Abstract
Maternal smoking increases mortality and morbidity risks for both mother and infant. The First Breath Wisconsin study examined the cost-effectiveness of providing incentives to pregnant women who smoked to engage in stop smoking treatment. Participants (N = 1014) were Medicaid-enrolled pregnant women recruited from September 2012 to April 2015 through public health departments, private, and community health clinics in Wisconsin. The incentive group (n = 505) could receive $460 for completing pre-birth visits ($25 each), post-birth home visits ($40, $25, $25, $40 for 1-week, 2-month, 4-month and 6-month visits), monthly smoking cessation phone calls post-birth ($20 each), and biochemically-verified tobacco abstinence at 1-week ($40) and 6-months ($40) post-birth. The control group (n = 509) received up to $80 for 1-week ($40) and 6-month ($40) post-birth assessments. Intervention costs included incentive payments to participants, counselor and administrative staff time, and smoking cessation medications. Cost-effectiveness analysis calculated the incremental cost-effectiveness ratio (ICER) per one additional smoker who quit. The incentive group had higher 6-month post-birth biochemically-confirmed tobacco abstinence than the control group (14.7% vs. 9.2%). Incremental costs averaged $184 per participant for the incentive group compared to controls ($317 vs $133). The ICER of financial incentives was $3399 (95% CI $2228 to $8509) per additional woman who was tobacco abstinent at 6 months post-birth. The ICER was lower ($2518 vs $4760) for women who did not live with another smoker. This study shows use of financial incentives for stop smoking treatment is a cost-effective option for low-income pregnant women who smoke.
Keywords: clinical trials, cost-effectiveness, Medicaid, smoking and tobacco
Introduction
Approximately 7.2% of US women report cigarette smoking during pregnancy, and 85% of women who quit smoking while pregnant relapse in the first six months postpartum (Drake, Driscoll, & Mathews, 2018). Maternal smoking increases mortality and morbidity risks for both mother and infant including pre-term delivery, low birthweight, Sudden Infant Death Syndrome (SIDS) (US Department of Health and Human Services, 2014), asthma, wheezing and cognitive deficits (Bakker & Jaddoe, 2011; Dietz et al., 2010; Holz et al., 2014; Nichter et al., 2008). Furthermore, socio-economically disadvantaged (low-SES) women smoke during pregnancy at disproportionally higher rates, which puts them at higher risk for catastrophic pregnancy complications as well as adverse health effects for their infants that may last a lifetime (Higgins et al., 2010; Higgins et al., 2014; Kandel, Griesler, & Schaffran, 2009).
One strategy for reducing smoking prevalence rates among low-SES pregnant women is to increase their participation in evidence-based smoking cessation treatment. Recent research has shown that modest monetary incentives for participating in smoking treatment can increase both treatment engagement and smoking cessation among low-SES populations (Notley et al., 2019). Incentives have typically been used to incentivize smoking abstinence (Higgins et al., 2012). However, incentivizing abstinence usually involves burdensome methods to confirm abstinence at multiple time points. Incentivizing evidence-based treatment engagement removes some barriers for participation as well as the expense of biochemically confirming abstinence for incentive delivery (Dallery et al., 2017; Sigmon & Patrick, 2012). The reasoning for incentivizing treatment engagement is that such incentives increase exposure to smoking treatment, and that increased treatment exposure has the potential to increase abstinence rates (Fiore et al., 2008).
The present study is a cost-effectiveness analysis of the First Breath Wisconsin study, a randomized controlled trial of financial incentives to Medicaid-enrolled pregnant women who smoked to engage in smoking cessation treatment during pregnancy and post-partum. Medicaid has unrealized potential to improve treatment reach markedly by using financial incentives to encourage engagement in evidence-based smoking treatments for pregnant and postpartum Medicaid enrollees who smoke. A Cochrane review demonstrates that financial incentives are ‘the single most effective intervention’ for achieving abstinence during pregnancy (Boyd, Briggs, Bauld, Sinclair, & Tappin, 2016; Lumley et al., 2009; Notley et al., 2019). The First Breath Wisconsin outcome study showed that financial incentives to engage with an evidence-based tobacco use intervention was efficacious in smoking cessation for Medicaid-enrolled pregnant women by encouraging enrollees to seek out and engage in smoking treatment adherently, and in accord with their own best interests (Baker et al., 2018).
Despite evidence from the First Breath Wisconsin study, limited evidence exists on the cost-effectiveness of financial incentives for pregnant and postpartum Medicaid-enrolled women who smoke to engage with cessation counseling. Cost-effectiveness evaluations are necessary for Medicaid decision makers and other stakeholders to consider adoption of new treatments (Neumann, Sanders, Russell, Siegel, & Ganiats, 2016; Ryder, McDonough, Tosteson, & Lurie, 2009). Thus, the purpose of this undertaking is to conduct a cost-effectiveness evaluation of financial incentives for low-income pregnant women who smoke to engage in tobacco cessation counseling with First Breath Wisconsin, a free, statewide program that helps pregnant women, new moms, and members of their families, quit smoking (Baker et al., 2018). The cost-effectiveness is expressed in terms of the costs necessary to gain one additional pregnant smoker who is abstinent at six months postpartum.
Methods
This study is a cost-effectiveness analysis of the First Breath Wisconsin randomized controlled trial that enrolled pregnant Medicaid recipients who smoked into the trial from September 2012 to April 2015 (Baker et al., 2018). Medicaid is a public insurance program jointly funded by U.S. states and the federal government that provides health coverage to low-income families and individuals. Each state operates its own Medicaid program, and as a result, Medicaid eligibility and benefits vary from state to state. States are required to provide coverage to all women who are pregnant and have income below 138% of the poverty line ($29,974 for a family of three in 2020).
Eligibility criteria included smoking at least one cigarette each day for at least one week in the past 6 months. Women who smoked, had cut down on smoking, or had quit smoking in the past 6 months, which may have been before or during pregnancy, were enrolled in the randomized control trial. Tobacco cessation outcomes during pregnancy were self-reported, not biochemically verified data that were collected at different gestational points of pregnancy depending on the study enrollment date.
The analysis was conducted from a health care system perspective based on costs and effectiveness from enrollment to six months post-birth. Eligible participants were randomized to either the incentive (n = 505) or the control group (n = 509) and offered up to 6 pre-birth visits, 4 post-birth home visits, 5 monthly post-birth phone calls, and smoking cessation medications. Participants in both arms received financial incentives: incentive group participants received financial incentives for completing pre-birth visits ($25 per visit), post-birth home visits at 1-week ($40), 2-month ($25), 4-month ($25) and 6-month ($40) follow-up, monthly tobacco cessation counseling calls post-birth ($20 per call), as well as for biochemically-verified tobacco abstinence at 1-week ($40) and 6-month home visit ($40), for a maximum total incentive of $460; control group participants received $40 each for participating in 1-week and 6-month post-birth assessments, for a maximum incentive of $80. Incentive costs included the cost of the incentives plus administrative charges for providing the incentives. Participants were given the choice to receive the incentive in the form of one of four gift cards: Visa, Wal-Mart, Target, or Walgreens. Incentive payments were distributed either by mail for pre-birth visits and postpartum calls or in person at home visits. The study was approved by the University of Wisconsin Health Sciences Institutional Review Board and registered at clinicaltrials.gov, https://www.clinicaltrials.gov/ct2/show/NCT01569490?term=NCT01569490&draw=2&rank=1, in March 2012 as NCT01569490. Full details on the study protocol can be accessed in the initial report (Baker et al., 2018).
Data
The primary outcome was biochemically confirmed 7-day point-prevalence abstinence at the 6-month post-birth follow-up visit, verified by a carbon monoxide (CO) breath test. The result was recorded dichotomously as either abstinent (CO < 7 ppm) or smoking. Participants who did not complete the 6-month post-birth follow-up visit were assumed to be smoking.
Total costs of the intervention were calculated based on the incentive costs actually received by study participants, service costs for staff counseling and testing, staff costs connected with intervention scheduling and follow-up, and medication costs for smoking cessation medications purchased by Medicaid during the 6-month study period. Pre-birth counseling was delivered at the participants’ visits to First Breath agencies. In addition to smoking counseling, First Breath providers (nurses, medical assistants and health educators) also discussed women’s social support, their stress level, and the importance of breastfeeding. Staff costs for pre-birth visits, phone calls, and home visits were determined by applying a median counselor salary to contacts completed. Counselors tracked logs of time spent with each study participant. Medication costs were calculated using Medicaid pharmacy records for smoking cessation medication prescriptions filled by study participants up to 6 months post-partum. Medicaid weighted average reimbursement rates, as of 01/01/2016, were drawn from the Medicaid website and applied to each medication (Medicaid Services, 2016). All costs were calculated in 2016 dollars and inflated to 2020 dollars using the Consumer Price Index (Bureau of Labor Statistics, 2020).
Analyses
Cost-effectiveness is reported in terms of the incremental cost-effectiveness ratio (ICER) (Sanders et al., 2016). The ICER is calculated as:
To determine the ICER, the proportion of women receiving from 0 up to 4 home visits and from 0 up to 5 counseling calls was computed for the incentive and the control groups (see Figure 1 for the pathway probabilities). Cumulative costs and the cumulative probability of 6-month smoking abstinence by study group were determined by calculating the weighted sum of the individual pathways for each study arm. Confidence intervals for the ICER were determined using 10,000 Monte Carlo simulations (Briggs, Mooney, & Wonderling, 1999). (See Fig. 2)
Fig. 1:
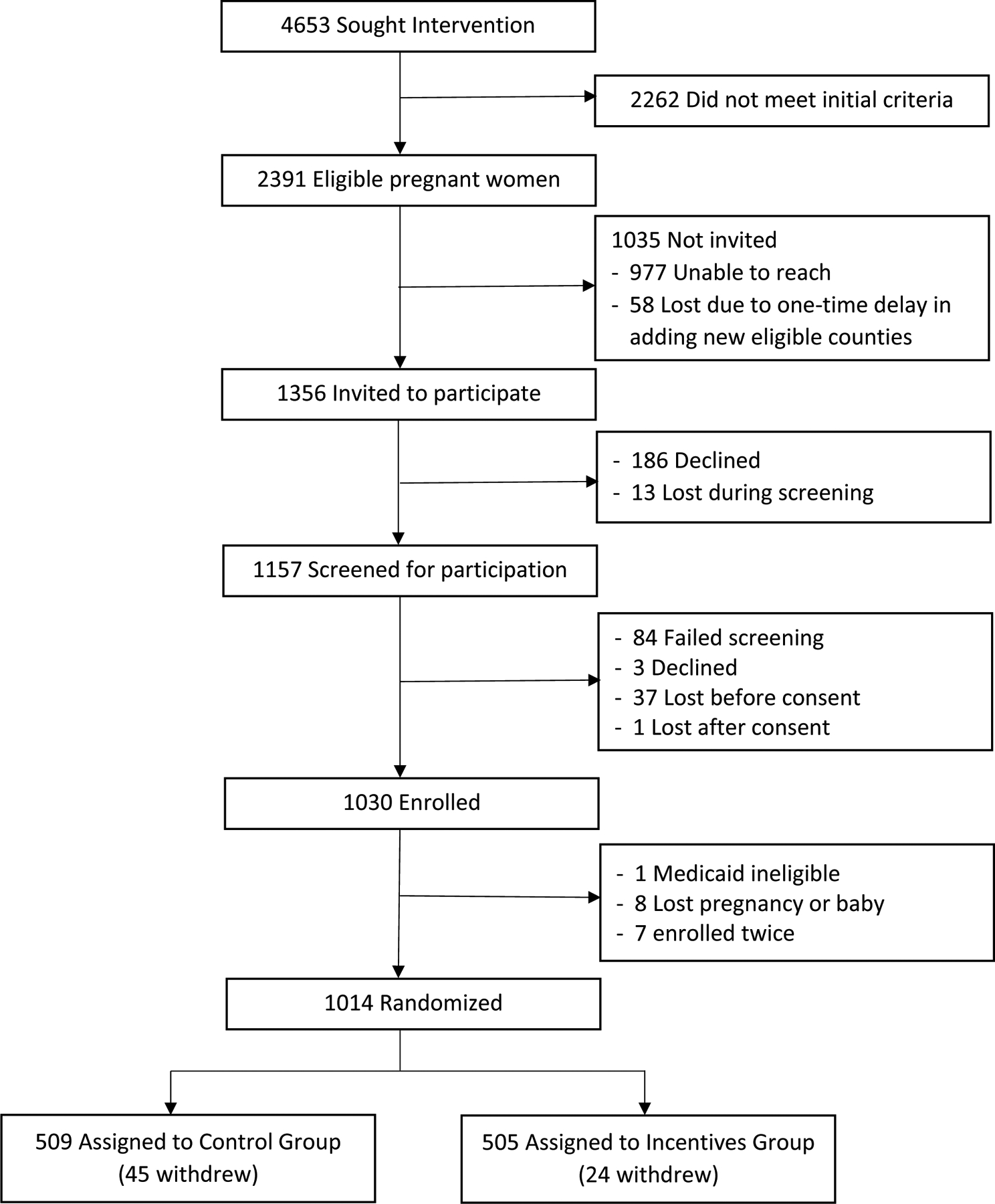
Consort Diagram of First Breath Wisconsin Randomized Controlled Trial
Fig. 2:

Cost-effectiveness decision tree for incentiving First Breath engagement based on number of post-partum home visits and post-partum phone consults completed.
The First Breath Wisconsin trial included incentives for pre-birth visits; however, few of the study participants enrolled early in their pregnancies and there were no significant differences between incentive and control participants in the number of pre-birth visits (1.2 visits for incentive group vs 0.9 visits for control group, on average). As a result, the analysis focused on postpartum home visits and counseling calls. Pre-birth visit incentives were included in the overall cost of the intervention.
Subgroup Analyses
Variation in the cost-effectiveness of the intervention was explored for various subgroups of participants. Subgroup analyses included differences in cost-effectiveness by age, race, ethnicity, education, prior attempts to quit smoking, tobacco dependence, motivation to quit smoking, confidence in being able to quit smoking, and living with another smoker.
Results
Study Sample
Table 1 provides the demographic and smoking history data of the sample by randomization group. The treatment groups did not differ significantly at baseline. Overall, 46% of the study participants self-identified as White and 39% self-identified as Black or African American. Participants’ mean age was 26.4 years. More than half of the study participants reported living with someone who smoked. Study participants were highly motivated to quit smoking (mean of 4.0 on a 1–5 scale) and were highly confident that they could quit (mean of 4.3 on a 1–5 scale).
Table 1:
Sociodemographic and Smoking-Related Variables by Treatment Group
| Control (n=509) |
Incentive (n=505) |
|
|---|---|---|
| Age, mean (SD) | 26.1 (5.1) | 26.7 (5.4) |
| Gestational age at enrollment, n (%) | ||
| 1st trimester (1–12 weeks) | 212 (41.6) | 218 (43.2) |
| 2nd trimester (13–26 weeks) | 227 (44.6) | 213 (42.2) |
| 3rd trimester (27+weeks) | 70 (13.8) | 74 (14.6) |
| Race, n (%) | ||
| White | 240 (47.2) | 229 (45.4) |
| Black or African American | 188 (36.9) | 201 (39.8) |
| Asian | 4 (0.8) | 1 (0.2) |
| American Indian/Alaska Native | 10 (2.0) | 5 (1.0) |
| Other | 14 (2.8) | 5 (1.0) |
| Refused/missing | 38 (7.5) | 43 (8.5) |
| Ethnicity, n (%) | ||
| Hispanic | 27 (5.3) | 24 (4.8) |
| Non-Hispanic | 416 (81.7) | 413 (81.8) |
| Refused/missing | 66 (13.0) | 68 (13.5) |
| Education, n (%) | ||
| Less than high school | 19 (3.7) | 21 (4.2) |
| Some high school | 105 (20.6) | 104 (20.6) |
| High school or GED | 174 (34.2) | 173 (34.3) |
| Some college or 2-year degree | 130 (25.5) | 111 (22.0) |
| College degree | 15 (3.0) | 27 (5.4) |
| Refused/missing | 66 (13.0) | 69 (13.7) |
| Marital status, n (%) | ||
| Single | 162 (31.8) | 163 (32.3) |
| In a relationship | 142 (27.9) | 135 (26.7) |
| Living with a partner | 82 (16.1) | 74 (14.7) |
| Married | 40 (7.9) | 43 (8.5) |
| Widowed/divorced/other | 9 (1.8) | 17 (3.4) |
| Refused/missing | 74 (14.5) | 73 (14.5) |
| Baseline heaviest cigarettes per day, n (%) | ||
| 1–10 cigarettes | 200 (39.3%) | 194 (38.4) |
| 11–20 cigarettes | 199 (39.1) | 199 (39.4) |
| >20 cigarettes | 89 (17.5) | 98 (19.4) |
| Refused/missing | 21 (4.1) | 14 (2.8) |
| Living with a smoker, n (%) | 265 (52.1) | 253 (50.1) |
| Prior use of nicotine replacement therapy, n (%) | 69 (13.6) | 61 (12.1) |
| Prior use of varenicline, n (%) | 13 (2.6) | 13 (2.6) |
| Prior use of bupropion, n (%) | 6 (1.2) | 7 (1.4) |
| Tried to quit on own, n (%) | 81 (15.9) | 62 (12.3) |
| Tried reduction in smoking, n (%) | 118 (23.2) | 132 (26.1) |
| Confidence in quitting, mean (SD) | 4.0 (1.1) | 4.1 (1.1) |
| Motivation to quit, mean (SD) | 4.3 (1.0) | 4.3 (1.1) |
Confidence in Quitting was rated on a 1 to 5 scale (1=not at all; 5=extremely confident about quitting); Motivation to be Tobacco Free was rated on a 1 to 5 scale (1=not at all; 5=extremely motivated).
Resource Costs
Table 2 demonstrates the average cost per unit of resources used by participants. Incentive group participants could receive up to $500 if they completed all components of the study and were abstinent at 1-week and 6-month follow-up. Phone calls averaged 10 min per call. Home visits ranged between 30 and 60 min per visit. Most of the counselors were bachelor’s degree trained. The average cost of staff time was estimated at $19 per home visit and $8 per counseling call completed. Fixed costs of operation (e.g. telephone service, office space, other overhead) were excluded from the analysis. Participants could obtain tobacco cessation medications from their primary care providers and 16% of participants used at least one cessation medication during the study. Medication costs ranged from $0.38 to $5.14 per dose, depending on the medication (Medicaid Services, 2016).
Table 2:
Unit Cost of Resources for Study
| Resource Costs | Cost per Unit ($/unit) |
|---|---|
| Incentives (Incentive group participants only) | |
| Pre-birth visits (up to 6) | $25 each |
| 2-month post-birth home visit | $25 |
| 4-month post-birth home visit | $25 |
| Post-birth counselor phone call (up to 5) | $20 each |
| Biochemically verified tobacco abstinence (1-week home visit) | $40 |
| Biochemically verified tobacco abstinence (6-month home visit) | $40 |
| Incentives (all participants) | |
| 1-week post-birth home visit | $40 |
| 6-month post-birth home visits | $40 |
| Service Costs | |
| Staff time per home visit | $18 |
| Staff time per follow-up phone call | $9 |
| Medication Costs | |
| Bupropion, per dose | $0.38 |
| Chantix, per dose | $5.14 |
| Nicoderm, per patch | $2.98 |
| Nicorette, per dose | $0.41 |
| Nicotrol, per cartridge | $1.67 |
Source Authors’ analysis of randomized controlled trial data.
Quit Rate Effectiveness
Overall, 14.7% (74/505, 95% CI: 11.7%, 18.0%) of incentive group participants and 9.2% (47/509, 95% CI: 6.9%, 12.1%) of control group participants had biochemically verified tobacco abstinence at 6-month follow-up. The differential effectiveness of the financial incentive intervention was 5.5% (95% CI: 1.5%, 9.5%, Table 3).
Table 3:
Costs and Effectiveness of Tobacco Quit Counseling Incentives by Treatment Group
| Incentive Group (n=505) | Control Group (n=509) | Incentive-Control difference | |
|---|---|---|---|
| Cost, mean (95% CI) | |||
| Incentive cost | $214 ($204, $224) | $54 ($51, $57) | $160 ($150, $170) |
| Service cost | $84 ($81, $87) | $62 ($59, $65) | $22 ($17, $27) |
| Medication cost | $19 ($12, $26) | $17 ($11, $23) | $2 (−$8, $12) |
| Total Cost, mean (95% CI) | $317 ($302, $333) | $133 ($124, $142) | $184 ($166, $202) |
| Effectiveness | |||
| Biochemically-verified tobacco abstinence, n (%, 95% CI) | 74 (14.7%, 11.7%–18.0%) | 47 (9.2%, 6.9%–12.1%) | 5.5% (1.4%–9.4%) |
| Incremental Cost-Effectiveness Ratio (95% CI) | $3399 ($2228, $8509) | ||
Source Authors’ analysis of randomized controlled trial data.
Engagement in First Breath Home Visits and Tobacco Counseling Calls Post-Birth
Fig. 1 provides the decision tree probabilities of 6-month tobacco abstinence by randomization group and by number of post-birth home visits and follow-up counseling calls taken. As seen in Fig. 1, 54.1% of incentive group participants completed all 4 home visits compared to 30.5% of control group participants. Of those who completed all 4 home visits, over 90% engaged in at least 4 of the 5 postpartum follow-up calls as well, compared to only 68% of the control group participants who had completed all 4 home visits. While the abstinence rate was similar among those who completed all of the home visits and follow-up calls (20.3% abstinent in the incentive group compared to 21.7% in the control group), the increased exposure to treatment among the incentive group participants meant that significantly more were abstinent at 6-month follow-up.
Fig. 1 shows that the number of home visits completed was significantly associated with being in the incentive group (r = 0.231, p < .001) and with smoking cessation at 6-month follow-up (r = 0.252, p < .001). The number of post-birth home visits averaged 3.0 visits (95% CI: 2.88, 3.12) per incentive group participant versus 2.3 visits (95% CI: 2.17, 2.43) per control group participant. Across both randomization groups, subjects who completed all 4 post-birth home visits had biochemically verified abstinence rates at 6-month follow-up of 20.6%, compared to 9.9% when completing 2–3 home visits and 0.7% when completed 0 or 1 home visit.
Table 4 provides the cost per participant based on the intervention group and number of home visits and follow-up calls completed. As more resources were used for home visits, follow-up calls, and incentives, the mean cost increased, up to an average of $467 (95% CI: $445, $489) per participant in the incentive group who completed 4 home visits and 4–5 follow-up calls. The overall mean cost per incentive group participant was $317 (95% CI: $302, $333). The overall mean cost per control group participant was $133 (95% CI: $124, $142).
Table 4:
Estimated Cost per Study Participant
| Randomization | Postpartum Home Visits | Postpartum Phone Consults | Biochemically Confirmed Abstinence | N | Mean Total Cost | Standard Deviation |
|---|---|---|---|---|---|---|
| Incentive Group | 0–1 | 0–1 | Yes | 1 | $124 | . |
| No | 79 | $49 | $56 | |||
| 2–3 | Yes | 0 | . | . | ||
| No | 14 | $138 | $69 | |||
| 4–5 | Yes | 0 | . | . | ||
| No | 2 | $211 | $23 | |||
| 2–3 | 0–1 | Yes | 2 | $246 | $71 | |
| No | 12 | $165 | $42 | |||
| 2–3 | Yes | 7 | $277 | $50 | ||
| No | 48 | $236 | $53 | |||
| 4–5 | Yes | 8 | $365 | $49 | ||
| No | 59 | $298 | $95 | |||
| 4 | 0–1 | Yes | 0 | . | . | |
| No | 3 | $278 | $22 | |||
| 2–3 | Yes | 6 | $388 | $21 | ||
| No | 18 | $356 | $75 | |||
| 4–5 | Yes | 50 | $467 | $79 | ||
| No | 196 | $431 | $144 | |||
| Control Group | 0–1 | 0–1 | Yes | 1 | $59 | . |
| No | 135 | $44 | $92 | |||
| 2–3 | Yes | 0 | . | . | ||
| No | 36 | $65 | $48 | |||
| 4–5 | Yes | 0 | . | . | ||
| No | 5 | $114 | $50 | |||
| 2–3 | 0–1 | Yes | 3 | $114 | $31 | |
| No | 47 | $141 | $108 | |||
| 2–3 | Yes | 7 | $141 | $25 | ||
| No | 87 | $151 | $93 | |||
| 4–5 | Yes | 4 | $184 | $24 | ||
| No | 39 | $179 | $57 | |||
| 4 | 0–1 | Yes | 1 | $164 | . | |
| No | 2 | $240 | $107 | |||
| 2–3 | Yes | 8 | $181 | $17 | ||
| No | 38 | $193 | $53 | |||
| 4–5 | Yes | 23 | $203 | $36 | ||
| No | 83 | $205 | $38 |
Total cost=Incentive costs+Service costs+Medication Costs
Pre-birth visits were incentivized in the study, but the number of pre-birth visits did not differ greatly between the incentive and the control groups (1.2 for incentive vs 0.9 for control, on average) and abstinence at 6-month post-birth follow-up was not significantly associated with number of pre-birth visits (r = 0.02, p = .551).
Average and Incremental Costs
Table 3 shows the average and incremental costs for the incentive group participants relative to the control group participants. The incentive group participants, who could have gained $460 each for completing all aspects of the study, ended up receiving $214 (95% CI: $204, $224) in incentives on average. Compared to the average control participant incentive of $54 (95% CI: $51, $57), the differential cost of the financial payments to the incentive group averaged $160 (95% CI: $150, $170) per participant. Added service costs based on counselor time averaged $22 (95% CI: $17, $27) per participant. The total cost per participant, based on treatments utilized and incentives received, was $317 (95% CI: $302, $333) for the incentive group and $133 (95% CI: $124, $142) for the control group, resulting in an incremental cost per participant of $184 (95% CI: $166, $202).
Incremental Cost-Effectiveness Ratio (ICER)
Using Monte Carlo based sensitivity analyses, we calculated the ICER and its 95% confidence interval based on the distribution of costs of the intervention and effectiveness from the decision tree. With an incremental cost of $184 per participant and an incremental effectiveness of 0.055 per participant, the ICER (i.e., cost of one additional biochemically confirmed tobacco abstinent participant at 6 months postpartum) was calculated as $3399 (95% CI $2228 to $8509) per additional smoker who quit. Participants had varying numbers of pre-birth visits, ranging from 0 to 11+ pre-birth visits, which skews the cost data between randomization arms of the study. Nevertheless, we have added pre-birth visit costs to our analysis to check robustness of our ICER calculations and the incremental cost-effectiveness ratio per quitter is $3420. This analysis is available upon request.
Subgroup Analyses
Subgroup analyses revealed that the ICER per one additional smoker who quit was higher for participants who lived with another smoker ($4760; 95% CI $3456 to $9881) than for participants who did not live with another smoker ($2518; 95% CI $1925 to $3523). There were no significant differences in ICERs based on age, race, education, tobacco dependence, confidence in quitting or motivation to quit.
Discussion
This health economic evaluation sought to assess whether financial incentives to engage with tobacco cessation treatment are cost-effective for pregnant Medicaid enrollees who smoke. Our overall finding was that financial incentives for smoking cessation treatment engagement are cost-effective for socio-economically disadvantaged pregnant women who smoke.
The ICER of financial incentives to engage in tobacco cessation counseling in the First Breath Wisconsin study was $3399 per additional smoker who quit, attributable to a $184 difference in costs per person between the incentive group and the control group and a 5.5% difference in tobacco abstinence rates between the two arms of the study at 6-month follow-up. It is important to note that the incentives in the trial were primarily for treatment engagement, not for the abstinence itself, under the assumption that increased treatment exposure has the potential to increase abstinence rates. Over half of the incentive group participants completed all 4 scheduled post-birth home visits, compared to less than a third of control group participants, and a greater number of post-birth home visits was directly associated with increased abstinence at 6 months post-birth.
This $3399 ICER in the First Breath Wisconsin study compares to an inflation-adjusted incentive cost of $2037 per smoker who quit during late pregnancy in a study reported by Boyd and colleagues (Boyd et al., 2016). The Boyd et al study provided incentives for smoking cessation during pregnancy and the mean gestation at enrollment was 12 weeks, which is earlier than the mean gestation at enrollment of 15 weeks in the First Breath Wisconsin study (Boyd et al., 2016). Smoking cessation was assessed in Boyd et al at 34–38 weeks of gestation, which is earlier than the First Breath of Wisconsin tobacco cessation evaluation at 6 months post-partum (Boyd et al., 2016).
A large proportion of the ICER ($2957) per smoker who quit in the First Breath Wisconsin study comes from the difference in financial incentive payments to the low-income pregnant women who smoke. Increased use of cessation counselor time and use of smoking cessation medications contributed the remaining portion of the ICER ($442) per smoker who quit. Our ICER results illustrate that incentivizing engagement with First Breath Wisconsin is a viable option to increase the number of abstainers among low-income pregnant women who smoke and are enrolled in Medicaid.
This study is based on a predominantly post-natal intervention with new mothers, which precludes us from estimating the mortality and neonatal health care costs related to infants’ pre-term and low birthweight delivery due to a lack of study data availability for pregnancy outcomes and infant health outcomes. More studies are needed to determine women’s likelihood of engaging in and benefiting from incentivized smoking cessation treatment if it is offered to them earlier in their pregnancies where data are collected on pregnancy delivery outcomes and children’s health. The excess neonatal costs for infants of mothers who smoke is estimated to exceed $880 per child, which clearly indicates the need to continue efforts to reduce smoking during pregnancy (Ayadi et al., 2006).
It could be argued that the effectiveness in postpartum smoking reductions alone, however, could have important downstream health and economic benefits to mother and infant/child. As an example, asthma risk for infants ages 2 or younger with mothers who smoke is 85% higher than for offspring of mothers who do not smoke (Zacharasiewicz, 2016). The average medical cost to Medicaid for a child with treated asthma is $3279 per year (Perry, Braileanu, Palmer, & Stevens, 2019). From this perspective, the incremental cost of $3399 per mother who is abstinent at 6 months post-partum could potentially be offset by reductions in Medicaid covered medical cost for treatment of asthma and other childhood outcomes related to maternal smoking. Future studies are needed to explore the cost offsets and health gains of successful smoking interventions in the post-partum period where data on pregnancy delivery and child health outcomes are collected over a long-term period. Future research could also examine the differential cost-effectiveness of incentivizing primarily treatment engagement as opposed to abstinence. Technological advances, such as app-based CO verification, could reduce the burden of biochemical confirmation of abstinence.
The study results should be viewed in light of some limitations. Lack of biochemically verified data on how many women quit during pregnancy precludes us from a definitive exploration of how many women reliably quit tobacco during pregnancy and how that might have related to post-partum tobacco cessation. Future research may wish to explore this further. Dropout rates from the study were quite high: 29% for the intervention and 38% control conditions. Participants who did not complete the 6-month home visit were assumed to be smoking. While this may potentially bias the results toward the incentive group, the high rates of smoking among those completing the 6-month follow-up suggest that this was a safe assumption. This research also does not reveal whether the prospect of financial incentives may have enhanced overall recruitment into this project. The control group was not a true standard-of-care/no-intervention control (control participants received $40 for completing 1-week and 6-month assessments). While the provision of incentives to the control group participants increased the average cost for the control group relative to a no-incentive control, it may also have increased the adherence and participation of the control group women in the study, and therefore their abstinence rates. It should be noted that contact with the study personnel in and of itself could provide motivation for increased cessation. Tobacco abstinence was verified with CO breath test, which likely only represents smoking status in the past 24 h. Finally, cost offsets for health care visits, ER use, and hospital stays for mother and infant could not be measured due to short-term follow-up, but future studies may wish to explore these issues further.
Conclusions
Financial incentives for engagement with evidence-based smoking cessation treatments increase post-partum abstinence and are a cost-effective option to encourage smoking cessation among low-income pregnant women who smoke.
Highlights.
Maternal smoking increases mortality and morbidity risk for both mother and infant
Low-SES pregnant women smoke at disproportionately higher rates
Financial incentives reduce smoking among low-SES women at 6 months post-partum
Incremental cost-effectiveness of incentives is $3399 per smoker who quits
Financial incentives are cost-effective in reducing smoking post-partum
Funding Support:
The authors would like to thank the University of Wisconsin Carbone Cancer Center’s (UWCCC) philanthropic donors for their support of this project. This work is supported in part by NIH/NCI P30 CA014520‐UW Comprehensive Cancer Center Support (Mundt) and NIH/NCI R35 CA197573 (Fiore, Baker). This research was also supported by Funding Opportunity Number 1B1CMS330876 from the Centers for Medicare & Medicaid Services (Fiore, Baker). The article is solely the responsibility of the authors and does not necessarily represent the views of the funding agencies.
References
- Ayadi MF, Adams EK, Melvin CL, Rivera CC, Gaffney CA, Pike J, … Ferguson JN (2006). Costs of a smoking cessation counseling intervention for pregnant women: comparison of three settings. Public Health Rep, 121(2), 120–126. doi: 10.1177/003335490612100204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Fraser DL, Kobinsky K, Adsit R, Smith SS, Khalil L, … Fiore MC (2018). A randomized controlled trial of financial incentives to low income pregnant women to engage in smoking cessation treatment: Effects on post-birth abstinence. J Consult Clin Psychol, 86(5), 464–473. doi: 10.1037/ccp0000278 [DOI] [PubMed] [Google Scholar]
- Bakker H, & Jaddoe VW (2011). Cardiovascular and metabolic influences of fetal smoke exposure. Eur J Epidemiol, 26(10), 763–770. doi: 10.1007/s10654-011-9621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd KA, Briggs AH, Bauld L, Sinclair L, & Tappin D (2016). Are financial incentives cost-effective to support smoking cessation during pregnancy? Addiction, 111(2), 360–370. doi: 10.1111/add.13160 [DOI] [PubMed] [Google Scholar]
- Briggs AH, Mooney CZ, & Wonderling DE (1999). Constructing confidence intervals for cost-effectiveness ratios: an evaluation of parametric and non-parametric techniques using Monte Carlo simulation. Stat Med, 18(23), 3245–3262. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10602149 [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. (2020). Consumer price indexes. Retrieved from http://www.bls.gov/data
- Dallery J, Raiff BR, Kim SJ, Marsch LA, Stitzer M, & Grabinski MJ (2017). Nationwide access to an internet-based contingency management intervention to promote smoking cessation: a randomized controlled trial. Addiction, 112(5), 875–883. doi: 10.1111/add.13715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, & Callaghan WM (2010). Infant morbidity and mortality attributable to prenatal smoking in the U.S. Am J Prev Med, 39(1), 45–52. doi: 10.1016/j.amepre.2010.03.009 [DOI] [PubMed] [Google Scholar]
- Drake P, Driscoll AK, & Mathews TJ (2018). Cigarette Smoking During Pregnancy: United States, 2016. NCHS Data Brief(305), 1–8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29528282 [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Bennett G, Benowitz NL, … Treati CPG (2008). A Clinical Practice Guideline for Treating Tobacco Use and Dependence: 2008 Update - A US Public Health Service report. American Journal of Preventive Medicine, 35(2), 158–176. doi: 10.1016/j.amepre.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Bernstein IM, Washio Y, Heil SH, Badger GJ, Skelly JM, … Solomon LJ (2010). Effects of smoking cessation with voucher-based contingency management on birth outcomes. Addiction, 105(11), 2023–2030. doi: 10.1111/j.1360-0443.2010.03073.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Washio Y, Heil SH, Solomon LJ, Gaalema DE, Higgins TM, & Bernstein IM (2012). Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med, 55 Suppl, S33–40. doi: 10.1016/j.ypmed.2011.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Washio Y, Lopez AA, Heil SH, Solomon LJ, Lynch ME, … Bernstein IM (2014). Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med, 68, 51–57. doi: 10.1016/j.ypmed.2014.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz NE, Boecker R, Baumeister S, Hohm E, Zohsel K, Buchmann AF, … Laucht M (2014). Effect of prenatal exposure to tobacco smoke on inhibitory control: neuroimaging results from a 25-year prospective study. JAMA Psychiatry, 71(7), 786–796. doi: 10.1001/jamapsychiatry.2014.343 [DOI] [PubMed] [Google Scholar]
- Kandel DB, Griesler PC, & Schaffran C (2009). Educational attainment and smoking among women: risk factors and consequences for offspring. Drug Alcohol Depend, 104 Suppl 1, S24–33. doi: 10.1016/j.drugalcdep.2008.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, & Watson L (2009). Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev(3), CD001055. doi: 10.1002/14651858.CD001055.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicaid Services, H. H. S. (2016). Drug Pricing and Payment National Average Drug Acquisition Cost. Retrieved from https://data.medicaid.gov/Drug-Pricing-and-Payment/NADAC-as-of-2016-01-06/p8pu-f52w
- Neumann PJ, Sanders GD, Russell LB, Siegel JE, & Ganiats TG (2016). Cost-Effectiveness in Health and Medicine (Second Edition ed.). New York: Oxford University Press. [Google Scholar]
- Nichter M, Nichter M, Adrian S, Goldade K, Tesler L, & Muramoto M (2008). Smoking and harm-reduction efforts among postpartum women. Qualitative Health Research, 18(9), 1184–1194. doi: 10.1177/1049732308321738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notley C, Gentry S, Livingstone-Banks J, Bauld L, Perera R, & Hartmann-Boyce J (2019). Incentives for smoking cessation. Cochrane Database Syst Rev, 7, CD004307. doi: 10.1002/14651858.CD004307.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R, Braileanu G, Palmer T, & Stevens P (2019). The Economic Burden of Pediatric Asthma in the United States: Literature Review of Current Evidence. Pharmacoeconomics, 37(2), 155–167. doi: 10.1007/s40273-018-0726-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder HF, McDonough C, Tosteson AN, & Lurie JD (2009). Decision Analysis and Cost-effectiveness Analysis. Semin Spine Surg, 21(4), 216–222. doi: 10.1053/j.semss.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, … Ganiats TG (2016). Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA, 316(10), 1093–1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- Sigmon SC, & Patrick ME (2012). The use of financial incentives in promoting smoking cessation. Prev Med, 55 Suppl, S24–32. doi: 10.1016/j.ypmed.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. (2014). In The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta (GA). [Google Scholar]
- Zacharasiewicz A (2016). Maternal smoking in pregnancy and its influence on childhood asthma. ERJ Open Res, 2(3). doi: 10.1183/23120541.00042-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]


