Abstract
As the exploration of Mars and other worlds for signs of life has increased, the need for a common nomenclature and consensus has become significantly important for proper identification of nonterrestrial/non-Earth biology, biogenic structures, and chemical processes generated from biological processes. The fact that Earth is our single data point for all life, diversity, and evolution means that there is an inherent bias toward life as we know it through our own planet's history. The search for life “as we don't know it” then brings this bias forward to decision-making regarding mission instruments and payloads. Understandably, this leads to several top-level scientific, theoretical, and philosophical questions regarding the definition of life and what it means for future life detection missions. How can we decide on how and where to detect known and unknown signs of life with a single biased data point? What features could act as universal biosignatures that support Darwinian evolution in the geological context of nonterrestrial time lines? The purpose of this article is to generate an improved nomenclature for terrestrial features that have mineral/microbial interactions within structures and to confirm which features can only exist from life (biotic), features that are modified by biological processes (biogenic), features that life does not affect (abiotic), and properties that can exist or not regardless of the presence of biology (abiogenic). These four categories are critical in understanding and deciphering future returned samples from Mars, signs of potential extinct/ancient and extant life on Mars, and in situ analyses from ocean worlds to distinguish and separate what physical structures and chemical patterns are due to life and which are not. Moreover, we discuss hypothetical detection and preservation environments for extant and extinct life, respectively. These proposed environments will take into account independent active and ancient in situ detection prospects by using previous planetary exploration studies and discuss the geobiological implications within an astrobiological context.
Key Words: Biogenicity, Nomenclature, Geobiology, Mars Sample Return, Evaporites. Astrobiology 21, 954–967
1. Introduction and Motivation
As the discipline of astrobiology is increasing in its parameters and practice for planetary missions, the definitions and usage of terminology that allows for proper differentiation of features from life or modified from life do not yet exist. The broad term of “biosignatures” has been increasingly applied to features on Earth where the burden-of-proof for life is significantly lower than Mars, Europa, and other solar system bodies that evidence past or currently habitable chemistries and liquid water as the solvent that potential life could utilize (Benner, 2010).
Over the last three decades, searching for the oldest preserved signs of life has led to misinterpretations of features preserved in ancient rocks that cannot take into account terrestrial in situ “contamination,” in the sense of “younger” biological features inhabiting older features in the rock record (Westall and Cavalazzi, 2011). However, if an independent origin of life did indeed start separately on Mars, a planet without plate tectonics for most of its history, these contamination caveats would be used as supporting arguments for the preservation of ancient organics or perhaps extant life in the subsurface, providing we were able to prove that a positive signature and/or marker were indeed indigenous to the sample and/or site. This is not to say that these efforts are unwarranted, quite the contrary.
However, the robustness of biology on our own planet makes life detection much easier owing to the access of complete laboratory facilities devoted to such analyses and sample handling. The difficulty of life detection and separation of potential “younger” contamination for samples increases in the ancient rock record to degrees where it may not be possible. The significant difference between the burdens-of-proof for the Earth and other solar system bodies requires both independent observations of biogenicity and an agreed-upon nomenclature, encompassing the geological context and the biological feedback.
Clarity for this field is crucial for proper identification of what definitely is created only by biological processes and preserved within rocks and minerals. Many landed robotic planetary missions are focused on signs of ancient or present aqueous activity on planetary bodies or moons (Squyres et al., 2005; Murchie et al., 2009a, 2009b). Mars exploration since Pathfinder in the late 1990s was a proof-of-concept that a rover could indeed land safely on the planet.
It was not until the gamma ray spectrometer and the thermal emission spectrometer (TES) onboard Mars Odyssey detected the Fe-oxide hematite (Fe2O3) in the plains of Meridiani Planum that the Mars Exploration Rover Opportunity was sent to that site in 2004 (McLennan et al., 2005; McLennan, 2012). It was during the first 6 weeks of that mission that several layered outcrops and associated sulfate eolianites along the rim were observed in situ in the Endurance Crater. A short time after Opportunity made this discovery, the Mars Reconnaissance Orbiter Compact Reconnaissance Imaging Spectrometer for Mars (CRISM) started its global campaign to observe planet-wide signs of ancient aqueous environment as potential sites of habitability in late Noachian/early Hesperian waters and the minerals precipitated or modified by in situ fluids (Squyres and Knoll, 2005).
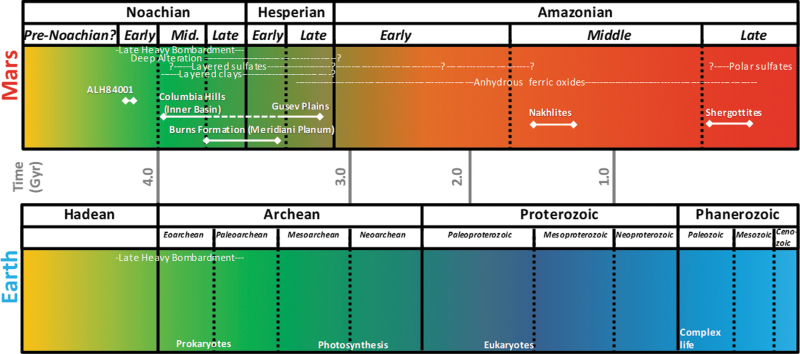
How can we constrain habitability on another planet other than Earth without knowing how life would have evolved in a planetary ecosystem? How early in the terrestrial Darwinian evolution should we consider extraterrestrial life even having the ability to adapt to ancient planetary environments (Fig. 1 and Table 1) and their associated terrestrial analogues on Earth? What features of evolution should we consider when assessing life detection? These questions have led the planetary geology community down pathways of directly associating habitability with ancient signs of water on Mars and with potential active subsurface water on Europa.
FIG. 1.
Comparisons of the origins of life on Earth and history of sedimentary features on Mars. Relationship between habitable periods on Earth and Mars. During the periods when the first life evolved on Earth, the martian surface was warmer, wetter, and habitable. The age of sedimentary rocks from the Opportunity rover landing site is ∼3.5 Gyr (late Noachian/early Hesperian). It is also in this time period where the surface of Mars likely had water stable on the surface (McLennan et al., 2005; Squyres et al., 2005) in liquid form versus recent discoveries of surface brine fluids on present-day slopes (Ohja et al., 2015).
Table 1.
The Terrestrial Extreme Environments Where Conditions for Life Are at Its Limit
| Parameter limits | Parameters |
||||||
|---|---|---|---|---|---|---|---|
| Temperature | pH | Salinity | O2 | Desiccation | Radiation | Pressure | |
| Hot (growth >80°C) | Hyperthermophile (Methanopyrus kandleri) | ||||||
| Warmer (growth 60° to 80°C) | Thermophile (Pyrolobus fumarii) | ||||||
| Frozen (growth <15°C, active at - 18°C) | Psychrophile (Synechococcus lividus, Cowellia) | ||||||
| Low pH (<5) | Acidophile (Ferroplasma acidarmanus) | ||||||
| High pH (>9) | Alkaliphile (Alkaliphilus transvaalensis) | ||||||
| 2 to 5 M NaCl | Halophile (Halobacteriaceae) | ||||||
| Requires O2 | Aerobe | ||||||
| Tolerates some O2 | Microaerophile | ||||||
| Does not require O2 | Anaerobe | ||||||
| Anhydrobio tic | Xerophile | ||||||
| Ionizing radiation to 15 kGy | Radiophile | ||||||
| Pressure-tolerant | Piezophile | ||||||
Examples of extreme environment microorganisms that can potentially survive planetary transit from Earth to Mars, Europa, or other planetary body. Moreover, these metabolisms also have the ability to survive in these solar system environments and would provide useful metagenomic insight into survival strategies for life as we do not know it yet (Adopted from the National Academy of Sciences and Rothschild and Mancinelli (2001).
Life as we know it on Earth can survive in all known climates and extreme ecosystems despite the highest and lowest temperature and pressure endmembers. Life “as we know it” (Table 1) persists in settings that it can adapt to and eventually, over geologic time, can thrive in.
An astrobiology nomenclature needs to consider the availability of the one single data point of life and evolutionary processes that we know of on Earth. This article discusses the shortcomings of life detection and provides a framework for how to define extraterrestrial biology. Moreover, we formulate and discuss the proper definitions for biogenic and abiogenic processes that can lead to better assessments of planetary habitability (Cockell, 2014a, 2014b). This proposed nomenclature will reflect life as we know it and leave enough ambiguity for life as we are yet to discover it.
2. Nomenclature and Definition Sets
The definition of life from a textbook will vary depending on the interpretation of the discipline. In biology, if something is alive, then it can respond to its current environment, has a cellular composition, is able to gain energy from chemical reactions (metabolism), has the potential for growth, can replicate itself, can maintain homeostasis, and can inherit properties or traits from previous generations.
Extreme environments that yield high salinity, low aw, and radiation tolerance, among others (Table 1), will have specialized gene expression that tolerates the aforementioned extreme properties. Typically, these ecosystems have a lower microbial diversity than nominal/nonextreme settings due to lower adaptability of microorganisms and tolerant gene expressions that would allow the ongoing maintenance of these biological processes (Summons et al., 2008; Msarah et al., 2018; Chaya et al., 2019; Fagorzi et al., 2019; Gugliandolo and Maugeri, 2019).
The current broad nomenclature for detecting these features, however, does not capture temporal and evolutionary features in the general “biosignatures” terminology that many in the planetary science communities use for advocating for mission landing sites and future instrument payloads.
Chemical Biomarkers are quantified only by instrumentation and unable to be seen with the naked eye. They are macromolecules from the chemistry of biological processes and interactions with minerals. These biomarkers are usually not arranged in any well-ordered set concerning volume and retention time (Anything else besides volume and retention time?). They are only observable if active biology is present or if the remnants of extinct biology have remained intact since the point of mineral precipitation.
Examples from terrestrial life include nucleic acids (deoxyribonucleic acid, ribonucleic acid), hopanes (Summons et al., 1999), cellulose, lipids (Eigenbrode, 2008; Georgiou and Deamer, 2014) (specifically fatty acids), proteins (specifically polypeptides and repeating monomer units), carotenoids (Perl and Baxter, 2020), among others. It should be noted that the chemistry that forms carotenoid pigments that are visible to the naked eye (in high enough concentrations) are simultaneously chemical in their composition and also presented as physical features.
Physical Biosignatures are physically observable formations that can be imaged. Such examples include non-Brownian and independent motion of cellular life (Bedrossian et al., 2017), microfossils (Schopf, 1993), and visible pigments (Fendrihan et al., 2009; Lowenstein et al., 2011; Winters et al., 2013; Perl and Baxter, 2020). It should be noted that pigment components are both a visible feature and have a chemistry that would be a biomarker, and so, these satisfy both categories simultaneously (Perl and Baxter, 2020). These visible features can be seen with the naked eye, μ-scale to mm-scale images, and do not require instrumentation other than images or video. For extinct biology, these physical signs of life can be evident if the preservation medium has been maintained and if no physical or chemical modification occurred after the last instance of biology or a biological process took place. For extant life, these features should be able to respond to forms of chemotaxis, phototaxis, or pigment generation.
In order for the evidence of life as we know it to be significant, both chemical biomarkers and physical biosignatures would need to be independently measured in multiple parts of a sample. This is taking into account extinct (ancient) life where the preservation medium plays a significant role and extant (active) life where availability of sample is less of an abundance concern. Evidence of ancient life relies heavier on the preservation medium for any chemical biomarker and physical biosignature, whereas chemistry and physical features of active life could be readily available.
These two overarching definitions need widely different lines of evidence before a burden of proof is established. If either the chemical biomarker or the physical biosignature in question in an unknown sample can be established, and not both, the evidence will likely fall short for the burden of proof needed for astrobiology and a second sign of life in our solar system. Should these physical and chemical features co-occur and remain preserved over geologic time, then the interpretation, with respect to their visual/physical and chemical analysis, should fall into the following four categories:
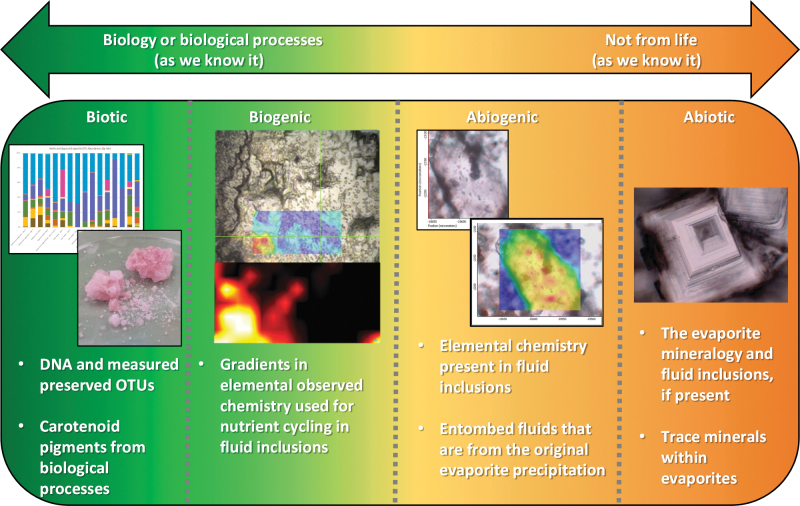
Biotic: a feature or measurement that would only exist if biology generated it or it was undoubtedly modified by life. Without the processes from life, this measurement or feature would not exist. Notable examples of this would include nucleic acids RNA and DNA.
Biogenic: a feature or measurement that is found with relationships to biological processes but may exist (or be consumed by) without the influence of life. This feature would look different depending on the type of biological processes involved.
The difference between biotic and biogenic would be a measurement of life directly and a measurement of a process generated by life, respectively. An example of biotic would be the visual and chemical measurement of bacteria, while an example of biogenic would the calcium carbonate (CaCO3) precipitated onto bacterial extracellular polymers (Tourney and Ngwenya, 2009) by life.
Abiogenic: a feature or measurement that is found equivalently with and without associations to biological processes with its existence not contributing to any biomarker or biosignature. This is often difficult to quantify for Earth due to the abundance of extant life and what is preserved in the rock record.
Abiotic: a feature or measurement that has no relationship to life at all and whose existence would be visually and/or chemically interchangeable with or without the presence of biology or biological processes and has undeniably no relationship with biology, past or present.
An example of an abiogenic measurement would be the presence of methane (CH4) in a measurable source without associations to biology, such as when these natural gases are generated and trapped in Earth's mantle (Scott et al., 2004). A positive CH4 measurement does not conclude that its sources are from life's processes, only that it is present. However, an abiogenic measurement can become biogenic depending on the relationships between positive detections and their biological sources. With regard to the aforementioned methane example, should the carbon source of the methane be higher in the lighter 12C isotope and alongside formaldehyde, methanol, and other trace gases, then these would have a higher likelihood of having a biological origin.
Finally, should these measurements have an energetic flux [i.e., increasing volumes due to biological activity during daytime because of cellular energy consumption via photosynthesis (Westall et al., 2011; Bansal et al., 2018)], then it would further add to responding to a high burden of proof-for-life validation (Kiang et al., 2007).
An example of an abiotic component can be treated spatially, chemically, or temporally. An example of metal-reducing bacteria can be used to illustrate all three. Fe-reducing organisms are able to utilize Fe(III), Mn(IV), or other electron acceptors depending on the abundance and proximity. In the case of these terminal electron acceptors being part of the rock record, the host rock itself could contain Fe(III) and would be utilized by the iron reducers.
The parent rock itself has no impact on this electron transport chain, but its spatial position in the rock record is necessary to compartmentalize the energy source. Chemically, as long as the parent rock is stable (and potentially helps preserve the electron acceptors), it does not provide any redox reaction to the nutrient chain. Consequently, if the parent rock is not stable over time and does fracture, the addition of younger material to this hypothesized system, should it not influence any of the aforementioned processes and/or occurs after extant life has perished, would be abiotic due to not overlapping with any of the electron transport chains.
Ironically, the further away from biotic you perceive these examples to be the more difficult it is to describe. If features on Earth are indeed from life, it is quite simple to make in situ measurements and study the operational taxonomic units and metabolomics of a system (Seyler et al., 2020). As we live on a microbially diverse planet teeming with life, the search for uninhabited regions still within the thermodynamic, pressure, and temperature endmembers for supporting metabolic processes is a more difficult endeavor.
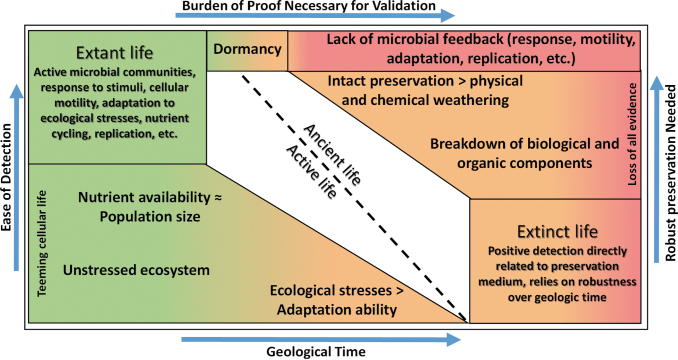
These categories can be heavily dependent on the terrestrial in situ setting that life and its habitats would utilize for nutrient cycling (i.e., the Fe mineralogy used by Fe-reducing bacteria (Luef et al., 2013), elemental carbon or sulfur in fluid inclusions for entombed halobacteria, being some examples, Mancinelli et al. 2004; Perl and Baxter, 2020). For planetary exploration on Mars into outcrops and features laid down by flowing ancient waters, these features could have contributed to extant life on the planet (if it existed in the first place), but by themselves do not provide enough evidence to establish it is biotic (Fig. 2).
FIG. 2.
Conceptual probabilities of validation of extinct and extant life. Astrobiological mission strategies need to move toward life validation alongside life detection. For extinct (ancient) life, the probability of detection of former biological components or other true biological features are at the mercy of the preservation medium and its robustness over geologic time. For extant (active) life, the availability of “positive” detections is only limited by the instrumentation and physical proximity to the microbial communities.
3. Probability of Life Detections: Moving Toward Life Validation
The focus of the Mars program at the beginning of the Spirit and Opportunity rover missions had been to “follow the water.” The discovery of layered bedrock, the presence of spherical Fe-oxide concretions (“blueberries”), and a host of other water/rock features at Meridiani Planum showed that this site was host to several groundwater recharge events (McLennan et al., 2005; McLennan, 2012) and had a stagnant paleo-groundwater table for a significant amount of time (Ehlmann et al., 2011). Later with the Curiosity rover mission to Gale crater (Hurowitz et al., 2017) and the upcoming Perseverance rover mission to Jezero crater (Lapôtre and Ielpi, 2020), the shift to habitable environments on Mars has linked the ancient stable surface waters of the late Noachian to areas where sedimentary outcrop could reveal signs of life (Westall et al., 2015).
Should an independent origin of life have occurred on Mars, and if it utilized these stable surface and subsurface waters as its solvent for metabolic processes, these sites would have the highest probability of detection due to direct water/mineral interactions with the potential to be preserved in a layered sedimentary deposit (Cockell, 2014a). Up until recently, the focus for Mars mission objectives was the search for extinct life and how it could be preserved (Summons et al., 2014). Carrier et al. (2020) noted a new interest in extant life on Mars with sites and features that could harbor active microbial communities.
For Europa, Titan, and Enceladus, the focus is on extant life due to active solvents on these moons (water, liquid ethane, and methane) (Cable et al., 2012) buried beneath kilometers of ice. Moreover, Enceladus is being looked at for a prebiotic potential (Kahana et al., 2019). The flyby missions of Galileo and Cassini have shown active liquid plumes erupting from the surface and observations by the Hubble Space Telescope have even captured these features from the Earth (Sparks et al., 2017).
Later this decade, part of the Europa Clipper mission (Howell and Pappalardo, 2020) will hopefully quantify and observe these features with future landed science payloads targeting ways to dig beneath the ice layers to get to potentially active microbial life in the subsurface ocean (Priscu et al., 1999). Current mission concepts and studies include ESA's Jupiter Icy Moon Explorer, the Europa Lander concept, NASA's Scientific Exploration Subsurface Access Mechanism for Europa, and the Honeybee Robotics Search for Life Using Submersible Heated Drill, among others.
The common habitable environments and life detection payloads of both extinct and extant life mission objectives tend to yield overlapping elements. Some sort of visual imager or camera [Pancam, MastCam, MastCamZ, Europa Imaging System (Squyres et al., 2005; Wellington et al., 2017; Centurelli et al., 2018); a chemical analyzer for elemental and mineralogical analyses (VNIR and IR spectrometers (CRISM) (Murchie et al., 2009a, 2009b; Viviano-Beck et al., 2014))], laser-induced mass spectrometer (CheMin) (Blake et al., 2012), alpha particle X-ray spectrometer (Rieder et al., 2003), Mini-TES (Christensen et al., 2003), Europa-UVS, Mapping Imaging Spectrometer for Europa (Retherford et al., 2015; Bender et al., 2019); a gas chromatography suite [Sample Analysis at Mars (Eigenbrode et al., 2018), Mass SPectrometer for Planetary EXploration/Europa (Brockwell et al., 2016); and a radar system (Radar for Europa Assessment and Sounding: Ocean to Near-surface (Pappalardo et al., 2013), Mars SHAllow RADar sounder (Nunes et al., 2011))]. On Earth these instruments can be used to document distinct features of life after we have already confirmed where it has thrived or impacted the mineral and rock records.
The measurements of in situ organic compounds on both Mars (Eigenbrode et al., 2018) and the ocean worlds (Waite et al., 2017; Kaplan et al., 2018) are steps in the right direction for potential biological detection with future mission payloads, but how would we design a mission and its architecture to validate life as we do not know it? Would it benefit us to send a DNA extraction system to a martian recurring slope lineae site or a plume eruption from Europa? If we intend to prove that life as we do not know it did not come from terrestrial evolution, but still has properties of Darwinian evolution, it may behoove us to focus on the utility of biological compounds (Lovelock, 1965). Their function on Earth and through terrestrial geological time could still apply (Kish and DiRuggiero, 2012).
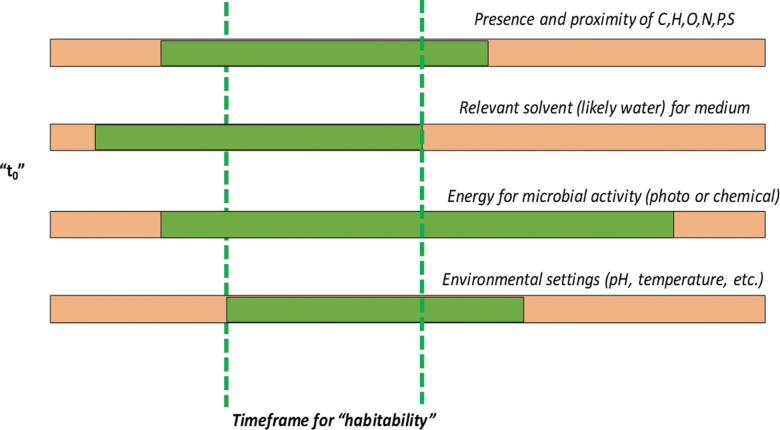
The evolutionary need for transfer of genetic information and surviving gene expressions, the ability to replicate, reactions to stimuli, adaption to ecological stresses (Jones and Baxter, 2016), maintenance of homeostasis, and organization of cellular compartments—these are all characteristics of life as we know it and potentially could be used as universal biomarkers (chemical) and biosignatures (physical) due to their measurable presence and independent of being locked into issues of contamination versus in situ signal. Being able to incorporate these utility-driven features into future instrument payloads sidesteps the terrestrial bias of life as we know it. If an independent tree-of-life started elsewhere in our solar system and life thrived there, the aforementioned traits would need to exist in parallel with each other (Fig. 5).
FIG. 5.
A proposed update to the classical perspective of habitability for planetary systems. The classic Venn diagram of energy sources, solvents, climate conditions, and C, H, O, N, P, and S has been used for discussions into martian habitability and taking global and local measurements from orbiters, rovers, and landers to determine how “habitable” a location was. That assessment did not take into account the need for the four environmental and aqueous features to overlap in time. It would only be the time frame of the combined overlap of all four products that life, as we know it, would have the highest probability for survival after a separate origin and last universal common ancestor should microbial evolution took place outside of Earth. Box 2.2 of the National Academy of Sciences Dynamic Habitability chapter in An Astrobiology Strategy for the Search for Life in the Universe discusses these features in further detail.
3.1. Extinct (ancient) life vs. extant (active) life
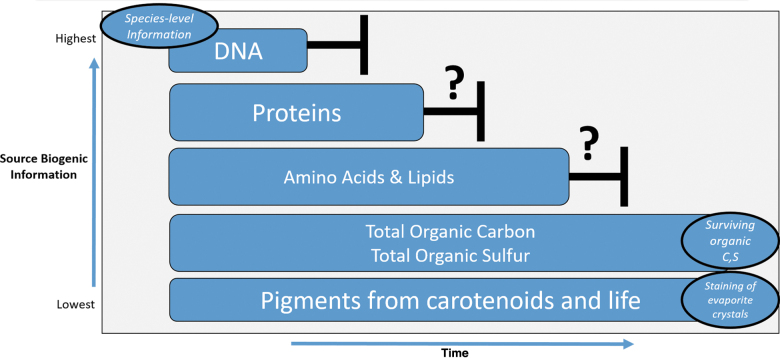
The question sets of validating extinct life and extant life do overlap in content but differ in volume, molecular complexity, and spatial distribution (Marshall et al., 2017). Contamination concerns notwithstanding, a positive detection of extinct life in the form of a robust lipid, layered stromatolite, or other true biological features are at the mercy of the preservation medium (Fig. 3).
FIG. 3.
Biogenic preservation and biotic information over modern and geologic time. High-level investigation space using terrestrial life (“as we know it”) and the loss of biotic information within biogenic preserved settings with respect to time.
Summons et al. (2014) discussed the probability of lacustrine environments (Lynch et al., 2015) and its features preserving signs of life for the planned Mars sample return campaigns. These features on Mars would have had their “best” preservation abilities when water was abundant and stable and the desiccated surface was not exposed to UVC radiation that allows for cell lysis. There are physical considerations where even within such a hostile setting, specific minerals and sedimentary outcrop features can protect against such detrimental processes (Perl et al., 2019). Aside from the natural degradation of sensitive nucleic acids and other more robust biological compounds, these features need to continually be examined for preservation metrics over geologic time since they would allow for the best defense against irradiation and desiccation.
If we assume that extant life in a martian or europan environment follows Darwinian evolution and it is in a “steady-state” form (implying that it has adapted to the majority of ecological stresses that have naturally occurred in its environment), then any extant biology inhabiting an environment would have an abundant presence only constrained by stresses too hostile for their maintenance of metabolic processes and homeostasis (Fig. 2). Preservation metrics in the extant life case could isolate some microbial communities and create population differences with a common ancestor occurring at the temporal point before separation (Hedge and Wilson, 2016) but would not be a direct influencer of a potential biological measurement due to the ongoing microbial activity.
3.2. Use case: preservation of nucleic acids and carotenoids in evaporites
Features from mineral/microbe interactions in modern and former hypersaline settings can simultaneously act as both a marker for aqueous environments and signs of halophilic extant or extinct life. Evaporite minerals can capture and entomb organic matter within their intercrystalline and intracrystalline structure as inclusions because they precipitate relatively quickly (nomenclature adopted from Schopf et al., 2012 and in further detail in Perl et al., 2020) and can further preserve metabolic processes within the fluidic structures. Thus, evaporite minerals constitute a target for biosignature investigation on Earth and Mars, where evaporitic deposits exist. However, little is known about the process of organic preservation and detection limits in evaporites, or the stability of such molecules when exposed to significant UV radiation (as would be present on the surface of Mars).
Previous results (e.g., Vítek et al. 2009; Jehlička and Oren, 2013; Winters et al., 2013; Jehlička et al., 2014; Perl and Baxter, 2020; Perl et al., 2020) show that β-carotene has a strong Raman signature that remains strong even when entombed in halite. One study shows that the β-carotene trapped in fluid inclusions and subjected to intense UVC radiation changed little even after several weeks, while β-carotene not trapped in halite degraded quickly. The authors' (Perl and Baxter, 2020) results reveal that complex organic molecules such as β-carotene should be preserved well in halite, especially in fluid inclusions, and that halite does provide some protection from organic matter degradation from UVC radiation.
Isolated pockets of brine trapped in halite crystalline structures have been used to study the ancient environments of where fluids originated as well as microorganisms from ancient waters (Satterfield et al., 2005; Benison et al., 2008; Lowenstein et al., 2011). Thus, evaporites constitute a good target for the search for biomarkers on Mars. These findings will allow for proper criteria (Fig. 4) for the discovery of any potential physical biosignature and chemical biomarker that would be on active ocean worlds (Europa, Enceladus) and for future Mars subsurface roving or drilling missions (Lunine et al., 2015).
FIG. 4.
“Pendulum” diagram showing examples and definitions of the proposed astrobiology nomenclature for an NaCl hopper crystal. The example above is for a single pigmented halite hopper crystal (biogenic) and then brought from left-to-right showing how features lose their biological validity due to the definitions proposed in this aricle (Original credit: Frank A. Corsetti).
3.3. Use case: differences in total organic species from biological and nonbiological sources
Many planetary missions, including Cassini and the Mars Science Laboratory, have been or are equipped with the ability to detect organic species. Organic molecules are necessary for life; however, it is well accepted that there are multiple abiotic routes to the synthesis of organic compounds such as amino acids and nucleotides (McDonald and Storrie-Lombardi, 2006), and even of more complex molecules such as oligomers. The combination of biotic and abiotic synthesis routes makes the use of individual organic compounds as biosignatures challenging. Some researchers have considered the ratio of different biologically relevant compounds to be a biosignature, able to distinguish between the biotic and abiotic syntheses of the organics (McKay, 2004, 2011); however, changing environmental parameters can also affect organic distribution patterns in abiotic systems (Barge et al., 2019).
More laboratory-based experiments would help elucidate the ability of abiotic chemistry under diverse geological settings (Georgiou, 2018). Better understanding of the abiotic ratios of organic molecules will limit the number of false positives observed on planetary science missions especially as we continue to explore worlds that we believe could be habitable.
3.4. Caveats and false positives
The burden of proof for life (as we do not know it) is compounded by the methods we seek to detect and validate such evidence. If we had the capability of terrestrial research laboratories on Mars, Europa, and the other ocean worlds, then multiple experiments and limitless sample return opportunities would be present. Budgetary, sample mass, rover power source, rover size, flight time, and a whole host of other technical issues will always prevent this until planetary exploration missions have the capability to have sustained human presence on these astrobiologically relevant bodies. Until then, we will rely on robotic missions, remote observations, and in situ analyses of regolith, mineral, and liquid samples.
One of the recommendations from the National Academy of Sciences Astrobiology Strategy (National Academies of Sciences, Engineering, and Medicine, 2019) for the Exploration of Mars was “selection of samples for analysis (either in situ or samples returned from Mars to Earth, McCubbin et al., 2017) should emphasize those having the best chance of retaining biosignatures.” This decision process must consider eliminating false positives from the beginning of in situ sample collection onward to when these samples are returned to Earth for laboratory study.
Given the ambiguity of results from martian meteorite ALH 84001 (McKay, 1997; Thomas-Keprta et al., 1998), one key lesson learned was that morphology alone is not enough to determine biogenicity (García Ruiz et al., 2002). While this may be self-evident at present, this fact can be used as an argument for sample collection based on unique empirical observations on the mm- or meter-scale in martian (e.g., stromatolite-type features, evaporite mineral pigments) or europan/enceladen ice features (e.g., ambiguous cell motility, unique/patterned ice layering). Distinguishing between false positives in terrestrial samples (Cady et al., 2003; Cady and Noffke, 2009; Emerson et al., 2017; Reinhard et al., 2017; Harman and Domagal-Goldman, 2018; Neveu et al., 2018; Cockell et al., 2019) continues to be necessary for planetary analogues.
Should returned samples from Mars yield no positive sign of life, there would be several distinct possibilities for this (Cockell and McMahon, 2019) and would not be representative of the entire planet. Some of these prospects include if the sample collected from Mars did not originate on Mars itself, if biosignatures within the sample did not originate from within the sample and endure to present-day discovery, if the signs of life cannot be separated from its abiogenic and abiotic components, or if the volume of the preserved life cannot be detected.
4. Validation of Life and Removing the Terrestrial Biases of Life Detection
While the official statement from Lourens Baas Becking may have been lost to time, he is loosely quoted (Wit and Bouvier, 2006) as saying for life, “Everything is everywhere, but the environment selects.” While this was largely meant for terrestrial biology, the application of this to our solar system and the Universe provides an interesting paradox for life detection and our perspectives for searching for life as we do not know it. Terrestrial microbial evolution at present is not a sufficient metric for life elsewhere, due to likely evolutionary differences between Earth's geologic and climate histories and other habitable solar system bodies. Stated differently, we should not focus our search on macromolecules (such as DNA) outside of Earth. DNA is a product of Earth evolution and the eventual output of the original Last Universal Common Ancestor (LUCA) on our own world.
Rather than DNA alone, the utility of nucleic acid oligomers as an information transfer component should be considered. Within the search for life in our solar system, the ability to search for a “DNA-like” compound that fulfills the transfer of genetic information between generations could fulfill both the metric for validation of an independent biology and being able to place it in a second tree-of-life. The more specific and closer to our present-day evolutionary markers we get, the more distant we would be from a separate evolutionary pathway or separate tree-of-life whose evolution would be different both from the standpoint of a non-Earth LUCA and from different temporal, biogeochemical, and planet-wide trajectories (Scharf et al., 2015; Hug et al., 2016).
In short, life has evolved on Earth due to the geological, chemical, and environmental histories that our planet experienced. During these events, early cellular life as we know it evolved and responded to changes to our planet over time and led to the genetic makeups and ecological systems as we know them today. To try to search for those same systems outside of Earth makes the incorrect assumption that other planets and moons shared the same LUCA, the same planetary evolution, and the same microbial response over geologic time.
5. Discussion
The hybrid nature of geobiology has allowed for interpretations into mineral/microbial interactions from the perspectives of terrestrial microbial ecology and evolution to be studied as a reference for life as we know it. Until now the lack of a utilized nomenclature has led many abiotic and abiogenic features to be misclassified as potentially modified from life, younger contaminated features into ancient mineralogy (Vreeland et al., 2000), or unitless measurements of habitability that do not take into account evolution and adaptation (Ehrenfreund et al., 2011). These previous studies are critical for understanding and constraining biogenicity in fluid-precipitated (evaporite) samples and can be used as baseline constraints for future Mars Sample Return studies.
On Earth and other habitable solar system bodies that could harbor life, it would be valid to state that biology, acting faster than geology, can adapt to planetary changes that would occur over geologic time (Fig. 1). Should those changes continue to propagate over geologic periods and globally those differences in habitability not change too significantly, life as we do not know it should be observable on a larger scale (Seager et al., 2005) than the approach that planetary missions have taken in the last five decades.
The need for nomenclature to study astrobiological features potentially generated or modified by non-Earth biological processes is paramount for the proper interpretation needed for future data analysis of unknown samples that are planned to be returned from Mars or ocean worlds within the next decades. While no concrete plans have been set for how to return samples from Mars and how (and which) laboratory analyses on Earth will be conducted within a modified BSL environment, significant thought to preventing contamination needs to be undertaken.
Following the first “stage” of sample collection by the Perseverance rover, samples will likely be cached and left on the martian surface. Should any organic components be preserved in these sedimentary deposits, the quantitative yield between in situ organics would be very sensitive to any modern contamination. Moreover, should any less robust biological components be present (extant life), the risk of contamination and the inability to decipher between terrestrial contamination and that biology are significantly higher.
It is still unknown how these samples would be analyzed if they can make it back to Earth intact and what types of geobiological and microbiological laboratory work would be conducted to determine whether life ever was present on Mars. However, the types of analyses that should be done would be very similar to what we currently do, and these analyses may not yield the results that we would expect. Our modern-day laboratories and analytical strategies are geared toward life as we know it.
Before any Earth analyses on martian samples, these soil (and hopefully evaporite mineral) samples should be inspected nondestructively and visually for any physical biosignatures that may have modified the mineral or sediment. Only after visual inspection and μ-scale assessment have been made should destructive chemical biomarker analysis take place. This can include liquid chromatography for any lipid preservation and potential metagenomics to see whether we have contaminated the samples with any terrestrial by-products of the initial sample capture on Mars.
Would nonterrestrial life have the same common elemental chemistry as we know of on Earth? Is something as simple as organic carbon in a unique looking microstructure to prove that a feature is indeed biogenic and not just organic? If we go back to the Baas Becking hypothesis, we should conclude that we would not have to look very hard if we had a hand sample from another planet that was teeming with life. We should see life's unique properties all over the sample, much like algae atop a pond or worms underneath a rock. Does this mean that since we see nothing alive on the surface of Mars that there is no life there now, or nothing biological was ever present?
The lack of evidence on Mars's surface does not infer anything for the shallow subsurface or even deeper subsurface regions, kilometers below the martian crust. We do know that groundwater at pH ranging from ∼2 to 4 to near-neutral levels in Meridiani Planum (Knoll et al., 2005; Tosca et al., 2005; Filiberto and Schwenzer, 2018) and Gale Crater (Meslin et al., 2013; Rapin et al., 2019), respectively, had the ability and vertical range to make way through permeable sedimentary rock and breach the crust. We also know that some of these fluids' sources were closed basin lake systems, where salinity was likely 10-fold higher than Earth marine waters are now.
If life was ever present on Mars and it resided in these closed basin systems, then the eventual downward movement of these ancient waters after the loss of the martian atmosphere would have provided a haven from the UVC and global desiccation that would eventually occur over the next ∼3.5 Gyr into the Amazonian (Michalski et al., 2013). Given the duration of this groundwater, downwelling waters still exposed to the surface would have eventually frozen.
This aqueous downwelling and likely hypersaline values in these waters would have led to significant precipitation of subcrustal evaporite layers where the water became stagnant and even deeper still. If cellular life utilized the current categories of habitability and overlapped each other (Fig. 5), that would yield a high probability that preservation could have occurred.
6. Conclusion and Pathways Forward
The best way forward for astrobiology is to integrate planetary geology with terrestrial microbiology so that the communities understand how each discipline formulates the research questions for the joint in situ sample analysis and continued global observations of Mars. Should we ever find something in situ that fulfills the requirements both for a physical biosignature and a chemical biomarker, the next question will involve identification and classification.
In turn, this will lead to noteworthy questions involving martian cellular life, metabolisms in the deep subsurface of Mars, and evolution outside of Earth. For the ocean worlds and the prospect of extant life, the burden of proof needed is not limited by a preservation medium. Moreover, the volumes of extant biological samples would likely be magnitudes higher than in a preserved state after life has died out. However, if life were ever present on a rocky planet that became inhabitable over geologic time, the ability for biology to evolve and adapt strategies for survival in subsurface ecosystems (in the case for Mars) could have been utilized by life via permeable sedimentary rocks and groundwater downwelling into shallow subsurface aquifers.
The burden of proof needed for validating a second sign of life in our solar system is significantly higher for ancient/extinct life than active/extant life (Fig. 2). Focusing our efforts toward what Darwinian evolution would yield over geologic time allows our science mission objectives to be designed with validating life rather than limited to life detection, which can inherently contain biases from terrestrial biology. Robotic missions, which are inherently limited with respect to laboratory capabilities due to power and mass constraints, compound these issues.
Should an independent origin of life have occurred on Mars ∼3.5 Gyr, the genomic expressions that would have been necessary for survival would have had to include the halotolerance as well as low aw settings, while still maintaining metabolic processes. Photobiological feedback in the form of pigments would be an ideal survival strategy for halophilic microorganisms as the planet became more irradiated with UVC (Litchfield, 1998; Perl and Baxter, 2020). As the focus for Mars starts to include extant life studies, the late Noachian preservation of these features is of paramount importance (Carrier et al., 2020).
For the icy moons where present-day subsurface oceans may exist (Pappalardo et al., 2013), we would consider cellular motility (Bedrossian et al., 2017) and the presence of complex molecules that could be the framework for life as we do not know it. If we assume some form of Darwinian evolution to be the same for nonterrestrial life, then information transmission between growing microbial communities would allow the adaptation of cellular life in these subglacial oceans.
Strategies would then need to focus on traversing beneath the ice for ocean world exploration and in situ sample analyses. Similar strategies could be used for rocky planet subsurface exploration on Mars where many of the halotolerant survival strategies could be utilized far away from surface UVC irradiation.
Acknowledgments
The research was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration (80NM0018D0004).
Abbreviations Used
- CRISM
Compact Reconnaissance Imaging Spectrometer for Mars
- LUCA
Last Universal Common Ancestor
- TES
thermal emission spectrometer
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Funding for work provided in this manuscript was provided by the NASA Jet Propulsion Laboratory and Caltech Presidents and Directors Research Development Fund Grant # 105275/18AW0074.
References
- Bansal S, Tangen B, Finocchiaro R (2018) Diurnal patterns of methane flux from a seasonal wetland: mechanisms and methodology. Wetlands 38, doi: 10.1007/s13157-018-1042-5 [DOI] [Google Scholar]
- Barge L, Flores E, VanderVelde D, et al. (2019) Redox and pH gradients drive amino acid synthesis in iron oxyhydroxide mineral systems. Proc Natl Acad Sci U S A 116:4828–4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrossian M, Lindensmith C, and Nadeau JL (2017) Digital holographic microscopy, a method for detection of microorganisms in plume samples from Enceladus and other icy worlds. Astrobiology 17:913–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender HA, Blaney DL, Mouroulis P, et al. (2019) Optical design of the Mapping Imaging Spectrometer for Europa (MISE). In Proceedings of SPIE 11130, Imaging Spectrometry XXIII: Applications, Sensors, and Processing, 111300C; doi: 10.1117/12.2530464 [DOI] [Google Scholar]
- Benison KC, Jagniecki EA, Edwards TB, et al. (2008) “Hairy blobs”: microbial suspects preserved in modern and ancient extremely acid lake evaporites. Astrobiology 8:807–821 [DOI] [PubMed] [Google Scholar]
- Benner SA (2010) Defining life. Astrobiology 10:1021–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D, Vaniman D, Achilles C, et al. (2012) Characterization and calibration of the CheMin mineralogical instrument on mars science laboratory. Space Sci Rev 170:341–399 [Google Scholar]
- Brockwell T, Meech K, Pickens K, et al. (2016) The mass spectrometer for planetary exploration (MASPEX). In 2016 IEEE Aerospace Conference, Big Sky, MT, pp 1–17, doi: 10.1109/AERO.2016.7500777 [DOI] [Google Scholar]
- Cable ML, Horst SM, Hodyss R, et al. (2012) Titan tholins: simulating Titan organic chemistry in the Cassini-Huygens era. Chem Rev 112:1882–1909 [DOI] [PubMed] [Google Scholar]
- Cady SL, Farmer JD, Grotzinger JP, et al. (2003) Morphological biosignatures and the search for life on Mars. Astrobiology 3:351–368 [DOI] [PubMed] [Google Scholar]
- Cady SL and Noffke N (2009) Geobiology: evidence for early life on Earth and the search for life on other planets. GSA Today 19:4–10 [Google Scholar]
- Carrier BL, Beaty DW, Meyer MA, et al. (2020) Mars extant life: what's next? Conference Report. Astrobiology 20:785-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centurelli JL, Turtle ZP, Osterman SN, et al. (2018) Europa imaging system wide angle camera: the effect of gamma radiation on the refractive index and transmission of radiation resistant glasses. Proc. SPIE 10698, Space Telescopes and Instrumentation 2018: Optical, Infrared, and Millimeter Wave, 106984D, doi: 10.1117/12.2313596 [DOI] [Google Scholar]
- Chaya A, Kurosawa N, Kawamata A, et al. (2019) Community Structures of Bacteria, Archaea, and Eukaryotic Microbes in the Freshwater Glacier Lake Yukidori-Ike in Langhovde, East Antarctica. Diversity 11:105 [Google Scholar]
- Christensen PR, Mehall GL, Silverman SH, et al. (2003) Miniature thermal emission spectrometer for the Mars exploration Rovers. J Geophys Res 108:8064 [Google Scholar]
- Cockell CS (2014a) The subsurface habitability of terrestrial rocky planets: Mars. In Microbial Life of the Deep Biosphere, edited by J Kallmeyer and D Wagner, Life in Extreme Environments, Vol. 1. Walter de Gruyter GmbH, Berlin, pp 225–259 [Google Scholar]
- Cockell CS (2014b) Trajectories of martian habitability. Astrobiology 14:182–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell CS and McMahon S (2019) Lifeless Martian samples and their significance. Nat Astron 3:468–470 [Google Scholar]
- Cockell CS, Mc Mahon S, Lim DSS, et al. (2019) Sample collection and return from Mars: Optimising sample collection based on the microbial ecology of terrestrial volcanic environments, Space Sci Rev 215:44 [Google Scholar]
- Ehlmann BL, Mustard JF, Murchie SL, et al. (2011) Subsurface water and clay mineral formation during the early history of Mars. Nature 479:53–60 [DOI] [PubMed] [Google Scholar]
- Ehrenfreund P, Röling WFM, Thiel CS, et al. (2011) Astrobiology and habitability studies in preparation for future Mars missions: trends from investigating minerals, organics and biota. Int J Astrobiol 10:239–253 [Google Scholar]
- Eigenbrode JL (2008) Fossil lipids for life-detection: a case study from the early Earth record. Space Sci Rev 135:161–185 [Google Scholar]
- Eigenbrode JL, Summons RE, Steele A, et al. (2018) Organic matter preserved in 3-billion-year-old mudstones at Gale crater, Mars. Sci 360:1096–1101 [DOI] [PubMed] [Google Scholar]
- Emerson JB, Adams RI, Román CMB, et al. (2017) Schrödinger's microbes: tools for distinguishing the living from the dead in microbial ecosystems. Microbiome 5:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagorzi C, Del Duca S, Venturi S, et al. (2019) Bacterial communities from extreme environments: Vulcano Island. Diversity 11:140 [Google Scholar]
- Fendrihan S, Musso M, and Stan-Lotter H (2009) Raman spectroscopy as a potential method for the detection of extremely halophilic archaea embedded in halite in terrestrial and possibly extraterrestrial samples. Journal of Raman Spectroscopy: JRS 40:1996–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiberto J and Schwenzer SP (2018) Volatiles in the Martian Crust. Elsevier, doi: 10.1016/B978-0-12-804191-8.00017-9 [DOI] [Google Scholar]
- García Ruiz JM, Carnerup A, Christy AG, et al. (2002) Morphology: an ambiguous indicator of biogenicity. Astrobiology 2:353–369 [DOI] [PubMed] [Google Scholar]
- Georgiou CD (2018) Functional properties of amino acid side chains as biomarkers of extraterrestrial life. Astrobiology 18:1479-1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou CD and Deamer DW (2014) Lipids as universal biomarkers of extraterrestrial life. Astrobiology 14:541–549 [DOI] [PubMed] [Google Scholar]
- Gugliandolo C and Maugeri TL (2019) Phylogenetic diversity of archaea in shallow hydrothermal vents of Eolian Islands, Italy. Diversity 11:156 [Google Scholar]
- Harman CE and Domagal-Goldman SD (2018) Biosignature false positives. Geology, doi: 10.1007/978-3-319-30648-3_71-1 [DOI] [Google Scholar]
- Hedge J and Wilson DJ (2016) Practical approaches for detecting selection in microbial genomes. PLoS Comput Biol 12:e1004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell SM and Pappalardo RT (2020) NASA's Europa Clipper—a mission to a potentially habitable ocean world. Nat Commun 11:1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug LA, Baker BJ, Anantharaman K, et al. (2016) A new view of the tree of life. Nat Microbiol 1. doi: 10.1038/nmicrobiol.2016.48 [DOI] [PubMed] [Google Scholar]
- Hurowitz JA, Grotzinger JP, Fischer WW, et al. (2017) Redox stratification of an ancient lake in Gale crater, Mars. Science 356:922. [DOI] [PubMed] [Google Scholar]
- Jehlička J and Oren A (2013) Raman spectroscopy in halophile research. Front Microbiol 4:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehlička J, Edwards HG, and Oren A (2014) Raman spectroscopy of microbial pigments. Appl Environ Microbiol 80:3286–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL and Baxter BK (2016) Bipyrimidine signatures as a photoprotective genome strategy in G+ C-rich Halophilic Archaea. Life 6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana A, Schmitt- Kopplin P, and Lancet D (2019) Enceladus: first observed primordial soup could arbitrate origin-of-life debate. Astrobiology 19, 10:1263–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HH, Milliken RE, and Alexander CMO'D (2018) New constraints on the abundance and composition of organic matter on Ceres. Geophys Res Lett 45:5274–5282 [Google Scholar]
- Kiang NY, Segura A, Tinetti G, et al. (2007) Spectral signatures of photosynthesis. Coevolution with other stars and the atmosphere on extrasolar worlds. Astrobiology 7:252–274 [DOI] [PubMed] [Google Scholar]
- Kish A and DiRuggiero J (2012) DNA replication and repair in halophiles. In Advances in Understanding the Biology of Halophilic Microorganisms, edited by RH Vreeland, Springer, Dordrecht, Netherlands, pp 163–198 [Google Scholar]
- Knoll AH, Carr M, and Clark B (2005) An astrobiological perspective on Meridiani Planum. Earth Planet Sci Lett 240:179–189 [Google Scholar]
- Lapôtre MGA and Ielpi A (2020) The pace of fluvial meanders on Mars and implications for the western delta deposits of Jezero crater. AGU Adv 1:e2019AV000141 [Google Scholar]
- Litchfield CD (1998) Survival strategies for microorganisms in hypersaline environments and their relevance to life on early Mars. Meteorit Planet Sci 33:813–819 [DOI] [PubMed] [Google Scholar]
- Lovelock JE (1965) A physical basis for life detection experiments. Nature 207:568–570 [DOI] [PubMed] [Google Scholar]
- Lowenstein TK, Schubert BA, and Timofeeff MN (2011) Microbial communities in fluid inclusions and long-term survival in halite. Geol Soc Am Today 21:4–9 [Google Scholar]
- Luef B, Fakra S, Csencsits R, et al. (2013) Iron-reducing bacteria accumulate ferric oxyhydroxide nanoparticle aggregates that may support planktonic growth. ISME J 7:338–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunine JI, Waite JH, Postberg F, et al. (2015) Enceladus Life Finder: the search for life in a habitable moon [EGU2015-14923]. In Geophysical Research Abstracts, vol. 17, EGU General Assembly [Google Scholar]
- Lynch KL, Horgan BH, Munakata-Marr J, et al. (2015) Near-infrared spectroscopy of lacustrine sediments in the Great Salt Lake Desert: an analog study for Martian paleolake basins. J Geophys Res Planets 120, 599–623 [Google Scholar]
- Mancinelli RL, Fahlen TF, Landheim R, et al. (2004) Brines and evaporites: analogs for Martian life. Adv Space Res 33:1244–1246 [Google Scholar]
- Marshall SM, Murray ARG, and Cronin L (2017) A probabilistic calibrated framework for identifying biosignatures using Pathway Complexity. Philos Trans A Math Phys Eng Sci 375, doi: 10.1098/rsta.2016.0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubbin FM, Allton JH, Barnes JJ, et al. (2017) Priority science targets for future sample return missions within the Solar System out to the year 2050 [abstract #8224]. In Planetary Science Vision 2050. Workshop, Lunar and Planetary Institute, Houston, LPI Contribution 1989 [Google Scholar]
- McDonald GD and Storrie-Lombardi MC (2006) Amino acid distribution in meteorites: diagenesis, extraction methods, and standard metrics in the search for extraterrestrial biosignatures. Astrobiology 6:17–33 [DOI] [PubMed] [Google Scholar]
- McKay CP (1997) The search for life on Mars. In Planetary and Interstellar Processes Relevant to the Origins of Life, edited by DCB Whittet, Springer, Dordrecht [Google Scholar]
- McKay CP (2004) What is life—and how do we search for it in other worlds? PLoS Biol 2, doi: 10.1371/journal.pbio.0020302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay CP (2011). The search for life in our Solar System and the implications for science and society. Phil Trans R Soc A369:594–606 [DOI] [PubMed] [Google Scholar]
- McLennan SM (2012) Geochemistry of sedimentary processes on Mars. In Sedimentary Geology of Mars, edited by JP Grotzinger, and RE Milliken, doi: 10.2110/pec.12.102.0119 [DOI] [Google Scholar]
- McLennan SM, Bell JF, Calvin WM, et al. (2005) Provenance and diagenesis of the evaporite-bearing Burns Formation, Meridiani Planum, Mars. Earth Planet Sci Lett 240:95–121 [Google Scholar]
- Meslin P, Gasnault O, Forni O, et al. (2013) Soil diversity and hydration as observed by ChemCam at Gale Crater, Mars. Science 341:123; 8670 [DOI] [PubMed] [Google Scholar]
- Michalski JR, Cuadros J, Niles PB, et al. (2013) Groundwater activity on Mars and implications for a deep biosphere. Nat Geosci 6:133–138 [Google Scholar]
- Msarah MJ, Yusoff MF, Samion SN, et al. (2018) Extreme environment: biofilms and microbial diversity. Malaysian J Microbiol 14:435–443 [Google Scholar]
- Murchie SL, Mustard JF, Ehlmann BL, et al. (2009a) A synthesis of Martian aqueous mineralogy after 1 Mars year of observations from the Mars Reconnaissance Orbiter. J Geophys Res 114, doi: 10.1029/2009JE003342 [DOI] [Google Scholar]
- Murchie SL, Seelos FP, Hash CD, et al. (2009b) Compact Reconnaissance Imaging Spectrometer for Mars investigation and data set from the Mars Reconnaissance Orbiter's primary science phase. J Geophys Res 114:E00D07 [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (2019) An Astrobiology Strategy for the Search for Life in the Universe. The National Academies Press, Washington, DC, doi: 10.17226/5252 [DOI] [Google Scholar]
- Neveu M, Hays LE, Voytek MA, et al. (2018) The ladder of life detection. Astrobiology 18:1375-1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes DC, Smrekar SE, Fisher B, et al. (2011) Shallow Radar (SHARAD), pedestal craters, and the lost Martian layers: initial assessments. J Geophys Res 116:E04006 [Google Scholar]
- Ohja L, Wilhelm M, Murchie S, et al. (2015) Spectral evidence for hydrated salts in recurring slope lineae on Mars. Nature Geosci 8:829–832 [Google Scholar]
- Pappalardo RT, Vance S, Bagenal F, et al. (2013) Science potential from a Europa Lander. Astrobiology 13:8, 740–773 [DOI] [PubMed] [Google Scholar]
- Perl SM and Baxter BK (2020) Great Salt Lake as an astrobiology analogue for ancient martian hypersaline aqueous systems. In Great Salt Lake Biology, edited by B Baxter, J Butler, Springer, Cham. pp 487–514. doi: 10.1007/978-3-030-40352-2_16 [DOI] [Google Scholar]
- Perl SM, Baxter BK, Celestian AJ, et al. (2019) Mineral preservation and photoprotective attributes of halophilic organisms in planetary analogue lake bed environments [abstract #479957]. In Astrobiology Science Conference, Bellevue, Washington [Google Scholar]
- Perl SM, Celestian AJ, Seuylemezian A, et al. (2020) Evaporitic preservation of modern carotenoid biomarkers and halophilic microorganisms in Martian analogue hypersaline environments. Astrobiology (in revision) [Google Scholar]
- Priscu JC, Adams EE, Lyons WB, et al. (1999) Geomicrobiology of subglacial ice above Lake Vostok, Antarctica. Science 286:2141–2144 [DOI] [PubMed] [Google Scholar]
- Rapin W, Ehlmann BL, Dromart G. et al. (2019) An interval of high salinity in ancient Gale crater lake on Mars. Nat Geosci 12:889–895 [Google Scholar]
- Reinhard CT, Olson SL, Schwieterman EW, et al. (2017) False negatives for remote life detection on ocean-bearing planets: lessons from the early Earth. Astrobiology 17:287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retherford KD, Gladstone R, Greathouse TK, et al. (2015) The Ultraviolet Spectrograph on the Europa Mission (Europa-UVS) (2015) AGU Fall Meeting Abstracts [Google Scholar]
- Rieder R, Gellert R, Brückner J, et al. (2003) The new Athena alpha particle X-ray spectrometer for the Mars Exploration Rovers. J Geophys Res 108:8066 [Google Scholar]
- Rothschild LJ and Mancinelli RL (2001) Life in extreme environments. Nature 409:1092–1101 [DOI] [PubMed] [Google Scholar]
- Satterfield CL, Lowenstein TK, Vreeland RH, et al. (2005) Paleobrine temperatures, chemistries, and paleonvironments of Silurian Salina Formation F-1 salt, Michigan Basin U.S.A., from petrography and fluid inclusions in halite. J Sediment Res 75:534–546 [Google Scholar]
- Scharf C, Virgo N, Cleaves H. et al. (2015) A strategy for origins of life research. Astrobiology 15:1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf J, Farmer J, Foster IS, et al. (2012) Gypsum-permineralized microfossils and their relevance to the search for life on Mars. Astrobiology 12:619–633 [DOI] [PubMed] [Google Scholar]
- Schopf JW (1993) Microfossils of the EarlyArchean Apex chert: new evidence of the antiquity of life. Science 260:640–646 [DOI] [PubMed] [Google Scholar]
- Scott HP, Hemley RJ, Mao HK, et al. (2004) Generation of methane in the Earth's mantle: in situ high pressure-temperature measurements of carbonate reduction. Proc Natl Acad Sci U S A 101:14023–14026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seager S, Turner EL, Schafer J, et al. (2005) Vegetation's red edge: a possible spectroscopic biosignature of extraterrestrial plants. Astrobiology 5:372–390 [DOI] [PubMed] [Google Scholar]
- Seyler LM, Kujawinski E, Marlow J, et al. (2020) Metabolomics as an emerging tool in the search for astrobiologically-relevant biomarkers. Astrobiology 20:1251–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks W, Schmidt B, McGrath M, et al. (2017) Active Cryovolcanism on Europa?. Astrophys J Lett 839, doi: 10.3847/2041-8213/aa67f8 [DOI] [Google Scholar]
- Squyres SW and Knoll AH (2005) Sedimentary rocks at Meridiani Planum: origin, diagenesis, and implications for life on Mars, Earth Planet. Sci Lett 240:1–10 [Google Scholar]
- Squyres S, Arvidson R, Bell JF, et al. (2005) The Opportunity Rover's Athena Science Investigation at Meridiani Planum, Mars. Science (New York, N.Y.) 306:1698–1703 [DOI] [PubMed] [Google Scholar]
- Summons RE, Jahnke LL, Hope JM, et al. (1999) 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature 400:554–557 [DOI] [PubMed] [Google Scholar]
- Summons RE, Albrecht P, McDonald G, et al. (2008) Molecular biosignatures. Space Sci Rev 135:133–159 [Google Scholar]
- Summons RE, Sessions AL, Allwood AC, et al. (2014) Planning considerations related to the organic contamination of martian samples and implications for the Mars 2020 rover. Astrobiology 14:969–1027 [DOI] [PubMed] [Google Scholar]
- Thomas-Keprta KL, McKay DS, Wentworth SJ, et al. (1998) Bacterial mineralization patterns in basaltic aquifers: implications for possible life in martian meteorite ALH84001. Geology 26:1031–1034 [DOI] [PubMed] [Google Scholar]
- Tosca, Nicholas, McLennan SM, Clark BC, et al. (2005) Geochemical modeling of evaporation processes on Mars: insight from the sedimentary record at Meridiani Planum. Earth Planet Sci Lett 240:122–148 [Google Scholar]
- Tourney J and Ngwenya BT (2009) Bacterial extracellular polymeric substances (EPS) mediate CaCO3 morphology and polymorphism. Chem Geol 262:138–146 [Google Scholar]
- Vítek P, Jehlicka J, Edwards HG, et al. (2009) Identification of beta-carotene in an evaporitic matrix—evaluation of Raman spectroscopic analysis for astrobiological research on Mars. Anal Bioanal Chem 393:1967–1975 [DOI] [PubMed] [Google Scholar]
- Viviano-Beck CE, Seelos F, Murchie S, et al. (2014) Revised CRISM spectral parameters and summary products based on the currently detected mineral diversity on Mars. J Geophys Res Planets 119:1403–1431 [Google Scholar]
- Vreeland RH, Rosenzweig WD, and Powers DW (2000) Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature 407:897–900 [DOI] [PubMed] [Google Scholar]
- Waite JH, Glein CR, Perryman RS, et al. (2017) Cassini finds molecular hydrogen in the Enceladus plume: evidence for hydrothermal processes. Science 356:155–159 [DOI] [PubMed] [Google Scholar]
- Wellington DF, Bell JF, Johnson JR, et al. (2017) Visible to near-infrared MSL/Mastcam multispectral imaging: initial results from select high-interest science targets within Gale Crater, Mars. Am Mineral 102:1202 [Google Scholar]
- Westall F and Cavalazzi B (2011) Biosignatures in rocks. In Encyclopedia of Geobiology, edited by J. Reitner and V. Thiel, Springer, Dordrecht, the Netherlands, pp 189–201 [Google Scholar]
- Westall F, Cavalazzi B, Lemelle L, et al. (2011) Implications of in situ calcification for photosynthesis in a ∼3.3 Ga-old microbial biofilm from the Barberton greenstone belt, South Africa. Earth Planet Sci Lett 310:468–479 [Google Scholar]
- Westall F, Foucher F, Bost N, et al. (2015) Biosignatures on Mars: what, where, and how? Implications for the search for martian life. Astrobiology 15:998–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters YD, Lowenstein TK, and Timofeeff MN (2013) Identification of carotenoids in ancient salt from Death Valley, Saline Valley, and Searles Lake, California, using Laser Raman Spectroscopy. Astrobiology 13:1065–1080 [DOI] [PubMed] [Google Scholar]
- Wit RD and Bouvier T (2006) ‘Everything is everywhere, but, the environment selects'; what did Baas Becking and Beijerinck really say? Environ Microbiol, doi: 10.1111/j.1462-2920.2006.01017 [DOI] [PubMed] [Google Scholar]