Abstract
Objectives
The present pilot study analyzed two abutment types (a retentive ball and a non‐retentive dome) in implant‐assisted removable partial dentures (IARPDs) on 6 mm short implants with respect to clinical, radiological, and patient‐reported outcomes (PROs), during the first year.
Materials and Methods
Two implants were placed bilaterally in mandibular molar sites, converting existing free‐end removable partial dentures (RPDs) to IARPDs. Twelve subjects were randomized to initially receive either the dome (Group A, n = 6) or the ball abutment (Group B, n = 6). After eight weeks, the abutments were exchanged. After another 8 weeks, the participants were given the choice of one of the abutments. Mean values and standard deviations (SD) were calculated, and random‐effect linear regression analyses were applied to analyze marginal bone level alterations and PROs (α < .05).
Results
Twelve participants were included in the study; however, one dropout occurred. Patient ratings increased significantly in both study groups. The majority of the participants (82%) ultimately chose the ball abutment. The implant survival rate was 100%, and the success rate was 90.9% twelve months after implant placement (mean peri‐implant bone‐loss: −1.2; SD: 0.6 mm) without a statistically significant difference between the study groups, in terms of clinical‐ and radiological outcomes.
Conclusion
Placing 6 mm short implants at mandibular molar sites of RPD wearers seems to be a viable treatment option, based on this investigation with a short‐term follow‐up. Although only minor differences between the two abutments were observed, patients seem to prefer the ball over the dome abutment.
Keywords: abutment, anchor, bone‐remodeling, implant overdenture, OHRQoL, Short implant, strategic implant
1. INTRODUCTION
Due to significant oral health improvements in industrialized countries over the past decade, this will result in an increased number of partially edentulous individuals (Dye et al., 2007; Jordan et al., 2014). Accordingly, the demand for tooth replacement in partially dentate scenarios will increase (Douglass & Watson, 2002). Removable partial dentures (RPDs) represent an economical and conservative treatment option, especially in sites with multiple or extended edentulous ridges. Several authors have considered well‐designed RPDs as a cost‐effective and acceptable alternative treatment option for the rehabilitation of partially edentulous patients (Bassetti et al., 2016; Bergman et al., 1995).
The most commonly missing teeth are the molars, both in periodontally untreated and treated patients (Jordan et al., 2014; Mcfall, 1982). However, rehabilitating patients with missing molars using bilateral or unilateral extension RPDs (Kennedy Class I and II) (Kennedy, 1932) presents specific challenges, especially in the mandible. Free‐end RPDs are associated with several problems related to their limited stability, retention, and chewing efficiency (Brudvik, 1999; Gonçalves et al., 2014; Zancopé et al., 2015). One chiefly apparent problem is the distal sinking of the RPDs in the soft tissues due to its resilience, which transmits increased occlusal forces to the soft tissues (El Mekawy et al., 2012; ELsyad et al., 2017). Consequently, accelerated bone resorption in the edentulous alveolar ridge may occur, leading to changes in RPD occlusion, which promotes further bone resorption due to early contacts and unbalanced occlusal forces (Ozan et al., 2013). Furthermore, it has been demonstrated that patients with Kennedy class I RPDs show a low chewing efficiency, which can be attributed to the aforementioned changes (Schimmel et al., 2017). This might be one of the main reasons why distal extension RPDs represent the least worn type of RPDs (Wetherell & Smales, 1980). Therefore, transforming a Kennedy class I RPD into a Kennedy class III implant‐assisted removable partial denture (IARPD) by placing single implants bilaterally in the molar regions may be beneficial and relatively cost‐efficient. (Kaufmann et al., 2009; Minoretti et al., 2009). Furthermore, positive clinical (Payne et al., 2017) and patient‐reported outcomes (PROs) (Mitrani et al., 2003) using different abutment types have been reported. Depending on the choice of implant abutment, bilateral implant placement can improve denture stability and/or retention and prevent the distal extension from sinking into the soft tissues (Gonçalves et al., 2014; Ohkubo et al., 2008; Zancopé et al., 2015). Furthermore, implant support can reduce the resorption of the alveolar ridge and therefore the need for relining procedures in subsequent years (De Freitas et al., 2012; Kremer et al., 2016). In a finite element analysis, it has also been demonstrated that placing posterior implants, assisting free‐end RPDs improves the occlusal support and reduces the stress in the temporomandibular joint (Maeda et al., 2005). However, the vertical bone quantity in molar sites is frequently insufficient for placing standard‐length implants (≥8 mm) and may require extended bone augmentation procedures (Al‐Nawas & Schiegnitz, 2014; Jaffin & Berman, 1991). This procedure is accompanied by an increased risk of biological complications, particularly in medically compromised or elderly people (Schimmel et al., 2018). In these cases, short implants (≤6 mm) present a possible alternative, avoiding augmentation procedures and further reducing the cost (Al‐Nawas & Schiegnitz, 2014; Heitz‐Mayfield et al., 2014; Mundt et al., 2015). A systematic review demonstrated a mean survival rate of 96% in implants with a length of ≤6 mm after 1–5 years in place (Papaspyridakos et al., 2018). Short and/or small‐diameter implants supporting IARPDs have been successfully applied (Gates et al., 2014). However, the benefits of a specific abutment type in short implants on clinical outcomes and PROs have not been evaluated. In this pilot randomized crossover study, we compared two abutment types (dome and ball) in IARPDs on 6 mm implants in terms of clinical outcomes and PROs. The null hypothesis was, that patient‐reported outcomes are not different between the two abutment types. Furthermore, clinical and radiological outcomes were evaluated. The primary endpoint was the participants’ final abutment choice.
2. MATERIALS AND METHODS
2.1. Study design
The study was designed as a pilot randomized crossover study comparing two types of attachments on 6 mm short implants in the posterior mandible supporting mandibular RPDs in terms of clinical, radiological, and patient‐reported outcomes. It was conducted according to the standards of the Declaration of Helsinki (General Assembly of the World Medical Association, 2014). Ethical approval was granted by the Cantonal Ethics Committee Bern (CEC; No. 223/13). All participants gave their written informed consent. The study was conducted at the University of Bern, School of Dental Medicine.
2.2. Material
All applied implants (SIC ace; SIC invent AG, Basel, Switzerland) had a length of 6 mm and a diameter of 4.0 or 4.5 mm. Except for the implant length, the design of the implant corresponds to the standard‐length implants of the same manufacturer (Enkling et al., 2018). The implant was designed as a bone‐level type titanium grade 4 implant with an integrated 45° platform and a hexagonal, internal implant‐abutment connection. The overall implant surface is ZrO2‐blasted and acid‐etched, resulting in surface roughness of 1 µm. The implant neck is non‐threaded, and the first thread starts 1.5 mm below the implant platform. Due to this neck design, the implant can be inserted with an intrabony depth of 4.5 to 6 mm. In this study, the implant shoulders were placed epicrestally relative to the buccal crest.
Two types of titanium grade 5 stock abutments (ball and dome abutments), as demonstrated in Figure 1 were used. The dome abutment is non‐retentive, and therefore only served as a posterior support, preventing the RPDs from sinking into the distally extending soft tissues. The dome attachment shows an internal hex‐connection. Therefore, it consists of two parts: the dome abutment and an occlusal screw. This concept is similar to the concept of using an individual healing abutment, described by Brudvik (1999). In contrast, the ball abutment and its corresponding gold‐platinum matrix (SIC invent AG) provide direct, screwdriver‐adjustable retention. The ball abutment is a one‐piece attachment with an integrated occlusal screw. Both abutment types were available in heights of 2 and 4 mm. The abutment height was selected so as to result in an approximately 1–2 mm supramucosal position of the abutments’ non‐retentive portion, in order to achieve a minimal vertical space requirement within the implant overdenture. For the second abutment type, the same height as for the first type was always selected.
FIGURE 1.
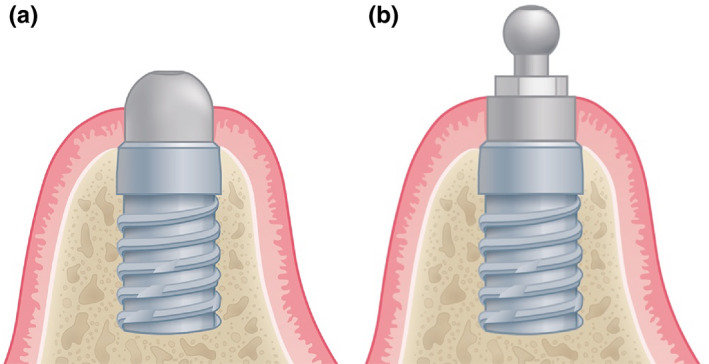
Schematic of the applied abutments: (a) the dome and (b) the ball abutment on short implants. For the dome attachment, less vertical space is required
2.3. Eligibility criteria
The present study included RPD‐wearing patients with mandibular bilateral free‐end situations (Kennedy class I). All patients considered for the study had to be dissatisfied with their existing RPD. Minimally, the second premolars and first molars had to be replaced by the RPD. All subjects intending to participate completed a general and dental history, and were subsequently clinically and radiographically evaluated. For the radiographic evaluation, 5 mm steel balls were temporarily luted to the existing dentures in order to determine the vertical bone height in an orthopantomogram (OPT). The eligibility criteria were as follows:
2.3.1. Inclusion
Minimum age of 18 years
Anterior residual dentition or implants
Bilateral free‐end saddle situation missing at least the second premolars and molars
Minimum vertical and horizontal bone quantity of 7 mm
Acceptable mandibular RPD according to the criteria of the Department of Reconstructive Dentistry and Gerodontology, University of Bern
Periodontally healthy
Natural/artificial antagonistic dentition, at least up to the first molar
2.3.2. Exclusion
Psychological:
No willingness to follow the study curriculum
Alcohol‐ or drug‐ dependence
Pathologically increased dental anxiety
Systemic:
Pregnant or breastfeeding patients
Presence of any systemic medical conditions that are contraindications for implant placement/therapy
Uncontrolled diabetes mellitus
Radiation in the head and neck area
Infectious diseases such as active tuberculosis, acute hepatitis, HIV
Drug immunosuppressive therapy
Osteopathy, for example, Paget's disease
Medication that can negatively influence bone metabolism, for example, Bisphosphonates
General illnesses which do not allow the described treatment procedure
Physiological:
Pronounced hyposalivation
Aggressive or severe periodontitis
Insufficient bone quantity
Painful temporomandibular disorders
Inadequate oral hygiene
Extractions in the area of implants more recent than 6 months
2.4. Clinical workflow
Two 6 mm short implants were placed bilaterally in the posterior mandibular molar sites. The desired implant positions were the first molar sites, due to the superior biomechanical behavior of the IARPD, compared to implant placement in the second premolar or second molar sites (Ortiz‐Puigpelat,et al., 2019). However, if local defects were present at the first molar sites, implant placement at the second molar sites was considered. If the implants were placed in the region of the first molar, the second molar was not replaced by the prosthesis, as this would have contradicted the protocol of converting a Kennedy Class I to a Kennedy Class III. At the first visit, all participants were evaluated clinically, verifying their eligibility according to the inclusion and exclusion criteria. An OPT was taken, evaluating the vertical bone height. The bone width was estimated via palpation by a board‐specified oral surgeon. If the minimum bone width of 7 mm was not evident, a cone beam computed tomography (CBCT) instead of the OPT was recorded. Furthermore, impressions of the maxilla and the mandible were taken in order to fabricate a customized radiographic splint (Figure 2), which was hollowed out in the desired implant regions. At the second visit (BL), the implants were placed. A terminal infiltration anesthesia (Ultracain D‐S forte, 4.0 ml) was applied buccally and lingually to the surgical area. Afterward, mucoperiosteal flaps with vertical releasing incisions were prepared, exposing the bone ridge. The implant site was prepared according to a standardized procedure following the surgical protocol of the manufacturer. The implants were placed at bone level, relative to the buccal bone crest. Afterward, a cover screw was mounted for a submerged healing period of three months. At implant uncovering, the participants were randomized into the two study groups (allocation ratio 1:1). The randomization was performed without any restrictions, by a clinician not involved in the clinical treatment of the participants, based on a randomly computer‐generated list. Study group allocation was kept in sealed, opaque envelopes that were opened upon implant uncovering. Consequently, the surgeon was not aware of the participants’ allocation during surgery. After a soft tissue healing period of 4 weeks, an open‐tray polyether implant impression, using the existing denture as an impression tray, was performed. A gypsum master cast including implant analogues, which was used for all subsequent denture transformations during the study, was produced. According to the allocation, 4 months after implant placement, the implants were loaded: either the dome (Group A) or the ball abutments (Group B) were mounted to the implants with a torque‐controlled wrench, applying a torque of 20 Ncm. In the dental laboratory, the dentures were either relined (Group A), or matrices (Group B) integrated (Figure 3), both using a cold‐polymerizing polymethyl‐methacrylate (PMMA). Follow‐up visits were conducted 4 and 8 weeks after abutment connection. At the 8‐week follow‐up, that is, 6 months after implant insertion, the abutments were changed to the second option (Group A: ball; Group B: dome) and the dentures were indirectly modified by a dental technician as described before. For ethical reasons, there was no washout period before the exchange to the second abutment option. Two further follow‐up appointments were carried out after additional 4 and 8 weeks. Afterward, the participants were asked to choose their preferred treatment option; if necessary, the dentures were modified a third time. A final clinical appointment was scheduled four months after the participants’ final choice (1 year after implant placement). Figure 4 gives a chronological overview of the study procedures.
FIGURE 2.

Customized radiographic splint supported by anterior teeth, for standardized x‐ray recording. The rough surfaces of the splint on the x‐ray film holder and in the molar region were used for reproducible repositioning
FIGURE 3.

Intraoral view and intaglio denture surface of a participant with the dome abutments (upper) and another participant with the ball abutments (lower)
FIGURE 4.

Study flowchart, summarizing the randomization, clinical, and follow‐up procedures
2.5. Data acquisition
Clinical peri‐implant parameters were recorded at BL, 6 months, and 12 months after implant placement. A periodontal probe was used to measure probing depths (PD) around each implant at four sites (mesial, buccal, distal, lingual). Bleeding on probing (yes/no), and the presence of plaque (yes/no) was evaluated for each implant. Furthermore, technical complications, for example, abutment screw loosening or denture fractures were recorded. Complication‐free IODs were rated as prosthetic successes. Implant success was defined according to the PISA consensus conference (Misch et al., 2008): Radiographs were recorded at the same time points, using the customized radiographic splints with a paralleling technique. The six‐month follow‐up was identical with the change of the abutment according to the crossover study design. Radiograph evaluation was performed independently by two calibrated dentists, who were not involved in the patient treatment, using a software application (DBS‐Win 4.5; Dürr Dental AG; Bietigheim‐Bissingen) with a digital 20‐fold magnification. Calibration was done, by identifying the position of the first bone to implant contact (BIC) in 20 randomly selected x‐rays, together with the senior author. The distance from implant shoulder to the apex was defined to be 6 mm, resulting in the correct dimension for the measurements. The distances from the implant shoulder to the first BIC were measured separately at the mesial and distal aspects of the implants and defined as the marginal bone level (MBL). The evaluation was performed at two separate timepoints. For instances of interrater discrepancies >0.2 mm, the position of the first BIC was inspected together by the two clinicians, to reach a consensus. Afterward, the measurements were repeated independently. The marginal bone level alteration (ΔMBL) was calculated by subtracting the distance at follow‐up visits from the BL values.
Numerical rating scales (NRS) were used, analyzing PROs. After wearing each type of abutment for 2 months, the participants completed an NRS‐based questionnaire with four items asking for changes in denture stability, painfulness when wearing the denture, chewing ability, and cleanability to be answered on a scale ranging from −10 to 10. −10 represented the maximum worsening and +10 the maximum improvement relative to the previous intraoral situation, which was either the RPD without implant support for the evaluation at 8 weeks after loading, or the IARPD with the first type of abutment at 16 weeks after loading. A score of 0 represented no change.
2.6. Statistics
The sample size calculation was based on ΔMBL reported in previous studies on IARPDs (Zancopé et al., 2015). Assuming a ΔMBL of 0.3 (SD: 0.5 mm), resulted in six participants per group with a total number of 24 MBLs, resulting from the mesial and distal aspects of each implant (two‐sided t‐test for two dependent samples, level of significance 0.05, power 0.8). Furthermore, the assumption that a difference in mean ΔMBL of 0.4 mm between the study groups would be significant (Astrand et al., 1999) also resulted in six participants per group to show a significant difference (sample size estimation for repeated measurement analysis, correlation between repeated measures 0.5, between‐group variance 0.11, variance of error 1). The MBLs at implant sides at different points and ΔMBL were described by means and standard deviations (SDs). For months 0 and 6 mean differences and 95% confidence intervals between randomization groups were estimated at implant site level by linear regression, in case of group differences with respect to ΔMBL by adjusting for MBL at baseline. Random‐effects linear regression with random effect patient, fixed effect implant site, and MBL at baseline was used to analyze whether ΔMBL from months 0 to 6 was different in groups A and B. The same approach was used to compare ΔMBL from months 6 to 12 of those patients who finally chose the dome or ball abutment (further adjustment for randomization group). Furthermore, it was applied to investigate whether ΔMBL between months 0 to 6 was different from ΔMBL between months 6 to 12 (further adjustment for randomization group and abutment at the time of measurement). The changes in PROs were analyzed relative to the previous intraoral situation for time points 8‐ and 16 weeks post‐loading. Mean values with 95%‐CI were estimated for each randomization group by random‐effects linear regression (random effect patient) with fixed effects time of measurement (week 8, 16), randomization group (A, B), and interaction (time × group). All statistical tests were two‐sided with α < 0.05. Stata/IC 16.1 for Unix was used for statistical analysis. The Bland‐Altman analysis was used to analyze the interrater reliability, using all individual measurements (n = 140).
3. RESULTS
3.1. Description of participants
In total, 12 participants (six males and six females) with a mean age of 67.2 ± 8.2 years were included. Six participants were each randomly assigned to one of the two study groups. Group A included three male and three female participants with a mean age of 67.8 ± 9.1 years wearing removable dentures for a mean time of 9.1 years. Group B included three male and three female participants with a mean age of 66.8 ± 9.2 years wearing removable dentures for a mean time of 15.2 years. One female participant from Group B withdrew her consent to participate in the study after implant placement and was therefore excluded from the analyses, resulting in a final study size of 11 included participants (six in Group A and five in Group B). Fourteen implants were placed in the first, and eight in the second molar sites. An overview of the participants’ intraoral status is presented in Table 1.
TABLE 1.
Dental status of study participants
| Patient number | Study group | Time of removable denture wearing [years] | Intraoral status maxilla | Intraoral status mandible |
|---|---|---|---|---|
| 1 | A | 3 | CD | Clasp retained RPD, remaining (abutment teeth: 33,43,44) |
| 2 | B | 45 | Root‐retained OD (cast post‐and‐cores on 14,11,21,23) | Root‐retained OD (cast post‐and‐cores on 43,33) |
| 3 | A | 30 | Telescopic‐crown retained OD (abutment teeth: 15,11,21, 22, 24) | Telescopic‐crown retained RPD (abutment teeth 43,33,34) |
| 4 | B | 4 | CD | Clasp retained RPD, remaining (abutment teeth: 44,43,33,34) |
| 5 | A | 6 | CD | IOD (round bar; implants 43,33) |
| 6 | A | 10 | IOD (parallel‐walled bar; implants 14,12,22,24) | Clasp retained RPD, remaining (abutment teeth: 33,34,43) |
| 7 | A | 0.8 | CD | Clasp retained RPD, remaining (abutment teeth: 44,43,33) |
| 8 | A | 9.9 | CD | Clasp retained RPD, remaining (abutment teeth: 33,34,43) |
| 9 | B | 31 | Clasp retained RPD, remaining (abutment teeth: 13,14,23,24,25) | IARPD (implants wit ball abutments: 34, 44; clasps: 33,34) |
| 11 | B | 1.2 | CD | Root‐retained OD (cast post‐and‐cores on 43,33) |
| 12 | B | 4 | IOD (parallel‐walled bar; implants 14,12,22,24) | Clasp retained RPD, remaining (abutment teeth: 33, 43) |
Abbreviations: CD, complete denture; IARPD, implant‐assisted removable partial denture; IOD, implant overdenture; OD, overdenture; RPD, removable partial denture.
Overview of participants’ intraoral status in the maxilla and mandible.
3.2. Final abutment choice
Of the eleven participants, nine participants chose the ball abutments (82%) and two chose the dome abutments (18%) as their final treatment option. Five participants who chose the ball abutments had been randomized to Group A and four to Group B. Consequently, the dentures of five participants (four from dome to ball and one from ball to dome) were re‐adapted after exchanging the abutments in an additional clinical visit.
3.3. Patient‐reported outcomes
Relative to baseline, the subjective denture stability increased significantly in both study groups (both p < .001) after 8 weeks. After changing from dome to the ball abutments (Group A), an additional statistically significant increase in subjective denture stability (p < .001) was observed, whereas no further changes could be observed after changing from ball to dome abutments (Group B; p = .611). The participant reported changes in denture stability were not significantly between the two groups (p= .722).
After 8 weeks, the subjective chewing ability increased significantly in both study groups (both p < .001). An additional statistically significant increase was observed in Group A after switching the abutment type (dome to ball, p = .010), whereas no significant change was found in Group B (ball to dome, p = .573). Comparing the two study groups, the changes of the chewing ability were not significantly different (p = .775).
According to the participants, painfulness was significantly reduced in Group A (p = 0.002) but not in Group B (p = .324), when wearing the dentures with the first abutment type. After exchanging the abutments, a statistically significant pain reduction could be observed in Group A (dome to ball, p = .003) but not in Group B (ball to dome, p = .276). The feeling of pain was significantly more improved in group A than in group B (p = .021).
The denture cleanability significantly improved for Group A (p = .003) but not for Group B (p = .203), after 8 weeks. Between 8‐ and 16 weeks post‐loading, a statistically significant increase of the denture cleanability was observed for Group A (p = .003) but not for Group B (p = .116). Comparing the two study groups, the changes in terms of denture cleanability were not significantly different (p = .149). An overview of the patient‐reported outcomes is shown in Figure 5.
FIGURE 5.
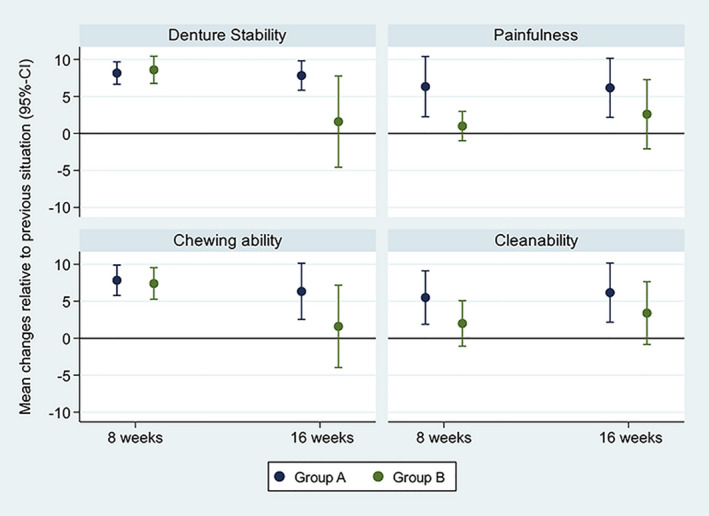
Patient ratings: Mean values and 95% confidence intervals of changes in terms of denture stability, painfulness, chewing ability, and cleanability at 8 (evaluating the first abutment type) and 16 weeks (evaluating the second abutment type) after loading; a score of +10 being the maximum possible improvement in each period
3.4. Clinical and radiological outcomes
After 1 year, the overall mean ΔMBLs were −1.2 (SD: 0.6 mm), −1.1 (SD: 0.9 mm) in the implants placed at the left, and −1.3 (SD: 0.9 mm) at the right molar sites, respectively. Figure 6a–c depicts X‐rays of an implant at the BL, 6‐month, and 12‐month follow‐up visits. At 0 months, there was no significant difference in the MBL between the two study groups (all ns). Detailed information on the MBLs and ΔMBLs at implant placement and the 6‐month follow‐up are presented in Table 2. The overall ΔMBLs were significant in Group A (−0.9 mm [95% CI: −1.1 to −0.6]; p < .001), and Group B (−0.7 mm [−0.9 to 0.4]; p < .001), without a difference between the two groups (estimated mean difference: 0.2 mm [95% CI: −0.2 to 0.6]; p = .339). Table 3 provides detailed information on the MBLs and ΔMBLs at implant placement and the 12‐month follow‐up, based on the participants’ final abutment choice. The ΔMBLs were significantly smaller in participants who finally chose the dome abutment (p < .001; Table 4). No difference was found between the study groups (p = .246) and the interaction of the study group and final abutment choice (p = .998) (Table 4). The overall mean ΔMBL was significantly greater within the first 6 months (−0.8 mm [95% CI: −1.0 to −0.6] compared with the subsequent 6 months (−0.4 mm [95% CI: −0.6 to −0.1]; p = .011). Analyzing the radiographs, the mean interobserver difference was 0 mm (95% CI: −0.01 to 0.02 mm), and the intraobserver difference 0.02 mm (95% CI: −0.05 to 0.10 mm). The limit of agreement between the two reviewers ranged from −0.15 to 0.16 mm (Figure 7).
FIGURE 6.

X‐ray of an implant in the right molar site (a) at baseline with the healing abutment, (b) at the 6‐month follow‐up with the dome abutment, and (c) at the 12‐month follow‐up with the ball abutment. At the mesial aspect, a slight decrease in the MBL to the first implant thread can be observed. (SIC ace, 6.0 mm × 4.5 mm, SIC invent AG, Basel, CH)
TABLE 2.
Radiographic evaluation after 6 months
| Implant site |
Follow‐up [months] |
Mean MBL, SD [mm] |
Estimated mean difference between groups [mm] (B vs. A) (95%‐CI) |
|
|---|---|---|---|---|
|
Group A (n = 6) |
Group B (n = 5) |
|||
| Left molar mesial | 0 | 0.5, 0.7 | −0.2, 0.7 | −0.7 (−1.6; 0.2) |
| 6 | 1.4, 0.8 | 0.8, 0.7 | −0.5 (−1.5; 0.4) | |
| ΔMBL | −0.8, 0.5 | −1.0, 0.6 | 0.0 (−0.7; 0.7) | |
| Right molar mesial | 0 | 0.4, 1.0 | 0.5, 1.1 | 0.2 (−1.2; 1.6) |
| 6 | 1.4, 1.0 | 1.2, 0.9 | −0.2 (−1.5; 1.0) | |
| ΔMBL | −1.1, 0.6 | −0.7, 0.6 | 0.4 (−0.4; 1.1) | |
| Left molar distal | 0 | 0.2, 0.7 | −0.6, 1.0 | −0.9 (−2.0; 0.3) |
| 6 | 0.9, 0.7 | 0.1, 1.0 | −0.8 (−2.0; 0.4) | |
| ΔMBL | −0.6, 0.4 | −0.7, 0.6 | 0.0 (−0.7; 0.7) | |
| Right molar distal | 0 | −0.1, 0.9 | −0.0, 1.4 | 0.1 (−1.6; 1.7) |
| 6 | 0.7, 0.9 | 0.5, 1.1 | −0.2 (−1.6; 1.2) | |
| ΔMBL | −0.8, 0.6 | −0.5, 0.4 | 0.2 (−0.3; 0.8) | |
Mean marginal bone levels (MBLs, negative values indicating an implant position below the marginal crest), bone level alterations (ΔMBLs) and standard deviations (SD), and the estimated mean differences between the groups, including and 95% confidence intervals (95%‐CI) at implant placement, and the 6‐month follow‐up. The mean differences between groups in terms of ΔMBLs were estimated by a linear regression model, adjusted for MBL at baseline (mean differences and difference of mean may deviate due to rounding).
TABLE 3.
Radiographic evaluation after 12 months
| Implant site |
Follow‐up [months] |
Mean MBL, SD [mm] | |
|---|---|---|---|
|
Choice dome (n = 2) |
Choice ball (n = 9) |
||
| Left molar mesial | 0 | 0.3, 1.2 | 0.2, 0.7 |
| 12 | 0.9, 1.0 | 1.5, 0.9 | |
| ΔMBL | −0.6, 0.2 | −1.3, 0.7 | |
| Right molar mesial | 0 | 0.5, 0.3 | 0.4, 1.1 |
| 12 | 1.3, 1.1 | 1.7, 0.8 | |
| ΔMBL | −0.8, 0.8 | −1.3, 0.6 | |
| Left molar distal | 0 | −0.7, 1.2 | −0.0, 0.9 |
| 12 | −0.1, 1.7 | 1.1, 0.6 | |
| ΔMBL | −0.6, 0.5 | −1.1, 0.5 | |
| Right molar distal | 0 | −0.4, 0.2 | 0.0, 1.2 |
| 12 | 0.1, 0.3 | 1.2, 1.0 | |
| ΔMBL | −0.5, 0.5 | −1.2, 0.7 | |
Mean marginal bone levels (MBLs, negative values indicating an implant position below the marginal crest), and bone level alterations (ΔMBLs) and standard deviations (SD), at implant placement, and the 12‐month follow‐up (mean differences and difference of mean may deviate due to rounding).
TABLE 4.
Comparison of overall ΔMBL (n = 44) after 12 months
| ΔMBL (95%‐CI) | p‐Value | |
|---|---|---|
| Choice dome (n = 8) | −0.6 (−0.6; −0.5) | <.001 |
| Choice ball (n = 36) | −1.2 (−1.5; −1.0) | <.001 |
| Ball vs. Dome | −0.7 (−1.0; −0.4) | <.001 |
| Group A (n = 24) | −1.1 (−1.3; −0.8) | <.001 |
| Group B (n = 20) | −1.2 (−1.6; −0.8) | <.001 |
| B vs. A | −0.1 (−0.4; 0.1) | .246 |
| Interaction choice × Group | 0.001 (0.6–0.6) | .998 |
Mean marginal bone level alterations (ΔMBLs) and corresponding 95% confidence intervals, relative to the participants’ final abutment choice, study group allocation, and the interaction of study group and final choice. The differences and p‐values resulted from a random effects linear regression analysis adjusted for implant site and MBL at baseline
FIGURE 7.

Bland–Altman plot, demonstrating differences (y‐axis) of the individually determined MBLs (x‐axis) of the two reviewers. The limits of agreement ranged from −0.15 to 0.16 mm
The implant survival rate was 100%. The implant success rate according to the PISA consensus was 90.9%, as the ΔMBLs of both implants in one patient were higher than 2 mm (2.2 mm and 2.4 mm). The clinical peri‐implant parameters at 0, 6, and 12 months are presented in Tables 5 and 6: no biological complications occurred. Additionally, there were no technical complications in any of the study groups; consequently, the prosthetic success rate was 100%.
TABLE 5.
Probing depths
| Implant site | Follow‐up [months] | Mv [mm] | SD [mm] | Implant site | Mv [mm] | SD [mm] | |
|---|---|---|---|---|---|---|---|
| Left molar mesial | 4 | Group A | 2.2 | 1.1 | Right molar mesial | 2.4 | 1.1 |
| Group B | 2 | 1.7 | 2.3 | 1.4 | |||
| 6 | Group A | 2.6 | 0.6 | 2.8 | 0.8 | ||
| Group B | 1.5 | 1.1 | 1.8 | 1.0 | |||
| 12 | Group A | 2.4 | 0.6 | 3.4 | 0.9 | ||
| Group B | 1.8 | 1.5 | 2.2 | 1.7 | |||
| Left molar distal | 4 | Group A | 2.6 | 0.9 | Right molar distal | 2.6 | 0.6 |
| Group B | 2.8 | 1.6 | 2.3 | 1.5 | |||
| 6 | Group A | 3 | 1.6 | 3.4 | 1.5 | ||
| Group B | 1.7 | 1.6 | 1.5 | 1.4 | |||
| 12 | Group A | 2.6 | 0.9 | 3.6 | 0.9 | ||
| Group B | 1.8 | 1.5 | 1.8 | 1.5 | |||
| Left molar buccal | 4 | Group A | 2.6 | 0.9 | Right molar buccal | 2.8 | 0.8 |
| Group B | 2.3 | 1.7 | 2.3 | 1.4 | |||
| 6 | Group A | 2.4 | 0.6 | 3 | 0.7 | ||
| Group B | 1.5 | 1.1 | 1.8 | 1.0 | |||
| 12 | Group A | 2.6 | 0.6 | 3 | 0.8 | ||
| Group B | 1.8 | 1.7 | 2.5 | 1.3 | |||
| Left molar lingual | 4 | Group A | 2.8 | 0.8 | Right molar lingual | 2.40 | 0.5 |
| Group B | 2.7 | 1.4 | 2.2 | 1.4 | |||
| 6 | Group A | 2.6 | 1.1 | 3.2 | 0.8 | ||
| Group B | 1.8 | 1.3 | 1.5 | 1.1 | |||
| 12 | Group A | 3.2 | 0.8 | 3 | 0.7 | ||
| Group B | 2.3 | 1.2 | 2 | 1.2 | |||
Mean probing depths (Mv) and standard deviations (SD) at four sites per implant, separated for the two study groups.
TABLE 6.
Plaque and bleeding on probing (BOP) scores
| Follow‐up [months] | Group A | Group B | |
|---|---|---|---|
| Plaque | |||
| Left molar | 4 | 0 (0%) | 1 (20%) |
| 6 | 2 (33.3%) | 0 (0%) | |
| 12 | 1 (16.7.0%) | 2 (40%) | |
| Right molar | 4 | 0 (0%) | 1 (20%) |
| 6 | 3 (50.0%) | 1 (20%) | |
| 12 | 2 (33.3%) | 2 (40%) | |
| BOP | |||
| Left molar | 4 | 2 (33.3%) | 3 (60.0%) |
| 6 | 3 (50.0%) | 2 (40%) | |
| 12 | 2 (33.3%) | 2 (40%) | |
| Right molar | 4 | 2 (33.3%) | 2 (40%) |
| 6 | 3 (50.0%) | 2 (40%) | |
| 12 | 3 (50.0%) | 1 (20%) | |
Number and relative frequency of plaque and bleeding on probing (BOP)‐positive implant sites at evaluated timepoints. 100% indicates n = 6 in Group A and n = 5 in Group B.
4. DISCUSSION
In this randomized crossover pilot study, two abutment types (dome and ball) on 6 mm implants were compared in terms of clinical, radiological, and patient‐reported outcomes. At the completion of the study, the majority of the participants (nine) chose the ball abutment, whereas only two opted for the dome abutments. Thus, in the indication of changing Kennedy Class I to Class III mandibles by means of strategic implants, the ball attachment was more popular. In terms of patient‐reported outcomes, a significant difference was found at 8 weeks regarding painfulness, with advantages for the abutment sequence dome‐ball. Therefore, the 0‐hypothesis of equal PROs, using the two abutments was rejected. Significant ΔMBLs were observed in both study groups with no difference between the two groups.
Regarding the participants’ final choice, it seems that they valued higher retention rather than only posterior denture support. This result is consistent with the findings from another randomized controlled trial, analyzing ball attachments and healing abutments in molar sites of patients rehabilitated with IARPDs (Suzuki et al., 2017). The study demonstrated that the participants preferred the ball attachments over the healing abutments, which was attributed to the higher retention provided by the ball attachments. Due to the unequal distribution of the final abutment choice and the small number of participants finally choosing the dome abutment, factors influencing the final abutment choice were not analyzed.
Although the final abutment choice demonstrated a preference for the ball abutments, all participants seemed similarly satisfied with both abutments, according to the PROs. Only in terms of painfulness, a significant difference between the study groups could be demonstrated. Nevertheless, after 8 weeks, the subjective denture stability and chewing ability improved for both study groups, whereas the painfulness and denture cleanability only improved for Group A. Furthermore, in Group A, the parameters cleanability and painfulness were also improved after changing the dome to the ball abutments. After exchanging the abutments in Group B, no significant changes of these parameters were observed. Because the ball matrices incorporated into the dentures are small, uneven, and consequently more difficult to clean, one may have expected responses regarding the parameter “denture cleanability” to favor the dome abutments. However, no disadvantage of the ball vs. dome regarding subjective cleanability was observed. The difference in pain perception might be a direct consequence of the non‐retentive properties of the dome abutment, relieving the posterior soft tissues, especially in the early stages after implant uncovering surgery. Previous studies have demonstrated that loading of ball abutments in early stages after surgery provokes more pain relative to delayed loading (Mundt et al., 2017). As patients were similarly satisfied in terms of denture stability and chewing ability with the non‐retentive dome abutments, using comparable abutment types, might be preferable from a patient's point of view in early stages when converting a Kennedy Class I RPD to a Kennedy Class III IARPD. After an additional 8 weeks, a second statistically significant increase for all evaluated PROs was observed when the dome abutments were exchanged to the ball abutments, but not when the ball abutments were exchanged to the dome abutments. The further increase in the PROs in Group A may explain why the majority of participants finally chose the ball abutment and corroborates the theory that patients rehabilitated with IARPDs seek increased retention and not only posterior denture support. The beneficial effects of implant placement in posterior sites in distal extension RPDs in terms of PROs, as reported in previous studies, could be confirmed (Bortolini et al., 2011; Gonçalves et al., 2014; Ohkubo et al., 2008; Swelem et al., 2014).
Clinical advantages of the dome compared with retentive attachments include the following parameters: a decreased vertical space requirement, as there is no retentive element on the male part, and no female part exists. This gives the opportunity to integrate the dome into existing RPDs or to use it in cases where the vertical space is insufficient. Next, because a precision matrix can be waived, initial costs, as well as follow‐up costs due to the inevitable loss of retention over time (Payne et al., 2017), are reduced. Compared with the concept of using an individual healing abutment as described by Brudvik (Brudvik), the dome abutment is a stock abutment. Therefore, a precise fit of the abutment, as well as lower costs can be expected due to the standardized manufacturing process. The advantage of a dome abutment relative to a standard healing abutment is that the dome consists of two parts and the internal hex‐connections are still available. The risk described by Brudvik of the abutment screw engaging with the prosthesis (Brudvik), which could lead to distortion of the screw head, does not exist, as the screw head lies minimally below the top of the abutment. This design enables higher mucosal heights of the abutment and leads to a reduced risk of abutment loosening, compared to a standard healing abutment. Payne et al. demonstrated a high frequency of healing abutment loosening in IARPDs (Payne et al., 2017), whereas loosening did not occur in any of the participants in the current study. In cases with angled implant axes, the round head of the dome avoids undercuts in relation to the dentures’ insertion direction and guarantees vertical support to the denture. In addition, frequent complications were described with the use of retentive abutments, with matrix loosening being the most frequent complication (Assaf et al., 2017; Payne et al., 2017). In contrast, no complications occurred in the present study regardless of the final abutment type selected, which is most likely due to the short observation period. However, it must be assumed that in the future the exchange of matrices will be a complication in the ball abutments, which will lead to an additional financial cost for the patients.
The survival rate of the applied 6 mm implants was 100%, and the success rate was 90.9% according to the PISA criteria (Misch et al., 2008). A systematic review showed that the majority of implant failures in 6 mm implants are early failures (76%) and therefore would have been observed in the one‐year follow‐up of the present study (Srinivasan et al., 2014). Systematic reviews have demonstrated that the survival rates of implants ≤6 mm are similar to those of longer implants, but with a higher outcome variability (Nisand et al., 2015; Papaspyridakos et al., 2018).
The MBLs decreased steadily during the 12 months following implant placement. It should be noted that the decrease significantly decelerated after 6 months. The decrease was to be expected and can also be similarly observed in other studies on longer implants (Enkling et al., 2013; Maló et al., 2007). Applying the implant success criteria from the PISA consensus conference (Misch et al., 2008), defining implants with ΔMBLs of ≤2mm after 1 year as a success, 90.9% of the implants were rated as successful. The amount of marginal bone loss around implants is a frequently used and decisive parameter in terms of success (Misch et al., 2008; Monje et al., 2014; Salvi & Lang, 2004). It was shown that the determination of bone loss from radiographs is subject to large variations (Walton & Layton, 2018). However, in the aforementioned study, low median intra‐ and interrater reliabilities were shown (kappa =0.58 and 0.54, respectively). The rather small intra‐ and interrater discrepancies in the present study are probably due to the calibration of the two evaluators, as well as the standardization of the radiographs, and can therefore be considered relatively reliable. Nevertheless, the possible variations in the interpretation of the radiographs should not be neglected.
It should be noted, that the currently applied success criteria are defined for standard‐length implants. Therefore, it questionable whether these criteria can be equally applied to short implants, because a ΔMBL of 2 mm represents one‐third of the entire implant length in a 6 mm implant. A systematic review on implant ΔMBLs when converting a Kennedy class I RPD to a Kennedy class III IARPD calculated mean ΔMBLs between 0 and 1.4 mm after 0–120 months (Zancopé et al., 2015). However, most of the implants were standard‐length implants. In the present study, the mean ΔMBLs were smaller than 1.4 mm at both implant sites, but with only a follow‐up of 12 months. When using short implants to support an RPD, a higher variability of the outcomes may be expected (Papaspyridakos et al., 2018). However, comparing ΔMBLs between Groups A and B at the 6‐ and 12‐month follow‐up visits, no statistically significant differences were observed. When using ball attachments, more load is applied to the implants than for dome attachments. For short implants in cortical bone, this might be a disadvantage, leading to a reduced implant survival and implant success. The possible benefit of a reduced load on short implants using non‐retentive abutments like the dome abutment was similarly hypothesized in classic textbooks on the IARPD concept (Brudvik; Carr & Brown, 2016). This could also explain the lower ΔMBLs at 12 months in the participants who finally opted for the dome abutment. However, this result should not be overinterpreted, as the data from the dome group are based on eight measurements in two participants only. Based on the available knowledge, the most decisive criterion for the choice of abutment still seems to be the patient's desire for improved retention, or improved comfort through a mere support of the prosthesis (Carr & Brown, 2016).
The limitations of the study are the small number of participants and the short follow‐up time. Additionally, there was a dropout despite the limited, one‐year follow‐up period. Although the number of participants was selected according to the sample size calculation, it was based on studies reporting ΔMBLs in IARPDs with various implant lengths. However, the number of included study participants was similar to other studies analyzing PROs in IARPD wearers, including twelve and ten participants, respectively (Gonçalves et al., 2014; Mitrani et al., 2003). A specific weakness of the crossover design used in this study is the lack of a washout period, which may have led to a carryover effect of treatment. This may have caused the effect of the first treatment option to carry over into the second treatment (Dwan et al., 2019). Furthermore, there was no control group with longer implants to compare implants in terms of success.
5. CONCLUSION
Short implants placed in the posterior mandible to convert Kennedy Class I RPDs to Kennedy Class III IARPDs seems to be a suitable treatment concept from a clinician's as well as from a patient's point of view, after a short‐term period. Although non‐retentive abutments initially lead to similar improvements as retentive abutments, patients may finally prefer retentive abutments. For future research, more studies with increased sample sizes and follow‐up periods are needed to determine the suitability of this treatment concept, especially focusing on bone level alterations around short implants and long‐term complications.
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHOR CONTRIBUTION
Norbert Enkling: Conceptualization (lead); Data curation (equal); Formal analysis (equal); Funding acquisition (lead); Investigation (equal); Methodology (equal); Project administration (equal); Validation (equal); Writing‐review & editing (equal). Joel Nauli: Data curation (equal); Investigation (equal); Validation (supporting); Writing‐review & editing (supporting). Dominik Kraus: Data curation (supporting); Investigation (supporting); Writing‐original draft (supporting); Writing‐review & editing (equal). Julia Gabriela Wittneban: Data curation (supporting); Investigation (supporting); Writing‐original draft (supporting); Writing‐review & editing (equal). Martin Schimmel: Data curation (equal); Formal analysis (supporting); Methodology (supporting); Project administration (lead); Resources (lead); Writing‐review & editing (equal). Samir Abou Ayash: Data curation (equal); Investigation (supporting); Project administration (supporting); Resources (supporting); Supervision (equal); Writing‐original draft (lead); Writing‐review & editing (lead).
Supporting information
CONSORT checklist
ACKNOWLEDGEMENTS
The authors thank Mrs. Hiltrud Niggemann for the statistical analyses. Open Access funding provided by Universitat Bern.
Enkling, N. , Nauli, J. , Kraus, D. , Wittneben, J. G. , Schimmel, M. , & Abou‐Ayash, S. (2021). Short strategic implants for mandibular removable partial dentures: One‐year results from a pilot randomized crossover abutment type study. Clinical Oral Implants Research, 32, 1176–1189. 10.1111/clr.13815
Trial Registration: German Clinical Trials Register (DRKS); Number: DRKS00024147
Funding information
The study was funded by Innosuisse – Swiss Innovation Agency (KTI‐Nr. 16012.2 PFLS‐LS), and SIC invent AG.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- Al‐Nawas, B. , & Schiegnitz, E. (2014). Augmentation procedures using bone substitute materials or autogenous bone ‐ a systematic review and meta‐analysis. European Journal of Oral Implantology, 7, 219–234. [PubMed] [Google Scholar]
- Assaf, A. , Daas, M. , Boittin, A. , Eid, N. , & Postaire, M. (2017). Prosthetic maintenance of different mandibular implant overdentures: A systematic review. Journal of Prosthetic Dentistry, 118, 144–152. 10.1016/j.prosdent.2016.10.037 [DOI] [PubMed] [Google Scholar]
- Astrand, P. , Engquist, B. , Dahlgren, S. , Engquist, E. , Feldmann, H. , & Gröndahl, K. (1999). Astra Tech and Brånemark System implants: A prospective 5‐year comparative study. Results after one year. Clinical Implant Dentistry and Related Research, 1, 17–26. 10.1111/j.1708-8208.1999.tb00087.x [DOI] [PubMed] [Google Scholar]
- Bassetti, R. G. , Mericske‐Stern, R. , & Enkling, N. (2016). Are there differences in the changes in oral‐health‐related quality of life (OHRQoL) depending on the type (rigidity) of prosthetic treatment? Quintessence International, 47, 749–757. [DOI] [PubMed] [Google Scholar]
- Bergman, B. , Hugoson, A. , & Olsson, C. O. (1995). A 25 year longitudinal study of patients treated with removable partial dentures. Journal of Oral Rehabilitation, 22, 595–599. 10.1111/j.1365-2842.1995.tb01055.x [DOI] [PubMed] [Google Scholar]
- Bortolini, S. , Natali, A. , Franchi, M. , Coggiola, A. , & Consolo, U. (2011). Implant‐retained removable partial dentures: An 8‐year retrospective study. Journal of Prosthodontics, 20, 168–172. 10.1111/j.1532-849X.2011.00700.x [DOI] [PubMed] [Google Scholar]
- Brudvik, J. S. (1999). Implants and removable partial dentures. In Advanced removable partial dentures (1st ed., pp. 153–159). Quitnessence Publishing Co. [Google Scholar]
- Carr, A. B. , & Brown, D. T. (2016). Considerations for the use of dental implants with removable partial dentures. Mc Cracken’s removable partial prosthodontics (13th ed., pp. 146–153). Elsevier, Inc. [Google Scholar]
- De freitas, R. F. C. P. , De carvalho dias, K. , Da fonte porto carreiro, A. , Barbosa, G. A. S. , & Ferreira, M. â. F. (2012). Mandibular implant‐supported removable partial denture with distal extension: A systematic review. Journal of Oral Rehabilitation, 39, 791–798. 10.1111/j.1365-2842.2012.02326.x [DOI] [PubMed] [Google Scholar]
- Douglass, C. W. , & Watson, A. J. (2002). Future needs for fixed and removable partial dentures in the United States. The Journal of Prosthetic Dentistry, 87, 9–14. 10.1067/mpr.2002.121204 [DOI] [PubMed] [Google Scholar]
- Dwan, K. , Li, T. , Altman, D. G. , & Elbourne, D. (2019). CONSORT 2010 statement: Extension to randomised crossover trials. BMJ, 366, l4378. 10.1136/bmj.l4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye, B. A. , Tan, S. , Smith, V. , & Lewis, B. G. (2007). Trends in oral health status: United States, 1988–1994 and 1999–2004. National Centre for Health Statistics. Vital Health Statatistics, 11, 1–92. [PubMed] [Google Scholar]
- El Mekawy, N. H. , Abd El‐Raof, E. N. S. , El‐Awady, G. M. , & El‐Hawary, Y. M. (2012). Intracoronal Mandibular Kennedy Class I implant‐tooth–supported removable partial overdenture: A 2‐year multicenter prospective study. The International Journal of Oral & Maxillofacial Implants, 27, 677–683. [PubMed] [Google Scholar]
- ELsyad, M. A. , Omran, A. O. , & Fouad, M. M. (2017). Strains around abutment teeth with different attachments used for implant‐assisted distal extension partial overdentures: An in vitro study. Journal of Prosthodontics, 26, 42–47. 10.1111/jopr.12370 [DOI] [PubMed] [Google Scholar]
- Enkling, N. , Jöhren, P. , Katsoulis, J. , Bayer, S. , Jervøe‐Storm, P. M. , Mericske‐Stern, R. , & Jepsen, S. (2013). Influence of platform switching on bone‐level alterations: A three‐year randomized clinical trial. Journal of Dental Research, 92, 139S–145S. 10.1177/0022034513504953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkling, N. , Kraus, D. , Stark, H. , & Schimmel, M. (2018). Equivalent bone‐level‐alterations at implants with platform‐switching and implants with matching‐platforms: RCT‐ 5 years‐results. Journal of Dental Research, 97 (Spec Iss B): abstract number/ presentation ID: 1257. [Google Scholar]
- Gates, W. D. , Cooper, L. F. , Sanders, A. E. , Reside, G. J. , & De Kok, I. J. (2014). The effect of implant‐supported removable partial dentures on oral health quality of life. Clinical Oral Implants Research, 25, 207–213. 10.1111/clr.12085 [DOI] [PubMed] [Google Scholar]
- General Assembly of the World Medical Association . (2014). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. The Journal of the American College of Dentists, 81, 14–18. [PubMed] [Google Scholar]
- Gonçalves, T. M. , Campos, C. H. , & Garcia, R. C. (2014). Implant retention and support for distal extension partial removable dental prostheses: Satisfaction outcomes. Journal of Prosthetic Dentistry, 112, 334–339. 10.1016/j.prosdent.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. , Needleman, I. , Salvi, G. E. , & Pjetursson, B. E. (2014). Consensus statements and clinical recommendations for prevention and management of biologic and technical implant complications. The International Journal of Oral and Maxillofacial Implants, 29, 346–350. 10.11607/jomi.2013.g5 [DOI] [PubMed] [Google Scholar]
- Jaffin, R. A. , & Berman, C. L. (1991). The excessive loss of Brånemark fixture in type IV bone: A 5‐year analysis. Journal of Periodontology, 62, 2–4. 10.1902/jop.1991.62.1.2 [DOI] [PubMed] [Google Scholar]
- Jordan, R. A. , Bodechtel, C. , Hertrampf, K. , Hoffmann, T. , Kocher, T. , Nitschke, I. , Schiffner, U. , Stark, H. , Zimmer, S. , & Micheelis, W. (2014). DMS V Surveillance Investigators Group. The Fifth German Oral Health Study (Fünfte Deutsche Mundgesundheitsstudie, DMS V) – rationale, design, and methods. BMC Oral Health, 14, 161. 10.1186/1472-6831-14-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann, R. , Friedli, M. , Hug, S. , & Mericske‐Stern, R. (2009). Removable dentures with implant support in strategic positions followed for up to 8 years. The International Journal of Prosthodontics, 22, 233–242. [PubMed] [Google Scholar]
- Kennedy, E. (1932). Zahnprothesen und Ihre Herstellung. Meusser. [Google Scholar]
- Kremer, U. , Schindler, S. , Enkling, N. , Worni, A. , Katsoulis, J. , & Mericske‐Stern, R. (2016). Bone resorption in different parts of the mandible in patients restored with an implant overdenture. A retrospective clinical analysis. Clinical Oral Implants Research, 27, 267–272. [DOI] [PubMed] [Google Scholar]
- Maeda, Y. , Sogo, M. , & Tsutsumi, S. (2005). Efficacy of a posterior implant support for extra shortened dental arches: A biomechanical model analysis. Journal of Oral Rehabilitation, 32, 656‐660. 10.1111/j.1365-2842.2005.01478.x [DOI] [PubMed] [Google Scholar]
- Maló, P. , De Araújo, N. M. , & Rangert, B. (2007). Short implants placed one‐stage in maxillae and mandibles: A retrospective clinical study with 1 to 9 years of follow‐up. Clinical Implant Dentistry and Related Research, 9, 15–21. 10.1111/j.1708-8208.2006.00027.x [DOI] [PubMed] [Google Scholar]
- Mcfall, W. T. Jr (1982). Tooth loss in 100 treated patients with periodontal disease: A long‐term Study. Journal of Periodontology, 53, 539–549. 10.1902/jop.1982.53.9.539 [DOI] [PubMed] [Google Scholar]
- Minoretti, R. , Triaca, A. , & Saulacic, N. (2009). The use of extraoral implants for distal‐extension removable dentures: A clinical evaluation up to 8 years. The International Journal of Oral & Maxillofacial Implants, 24, 1129–1137. [PubMed] [Google Scholar]
- Misch, C. E. , Perel, M. L. , Wang, H. L. , Sammartino, G. , Galindo‐Moreno, P. , Trisi, P. , Steigmann, M. , Rebaudi, A. , Palti, A. , Pikos, M. A. , Schwartz‐Arad, D. , Choukroun, J. , Gutierrez‐Perez, J. L. , Marenzi, G. , & Valavanis, D. K. (2008). Implant success, survival, and failure: The international congress of oral implantologists (ICOI) pisa consensus conference. Implant Dentistry, 17, 5–15. 10.1097/ID.0b013e3181676059 [DOI] [PubMed] [Google Scholar]
- Mitrani, R. , Brudvik, J. S. , & Phillips, K. M. (2003). Posterior implants for distal extension removable prostheses: A retrospective study. International Journal of Periodontics and Restorative Dentistry, 23, 353–359. [PubMed] [Google Scholar]
- Monje, A. , Suarez, F. , Galindo‐Moreno, P. , García‐Nogales, A. , Fu, J.‐H. , & Wang, H.‐L. (2014). A systematic review on marginal bone loss around short dental implants (<10 mm) for implant‐supported fixed prostheses. Clinical Oral Implants Research, 25, 1119–1124. 10.1111/clr.12236 [DOI] [PubMed] [Google Scholar]
- Mundt, T. , Passia, N. , Att, W. , Heydecke, G. , Freitag‐Wolf, S. , Luthardt, R. G. , Kappel, S. , Konstantinidis, I. K. , Stiesch, M. , Wolfart, S. , & Kern, M. (2017). Pain and discomfort following immediate and delayed loading by overdentures in the single mandibular implant study (SMIS). Clinical Oral Investigations, 21, 635–642. 10.1007/s00784-016-1930-0 [DOI] [PubMed] [Google Scholar]
- Mundt, T. , Schwahn, C. , Stark, T. , & Biffar, R. (2015). Clinical response of edentulous people treated with mini dental implants in nine dental practices. Gerodontology, 32, 179–187. 10.1111/ger.12066 [DOI] [PubMed] [Google Scholar]
- Nisand, D. , Picard, N. , & Rocchietta, I. (2015). Short implants compared to implants in vertically augmented bone: A systematic review (EAO). Clinical Oral Implants Research, 26, 170–179. 10.1111/clr.12632 [DOI] [PubMed] [Google Scholar]
- Ohkubo, C. , Kobayashi, M. , Suzuki, Y. , & Hosoi, T. (2008). Effect of implant support on distal‐extension removable partial dentures: In vivo assessment. The International Journal of Oral & Maxillofacial Implants, 23, 1095–1101. [PubMed] [Google Scholar]
- Ortiz‐Puigpelat, O. , Lázaro‐Abdulkarim, A. , de Medrano‐Reñé, J. M. , Gargallo‐Albiol, J. , Cabratosa‐Termes, J. , & Hernández‐Alfaro, F. (2019). Influence of implant position in implant‐assisted removable partial denture: A three‐dimensional finite element analysis. Journal of Prosthodontics, 28, e675–e681. 10.1111/jopr.12722 [DOI] [PubMed] [Google Scholar]
- Ozan, O. , Orhan, K. , Aksoy, S. , Icen, M. , Bilecenoglu, B. , & Sakul, B. U. (2013). The effect of removable partial dentures on alveolar bone resorption: A retrospective study with cone‐beam computed tomography. Journal of Prosthodontics, 22, 42–48. 10.1111/j.1532-849X.2012.00877.x [DOI] [PubMed] [Google Scholar]
- Papaspyridakos, P. , De Souza, A. , Vazouras, K. , Gholami, H. , Pagni, S. , & Weber, H. P. (2018). Survival rates of short dental implants (≤6 mm) compared with implants longer than 6 mm in posterior jaw areas: A meta‐analysis. Clinical Oral Implants Research, 16, 8–20. 10.1111/clr.13289 [DOI] [PubMed] [Google Scholar]
- Payne, A. G. , Tawse‐Smith, A. , Wismeijer, D. , De Silva, R. K. , & Ma, S. (2017). Multicentre prospective evaluation of implant‐assisted mandibular removable partial dentures: Surgical and prosthodontic outcomes. Clinical Oral Implants Research, 28, 116–125. 10.1111/clr.12769 [DOI] [PubMed] [Google Scholar]
- Salvi, G. E. , & Lang, N. P. (2004). Diagnostic parameters for monitoring peri‐implant conditions. The International Journal of Oral & Maxillofacial Implants, 19, 116–127. [PubMed] [Google Scholar]
- Schimmel, M. , Memedi, K. , Parga, T. , Katsoulis, J. , & Muller, F. (2017). Masticatory performance and maximum bite and lip force depend on the type of prosthesis. The International Journal of Prosthodontics, 30, 565–572. 10.11607/ijp.5289 [DOI] [PubMed] [Google Scholar]
- Schimmel, M. , Srinivasan, M. , Mc Kenna, G. , & Müller, F. (2018). Effect of advanced age and/or systemic medical conditions on dental implant survival: A systematic review and meta‐analysis. Clinical Oral Implants Research, 29, 311–330. 10.1111/clr.13288 [DOI] [PubMed] [Google Scholar]
- Srinivasan, M. , Vazquez, L. , Rieder, P. , Moraguez, O. , Bernard, J.‐P. , & Belser, U. C. (2014). Survival rates of short (6 mm) micro rough surface implants: A meta‐analysis. Clinical Oral Implants Research, 25, 539–545. 10.1111/clr.12125 [DOI] [PubMed] [Google Scholar]
- Suzuki, Y. , Kono, K. , Shimpo, H. , Sato, Y. , & Ohkubo, C. (2017). Clinical evaluation of implant‐supported removable partial dentures with a stress‐breaking attachment. Implant Dentistry, 26, 516–523. 10.1097/ID.0000000000000592 [DOI] [PubMed] [Google Scholar]
- Swelem, A. A. , Gurevich, K. G. , Fabrikant, E. G. , Hassan, M. H. , & Aqou, S. (2014). Oral health‐related quality of life in partially edentulous patients treated with removable, fixed, fixed‐removable, and implant‐supported prostheses. The International Journal of Prosthodontics, 27, 338–347. 10.11607/ijp.3692 [DOI] [PubMed] [Google Scholar]
- Walton, T. R. , & Layton, D. M. (2018). Intra‐ and inter‐examiner agreement when assessing radiographic implant bone levels: Differences related to brightness, accuracy, participant demographics and implant characteristics. Clinical Oral Implants Research 29, 756‐771. 10.1111/clr.13290 [DOI] [PubMed] [Google Scholar]
- Wetherell, J. D. , & Smales, R. J. (1980). Partial denture failures: A long‐term clinical survey. Journal of Dentistry, 8, 333–340. 10.1016/0300-5712(80)90049-4 [DOI] [PubMed] [Google Scholar]
- Zancopé, K. , Abrão, G. M. , Karam, F. K. , & Neves, F. D. (2015). Placement of a distal implant to convert a mandibular removable Kennedy class I to an implant‐supported partial removable Class III dental prosthesis: A systematic review. Journal of Prosthetic Dentistry, 113, 528–533. 10.1016/j.prosdent.2014.12.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT checklist
Data Availability Statement
Data available on request from the authors.


