Abstract
Calcineurin, also known as PP2B or PPP3, is a member of the PPP family of protein phosphatases that also includes PP1 and PP2A. Together these three phosphatases carryout the majority of dephosphorylation events in the heart. Calcineurin is distinct in that it is activated by the binding of calcium/calmodulin (Ca2+/CaM) and therefore acts as a node for integrating Ca2+ signals with changes in phosphorylation, two fundamental intracellular signaling cascades. In the heart, calcineurin is primarily thought of in the context of pathological cardiac remodeling, acting through the Nuclear Factor of Activated T-cell (NFAT) family of transcription factors. However, calcineurin activity is also essential for normal heart development and homeostasis in the adult heart. Furthermore, it is clear that NFAT-driven changes in transcription are not the only relevant processes initiated by calcineurin in the setting of pathological remodeling. There is a growing appreciation for the diversity of calcineurin substrates that can impact cardiac function as well as the diversity of mechanisms for targeting calcineurin to specific sub-cellular domains in cardiomyocytes and other cardiac cell types. Here, we will review the basics of calcineurin structure, regulation, and function in the context of cardiac biology. Particular attention will be given to: the development of improved tools to identify and validate new calcineurin substrates; recent studies identifying new calcineurin isoforms with unique properties and targeting mechanisms; and the role of calcineurin in cardiac development and regeneration.
Keywords: Calcineurin, intracellular signaling, calcium signaling, hypertrophy, cardiac development, heart failure
Graphical Abstract:
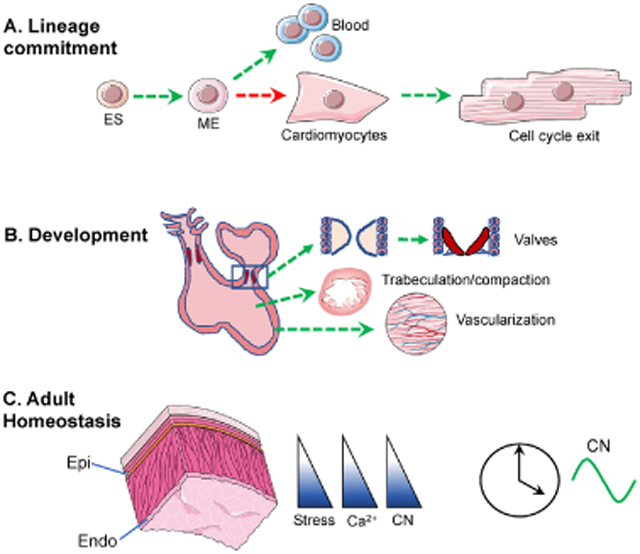
Calcineurin (CN) signaling plays diverse roles in cardiac development, from (A) early steps in lineage commitment to (B) critical processes during cardiac morphogenesis. (C) In the adult heart, calcineurin activity is heterogeneous across the ventricular wall following a gradient of increasing stress from the epicardium (Epi) to the endocardium (Endo), as well as displaying a robust 24-hour oscillation. Green arrows indicate steps dependent on CN. Red arrows indicate steps inhibited by CN. embryonic stem cells (ES), mesoderm (ME).
1. Introduction:
Calcineurin was first isolated as the most abundant Ca2+/CaM-binding protein present in the brain [1] and was soon shown to function as a Ca2+/CaM-activated protein phosphatase [2]. Calcineurin is also abundant in skeletal muscle and heart where it has been shown capable of dephosphorylating an array of sarcolemmal proteins that are phosphorylated by cAMP-dependent kinase (PKA) [3], suggesting a role for calcineurin in the coordination of Ca2+ and cAMP signaling cascades that mediate cardiac adaptation. Interest in calcineurin grew exponentially after seminal experiments by Jeff Molkentin and Eric Olson demonstrated that sustained calcineurin activity in cardiomyocytes was sufficient to drive hypertrophic growth of the heart that progressed to decompensated failure and fatal arrhythmias [4]. Numerous investigators have since validated these findings and demonstrated that inhibiting calcineurin will both blunt hypertrophic growth and preserve cardiac function under a variety of physiological and pathological conditions [5-11]. Despite thousands of papers describing a diversity of roles for calcineurin in the cardiovascular system, much remains unknown regarding how this Ca2+-responsive enzyme functions in the Ca2+-rich environment of a continuously contracting heart without pathological consequences. Multiple mechanisms mediate control and specificity. Some are inherent to the structure and regulation of calcineurin itself, others are mediated by selective targeting of calcineurin and its substrates to subcellular signaling domains.
2. Calcineurin structure and regulation:
Calcineurin is a heterodimer composed of a 62 kilodalton catalytic subunit (CnA) and a 19-kilodalton regulatory subunit (CnB) (Fig. 1). The catalytic domain at the N-terminus of CnA is followed by a binding domain for CnB (BBD) and a binding domain for a Ca2+/CaM complex (CBD). An autoinhibitory sequence (AIS) lies immediately adjacent to and overlapping with the CBD. In the absence of Ca2+/CaM the AIS blocks one of the substrate docking sites on calcineurin [12]. An autoinhibitory domain (AID) at the C-terminus of canonical CnA subunits engages and blocks the catalytic site when Ca2+ levels are low (<100nM) or availability of CaM is limited. Truncations of CnA that remove the CBD/AIS and AID yield CnA*, a constitutively active form that no longer requires Ca2+/CaM. It is this truncated form, under the control of the cardiomyocyte-specific alpha myosin heavy chain promoter (αMHC-CnA*) that was initially used to demonstrate the pro-hypertrophic properties of calcineurin [4].
Figure 1: Schematic shows the domain details of calcineurin catalytic and regulatory subunits, as well conserved domains in RCAN proteins using the RCAN1.4 isoform as an example.
The location of calpain cleavage sites are indicated by red arrow points below CnAα. CnAα* depicts the constitutively active calcineurin expressed in αMHC-CnA* transgenic mice. CnB binding domain (BBD), calmodulin binding domain (CBD), autoinhibitory sequence (AIS), Autoinhibitory domain (AID), polyproline rich domain (PP), membrane localization sequence 9MLS), LxVP calcineurin docking motif (LxVP), PxIxIT calcineurin docking motif (PxIxIT), EF-hand calcium-binding domain (EF), RCAN1 motif (SPPASPP), RCAN1 calcineurin inhibitory domain (TxxP).
Calcineurin binds Ca2+ both directly, through CnB, and indirectly, through CaM (Fi. 2). The structure of the regulatory subunit, CnB, is similar to CaM, containing four EF-hand Ca2+-binding sites. Two sites are high affinity structural sites that are always occupied, and two are lower affinity regulatory sites [13, 14]. Binding of Ca2+ to the regulatory sites on CnB causes a conformational change in the holoenzyme allowing subsequent binding of the Ca2+/CaM complex. Binding of Ca2+/CaM to CnA then displaces the AIS from the substrate-docking site and the AID from the catalytic site, allowing substrates access [12, 15]. On average, during the course of a single cardiac cycle, cytosolic Ca2+ raises from 0.1 μM to 1 μM [16], sufficient to activate calcineurin, which requires only 0.6 μM Ca2+ for half maximal activity in the presence of 20 μM CaM [17]. The concentration of Ca2+ in defined microdomains can raise even higher. Initial influx through L-type voltage gated Ca2+ channels (LTCC) increases Ca2+ levels in the dyadic cleft between the sarcolemma and the sarcoplasmic reticulum (SR) to as high as 10 μM [18]. Subsequent Ca2+-induced Ca2+ release from ryanodine receptors (RyR2) in the SR may then raise Ca2+ above 100 μM in the dyadic cleft [19].
Calcineurin’s ability to evade beat-to-beat activation likely depends on a number of factors. Among them are the rate of association with Ca2+/CaM, regional differences in CaM distribution, competition with other Ca2+/CaM-binding proteins, and association with endogenous inhibitors [20]. Signaling microdomains are determined by local concentrations of calcineurin, Ca2+, and CaM, as well as the availability of specific substrates [21-23]. Free CaM in adult cardiomyocytes averages between 50 to 100 nM [24] but variations in CaM across microdomains can alter the concentration of Ca2+ required to activate calcineurin. As an example, at 20 nM CaM, the level of Ca2+ required for half maximal activation more than doubles to 1.3 μM [17]. Thus, higher Ca2+ levels are required to activate calcineurin in a domain where CaM is limited or being competed away. Theoretical models of competitive binding between calcineurin and CaM Kinase II (CaMKII), another Ca2+/CaM-activated enzyme involved in pathological remodeling of the heart, predict that activation shifts away from calcineurin in favor of CaMKII as the frequency of Ca2+ transients increases [25, 26].
2.1. Genes encoding calcineurin:
Three mammalian genes encode CnA catalytic subunits: PPP3CA/CnAα, PPP3CB/CnAβ, and PPP3CC/CnAγ (Fig 1). Two genes encode CnB regulatory subunits: PPP3R1/CnB1 and PPP3R2/CnB2. Both CnAα and CnAβ are abundant in the heart, with transcript and protein levels of the CnAβ isoform increasing in response to hypertrophic agonists or mechanical stress in a calcineurin-dependent fashion [11, 27, 28]. Mice lacking CnAβ have smaller hearts and a blunted hypertrophic response to pressure overload [29], but sustain greater damage following ischemia reperfusion (I/R) [30]. Less is known regarding the cardiac phenotype of mice lacking CnAα as homozygous KO mice die between 2 and 4 weeks of age [31] and conditional alleles have not yet been studied. As a consequence, the CnAβ isoform is generally considered the primary isoform involved in pathological remodeling of the adult heart, potentially forming a feed-forward amplification loop.
The CnAβ isoform contains a unique, N-terminal proline-rich domain not present on CnAα or CnAγ. There are also isoform variants of CnAβ with distinct regulatory mechanisms. The structure and regulation of the CnAβ2 isoform is typical of other CnA subunits, whereas the CnAβ1 isoform lacks the canonical AID. The unique properties of CnAβ play important roles in substrate targeting and regulation of catalytic activity. These will be discussed later in greater detail.
The CnAγ subunit is often quoted as being testis-specific. However, it is involved in both early fate decisions in stem cells [32] and synaptic vesicle endocytosis in the brain [33]. Furthermore, polymorphisms in CnAγ are associated with responsiveness of the heart to exercise training [34] and post-translational regulation of proBNP [35]. However, the specific molecular events mediated by the CnAγ isoform in the adult heart are as yet unknown.
All of the CnA isoforms are susceptible to cleavage by Ca2+-activated proteases, or calpains, which can yield constitutively active forms (CnA*) lacking both the CBD/AIS and AID [36-38]. Various other forms generated by calpain cleavage may retain aspects of Ca2+ responsivity. Calpain-dependent cleavage of CnA occurs in cardiomyocytes in vitro in response to angiotensin II (AngII), followed by translocation of the cleaved product into the nucleus [39]. In vivo, CnA* has been detected in failing human myocardium [40] and hearts of patients with atrial fibrillation [41]. The vast majority of calcineurin is cytosolic. The role of nuclear calcineurin is not fully understood, however, it has been shown capable of responding to Ca2+-transients released from inositol-3-phosphate receptors (IP3R) in the nuclear envelope [42]. Interestingly, caspase-3 has also been shown to cleave calcineurin to produce CnA* in immune cells [43] and in models of Alzheimer’s disease [44], raising the possibility that activation of caspase-3 in the heart is another avenue for generating constitutively active calcineurin.
CnB1 is the only calcineurin regulatory subunit expressed in the heart. Because of this, cardiomyocyte-specific deletion of CnB1 has been used to examine the consequences of eliminating calcineurin activity in the myocardium [45, 46]. Mice with a conditional deletion of CnB1 using an αMHC-Cre driver manifest metabolic dysregulation and declines in cardiac function, ultimately dying of arrhythmias around 3 months of age [45, 46]. These studies clearly demonstrate the essential nature of CnB, however, given that the catalytic subunits of calcineurin are still expressed and potentially subject to proteolytic cleavage, it would be wise to use caution extending interpretation of these studies to all calcineurin-dependent processes.
2.2. Unique properties of the CnAβ1 isoform:
The PPP3CB gene produces two different CnA proteins; CnAβ1 and CnAβ2, both of which contain an N-terminal, polyproline domain unique to CnAβ (Fig 1). This domain helps target CnAβ to specific subcellular compartments [47] and assists in the selection of some substrates [48]. The β1 and β2 isoforms have unique C-termini that are generated as a consequence of differences in the site chosen for polyadenylation at the 3’ end of their respective transcripts. The polyadenylation site for the β1 isoform is located upstream of the one used by β2 such that the last two exons in β2 are excluded from β1, which continues translation into the intronic sequence. As a consequence, the CnAβ1 isoform lacks an AID. Instead, the C-terminus of CnAβ1 contains a membrane localization sequence (MLS) that targets CnAβ1 to Golgi membranes through an interaction with COG8 (component of oligomeric goli complex 8) [49]. In addition, CnAβ1 contains an LxVP motif, that blocks access of substrates to one of the substrate -binding domains on calcineurin [50]. The canonical β2 isoform had a canonical AID, but does not carry either the MLS or LxVP motif found in the β1 isoform. Detailed biochemical analysis demonstrates that calcineurin enzyme complexes containing the CnAβ1 subunit have basal activity even in the absence of Ca2+/CaM [50]. Although CnAβ1 activity is still increased in response to Ca2+/CaM, maximal activity is limited by LxVP-mediated autoinhibition. CnAβ1 is highly expressed in progenitor cells and regenerating tissues, and its expression is induced by insulin-like growth factor 1 [51]. Developmental control of CnAβ isoforms is mediated by Muscleblind Like Splicing Regulator 1 (MBNL1) [49]. CnAβ1 levels are low in adult heart and skeletal muscle but are elevated in mouse models of diabetes [52].
The hearts of mice carrying an αMHC-CnAβ1 transgene are not hypertrophic at base line, demonstrating that the Ca2+-independent basal activity of CnAβ1 is not pathological. Remarkably, the αMHC-CnAβ1 transgene reduces pathological remodeling of the heart following myocardial infarction (MI), pressure overload, or ischemia reperfusion (I/R) [53-55]. Mice with targeted deletion of just the LxVP/MLS C-terminal domain unique to CnAβ1 develop age-related pathological hypertrophy and have an exaggerated response to pressure overload suggesting that the CnAβ1 mediates protective functions [55]. Recent studies link the mode of action of CnAβ1 with activation of protein kinase B (Akt) and mechanistic target of rapamycin (mTOR). Targeting of CnAβ1 to Golgi by its MLS domain is required for recruitment of mTORC2 to Golgi and its activation of an Akt/ATF4 cardioprotective, metabolic pathway [49, 53, 55]. Remarkably, a catalytically-dead CnAβ1 mutant was similarly able to activate Akt [51] suggesting that, in this setting, CnAβ1 may be acting as an adaptor scaffold, rather than a phosphatase. Many questions remain regarding this intriguing isoform, its mode of action, its potential substrates, and its relationship to canonical forms of calcineurin.
2.3. Motifs targeting substrates to calcineurin:
The catalytic cleft of calcineurin is shallow, allowing accommodation of a wide range of phospho - serine, -threonine and -tyrosine substrates [56, 57]. As a consequence, there are no conserved recognition motifs surrounding target residues that can be used to predict target sites, although there is a reported preference for sites with a basic residue in the −3 position and lacking an acidic residue immediately adjacent on the C-terminal side [57]. Crystal structures show that key residues in the AID of CnA (481ERMP484) engage the catalytic cleft of calcineurin in such a way that the proline at P484 binds a hydrophobic pocket in the catalytic cleft and E481 mimics a phosphorylated substrate residue thereby blocking the active site [58]. Phospho-substrates with a proline in the +3 position show enhanced affinity for calcineurin over other PPP family phosphatases, which lack calcineurin’s hydrophobic, proline-binding pocket [59].
Instead of a defined phosphosite recognition sequence, the primary determinants for calcineurin substrates are the presence of two short linear motifs (SLIMs), PxIxIT and LxVP, located elsewhere on the protein, that act as recognition motifs to dock and position the substrate protein to facilitate access to the catalytic site of calcineurin [60, 61]. SLIMs are short stretches of protein sequence that mediate protein-protein interactions. Both PxIxIT and LxVP were initially identified as conserved features of the Nuclear Factor of Activated T Cells (NFAT) family of transcription factors, the best understood of calcineurin substrates. The PxIxIT motif binds to a docking domain on CnA, regardless of whether calcineurin is active or inactive [62-64]. Substrates compete with one another for access to calcineurin based on their local concentration and the binding affinity of their specific PxIxIT motif. Over expression of a PxIxIT containing peptide is sufficient to inhibit dephosphorylation of calcineurin substrates in vivo. Over expression of any protein containing a PxIxIT domain will compete with native proteins for docking with calcineurin, because of this, in the literature there are several examples of PxIxIT containing proteins probably erroneously proposed as calcineurin inhibitors based solely on co-immunoprecipitation and over expression studies. By extension, one might predict that cardiomyocyte-specific overexpression of any PxIxIT containing protein would have the potential to suppress calcineurin-dependent hypertrophy regardless of its normal in vivo function.
In contrast to the PxIxIT motif, the LxVP motif binds at the CnA/CnB interface, which is only accessible when calcineurin is in its active conformation [12, 65]. Cocrystal structures of calcineurin with selected substrates demonstrate docking of both motifs, which act together to mediate calcineurin substrate recognition and specificity [59]. Many calcineurin substrates contain both docking motifs, while others appear to carry only one.
2.4. Calcineurin inhibitors:
2.4.1. Pharmacological inhibitors:
The calcineurin inhibitors Cyclosporin A (CsA) and FK506 (or Tacrolimus) are used clinically as immunosuppressives. Each acts by forming a complex with a different class of endogenous proteins called immunophilins: cyclophilins and FK506 binding proteins (FKBPs) respectively [66, 67]. The drug-immunophilin complex engages calcineurin at the docking site for LxVP, thereby preventing docking of substrates [58, 65, 66, 68, 69]. They do not directly block the active site or inhibit calcineurin enzymatic activity. Recombinant calcineurin bound by a CsA/FK506-immunophilin complex is still capable of dephosphorylating small, non-proteinaceous molecules such as p-nitrophenyl phosphate (pNPP), which is often used as a substrate in calcineurin assays [66]. Whether calcineurin might encounter and act on a non-protein, small molecule substrate in vivo is not known, but if so, neither CsA nor FK506 would inhibit this activity.
CsA and FK506 have distinctly different off-target effects based on sequestration of their respective immunophilins. Notably, CsA inhibits opening of the mitochondrial permeability transition pore (MPTP) by binding to cyclophilin D, an essential component of the MPTP [70]. FK506, on the other hand, can impact Ca2+ release from RyR2 and IP3R [71, 72], although this occurs at FK506 concentrations well above those required for calcineurin inhibition. Best practice for determining whether a process is calcineurin-dependent is to test outcomes with each drug separately. Sanglifehrin A, which inhibits MPTP but not calcineurin, can also be used as a control for off target effects of CsA [73].
Short peptide inhibitors of calcineurin act by mimicking PxIxIT, LxVP, or AID motifs [74, 75]. A cell permeable version of a PxIxIT peptide was shown capable of inhibiting pressure overload hypertrophy in vivo [10, 76]. Several reviews focused on pharmacological inhibitors of calcineurin are available for further details [77, 78].
2.4.2. Endogenous inhibitors:
The AID of calcineurin itself is the primary endogenous inhibitor of calcineurin activity. Studies of posttranslational modifications that regulate calcineurin activity are limited. One notable exception is the recent finding that in resting T-cells a conserved lysine in the PxIxIT binding pocket of CnA is mono-ubiquitinated with a K-29-linkage, impairing recruitment of NFAT [79]. Upon Ca2+ stimulation, the deubiquitinase USP16 rapidly removes ubiquitin from both CnAβ and CnAγ, allowing substrate access. USP16 does not engage CnAα and none of the other over 40 deubiquitinases tested was shown to be capable of deubiquitinating calcineurin. Whether this form of regulation impacts calcineurin activity in the heart awaits further investigation.
2.4.2.1. Regulators of calcineurin (RCAN1, RCAN2, and RCAN3):
RCANs, also known as DSCR1/MCIP1/calcipressin [80], are endogenous proteins that bind and inhibit calcineurin [81-83]. There are three mammalian genes: RCAN1, RCAN2, and RCAN3 [84]. Both RCAN1 and RCAN2 are abundant in the heart. RCAN1 is the most widely studied of the three genes, in part, because expression of the RCAN1.4 isoform is under direct control of calcineurin/NFAT and thus provides feedback inhibition of calcineurin activity [82], but also because of its location on human chromosome 21 and the potential for contributing to Down syndrome pathologies. Recombinant RCAN1 inhibits the ability of calcineurin to dephosphorylate both peptide substrates and pNPP [85, 86], making it distinct from inhibition by CsA or FK506.
RCAN proteins use three independent mechanisms to inhibit calcineurin (Fig. 1 and 2). First, they contain both a PxIxIT and an LxVP motif that compete with substrates for docking to calcineurin. Second, they have a C-terminal TxxP motif that engages and blocks the catalytic site of calcineurin in a way that resembles binding of 481ERMP484 in the calcineurin AID [85-88]. Third, their highly conserved SPPASPP motif is a calcineurin substrate that can function as a pseudosubstrate inhibitor [88, 89]. Various amino acids in RCAN1 have been shown to be phosphorylated in vivo and proposed to modify RCAN1 activity [90-96]. Of these, only phosphorylation of Thr153 by the p38 mitogen-activated protein kinase has undergone rigorous biochemical assessment demonstrating that phosphorylation of this site, located adjacent to the PxIxIT motif in RCAN1, reduces the affinity of RCAN1 for calcineurin 30-fold [88].
Figure 2: Model of calcineurin domain interactions.
in the inactive state (A), activated following binding of Ca2+ and Ca2+/Calmodulin to disengage the AID from the catalytic site (B), NFAT docked with the activated form of calcineurin, small red circles indicate phosphorylated amino acids in the NFAT regulatory domain that are dephosphorylated by calcineurin (C), RCAN1 docked with calcineurin showing the TxxP motif blocking the catalytic site and inhibiting calcineurin activity (D), and docking of immunophilin bound drug complexes (E).
Mice carrying a cardiomyocyte-specific αMHC-Rcan1 transgene have a blunted hypertrophic response to both pathological and physiological stimuli [6]. They are also protected from I/R and pathological remodeling following MI [7, 97]. Damage from I/R is greater in mice with a whole-body knockout of Rcan1 [97, 98]. Paradoxically, these mice have smaller hearts and a blunted hypertrophic response to pressure overload [98, 99]. This has been used to support the proposal that at very low levels RCAN1 acts in some way to facilitate calcineurin signaling, perhaps as a chaperone or targeting protein, however, rigorous biochemical evidence of this is still lacking [100, 101]. A recent review provides in depth discussion of RCAN1 in cardiovascular disease and includes discussion of pathways, in addition to calcineurin suppression, through which RCANs may act [102].
Information regarding the impact of RCAN2 and RCAN3 on cardiac biology is much more limited. The hearts of Rcan1−/−/Rcan2−/− double knockout mice are phenotypically similar to those of Rcan1−/− mice [98]. A recent intriguing study reports the generation of circular RCAN2 transcripts (circ-RCAN2) in cardiac progenitor cells and a significant decrease in circ-RCAN2 levels in pig heart following I/R in vivo [103]. Nothing is known regarding the function or physiological impact of these unique transcripts.
2.4.2.2. TBC1D10C (Carabin/EP164C):
TBC1D10C belongs to the EPI64/TBC1D10 GTPase-activating protein (GAP) family based on the presence of a conserved catalytic Tre2/Bub2/Cdc16 (TBC) at its N-terminus. It was first identified as a negative regulator of T-cell activation via its ability to inhibit both Ras and calcineurin [104]. Pressure overload-induced hypertrophy is enhanced in mice with either whole-body [105]or cardiomyocyte-specific deletion of TBC1D10C [105, 106]. Conversely, forced expression blunts hypertrophic growth and repress the activities of calcineurin, Ras/extracellular signal-regulated kinase (ERK), and CaMKII after either cardiac pressure overload or adrenergic stimulation. TBC1D10C levels are reduced in animal models of heart failure and in patients with either dilated or hypertrophic cardiomyopathy [106], suggesting that a reduction in TBC1D10C levels could contribute to severity of disease. The C-terminus of EPI64C binds to calcineurin near to where PxIxIT motifs dock [106] and therefore may act by competing with substrates for binding at this site. It is worth noting that the GTPase activity of TBC1D10C is specific for Rab35 and has been shown to impair exosome release from oligodendrocytes [107]. Given emerging evidence that Rab35 is involved in the release of extracellular vesicles from the heart in response to exercise [108], defining the interaction of this process with calcineurin-mediated signaling will be an important avenue for future research.
2.5. Methods for assessing calcineurin activity
Quantitative measures of in vivo calcineurin activity are challenging due to the dynamic nature of Ca2+-mediated activation. Biochemical assays of tissue extracts are carried out in the presence of excess Ca2+ and CaM [109]. Because of this they only assess the total amount of calcineurin in the extract, not what fraction of it was active in vivo. Co-immunoprecipitation of calcineurin with CaM is similarly limited as the outcome is entirely dependent on the level of Ca2+ present during extraction and washing steps. Transcript and protein levels of RCAN1.4 have proven to be one of the most reliable indications of calcineurin activity and have been used in a wide range of tissues. However, it is important to note that changes in RCAN1.4 are dependent on NFAT-mediated transcription, and therefore may not accurately reflect calcineurin activity directed toward other substrates.
Fluorescent-tagged NFAT proteins and NFAT-driven luciferase reporters have proven useful but are likewise directly tied to an NFAT response [110-113]. Genetically-encoded reporters, such as CaNAR, that use fluorescence resonance energy transfer (FRET) as a dynamic read-out of calcineurin activity, can be targeted to specific subcellular domains [20]. When combined with a Ca2+ sensor, these have been used to demonstrate that activation of calcineurin is impacted by differences in CaM availability and local rates of rephosphorylation even when the magnitude of the Ca2+ transient is the same, [20]. The CaNAR reporter is also based on NFAT and therefore still an indirect, substrate-specific assessment of calcineurin activity.
The FRET-based reporters DuoCaN and UniCaN provide a more direct readout of calcineurin activation [114]. Elegant experiments using these reporters reveal fundamental differences in the localization and activation of calcineurin in neonatal verses adult cardiomyocytes. In neonatal cardiomyocytes they distribute homogeneously throughout the cell and respond directly to individual Ca2+ pulses, achieving sustained activity at higher stimulation frequencies. In adult cardiomyocytes the reporters localize to T-tubules, consistent with the pattern described for endogenous calcineurin [115, 116]. Here, they are unresponsive to either single Ca2+ transients or low frequency pacing, activating only at pacing frequencies sufficient to raise diastolic Ca2+ levels. Therefore, studies from neonatal cardiomyocytes may not always be directly applicable to calcineurin signaling in the adult heart. During failure, adult cardiomyocytes develop a number of characteristics typical of immature cardiomyocytes and this may include aspects of calcineurin activation. It is important to be aware that adult cardiomyocytes isolated from mouse begin to dedifferentiate rapidly in culture, making the time required for adenoviral infection often problematic, although approaches have been developed that may help slow the rate of dedifferentiation [117]. Finally, the DuoCaN and UniCaN FRET reporters are based on CnAα and therefore may not accurately reflect properties unique to CnAβ or CnAγ.
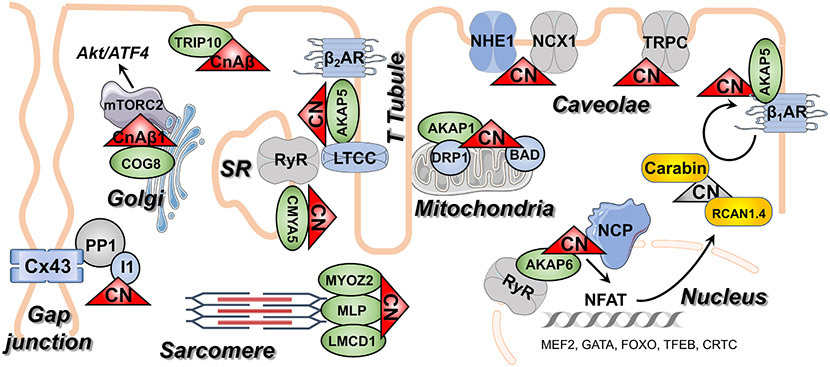
3. Calcineurin substrates relevant to cardiac function and disease (Fig 3):
Figure 3: Schematic of calcineurin (CN) functional and physical distribution in adult cardiomyocytes.
AKAPs and other proteins that act as calcineurin scaffolds are depicted in green. Interactions that are isoform-specific are indicated. Direct and indirect calcineurin substrates are depicted in blue. Calcineurin inhibitors are yellow. Note that CN is primarily tethered near known sites of Ca2+ release. A kinase anchor proteins (AKAP), β-adrenergic receptor (β-AR), BCL2 associated agonist of cell death (BAD), calcineurin (CN), carabin/TBC1D10C (Carabin), cardiomyopathy associated 5 (CMYA5), component of oligomeric goli complex 8 (COG8), connexin 43 (Cx43), dynamin related protein 1 (DRP1), inhibitor 1 (I1), LIM and cysteine-rich domains 1 (LMCD1), L-type calcium channel (LTCC), mammalian target of rapamycin complex 2 (mTROC2), muscle LIM protein (MLP), Myozenin 2 (MYOZ2), nuclear factor of activated T cells (NFAT), nuclear pore complex (NCP), protein phosphatase 1 (PP1), regulator of calcineurin 1 (RCAN1.4), ryanodine receptors (RyR), sodium-calcium exchanger (NCX1), sodium-potassium exchanger (NHE1), thyroid hormone receptor interactor 10 (TRIP10), transient receptor potential canonical (TRPC).
Calcineurin signaling mediates both immediate and long-term responses. Rapid responses may involve changes in protein activity, subcellular location, and/or protein-protein interactions, many of which could be rapidly reversed by rephosphorylation. In contrast, calcineurin-dependent changes in transcription have the potential for mediating longer-lasting consequences.
3.1. Key transcription factors controlled by calcineurin:
3.1.1. NFAT:
Four of the five known NFATs are activated by calcineurin; NFATc1 (a.k.a. NFAT2), NFATc2 (a.k.a. NFAT1), NFATc3 (a.k.a. NFAT4) and NFATc4 (a.k.a. NFAT3). Dephosphorylation by calcineurin causes translocation of NFAT to the nucleus where it acts in conjunction with a diversity of transcription factors, thereby facilitating the generation of a specific transcriptional response to a more generalized signal initiated by a change in sytosolic Ca2+. Both the MEF2 [118] and GATA [119-121] family of transcription factors are activated by calcineurin, work cooperatively with NFAT, and play prominent roles in cardiac gene expression [122-124]. A direct interaction between NFAT and NFκB during hypertrophy has also been reported [125]. Deletion of either NFATc2 or NFATc3 blunts hypertrophic remodeling [126, 127], although the two proteins show clear differences in the dynamics of their response to calcineurin [128-131]. Despite the prominence of NFAT-dependent transcription in the cardiac literature, the identity of the most relevant target genes remains a critical question.
3.1.2. CRTC:
Similar to NFATs, dephosphorylation of cAMP response element binding protein (CREB)-regulated transcriptional coactivators (CRTCs) by calcineurin promotes their translocation to the nucleus where they act in conjunction with CREB to drive gene expression. In other tissues CRTCs have been shown to mediate metabolic responses to hormonal stimuli [132-134] and promote mitochondrial biogenesis [135-137]. β-adrenergic stimulation of neonatal cardiomyocytes increases phosphorylation of CRTC1, and this is further enhanced with pharmacological inhibition of calcineurin [138]. The hearts of mice lacking CRTC1 are hypertrophic, suggesting that CRTC1 activity may mediate compensatory mechanisms that delay disease progression [138].
3.1.3. FOXO1:
The forkhead box protein family of transcription factors mediate metabolic remodeling of the heart in diabetic cardiomyopathy and post-ischemic heart failure [139-141]. Dephosphorylation of FOXO1 by calcineurin triggers its translocation to the nucleus, where it can cooperate with NFAT to drive gene expression [142]. Sustained FOXO1 activity provides feedback inhibition of calcineurin activity in the heart [143-145], suggesting a close interdependence of these two pathways.
3.1.4. TFEB:
Transcription Factor EB (TFEB) is a master regulator of lysosomal biogenesis and autophagy [146, 147]. Calcineurin dephosphorylates TFEB triggering its translocation to the nucleus and activation of target genes [148]. Proteotoxic stress in either the ER or cytosol can lead to calcineurin-dependent activation of TFEB [149, 150]. Thus, calcineurin control over lysosomal biogenesis via TFEB may help coordinate degradative and growth processes during structural and metabolic remodeling of the myocardium [151, 152].
3.2. Integrated regulation with sodium exchangers NHE1 and NCX1.
Calcineurin participates in regulation of the sodium-potassium (NHE1) and the sodium-calcium (NCX1) exchangers as well as being activated downstream of their joint activities. The cytoplasmic tail of NHE1 contains both a PxIxIT and LxVP motif for calcineurin docking [153, 154]. NHE1 activity is increased by phosphorylation at Thr779 and calcineurin dephosphorylates this site, thereby reducing exchanger activity [153]. Calcineurin is also reported to inhibit NCX1, although the specific mechanism of action is not yet defined [155, 156]. In the adult myocardium NHE1 and NCX1 can be found colocalized in caveolae-positive lipid rafts [156-158] providing a microdomain platform through which NHE1/NCX1 activity can activate calcineurin/NFAT to promote hypertrophic growth [154, 159] and calcineurin in turn can regulate exchanger activity. Activation of NHE1 causes a sustained increase in cytosolic Ca2+ to activate calcineurin by either decreasing NCX1 activity or increasing its reverse mode of action [160]. During ischemia, in response to a build-up of lactic acid, the combined actions of NHE1 and reverse-mode NCX1 lead to cytosolic Ca2+ overload, however, calcineurin remains inactive under ischemic conditions due to the acidic environment. Upon reperfusion, physiological pH is restored allowing calcineurin activity to then reduce the activities of NHE1 and NCX1, as well as acting on other potential substrates.
3.3. Connexin 43 (Cx43) and gap junction conductance:
The phosphorylation status of Cx43 regulates cardiac gap junction conductance and propagation of the action potential (AP) between adjacent cardiomyocytes [161, 162]. A reduction in the speed at which APs are propagated (conduction velocity) can lead to re-entrant arrhythmias. In a healthy heart Cx43 is phosphorylated at Ser365 and localized to intercalated disks. Calcineurin inhibitors prevent dephosphorylation of Cx43 during ischemia in both cardiomyocytes and astrocytes [163, 164]. In this context calcineurin acts indirectly by dephosphorylating inhibitor 1 (I1) thereby releasing its inhibition of PP1 [165]. PP1 then dephosphorylates Ser365 of Cx43, causing disassembly of gap junction channels and promoting redistribution Cx43 to lateral membranes [161, 162, 166].
3.4. Potential mechanism for cross-talk with JNK:
The dual specificity mitogen-activated protein kinase kinase 7 (MKK7) in the c-Jun NH(2)-terminal kinase (JNK) pathway has been implicated in both hypertrophy and arrhythmogenesis [167-169]. Calcineurin specifically suppress MKK7γ activity via a PxIxIT motif specific to the γ isoform but not present on the MKK7α and β splice variants [170]. The distribution of individual MKK7 isoforms in the heart has not been reported, however, it is possible that this mechanism may in part mediate previously reported crosstalk between calcineurin and JNK during cardiac remodeling [171, 172].
3.5. Identifying new calcineurin substrates using SLIMs:
Recently, two groups undertook systematic approaches to identify new calcineurin substrates that both expand our knowledge of calcineurin-mediated processes and provide data-base resources for investigators to query potential new targets [173] http://slim.icr.ac.uk/motifs/calcineurin/. These groups combined in silico proteome-wide identification of potential PxIxIT and LxVP motifs with a variety of in vitro and in vivo affinity capture methods to identify a diversity of new calcineurin substrates, increasing the known calcineurin interactome by an order of magnitude in both cardiomyocytes and neurons [173, 174]. A key finding from both of these groups was that multiple components of the nuclear pore complex (NCP) are calcineurin substrates. In addition to gating nucleocytoplasmic transport, the NCP is involved in diverse functions including chromatin organization, regulation of gene expression and DNA repair [175]. In humans, it is a massive complex of ~125 megaDaltons and composed of multiple (8-64) copies of 30 different nuclear pore proteins called nucleoporins (NUPs). Several NUP proteins were verified as calcineurin substrates and pharmacological inhibition of calcineurin was shown to alter nuclear import of model proteins. Thus, calcineurin may influence nuclear translocation of a much broader range of proteins and transcription factors than previously thought. Other key proteins verified as calcineurin substrates include NOTCH1 and MDM2, although the functional consequences of calcineurin-mediated modification of these proteins remain to be explored. The extensive supplemental data linked to these three manuscripts are a valuable resource for investigators seeking to determine whether their protein of interest may be modified by calcineurin [173, 174, 176].
4. Targeting of calcineurin to subcellular domains:
Immunohistochemistry indicates that in adult cardiomyocytes the majority of calcineurin is localized to the T-tubules, which are deep invaginations of the sarcolemma (or plasma membrane) that bring voltage-activated LTCC in the plasma membrane into close association with RyR2 in the sarcoplasmic reticulum (SR). Ca2+-induced Ca2+-release in this microdomain links depolarization of the plasma membrane with activation of cardiomyocyte contraction (excitation-contraction coupling). In addition to LTCC, a diversity of membrane channels and receptors are also found at T-tubules. Thus, calcineurin is found in close proximity to a wide array of proteins involved in receptor-mediated signaling, trafficking of ions, and mechanotransduction. Although there is also evidence for calcineurin, either free or tethered, responding to specific Ca2+ microdomains elsewhere in cardiomyocytes.
4.1. A kinase anchor proteins (AKAPs):
AKAPs are scaffolding proteins that form multimolecular signaling complexes that by definition include PKA. Many, but not all, also bind calcineurin, facilitating reversal of specific PKA-mediated phosphorylation events by calcineurin. More than 30 AKAPs are expressed in the heart [177], however, direct interactions with calcineurin have been described for only a handful of these, and include; AKAP5 (AKAP79/AKAP150), at the plasma membrane of T-tubles; AKAP6 (AKAP6β/mAKAPβ), at the nuclear envelop; AKAP1 (AKAP121/AKAP84), at the outer mitochondrial membrane; and Cypher/ZASP (LIM domain binding 3, LDB3), at the plasma membrane associated with LTCCs. Here we will describe AKAP5 in depth to illustrate the challenges of understanding the mechanism and regulation of calcineurin-AKAP interactions; what is known and what is not known regarding this fundamental aspect of calcineurin localization and substrate targeting. For additional details we recommend several comprehensive reviews covering AKAP structure, function, and cardiovascular phenotypes [113, 178-181].
4.1. AKAP5 (AKAP79/AKAP150)
AKAP5 was the first AKAP shown to bind calcineurin [182]. In addition to acting as a scaffold for PKA and calcineurin signaling, AKAP5 binds adenylyl cyclases (AC), phosphodiesterase (PDE4D), and protein kinase C (PKC) [178, 183, 184]. It forms complexes with numerous plasma membrane channels and receptors, including LTCC, transient receptor potential channels (TRPV1 and 4)[185, 186], N-methyl-D-aspartic acid receptors (NMDAR), α-amino-3-hydroxy-5- methyl-4-isoxazoleproprionic acid receptors (AMPAR), β-adrenergic receptors 1 and 2 (β-AR) and voltage-gated potassium channels including A-type (Kv4.2) [187-189] and M-type (KCNQ2-5) [190]. Notably, AKAP5 doesn’t directly bind KCNQ1, mutation of which causes Long Qt Syndrome [191]. Instead, KCNQ1 associates with the Yotiano form of AKAP9, which is not known to interact directly with calcineurin [192, 193] but may interact indirectly through TRIP10 (see later section for details). AKAP5 complexes also contain postsynaptic density protein 95 (PSD-95) and synapse-associated protein 97 (SAP97), members of the membrane-associated guanylate kinase (MAGUK) family that are involved in trafficking of transmembrane receptors [194]. Caveolin 3 (CAV3), enriched in striated muscle, is also an integral component of many AKAP5 complexes in cardiomyocytes [184]. The specific composition of a AKAP5/calcineurin complex depends on cell type, subcellular location, and physiological state. Studies in neurons have yielded important insights into the mechanism of action and physiological function of these complexes, some of which may, or may not, be directly transferable to signaling in the heart.
4.1.1. Engagement of AKAP5 with calcineurin:
AKAP5 binds calcineurin using a PxIxIT motif [195]. This leaves the LxVP docking site of calcineurin still available to engage substrates with LxVP motifs. This is the case for LTCC, which has an LxVP but no PxIxIT motif. A direct interaction between LTCC and AKAP5 helps hold LTCC in proximity of calcineurin (and PKA) [196, 197] and mediates Ca2+-dependent inactivation of LTCC in neurons [198]. Similarly, NFAT also interacts directly with AKAP5 through a C-terminal leucine zipper (LZ) domain that is required for NFAT activation in response to membrane depolarization in neurons [199]. Thus, AKAPs provide a scaffold to maintain both calcineurin and its substrates in a specific subcellular domain, often close to a site of Ca2+ entry. Variability in the binding affinities of PxIxIT motifs plays an important regulatory role in substrate selection and release from the complex. Replacing the PxIxIT motif on AKAP5 with a higher affinity site increases recruitment of calcineurin to AKAP5 but impairs NFAT activation [195]. Conversely, too weak of a PxIxIT domain on AKAP5 also impairs NFAT activation, potentially by losing tethering of calcineurin near a site of Ca2+ release. Recently, AKAP5 has been shown to also contain an LxVP motif [200]. This may help explain how isolated AKAP5 complexes contain four copies of calcineurin per AKAP5 dimer [201]. Taken as a whole, the AKAP5 complex orchestrates the remarkable feat of facilitating calcineurin as a positive transducer of LTCC Ca2+ signaling to NFAT as well as providing feedback inhibition of LTCC through calcineurin-mediated reversal of PKA-mediated phosphorylation of LTCC.
4.1.2. AKAP5/calcineurin in adrenergic signaling:
AKAP5/CAV3 complexs link a subset of LTCC with β-ARs, thereby providing a platform for adrenergic enhancement of LTCC Ca2+ release, activation of NFAT-dependent transcriptional responses, and modification of β-AR activity by calcineurin. Neonatal cardiomyocytes lacking AKAP5, or expressing an AKAP5 lacking a PxIxIT motif, do not activate NFAT3 in response to an adrenergic stimulus [202]. Whether AKAP5 is specific for NFAT or can act as a platform for other calcineurin-responsive transcription factors is not known. There is evidence that only AKAP5 complexes associated with CAV3 are able to mobilize NFAT in response to an adrenergic stimulus, however, this remains controversial [203, 204]. In heart failure and ischemic heart disease the structure of the T-tubule system degrades, channel microdomains are remodeled, and spontaneous Ca2+ release events increase [205-210]. As a consequence, the nature of NFAT mobilization at LTCC/AKAP5/calcineurin complexes is likely very different in a failing heart than in a healthy one.
In adult cardiomyocytes lacking AKAP5, β-AR agonists do not increase either the magnitude of Ca2+ transients or the phosphorylation of LTCC, RyR2 and phospholamban [184, 211]. Only the pool of LTCC associated with CAV3 containing AKAP5 complexes responds to β-adrenergic stimulation in the heart [184]. In neurons, AKAP5-bound calcineurin inactivates neuronal LTCC following adrenergic activation [197, 198], however, whether calcineurin does this in cardiomyocytes has not yet been rigorously tested.
Persistent activation of β-ARs causes desensitization and recycling, which involves internalization of the receptor into endosomes followed by degradation or return to the plasma membrane. Recycling of β1-AR, the primary cardiac β-AR subtype [212], is dependent on AKAP5 and has been shown to require binding of both PKA and calcineurin [213]. SAP97 targets AKAP5 to the C-terminal tail of β1-AR, facilitating phosphorylation at Ser312, which is required for receptor cycling [214, 215]. Calcineurin promotes return of the receptor to the plasma membrane and may act by dephosphorylating this same site, but this remains to be definitively demonstrated. Treating cardiomyocytes with FK506 to inhibit calcineurin has no impact on internalization of either β1-AR or β2-AR, however, it prevents recycling of β1-AR, but not β2-AR, back to the cell membrane following the removal of agonist [216], thus, calcineurin appears to be involved in trafficking of β1-AR but not β2-AR in cardiomyocytes. It is relevant to note that, in a healthy heart, β1-ARs are distributed throughout the sarcolemma, whereas, β2-ARs are restricted to caveolae and found in association with CAV3 and LTCC [217-219]. AKAP5/calcineurin/CAV3 complexes [220] are required for β2-AR activation of the LTCC [184, 217] and mediate β2-AR activation of MAPK kinase [221].
4.1.3. AKAP5/calcineurin remodeling of potassium channels:
In pathological hypertrophy and failure the action potential is prolonged, in part, due to a reduction in repolarizing K+ currents, including the fast transient outward K+ current (Ito) carried by Kv4 channels [222]. In mice lacking AKAP5 Ito is not decreased following MI [202]. In both neurons and reconstituted Cos7 cells, AKAP5-anchored PKA promotes phosphorylation and internalization of Kv4.2, reducing current density. Conversely, AKAP5-anchored calcineurin promotes trafficking back to the surface [187]. If similar mechanisms control surface expression of Kv4-type channels in cardiomyocytes, it would suggest that during heart failure, PKA activity dominates AKAP5-dependent reductions in Ito, despite concurrent activation of AKAP5-achored calcineurin. Ito is increased in neonatal rat ventricular myocytes (NRVMs) overexpressing CnA* [223], consistent with calcineurin facilitating K+ channel activity. β-adrenergic-stimulation of NRVMs reduces Ito which is further reduced by the addition of a calcineurin inhibitor, also consistent with a positive effect for calcineurin [224]. Finally, Ito density is elevated in young αMHC-CnA* mice prior to a decline in cardiac function [223]. This suggests that, although Ito is reduced in older αMHC-CnA* mice [225], this is likely secondary to heart failure rather than a direct consequence of calcineurin’s impact on K+ channel activity.
NFAT-dependent repression of K+ channel gene expression has been proposed as a mechanism through which calcineurin activity could reduce Ito [202, 226, 227], and indeed, Ito is not reduced following MI in mice lacking NFATc3, similar to Akap5 KO mice [227]. However, the fact that calcineurin activity increases K+ channel transcript levels in NRVMs and in αMHC-CnA* transgenic mice prior to heart failure argues against transcriptional repression as a mechanism of action [223]. Thus, the specifics of a potential NFATc3-dependent mechanism remain to be deciphered.
4.1.4. AKAP5/PKA/calcineurin in vascular smooth muscle cells:
An AKAP5/PKA/calcineurin signaling complex also mediates fundamental signaling responses in vascular smooth muscle cells, interfacing with specific pools of LTCC, K+ channels, TRPV4 channels, purinergic receptors, and β1-ARs (reviewed in [228, 229]). Many of these interactions are relevant to control of vascular tone and mice lacking AKAP5 are hypotensive [230].
Finally, it is important to note that deletion of AKAP5 in mice causes hypertrophy, pathological remodeling, and increased calcineurin/NFAT activity [211, 216, 231]. This would suggest that an association between calcineurin and AKAP5 is not required to mediate hypertrophic remodeling and may instead be cardioprotective by restraining pathological calcineurin signaling [213].
4.2. Other cardiac AKAPs anchoring calcineurin:
4.2.1. AKAP6 (AKAP6β/mAKAPβ),
located at the nuclear envelope, provides a platform for integrating calcineurin signaling with a diversity of proteins that mediate transcriptional responses to cardiac stress. Ca2+ released from a select pool of perinuclear RyRs provides localized activation of calcineurin and subsequent stimulation of NFAT and MEF2 dependent gene expression [232-234]. AKAP6 is required for agonist induced nuclear translocation of NFAT in neonatal cardiomyocytes [232, 235], however, this has also been demonstrated for AKAP5 [202, 203]. Whether the two AKAP complexes work in conjunction with one another or mobilize distinct pools of NFAT is not known. There is a precedent for AKAP cooperativity as AKAP6 and AKAP9 have been shown to work in conjunction to tether Golgi to the nucleus [236]. Mice with a cardiomyocyte-specific deletion of AKAP6 are resistant to both pressure overload and agonist induced heart failure [234]. Extensive details of the many signaling processes mediated through AKAP6 and their relevance to cardiac remodeling can be found in an excellent recent review [237].
4.2.2. AKAP1 (AKAP121/AKAP84),
located at the outer mitochondrial membrane (OMM), acts as a coordinating hub for mitochondrial dynamics driven by changes in phosphorylation of the Dynamin Related Protein 1 (DRP1/DNM1L). AKAP1-bound DRP1 is phosphorylated by PKA [238-240]. Dephosphorylated of DRP1 by calcineurin allows it to engage receptors on the OMM to initiate mitochondrial fission [241, 242]. Remarkably, preventing mitochondria fission blocks hypertrophic growth in both neonatal and adult cardiomyocytes [243, 244] suggesting that DRP1 is an essential calcineurin substrate in the hypertrophic response. Loss of AKAP1 increases dephosphorylation of Drp1 and mitochondrial fission [245]. Total loss of AKAP1, or disruption of its ability to bind mitochondria, increases hypertrophic growth in neonatal and adult cardiomyocytes [246-248]. PTEN-induced kinase (PINK1), which is stabilized upon loss of mitochondria membrane potential, reduces PKA’s affinity for AKAP1 [249], thereby increasing calcineurin/DRP1-mediated mitochondrial fission and providing a mechanism for coupling PINK1-mediated mitophagy with mitochondrial fission.
Calcineurin can activate intrinsic cell death pathways by dephosphorylating the pro-apoptotic protein BAD, sending it to the OMM where it promotes MPTP opening [250-252]. AKAP1 coordinates the opposing rephosphorylation of BAD by PKA [253]. In addition to coordinating PKA and calcineurin activities, AKAP1 contains an RNA binding domain that binds mRNAs encoding electron transport chain and TCA cycle proteins, thereby gating local translation of mitochondrial proteins at the OMM [254-256]. Many important questions remain unanswered, such as; Is calcineurin involved in AKAP1-dependent control over mitochondrial protein translation and import? Can Ca2+ released from mitochondria during MPTP opening activate calcineurin localized nearby? and Does AKAP1 act as a platform for mobilizing other calcineurin substrates?
4.2.3. LDB3 (LIM Binding Domain 3/Cypher/Zasp)
is a calcineurin binding AKAP, specific to striated muscle and required for sarcomere integrity [257, 258]. Mutations in LDB3 cause dilated heart failure in humans [259] and cardiomyocyte-specific KO of Ldb3 causes severe dilated failure [260]. In adult cardiomyocytes lacking LDB3, protein levels of LTCC and calcineurin are significantly elevated, yet, both basal and isoproterenol-stimulated LTCC currents are reduced [261], suggesting that LDB3 is involved in β-adrenergic regulation of LTCC. Calcineurin’s role in the LDB3 complex is yet to be fully defined.
4.2.4. CMYA5 (cardiomyopathy associated 5/Myospryn)
is an extremely large, 500 kDa calcineurin-binding AKAP found in association with the intermediate filament protein desmin [262, 263]. In adult cardiomyocytes CMYA5 co-localizes with RyR2 at junctional SR and when it is expressed in heterologous cells it promotes clustering of RyR2 [264]. CMYA5 polymorphisms are associated with increased cardiac wall thickness in hypertension [265] and mutations are associated with sporadic hypertrophic cardiomyopathy [266]. Little is known regarding calcineurin’s role in CMYA5 functions. It is intriguing to note that calpain-3 also binds CMYA5 in the same general region as calcineurin [263, 267], making it a potential platform for calpain cleavage of calcineurin to produce the unregulated, constitutively active CnA* form.
4.3. Calcineurin’s impact on PP1 via I1:
Calcineurin can indirectly increase the activity of other AKAP-associated phosphatases through its control of I1. Phosphorylation of I1 by PKA causes selective inhibition of PP1 [268], which is released when calcineurin dephosphorylates I1 [165]. Key cardiac AKAPs containing PP1, include AKAP7 (AKAP15/AKAP18)[269] and Yotiao[270].
4.4. Calcineurin’s impact on AKAP/PKA via RII:
The RII regulatory subunit of PKA contains an LxVP motif and was one of the first identified calcineurin substrates [65]. AKAPs have binding sites for either RI or RII regulatory subunits which act to tether the catalytic subunits of PKA to the complex in an inactive state. Binding of cAMP to the R subunits releases the now active catalytic subunit from the complex. Phosphorylation of RII at Ser-96 can both increase its affinity for AKAP [271] and decrease its affinity for the PKA catalytic subunits [272, 273]. Dissociation of the catalytic subunit allows phosphatases access to RII. Thus, in AKAP complexes containing both RII and calcineurin, the phosphatase is poised to facilitate reassociation of RII with the catalytic subunit and terminate PKA activity [274]. The abundance and phosphorylation level of RII is decreased in the hearts of patients with dilated cardiomyopathy [271].
4.5. Additional calcineurin scaffolds:
TRIP10 (thyroid hormone receptor interactor 10), also called CIP4 (CDC42 interacting protein 4) binds the polyproline domain unique to CnAβ, targeting it to the sarcolemma [47, 275]. TRIP10 is an adaptor protein involved in coordinating both actin polymerization and membrane curvature and is required for hypertrophic growth of neonatal cardiomyocytes [276]. Cardiomyocyte-specific deletion of TRIP10, or peptides designed to prevent binding of CnAβ to TRIP10, blunts pathological remodeling in response to pressure overload. Remarkably, this pool of calcineurin responds robustly to pacing but only minimally to neurohormonal stimulation [47]. TRIP10 is phosphorylated by AKAP9/PKA complexes localized to Golgi [277, 278], thereby indirectly associating calcineurin with AKAP9. Whether this also occurs in the Yotiao form of AKAP9 [192, 279] and if it is involved in the role of Golgi in cardiovascular disease [280] remain to be investigated.
5. Calcineurin responses to mechanical stress
5.1. Mechanical stress at the plasma membrane:
The concept of mechanosensitive Ca2+ microdomains is central to our understanding of how mechanical stress is translated to actionable signaling events in the heart [281-283]. Less well understood is how, where, and when calcineurin engages with this system. Stress receptors at the plasma membrane include transient receptor potential canonical (TRPC) channels composed of homo or heterotetramers of either TRPC1/4/5 (stretch activated) or TRPC3/6/7 (activated by diacylglycerol downstream of G protein coupled receptors) [284, 285]. Inhibition of either subtype blunts hypertrophy and failure in response to sustained agonist or pressure overload [286]. Activation of calcineurin/NFAT under conditions of pressure overload increases TRPC6 expression creating feed-forward amplification of hypertrophic signaling [287].
Polycystin-1 (PC-1), another member of the TRPC family of Ca2+ transporters is a calcineurin/NFAT coupled mechanosensor [288]. Pressure overload hypertrophy is blunted in mice lacking PC-1 [289]. TRPC-dependent stretch activation of calcineurin/NFAT also occurs in myofibroblasts [290] promoting production of extracellular matrix and differentiation into activated myofibroblasts, thereby increasing myocardial stiffness [291-293]. Syndacan-4, a transmembrane heparin sulfate, acts as an essential coordinating center for TRPC6/calcineurin-mediated activation of myofibroblasts [294]. In atrial myocytes calcineurin activity is also required to increase matrix metalloproteases in response to static stretch [295]
5.2. Mechanical stress at the Z-disk:
Muscle LIM protein (MLP/CSRP3) tethers calcineurin to the Z-disk and is required for both stretch and adrenergic activation of NFAT [296]. Glutaredoxin 3 (GLRX3/PICOT), a positive regulator of DNA damage repair [297], disrupts calcineurin’s interaction with MLP [298]. Overexpression of GLRX3/PICOT blunts pressure overload-induced hypertrophy, whereas mice with reduced GLRX3/PICOT have an exacerbated response to pressure overload [299]. The Z-band protein Myozenin 2 (MYOZ2/calsarcin) also interacts with calcineurin [116, 300]. Loss of MYOZ2 increases activation of calcineurin by mechanical stress but has no effect on adrenergic activation of calcineurin. LMCD1 (LIM and cysteine-rich domains 1/dyxin), is another Z-disc protein that signals through the calcineurin pathway [301]. Over expressing LMCD1 increases calcineurin activity in response to pressure overload and accentuates pathological remodeling in vivo, whereas depletion of LMCD1 blunts NFAT activation in vitro [302].
Finally, the muscle-specific RING finger 1 (MuRF1), an E3 ubiquitin ligase localized to the Z-disk, targets CnA for degradation via the proteasome [303]. Mice lacking MuRF1 have increased fibrosis and hypertrophy in response to pressure overload that can be normalized by inhibiting calcineurin.
6. Calcineurin signaling during development and regeneration.
Given the importance of Ca2+ signals during development, it is not surprising that calcineurin is activated at diverse steps over the course of differentiation from a single cell to a fully mature adult heart.
6.1. Early commitment:
Calcineurin signaling is involved at several steps during the progression from embryonic stem cells (ESCs) to cardiac progenitors. During early differentiation of ESCs calcineurin/NFATc3 promotes epithelial-to-mesenchymal transition in both mice and humans [32].[32] Knock down of CnAγ, the gamma isoform of the catalytic subunit, increases the capacity of ESCs for self-renewal, suggesting that a calcineurin-dependent signal is required to exit pluripotency. Multipotent, mesodermal Flk1+ (VEGF receptor 2) cells subsequently become committed to either hematopoietic or cardiac progenitor lineages. Calcineurin/NFATc3 again plays a role in this cell fate discission by driving expression of Etv2 (ETS Variant Transcription Factor 2), which promotes differentiation toward hematopoiesis away from a cardiac lineage [304]. Thus, calcineurin provides both positive and negative signals along the progression from ESC to committed cardiomyocyte depending on the developmental step.
6.2. Cardiac valve morphogenesis:
Calcineurin/NFAT signaling is involved in at least three steps during cardiac valve formation. Endocardial to mesenchymal transformation (EMT) gives rise to valve mesenchyme during cushion formation. Endocardial cells with high levels of NFATc1 remain as endocardium and then proliferate to form elongated valve leaflets. Severe valve defects occur in mice lacking Nfatc1 [305, 306]. Lineage tracing studies suggest that Nfatc1 suppresses EMT by repressing expression of the Snail family of transcriptional repressors, SNAI1 and SNAI2, key factors required for EMT [307]. In endothelial cells where NFATc1 remains high, VEGF activates calcineurin/NFATc1 to work in conjunction with MEK1-ERK1/2 and promote endothelial cell proliferation. Later in maturation, RANKL (receptor activator of NFκB ligand) activates calcineurin/NFATc1 to work in conjunction with JNK1/2 signaling to activate expression of cathepsin K, an enzyme involved in extracellular matrix remodeling [308].
6.3. CRELD1 and atrioventricular septal defects (AVSDs):
Mutations in Cysteine-Rich with EGF-Like Domains 1 (CRELD1) have been linked to AVSDs in humans [309-311]. CRELD1 forms a complex with the CnB subunit of calcineurin at the ER and forced expression of CRELD1 is sufficient to activate NFATc1 [312]. CRELD1 is required for VEGF-dependent proliferation of endocardial cells and expression of NFATc1 target genes at E10.5 [312]. Cardiomyocyte-specific deletion of Creld1 during development causes hypoplasia and trabeculation defects caused by altered NOTCH1 signaling [313]. Increased calcineurin and NOTCH1 activity have been linked to cardiac hypoplasia caused by inhibition of mitochondrial fusion in cardiomyocytes during development [314]. It is relevant to note that NOTCH1 was recently validated as a calcineurin substrate [173]. Therefore, understanding the mechanism of CRELD1/calcineurin/NOTCH1 function may yield new insights into AVSD, one of the most common congenital heart defects.
6.4. Calcineurin signaling is different in neonatal cardiomyocytes compared to adult cardiomyocytes:
There are significant structural, metabolic, and functional differences between neonatal and adult cardiomyocytes, many of which impact the character and duration of the Ca2+ signal encountered by calcineurin [315-317]. In embryonic cardiomyocytes Ca2+ is released primarily from the perinuclear SR through the cooperative action of IP3R and RyR2. Mature T-tubules and dyads are not established until many days after birth. As a consequence, there is a slower time to peak and decay of both the action potential and the Ca2+ transient in neonatal cardiomyocytes compared to adult. The capacity for buffering cytosolic Ca2+ also increases dramatically after birth, thereby increasing the potential for establishing confined Ca2+ microdomains where specific pools of calcineurin may be shielded. The shift in calcineurin localization from diffuse, cytosolic in neonatal cardiomyocytes to a T-tubule/Z-disk pattern adult cells is coincident with the postnatal development of T-tubules, but probably also depends on changes in expression of specific calcineurin isoforms such as CnAβ with its unique N-terminal polyproline domain. In general, the changes that occur during maturation increase the total potential capacity for calcineurin activity while establishing micro-environments that restrain activation and protect against uncontrolled signaling. During failure, we postulate that loss of the T-tubule system releases these domain constraints on calcineurin contributing to a pathological feed-forward signaling cascade.
6.5. Vascularization of the myocardium:
Calcineurin/NFATC1 signaling also contributes to formation of the cardiac vascular system. During embryogenesis RANKL activation of calcineurin/Nfatc1 in the epicardium drives expression of cathepsin K to degrade extracellular matrix, thereby facilitating migration of epicardial cells into the myocardium where they contribute to formation of coronary vessels and the fibrous matrix of the mature heart [318].
6.6. Postnatal cardiomyocyte proliferation:
Soon after birth mammalian cardiomyocytes exit cell cycle and lose the capacity to regenerate. We recently demonstrated that postnatal activation of calcineurin contributes to postnatal cell cycle withdrawal by dephosphorylating the homeodomain transcription factor HOXb13, promoting its nuclear translocation to act in conjunction with the TALE family protein MEIS1 to mediate cell cycle arrest [319]. Remarkably, there is evidence of increased proliferation of adult cardiomyocytes following MI in αMHC-RCAN1 transgenic mice compared to in wild type animals post-MI, suggesting that calcineurin actively contributes to suppression of mitosis in the adult myocardium following damage.
7. Special considerations:
7.1. Not all calcineurin activity is pathological:
Much of the study of calcineurin signaling in the heart has focused on pathological outcomes, yet the high level of calcineurin present in heart tissue is a strong indication that calcineurin is central to normal cardiac function. Indeed, cardiac metabolism and function are severely compromised in mice with a cardiomyocyte-specific deletion of CnB1, demonstrating that calcineurin activity is essential for maintaining heart health [45, 46]. Although some investigators have suggested that calcineurin signaling only participates in pathological hypertrophy and is not engaged during physiological, hypertrophic growth [112], it is more likely a matter of signal intensity and duration. Consistent with a role for calcineurin in physiological hypertrophic growth exercise-induced hypertrophy is blunted in αMHC-RCAN1 transgenic mice [6].
The mechanisms activating calcineurin during physiological verses pathological hypertrophy may be vastly different, particularly given the changes in the T-tubule system, dyadic junction, and Ca2+ handling that occur with failure. Some downstream substrates may be common to both processes while others are unique. Equally important are the activities of other cell signaling cascades with which calcineurin converges. An elegant illustration of this can be seen in studies of human cardiac troponin C (cTnC) mutations that lead either to hypertrophic cardiomyopathy (HCM) or dilated cardiomyopathy (DCM). Calcineurin was activated in the hearts of mouse models carrying either type of mutation [320]. However, the HCM mutations increased sarcomeric tension, leading to concentric hypertrophy, whereas the DCM mutations decreased sarcomeric tension, resulting in eccentric hypertrophy. Thus, even under pathological conditions, activation of calcineurin may occur in response to different inputs and have different outcomes. Similarly, we propose that, although there are certain to be some aspects of similarity, the behavior and targets of calcineurin signaling in a healthy heart are likely distinct from those set in motion by pathological stresses.
7.2. There is a gradient in calcineurin activity across the ventricular wall:
In a normal healthy heart there is evidence of a gradient in calcineurin activity across the ventricular wall such that calcineurin and NFAT activity are both lower in the epicardium (EPI) than in the endocardium (ENDO) [227]. This parallels the transmural gradients in mechanical stress [321], diastolic Ca2+, systolic Ca2+ [227], and action potential duration (APD) [322], each of which is lower in magnitude in EPI than in ENDO. The transmural APD gradient allows ventricular repolarization to proceed in a synchronized wave from EPI to ENDO, supporting efficient pump function and preventing arrhythmias. The APD gradient is blunted in both human heart failure [323] and mouse models of failure [324]. Studies in mice suggest that NFATc3 contributes to the APD gradient by suppressing Kv4.2 expression in the endocardium, thereby decreasing Ito to prolong APD in the endocardium [227, 325]. However, the mechanism of repression is not understood and the relevance to human pathology is not clear as Kv4.2 is negligible in human heart [326, 327]. Given the ability of calcineurin to impact the activity and/or trafficking of a diversity of membrane proteins, calcineurin is just as likely to shape transmural gradients and their remodeling through transcription-independent control over other target channels that contribute to APD, such as LTCC and the sodium/potassium ATPase. Alternatively, the transmural gradient in calcineurin activity could be a consequence rather than the cause of the gradients in the activities of these channels. This complicated relationship requires further study.
8.3. Calcineurin activity changes throughout the day in healthy hearts:
Many physiological processes of the cardiovascular system display robust circadian rhythms and disruption of normal circadian rhythms predisposes humans to cardiovascular disease [328-333]. We have shown that calcineurin activity cycles daily in the hearts of healthy mice, with a peak in activity at the animal’s transition to rest and the lowest activity at the transition to waking [334]. In wild type mice there is as much as a 20-fold change in nuclear translocation of NFAT, binding of NFAT to chromatin, and expression of Rcan1.4, over the course of twenty-four hours. This suggests that, although either sustained activation of calcineurin [4] or its complete absence [45] is damaging, daily, periodic activation of calcineurin is compatible with heart health. Damage to the heart from ischemia reperfusion (I/R) is also time of day dependent both in humans and mice, with the greatest damage occurring near the transition to waking [97, 335, 336]. Administering FK506 to at the time of reperfusion reduced damage when reperfusion occurred near the transition to waking, when the heart is at greater risk, but had no additional benefit if reperfusion occurred at the transition to rest, when the heart is already inherently protected [97]. This suggests not that all damage to the heart from I/R is calcineurin-dependent, but, that the component that is dependent on time-of-day.
Circadian activation of calcineurin is not unique to the heart and has also been documented in skeletal muscle [337], as evidenced by changes in Rcan1.4 transcript levels and activity of NFAT reporters. In a series of elegant experiments these investigators demonstrated that circadian activation of calcineurin/NFAT in skeletal was independent of the transcriptional circadian clock control and was driven instead by muscle innervation and contractile activity. By extension, we postulate that circadian activation of calcineurin in the heart is being driven by changes in cardiac demand.
9. Conclusion
The impact of calcineurin signaling on cardiac health and disease is far more complex than simple activation of NFAT-dependent hypertrophic gene expression. The calcineurin targets highlighted here are by no means exhaustive of all known calcineurin substrates and targeting molecules that may be relevant to cardiovascular biology, however, we have tried to cover the majority of those for which molecular studies have been carried out in the context of cardiomyocytes. However, calcineurin mediates process in all cell types and tissues of the cardiovascular system. Many of these may be equally important to maintaining a healthy heart. Key points to keep in mind are: (1) Calcineurin signaling in neonatal cardiomyocytes can be very different than that found in adult cardiomyocytes and changes in heart failure. (2) Targeting of calcineurin and its substrates to specific sites of Ca2+ release creates signaling microdomains. (3) Activation of calcineurin is no not always pathological. (4) In a healthy heart calcineurin activity is heterogeneous relative to location in the ventricular wall and time of day. (5) Sustained activation of calcineurin leads to fundamental changes in a number of substrates that often act in a feed-forward fashion that accelerates cardiac decline.
Highlights:
Calcineurin activity is not always pathological.
Calcineurin and its substrates are anchored near specific Ca2+ release microdomains.
Mechanisms of activation and substrate choice depend on developmental stage and cardiac health.
Calcineurin activity is heterogeneous relative to location and timing.
Sustained calcineurin activity accelerates cardiac decline.
Acknowledgements
This work was supported by the National Institutes of Health [grant numbers HD101544, HL147276, R01HL147276, and P51 HD087351], the American Heart Association [19TPA34920001], and the Australian National Health and Medical Research Council.
Abbreviations
- AID
autoinhibitory domain
- AIS
autoinhibitory sequence
- AKAP
PKA kinase anchor proteins
- Akt
protein kinase B
- Ang II
angiotensin II
- AP
action potential
- β-AR
β-adrenergic receptors
- Ca2+/CaM
calcium/calmodulin
- CamKII
CaM Kinase II
- CAV3
Caveolin 3
- CBD
CaM binding domain
- CMYA5
cardiomyopathy associated 5
- CnA
catalytic subunit of calcineurin
- CnA*
constitutively active, truncated form of calcineurin
- CnB
regulatory subunit of calcineurin
- CREB
cAMP response element binding protein
- CRELD1
Cysteine-Rich with EGF-Like Domains 1
- CRTC
CREB-regulated transcriptional coactivators
- CsA
Cyclosporin A
- Cx43
connexin 43
- DRP1
Dynamin Related Protein 1
- ENDO
endocardium
- EPI
epicardium
- ERK
extracellular signal-regulated kinase
- FOXO1
Forkhead box protein O1
- IP3R
inositol-3-phosphate receptors
- I1
inhibitor 1
- I/R
ischemia reperfusion
- Ito
fast transient outward K+ current
- JNK
c-Jun NH(2)-terminal kinase
- LDB3
LIM Binding Domain 3
- LTCC
L-type voltage gated Ca2+ channel
- LxVP
calcineurin docking motif
- MI
myocardial infarction
- MKK7
mitogen-activated protein kinase kinase 7
- MPTP
mitochondrial permeability transition pore
- MTOR
mechanistic target of rapamycin
- NFAT
Nuclear Factor of Activated T-cells
- NCX1
sodium-calcium exchanger
- NHE1
sodium-potassium exchanger
- NRVM
neonatal rat ventricular myocyte
- OMM
outer mitochondrial membrane
- PC-1
Polycystin-1
- PxIxIT
calcineurin docking motif
- PKA
cAMP-dependent kinase
- RII
regulatory subunit II of PKA
- RANKL
receptor activator of NFκB ligand
- RCAN
Regulator of calcineurin
- RyR
ryanodine receptor
- SAP97
synapse-associated protein 97
- SLIMs
short linear motifs
- SR
sarcoplasmic reticulum
- TBC1D10C
EPI64/TBC1D10 GTPase-activating protein
- TFEB
Transcription Factor EB
- TRIP10
thyroid hormone receptor interactor 10
- TRPC
transient receptor potential canonical channels
- TRPV
transient receptor potential channels
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Klee CB, Crouch TH, Krinks MH, Calcineurin: a calcium- and calmodulin-binding protein of the nervous system, Proc Natl Acad Sci U S A 76(12) (1979) 6270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stewart AA, Ingebritsen TS, Manalan A, Klee CB, Cohen P, Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80), FEBS Lett 137(1) (1982) 80–4. [DOI] [PubMed] [Google Scholar]
- [3].Manalan AS, Klee CB, Affinity selection of chemically modified proteins: role of lysyl residues in the binding of calmodulin to calcineurin, Biochemistry 26(5) (1987) 1382–90. [DOI] [PubMed] [Google Scholar]
- [4].Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN, A calcineurin-dependent transcriptional pathway for cardiac hypertrophy, Cell 93(2) (1998) 215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, Colbert MC, Gualberto A, Wieczorek DF, Molkentin JD, Prevention of cardiac hypertrophy in mice by calcineurin inhibition, Science 281(5383) (1998) 1690–3. [DOI] [PubMed] [Google Scholar]
- [6].Rothermel BA, McKinsey TA, Vega RB, Nicol RL, Mammen P, Yang J, Antos CL, Shelton JM, Bassel-Duby R, Olson EN, Williams RS, Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo, Proc Natl Acad Sci U S A 98(6) (2001) 3328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].van Rooij E, Doevendans PA, Crijns HJ, Heeneman S, Lips DJ, van Bilsen M, Williams RS, Olson EN, Bassel-Duby R, Rothermel BA, De Windt LJ, MCIP1 overexpression suppresses left ventricular remodeling and sustains cardiac function after myocardial infarction, Circ Res 94(3) (2004) e18–26. [DOI] [PubMed] [Google Scholar]
- [8].Hill JA, Rothermel B, Yoo KD, Cabuay B, Demetroulis E, Weiss RM, Kutschke W, Bassel-Duby R, Williams RS, Targeted inhibition of calcineurin in pressure-overload cardiac hypertrophy. Preservation of systolic function, J Biol Chem 277(12) (2002) 10251–5. [DOI] [PubMed] [Google Scholar]
- [9].De Windt LJ, Lim HW, Bueno OF, Liang Q, Delling U, Braz JC, Glascock BJ, Kimball TF, del Monte F, Hajjar RJ, Molkentin JD, Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo, Proc Natl Acad Sci U S A 98(6) (2001) 3322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yu H, van Berkel TJ, Biessen EA, Therapeutic potential of VIVIT, a selective peptide inhibitor of nuclear factor of activated T cells, in cardiovascular disorders, Cardiovasc Drug Rev 25(2) (2007) 175–87. [DOI] [PubMed] [Google Scholar]
- [11].Taigen T, De Windt LJ, Lim HW, Molkentin JD, Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy, Proc Natl Acad Sci U S A 97(3) (2000) 1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li SJ, Wang J, Ma L, Lu C, Wang J, Wu JW, Wang ZX, Cooperative autoinhibition and multi-level activation mechanisms of calcineurin, Cell Res 26(3) (2016) 336–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gallagher SC, Gao ZH, Li S, Dyer RB, Trewhella J, Klee CB, There is communication between all four Ca(2+)-bindings sites of calcineurin B, Biochemistry 40(40) (2001) 12094–102. [DOI] [PubMed] [Google Scholar]
- [14].Kakalis LT, Kennedy M, Sikkink R, Rusnak F, Armitage IM, Characterization of the calcium-binding sites of calcineurin B, FEBS Lett 362(1) (1995) 55–8. [DOI] [PubMed] [Google Scholar]
- [15].Li H, Rao A, Hogan PG, Interaction of calcineurin with substrates and targeting proteins, Trends Cell Biol 21(2) (2011) 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bers DM, Calcium cycling and signaling in cardiac myocytes, Annu Rev Physiol 70 (2008) 23–49. [DOI] [PubMed] [Google Scholar]
- [17].Stemmer PM, Klee CB, Dual calcium ion regulation of calcineurin by calmodulin and calcineurin B, Biochemistry 33(22) (1994) 6859–66. [DOI] [PubMed] [Google Scholar]
- [18].Fearnley CJ, Roderick HL, Bootman MD, Calcium signaling in cardiac myocytes, Cold Spring Harb Perspect Biol 3(11) (2011) a004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bers DM, Guo T, Calcium signaling in cardiac ventricular myocytes, Ann N Y Acad Sci 1047 (2005) 86–98. [DOI] [PubMed] [Google Scholar]
- [20].Mehta S, Aye-Han NN, Ganesan A, Oldach L, Gorshkov K, Zhang J, Calmodulin-controlled spatial decoding of oscillatory Ca2+ signals by calcineurin, Elife 3 (2014) e03765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Heineke J, Ritter O, Cardiomyocyte calcineurin signaling in subcellular domains: from the sarcolemma to the nucleus and beyond, J Mol Cell Cardiol 52(1) (2012) 62–73. [DOI] [PubMed] [Google Scholar]
- [22].Fiedler B, Wollert KC, Targeting calcineurin and associated pathways in cardiac hypertrophy and failure, Expert Opin Ther Targets 9(5) (2005) 963–73. [DOI] [PubMed] [Google Scholar]
- [23].Despa S, Shui B, Bossuyt J, Lang D, Kotlikoff MI, Bers DM, Junctional cleft [Ca(2)(+)]i measurements using novel cleft-targeted Ca(2)(+) sensors, Circ Res 115(3) (2014) 339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu X, Bers DM, Free and bound intracellular calmodulin measurements in cardiac myocytes, Cell Calcium 41(4) (2007) 353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li L, Stefan MI, Le Novere N, Calcium input frequency, duration and amplitude differentially modulate the relative activation of calcineurin and CaMKII, PLoS One 7(9) (2012) e43810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Romano DR, Pharris MC, Patel NM, Kinzer-Ursem TL, Competitive tuning: Competition's role in setting the frequency-dependence of Ca2+-dependent proteins, PLoS Comput Biol 13(11) (2017) e1005820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Haq S, Choukroun G, Lim H, Tymitz KM, del Monte F, Gwathmey J, Grazette L, Michael A, Hajjar R, Force T, Molkentin JD, Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure, Circulation 103(5) (2001) 670–7. [DOI] [PubMed] [Google Scholar]
- [28].Oka T, Dai YS, Molkentin JD, Regulation of calcineurin through transcriptional induction of the calcineurin A beta promoter in vitro and in vivo, Mol Cell Biol 25(15) (2005) 6649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD, Impaired cardiac hypertrophic response in Calcineurin Abeta -deficient mice, Proc Natl Acad Sci U S A 99(7) (2002) 4586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bueno OF, Lips DJ, Kaiser RA, Wilkins BJ, Dai YS, Glascock BJ, Klevitsky R, Hewett TE, Kimball TR, Aronow BJ, Doevendans PA, Molkentin JD, Calcineurin Abeta gene targeting predisposes the myocardium to acute ischemia-induced apoptosis and dysfunction, Circ Res 94(1) (2004) 91–9. [DOI] [PubMed] [Google Scholar]
- [31].Zhang BW, Zimmer G, Chen J, Ladd D, Li E, Alt FW, Wiederrecht G, Cryan J, O'Neill EA, Seidman CE, Abbas AK, Seidman JG, T cell responses in calcineurin A alpha-deficient mice, J Exp Med 183(2) (1996) 413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen H, Zeng Y, Shao M, Zhao H, Fang Z, Gu J, Liao B, Jin Y, Calcineurin A gamma and NFATc3/SRPX2 axis contribute to human embryonic stem cell differentiation, J Cell Physiol (2021). [DOI] [PubMed] [Google Scholar]
- [33].Cottrell JR, Li B, Kyung JW, Ashford CJ, Mann JJ, Horvath TL, Ryan TA, Kim SH, Gerber DJ, Calcineurin Agamma is a Functional Phosphatase That Modulates Synaptic Vesicle Endocytosis, J Biol Chem 291(4) (2016) 1948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].He ZH, Hu Y, Li YC, Bao DP, Ruiz JR, Lucia A, Polymorphisms in the calcineurin genes are associated with the training responsiveness of cardiac phenotypes in Chinese young adults, Eur J Appl Physiol 110(4) (2010) 761–7. [DOI] [PubMed] [Google Scholar]
- [35].Salo PP, Havulinna AS, Tukiainen T, Raitakari O, Lehtimaki T, Kahonen M, Kettunen J, Mannikko M, Eriksson JG, Jula A, Blankenberg S, Zeller T, Salomaa V, Kristiansson K, Perola M, Genome-Wide Association Study Implicates Atrial Natriuretic Peptide Rather Than B-Type Natriuretic Peptide in the Regulation of Blood Pressure in the General Population, Circ Cardiovasc Genet 10(6) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shioda N, Moriguchi S, Shirasaki Y, Fukunaga K, Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia, J Neurochem 98(1) (2006) 310–20. [DOI] [PubMed] [Google Scholar]
- [37].Wu HY, Tomizawa K, Oda Y, Wei FY, Lu YF, Matsushita M, Li ST, Moriwaki A, Matsui H, Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration, J Biol Chem 279(6) (2004) 4929–40. [DOI] [PubMed] [Google Scholar]
- [38].Liu F, Grundke-Iqbal I, Iqbal K, Oda Y, Tomizawa K, Gong CX, Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain, J Biol Chem 280(45) (2005) 37755–62. [DOI] [PubMed] [Google Scholar]
- [39].Burkard N, Becher J, Heindl C, Neyses L, Schuh K, Ritter O, Targeted proteolysis sustains calcineurin activation, Circulation 111(8) (2005) 1045–53. [DOI] [PubMed] [Google Scholar]
- [40].Ritter O, Hack S, Schuh K, Rothlein N, Perrot A, Osterziel KJ, Schulte HD, Neyses L, Calcineurin in human heart hypertrophy, Circulation 105(19) (2002) 2265–9. [DOI] [PubMed] [Google Scholar]
- [41].Zhao Y, Cui GM, Zhou NN, Li C, Zhang Q, Sun H, Han B, Zou CW, Wang LJ, Li XD, Wang JC, Calpain-Calcineurin-Nuclear Factor Signaling and the Development of Atrial Fibrillation in Patients with Valvular Heart Disease and Diabetes, J Diabetes Res 2016 (2016) 4639654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Olivares-Florez S, Czolbe M, Riediger F, Seidlmayer L, Williams T, Nordbeck P, Strasen J, Glocker C, Jansch M, Eder-Negrin P, Arias-Loza P, Muhlfelder M, Plackic J, Heinze KG, Molkentin JD, Engelhardt S, Kockskamper J, Ritter O, Nuclear calcineurin is a sensor for detecting Ca(2+) release from the nuclear envelope via IP3R, J Mol Med (Berl) 96(11) (2018) 1239–1249. [DOI] [PubMed] [Google Scholar]
- [43].Mukerjee N, McGinnis KM, Gnegy ME, Wang KK, Caspase-mediated calcineurin activation contributes to IL-2 release during T cell activation, Biochem Biophys Res Commun 285(5) (2001) 1192–9. [DOI] [PubMed] [Google Scholar]
- [44].D'Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, Diamantini A, De Zio D, Carrara P, Battistini L, Moreno S, Bacci A, Ammassari-Teule M, Marie H, Cecconi F, Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease, Nat Neurosci 14(1) (2011) 69–76. [DOI] [PubMed] [Google Scholar]
- [45].Maillet M, Davis J, Auger-Messier M, York A, Osinska H, Piquereau J, Lorenz JN, Robbins J, Ventura-Clapier R, Molkentin JD, Heart-specific deletion of CnB1 reveals multiple mechanisms whereby calcineurin regulates cardiac growth and function, J Biol Chem 285(9) (2010) 6716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schaeffer PJ, Desantiago J, Yang J, Flagg TP, Kovacs A, Weinheimer CJ, Courtois M, Leone TC, Nichols CG, Bers DM, Kelly DP, Impaired contractile function and calcium handling in hearts of cardiac-specific calcineurin b1-deficient mice, Am J Physiol Heart Circ Physiol 297(4) (2009) H1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li X, Li J, Martinez EC, Froese A, Passariello CL, Henshaw K, Rusconi F, Li Y, Yu Q, Thakur H, Nikolaev VO, Kapiloff MS, Calcineurin Abeta-Specific Anchoring Confers Isoform-Specific Compartmentation and Function in Pathological Cardiac Myocyte Hypertrophy, Circulation 142(10) (2020) 948–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chen J, Balakrishnan-Renuka A, Hagemann N, Theiss C, Chankiewitz V, Chen J, Pu Q, Erdmann KS, Brand-Saberi B, A novel interaction between ATOH8 and PPP3CB, Histochem Cell Biol 145(1) (2016) 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gomez-Salinero JM, Lopez-Olaneta MM, Ortiz-Sanchez P, Larrasa-Alonso J, Gatto A, Felkin LE, Barton PJR, Navarro-Lerida I, Angel Del Pozo M, Garcia-Pavia P, Sundararaman B, Giovinazo G, Yeo GW, Lara-Pezzi E, The Calcineurin Variant CnAbeta1 Controls Mouse Embryonic Stem Cell Differentiation by Directing mTORC2 Membrane Localization and Activation, Cell Chem Biol 23(11) (2016) 1372–1382. [DOI] [PubMed] [Google Scholar]
- [50].Bond R, Ly N, Cyert MS, The unique C terminus of the calcineurin isoform CNAbeta1 confers non-canonical regulation of enzyme activity by Ca(2+) and calmodulin, J Biol Chem 292(40) (2017) 16709–16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lara-Pezzi E, Winn N, Paul A, McCullagh K, Slominsky E, Santini MP, Mourkioti F, Sarathchandra P, Fukushima S, Suzuki K, Rosenthal N, A naturally occurring calcineurin variant inhibits FoxO activity and enhances skeletal muscle regeneration, J Cell Biol 179(6) (2007) 1205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nutter CA, Jaworski E, Verma SK, Perez-Carrasco Y, Kuyumcu-Martinez MN, Developmentally regulated alternative splicing is perturbed in type 1 diabetic skeletal muscle, Muscle Nerve 56(4) (2017) 744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Felkin LE, Narita T, Germack R, Shintani Y, Takahashi K, Sarathchandra P, Lopez-Olaneta MM, Gomez-Salinero JM, Suzuki K, Barton PJ, Rosenthal N, Lara-Pezzi E, Calcineurin splicing variant calcineurin Abeta1 improves cardiac function after myocardial infarction without inducing hypertrophy, Circulation 123(24) (2011) 2838–47. [DOI] [PubMed] [Google Scholar]
- [54].Lopez-Olaneta MM, Villalba M, Gomez-Salinero JM, Jimenez-Borreguero LJ, Breckenridge R, Ortiz-Sanchez P, Garcia-Pavia P, Ibanez B, Lara-Pezzi E, Induction of the calcineurin variant CnAbeta1 after myocardial infarction reduces post-infarction ventricular remodelling by promoting infarct vascularization, Cardiovasc Res 102(3) (2014) 396–406. [DOI] [PubMed] [Google Scholar]
- [55].Padron-Barthe L, Villalba-Orero M, Gomez-Salinero JM, Acin-Perez R, Cogliati S, Lopez-Olaneta M, Ortiz-Sanchez P, Bonzon-Kulichenko E, Vazquez J, Garcia-Pavia P, Rosenthal N, Enriquez JA, Lara-Pezzi E, Activation of Serine One-Carbon Metabolism by Calcineurin Abeta1 Reduces Myocardial Hypertrophy and Improves Ventricular Function, J Am Coll Cardiol 71(6) (2018) 654–667. [DOI] [PubMed] [Google Scholar]
- [56].Griffith JP, Kim JL, Kim EE, Sintchak MD, Thomson JA, Fitzgibbon MJ, Fleming MA, Caron PR, Hsiao K, Navia MA, X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex, Cell 82(3) (1995) 507–22. [DOI] [PubMed] [Google Scholar]
- [57].Donella-Deana A, Krinks MH, Ruzzene M, Klee C, Pinna LA, Dephosphorylation of phosphopeptides by calcineurin (protein phosphatase 2B), Eur J Biochem 219(1-2) (1994) 109–17. [DOI] [PubMed] [Google Scholar]
- [58].Kissinger CR, Parge HE, Knighton DR, Lewis CT, Pelletier LA, Tempczyk A, Kalish VJ, Tucker KD, Showalter RE, Moomaw EW, et al. , Crystal structures of human calcineurin and the human FKBP12-FK506-calcineurin complex, Nature 378(6557) (1995) 641–4. [DOI] [PubMed] [Google Scholar]
- [59].Hendus-Altenburger R, Wang X, Sjogaard-Frich LM, Pedraz-Cuesta E, Sheftic SR, Bendsoe AH, Page R, Kragelund BB, Pedersen SF, Peti W, Molecular basis for the binding and selective dephosphorylation of Na(+)/H(+) exchanger 1 by calcineurin, Nat Commun 10(1) (2019) 3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Liu JO, Nacev BA, Xu J, Bhat S, It takes two binding sites for calcineurin and NFAT to tango, Mol Cell 33(6) (2009) 676–8. [DOI] [PubMed] [Google Scholar]
- [61].Rodriguez A, Roy J, Martinez-Martinez S, Lopez-Maderuelo MD, Nino-Moreno P, Orti L, Pantoja-Uceda D, Pineda-Lucena A, Cyert MS, Redondo JM, A conserved docking surface on calcineurin mediates interaction with substrates and immunosuppressants, Mol Cell 33(5) (2009) 616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Aramburu J, Garcia-Cozar F, Raghavan A, Okamura H, Rao A, Hogan PG, Selective inhibition of NFAT activation by a peptide spanning the calcineurin targeting site of NFAT, Mol Cell 1(5) (1998) 627–37. [DOI] [PubMed] [Google Scholar]
- [63].Garcia-Cozar FJ, Okamura H, Aramburu JF, Shaw KT, Pelletier L, Showalter R, Villafranca E, Rao A, Two-site interaction of nuclear factor of activated T cells with activated calcineurin, J Biol Chem 273(37) (1998) 23877–83. [DOI] [PubMed] [Google Scholar]
- [64].Li H, Rao A, Hogan PG, Structural delineation of the calcineurin-NFAT interaction and its parallels to PP1 targeting interactions, J Mol Biol 342(5) (2004) 1659–74. [DOI] [PubMed] [Google Scholar]
- [65].Grigoriu S, Bond R, Cossio P, Chen JA, Ly N, Hummer G, Page R, Cyert MS, Peti W, The molecular mechanism of substrate engagement and immunosuppressant inhibition of calcineurin, PLoS Biol 11(2) (2013) e1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Liu J, Farmer JD Jr., Lane WS, Friedman J, Weissman I, Schreiber SL, Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes, Cell 66(4) (1991) 807–15. [DOI] [PubMed] [Google Scholar]
- [67].Cardenas ME, Muir RS, Breuder T, Heitman J, Targets of immunophilin-immunosuppressant complexes are distinct highly conserved regions of calcineurin A, EMBO J 14(12) (1995) 2772–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Huai Q, Kim HY, Liu Y, Zhao Y, Mondragon A, Liu JO, Ke H, Crystal structure of calcineurin-cyclophilin-cyclosporin shows common but distinct recognition of immunophilin-drug complexes, Proc Natl Acad Sci U S A 99(19) (2002) 12037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jin L, Harrison SC, Crystal structure of human calcineurin complexed with cyclosporin A and human cyclophilin, Proc Natl Acad Sci U S A 99(21) (2002) 13522–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Waldmeier PC, Feldtrauer JJ, Qian T, Lemasters JJ, Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811, Mol Pharmacol 62(1) (2002) 22–9. [DOI] [PubMed] [Google Scholar]
- [71].Ozawa T, Effects of FK506 on ca release channels (review), Perspect Medicin Chem 2 (2008) 51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bultynck G, De Smet P, Rossi D, Callewaert G, Missiaen L, Sorrentino V, De Smedt H, Parys JB, Characterization and mapping of the 12 kDa FK506-binding protein (FKBP12)-binding site on different isoforms of the ryanodine receptor and of the inositol 1,4,5-trisphosphate receptor, Biochem J 354(Pt 2) (2001) 413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Clarke SJ, McStay GP, Halestrap AP, Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A, J Biol Chem 277(38) (2002) 34793–9. [DOI] [PubMed] [Google Scholar]
- [74].Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A, Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A, Science 285(5436) (1999) 2129–33. [DOI] [PubMed] [Google Scholar]
- [75].Martinez-Martinez S, Rodriguez A, Lopez-Maderuelo MD, Ortega-Perez I, Vazquez J, Redondo JM, Blockade of NFAT activation by the second calcineurin binding site, J Biol Chem 281(10) (2006) 6227–35. [DOI] [PubMed] [Google Scholar]
- [76].Kuriyama M, Matsushita M, Tateishi A, Moriwaki A, Tomizawa K, Ishino K, Sano S, Matsui H, A cell-permeable NFAT inhibitor peptide prevents pressure-overload cardiac hypertrophy, Chem Biol Drug Des 67(3) (2006) 238–43. [DOI] [PubMed] [Google Scholar]
- [77].Sieber M, Baumgrass R, Novel inhibitors of the calcineurin/NFATc hub - alternatives to CsA and FK506?, Cell Commun Signal 7 (2009) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Erdmann F, Weiwad M, Calcineurin inhibitors: status quo and perspectives, Biomol Concepts 2(1-2) (2011) 65–78. [DOI] [PubMed] [Google Scholar]
- [79].Zhang Y, Liu RB, Cao Q, Fan KQ, Huang LJ, Yu JS, Gao ZJ, Huang T, Zhong JY, Mao XT, Wang F, Xiao P, Zhao Y, Feng XH, Li YY, Jin J, USP16-mediated deubiquitination of calcineurin A controls peripheral T cell maintenance, J Clin Invest 129(7) (2019) 2856–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Davies KJ, Ermak G, Rothermel BA, Pritchard M, Heitman J, Ahnn J, Henrique-Silva F, Crawford D, Canaider S, Strippoli P, Carinci P, Min KT, Fox DS, Cunningham KW, Bassel-Duby R, Olson EN, Zhang Z, Williams RS, Gerber HP, Perez-Riba M, Seo H, Cao X, Klee CB, Redondo JM, Maltais LJ, Bruford EA, Povey S, Molkentin JD, McKeon FD, Duh EJ, Crabtree GR, Cyert MS, de la Luna S, Estivill X, Renaming the DSCR1/Adapt78 gene family as RCAN: regulators of calcineurin, FASEB J 21(12) (2007) 3023–8. [DOI] [PubMed] [Google Scholar]
- [81].Kingsbury TJ, Cunningham KW, A conserved family of calcineurin regulators, Genes Dev 14(13) (2000) 1595–604. [PMC free article] [PubMed] [Google Scholar]
- [82].Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS, Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles, Circ Res 87(12) (2000) E61–8. [DOI] [PubMed] [Google Scholar]
- [83].Strippoli P, Lenzi L, Petrini M, Carinci P, Zannotti M, A new gene family including DSCR1 (Down Syndrome Candidate Region 1) and ZAKI-4: characterization from yeast to human and identification of DSCR1-like 2, a novel human member (DSCR1L2), Genomics 64(3) (2000) 252–63. [DOI] [PubMed] [Google Scholar]
- [84].Lee SK, Ahnn J, Regulator of Calcineurin (RCAN): Beyond Down Syndrome Critical Region, Mol Cells 43(8) (2020) 671–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Chan B, Greenan G, McKeon F, Ellenberger T, Identification of a peptide fragment of DSCR1 that competitively inhibits calcineurin activity in vitro and in vivo, Proc Natl Acad Sci U S A 102(37) (2005) 13075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Martinez-Martinez S, Genesca L, Rodriguez A, Raya A, Salichs E, Were F, Lopez-Maderuelo MD, Redondo JM, de la Luna S, The RCAN carboxyl end mediates calcineurin docking-dependent inhibition via a site that dictates binding to substrates and regulators, Proc Natl Acad Sci U S A 106(15) (2009) 6117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Aubareda A, Mulero MC, Perez-Riba M, Functional characterization of the calcipressin 1 motif that suppresses calcineurin-mediated NFAT-dependent cytokine gene expression in human T cells, Cell Signal 18(9) (2006) 1430–8. [DOI] [PubMed] [Google Scholar]
- [88].Li Y, Sheftic SR, Grigoriu S, Schwieters CD, Page R, Peti W, The structure of the RCAN1:CN complex explains the inhibition of and substrate recruitment by calcineurin, Sci Adv 6(27) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Vega RB, Yang J, Rothermel BA, Bassel-Duby R, Williams RS, Multiple domains of MCIP1 contribute to inhibition of calcineurin activity, J Biol Chem 277(33) (2002) 30401–7. [DOI] [PubMed] [Google Scholar]
- [90].Jung MS, Park JH, Ryu YS, Choi SH, Yoon SH, Kwen MY, Oh JY, Song WJ, Chung SH, Regulation of RCAN1 protein activity by Dyrk1A protein-mediated phosphorylation, J Biol Chem 286(46) (2011) 40401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hornbeck PV, Chabra I, Kornhauser JM, Skrzypek E, Zhang B, PhosphoSite: A bioinformatics resource dedicated to physiological protein phosphorylation, Proteomics 4(6) (2004) 1551–61. [DOI] [PubMed] [Google Scholar]
- [92].Pan C, Olsen JV, Daub H, Mann M, Global effects of kinase inhibitors on signaling networks revealed by quantitative phosphoproteomics, Mol Cell Proteomics 8(12) (2009) 2796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ma L, Tang H, Ren Y, Deng H, Wu J, Wang Z, p38alpha MAP kinase phosphorylates RCAN1 and regulates its interaction with calcineurin, Sci China Life Sci 55(7) (2012) 559–66. [DOI] [PubMed] [Google Scholar]
- [94].Lin HY, Michtalik HJ, Zhang S, Andersen TT, Van Riper DA, Davies KK, Ermak G, Petti LM, Nachod S, Narayan AV, Bhatt N, Crawford DR, Oxidative and calcium stress regulate DSCR1 (Adapt78/MCIP1) protein, Free Radic Biol Med 35(5) (2003) 528–39. [DOI] [PubMed] [Google Scholar]
- [95].Abbasi S, Lee JD, Su B, Chen X, Alcon JL, Yang J, Kellems RE, Xia Y, Protein kinase-mediated regulation of calcineurin through the phosphorylation of modulatory calcineurin-interacting protein 1, J Biol Chem 281(12) (2006) 7717–26. [DOI] [PubMed] [Google Scholar]
- [96].Liu Q, Busby JC, Molkentin JD, Interaction between TAK1-TAB1-TAB2 and RCAN1-calcineurin defines a signalling nodal control point, Nat Cell Biol 11(2) (2009) 154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Rotter D, Grinsfelder DB, Parra V, Pedrozo Z, Singh S, Sachan N, Rothermel BA, Calcineurin and its regulator, RCAN1, confer time-of-day changes in susceptibility of the heart to ischemia/reperfusion, J Mol Cell Cardiol 74 (2014) 103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sanna B, Brandt EB, Kaiser RA, Pfluger P, Witt SA, Kimball TR, van Rooij E, De Windt LJ, Rothenberg ME, Tschop MH, Benoit SC, Molkentin JD, Modulatory calcineurin-interacting proteins 1 and 2 function as calcineurin facilitators in vivo, Proc Natl Acad Sci U S A 103(19) (2006) 7327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Vega RB, Rothermel BA, Weinheimer CJ, Kovacs A, Naseem RH, Bassel-Duby R, Williams RS, Olson EN, Dual roles of modulatory calcineurin-interacting protein 1 in cardiac hypertrophy, Proc Natl Acad Sci U S A 100(2) (2003) 669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Mehta S, Li H, Hogan PG, Cunningham KW, Domain architecture of the regulators of calcineurin (RCANs) and identification of a divergent RCAN in yeast, Mol Cell Biol 29(10) (2009) 2777–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Shin SY, Yang HW, Kim JR, Heo WD, Cho KH, A hidden incoherent switch regulates RCAN1 in the calcineurin-NFAT signaling network, J Cell Sci 124(Pt 1) (2011) 82–90. [DOI] [PubMed] [Google Scholar]
- [102].Wang S, Wang Y, Qiu K, Zhu J, Wu Y, RCAN1 in cardiovascular diseases: molecular mechanisms and a potential therapeutic target, Mol Med 26(1) (2020) 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mester-Tonczar J, Einzinger P, Winkler J, Kastner N, Spannbauer A, Zlabinger K, Traxler D, Lukovic D, Hasimbegovic E, Goliasch G, Pavo N, Gyongyosi M, Novel Identified Circular Transcript of RCAN2, circ-RCAN2, Shows Deviated Expression Pattern in Pig Reperfused Infarcted Myocardium and Hypoxic Porcine Cardiac Progenitor Cells In Vitro, Int J Mol Sci 22(3) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Pan F, Sun L, Kardian DB, Whartenby KA, Pardoll DM, Liu JO, Feedback inhibition of calcineurin and Ras by a dual inhibitory protein Carabin, Nature 445(7126) (2007) 433–6. [DOI] [PubMed] [Google Scholar]
- [105].Bisserier M, Berthouze-Duquesnes M, Breckler M, Tortosa F, Fazal L, de Regibus A, Laurent AC, Varin A, Lucas A, Branchereau M, Marck P, Schickel JN, Delomenie C, Cazorla O, Soulas-Sprauel P, Crozatier B, Morel E, Heymes C, Lezoualc'h F, Carabin protects against cardiac hypertrophy by blocking calcineurin, Ras, and Ca2+/calmodulin-dependent protein kinase II signaling, Circulation 131(4) (2015) 390–400; discussion 400. [DOI] [PubMed] [Google Scholar]
- [106].Zhu X, Fang J, Gong J, Guo JH, Zhao GN, Ji YX, Liu HY, Wei X, Li H, Cardiac-Specific EPI64C Blunts Pressure Overload-Induced Cardiac Hypertrophy, Hypertension 67(5) (2016) 866–77. [DOI] [PubMed] [Google Scholar]
- [107].Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Gronborg M, Mobius W, Rhee J, Barr FA, Simons M, Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C, J Cell Biol 189(2) (2010) 223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bei Y, Xu T, Lv D, Yu P, Xu J, Che L, Das A, Tigges J, Toxavidis V, Ghiran I, Shah R, Li Y, Zhang Y, Das S, Xiao J, Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury, Basic Res Cardiol 112(4) (2017) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Fruman DA, Pai SY, Klee CB, Burakoff SJ, Bierer BE, Measurement of Calcineurin Phosphatase Activity in Cell Extracts, Methods 9(2) (1996) 146–54. [DOI] [PubMed] [Google Scholar]
- [110].Minami T, Yano K, Miura M, Kobayashi M, Suehiro J, Reid PC, Hamakubo T, Ryeom S, Aird WC, Kodama T, The Down syndrome critical region gene 1 short variant promoters direct vascular bed-specific gene expression during inflammation in mice, J Clin Invest 119(8) (2009) 2257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Rinne A, Blatter LA, A fluorescence-based assay to monitor transcriptional activity of NFAT in living cells, J Physiol 588(Pt 17) (2010) 3211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD, Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy, Circ Res 94(1) (2004) 110–8. [DOI] [PubMed] [Google Scholar]
- [113].Parra V, Rothermel BA, Calcineurin signaling in the heart: The importance of time and place, J Mol Cell Cardiol 103 (2017) 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bazzazi H, Sang L, Dick IE, Joshi-Mukherjee R, Yang W, Yue DT, Novel fluorescence resonance energy transfer-based reporter reveals differential calcineurin activation in neonatal and adult cardiomyocytes, J Physiol 593(17) (2015) 3865–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Santana LF, Chase EG, Votaw VS, Nelson MT, Greven R, Functional coupling of calcineurin and protein kinase A in mouse ventricular myocytes, J Physiol 544(Pt 1) (2002) 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Frey N, Richardson JA, Olson EN, Calsarcins, a novel family of sarcomeric calcineurin-binding proteins, Proc Natl Acad Sci U S A 97(26) (2000) 14632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Reddy GR, West TM, Jian Z, Jaradeh M, Shi Q, Wang Y, Chen-Izu Y, Xiang YK, Illuminating cell signaling with genetically encoded FRET biosensors in adult mouse cardiomyocytes, J Gen Physiol 150(11) (2018) 1567–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, Bassel-Duby R, Williams RS, Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway, EMBO J 20(22) (2001) 6414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Musaro A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N, IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1, Nature 400(6744) (1999) 581–5. [DOI] [PubMed] [Google Scholar]
- [120].Wada H, Hasegawa K, Morimoto T, Kakita T, Yanazume T, Abe M, Sasayama S, Calcineurin-GATA-6 pathway is involved in smooth muscle-specific transcription, J Cell Biol 156(6) (2002) 983–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Iwai-Kanai E, Hasegawa K, Intracellular signaling pathways for norepinephrine- and endothelin-1-mediated regulation of myocardial cell apoptosis, Mol Cell Biochem 259(1-2) (2004) 163–8. [DOI] [PubMed] [Google Scholar]
- [122].Putt ME, Hannenhalli S, Lu Y, Haines P, Chandrupatla HR, Morrisey EE, Margulies KB, Cappola TP, Evidence for coregulation of myocardial gene expression by MEF2 and NFAT in human heart failure, Circ Cardiovasc Genet 2(3) (2009) 212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Cortes R, Rivera M, Rosello-Lleti E, Martinez-Dolz L, Almenar L, Azorin I, Lago F, Gonzalez-Juanatey JR, Portoles M, Differences in MEF2 and NFAT transcriptional pathways according to human heart failure aetiology, PLoS One 7(2) (2012) e30915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zarain-Herzberg A, Fragoso-Medina J, Estrada-Aviles R, Calcium-regulated transcriptional pathways in the normal and pathologic heart, IUBMB Life 63(10) (2011) 847–55. [DOI] [PubMed] [Google Scholar]
- [125].Liu Q, Chen Y, Auger-Messier M, Molkentin JD, Interaction between NFkappaB and NFAT coordinates cardiac hypertrophy and pathological remodeling, Circ Res 110(8) (2012) 1077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Bourajjaj M, Armand AS, da Costa Martins PA, Weijts B, van der Nagel R, Heeneman S, Wehrens XH, De Windt LJ, NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure, J Biol Chem 283(32) (2008) 22295–303. [DOI] [PubMed] [Google Scholar]
- [127].Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, Molkentin JD, Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth, Mol Cell Biol 22(21) (2002) 7603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kar P, Parekh AB, Distinct spatial Ca2+ signatures selectively activate different NFAT transcription factor isoforms, Mol Cell 58(2) (2015) 232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Rinne A, Kapur N, Molkentin JD, Pogwizd SM, Bers DM, Banach K, Blatter LA, Isoform- and tissue-specific regulation of the Ca(2+)-sensitive transcription factor NFAT in cardiac myocytes and heart failure, Am J Physiol Heart Circ Physiol 298(6) (2010) H2001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Calabria E, Ciciliot S, Moretti I, Garcia M, Picard A, Dyar KA, Pallafacchina G, Tothova J, Schiaffino S, Murgia M, NFAT isoforms control activity-dependent muscle fiber type specification, Proc Natl Acad Sci U S A 106(32) (2009) 13335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Shen T, Liu Y, Cseresnyes Z, Hawkins A, Randall WR, Schneider MF, Activity- and calcineurin-independent nuclear shuttling of NFATc1, but not NFATc3, in adult skeletal muscle fibers, Mol Biol Cell 17(4) (2006) 1570–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Altarejos JY, Montminy M, CREB and the CRTC co-activators: sensors for hormonal and metabolic signals, Nat Rev Mol Cell Biol 12(3) (2011) 141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Song Y, Altarejos J, Goodarzi MO, Inoue H, Guo X, Berdeaux R, Kim JH, Goode J, Igata M, Paz JC, Hogan MF, Singh PK, Goebel N, Vera L, Miller N, Cui J, Jones MR, Consortium C, Consortium G, Chen YD, Taylor KD, Hsueh WA, Rotter JI, Montminy M, CRTC3 links catecholamine signalling to energy balance, Nature 468(7326) (2010) 933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Mouchiroud L, Eichner LJ, Shaw RJ, Auwerx J, Transcriptional coregulators: fine-tuning metabolism, Cell Metab 20(1) (2014) 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Than TA, Lou H, Ji C, Win S, Kaplowitz N, Role of cAMP-responsive element-binding protein (CREB)-regulated transcription coactivator 3 (CRTC3) in the initiation of mitochondrial biogenesis and stress response in liver cells, J Biol Chem 286(25) (2011) 22047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Rahnert JA, Zheng B, Hudson MB, Woodworth-Hobbs ME, Price SR, Glucocorticoids Alter CRTC-CREB Signaling in Muscle Cells: Impact on PGC-1alpha Expression and Atrophy Markers, PLoS One 11(7) (2016) e0159181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, Bare O, Labow M, Spiegelman B, Stevenson SC, Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells, Proc Natl Acad Sci U S A 103(39) (2006) 14379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Morhenn K, Quentin T, Wichmann H, Steinmetz M, Prondzynski M, Sohren KD, Christ T, Geertz B, Schroder S, Schondube FA, Hasenfuss G, Schlossarek S, Zimmermann WH, Carrier L, Eschenhagen T, Cardinaux JR, Lutz S, Oetjen E, Mechanistic role of the CREB-regulated transcription coactivator 1 in cardiac hypertrophy, J Mol Cell Cardiol 127 (2019) 31–43. [DOI] [PubMed] [Google Scholar]
- [139].Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA, Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice, J Clin Invest 122(3) (2012) 1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kappel BA, Stohr R, De Angelis L, Mavilio M, Menghini R, Federici M, Posttranslational modulation of FoxO1 contributes to cardiac remodeling in post-ischemic heart failure, Atherosclerosis 249 (2016) 148–56. [DOI] [PubMed] [Google Scholar]
- [141].Qi Y, Zhu Q, Zhang K, Thomas C, Wu Y, Kumar R, Baker KM, Xu Z, Chen S, Guo S, Activation of Foxo1 by insulin resistance promotes cardiac dysfunction and beta-myosin heavy chain gene expression, Circ Heart Fail 8(1) (2015) 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Shioda N, Han F, Moriguchi S, Fukunaga K, Constitutively active calcineurin mediates delayed neuronal death through Fas-ligand expression via activation of NFAT and FKHR transcriptional activities in mouse brain ischemia, J Neurochem 102(5) (2007) 1506–1517. [DOI] [PubMed] [Google Scholar]
- [143].Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, Kuro OM, Rothermel BA, Hill JA, FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases, Proc Natl Acad Sci U S A 104(51) (2007) 20517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu G, Morris DJ, Castrillon DH, Gerard RD, Rothermel BA, Hill JA, Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling, Circulation 114(11) (2006) 1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Yuan Y, Shi XE, Liu YG, Yang GS, FoxO1 regulates muscle fiber-type specification and inhibits calcineurin signaling during C2C12 myoblast differentiation, Mol Cell Biochem 348(1-2) (2011) 77–87. [DOI] [PubMed] [Google Scholar]
- [146].Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A, TFEB links autophagy to lysosomal biogenesis, Science 332(6036) (2011) 1429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Saftig P, Puertollano R, How Lysosomes Sense, Integrate, and Cope with Stress, Trends Biochem Sci 46(2) (2021) 97–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Puertollano R, Ferguson SM, Brugarolas J, Ballabio A, The complex relationship between TFEB transcription factor phosphorylation and subcellular localization, EMBO J 37(11) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Martina JA, Diab HI, Brady OA, Puertollano R, TFEB and TFE3 are novel components of the integrated stress response, EMBO J 35(5) (2016) 479–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Pan B, Li J, Parajuli N, Tian Z, Wu P, Lewno MT, Zou J, Wang W, Bedford L, Mayer RJ, Fang J, Liu J, Cui T, Su H, Wang X, The Calcineurin-TFEB-p62 Pathway Mediates the Activation of Cardiac Macroautophagy by Proteasomal Malfunction, Circ Res 127(4) (2020) 502–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Lavandero S, Chiong M, Rothermel BA, Hill JA, Autophagy in cardiovascular biology, J Clin Invest 125(1) (2015) 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Lavandero S, Troncoso R, Rothermel BA, Martinet W, Sadoshima J, Hill JA, Cardiovascular autophagy: concepts, controversies, and perspectives, Autophagy 9(10) (2013) 1455–66. [DOI] [PubMed] [Google Scholar]
- [153].Hendus-Altenburger R, Wang X, Sjøgaard-Frich LM, Pedraz-Cuesta E, Sheftic SR, Bendsøe AH, Page R, Kragelund BB, Pedersen SF, Peti W, Molecular basis for the binding and selective dephosphorylation of Na(+)/H(+) exchanger 1 by calcineurin, Nat Commun 10(1) (2019) 3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Hisamitsu T, Nakamura TY, Wakabayashi S, Na(+)/H(+) exchanger 1 directly binds to calcineurin A and activates downstream NFAT signaling, leading to cardiomyocyte hypertrophy, Mol Cell Biol 32(16) (2012) 3265–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Katanosaka Y, Iwata Y, Kobayashi Y, Shibasaki F, Wakabayashi S, Shigekawa M, Calcineurin inhibits Na+/Ca2+ exchange in phenylephrine-treated hypertrophic cardiomyocytes, J Biol Chem 280(7) (2005) 5764–72. [DOI] [PubMed] [Google Scholar]
- [156].Shigekawa M, Katanosaka Y, Wakabayashi S, Regulation of the cardiac Na+/Ca2+ exchanger by calcineurin and protein kinase C, Ann N Y Acad Sci 1099 (2007) 53–63. [DOI] [PubMed] [Google Scholar]
- [157].Cavalli A, Eghbali M, Minosyan TY, Stefani E, Philipson KD, Localization of sarcolemmal proteins to lipid rafts in the myocardium, Cell Calcium 42(3) (2007) 313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Harvey RD, Calaghan SC, Caveolae create local signalling domains through their distinct protein content, lipid profile and morphology, J Mol Cell Cardiol 52(2) (2012) 366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Nakamura TY, Iwata Y, Arai Y, Komamura K, Wakabayashi S, Activation of Na+/H+ exchanger 1 is sufficient to generate Ca2+ signals that induce cardiac hypertrophy and heart failure, Circ Res 103(8) (2008) 891–9. [DOI] [PubMed] [Google Scholar]
- [160].Wakabayashi S, Hisamitsu T, Nakamura TY, Regulation of the cardiac Na+/H+ exchanger in health and disease, J Mol Cell Cardiol 61 (2013) 68–76. [DOI] [PubMed] [Google Scholar]
- [161].Hood AR, Ai X, Pogwizd SM, Regulation of cardiac gap junctions by protein phosphatases, J Mol Cell Cardiol 107 (2017) 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Rodríguez-Sinovas A, Sánchez JA, Valls-Lacalle L, Consegal M, Ferreira-González I, Connexins in the Heart: Regulation, Function and Involvement in Cardiac Disease, Int J Mol Sci 22(9) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Hatanaka K, Kawata H, Toyofuku T, Yoshida K, Down-regulation of connexin43 in early myocardial ischemia and protective effect by ischemic preconditioning in rat hearts in vivo, Jpn Heart J 45(6) (2004) 1007–19. [DOI] [PubMed] [Google Scholar]
- [164].Li WE, Nagy JI, Connexin43 phosphorylation state and intercellular communication in cultured astrocytes following hypoxia and protein phosphatase inhibition, Eur J Neurosci 12(7) (2000) 2644–50. [DOI] [PubMed] [Google Scholar]
- [165].El-Armouche A, Bednorz A, Pamminger T, Ditz D, Didie M, Dobrev D, Eschenhagen T, Role of calcineurin and protein phosphatase-2A in the regulation of phosphatase inhibitor-1 in cardiac myocytes, Biochem Biophys Res Commun 346(3) (2006) 700–6. [DOI] [PubMed] [Google Scholar]
- [166].Jabr RI, Hatch FS, Salvage SC, Orlowski A, Lampe PD, Fry CH, Regulation of gap junction conductance by calcineurin through Cx43 phosphorylation: implications for action potential conduction, Pflugers Arch 468(11-12) (2016) 1945–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Chowdhury SK, Liu W, Zi M, Li Y, Wang S, Tsui H, Prehar S, Castro S, Zhang H, Ji Y, Zhang X, Xiao R, Zhang R, Lei M, Cyganek L, Guan K, Millar CB, Liao X, Jain MK, Boyett MR, Cartwright EJ, Shiels HA, Wang X, Stress-Activated Kinase Mitogen-Activated Kinase Kinase-7 Governs Epigenetics of Cardiac Repolarization for Arrhythmia Prevention, Circulation 135(7) (2017) 683–699. [DOI] [PubMed] [Google Scholar]
- [168].Liu W, Zi M, Chi H, Jin J, Prehar S, Neyses L, Cartwright EJ, Flavell RA, Davis RJ, Wang X, Deprivation of MKK7 in cardiomyocytes provokes heart failure in mice when exposed to pressure overload, J Mol Cell Cardiol 50(4) (2011) 702–11. [DOI] [PubMed] [Google Scholar]
- [169].Wang Y, Su B, Sah VP, Brown JH, Han J, Chien KR, Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells, J Biol Chem 273(10) (1998) 5423–6. [DOI] [PubMed] [Google Scholar]
- [170].Gibson ES, Woolfrey KM, Li H, Hogan PG, Nemenoff RA, Heasley LE, Dell'Acqua ML, Subcellular Localization and Activity of the Mitogen-Activated Protein Kinase Kinase 7 (MKK7) gamma Isoform are Regulated through Binding to the Phosphatase Calcineurin, Mol Pharmacol 95(1) (2019) 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].De Windt LJ, Lim HW, Haq S, Force T, Molkentin JD, Calcineurin promotes protein kinase C and c-Jun NH2-terminal kinase activation in the heart. Cross-talk between cardiac hypertrophic signaling pathways, J Biol Chem 275(18) (2000) 13571–9. [DOI] [PubMed] [Google Scholar]
- [172].Maillet M, Lynch JM, Sanna B, York AJ, Zheng Y, Molkentin JD, Cdc42 is an antihypertrophic molecular switch in the mouse heart, J Clin Invest 119(10) (2009) 3079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Wigington CP, Roy J, Damle NP, Yadav VK, Blikstad C, Resch E, Wong CJ, Mackay DR, Wang JT, Krystkowiak I, Bradburn DA, Tsekitsidou E, Hong SH, Kaderali MA, Xu SL, Stearns T, Gingras AC, Ullman KS, Ivarsson Y, Davey NE, Cyert MS, Systematic Discovery of Short Linear Motifs Decodes Calcineurin Phosphatase Signaling, Mol Cell 79(2) (2020) 342–358 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Brauer BL, Moon TM, Sheftic SR, Nasa I, Page R, Peti W, Kettenbach AN, Leveraging New Definitions of the LxVP SLiM To Discover Novel Calcineurin Regulators and Substrates, ACS Chem Biol 14(12) (2019) 2672–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Beck M, Hurt E, The nuclear pore complex: understanding its function through structural insight, Nat Rev Mol Cell Biol 18(2) (2017) 73–89. [DOI] [PubMed] [Google Scholar]
- [176].Nguyen HQ, Roy J, Harink B, Damle NP, Latorraca NR, Baxter BC, Brower K, Longwell SA, Kortemme T, Thorn KS, Cyert MS, Fordyce PM, Quantitative mapping of protein-peptide affinity landscapes using spectrally encoded beads, Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Diviani D, Dodge-Kafka KL, Li J, Kapiloff MS, A-kinase anchoring proteins: scaffolding proteins in the heart, Am J Physiol Heart Circ Physiol 301(5) (2011) H1742–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Gildart M, Kapiloff MS, Dodge-Kafka KL, Calcineurin-AKAP interactions: therapeutic targeting of a pleiotropic enzyme with a little help from its friends, J Physiol 598(14) (2020) 3029–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Flynn R, Altier C, A macromolecular trafficking complex composed of beta(2)-adrenergic receptors, A-Kinase Anchoring Proteins and L-type calcium channels, J Recept Signal Transduct Res 33(3) (2013) 172–6. [DOI] [PubMed] [Google Scholar]
- [180].Qasim H, McConnell BK, AKAP12 Signaling Complex: Impacts of Compartmentalizing cAMP-Dependent Signaling Pathways in the Heart and Various Signaling Systems, J Am Heart Assoc 9(13) (2020) e016615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Redden JM, Dodge-Kafka KL, AKAP phosphatase complexes in the heart, J Cardiovasc Pharmacol 58(4) (2011) 354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WM, Scott JD, Association of protein kinase A and protein phosphatase 2B with a common anchoring protein, Science 267(5194) (1995) 108–11. [DOI] [PubMed] [Google Scholar]
- [183].Guo Y, Bo T, Zhou X, Gao L, Wang Y, Zhao J, AKAP5 signaling complexes: focal points and functional properties, Neuro Endocrinol Lett 36(1) (2015) 7–14. [PubMed] [Google Scholar]
- [184].Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, McKnight GS, Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels, Circ Res 107(6) (2010) 747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Mercado J, Baylie R, Navedo MF, Yuan C, Scott JD, Nelson MT, Brayden JE, Santana LF, Local control of TRPV4 channels by AKAP150-targeted PKC in arterial smooth muscle, J Gen Physiol 143(5) (2014) 559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA, A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1, Pain 146(3) (2009) 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Lin L, Sun W, Kung F, Dell'Acqua ML, Hoffman DA, AKAP79/150 impacts intrinsic excitability of hippocampal neurons through phospho-regulation of A-type K+ channel trafficking, J Neurosci 31(4) (2011) 1323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Vacher H, Trimmer JS, Diverse roles for auxiliary subunits in phosphorylation-dependent regulation of mammalian brain voltage-gated potassium channels, Pflugers Arch 462(5) (2011) 631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Sanderson JL, Dell'Acqua ML, AKAP signaling complexes in regulation of excitatory synaptic plasticity, Neuroscientist 17(3) (2011) 321–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Bal M, Zhang J, Hernandez CC, Zaika O, Shapiro MS, Ca2+/calmodulin disrupts AKAP79/150 interactions with KCNQ (M-Type) K+ channels, J Neurosci 30(6) (2010) 2311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Huang H, Kuenze G, Smith JA, Taylor KC, Duran AM, Hadziselimovic A, Meiler J, Vanoye CG, George AL Jr., Sanders CR, Mechanisms of KCNQ1 channel dysfunction in long QT syndrome involving voltage sensor domain mutations, Sci Adv 4(3) (2018) eaar2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS, Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel, Science 295(5554) (2002) 496–9. [DOI] [PubMed] [Google Scholar]
- [193].Terrenoire C, Houslay MD, Baillie GS, Kass RS, The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3, J Biol Chem 284(14) (2009) 9140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD, Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex, Neuron 27(1) (2000) 107–19. [DOI] [PubMed] [Google Scholar]
- [195].Li H, Pink MD, Murphy JG, Stein A, Dell'Acqua ML, Hogan PG, Balanced interactions of calcineurin with AKAP79 regulate Ca2+-calcineurin-NFAT signaling, Nat Struct Mol Biol 19(3) (2012) 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Murphy JG, Sanderson JL, Gorski JA, Scott JD, Catterall WA, Sather WA, Dell'Acqua ML, AKAP-anchored PKA maintains neuronal L-type calcium channel activity and NFAT transcriptional signaling, Cell Rep 7(5) (2014) 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Dittmer PJ, Dell'Acqua ML, Sather WA, Ca2+/calcineurin-dependent inactivation of neuronal L-type Ca2+ channels requires priming by AKAP-anchored protein kinase A, Cell Rep 7(5) (2014) 1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Oliveria SF, Dittmer PJ, Youn DH, Dell'Acqua ML, Sather WA, Localized calcineurin confers Ca2+-dependent inactivation on neuronal L-type Ca2+ channels, J Neurosci 32(44) (2012) 15328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Murphy JG, Crosby KC, Dittmer PJ, Sather WA, Dell'Acqua ML, AKAP79/150 recruits the transcription factor NFAT to regulate signaling to the nucleus by neuronal L-type Ca(2+) channels, Mol Biol Cell 30(14) (2019) 1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Nygren PJ, Mehta S, Schweppe DK, Langeberg LK, Whiting JL, Weisbrod CR, Bruce JE, Zhang J, Veesler D, Scott JD, Intrinsic disorder within AKAP79 fine-tunes anchored phosphatase activity toward substrates and drug sensitivity, Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Gold MG, Stengel F, Nygren PJ, Weisbrod CR, Bruce JE, Robinson CV, Barford D, Scott JD, Architecture and dynamics of an A-kinase anchoring protein 79 (AKAP79) signaling complex, Proc Natl Acad Sci U S A 108(16) (2011) 6426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Nieves-Cintron M, Hirenallur-Shanthappa D, Nygren PJ, Hinke SA, Dell'Acqua ML, Langeberg LK, Navedo M, Santana LF, Scott JD, AKAP150 participates in calcineurin/NFAT activation during the down-regulation of voltage-gated K(+) currents in ventricular myocytes following myocardial infarction, Cell Signal 28(7) (2016) 733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Makarewich CA, Correll RN, Gao H, Zhang H, Yang B, Berretta RM, Rizzo V, Molkentin JD, Houser SR, A caveolae-targeted L-type Ca(2)+ channel antagonist inhibits hypertrophic signaling without reducing cardiac contractility, Circ Res 110(5) (2012) 669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Correll RN, Makarewich CA, Zhang H, Zhang C, Sargent MA, York AJ, Berretta RM, Chen X, Houser SR, Molkentin JD, Caveolae-localized L-type Ca2+ channels do not contribute to function or hypertrophic signalling in the mouse heart, Cardiovasc Res 113(7) (2017) 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Glukhov AV, Balycheva M, Sanchez-Alonso JL, Ilkan Z, Alvarez-Laviada A, Bhogal N, Diakonov I, Schobesberger S, Sikkel MB, Bhargava A, Faggian G, Punjabi PP, Houser SR, Gorelik J, Direct Evidence for Microdomain-Specific Localization and Remodeling of Functional L-Type Calcium Channels in Rat and Human Atrial Myocytes, Circulation 132(25) (2015) 2372–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Hong T, Shaw RM, Cardiac T-Tubule Microanatomy and Function, Physiol Rev 97(1) (2017) 227–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Horiuchi-Hirose M, Kashihara T, Nakada T, Kurebayashi N, Shimojo H, Shibazaki T, Sheng X, Yano S, Hirose M, Hongo M, Sakurai T, Moriizumi T, Ueda H, Yamada M, Decrease in the density of t-tubular L-type Ca2+ channel currents in failing ventricular myocytes, Am J Physiol Heart Circ Physiol 300(3) (2011) H978–88. [DOI] [PubMed] [Google Scholar]
- [208].Medvedev RY, Sanchez-Alonso JL, Mansfield CA, Judina A, Francis AJ, Pagiatakis C, Trayanova N, Glukhov AV, Miragoli M, Faggian G, Gorelik J, Local hyperactivation of L-type Ca(2+) channels increases spontaneous Ca(2+) release activity and cellular hypertrophy in right ventricular myocytes from heart failure rats, Sci Rep 11(1) (2021) 4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Sanchez-Alonso JL, Bhargava A, O'Hara T, Glukhov AV, Schobesberger S, Bhogal N, Sikkel MB, Mansfield C, Korchev YE, Lyon AR, Punjabi PP, Nikolaev VO, Trayanova NA, Gorelik J, Microdomain-Specific Modulation of L-Type Calcium Channels Leads to Triggered Ventricular Arrhythmia in Heart Failure, Circ Res 119(8) (2016) 944–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Heinzel FR, Bito V, Biesmans L, Wu M, Detre E, von Wegner F, Claus P, Dymarkowski S, Maes F, Bogaert J, Rademakers F, D'Hooge J, Sipido K, Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium, Circ Res 102(3) (2008) 338–46. [DOI] [PubMed] [Google Scholar]
- [211].Li L, Li J, Drum BM, Chen Y, Yin H, Guo X, Luckey SW, Gilbert ML, McKnight GS, Scott JD, Santana LF, Liu Q, Loss of AKAP150 promotes pathological remodelling and heart failure propensity by disrupting calcium cycling and contractile reserve, Cardiovasc Res 113(2) (2017) 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Woo AY, Xiao RP, beta-Adrenergic receptor subtype signaling in heart: from bench to bedside, Acta Pharmacol Sin 33(3) (2012) 335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Li X, Nooh MM, Bahouth SW, Role of AKAP79/150 protein in beta1-adrenergic receptor trafficking and signaling in mammalian cells, J Biol Chem 288(47) (2013) 33797–33812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Gardner LA, Naren AP, Bahouth SW, Assembly of an SAP97-AKAP79-cAMP-dependent protein kinase scaffold at the type 1 PSD-95/DLG/ZO1 motif of the human beta(1)-adrenergic receptor generates a receptosome involved in receptor recycling and networking, J Biol Chem 282(7) (2007) 5085–5099. [DOI] [PubMed] [Google Scholar]
- [215].Gardner LA, Tavalin SJ, Goehring AS, Scott JD, Bahouth SW, AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the beta1-adrenergic receptor promotes recycling and functional resensitization of the receptor, J Biol Chem 281(44) (2006) 33537–53. [DOI] [PubMed] [Google Scholar]
- [216].Li X, Matta SM, Sullivan RD, Bahouth SW, Carvedilol reverses cardiac insufficiency in AKAP5 knockout mice by normalizing the activities of calcineurin and CaMKII, Cardiovasc Res 104(2) (2014) 270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ, Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation, Proc Natl Acad Sci U S A 103(19) (2006) 7500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Best JM, Kamp TJ, Different subcellular populations of L-type Ca2+ channels exhibit unique regulation and functional roles in cardiomyocytes, J Mol Cell Cardiol 52(2) (2012) 376–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].George MS, Pitt GS, The real estate of cardiac signaling: location, location, location, Proc Natl Acad Sci U S A 103(20) (2006) 7535–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Fraser ID, Cong M, Kim J, Rollins EN, Daaka Y, Lefkowitz RJ, Scott JD, Assembly of an A kinase-anchoring protein-beta(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling, Curr Biol 10(7) (2000) 409–12. [DOI] [PubMed] [Google Scholar]
- [221].Tao J, Malbon CC, G-protein-coupled receptor-associated A-kinase anchoring proteins AKAP5 and AKAP12: differential signaling to MAPK and GPCR recycling, J Mol Signal 3 (2008) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Yang KC, Nerbonne JM, Mechanisms contributing to myocardial potassium channel diversity, regulation and remodeling, Trends Cardiovasc Med 26(3) (2016) 209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Gong N, Bodi I, Zobel C, Schwartz A, Molkentin JD, Backx PH, Calcineurin increases cardiac transient outward K+ currents via transcriptional up-regulation of Kv4.2 channel subunits, J Biol Chem 281(50) (2006) 38498–506. [DOI] [PubMed] [Google Scholar]
- [224].Panama BK, Korogyi AS, Aschar-Sobbi R, Oh Y, Gray CB, Gang H, Brown JH, Kirshenbaum LA, Backx PH, Reductions in the Cardiac Transient Outward K+ Current Ito Caused by Chronic beta-Adrenergic Receptor Stimulation Are Partly Rescued by Inhibition of Nuclear Factor kappaB, J Biol Chem 291(8) (2016) 4156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Dong D, Duan Y, Guo J, Roach DE, Swirp SL, Wang L, Lees-Miller JP, Sheldon RS, Molkentin JD, Duff HJ, Overexpression of calcineurin in mouse causes sudden cardiac death associated with decreased density of K+ channels, Cardiovasc Res 57(2) (2003) 320–32. [DOI] [PubMed] [Google Scholar]
- [226].Perrier E, Perrier R, Richard S, Benitah JP, Ca2+ controls functional expression of the cardiac K+ transient outward current via the calcineurin pathway, J Biol Chem 279(39) (2004) 40634–9. [DOI] [PubMed] [Google Scholar]
- [227].Rossow CF, Dilly KW, Santana LF, Differential calcineurin/NFATc3 activity contributes to the Ito transmural gradient in the mouse heart, Circ Res 98(10) (2006) 1306–13. [DOI] [PubMed] [Google Scholar]
- [228].Man KNM, Navedo MF, Horne MC, Hell JW, beta2 Adrenergic Receptor Complexes with the L-Type Ca(2+) Channel CaV1.2 and AMPA-Type Glutamate Receptors: Paradigms for Pharmacological Targeting of Protein Interactions, Annu Rev Pharmacol Toxicol 60 (2020) 155–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Nieves-Cintron M, Flores-Tamez VA, Le T, Baudel MM, Navedo MF, Cellular and molecular effects of hyperglycemia on ion channels in vascular smooth muscle, Cell Mol Life Sci 78(1) (2021) 31–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF, AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension, Circ Res 102(2) (2008) e1–e11. [DOI] [PubMed] [Google Scholar]
- [231].Hulme JT, Westenbroek RE, Scheuer T, Catterall WA, Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during beta1-adrenergic regulation, Proc Natl Acad Sci U S A 103(44) (2006) 16574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Pare GC, Bauman AL, McHenry M, Michel JJ, Dodge-Kafka KL, Kapiloff MS, The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling, J Cell Sci 118(Pt 23) (2005) 5637–46. [DOI] [PubMed] [Google Scholar]
- [233].Li J, Vargas MA, Kapiloff MS, Dodge-Kafka KL, Regulation of MEF2 transcriptional activity by calcineurin/mAKAP complexes, Exp Cell Res 319(4) (2013) 447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Kritzer MD, Li J, Passariello CL, Gayanilo M, Thakur H, Dayan J, Dodge-Kafka K, Kapiloff MS, The scaffold protein muscle A-kinase anchoring protein beta orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure, Circ Heart Fail 7(4) (2014) 663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Li J, Negro A, Lopez J, Bauman AL, Henson E, Dodge-Kafka K, Kapiloff MS, The mAKAPbeta scaffold regulates cardiac myocyte hypertrophy via recruitment of activated calcineurin, J Mol Cell Cardiol 48(2) (2010) 387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Vergarajauregui S, Becker R, Steffen U, Sharkova M, Esser T, Petzold J, Billing F, Kapiloff MS, Schett G, Thievessen I, Engel FB, AKAP6 orchestrates the nuclear envelope microtubule-organizing center by linking golgi and nucleus via AKAP9, Elife 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Dodge-Kafka K, Gildart M, Tokarski K, Kapiloff MS, mAKAPbeta signalosomes - A nodal regulator of gene transcription associated with pathological cardiac remodeling, Cell Signal 63 (2019) 109357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Dickey AS, Strack S, PKA/AKAP1 and PP2A/Bbeta2 regulate neuronal morphogenesis via Drp1 phosphorylation and mitochondrial bioenergetics, J Neurosci 31(44) (2011) 15716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Merrill RA, Dagda RK, Dickey AS, Cribbs JT, Green SH, Usachev YM, Strack S, Mechanism of neuroprotective mitochondrial remodeling by PKA/AKAP1, PLoS Biol 9(4) (2011) e1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Dagda RK, Gusdon AM, Pien I, Strack S, Green S, Li C, Van Houten B, Cherra SJ 3rd, Chu CT, Mitochondrially localized PKA reverses mitochondrial pathology and dysfunction in a cellular model of Parkinson's disease, Cell Death Differ 18(12) (2011) 1914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L, Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria, Proc Natl Acad Sci U S A 105(41) (2008) 15803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Slupe AM, Merrill RA, Flippo KH, Lobas MA, Houtman JC, Strack S, A calcineurin docking motif (LXVP) in dynamin-related protein 1 contributes to mitochondrial fragmentation and ischemic neuronal injury, J Biol Chem 288(17) (2013) 12353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Pennanen C, Parra V, Lopez-Crisosto C, Morales PE, Del Campo A, Gutierrez T, Rivera-Mejias P, Kuzmicic J, Chiong M, Zorzano A, Rothermel BA, Lavandero S, Mitochondrial fission is required for cardiomyocyte hypertrophy mediated by a Ca2+-calcineurin signaling pathway, J Cell Sci 127(Pt 12) (2014) 2659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC, Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure, PLoS One 7(3) (2012) e32388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Edwards G, Perkins GA, Kim KY, Kong Y, Lee Y, Choi SH, Liu Y, Skowronska-Krawczyk D, Weinreb RN, Zangwill L, Strack S, Ju WK, Loss of AKAP1 triggers Drp1 dephosphorylation-mediated mitochondrial fission and loss in retinal ganglion cells, Cell Death Dis 11(4) (2020) 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Abrenica B, AlShaaban M, Czubryt MP, The A-kinase anchor protein AKAP121 is a negative regulator of cardiomyocyte hypertrophy, J Mol Cell Cardiol 46(5) (2009) 674–81. [DOI] [PubMed] [Google Scholar]
- [247].Perrino C, Feliciello A, Schiattarella GG, Esposito G, Guerriero R, Zaccaro L, Del Gatto A, Saviano M, Garbi C, Carangi R, Di Lorenzo E, Donato G, Indolfi C, Avvedimento VE, Chiariello M, AKAP121 downregulation impairs protective cAMP signals, promotes mitochondrial dysfunction, and increases oxidative stress, Cardiovasc Res 88(1) (2010) 101–10. [DOI] [PubMed] [Google Scholar]
- [248].Schiattarella GG, Boccella N, Paolillo R, Cattaneo F, Trimarco V, Franzone A, D'Apice S, Giugliano G, Rinaldi L, Borzacchiello D, Gentile A, Lombardi A, Feliciello A, Esposito G, Perrino C, Loss of Akap1 Exacerbates Pressure Overload-Induced Cardiac Hypertrophy and Heart Failure, Front Physiol 9 (2018) 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Pryde KR, Smith HL, Chau KY, Schapira AH, PINK1 disables the anti-fission machinery to segregate damaged mitochondria for mitophagy, J Cell Biol 213(2) (2016) 163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC, Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD, Science 284(5412) (1999) 339–43. [DOI] [PubMed] [Google Scholar]
- [251].Springer JE, Azbill RD, Nottingham SA, Kennedy SE, Calcineurin-mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury, J Neurosci 20(19) (2000) 7246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Shou Y, Li L, Prabhakaran K, Borowitz JL, Isom GE, Calcineurin-mediated Bad translocation regulates cyanide-induced neuronal apoptosis, Biochem J 379(Pt 3) (2004) 805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Affaitati A, Cardone L, de Cristofaro T, Carlucci A, Ginsberg MD, Varrone S, Gottesman ME, Avvedimento EV, Feliciello A, Essential role of A-kinase anchor protein 121 for cAMP signaling to mitochondria, J Biol Chem 278(6) (2003) 4286–94. [DOI] [PubMed] [Google Scholar]
- [254].Gabrovsek L, Collins KB, Aggarwal S, Saunders LM, Lau HT, Suh D, Sancak Y, Trapnell C, Ong SE, Smith FD, Scott JD, A-kinase-anchoring protein 1 (dAKAP1)-based signaling complexes coordinate local protein synthesis at the mitochondrial surface, J Biol Chem 295(31) (2020) 10749–10765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Rogne M, Landsverk HB, Van Eynde A, Beullens M, Bollen M, Collas P, Kuntziger T, The KH-Tudor domain of a-kinase anchoring protein 149 mediates RNA-dependent self-association, Biochemistry 45(50) (2006) 14980–9. [DOI] [PubMed] [Google Scholar]
- [256].Merrill RA, Strack S, Mitochondria: a kinase anchoring protein 1, a signaling platform for mitochondrial form and function, Int J Biochem Cell Biol 48 (2014) 92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Zhou Q, Ruiz-Lozano P, Martone ME, Chen J, Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to alpha-actinin-2 and protein kinase C, J Biol Chem 274(28) (1999) 19807–13. [DOI] [PubMed] [Google Scholar]
- [258].Lin C, Guo X, Lange S, Liu J, Ouyang K, Yin X, Jiang L, Cai Y, Mu Y, Sheikh F, Ye S, Chen J, Ke Y, Cheng H, Cypher/ZASP is a novel A-kinase anchoring protein, J Biol Chem 288(41) (2013) 29403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Vatta M, Mohapatra B, Jimenez S, Sanchez X, Faulkner G, Perles Z, Sinagra G, Lin JH, Vu TM, Zhou Q, Bowles KR, Di Lenarda A, Schimmenti L, Fox M, Chrisco MA, Murphy RT, McKenna W, Elliott P, Bowles NE, Chen J, Valle G, Towbin JA, Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction, J Am Coll Cardiol 42(11) (2003) 2014–27. [DOI] [PubMed] [Google Scholar]
- [260].Zheng M, Cheng H, Li X, Zhang J, Cui L, Ouyang K, Han L, Zhao T, Gu Y, Dalton ND, Bang ML, Peterson KL, Chen J, Cardiac-specific ablation of Cypher leads to a severe form of dilated cardiomyopathy with premature death, Hum Mol Genet 18(4) (2009) 701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Yu H, Yuan C, Westenbroek RE, Catterall WA, The AKAP Cypher/Zasp contributes to beta-adrenergic/PKA stimulation of cardiac CaV1.2 calcium channels, J Gen Physiol 150(6) (2018) 883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Kielbasa OM, Reynolds JG, Wu CL, Snyder CM, Cho MY, Weiler H, Kandarian S, Naya FJ, Myospryn is a calcineurin-interacting protein that negatively modulates slow-fiber-type transformation and skeletal muscle regeneration, FASEB J 25(7) (2011) 2276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Tsoupri E, Capetanaki Y, Muyospryn: a multifunctional desmin-associated protein, Histochem Cell Biol 140(1) (2013) 55–63. [DOI] [PubMed] [Google Scholar]
- [264].Benson MA, Tinsley CL, Waite AJ, Carlisle FA, Sweet SMM, Ehler E, George CH, Lai FA, Martin-Rendon E, Blake DJ, Ryanodine receptors are part of the myospryn complex in cardiac muscle, Sci Rep 7(1) (2017) 6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Nakagami H, Kikuchi Y, Katsuya T, Morishita R, Akasaka H, Saitoh S, Rakugi H, Kaneda Y, Shimamoto K, Ogihara T, Gene polymorphism of myospryn (cardiomyopathy-associated 5) is associated with left ventricular wall thickness in patients with hypertension, Hypertens Res 30(12) (2007) 1239–46. [DOI] [PubMed] [Google Scholar]
- [266].Xu J, Li Z, Ren X, Dong M, Li J, Shi X, Zhang Y, Xie W, Sun Z, Liu X, Dai Q, Investigation of Pathogenic Genes in Chinese sporadic Hypertrophic Cardiomyopathy Patients by Whole Exome Sequencing, Sci Rep 5 (2015) 16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Sarparanta J, Blandin G, Charton K, Vihola A, Marchand S, Milic A, Hackman P, Ehler E, Richard I, Udd B, Interactions with M-band titin and calpain 3 link myospryn (CMYA5) to tibial and limb-girdle muscular dystrophies, J Biol Chem 285(39) (2010) 30304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Huang FL, Glinsmann WH, Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle, Eur J Biochem 70(2) (1976) 419–26. [DOI] [PubMed] [Google Scholar]
- [269].Singh A, Redden JM, Kapiloff MS, Dodge-Kafka KL, The large isoforms of A-kinase anchoring protein 18 mediate the phosphorylation of inhibitor-1 by protein kinase A and the inhibition of protein phosphatase 1 activity, Mol Pharmacol 79(3) (2011) 533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Li Y, Chen L, Kass RS, Dessauer CW, The A-kinase anchoring protein Yotiao facilitates complex formation between adenylyl cyclase type 9 and the IKs potassium channel in heart, J Biol Chem 287(35) (2012) 29815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Zakhary DR, Moravec CS, Bond M, Regulation of PKA binding to AKAPs in the heart: alterations in human heart failure, Circulation 101(12) (2000) 1459–64. [DOI] [PubMed] [Google Scholar]
- [272].Rangel-Aldao R, Kupiec JW, Rosen OM, Resolution of the phosphorylated and dephosphorylated cAMP-binding proteins of bovine cardiac muscle by affinity labeling and two-dimensional electrophoresis, J Biol Chem 254(7) (1979) 2499–508. [PubMed] [Google Scholar]
- [273].Granot J, Mildvan AS, Hiyama K, Kondo H, Kaiser ET, Magnetic resonance studies of the effect of the regulatory subunit on metal and substrate binding to the catalytic subunit of bovine heart protein kinase, J Biol Chem 255(10) (1980) 4569–73. [PubMed] [Google Scholar]
- [274].Manni S, Mauban JH, Ward CW, Bond M, Phosphorylation of the cAMP-dependent protein kinase (PKA) regulatory subunit modulates PKA-AKAP interaction, substrate phosphorylation, and calcium signaling in cardiac cells, J Biol Chem 283(35) (2008) 24145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Woulfe KC, Travers JG, McKinsey TA, A Phosphatase Anchor Weighs on the Heart, Circulation 142(10) (2020) 963–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Rusconi F, Thakur H, Li J, Kapiloff MS, CIP4 is required for the hypertrophic growth of neonatal cardiac myocytes, J Biomed Sci 20 (2013) 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Tonucci FM, Almada E, Borini-Etichetti C, Pariani A, Hidalgo F, Rico MJ, Girardini J, Favre C, Goldenring JR, Menacho-Marquez M, Larocca MC, Identification of a CIP4 PKA phosphorylation site involved in the regulation of cancer cell invasiveness and metastasis, Cancer Lett 461 (2019) 65–77. [DOI] [PubMed] [Google Scholar]
- [278].Larocca MC, Shanks RA, Tian L, Nelson DL, Stewart DM, Goldenring JR, AKAP350 interaction with cdc42 interacting protein 4 at the Golgi apparatus, Mol Biol Cell 15(6) (2004) 2771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Witczak O, Skalhegg BS, Keryer G, Bornens M, Tasken K, Jahnsen T, Orstavik S, Cloning and characterization of a cDNA encoding an A-kinase anchoring protein located in the centrosome, AKAP450, EMBO J 18(7) (1999) 1858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Lu L, Zhou Q, Chen Z, Chen L, The significant role of the Golgi apparatus in cardiovascular diseases, J Cell Physiol 233(4) (2018) 2911–2919. [DOI] [PubMed] [Google Scholar]
- [281].Schonleitner P, Schotten U, Antoons G, Mechanosensitivity of microdomain calcium signalling in the heart, Prog Biophys Mol Biol 130(Pt B) (2017) 288–301. [DOI] [PubMed] [Google Scholar]
- [282].Caffarra Malvezzi C, Cabassi A, Miragoli M, Mitochondrial mechanosensor in cardiovascular diseases, Vasc Biol 2(1) (2020) R85–R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Chen-Izu Y, Izu LT, Mechano-chemo-transduction in cardiac myocytes, J Physiol 595(12) (2017) 3949–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, Olson EN, McKinsey TA, Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling, J Biol Chem 281(44) (2006) 33487–96. [DOI] [PubMed] [Google Scholar]
- [285].Yue Z, Xie J, Yu AS, Stock J, Du J, Yue L, Role of TRP channels in the cardiovascular system, Am J Physiol Heart Circ Physiol 308(3) (2015) H157–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Wu X, Eder P, Chang B, Molkentin JD, TRPC channels are necessary mediators of pathologic cardiac hypertrophy, Proc Natl Acad Sci U S A 107(15) (2010) 7000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN, TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling, J Clin Invest 116(12) (2006) 3114–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Puri S, Magenheimer BS, Maser RL, Ryan EM, Zien CA, Walker DD, Wallace DP, Hempson SJ, Calvet JP, Polycystin-1 activates the calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway, J Biol Chem 279(53) (2004) 55455–64. [DOI] [PubMed] [Google Scholar]
- [289].Pedrozo Z, Criollo A, Battiprolu PK, Morales CR, Contreras-Ferrat A, Fernandez C, Jiang N, Luo X, Caplan MJ, Somlo S, Rothermel BA, Gillette TG, Lavandero S, Hill JA, Polycystin-1 Is a Cardiomyocyte Mechanosensor That Governs L-Type Ca2+ Channel Protein Stability, Circulation 131(24) (2015) 2131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Lighthouse JK, Small EM, Transcriptional control of cardiac fibroblast plasticity, J Mol Cell Cardiol 91 (2016) 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Herum KM, Lunde IG, Skrbic B, Florholmen G, Behmen D, Sjaastad I, Carlson CR, Gomez MF, Christensen G, Syndecan-4 signaling via NFAT regulates extracellular matrix production and cardiac myofibroblast differentiation in response to mechanical stress, J Mol Cell Cardiol 54 (2013) 73–81. [DOI] [PubMed] [Google Scholar]
- [292].Herum KM, Lunde IG, Skrbic B, Louch WE, Hasic A, Boye S, Unger A, Brorson SH, Sjaastad I, Tonnessen T, Linke WA, Gomez MF, Christensen G, Syndecan-4 is a key determinant of collagen cross-linking and passive myocardial stiffness in the pressure-overloaded heart, Cardiovasc Res 106(2) (2015) 217–26. [DOI] [PubMed] [Google Scholar]
- [293].Kim EY, Roshanravan H, Dryer SE, Syndecan-4 ectodomain evokes mobilization of podocyte TRPC6 channels and their associated pathways: An essential role for integrin signaling, Biochim Biophys Acta 1853(10 Pt A) (2015) 2610–20. [DOI] [PubMed] [Google Scholar]
- [294].Herum KM, Lunde IG, McCulloch AD, Christensen G, The Soft- and Hard-Heartedness of Cardiac Fibroblasts: Mechanotransduction Signaling Pathways in Fibrosis of the Heart, J Clin Med 6(5) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Saygili E, Rana OR, Meyer C, Gemein C, Andrzejewski MG, Ludwig A, Weber C, Schotten U, Kruttgen A, Weis J, Schwinger RH, Mischke K, Rassaf T, Kelm M, Schauerte P, The angiotensin-calcineurin-NFAT pathway mediates stretch-induced up-regulation of matrix metalloproteinases-2/-9 in atrial myocytes, Basic Res Cardiol 104(4) (2009) 435–48. [DOI] [PubMed] [Google Scholar]
- [296].Heineke J, Ruetten H, Willenbockel C, Gross SC, Naguib M, Schaefer A, Kempf T, Hilfiker-Kleiner D, Caroni P, Kraft T, Kaiser RA, Molkentin JD, Drexler H, Wollert KC, Attenuation of cardiac remodeling after myocardial infarction by muscle LIM protein-calcineurin signaling at the sarcomeric Z-disc, Proc Natl Acad Sci U S A 102(5) (2005) 1655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Pandya P, Braiman A, Isakov N, PICOT (GLRX3) is a positive regulator of stress-induced DNA-damage response, Cell Signal 62 (2019) 109340. [DOI] [PubMed] [Google Scholar]
- [298].Jeong D, Kim JM, Cha H, Oh JG, Park J, Yun SH, Ju ES, Jeon ES, Hajjar RJ, Park WJ, PICOT attenuates cardiac hypertrophy by disrupting calcineurin-NFAT signaling, Circ Res 102(6) (2008) 711–9. [DOI] [PubMed] [Google Scholar]
- [299].Cha H, Kim JM, Oh JG, Jeong MH, Park CS, Park J, Jeong HJ, Park BK, Lee YH, Jeong D, Yang DK, Bernecker OY, Kim DH, Hajjar RJ, Park WJ, PICOT is a critical regulator of cardiac hypertrophy and cardiomyocyte contractility, J Mol Cell Cardiol 45(6) (2008) 796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Frey N, Barrientos T, Shelton JM, Frank D, Rutten H, Gehring D, Kuhn C, Lutz M, Rothermel B, Bassel-Duby R, Richardson JA, Katus HA, Hill JA, Olson EN, Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress, Nat Med 10(12) (2004) 1336–43. [DOI] [PubMed] [Google Scholar]
- [301].Frank D, Frauen R, Hanselmann C, Kuhn C, Will R, Gantenberg J, Fuzesi L, Katus HA, Frey N, Lmcd1/Dyxin, a novel Z-disc associated LIM protein, mediates cardiac hypertrophy in vitro and in vivo, J Mol Cell Cardiol 49(4) (2010) 673–82. [DOI] [PubMed] [Google Scholar]
- [302].Bian ZY, Huang H, Jiang H, Shen DF, Yan L, Zhu LH, Wang L, Cao F, Liu C, Tang QZ, Li H, LIM and cysteine-rich domains 1 regulates cardiac hypertrophy by targeting calcineurin/nuclear factor of activated T cells signaling, Hypertension 55(2) (2010) 257–63. [DOI] [PubMed] [Google Scholar]
- [303].Maejima Y, Usui S, Zhai P, Takamura M, Kaneko S, Zablocki D, Yokota M, Isobe M, Sadoshima J, Muscle-specific RING finger 1 negatively regulates pathological cardiac hypertrophy through downregulation of calcineurin A, Circ Heart Fail 7(3) (2014) 479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Wang YJ, Huang J, Liu W, Kou X, Tang H, Wang H, Yu X, Gao S, Ouyang K, Yang HT, IP3R-mediated Ca2+ signals govern hematopoietic and cardiac divergence of Flk1+ cells via the calcineurin-NFATc3-Etv2 pathway, J Mol Cell Biol 9(4) (2017) 274–288. [DOI] [PubMed] [Google Scholar]
- [305].de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, Potter J, Wakeham A, Marengere L, Langille BL, Crabtree GR, Mak TW, Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum, Nature 392(6672) (1998) 182–6. [DOI] [PubMed] [Google Scholar]
- [306].Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T, Mickanin C, Baldwin HS, Glimcher LH, The transcription factor NF-ATc is essential for cardiac valve formation, Nature 392(6672) (1998) 186–90. [DOI] [PubMed] [Google Scholar]
- [307].Wu B, Wang Y, Lui W, Langworthy M, Tompkins KL, Hatzopoulos AK, Baldwin HS, Zhou B, Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation, Circ Res 109(2) (2011) 183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Combs MD, Yutzey KE, VEGF and RANKL regulation of NFATc1 in heart valve development, Circ Res 105(6) (2009) 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Ghosh P, Bhaumik P, Ghosh S, Ozbek U, Feingold E, Maslen C, Sarkar B, Pramanik V, Biswas P, Bandyopadhyay B, Dey SK, Polymorphic haplotypes of CRELD1 differentially predispose Down syndrome and euploids individuals to atrioventricular septal defect, Am J Med Genet A 158A(11) (2012) 2843–8. [DOI] [PubMed] [Google Scholar]
- [310].Robinson SW, Morris CD, Goldmuntz E, Reller MD, Jones MA, Steiner RD, Maslen CL, Missense mutations in CRELD1 are associated with cardiac atrioventricular septal defects, Am J Hum Genet 72(4) (2003) 1047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Zhian S, Belmont J, Maslen CL, Specific association of missense mutations in CRELD1 with cardiac atrioventricular septal defects in heterotaxy syndrome, Am J Med Genet A 158A(8) (2012) 2047–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Mass E, Wachten D, Aschenbrenner AC, Voelzmann A, Hoch M, Murine Creld1 controls cardiac development through activation of calcineurin/NFATc1 signaling, Dev Cell 28(6) (2014) 711–26. [DOI] [PubMed] [Google Scholar]
- [313].Beckert V, Rassmann S, Kayvanjoo AH, Klausen C, Bonaguro L, Botermann DS, Krause M, Moreth K, Spielmann N, da Silva-Buttkus P, Fuchs H, Gailus-Durner V, de Angelis MH, Handler K, Ulas T, Aschenbrenner AC, Mass E, Wachten D, Creld1 regulates myocardial development and function, J Mol Cell Cardiol 156 (2021) 45–56. [DOI] [PubMed] [Google Scholar]
- [314].Kasahara A, Cipolat S, Chen Y, Dorn GW 2nd, Scorrano L, Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling, Science 342(6159) (2013) 734–7. [DOI] [PubMed] [Google Scholar]
- [315].Rapila R, Korhonen T, Tavi P, Excitation-contraction coupling of the mouse embryonic cardiomyocyte, J Gen Physiol 132(4) (2008) 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Ziman AP, Gomez-Viquez NL, Bloch RJ, Lederer WJ, Excitation-contraction coupling changes during postnatal cardiac development, J Mol Cell Cardiol 48(2) (2010) 379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Louch WE, Koivumaki JT, Tavi P, Calcium signalling in developing cardiomyocytes: implications for model systems and disease, J Physiol 593(5) (2015) 1047–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Combs MD, Braitsch CM, Lange AW, James JF, Yutzey KE, NFATC1 promotes epicardium-derived cell invasion into myocardium, Development 138(9) (2011) 1747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Nguyen NUN, Canseco DC, Xiao F, Nakada Y, Li S, Lam NT, Muralidhar SA, Savla JJ, Hill JA, Le V, Zidan KA, El-Feky HW, Wang Z, Ahmed MS, Hubbi ME, Menendez-Montes I, Moon J, Ali SR, Le V, Villalobos E, Mohamed MS, Elhelaly WM, Thet S, Anene-Nzelu CG, Tan WLW, Foo RS, Meng X, Kanchwala M, Xing C, Roy J, Cyert MS, Rothermel BA, Sadek HA, A calcineurin-Hoxb13 axis regulates growth mode of mammalian cardiomyocytes, Nature 582(7811) (2020) 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Davis J, Davis LC, Correll RN, Makarewich CA, Schwanekamp JA, Moussavi-Harami F, Wang D, York AJ, Wu H, Houser SR, Seidman CE, Seidman JG, Regnier M, Metzger JM, Wu JC, Molkentin JD, A Tension-Based Model Distinguishes Hypertrophic versus Dilated Cardiomyopathy, Cell 165(5) (2016) 1147–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Thomas JD, Popovic ZB, Assessment of left ventricular function by cardiac ultrasound, J Am Coll Cardiol 48(10) (2006) 2012–25. [DOI] [PubMed] [Google Scholar]
- [322].Wang Z, Kutschke W, Richardson KE, Karimi M, Hill JA, Electrical remodeling in pressure-overload cardiac hypertrophy: role of calcineurin, Circulation 104(14) (2001) 1657–63. [DOI] [PubMed] [Google Scholar]
- [323].Glukhov AV, Fedorov VV, Lou Q, Ravikumar VK, Kalish PW, Schuessler RB, Moazami N, Efimov IR, Transmural dispersion of repolarization in failing and nonfailing human ventricle, Circ Res 106(5) (2010) 981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Wang Y, Cheng J, Joyner RW, Wagner MB, Hill JA, Remodeling of early-phase repolarization: a mechanism of abnormal impulse conduction in heart failure, Circulation 113(15) (2006) 1849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Rossow CF, Dilly KW, Yuan C, Nieves-Cintron M, Cabarrus JL, Santana LF, NFATc3-dependent loss of I(to) gradient across the left ventricular wall during chronic beta adrenergic stimulation, J Mol Cell Cardiol 46(2) (2009) 249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].McKinnon D, Rosati B, Transmural gradients in ion channel and auxiliary subunit expression, Prog Biophys Mol Biol 122(3) (2016) 165–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Dixon JE, Shi W, Wang HS, McDonald C, Yu H, Wymore RS, Cohen IS, McKinnon D, Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current, Circ Res 79(4) (1996) 659–68. [DOI] [PubMed] [Google Scholar]
- [328].Smolensky MH, Portaluppi F, Manfredini R, Hermida RC, Tiseo R, Sackett-Lundeen LL, Haus EL, Diurnal and twenty-four hour patterning of human diseases: acute and chronic common and uncommon medical conditions, Sleep Med Rev 21 (2015) 12–22. [DOI] [PubMed] [Google Scholar]
- [329].Takeda N, Maemura K, The role of clock genes and circadian rhythm in the development of cardiovascular diseases, Cell Mol Life Sci 72(17) (2015) 3225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Virag JA, Lust RM, Circadian influences on myocardial infarction, Front Physiol 5 (2014) 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Morris CJ, Purvis TE, Hu K, Scheer FA, Circadian misalignment increases cardiovascular disease risk factors in humans, Proc Natl Acad Sci U S A 113(10) (2016) E1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Aziz IS, McMahon AM, Friedman D, Rabinovich-Nikitin I, Kirshenbaum LA, Martino TA, Circadian influence on inflammatory response during cardiovascular disease, Curr Opin Pharmacol 57 (2020) 60–70. [DOI] [PubMed] [Google Scholar]
- [333].Martino TA, Harrington ME, The Time for Circadian Medicine, J Biol Rhythms 35(5) (2020) 419–420. [DOI] [PubMed] [Google Scholar]
- [334].Sachan N, Dey A, Rotter D, Grinsfelder DB, Battiprolu PK, Sikder D, Copeland V, Oh M, Bush E, Shelton JM, Bibb JA, Hill JA, Rothermel BA, Sustained hemodynamic stress disrupts normal circadian rhythms in calcineurin-dependent signaling and protein phosphorylation in the heart, Circ Res 108(4) (2011) 437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME, Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock, Circ Res 106(3) (2010) 546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, Potelle C, Berthier A, Gheeraert C, Piveteau C, Deprez R, Eeckhoute J, Duez H, Lacroix D, Deprez B, Jegou B, Koussa M, Edme JL, Lefebvre P, Staels B, Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbalpha antagonism: a single-centre propensity-matched cohort study and a randomised study, Lancet 391(10115) (2018) 59–69. [DOI] [PubMed] [Google Scholar]
- [337].Dyar KA, Ciciliot S, Tagliazucchi GM, Pallafacchina G, Tothova J, Argentini C, Agatea L, Abraham R, Ahdesmaki M, Forcato M, Bicciato S, Schiaffino S, Blaauw B, The calcineurin-NFAT pathway controls activity-dependent circadian gene expression in slow skeletal muscle, Mol Metab 4(11) (2015) 823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]