen. Microbiol 52 (1), 45–54. [Google Scholar]
- (33).Bayer ME, and Sloyer JL Jr (1990) The electrophoretic mobility of Gram-negative and Gram-positive bacteria: an electrokinetic analysis. J. Gen. Microbiol 136 (5), 867–874. [DOI] [PubMed] [Google Scholar]
- (34).Siegbahn PE, and Blomberg MR (2000) Transition-metal systems in biochemistry studied by high-accuracy quantum chemical methods. Chem. Rev 100 (2), 421–438. [DOI] [PubMed] [Google Scholar]
- (35).Kittler S, Greulich C, Diendorf J, Koller M, and Epple M (2010) Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater 22 (16), 4548–4554. [Google Scholar]
- (36).Baek Y-W, and An Y-J (2011) Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci. Total Environ 409 (8), 1603–1608. [DOI] [PubMed] [Google Scholar]
- (37).Horie M, Fujita K, Kato H, Endoh S, Nishio K, Komaba LK, Nakamura A, Miyauchi A, Kinugasa S, Hagihara Y, et al. (2012) Association of the physical and chemical properties and the cytotoxicity of metal oxidenanoparticles: metal ion release, adsorption ability and specific surface area. Metallomics 4 (4), 350–360. [DOI] [PubMed] [Google Scholar]
- (38).Seil JT, and Webster TJ (2012) Antimicrobial applications of nanotechnology: methods and literature. Int. J. Nanomed 7, 2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, and Mohamad D (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 7 (3), 219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Leung YH, Xu X, Ma AP, Liu F, Ng AM, Shen Z, Gethings LA, Guo MY, Djurišić AB, Lee PK, et al. (2016) Toxicity of ZnO and TiO2 to Escherichia coli cells. Sci. Rep 6 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Huh AJ, and Kwon YJ (2011) Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Controlled Release 156 (2), 128–145. [DOI] [PubMed] [Google Scholar]
- (42).Dastjerdi R, and Montazer M (2010) A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids Surf., B 79 (1), 5–18. [DOI] [PubMed] [Google Scholar]
- (43).Chithrani BD, Ghazani AA, and Chan WC (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 6 (4), 662–668. [DOI] [PubMed] [Google Scholar]
- (44).Shang L, Nienhaus K, and Nienhaus GU (2014) Engineered nanoparticles interacting with cells: size matters. J. Nanobiotechnol 12 (1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Yohan D, Cruje C, Lu X, and Chithrani DB (2016) Size-dependent gold nanoparticle interaction at nano–micro interface using both monolayer and multilayer (tissue-like) cell models. Nano-Micro Lett 8 (1), 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Vukomanović M, Logar M, Škapin S, and Suvorov D (2014) Hydroxyapatite/gold/arginine: designing the structure to create antibacterial activity. J. Mater. Chem. B 2 (11), 1557–1564. [DOI] [PubMed] [Google Scholar]
- (47).Slavin YN, Asnis J, Häfeli UO, and Bach H (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol 15 (1), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Karakoti A, Hench L, and Seal S (2006) The potential toxicity of nanomaterials—the role of surfaces. JOM 58 (7), 77–82. [Google Scholar]
- (49).Mu H, Tang J, Liu Q, Sun C, Wang T, and Duan J (2016) Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Sci. Rep 6 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Wang L, Hu C, and Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed 12, 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Ivask A, ElBadawy A, Kaweeteerawat C, Boren D, Fischer H, Ji Z, Chang CH, Liu R, Tolaymat T, Telesca D, et al. (2014) Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano 8 (1), 374–386. [DOI] [PubMed] [Google Scholar]
- (52).Kim JS, Kuk E, Yu KN, Kim J-H, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang C-Y, et al. (2007) Antimicrobial effects of silver nanoparticles. Nanomedicine 3 (1), 95–101. [DOI] [PubMed] [Google Scholar]
- (53).AshaRani P, Low Kah Mun G, Hande MP, and Valiyaveettil S (2009) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3 (2), 279–290. [DOI] [PubMed] [Google Scholar]
- (54).Rai M, Yadav A, and Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv 21 (1), 76–83. [DOI] [PubMed] [Google Scholar]
- (55).Shaikh S, Nazam N, Rizvi SMD, Ahmad K, Baig MH, Lee EJ, and Choi I (2019) Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci 20 (10), 2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Makowski M, Silva ÍC, Pais do Amaral C, Gonçalves S, and Santos NC (2019) Advances in lipid and metal nanoparticles for antimicrobial peptide delivery. Pharmaceutics 11 (11), 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Javanbakht T, Laurent S, Stanicki D, and Wilkinson KJ (2016) Relating the surface properties of superparamagnetic iron oxide nanoparticles (SPIONs) to their bactericidal effect towards a biofilm of Streptococcus mutans. PLoS One 11 (4), e0154445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Sabale S, Kandesar P, Jadhav V, Komorek R, Motkuri RK, and Yu X-Y (2017) Recent developments in the synthesis, properties, and biomedical applications of core/shell superparamagnetic iron oxide nanoparticles with gold. Biomater. Sci 5 (11), 2212–2225. [DOI] [PubMed] [Google Scholar]
- (59).Ibelli T, Templeton S, and Levi-Polyachenko N (2018) Progress on utilizing hyperthermia for mitigating bacterial infections. Int. J. Hyperthermia 34 (2), 144–156. [DOI] [PubMed] [Google Scholar]
- (60).Chen J, Andler SM, Goddard JM, Nugen SR, and Rotello VM (2017) Integrating recognition elements with nanomaterials for bacteria sensing. Chem. Soc. Rev 46 (5), 1272–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Khan MR, Fromm KM, Rizvi TF, Giese B, Ahamad F, Turner RJ, Füeg M, and Marsili E (2020) Metal nanoparticle–microbe interactions: synthesis and antimicrobial effects. Part. Part. Syst. Charact 37 (5), 1900419. [Google Scholar]
- (62).Furneri PM, Fuochi V, and Pignatello R (2018) Lipid-based nanosized delivery systems for fluoroquinolones: A review. Curr. Pharm. Des 23 (43), 6696–6704. [DOI] [PubMed] [Google Scholar]
- (63).Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, and Braeckmans K (2014) Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Controlled Release 190, 607–623. [DOI] [PubMed] [Google Scholar]
- (64).Fang C-L, Aljuffali IA, Li Y-C, and Fang J-Y (2014) Delivery and targeting of nanoparticles into hair follicles. Ther. Delivery 5 (9), 991–1006. [DOI] [PubMed] [Google Scholar]
- (65).Hao N, Li L, and Tang F (2017) Roles of particle size, shape and surface chemistry of mesoporous silica nanomaterials on biological systems. Int. Mater. Rev 62 (2), 57–77. [Google Scholar]
- (66).Bernardos A, Piacenza E, Sancenón F, Hamidi M, Maleki A, Turner RJ, and Martínez-Máñez R (2019) Mesoporous silica-based materials with bactericidal properties. Small 15 (24), 1900669. [DOI] [PubMed] [Google Scholar]
- (67).Francia V, Montizaan D, and Salvati A (2020) Interactions at the cell membrane and pathways of internalization of nano-sized materials for nanomedicine. Beilstein J. Nanotechnol 11 (1), 338–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Aruguete DM, and Hochella MF (2010) Bacteria–nanoparticle interactions and their environmental implications. Environ. Chem 7 (1), 3–9. [Google Scholar]
- (69).Zhang L, Jiang Y, Ding Y, Povey M, and York D (2007) Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res 9 (3), 479–489. [Google Scholar]
- (70).Pareek V, Gupta R, and Panwar J (2018) Do physicochemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng., C 90, 739–749. [DOI] [PubMed] [Google Scholar]
- (71).Tamayo L, Zapata P, Vejar N, Azócar M, Gulppi M, Zhou X, Thompson G, Rabagliati F, and Páez M (2014) Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater. Sci. Eng., C 40, 24–31. [DOI] [PubMed] [Google Scholar]
- (72).Liu J, Aruguete DM, Murayama M, and Hochella MF Jr (2009) Influence of size and aggregation on the reactivity of an environmentally and industrially relevant nanomaterial (PbS). Environ. Sci. Technol 43 (21), 8178–8183. [DOI] [PubMed] [Google Scholar]
- (73).Kerisit S, and Liu C (2009) Molecular simulations of water and ion diffusion in nanosized mineral fractures. Environ. Sci. Technol 43 (3), 777–782. [DOI] [PubMed] [Google Scholar]
- (74).Dahl JA, Maddux BL, and Hutchison JE (2007) Toward greener nanosynthesis. Chem. Rev 107 (6), 2228–2269. [DOI] [PubMed] [Google Scholar]
- (75).Shankar SS, Rai A, Ahmad A, and Sastry M (2004) Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci 275 (2), 496–502. [DOI] [PubMed] [Google Scholar]
- (76).Lesniak A, Fenaroli F, Monopoli MP, Åberg C, Dawson KA, and Salvati A (2012) Effects of the Presence or Absence of a Protein Corona on Silica Nanoparticle Uptake and Impact on Cells. ACS Nano 6 (7), 5845–5857. [DOI] [PubMed] [Google Scholar]
- (77).Benfield AH, and Henriques ST (2020) Mode-of-Action of Antimicrobial Peptides: Membrane Disruption vs. Intracellular Mechanisms. Front. Med. Technol, DOI: 10.3389/fmedt.2020.610997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Raheem N, and Straus SK (2019) Mechanisms of action for antimicrobial peptides with antibacterial and antibiofilm functions. Front. Microbiol, DOI: 10.3389/fmicb.2019.02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Rahman MA, Bam M, Luat E, Jui MS, Ganewatta MS, Shokfai T, Nagarkatti M, Decho AW, and Tang C (2018) Macromolecular-clustered facial amphiphilic antimicrobials. Nat. Commun 9 (1), 5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Armstead AL, and Li B (2011) Nanomedicine as an emerging approach against intracellular pathogens. Int. J. Nanomed 6, 3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Zhang L, Pornpattananangkul D, Hu CMJ, and Huang CM (2010) Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem 17 (6), 585–594. [DOI] [PubMed] [Google Scholar]
- (82).Ramalingam V (2019) Multifunctionality of gold nanoparticles: Plausible and convincing properties. Adv. Colloid Interface Sci 271, 101989. [DOI] [PubMed] [Google Scholar]
- (83).Masi M, Réfregiers M, Pos KM, and Pagès J-M (2017) Mechanisms of envelope permeability and antibiotic influx and efflux in Gram-negative bacteria. Nat. Microbiol 2 (3), 17001. [DOI] [PubMed] [Google Scholar]
- (84).Vaara M (2010) Polymyxins and their novel derivatives. Curr. Opin. Microbiol 13 (5), 574–581. [DOI] [PubMed] [Google Scholar]
- (85).Bolla J-M, Alibert-Franco S, Handzlik J, Chevalier J, Mahamoud A, Boyer G, Kieć-Kononowicz K, and Pagès J-M (2011) Strategies for bypassing the membrane barrier in multidrug resistant Gram-negative bacteria. FEBS Lett. 585 (11), 1682–1690. [DOI] [PubMed] [Google Scholar]
- (86).Heidari Torkabadi H, Bethel CR, Papp-Wallace KM, de Boer PAJ, Bonomo RA, and Carey PR (2014) Following drug uptake and reactions inside Escherichia coli Cells by raman microspectroscopy. Biochemistry 53 (25), 4113–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Reuter A, Virolle C, Goldlust K, Berne-Dedieu A, Nolivos S, and Lesterlin C (2020) Direct visualisation of drug-efflux in live Escherichia coli cells. FEMS Microbiol. Rev 44 (6), 782–792. [DOI] [PubMed] [Google Scholar]
- (88).Pankuch GA, Lin G, Seifert H, and Appelbaum PC (2008) Activity of Meropenem with and without Ciprofloxacin and Colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents Chemother 52 (1), 333–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Aoki N, Tateda K, Kikuchi Y, Kimura S, Miyazaki C, Ishii Y, Tanabe Y, Gejyo F, and Yamaguchi K (2009) Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J. Antimicrob. Chemother 63 (3), 534–542. [DOI] [PubMed] [Google Scholar]
- (90).Humphries RM, Kelesidis T, Dien Bard J, Ward KW, Bhattacharya D, and Lewinski MA (2010) Successful treatment of pan-resistant Klebsiella pneumoniae pneumonia and bacteraemia with a combination of high-dose tigecycline and colistin. J. Med. Microbiol 59 (11), 1383–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Gordon NC, Png K, and Wareham DW (2010) Potent synergy and sustained bactericidal activity of a vancomycin-colistin combination versus multidrug-resistant trains of Acinetobacter baumannii. Antimicrob. Agents Chemother 54 (12), 5316–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Mamelli L, Petit S, Chevalier J, Giglione C, Lieutaud A, Meinnel T, Artaud I, and Pagès J-M (2009) New antibiotic molecules: bypassing the membrane barrier of Gram negative bacteria increases the activity of peptide deformylase inhibitors. PLoS One 4 (7) , e6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Gill AO, and Holley RA (2006) Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int. J. Food Microbiol 108 (1), 1–9. [DOI] [PubMed] [Google Scholar]
- (94).Hemaiswarya S, and Doble M (2009) Synergistic interaction of eugenol with antibiotics against Gram negative bacteria. Phytomedicine 16 (11), 997–1005. [DOI] [PubMed] [Google Scholar]
- (95).Nikaido H (2003) Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev 67 (4), 593–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Bush K, and Macielag MJ (2010) New β-lactam antibiotics and β-lactamase inhibitors. Expert Opin. Ther. Pat 20 (10), 1277–1293. [DOI] [PubMed] [Google Scholar]
- (97).Salmi C, Loncle C, Vidal N, Letourneux Y, Fantini J, Maresca M, Taïeb N, Pagès J-M, and Brunel JM (2008) Squalamine: an appropriate strategy against the emergence of multidrug resistant Gram-negative bacteria? PLoS One 3 (7), e2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Alhanout K, Malesinki S, Vidal N, Peyrot V, Rolain JM, and Brunel JM (2010) New insights into the antibacterial mechanism of action of squalamine. J. Antimicrob. Chemother 65 (8), 1688–1693. [DOI] [PubMed] [Google Scholar]
- (99).Lavigne J-P, Brunel J-M, Chevalier J, and Pagès J-M (2010) Squalamine, an original chemosensitizer to combat antibiotic-resistant Gram-negative bacteria. J. Antimicrob. Chemother 65 (4), 799–801. [DOI] [PubMed] [Google Scholar]
- (100).Epand RM, Epand RF, and Savage PB (2008) Ceragenins (cationic steroid compounds), a novel class of antimicrobial agents. Drug News Perspect. 21 (6), 307–311. [DOI] [PubMed] [Google Scholar]
- (101).Findlay B, Zhanel GG, and Schweizer F (2010) Cationic amphiphiles, a new generation of antimicrobials inspired by the natural antimicrobial peptide scaffold. Antimicrob. Agents Chemother 54 (10), 4049–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Costa JR, Silva NC, Sarmento B, and Pintado M (2015) Potential chitosan-coated alginate nanoparticles for ocular delivery of daptomycin. Eur. J. Clin. Microbiol. Infect. Dis 34 (6), 1255–1262. [DOI] [PubMed] [Google Scholar]
- (103).Hussein MZ, Al Ali S, Geilich B, El Zowalaty M, and Webster T (2014) Synthesis, characterization, and antimicrobial activity of an ampicillin-conjugated magnetic nanoantibiotic for medical applications. Int. J. Nanomed 9, 3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Wang L, Chen YP, Miller KP, Cash BM, Jones S, Glenn S, Benicewicz BC, and Decho AW (2014) Functionalised nanoparticles complexed with antibiotic efficiently kill MRSA and other bacteria. Chem. Commun 50 (81), 12030–12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Alabresm A, Chen YP, Wichter-Chandler S, Lead J, Benicewicz BC, and Decho AW (2020) Nanoparticles as antibiotic-delivery vehicles (ADVs) overcome resistance by MRSA and other MDR bacterial pathogens: The grenade hypothesis. J. Glob. Antimicrob. Resist 22, 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).McQuillan JS, Groenaga Infante H, Stokes E, and Shaw AM (2012) Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12. Nanotoxicology 6 (8), 857–866. [DOI] [PubMed] [Google Scholar]
- (107).Mukha IP, Eremenko AM, Smirnova NP, Mikhienkova AI, Korchak GI, Gorchev VF, and Chunikhin AY (2013) Antimicrobial activity of stable silver nanoparticles of a certain size. Appl. Biochem. Microbiol 49 (2), 199–206. [DOI] [PubMed] [Google Scholar]
- (108).Ramalingam B, Parandhaman T, and Das SK (2016) Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of Gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 8 (7), 4963–4976. [DOI] [PubMed] [Google Scholar]
- (109).Costerton JW, Geesey GG, and Cheng K-J (1978) How bacteria stick. Sci. Am 238 (1), 86–95. [DOI] [PubMed] [Google Scholar]
- (110).Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, and Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol 14 (9), 563–575. [DOI] [PubMed] [Google Scholar]
- (111).Flemming H-C, and Wingender J (2010) The biofilm matrix. Nat. Rev. Microbiol 8 (9), 623–633. [DOI] [PubMed] [Google Scholar]
- (112).Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GCL, and Parsek MR (2011) The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 7 (1), e1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Böckelmann U, Janke A, Kuhn R, Neu TR, Wecke J, Lawrence JR, and Szewzyk U (2006) Bacterial extracellular DNA forming a defined network-like structure. FEMS Microbiol. Lett 262 (1), 31–38. [DOI] [PubMed] [Google Scholar]
- (114).Böckelmann U, Manz W, Neu TR, and Szewzyk U (2002) Investigation of lotic microbial aggregates by a combined technique of fluorescent in situ hybridization and lectin-binding-analysis. J. Microbiol. Methods 49 (1), 75–87. [DOI] [PubMed] [Google Scholar]
- (115).Tang L, Schramm A, Neu TR, Revsbech NP, and Meyer RL (2013) Extracellular DNA in adhesion and biofilm formation of four environmental isolates: a quantitative study. FEMS Microbiol. Ecol 86 (3), 394–403. [DOI] [PubMed] [Google Scholar]
- (116).Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sørensen SR, Moser C, Kühl M, Jensen PØ, and Høiby N (2013) The in vivo biofilm. Trends Microbiol. 21 (9), 466–474. [DOI] [PubMed] [Google Scholar]
- (117).Hall-Stoodley L, Costerton JW, and Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol 2 (2), 95–108. [DOI] [PubMed] [Google Scholar]
- (118).Høiby N, Bjarnsholt T, Givskov M, Molin S, and Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35 (4), 322–332. [DOI] [PubMed] [Google Scholar]
- (119).Gunn JS, Bakaletz LO, and Wozniak DJ (2016) What’s on the Outside Matters: The role of the extracellular polymeric substance of Gram-negative biofilms in evading host Immunity and as a target for therapeutic intervention*. J. Biol. Chem 291 (24), 12538–12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Davies D (2003) Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discovery 2 (2), 114–122. [DOI] [PubMed] [Google Scholar]
- (121).Seviour T, Derlon N, Dueholm MS, Flemming H-C, Girbal-Neuhauser E, Horn H, Kjelleberg S, van Loosdrecht MCM, Lotti T, Malpei MF, et al. (2019) Extracellular polymeric substances of biofilms: Suffering from an identity crisis. Water Res. 151, 1–7. [DOI] [PubMed] [Google Scholar]
- (122).Stewart PS, and Franklin MJ (2008) Physiological heterogeneity in biofilms. Nat. Rev. Microbiol 6 (3), 199–210. [DOI] [PubMed] [Google Scholar]
- (123).Boudarel H, Mathias J-D, Blaysat B, and Grédiac M (2018) Towards standardized mechanical characterization of microbial biofilms: analysis and critical review. npj Biofilms Microbiomes 4 (1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Stewart PS, and Parker AE (2019) Measuring antimicrobial efficacy against biofilms: a meta-analysis. Antimicrob. Agents Chemother 63 (5), e00020–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Stewart PS, Zhang T, Xu R, Pitts B, Walters MC, Roe F, Kikhney J, and Moter A (2016) Reaction–diffusion theory explains hypoxia and heterogeneous growth within microbial biofilms associated with chronic infections. npj Biofilms Microbiomes 2 (1), 16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Haurat MF, Aduse-Opoku J, Rangarajan M, Dorobantu L, Gray MR, Curtis MA, and Feldman MF (2011) Selective sorting of cargo proteins into bacterial membrane vesicles*. J. Biol. Chem 286 (2), 1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Dong T, and Schellhorn HE (2010) Role of RpoS in virulence of pathogens. Infect. Immun 78 (3), 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Madsen JS, Burmølle M, Hansen LH, and Sørensen SJ (2012) The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol. Med. Microbiol 65 (2), 183–195. [DOI] [PubMed] [Google Scholar]
- (129).Crabbé A, Jensen PØ, Bjarnsholt T, and Coenye T (2019) Antimicrobial tolerance and metabolic adaptations in microbial biofilms. Trends Microbiol. 27 (10), 850–863. [DOI] [PubMed] [Google Scholar]
- (130).Khameneh B, Diab R, Ghazvini K, and Fazly Bazzaz BS (2016) Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog 95, 32–42. [DOI] [PubMed] [Google Scholar]
- (131).Penesyan A, Nagy SS, Kjelleberg S, Gillings MR, and Paulsen IT (2019) Rapid microevolution of biofilm cells in response to antibiotics. npj Biofilms Microbiomes 5 (1), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Decho AW (2015) Localization of quorum sensing by extracellular polymeric substances (EPS): considerations of in situ signaling. In The Physical Basis of Bacterial Quorum Communication; (Hagen SJ, Ed.) pp 105–121, Springer, New York, NY; DOI: 10.1007/978-1-4939-1402-9. [DOI] [Google Scholar]
- (133).Allison KR, Brynildsen MP, and Collins JJ (2011) Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473 (7346), 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Prax M, and Bertram R (2014) Metabolic aspects of bacterial persisters. Front. Cell. Infect. Microbiol, DOI: 10.3389/fcimb.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Kim W, Zhu W, Hendricks GL, Van Tyne D, Steele AD, Keohane CE, Fricke N, Conery AL, Shen S, Pan W, et al. (2018) A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 556 (7699), 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Sunde M, Serpell LC, Bartlam M, Fraser PE, Pepys MB, and Blake CC (1997) Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol 273 (3), 729–739. [DOI] [PubMed] [Google Scholar]
- (137).Larsen P, Nielsen JL, Dueholm MS, Wetzel R, Otzen D, and Nielsen PH (2007) Amyloid adhesins are abundant in natural biofilms. Environ. Microbiol 9 (12), 3077–3090. [DOI] [PubMed] [Google Scholar]
- (138).Jordal PB, Dueholm MS, Larsen P, Petersen SV, Enghild JJ, Christiansen G, Højrup P, Nielsen PH, and Otzen DE (2009) Widespread abundance of functional bacterial amyloid in mycolata and other gram-positive bacteria. Appl. Environ. Microbiol 75 (12), 4101–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Van Gerven N, Klein RD, Hultgren SJ, and Remaut H (2015) Bacterial amyloid formation: structural insights into curli biogensis. Trends Microbiol. 23 (11), 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Rasmussen HØ, Otzen DE, and Pedersen JS (2021) A multimethod approach for analyzing FapC fibrillation and determining mass per length. Biophys. J 120 (11), 2262–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Rasmussen CB, Christiansen G, Vad BS, Lynggaard C, Enghild JJ, Andreasen M, and Otzen D (2019) Imperfect repeats in the functional amyloid protein FapC reduce the tendency to fragment during fibrillation. Protein Sci. 28 (3), 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Christensen LFB, Jensen KF, Nielsen J, Vad BS, Christiansen G, and Otzen DE (2019) Reducing the amyloidogenicity of functional amyloid protein FapC increases its ability to inhibit α-synuclein fibrillation. ACS Omega 4 (2), 4029–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Najarzadeh Z, Pedersen JN, Christiansen G, Shojaosadati SA, Pedersen JS, and Otzen DE (2019) Bacterial amphiphiles as amyloid inducers: Effect of Rhamnolipid and Lipopolysaccharide on FapC fibrillation. Biochim. Biophys. Acta, Proteins Proteomics 1867 (11), 140263. [DOI] [PubMed] [Google Scholar]
- (144).Dueholm MS, Søndergaard MT, Nilsson M, Christiansen G, Stensballe A, Overgaard MT, Givskov M, Tolker-Nielsen T, Otzen DE, and Nielsen PH (2013) Expression of Fap amyloids in Pseudomonas aeruginosa, P. fluorescens, and P. putida results in aggregation and increased biofilm formation. MicrobiologyOpen 2 (3), 365–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Seviour T, Hansen SH, Yang L, Yau YH, Wang VB, Stenvang MR, Christiansen G, Marsili E, Givskov M, Chen Y, et al. (2015) Functional amyloids keep quorum-sensing molecules in check. J. Biol. Chem 290 (10), 6457–6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Yin H, Deng Y, Wang H, Liu W, Zhuang X, and Chu W (2015) Tea polyphenols as an antivirulence compound disrupt quorum-sensing regulated pathogenicity of Pseudomonas aeruginosa. Sci. Rep 5 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Rouse SL, Matthews SJ, and Dueholm MS (2018) Ecology and biogenesis of functional amyloids in Pseudomonas. J. Mol. Biol 430 (20), 3685–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Andreasen M, Meisl G, Taylor JD, Michaels TC, Levin A, Otzen DE, Chapman MR, Dobson CM, Matthews SJ, and Knowles TP (2019) Physical determinants of amyloid assembly in biofilm formation. mBio 10 (1), e02279–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Kuo J.-n., McPherson B, Soon A, Pasternak J, and Garrett C (2010) Environmental concentrations of methoprene and its transformation products after the treatment of Altosid® XR Briquets in the city of Richmond, British Columbia, Canada. Environ. Toxicol. Chem 29 (10), 2200–2205. [DOI] [PubMed] [Google Scholar]
- (150).Gloag ES, Fabbri S, Wozniak DJ, and Stoodley P (2020) Biofilm mechanics: Implications in infection and survival. Biofilm 2, 100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Miao L, Wang C, Hou J, Wang P, Ao Y, Li Y, Geng N, Yao Y, Lv B, Yang Y, et al. (2016) Aggregation and removal of copper oxide (CuO) nanoparticles in wastewater environment and their effects on the microbial activities of wastewater biofilms. Bioresour. Technol 216, 537–544. [DOI] [PubMed] [Google Scholar]
- (152).Slomberg DL, Lu Y, Broadnax AD, Hunter RA, Carpenter AW, and Schoenfisch MH (2013) Role of sze and shape on biofilm eradication for nitric oxide-releasing slica nanoparticles. ACS Appl. Mater. Interfaces 5 (19), 9322–9329. [DOI] [PubMed] [Google Scholar]
- (153).Yu Q, Li J, Zhang Y, Wang Y, Liu L, and Li M (2016) Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci. Rep 6 (1), 26667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Chifiriuc C, Grumezescu V, Grumezescu AM, Saviuc C, Lazăr V, and Andronescu E (2012) Hybrid magnetite nanoparticles/Rosmarinus officinalis essential oil nanobiosystem with antibiofilm activity. Nanoscale Res. Lett 7 (1), 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Banin E, Friedman A, Gedanken A, and Lellouche J (2012) Antibacterial and antibiofilm properties of yttrium fluoride nanoparticles. Int. J. Nanomed 7, 5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Markowska K, Grudniak AM, and Wolska KI (2013) Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim. Polym, DOI: 10.18388/abp.2013_2016. [DOI] [PubMed] [Google Scholar]
- (157).Hetrick EM, Shin JH, Paul HS, and Schoenfisch MH (2009) Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 30 (14), 2782–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Hajipour MJ, Fromm KM, Akbar Ashkarran A, Jimenez de Aberasturi D, Larramendi I. R. d., Rojo T, Serpooshan V, Parak WJ, and Mahmoudi M (2012) Antibacterial properties of nanoparticles. Trends Biotechnol 30 (10), 499–511. [DOI] [PubMed] [Google Scholar]
- (159).Ikuma K, Decho AW, and Lau BLT (2015) When nanoparticles meet biofilms—interactions guiding the environmental fate and accumulation of nanoparticles. Front. Microbiol, DOI: 10.3389/fmicb.2015.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Park H, Park H-J, Kim JA, Lee SH, Kim JH, Yoon J, and Park TH (2011) Inactivation of Pseudomonas aeruginosa PA01 biofilms by hyperthermia using superparamagnetic nanoparticles. J. Microbiol. Methods 84 (1), 41–45. [DOI] [PubMed] [Google Scholar]
- (161).Decho AW (1999) Imaging an alginate polymer gel matrix using atomic force microscopy. Carbohydr. Res 315 (3), 330–333. [Google Scholar]
- (162).Schmitt J, and Flemming H-C (1999) Water binding in biofilms. Water Sci. Technol 39 (7), 77–82. [Google Scholar]
- (163).Neu TR, Manz B, Volke F, Dynes JJ, Hitchcock AP, and Lawrence JR (2010) Advanced imaging techniques for assessment of structure, composition and function in biofilm systems. FEMS Microbiol. Ecol 72 (1), 1–21. [DOI] [PubMed] [Google Scholar]
- (164).Aldeek F, Schneider R, Fontaine-Aupart M-P, Mustin C, Lécart S, Merlin C, and Block J-C (2013) Patterned hydrophobic domains in the exopolymer matrix of Shewanella oneidensis MR-1 biofilms. Appl. Environ. Microbiol 79 (4), 1400–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Decho AW (2000) Exopolymer microdomains as a structuring agent for heterogeneity within microbial biofilms. Microbial sediments, 9–15. [Google Scholar]
- (166).Lawrence JRLR, Swerhone GDWSDW, Kuhlicke UK, and Neu TRNR (2007) In situ evidence for microdomains in the polymer matrix of bacterial microcolonies. Can. J. Microbiol 53 (3), 450–458. [DOI] [PubMed] [Google Scholar]
- (167).Peulen T-O, and Wilkinson KJ (2011) Diffusion of nanoparticles in a biofilm. Environ. Sci. Technol 45 (8), 3367–3373. [DOI] [PubMed] [Google Scholar]
- (168).Fabrega J, Renshaw JC, and Lead JR (2009) Interactions of silver nanoparticles with Pseudomonas putida bofilms. Environ. Sci. Technol 43 (23), 9004–9009. [DOI] [PubMed] [Google Scholar]
- (169).Mayer TG, Weingart R, Münstermann F, Kawada T, Kurzchalia T, and Schmidt RR (1999) Synthesis of labeled glycosyl phosphatidyl inositol (GPI) anchors. Eur. J. Org. Chem 1999 (10), 2563–2571. [Google Scholar]
- (170).Rodríguez-Suárez JM, Butler CS, Gershenson A, and Lau BLT (2020) Heterogeneous diffusion of polystyrene nanoparticles through an alginate matrix: The role of cross-linking and particle size. Environ. Sci. Technol 54 (8), 5159–5166. [DOI] [PubMed] [Google Scholar]
- (171).Jennings LK, Storek KM, Ledvina HE, Coulon C, Marmont LS, Sadovskaya I, Secor PR, Tseng BS, Scian M, Filloux A, et al. (2015) Pel is a cationic exopolysaccharide that crosslinks extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. U. S. A 112 (36), 11353–11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Larsen P, Nielsen JL, Otzen D, and Nielsen PH (2008) Amyloid-Like adhesins produced by floc-forming and filamentous bacteria in activated sludge. Appl. Environ. Microbiol 74 (5), 1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Otzen D, and Nielsen PH (2008) We find them here, we find them there: Functional bacterial amyloid. Cell. Mol. Life Sci 65 (6), 910–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Lau BLT, Hockaday WC, Ikuma K, Furman O, and Decho AW (2013) A preliminary assessment of the interactions between the capping agents of silver nanoparticles and environmental organics. Colloids Surf., A 435, 22–27. [Google Scholar]
- (175).Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang Y-Y, Cone R, and Hanes J (2007) Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. U. S. A 104 (5), 1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Ferry JL, Craig P, Hexel C, Sisco P, Frey R, Pennington PL, Fulton MH, Scott IG, Decho AW, Kashiwada S, et al. (2009) Transfer of gold nanoparticles from the water column to the estuarine food web. Nat. Nanotechnol 4 (7), 441–444. [DOI] [PubMed] [Google Scholar]
- (177).Nevius BA, Chen YP, Ferry JL, and Decho AW (2012) Surface-functionalization effects on uptake of fluorescent polystyrene nanoparticles by model biofilms. Ecotoxicology 21 (8), 2205–2213. [DOI] [PubMed] [Google Scholar]
- (178).Q. Mesquita M, J. Dias C, P. M. S. Neves MG, Almeida A, and F. Faustino MA (2018) Revisiting current photoactive materials for antimicrobial photodynamic therapy. Molecules 23 (10), 2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Duncan B, Li X, Landis RF, Kim ST, Gupta A, Wang L-S, Ramanathan R, Tang R, Boerth JA, and Rotello VM (2015) Nanoparticle-stabilized capsules for the treatment of bacterial biofilms. ACS Nano 9 (8), 7775–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Li M, Kang E-T, Chua KL, and Neoh KG (2019) Sugar-powered nanoantimicrobials for combating bacterial biofilms. Biomater. Sci 7 (7), 2961–2974. [DOI] [PubMed] [Google Scholar]
- (181).Ahangaran F, and Navarchian AH (2020) Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Colloid Interface Sci 286, 102298. [DOI] [PubMed] [Google Scholar]
- (182).Zhang L, Mazouzi Y, Salmain M, Liedberg B, and Boujday S (2020) Antibody-gold nanoparticle bioconjugates for biosensors: synthesis, characterization and selected applications. Biosens. Bioelectron 165, 112370. [DOI] [PubMed] [Google Scholar]
- (183).Borse VB, Konwar AN, Jayant RD, and Patil PO (2020) Perspectives of characterization and bioconjugation of gold nanoparticles and their application in lateral flow immunosensing. Drug Delivery Transl. Res 10 (4), 878–902. [DOI] [PubMed] [Google Scholar]
- (184).Algar WR, Dawson P, and Medintz IL (2017) Chemoselective and Bioorthogonal Ligation Reactions; Wiley Online Library, Germany. [Google Scholar]
- (185).Biju V (2014) Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev 43 (3), 744–764. [DOI] [PubMed] [Google Scholar]
- (186).Hore MJ (2019) Polymers on nanoparticles: structure & dynamics. Soft Matter 15 (6), 1120–1134. [DOI] [PubMed] [Google Scholar]
- (187).Pribyl J, and Benicewicz BC (2008) Surface and particle modification via the RAFT process: approach and properties (Ed. C. Barner-Kowollik W-V, Ed.), Weinheim, Germany. [Google Scholar]
- (188).Rana R, Awasthi R, Sharma B, and Kulkarni GT (2020) Nanoantibiotic formulations to combat antibiotic resistance - old wine in a new bottle. Recent Pat. Drug Delivery Formulation 13 (3), 174–183. [DOI] [PubMed] [Google Scholar]
- (189).Shahverdi AR, Fakhimi A, Shahverdi HR, and Minaian S (2007) Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine 3 (2), 168–171. [DOI] [PubMed] [Google Scholar]
- (190).Railean-Plugaru V, Pomastowski P, Rafinska K, Wypij M, Kupczyk W, Dahm H, Jackowski M, and Buszewski B (2016) Antimicrobial properties of biosynthesized silver nanoparticles studied by flow cytometry and related techniques. Electrophoresis 37 (5–6), 752–761. [DOI] [PubMed] [Google Scholar]
- (191).Weldrick PJ, Iveson S, Hardman MJ, and Paunov VN (2019) Breathing new life into old antibiotics: overcoming antibacterial resistance by antibiotic-loaded nanogel carriers with cationic surface functionality. Nanoscale 11 (21), 10472–10485. [DOI] [PubMed] [Google Scholar]
- (192).Doudna JA, and Mali P (2016) CRISPR-Cas: a laboratory manual; Cold Spring Harbor Laboratory Press. [Google Scholar]
- (193).Cho S, Shin J, and Cho B-K (2018) Applications of CRISPR/Cas system to bacterial metabolic engineering. Int. J. Mol. Sci 19 (4), 1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Citorik RJ, Mimee M, and Lu TK (2014) Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol 32 (11), 1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Bikard D, Euler CW, Jiang W, Nussenzweig PM, Goldberg GW, Duportet X, Fischetti VA, and Marraffini LA (2014) Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat. Biotechnol 32 (11), 1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (196).Carnes EC, Lopez DM, Donegan NP, Cheung A, Gresham H, Timmins GS, and Brinker CJ (2010) Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nat. Chem. Biol 6 (1), 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Cress BF, Jones JA, Kim DC, Leitz QD, Englaender JA, Collins SM, Linhardt RJ, and Koffas MAG (2016) Rapid generation of CRISPR/dCas9-regulated, orthogonally repressible hybrid T7-lac promoters for modular, tuneable control of metabolic pathway fluxes in Escherichia coli. Nucleic Acids Res. 44 (9), 4472–4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Singh V, Braddick D, and Dhar PK (2017) Exploring the potential of genome editing CRISPR-Cas9 technology. Gene 599, 1–18. [DOI] [PubMed] [Google Scholar]
- (199).Fuqua C, and Greenberg EP (2002) Listening in on bacteria: acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol 3 (9), 685–695. [DOI] [PubMed] [Google Scholar]
- (200).Rutherford ST, and Bassler BL (2012) Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harbor Perspect. Med 2 (11), a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Camilli A, and Bassler BL (2006) Bacterial small-molecule signaling pathways. Science 311 (5764), 1113–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Bassler BL, and Losick R (2006) Bacterially speaking. Cell 125 (2), 237–246. [DOI] [PubMed] [Google Scholar]
- (203).Diggle SP, Stacey RE, Dodd C, Cámara M, Williams P, and Winzer K (2006) The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa. Environ. Microbiol 8 (6), 1095–1104. [DOI] [PubMed] [Google Scholar]
- (204).Dietrich LEP, Price-Whelan A, Petersen A, Whiteley M, and Newman DK (2006) The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol 61 (5), 1308–1321. [DOI] [PubMed] [Google Scholar]
- (205).Kariminik A, Baseri-Salehi M, and Kheirkhah B (2017) Pseudomonas aeruginosa quorum sensing modulates immune responses: An updated review article. Immunol. Lett 190, 1–6. [DOI] [PubMed] [Google Scholar]
- (206).Tielker D, Hacker S, Loris R, Strathmann M, Wingender J, Wilhelm S, Rosenau F, and Jaeger K-E (2005) Pseudomonas aeruginosa lectin LecB is located in the outer membrane and is involved in biofilm formation. Microbiology 151 (5), 1313–1323. [DOI] [PubMed] [Google Scholar]
- (207).Colino CI, Millán CG, and Lanao JM (2018) Nanoparticles for signaling in biodiagnosis and treatment of infectious diseases. Int. J. Mol. Sci 19 (6), 1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (208).Sintim HO, Smith JA, Wang J, Nakayama S, and Yan L (2010) Paradigm shift in discovering next-generation anti-infective agents: targeting quorum sensing, c-di-GMP signaling and biofilm formation in bacteria with small molecules. Future Med. Chem 2 (6), 1005–1035. [DOI] [PubMed] [Google Scholar]
- (209).Defoirdt T, Miyamoto CM, Wood TK, Meighen EA, Sorgeloos P, Verstraete W, and Bossier P (2007) The natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone disrupts quorum sensing-regulated gene expression in Vibrio harveyi by decreasing the DNA-binding activity of the transcriptional regulator protein luxR. Environ. Microbiol 9 (10), 2486–2495. [DOI] [PubMed] [Google Scholar]
- (210).Beitelshees M, Hill A, Jones CH, and Pfeifer BA (2018) Phenotypic variation during biofilm formation: implications for antibiofilm therapeutic design. Materials 11 (7), 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Miller KP, Wang L, Chen Y-P, Pellechia PJ, Benicewicz BC, and Decho AW (2015) Engineering nanoparticles to silence bacterial communication. Front. Microbiol, DOI: 10.3389/fmicb.2015.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (212).Lee CK, de Anda J, Baker AE, Bennett RR, Luo Y, Lee EY, Keefe JA, Helali JS, Ma J, Zhao K, et al. (2018) Multigenerational memory and adaptive adhesion in early bacterial biofilm communities. Proc. Natl. Acad. Sci. U. S. A 115 (17), 4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (213).LaSarre B, and Federle MJ (2013) Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev 77 (1), 73–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (214).Hirakawa H, and Tomita H (2013) Interference of bacterial cell-to-cell communication: a new concept of antimicrobial chemotherapy breaks antibiotic resistance. Front. Microbiol, DOI: 10.3389/fmicb.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Schaefer AL, Val DL, Hanzelka BL, Cronan JE, and Greenberg EP (1996) Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc. Natl. Acad. Sci. U. S. A 93 (18), 9505–9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (216).Smith KM, Bu Y, and Suga H (2003) Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem. Biol 10 (1), 81–89. [DOI] [PubMed] [Google Scholar]
- (217).Smith KM, Bu Y, and Suga H (2003) Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem. Biol 10 (6), 563–571. [DOI] [PubMed] [Google Scholar]
- (218).Zhang H-B, Wang L-H, and Zhang L-H (2002) Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. U. S. A 99 (7), 4638–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Zhu J, Beaber JW, Moré MI, Fuqua C, Eberhard A, and Winans SC (1998) Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J. Bacteriol 180 (20), 5398–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (220).Singh BN, Prateeksha, Upreti DK, Singh BR, Defoirdt T, Gupta VK, De Souza AO, Singh HB, Barreira JCM, Ferreira ICFR, et al. (2017) Bactericidal, quorum quenching and anti-biofilm nanofactories: a new niche for nanotechnologists. Crit. Rev. Biotechnol 37 (4), 525–540. [DOI] [PubMed] [Google Scholar]
- (221).Ishida T, Ikeda T, Takiguchi N, Kuroda A, Ohtake H, and Kato J (2007) Inhibition of quorum sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides. Appl. Environ. Microbiol 73 (10), 3183–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Vannini A, Volpari C, Gargioli C, Muraglia E, Cortese R, De Francesco R, Neddermann P, and Di Marco S (2002) The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J. 21 (17), 4393–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (223).Geske GD, O’Neill JC, and Blackwell HE (2007) N-Phenylacetanoyl-l-homoserine lactones can strongly antagonize or superagonize quorum sensing in Vibrio fischeri. ACS Chem. Biol 2 (5), 315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Parsek MR, Val DL, Hanzelka BL, Cronan JE, and Greenberg EP (1999) Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. U. S. A 96 (8), 4360–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (225).Chung J, Goo E, Yu S, Choi O, Lee J, Kim J, Kim H, Igarashi J, Suga H, Moon JS, et al. (2011) Small-molecule inhibitor binding to an N-Acyl-homoserine lactone synthase. Proc. Natl. Acad. Sci. U. S. A 108 (29), 12089–12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (226).Xavier KB, and Bassler BL (2003) LuxS quorum sensing: more than just a numbers game. Curr. Opin. Microbiol 6 (2), 191–197. [DOI] [PubMed] [Google Scholar]
- (227).Dong S, McPherson SA, Wang Y, Li M, Wang P, Turnbough CL, and Pritchard DG (2010) Characterization of the enzymes encoded by the anthrose biosynthetic operon of Bacillus anthracis. J. Bacteriol 192 (19), 5053–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (228).Uroz S, Oger PM, Chapelle E, Adeline M-T, Faure D, and Dessaux Y (2008) A Rhodococcus qsdA-encoded enzyme defines a novel class of large-spectrum quorum-quenching lactonases. Appl. Environ. Microbiol 74 (5), 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (229).Wang W-Z, Morohoshi T, Ikenoya M, Someya N, and Ikeda T (2010) AiiM, a novel class of N-acylhomoserine lactonase from the Leaf-associated bacterium Microbacterium testaceum. Appl. Environ. Microbiol 76 (8), 2524–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (230).Schipper C, Hornung C, Bijtenhoorn P, Quitschau M, Grond S, and Streit WR (2009) Metagenome-derived clones encoding two novel lactonase family proteins involved in biofilm inhibition in Pseudomonas aeruginosa. Appl. Environ. Microbiol 75 (1), 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (231).Huang W, Lin Y, Yi S, Liu P, Shen J, Shao Z, and Liu Z (2012) QsdH, a novel AHL lactonase in the RND-type inner membrane of marine Pseudoalteromonas byunsanensis strain 1A01261. PLoS One 7 (10), e46587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (232).Tong R, Hemmati HD, Langer R, and Kohane DS (2012) Photoswitchable nanoparticles for triggered tissue penetration and drug delivery. J. Am. Chem. Soc 134 (21), 8848–8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (233).Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, and Wanker EE (2008) EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol 15 (6), 558–566. [DOI] [PubMed] [Google Scholar]
- (234).Stenvang M, Dueholm MS, Vad BS, Seviour T, Zeng G, Geifman-Shochat S, Søndergaard MT, Christiansen G, Meyer RL, Kjelleberg S, et al. (2016) Epigallocatechin gallate remodels overexpressed functional amyloids in Pseudomonas aeruginosa and increases biofilm susceptibility to antibiotic treatment. J. Biol. Chem 291 (51), 26540–26553. [DOI] [PMC free article] [PubMed] [Google Scholar]