Abstract
Purpose
To investigate the impact of delayed adjuvant imatinib on GIST patients with high risk of recurrence.
Method
Adult GIST patients were retrospectively collected from our hospital between 2011 and 2018, and patients having high risk of recurrence were included for subsequent analyses. The primary endpoint was recurrence-free survival (RFS).
Results
According to the interval between the radical surgery and the beginning of adjuvant imatinib, 222 patients were divided into three groups: group A (≤ 2 months, n = 41), group B (2–≤ 4 months, n = 113), and group C (4–≤ 6 months, n = 68). Univariate, multivariate, and survival analyses all showed that patients in group A had significantly more favorable RFS than those in group C but not group B, and patients taking adjuvant imatinib for over 12 months were also associated with longer RFS comparing to adjuvant imatinib of ≤ 12 months. When stratified by the duration of adjuvant imatinib, no significant differences were found in RFS among groups A, B, and C for adjuvant imatinib of ≤ 12 months. While for adjuvant imatinib of over 12 months, both groups A and B had significantly more favorable RFS than group C, and no significant difference in RFS was found between group A and B.
Conclusion
Delayed postoperative adjuvant imatinib for over 4 months in patients with high risk of recurrence of GIST may lead to worse RFS, and longer treatment with shorter delay has best results.
Keywords: Gastrointestinal stromal tumor, Imatinib, Recurrence-free survival
Introduction
Gastrointestinal stromal tumor (GIST) is the most common gastrointestinal soft-tissue malignancy (Joensuu et al. 2013; von Mehren and Joensuu 2018). Complete surgical resection is the standard treatment for localized, primary GIST, but approximately 40% of patients have disease recurrence (Joensuu 2012). Hirota et al. first found that the activating mutations in KIT resulted in GIST development, which significantly altered the biological understanding and management of this disease (Hirota et al. 1998; Mei et al. 2018). About 80% of GIST contain an activating mutation in the KIT oncogene, whereas 3–5% have a mutation in the gene encoding PDGFRα (Raut et al. 2018, Serrano and George 2020).
Imatinib, a small molecular tyrosine kinase inhibitor (TKI) of KIT and PDGFR, was applied originally in chronic myelogenous leukemia (Joensuu and DeMatteo 2012; Mantese 2019; Liu et al. 2020). During the pre-imatinib era, patients with localized, primary GIST undergoing macroscopically complete R0/R1 resections had high rate of recurrence, and the 5-year disease-specific survival was about 54% (DeMatteo et al. 2000). Furthermore, the 5-year survival for all patients diagnosed with GIST was 35%, and the median survival for those with metastatic disease was 12–19 months (Hemming et al. 2018). In 2000, imatinib was first used for treating metastatic GIST. Then, the efficacy of imatinib on GIST was confirmed by several studies (Joensuu et al. 2001, Demetri et al. 2002, Verweij et al. 2004, Blanke et al. 2008a, b, Blanke et al. 2008a, b). Compared with the pre-imatinib era, GIST patients in the imatinib era had prolonged OS across all presentations (Cavnar et al. 2019). Adjuvant imatinib has been recommended as the standard first-line agent in the treatment of GIST with high risk of recurrence, based on controlled randomized trials, which revolutionized the treatment of GIST (Dematteo et al. 2009; Joensuu et al. 2012, 2020). Furthermore, several studies indicated that even longer adjuvant imatinib might further prolong disease-free survival for GIST patients with high risk of recurrence (Zhao et al. 2017, Raut et al. 2018), and presently, two European randomized trials are investigating this issue further.
Although adjuvant imatinib significantly improved the survival of GIST patients, the availability of imatinib, particularly in underdeveloped and developing countries, is still limited because of financial considerations and lack of insurance coverage (Bengio et al. 2011; Kurtovic-Kozaric et al. 2016). Even in developed countries, the cost of imatinib is also a huge burden. In the US, the monthly pharmacy costs were 4340 $ based on a 30-day supply of imatinib 400 mg/day, and in The Netherlands, the 3-year and 1-year adjuvant imatinib drug costs were 74,631 € and 27,619 €, respectively (Majer et al. 2013; Rutkowski 2013). We noticed one study reporting that it had no difference in overall survival in patients who waited > 6 months for TKI therapy compared to those who received immediate treatment (Kurtovic-Kozaric et al. 2017). However, this study did not focus on the postoperative adjuvant imatinib therapy, and the safe and appropriate interval between radical surgery and the beginning of adjuvant imatinib for GIST patients with high risk of recurrence is still unclear. To investigate the impact of delayed adjuvant imatinib on GIST patients of high risk of recurrence after radical surgery, we retrospectively collected the data in our center and conducted this study.
Patients and methods
Patients
In this study, we retrospectively collected the GIST patients aged ≥ 18 years from the West China Hospital between 2011 and 2018. All GIST patients were confirmed by immunohistochemistry. The inclusion criteria were as follows: patients with KIT (CD117) positivity in immunohistochemistry; received radical surgery; received adjuvant imatinib after surgery and was tolerant to imatinib; with high risk of recurrence confirmed by the modified National Institutes of Health (NIH) consensus classification system (Fletcher et al. 2002; Joensuu 2008; Rutkowski et al. 2011). Patients were excluded if they had metastatic GIST, recurrent GIST, or other neoplasms, and patients receiving preoperative imatinib or having delayed adjuvant imatinib for over 6 months after surgery were also excluded.
Follow-up
All patients were followed postoperatively. For each patient, the CT or MRI scanning was performed at 3-month intervals in the first 2 years and 6-month intervals in the subsequent years. Peripheral blood cells and chemistries were examined at 3-month intervals during the period of the treatment. The primary endpoint was recurrence-free survival (RFS).
Statistical analyses
All statistical analyses were conducted using SPSS version 25.0. We used the Chi-square test to compare categorical data, and the t test or ANOVA was used to compare continuous data. The survival data were analyzed using the Kaplan–Meier survival curves, and the differences in survival curves between different groups were analyzed using log-rank tests. The univariate Cox proportional hazards model was used to estimate the hazard ratios (HR) and 95% confidence intervals (CI) for RFS. Then, variables with a P value of < 0.1 in the univariate analysis were analyzed in multivariate analysis. All P values were two-sided, and a P value of < 0.05 was regarded as having statistical significance.
Results
We identified 222 patients in this study, including 126 males and 96 females, and the mean age was 53.82 ± 10.48 years. The follow-up time ranged from 5 to 100 months, with a mean of 48.08 months. All patients were classified as having high risk of recurrence.
According to the interval between radical surgery and the beginning of adjuvant imatinib, we divided the 222 patients into three groups: group A (≤ 2 months), group B (2–≤ 4 months), and group C (4–≤ 6 months). There were 41, 113, and 68 patients in groups A, B, and C, respectively. About 68.3%, 38.1%, and 76.5% of patients in groups A, B, and C received adjuvant imatinib for more than 12 months, respectively (P < 0.001). There were no significant differences in gender, age, tumor size, tumor mitotic rate, tumor site, and tumor rupture among the three groups (Table 1).
Table 1.
Characteristics of study patients
| Characteristics | Group A (n = 41)a | Group B (n = 113) | Group C (n = 68) | P |
|---|---|---|---|---|
| Gender | 0.41 | |||
| Female | 19 | 44 | 33 | |
| Male | 22 | 69 | 35 | |
| Age (years) | 56.29 ± 10.88 | 52.96 ± 10.55 | 53.76 ± 10.04 | 0.22 |
| Duration of adjuvant imatinib (months) | < 0.001 | |||
| ≤ 12 | 13 | 70 | 16 | |
| > 12 | 28 | 43 | 52 | |
| Tumor size (cm) | 0.43 | |||
| ≤ 10 | 36 | 91 | 53 | |
| > 10 | 5 | 22 | 15 | |
| Tumor mitotic rate | 0.42 | |||
| ≤ 10 | 13 | 30 | 14 | |
| > 10 | 28 | 83 | 54 | |
| Tumor site | 0.99 | |||
| Stomach | 17 | 46 | 28 | |
| Non-stomach | 24 | 67 | 40 | |
| Tumor rupture | 0.18 | |||
| Yes | 18 | 54 | 23 | |
| No | 23 | 59 | 45 | |
aPatients were divided into three groups based on the interval between radical surgery and the beginning of adjuvant imatinib: group A (interval ≤ 2 months), group B (2 < interval ≤ 4 months), and group C (4 < interval ≤ 6 months)
The univariate analysis was conducted to investigate the impact of the interval between radical surgery and the beginning of adjuvant imatinib and other characteristics on RFS. Compared to those of ≤ 2 months, GIST patients in group B (2–≤ 4 months) had no significant differences in RFS (HR: 2.56, 95% CI: 0.90–7.29, P = 0.08), while patients in group C (4–≤ 6 months) had significantly worse RFS (HR: 1.97, 95% CI: 1.15–3.37, P = 0.01) (Table 2). For the duration of adjuvant imatinib, GIST patients taking adjuvant imatinib for over 12 months had significantly longer RFS than those with adjuvant imatinib of ≤ 12 months (HR: 0.47, 95% CI: 0.27–0.80, P = 0.006). Furthermore, the tumor size (≤ 10 cm vs. > 10 cm), tumor mitotic rate (≤ 10 vs. > 10), tumor site (stomach vs. non- stomach), and tumor rupture (no vs, yes) were also included in univariate analysis, but none of them was significantly associated with RFS in GIST patients with high risk of recurrence (Table 2). In multivariate analysis, GIST patients in group C (HR: 1.90, 95% CI: 1.10–3.28, P = 0.02) instead of group B (HR: 1.52, 95% CI: 0.49–4.70, P = 0.47) were associated with significantly poorer RFS than those in group A, and adjuvant imatinib of over 12 months was also associated with longer RFS comparing to adjuvant imatinib of ≤ 12 months (HR: 0.47, 95% CI: 0.28–0.82, P = 0.007) (Table 2).
Table 2.
Univariate and multivariate analyses of RFS
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Interval between radical surgery and the beginning of adjuvant imatinib (interval ≤ 2 months as ref) | ||||
| 2 < interval ≤ 4 months | 2.56 (0.90–7.29) | 0.08 | 1.52 (0.49–4.70) | 0.47 |
| 4 < interval ≤ 6 months | 1.97 (1.15–3.37) | 0.01 | 1.90 (1.10–3.28) | 0.02 |
| Duration of adjuvant imatinib (≤ 12 months as ref) | ||||
| > 12 months | 0.47 (0.27–0.80) | 0.006 | 0.47 (0.28–0.82) | 0.007 |
| Tumor size (≤ 10 cm as ref) | ||||
| > 10 cm | 1.09 (0.55–2.13) | 0.81 | ||
| Tumor mitotic rate (≤ 10 as ref) | ||||
| > 10 | 0.90 (0.52–1.55) | 0.69 | ||
| Tumor site (stomach as ref) | ||||
| Non-stomach | 0.82 (0.47–1.43) | 0.48 | ||
| Tumor rupture (no as ref) | ||||
| Yes | 1.31 (0.76–2.26) | 0.33 | ||
RFS recurrence-free survival; HR hazard ratios; 95% CI 95% confidence intervals
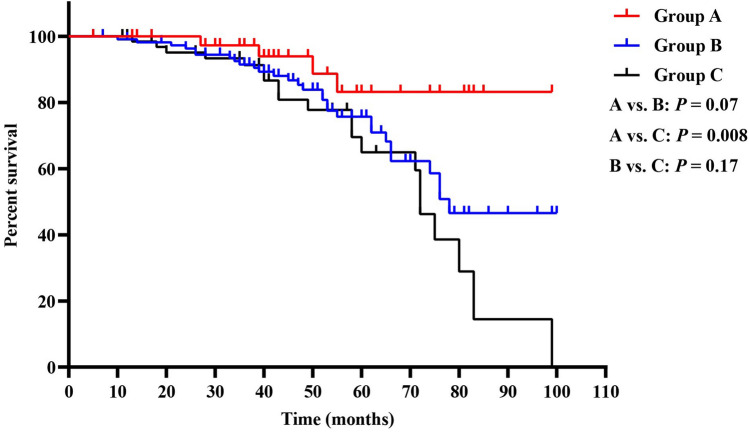
Because univariate and multivariate analyses showed that only the interval between radical surgery and the beginning of adjuvant imatinib and the duration of adjuvant imatinib were significantly associated with RFS, we further investigated how the interval and duration of adjuvant imatinib influence the RFS. Through Kaplan–Meier survival curves and log-rank tests, we found that patients in group A had significantly more favorable RFS than those in group C (P = 0.008), while no significant differences were found between groups A and B (P = 0.07) or groups B and C (P = 0.17) (Fig. 1). Besides, patients taking adjuvant imatinib for over 12 months were associated with significantly more favorable RFS than those with adjuvant imatinib of ≤ 12 months (P = 0.005) (Fig. 2).
Fig. 1.
Kaplan–Meier curves of recurrence-free survival for all patients in groups A, B, and C
Fig. 2.
Kaplan–Meier curves of recurrence-free survival for patients have different duration of adjuvant imatinib
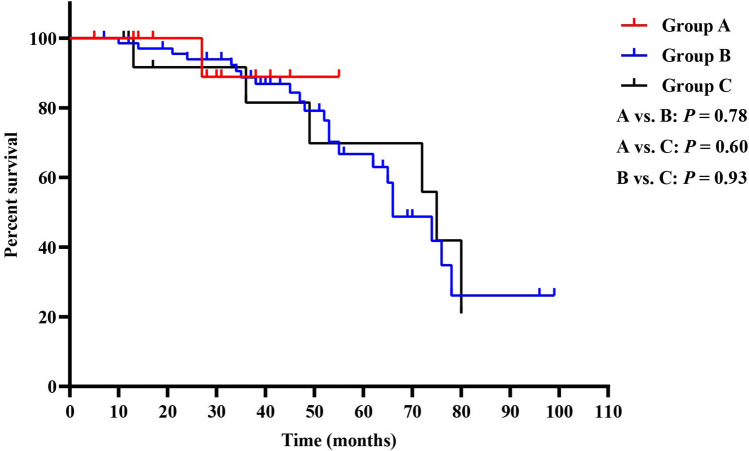
Because long-term adjuvant imatinib led to more favorable RFS, and patients in group C had a higher rate of taking adjuvant imatinib for over 12 months than those in group B. We further analyzed the impact of delayed adjuvant imatinib on patients with different duration of imatinib treatment. Interestingly, we found that when patients took adjuvant imatinib for ≤ 12 months, there were no significant differences in RFS among the three groups (Fig. 3). While in patients taking adjuvant imatinib for over 12 months, both group A and B had more favorable RFS than group C (A vs. C: P = 0.01, B vs. C: P = 0.02), and no significant difference in RFS was found between group A and B (P = 0.54) (Fig. 4).
Fig. 3.
Kaplan–Meier curves of recurrence-free survival for patients in groups A, B, and C (taking adjuvant imatinib for ≤ 12 months)
Fig. 4.
Kaplan–Meier curves of recurrence-free survival for patients in groups A, B, and C (taking adjuvant imatinib for > 12 months)
Discussion
Since the FDA first approving the use of imatinib for the treatment of patients with KIT-positive unresectable and/or metastatic malignant GIST, many clinical studies focused on the duration of imatinib treatment in GIST, while the impact of delayed adjuvant imatinib on GIST was seldom concerned. In this study, we investigated the effect of delayed adjuvant imatinib in patients with high risk of recurrence of GIST after radical surgery. We divided the included patients into three groups according to the interval between radical surgery and the beginning of adjuvant imatinib. Our findings showed that patients with an interval of > 4 months had poorer RFS than those of ≤ 4 months or ≤ 2 months when the duration of adjuvant imatinib was over 12 months. However, when patients took adjuvant imatinib for ≤ 12 months, the impact of delayed adjuvant imatinib on RFS might be diminished by the short duration of imatinib treatment, and there were no significant differences in RFS among group A, B, and C. The findings suggested that the impact of delayed imatinib treatment on RFS was significant only when patients taking long-term adjuvant imatinib after radical surgery. Because the recent studies (von Mehren and Joensuu 2018; Joensuu et al. 2020) demonstrated that patients with high risk of recurrence of GIST receiving 3 years of imatinib had more favorable survival compared with those taking imatinib for 1 year, we suggested that patients with high risk of recurrence of GIST should receive imatinib treatment as soon as possible after radical surgery. Furthermore, our study showed no significant difference on RFS between patients in group A and group B. Considering that patients in group B have a lower percentage of receiving adjuvant imatinib of over 12 months (38%) than those in group A (68%) and group C (76%), and the duration of imatinib treatment was a significant prognostic factor for RFS. Therefore, whether there are significant differences on RFS between group A and group B still needs more studies to verify.
The common risk stratification of GIST is the modified NIH consensus classification system, including tumor size, tumor mitotic rate, tumor site, and tumor rupture (Joensuu 2008; Khoo et al. 2018, Duan et al. 2020, Li et al. 2020). Interestingly, the univariate analysis in this study found that the tumor size, tumor mitotic rate, tumor site, and tumor rupture were not associated with RFS, which might be caused by that we only included GIST patients classified as high risk of recurrence, where the four parameters were comparable. Furthermore, the modified NIH system just predicted the risk of recurrence after radical surgery, and all patients in this study took imatinib after surgery, which could also diminish the impact of the four parameters on RFS.
The delayed time of imatinib treatment was diverse in GIST patients. In this study, about half of the included GIST patients (50.9%) received adjuvant imatinib with a delay of 3 to 4 months, and the number of patients with a delay of adjuvant imatinib for over 4 months (30.6%) was more than those with a delay of ≤ 2 months (18.5%). In our center, the delay of adjuvant imatinib after radical surgery was caused by multiple factors. First, current evidence demonstrated that patients with KIT exon 11 mutation responded more often and achieved longer responses compared with those who had KIT exon 9 mutation or wild-type GIST, and adjuvant imatinib cannot be recommended for patients with PDGFRA Asp842Val substitution (Joensuu 2012; Joensuu et al. 2020). Because GIST patients have different response rates to imatinib based on the tumor mutation status, gene detection before adjuvant imatinib is particularly useful for patients with high risk of recurrence. Although gene detection became more common, it also increased the interval between radical surgery and the beginning of adjuvant imatinib. Second, some patients lacked private insurances and could not afforded the price of imatinib. For these patients, they could apply for the imatinib through official charity projects, which required various disease proofs and a complex approval process. Therefore, the waiting time for imatinib was prolonged. Third, some patients received radical surgeries in subordinate hospitals, while the resected tumor samples were further confirmed by immunohistochemistry in our center, and these patients always had a long interval between radical surgery and the beginning of adjuvant imatinib.
There were several limitations in our study. First, this was a retrospective study without a large sample, and there were some censored cases, which might increase the risk of bias. Therefore, our results still need more cohort studies with larger sample to verify in the future. Second, the data of tumor gene detection and molecular analysis for KIT gene were incomplete, and we did not analyze the impact of different gene mutations on RFS. Third, a small part of the patients received radical surgeries in other hospitals, which might increase the risk of bias. There were also several strengths in our study. We focused on GIST patients classified as high risk of recurrence for whom postoperative imatinib treatment was extremely important. We investigated an interval within 6 months between radical surgery and the beginning of adjuvant imatinib, and we divided the patients into three groups with different intervals, which could be more helpful for understanding the impact of delayed imatinib treatment on RFS precisely. Furthermore, we analyzed the delayed time and the duration time of adjuvant imatinib together, which improved the clinical value of this study.
In summary, this study demonstrated that delayed postoperative adjuvant imatinib for over 4 months in patients with high risk of recurrence of GIST might lead to worse RFS, and longer treatment with shorter delay had best results. Considering the limitations of this study, more clinical studies with the prospective design, particularly for those including analyses of gene mutation, are required in the future.
Acknowledgements
None.
Author contributions
WQ, XM, and ZR contributed equally to this work. WX and ZR conceived together the study. All of the authors contributed to the research and development process that resulted in this article. WQ, XM, and ZR wrote the manuscript under the guidance of WX. All of the authors read the manuscript and approved the final manuscript.
Funding
None.
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
Declarations
Conflict of interest
There was no conflict of interest.
Ethics approval
The study was approved by the ethics committee of West China Hospital, Sichuan University. Patient records/information were anonymized and de-identified before analysis, and the methods were performed following the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bengio RM, Riva ME, Moiraghi B, Lanari E, Milone J, Ventriglia V, Bullorsky E, Tezanos Pinto M, Murro H, Bianchini M, Larripa I (2011) Clinical outcome of chronic myeloid leukemia imatinib-resistant patients: do BCR-ABL kinase domain mutations affect patient survival? First multicenter Argentinean study. Leuk Lymphoma 52(9):1720–1726 [DOI] [PubMed] [Google Scholar]
- Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CDM, Roberts PJ, Heinz D, Wehre E, Nikolova Z, Joensuu H (2008a) Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 26(4):620–625 [DOI] [PubMed] [Google Scholar]
- Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VHC, Baker LH, Maki RG, Tanaka M, Hecht JR, Heinrich MC, Fletcher CDM, Crowley JJ, Borden EC (2008b) Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 26(4):626–632 [DOI] [PubMed] [Google Scholar]
- Cavnar MJ, Seier K, Curtin C, Balachandran VP, Coit DG, Yoon SS, Crago AM, Strong VE, Tap WD, Gonen M, Antonescu CR, Brennan MF, Singer S, DeMatteo RP (2019) Outcome of 1000 patients with gastrointestinal stromal tumor (GIST) treated by surgery in the pre and post-imatinib eras. Ann Surg 273(1):128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231(1):51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S, McCarter MD, Polikoff JA, Tan BR, Owzar K, GST. American College of Surgeons Oncology Group Intergroup Adjuvant (2009) Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 373(9669):1097–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CDM, Joensuu H (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347(7):472–480 [DOI] [PubMed] [Google Scholar]
- Duan Y, Haybaeck J, Yang Z (2020) Therapeutic potential of PI3K/AKT/mTOR pathway in gastrointestinal stromal tumors: rationale and progress. Cancers (basel) 12(10):2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher CDM, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 33(5):459–465 [DOI] [PubMed] [Google Scholar]
- Hemming ML, Heinrich MC, Bauer S, George S (2018) Translational insights into gastrointestinal stromal tumor and current clinical advances. Ann Oncol 29(10):2037–2045 [DOI] [PubMed] [Google Scholar]
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Tunio GM, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279(5350):577–580 [DOI] [PubMed] [Google Scholar]
- Joensuu H (2008) Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 39(10):1411–1419 [DOI] [PubMed] [Google Scholar]
- Joensuu H (2012) Adjuvant treatment of GIST: patient selection and treatment strategies. Nat Rev Clin Oncol 9(6):351–358 [DOI] [PubMed] [Google Scholar]
- Joensuu H, DeMatteo RP (2012) The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med 63:247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman SL, Capdeville R, Dimitrijevic S, Druker B, Demetri GD (2001) Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 344(14):1052–1056 [DOI] [PubMed] [Google Scholar]
- Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schutte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE, Schlemmer M, Bauer S, Wardelmann E, Sarlomo-Rikala M, Nilsson B, Sihto H, Monge OR, Bono P, Kallio R, Vehtari A, Leinonen M, Alvegard T, Reichardt P (2012) One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 307(12):1265–1272 [DOI] [PubMed] [Google Scholar]
- Joensuu H, Hohenberger P, Corless CL (2013) Gastrointestinal stromal tumour. Lancet 382(9896):973–983 [DOI] [PubMed] [Google Scholar]
- Joensuu H, Eriksson M, Sundby Hall K, Reichardt A, Hermes B, Schutte J, Cameron S, Hohenberger P, Jost PJ, Al-Batran SE, Lindner LH, Bauer S, Wardelmann E, Nilsson B, Kallio R, Jaakkola P, Junnila J, Alvegard T, Reichardt P (2020) Survival outcomes associated with 3 years vs 1 year of adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: an analysis of a randomized clinical trial after 10-year follow-up. JAMA Oncol 6(8):1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo CY, Chai X, Quek R, Teo MCC, Goh BKP (2018) Systematic review of current prognostication systems for primary gastrointestinal stromal tumors. Eur J Surg Oncol 44(4):388–394 [DOI] [PubMed] [Google Scholar]
- Kurtovic-Kozaric A, Hasic A, Radich JP, Bijedic V, Nefic H, Eminovic I, Kurtovic S, Colakovic F, Kozaric M, Vranic S, Bovan NS (2016) The reality of cancer treatment in a developing country: the effects of delayed TKI treatment on survival, cytogenetic and molecular responses in chronic myeloid leukaemia patients. Br J Haematol 172(3):420–427 [DOI] [PubMed] [Google Scholar]
- Kurtovic-Kozaric A, Kugic A, Hasic A, Beslija S, Ceric T, Pasic A, Vranic S, Kopric D, Iljazovic E, Todorovic Barbuscia J, Kozaric M, Ibisevic N, Keskic L, Kurtovic S (2017) Long-term outcome of GIST patients treated with delayed imatinib therapy. Eur J Cancer 78:118–121 [DOI] [PubMed] [Google Scholar]
- Li S, Chen D, Li S, Zhao Z, Yang H, Wang D, Zhang Z, Fu W (2020) Novel prognostic nomogram for recurrence-free survival of patients with primary gastrointestinal stromal tumors after surgical resection: combination of prognostic nutritional index and basic variables. Front Oncol 10:581855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Tan F, Liu H, Li B, Lei T, Zhao X (2020) The use of molecular subtypes for precision therapy of recurrent and metastatic gastrointestinal stromal tumor. Onco Targets Ther 13:2433–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer IM, Gelderblom H, van den Hout WB, Gray E, Verheggen BG (2013) Cost-effectiveness of 3-year vs 1-year adjuvant therapy with imatinib in patients with high risk of gastrointestinal stromal tumour recurrence in the Netherlands; a modelling study alongside the SSGXVIII/AIO trial. J Med Econ 16(9):1106–1119 [DOI] [PubMed] [Google Scholar]
- Mantese G (2019) Gastrointestinal stromal tumor: epidemiology, diagnosis, and treatment. Curr Opin Gastroenterol 35(6):555–559 [DOI] [PubMed] [Google Scholar]
- Mei L, Smith SC, Faber AC, Trent J, Grossman SR, Stratakis CA, Boikos SA (2018) Gastrointestinal stromal tumors: the GIST of precision medicine. Trends Cancer 4(1):74–91 [DOI] [PubMed] [Google Scholar]
- Raut CP, Espat NJ, Maki RG, Araujo DM, Trent J, Williams TF, Purkayastha DD, DeMatteo RP (2018) Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: The PERSIST-5 clinical trial. JAMA Oncol 4(12):184060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski P, Gronchi A (2013) Efficacy and economic value of adjuvant imatinib for gastrointestinal stromal tumors. Oncologist 18(6):689–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski P, Bylina E, Wozniak A, Nowecki ZI, Osuch C, Matlok M, Switaj T, Michej W, Wronski M, Gluszek S, Kroc J, Nasierowska-Guttmejer A, Joensuu H (2011) Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour - the impact of tumour rupture on patient outcomes. Eur J Surg Oncol 37(10):890–896 [DOI] [PubMed] [Google Scholar]
- Serrano C, George S (2020) Gastrointestinal stromal tumor: challenges and opportunities for a new decade. Clin Cancer Res 26(19):5078–5085 [DOI] [PubMed] [Google Scholar]
- Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PCW, Van Glabbeke M, Bertulli R, Judson I, Grp ESTBS, Italian Sarcoma G, Australasian Gastrointestinal T (2004) Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364(9440):1127–1134 [DOI] [PubMed] [Google Scholar]
- von Mehren M, Joensuu H (2018) Gastrointestinal stromal tumors. J Clin Oncol 36(2):136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Wang Y, Huang Y, Cui Y, Xia L, Chen Y, Zhuang W, Zhou Y, Wu X (2017) Adjuvant imatinib for patients with high-risk gastrointestinal stromal tumors: a retrospective cohort study. Sci Rep 7(1):16834 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Not applicable.