Abstract
Although our understanding of axial spondyloarthropathy (axSpA) has increased recently, there has not been a concurrent improvement in patient diagnosis with delays contributing to patient morbidity. Imaging findings of axSpA can be subtle and may be dismissed often due to lack of understanding by reporters and importantly clinicians who do not suspect the disease. Recognition of the importance of imaging has led to the inclusion of MRI as part of the diagnostic criteria for axSpA. With this in mind, a number of advancements have been made in an attempt to increase our diagnostic accuracy on imaging. This article will give an overview of these techniques as well as a recap of the imaging features of axSpA.
Keywords: Spondyloarthropathy, Rheumatology, Structural lesions, Inflammatory lesions
1. Introduction
Spondyloarthropathy (SpA), can be thought of as an umbrella term to encompass a range of heterogenous but interlinked inflammatory processes. These include psoriatic arthritis (PsA), reactive arthritis (ReA), enteropathic arthritis, peripheral SpA and axial spondyloarthropathy (axSpA). Axial SpA can be further subdivided into its radiographic and non-radiographic (nr-axSpA) subtypes.1,2 The hallmark of SpA is enthesitis (Fig. 1, Fig. 2).
Fig. 1.
Demonstration of the different entheseal fibrocartilage at the Achilles tendon. (a) T1-weighted sagittal image of the attachment of the Achilles tendon on the calcaneum. (b) Diagrammatic representation of different fibrocartilage at the insertion of the Achilles tendon. Entheseal fibrocartilage is not only noted at the tendon-bone junction but also at the sesamoid and periosteal fibrocartilage (which form the boundaries of the bursa). All these areas are prone to entheseal inflammation. (c) Coronal STIR MRI image of a female patient demonstrating thickening and high signal intensity oedema/inflammation of the right hamstring tendon at its insertion on the right ischial tuberosity (DASHED WHITE ARROW). Adjacent bone marrow oedema is also noted.
Fig. 2.
a-d MRI STIR sequences demonstrating enthesitis at various sites. (a) Axial STIR MRI image demonstrating inflammation and oedema of the left iliopsoas muscle along with its myotendinous junction to its attachment on the left lesser trochanter. An iliopsoas bursa (WHITE ARROW) is noted with fluid deep to the iliacus muscle. Comparatively the right iliopsoas tendon is unremarkable. Note the very subtle bone marrow oedema (BMO) in the lesser trochanter enthesis with its surrounding increased adjacent soft tissue oedema. (b) Enthesitis of the right iliolumbar attachment with BMO and importantly soft tissue oedema (SOLID ARROW). (c) Axial oblique STIS sequences of the SIJ. At the inferior limits of the scan, florid enthesitis at the right gluteus medius attachment at the greater trochanter is noted. This highlights the importance of reviewing all sequences performed. (d) Sagittal MRI STIR sequences demonstrating calcaneal enthesitis at the plantar fascia attachment.
The lack of single diagnostic criteria, poor understanding of inflammatory back pain and insidious onset of the disease in the past have resulted in delays to diagnosis, contributing significantly to patient morbidity. In 2009, the Assessment of SpondyloArthritis International Society (ASAS) published new criteria for classification of axSpA with the recognition of imaging, specifically MRI, as an integral part of the diagnosis of axSpA. ASAS criteria revolutionised our understanding of SpA and acknowledged axSpA and peripheral SpA as two distinct phenotypes. Further dividing axSpA into ankylosing spondylitis (AS) and non-radiographic axSpA (nr-axSpA) has helped further our understanding of the disease and allowed earlier identification through MRI. Patients could be identified earlier in the course of their disease. The ability to identify axSpA patients without radiographic changes (nr-axSpA) has led to better understanding of the natural history and risk factors for axSpA, and greater appreciation for the burden of axSpA worldwide. Despite these advances, the delay to diagnosis has not significantly improved. Recent reports have suggested that clinical indicators such as Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) are poor markers for MR evidence of inflammatory change.3, 4, 5
MRI is the imaging modality of choice for axSpA, and has allowed identification of early disease where x-ray changes are minimal or absent. MRI can detect structural (erosions, fat metaplasia or ankylosis) or inflammatory (bone marrow oedema (BMO), capsulitis or enthesitis) (Table 1, Table 2) (Fig. 3, Fig. 4). The imaging arm of the ASAS Classification Criteria has a reported sensitivity and specificity of 66.2% and 97.3% respectively.6 The ASAS definition of MRI sacroiliitis is a BMO lesion in the SI joint on 2 consecutive slices, or more than one BMO lesions on a single slice. However, degenerative changes in the SI joints can also lead to similar BMO lesions, and they can be found in normal healthy individuals too.7, 8, 9 Recent advances in MRI and other imaging techniques have helped further improve the assessment of active inflammation and structural change. The aim of this paper is to review and provide an update on these imaging techniques.
Table 1.
Findings in the spine and sacroiliac joint (SIJ) on MRI using T1W and STIR sequences, can be broadly divided into structural and inflammatory lesions. Table 1 categorises common structural lesions in the spine and SIJ.
| Structural Lesions | MRI findings |
|---|---|
| Erosions | Loss of cortical bone associated with adjacent low bone marrow signal intensity best seen on T1W sequences. If active, manifests as a hyperintense lesion or with extensive adjacent BMO on STIR |
| Fat Infiltration | Juxta-articular – in contact with the articular surface. Geographical – sharp margins Signal – Uniform marrow signal intensity on T1W images. |
| Sclerosis | Low signal intensity on T1 and STIR imaging, in the subchondral region. |
| Ankylosis | Continuous marrow signal intensity across the joint. Can also manifest as marrow across parallel sclerotic tram-track lines believed to be residual joint lines from previous long erosive changes affecting the SIJ. |
| Syndesmophytes | Syndesmophytes are ossification of spinal ligaments or of the annulus fibrosus. Manifests as a linear continuous marrow signal between vertebral bodies on MRI. May occur on para sagittal slices and not on the central sagittal imaging. |
| Tissue Backfill | Tissue Backfill refers to fat metaplasia in excavated erosions seen as intra-articular high signal intensity on T1W images. Characterised as a complete loss of the cortical within the SIJ replaced by high-signal intensity tissue on T1W images clearly definable from the adjacent normal bone marrow signal by the irregular signal from the sclerotic border of the erosion. |
Table 2.
Findings in the spine and sacroiliac joint (SIJ) on MRI using T1W and STIR sequences, can be broadly divided into structural and inflammatory lesions. Table 2 categorises common inflammatory lesions in the spine and SIJ.
| Inflammatory Lesions | MRI manifestation |
|---|---|
| Corner Inflammatory Lesion (CIL) | Triangular or L shape BMO in one quadrant of the vertebra, commonly along the anterior or posterior margin on mid sagittal imaging. Related to the entheses of the ALL/PLL with the annulus fibrosis and the vertebral body. |
| Central Inflammatory Lesion (Andersson) | typically appears as a semi-circular area of BMO, related to the vertebral end plate adjacent to the intervertebral discs and can be associated with erosions. |
| Costotransverse Joint (CTJ) | Adjacent BMO on the far lateral sagittal images, related to the junction of the rib and the transverse process of the adjacent thoracic vertebra. Absent at T11 and T12. |
| Costovertebral Joint (CVJ) | Can affect any joint from T1 to T12. Circular pattern of bone marrow oedema related to the posterior intervertebral disc and middle column of the vertebral body. It can extend to the adjacent soft tissue, rib margin and posterior aspect of vertebral bodies. |
| Enthesitis of spinal ligaments | Supraspinous, interspinous ligament inflammation, seen along the spinous processes in the mid sagittal slices, along the posterior elements. |
Fig. 3.
Typical structural lesions seen in axial spondyloarthropathy. (a) Sagittal T1 image of the thoracolumbar spine demonstrating vertebral corner fat infiltration (SOLID ARROWS). (b) T1W coronal oblique image of the sacroiliac joint (SIJ). The solid arrows demonstrated typical tissue backfill representing fat metaplasia within an erosion. Notice the lesions are demarcated by the sclerotic border of the erosion and new cortical bone. Tissue backfill will be seen as high signal on STIR sequences (SOLID ARROWS in c). Note in (c) there are also subchondral inflammatory lesions bilaterally more so on the sacral side.
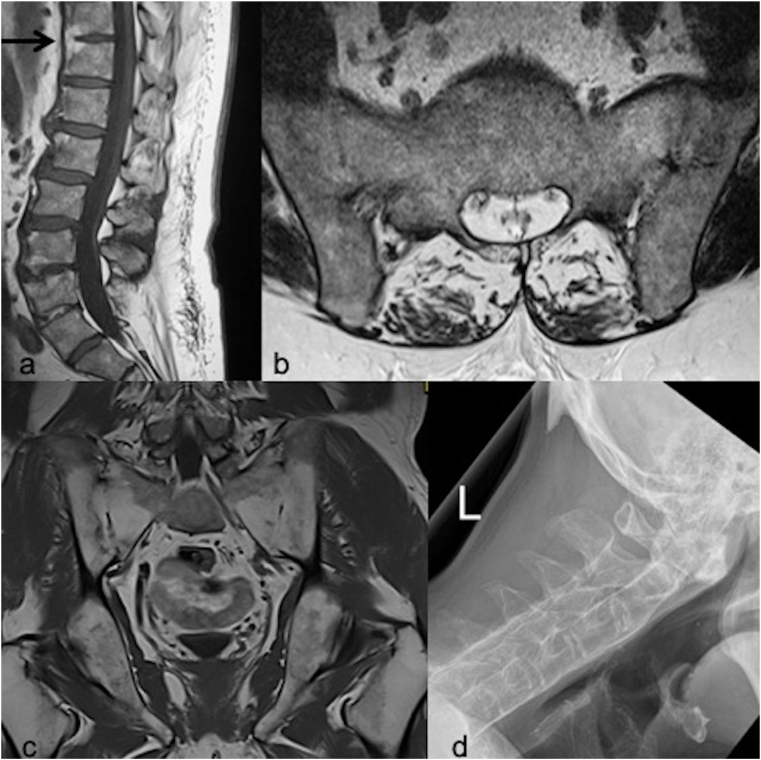
Fig. 4.
Typical examples of structural lesions of spondyloarthropathy. (a) T1W sagittal lumbar spine image showing fatty infiltration and subsequent syndesmophyte formation (BLACK ARROW). Fatty corner lesions are noted at multiple levels. (b) Axial oblique T1W image of the sacroiliac joint (SIJ) and (c) T1W coronal image of the SIJs demonstrating ankylosis of the SIJs. (d) This lateral view of the cervical spine shows a rigid cervical spine in a patient with severe, long-standing ankylosing spondylitis. The spine is completely ankylosed (“bamboo spine”) due to syndesmophytes, fused facet (apophyseal) joints, and paraspinal ligamentous calcification.
2. X-ray/CT
As previously mentioned, axSpA can be classified into radiographic, synonymous with ankylosing spondylitis (AS), and non-radiographic axSpA (nr-axSpA). The latter, nr-axSpA is described as a form of axial spondyloarthropathy that has not caused substantial erosive damage to the SIJs, i.e., the changes cannot be identified on an X-ray. The percentage of nr-SpA subjects is believed to be as high as 80% in the imaging arm and 20% in the clinical arm. The progression of nr-SpA to the end stage of radiographic ankylosis is, as yet, undetermined (Fig. 5). The Modified New Criteria classifies a positive radiograph for AS as either bilateral Grade 2–4 changes or unilateral Grade 3–4 change of the SIJ (Table 3).10 The early identification of axSpA has been facilitated by advances in MRI. The changes that can be seen in radiographic axSpA are sclerosis, erosions and eventually ankylosis, however bone changes often occur years after the associated inflammation, delaying diagnosis. Furthermore, due to the variability in morphology of the SIJ, positioning and squaring of the pelvis, they are poorly imaged in standard radiograph views, making interpretation challenging for even the most experienced.10 Ferguson's view, in which the x-ray source is positioned inferiorly compared to the standard anteroposterior (AP) pelvis view and angled cephalically, produces a more dedicated view of the SIJs. Multiple studies have looked at whether this view offers any advantage over a standard AP pelvis view, with their results demonstrating close agreement between standard AP pelvis and dedicated SIJ views.11,12 Despite its limitations, conventional radiography is relatively inexpensive and widely available making it a valuable modality when it returns positive findings.
Fig. 5.
Summary of the ASAS classification and the varying definitions of classification criteria. Radiographic spondyloarthropathy (SpA) refers to patients in the clinical or imaging arm with evidence of sacroiliitis on X-rays of the Sacroiliac joint (SIJ), whereas non-radiographic SpA can be defined as inflammatory back pain in the absence of structural damage on conventional radiographs. The percentage of nr-SpA subjects is believed to be as high as 80% in the Imaging arm and 20% in the clinical arm. The progression of nr-SpA to the end stage of radiographic ankylosis is, as yet, undetermined.
Table 3.
The modified New York Criteria for classification of ankylosing spondylitis.
| Grade | Findings | Radiograph |
|---|---|---|
| Grade 0 | Normal |  |
| Grade 1 | Suspicious Changes |  |
| Grade 2 |
Minimum Abnormality Small localized areas with erosion or sclerosis, without alteration in the joint width. |
 |
| Grade 3 |
Unequivocal Abnormality Moderate or advanced sacroiliitis with erosions, evidence of sclerosis, widening, narrowing, or partial ankylosis. |
 |
| Grade 4 |
Severe Abnormality Total ankylosis. |
 |
Computerised tomography (CT) is a good modality for identifying the structural changes associated with axSpA, as with the use of multi-planar reformatting, 3D image sets can be thoroughly interrogated in the appropriate planes.10 CT can also demonstrate other pathological changes including alterations to the bone trabecula, subarticular bone destruction and articular surface roughness.13 Further to this, while the high patient dose associated with CT has acted as a barrier to its more frequent use, there is promising data looking at the performance of low dose CT in the assessment bone proliferation in patients with AS.14 There have been previously been limitations with regards to the ability of conventional, single-energy CT to demonstrate bone BMO, suggesting active inflammation, however there are multiple studies that have investigated the performance of dual-energy CT (DECT) in detecting BMO, whilst using fluid sensitive MRI as the reference standard. By utilising the difference in attenuation of x-rays at difference energies, by different substances and post-processing techniques, these studies have suggested that DECT may have a role in detecting BMO in the SIJs. The limitations associated with these studies are that sample sizes were relatively small and because of the post-processing algorithm used mild BMO may be missed, as well as oedema directly adjacent to cortical bone.15, 16, 17 For this reason, although DECT has shown its ability to detect overt SIJ changes, more work may be needed before it can be used to detect the important subtle changes that affect a larger proportion of patients with SpA.
3. Conventional MRI
MRI is a non-invasive investigation and an imaging modality with the ability to interrogate anatomy in multiple planes with excellent spatial resolution. Moreover, different imaging sequences allow the study to be tailored dependent upon the clinical question, facilitating the identification of pathology that other modalities cannot. For these reasons, it has been widely accepted as a highly sensitive, highly specific method to diagnose and assess axSpA. The presence of bone marrow oedema (BMO)/osteitis, synovitis, enthesitis and capsulitis of the SIJs are MRI findings suggestive of active inflammation secondary to axSpA (Fig. 3b).18,19 Findings indicating structural damage consist of subchondral sclerosis, erosions, fat deposition and ankylosis, which are similar to those seen in radiographic axSpA.18 Recently there have been studies evaluating the use of contrast enhanced and functional MRI in assessing the effectiveness of treatment and patient's response to it.13 The contrast enhancement of tissues and behaviour of water molecules in local cellular environments vary with amount of inflammation. It is this variation that these recent techniques exploit in their appraisal of patients' disease states.
4. Quantitative MRI
Diffusion-weighted MR imaging (DWI) is based on the detection of water molecules which display different random motion depending on their cellular and tissue environments. The apparent diffusion coefficient (ADC) can be used to quantify the diffusion characteristics of water molecules in different tissues. Changes to ADC through serial imaging have been suggested to offer valuable insights into disease progression and treatment response. In context, this would offer a perception of the degree of juxta-articular bone destruction, synovitis and enthesitis in patients with axSpA (Fig. 6).20,21 ADCs derived from DWI have also been shown to have positive correlations with clinical scoring systems for disease activity, such as BASDAI, and functional status, such as Bath Ankylosing Spondylitis Functional Index (BASFI).22
Fig. 6.
(a) T1W coronal oblique image of the sacroiliac joints in a 47year old male demonstrates low signal intensity focus on the left iliac subchondral aspect (SOLID ARROW). The corresponding STIR image (b) demonstrates diffuse high signal intensity in keeping with bone marrow oedema (BMO) and an inflammatory component (SOLID ARROW) in axial spondyloarthropathy. (c) T1 Volumetric interpolated breath-hold examination (VIBE) (d) Diffusion weighted image (DWI) and (e) apparent diffusion coefficient (ADC) map. The Left SIJ BMO on T1W and STIR is better appreciated as an erosion on the T1 VIBE (c). A focus of apparent restricted diffusion on trace (d), with ADC (e) high signal demonstrates T2 shine through phenomenon. Probably due to the fluid component in the erosion.
Single-shot echo-planar imaging (ss-EPI), in contrast to conventional MRI sequences, has been used to produce diffusion-weighted MR images of the SIJs, however this technique is susceptible to artefacts (such as geometric distortion and signal intensity drop-out) due to slow traversal in the phase encoding direction. These artefacts are highlighted in the SIJs, due to the multiple tissue interfaces, however one study has suggested that readout-segmented EPI (rs-EPI), in which the readout direction of k-space is segmented, improves the image quality, diagnostic confidence, signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) in DWI of the SIJs.21 Whilst this study advocates the inclusion of rs-EPI DWI in routine clinical imaging, the study was retrospective with a small sample size.
There have been criticisms of the clinical use of DWI in axSpA, with arguments that other sequences can also be measured quantitatively, something that is not unique to DWI, and that the subjectiveness of choosing regions of interest (ROI) from the initial images makes standardisation difficult.23 Valid points have been made addressing these however, that while manually defining is ROI cumbrous, in future new methods may develop whereby the whole SIJ is sampled and analysed using thresholds to separate normal bone marrow from that which is inflamed.24 This could reduce or remove the subjective element that was previously criticised.
5. MRIc
Assessment of SIJs for active sacroiliitis is routinely performed without the use of intravenous (I.V.) contrast, with the ASAS classification criteria deeming non-contrast studies sufficient to make a diagnosis of axSpA. Several studies have questioned whether the use of I.V contrast would add value to diagnosis or disease monitoring in SpA. One study suggested that the assessment of pathological enhancement, implying osteitis, was more reliable in estimating the response to TNF antagonists than findings of BMO.25 Another found that using delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) indices, in assessment the metacarpophalangeal (MCP), proximal interphalangeal (PIP), and distal interphalangeal (DIP) joints of patients with PsA, demonstrated a relationship between proteoglycan loss in the articular cartilage and acute inflammation.26
Dynamic contrast enhanced MRI (DCE-MRI) offers functional information about the passage of body fluids within a specific region of interest, indirectly reflecting the blood flow and vascularisation status, which in turn facilitate assessment of inflammatory changes at specific sites. Because of the superior spatio-temporal resolution offered by MRI, relative to molecular imaging, the use of radionuclide tracer with DCE-MRI is a very active field of research.13
6. NM
The development of hybrid imaging utilising the sensitivity of nuclear medicine (NM) techniques to functional tissue changes, paired with the spatial resolution of modalities such as CT offers the possibility of this modality to be of great use in the early detection of axSpA. One study found that 18F-NaF PET/CT had 79.6% sensitivity and 84.2% specificity in diagnosing AS, when positive findings were defined as symmetric SI joint uptake suggesting sacroiliitis, spinal syndesmophytes and enthesopathy at any site, suggesting that there is a role for NM in the SpA.27 Another study compared the Heel Enthesitis MRI Scoring model (HEMRIS) with clinical and PET/CT outcomes in patients with cutaneous psoriasis (Pso), PsA or AS and found that whilst HEMRIS is sensitive for the detection of structural entheseal lesions and enthesitis, there were disparities between the MRI findings of enthesitis versus clinical and PET/CT identification. This would suggest that further research is needed to determine the clinical significance of the entheseal metabolic activity measured on PET/CT.28
7. DEXA
Whilst conventional radiography, and increasingly MRI, are the mainstay for diagnosis and monitoring of axSpA, osteoporosis has been demonstrated as a highly prevalent co-morbidity among these patients,29 inviting the use of dual-energy X-ray absorptiometry (DXA) to assess lumbar spine bone mineral density (BMD). In conventional DXA, where anteroposterior (AP) images of the lumbar spine are acquired with the patient in a supine position, it becomes difficult to assess for BMD loss, in axSpA patients, due to potentially superimposed osteoproliferation, producing seemingly normal BMD and therefore offering false reassurance.30
In one study, the use of lateral lumbar DXA, in addition to conventional DXA, was shown to increase detection of cases of low BMD from 35% to 56% when compared with conventional projections alone. This further increased to 58% when used in combination with hip data.30 Another study advocated the use of lateral projections when assessing BMD as a predictor of 2-year progression of spinal damage in patients with axSpA.31
8. US
The role of ultrasound imaging (US) in SpA includes the assessment of synovia, tendons and entheses, with the ability to discern the presence of pathology at a subclinical level. A further benefit of using US at an early stage of a patient's disease course is its higher sensitivity than clinical examination at differentiating SpA from other inflammatory arthropathies, such as rheumatoid arthritis, polymyalgia rheumatica and crystalline arthropathies, therefore reducing the number of differential diagnoses for the clinician. US findings in keeping with SpA include, but are not limited to, synovitis, tenosynovitis and enthesitis.32 Enthesitis is an important feature of SpA, seen less in other inflammatory arthropathies, and therefore US evaluation of the Achilles is a widely utilised tool. On US, enthesitis is characterised by hypoechogenicity and thickening of the tendon or ligament, cortical bone erosions, calcification and abnormal vascularity on power Doppler at the enthesis insertion site (Fig. 7). It is worth noting that erosions and bony proliferation can also be identified sonographically, however as previously discussed they are changes associated with chronic inflammation.33
Fig. 7.
MRI and Ultrasound (US) findings of Achilles enthesopathy in a patient confirmed to have axial spondyloarthritis. Longitudinal (a) and transverse (b) US section through the Achilles tendon demonstrating abnormal vascularisation of the cortical bone insertion on power Doppler imaging. The corresponding MRI (Sagittal T1 (c) and STIR (d) sequences) demonstrates fluid in the bursa (WHITE ARROW) and surrounding oedema and inflammation. Note the inflammation in the different fibrocartilage areas as previously discussed in Fig. 1.
9. Imaging in trauma
Trauma in patients with ankylosing spinal disorders (ASD) can cause fracture from even the most innocuous events. AS, the predominant form of axSpA, is one of the ASDs predisposing patients to this type of injury. The others include: diffuse idiopathic skeletal hyperostosis (DISH), degenerative spondylosis (DS), and a surgically fused spine however their discussion is beyond the scope of this paper. The biomechanical changes that occur in AS, increases the risk of fractures three-fold. Firstly, osseous fusion of the vertebral bodies removes the shock-absorbing properties of the intervertebral discs, increasing the load that the vertebral bodies have to bear. Secondly the spinal ankylosis produces a long, rigid lever which is more susceptible to fracture. Thirdly, kyphotic deformities are common in AS, shifting the centre of gravity anteriorly, increasing the shear stress forces.34 Early diagnosis and treatment may prevent disease burden of ankyloses and subsequent complex spinal fractures with significant associated morbidity.35
The most common mechanism for injury in patients with AS is reported as hyperextension, causing transverse fracture through the intervertebral disc space, due to the calcified disc being the weakest point along the ankylosed spine, with cervical fractures being more common than those of the thoracolumbar spine. There are however cases in which fractures can extend to involve the vertebral bodies and in severe injury multiple, non-contiguous fractures can occur. In the majority of these cases the cervical and thoracic spine are affected, followed by cases affecting the thoracic and lumbar regions.34 One study demonstrated that in the majority of patients with AS, complicated by spinal fracture, sustained three-column fractures, identified on CT. Of the patients in the study who underwent MRI, 36% demonstrated spinal cord injury, 59% saw vertebral fracture accompanied by BMO of the vertebral body and 31% had injury of the posterior ligamentous complex (PCL).36
There is little consensus on whether CT alone, or MRI in addition, should be performed in the context of trauma in AS however multiple sources advocate performing whole spine CT to look for multiple non-contiguous fractures, whilst having a low threshold for MRI spine for assessment of the PCL and spinal cord.34,35,37 One study found that when comparing spinal trauma patients with AS against those with DISH, MRI spine in AS patients, with CT confirmed vertebral body fracture, demonstrated posterior element injury in 71% of patients. These findings were considered to have clinical implications, with these AS patients undergoing surgical treatment, despite having no neurological deficit. Despite the small sample size, these findings promote the use of MRI in patients with AS.38 Another study, with a greater sample size, suggested that MRI should be reserved for assessment of patients with non-ankylosed spinal levels where disco-ligamentous injury is suspected and for those patients with neurological deficit. However, this study looked at ASD patients collectively and did not provide separate data on AS and DISH patients.39 Von der Höh et al. recommend performing MRI of the whole spine, to assess for non-displaced or occult fractures requiring stabilisation, which may not be apparent on CT.40
10. Artificial intelligence
Artificial intelligence (AI) is a method by which a machine is able to mimic human behaviour and make decisions based on its learning. Machine learning (ML) is a subset of AI, which is based on improving the machine's decision-making ability through statistical analysis of its experiences and learning, while deep learning (DL) is a further subset of ML which simulates neurons of the human brain in an attempt to learn high-level features from the data to which it is exposed.41 The use of ML in medical imaging is an emerging concept, which may have many roles from combating observer fatigue, by effectively screening data prior to its observation by a medical professional, to assisting with early diagnosis of cancers or degenerative bone diseases.
One study assessed the use of ML and DL in the early detection of AS, through analysis of CT images of the SIJs, assessing for erosions, sclerosis and/or fusion, taking the patients' ages into consideration. In this study, the ML and DL techniques made a CT diagnosis of AS with a raw sensitivity and specificity of 8.4% and 9.5% greater than an experienced musculoskeletal radiologist, in a fraction of the time. This being said however, the data-set used in the study was relatively-small, used images obtained from a single CT scanner at a single institution and the AI techniques were only compared to the findings of a single radiologist. Furthermore, AI techniques only assess predefined regions of interest (ROIs), which must first be set by the user, a process which may prove time-consuming, the sheer computing power required to perform this analysis may act as a barrier to this method's widespread use for the foreseeable future.42 Another study investigated the performance of various data-driven models in predicting AS progression, which took numerous variables into consideration, including clinical findings, laboratory tests and imaging features. Whilst the results showed reasonable prediction of progression with some of the models, the study sample size was small, from a single centre and had little ethnic diversity.43
A way to accurately, efficiently and consistently identify imaging features of early AS and its progression would be of great benefit to patients’ morbidity by decreasing delays to diagnosis and recognising the need for intervention, however further work is needed if these promising early advances are to offer solutions.
11. Conclusion
Our understanding of the disease spectrum of axSpA is continually evolving and its complexities require the same evolution of imaging techniques. Low specificity of the MRI finding of SIJ BME may lead to misclassification in populations with low axSpA prevalence. It is therefore important to research and develop novel modalities or techniques to improve the specificity of axSpA changes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial Support and sponsorship
Nil.
Declaration of competing interest
Nil.
References
- 1.Pathak H., Gaffney K. Spectrum of spondyloarthritis. Indian J Rheumatol. 2020;15(Suppl S1):34–39. [Google Scholar]
- 2.Shah A., Paramesparan K., Rennie W.J. Peripheral seronegative spondyloarthritis – updates on critical criteria. Indian J Musculoskelet Radiol. 2019;1(2):101–107. [Google Scholar]
- 3.Jain, N., Byravan, S., Stairs, J., Rennie, W.J., Moorthy, A. Rheum, Vol 60(S-1).
- 4.Ritchlin C., Adamopoulos I.E. Axial spondyloarthritis: new advances in diagnosis and management BMJ. 2021;372 doi: 10.1136/bmj.m4447. [DOI] [PubMed] [Google Scholar]
- 5.Chen M., Elawad A., Herregonds N., Jans L., Rennie W.J. Cutting edge technologies in the imaging of spondyloarthritis. Indian J Rheumatol. 2020;15(Suppl S1):27–33. [Google Scholar]
- 6.Rudwaleit M., van der Heijde D., Landewé R. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 7.Lambert R.G.W., Bakker P.A.C., van der Heijde D. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis. 2016;75:1958–1963. doi: 10.1136/annrheumdis-2015-208642. [DOI] [PubMed] [Google Scholar]
- 8.Rudwaleit M., Jurik A.G., Hermann K.A. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheumatic Dis. 2009;68:1520–1527. doi: 10.1136/ard.2009.110767. [DOI] [PubMed] [Google Scholar]
- 9.Weber U., Lambert R.G., Pedersen S.J., Hodler J., Østergaard M., Maksymowych W.P. Assessment of structural lesions in sacroiliac joints enhances diagnostic utility of magnetic resonance imaging in early spondylarthritis. Arthritis Care Res (Hoboken). 2010 Dec;62(12):1763–1771. doi: 10.1002/acr.20312. [DOI] [PubMed] [Google Scholar]
- 10.Robinson P.C., Sengupta R., Siebert S. Non-radiographic axial spondyloarthritis (nr-axSpA): advances in classification, imaging and therapy. Rheumatol Ther. 2019 Jun;6(2):165–177. doi: 10.1007/s40744-019-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omar A., Sari I., Bedaiwi M., Salonen D., Haroon N., Inman R.D. Analysis of dedicated sacroiliac views to improve reliability of conventional pelvic radiographs. Rheumatology (Oxford) 2017 Oct 1;56(10):1740–1745. doi: 10.1093/rheumatology/kex240. [DOI] [PubMed] [Google Scholar]
- 12.Battistone M.J., Manaster B.J., Reda D.J., Clegg D.O. Radiographic diagnosis of sacroiliitis--are sacroiliac views really better? J Rheumatol. 1998 Dec;25(12):2395–2401. [PubMed] [Google Scholar]
- 13.Shi Z., Han J., Qin J., Zhang Y. Clinical application of diffusion-weighted imaging and dynamic contrast-enhanced MRI in assessing the clinical curative effect of early ankylosing spondylitis. Medicine (Baltim) 2019 May;98(20) doi: 10.1097/MD.0000000000015227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Koning A., de Bruin F., van den Berg R. Low-dose CT detects more progression of bone formation in comparison to conventional radiography in patients with ankylosing spondylitis: results from the SIAS cohort. Ann Rheum Dis. 2018 Feb;77(2):293–299. doi: 10.1136/annrheumdis-2017-211989. [DOI] [PubMed] [Google Scholar]
- 15.Wu H., Zhang G., Shi L. Axial spondyloarthritis: dual-energy virtual noncalcium CT in the detection of bone marrow edema in the sacroiliac joints. Radiology. 2019 Jan;290(1):157–164. doi: 10.1148/radiol.2018181168. [DOI] [PubMed] [Google Scholar]
- 16.Carotti M., Benfaremo D., Di Carlo M. Dual-energy computed tomography for the detection of sacroiliac joints bone marrow oedema in patients with axial spondyloarthritis. Clin Exp Rheumatol. 2021 Jan 7 doi: 10.55563/clinexprheumatol/xdlfzb. Epub ahead of print, PMID: 33427625. [DOI] [PubMed] [Google Scholar]
- 17.Chen M., Herregods N., Jaremko J.L. Bone marrow edema in sacroiliitis: detection with dual-energy CT. Eur Radiol. 2020 Jun;30(6):3393–3400. doi: 10.1007/s00330-020-06670-7. [DOI] [PubMed] [Google Scholar]
- 18.Rudwaleit M., Jurik A.G., Hermann K.G. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009 Oct;68(10):1520–1527. doi: 10.1136/ard.2009.110767. [DOI] [PubMed] [Google Scholar]
- 19.Ramdin D., Moorthy A., Rennie W.J. Classification terminology and definitions in reporting of MRI in axial spondyloarthritis. J Belg Radiol. 2017;101(S2):11. doi: 10.5334/jbr-btr.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K., Park H.Y., Kim K.W. Advances in whole body MRI for musculoskeletal imaging: diffusion-weighted imaging. J Clin Orthop Trauma. 2019 Jul-Aug;10(4):680–686. doi: 10.1016/j.jcot.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H., Huang H., Zhang Y. Diffusion-weighted MRI to assess sacroiliitis: improved image quality and diagnostic performance of readout-segmented echo-planar imaging (EPI) over conventional single-shot EPI. AJR Am J Roentgenol. 2021 Jun 9:1–10. doi: 10.2214/AJR.20.23953. [DOI] [PubMed] [Google Scholar]
- 22.Lee K.H., Chung H.Y., Xu X., Lau V.W.H., Lau C.S. Apparent diffusion coefficient as an imaging biomarker for spinal disease activity in axial spondyloarthritis. Radiology. 2019 Apr;291(1):121–128. doi: 10.1148/radiol.2019180960. [DOI] [PubMed] [Google Scholar]
- 23.Lambert R.G., Maksymowych W.P. Diffusion-weighted imaging in axial spondyloarthritis: a measure of effusion or does it elicit confusion? J Rheumatol. 2018 Jun;45(6):729–730. doi: 10.3899/jrheum.171479. [DOI] [PubMed] [Google Scholar]
- 24.Hall-Craggs M.A., Bray T.J.P., Ciurtin C., Bainbridge A. Quantitative magnetic resonance imaging has potential for assessment of spondyloarthritis: arguments for its study and use. J Rheumatol. 2019 May;46(5):541–542. doi: 10.3899/jrheum.181049. [DOI] [PubMed] [Google Scholar]
- 25.Gentili F., Cantarini L., Fabbroni M. Magnetic resonance imaging of the sacroiliac joints in SpA: with or without intravenous contrast media? A preliminary report. Radiol Med. 2019 Nov;124(11):1142–1150. doi: 10.1007/s11547-019-01016-w. [DOI] [PubMed] [Google Scholar]
- 26.Abrar D.B., Schleich C., Nebelung S. Proteoglycan loss in the articular cartilage is associated with severity of joint inflammation in psoriatic arthritis-a compositional magnetic resonance imaging study. Arthritis Res Ther. 2020 May 29;22(1):124. doi: 10.1186/s13075-020-02219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son S.M., Kim K., Pak K., Kim S.J., Goh T.S., Lee J.S. Evaluation of the diagnostic performance of 18F-NaF positron emission tomography/computed tomography in patients with suspected ankylosing spondylitis according to the Assessment of SpondyloArthritis International Society criteria. Spine J. 2020 Sep;20(9):1471–1479. doi: 10.1016/j.spinee.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Kleinrensink N.J., Foppen W., Ten Katen I. Comparison of the Heel Enthesitis MRI Scoring System (HEMRIS) with clinical enthesitis and local metabolic activity on PET-CT. RMD Open. 2020 Nov;6(3) doi: 10.1136/rmdopen-2020-001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moltó A., Etcheto A., van der Heijde D. Prevalence of comorbidities and evaluation of their screening in spondyloarthritis: results of the international cross-sectional ASAS-COMOSPA study. Ann Rheum Dis. 2016 Jun;75(6):1016–1023. doi: 10.1136/annrheumdis-2015-208174. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald G., Anachebe T., McCarroll K., O'Shea F. Measuring bone density in axial spondyloarthropathy: time to turn things on their side? Int J Rheum Dis. 2020 Mar;23(3):358–366. doi: 10.1111/1756-185X.13765. [DOI] [PubMed] [Google Scholar]
- 31.Kim J.W., Chung M.K., Lee J. Low bone mineral density of vertebral lateral projections can predict spinal radiographic damage in patients with ankylosing spondylitis. Clin Rheumatol. 2019 Dec;38(12):3567–3574. doi: 10.1007/s10067-019-04743-7. [DOI] [PubMed] [Google Scholar]
- 32.Kaeley G.S., Bakewell C., Deodhar A. The importance of ultrasound in identifying and differentiating patients with early inflammatory arthritis: a narrative review. Arthritis Res Ther. 2020 Jan 2;22(1):1. doi: 10.1186/s13075-019-2050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mascarenhas S. A narrative review of the classification and use of diagnostic ultrasound for conditions of the Achilles tendon. Diagnostics (Basel) 2020 Nov 13;10(11):944. doi: 10.3390/diagnostics10110944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah N.G., Keraliya A., Nunez D.B. Injuries to the rigid spine: what the spine surgeon wants to know. Radiographics. 2019 Mar-Apr;39(2):449–466. doi: 10.1148/rg.2019180125. [DOI] [PubMed] [Google Scholar]
- 35.Caron T., Bransford R., Nguyen Q., Agel J., Chapman J., Bellabarba C. Spine fractures in patients with ankylosing spinal disorders. Spine (Phila Pa 1976) 2010 May 15;35(11):E458–E464. doi: 10.1097/BRS.0b013e3181cc764f. [DOI] [PubMed] [Google Scholar]
- 36.Chen L., Yang D., Yang T. Imaging findings of ankylosing spondylitis complicated with spinal fracture. Acta Med Mediterr. 2020 Jan 36;(5):2797–2802. [Google Scholar]
- 37.Vazan M., Ryang Y.M., Barz M., Török E., Gempt J., Meyer B. Ankylosing spinal disease-diagnosis and treatment of spine fractures. World Neurosurg. 2019 Mar;123:e162–e170. doi: 10.1016/j.wneu.2018.11.108. [DOI] [PubMed] [Google Scholar]
- 38.Shah N.G., Keraliya A., Harris M.B., Bono C.M., Khurana B. Spinal trauma in DISH and AS: is MRI essential following the detection of vertebral fractures on CT? Spine J. 2021 Apr;21(4):618–626. doi: 10.1016/j.spinee.2020.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Tavolaro C., Ghaffar S., Zhou H., Nguyen Q.T., Bellabarba C., Bransford R.J. Is routine MRI of the spine necessary in trauma patients with ankylosing spinal disorders or is a CT scan sufficient? Spine J. 2019 Aug;19(8):1331–1339. doi: 10.1016/j.spinee.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 40.von der Höh N.H., Henkelmann J., Jarvers J.S. Magnetic resonance tomography for the early detection of occult fractures of the spinal column in patients with ankylosing spondylitis. Eur Spine J. 2020 Apr;29(4):870–878. doi: 10.1007/s00586-020-06309-7. [DOI] [PubMed] [Google Scholar]
- 41.Bhatt C., Kumar I., Vijayakumar V. Multimedia Systems; 2020. The State of the Art of Deep Learning Models in Medical Science and Their Challenges. [Google Scholar]
- 42.Castro-Zunti R., Park E.H., Choi Y., Jin G.Y., Ko S.B. Early detection of ankylosing spondylitis using texture features and statistical machine learning, and deep learning, with some patient age analysis. Comput Med Imag Graph. 2020 Jun;82:101718. doi: 10.1016/j.compmedimag.2020.101718. [DOI] [PubMed] [Google Scholar]
- 43.Joo Y.B., Baek I.W., Park Y.J., Park K.S., Kim K.J. Machine learning-based prediction of radiographic progression in patients with axial spondyloarthritis. Clin Rheumatol. 2020 Apr;39(4):983–991. doi: 10.1007/s10067-019-04803-y. [DOI] [PubMed] [Google Scholar]