Abstract
Purpose:
To assess the prevalence of retinopathy and its association with systemic morbidity and laboratory indices of coagulation and inflammatory dysfunction in severe COVID-19.
Design:
Retrospective, observational cohort study.
Methods:
Adult patients hospitalized with severe COVID-19 who underwent ophthalmic examination from April to July 2020 were reviewed. Retinopathy was defined as one of the following: 1) Retinal hemorrhage; 2) Cotton wool spots; 3) Retinal vascular occlusion. We analyzed medical comorbidities, sequential organ failure assessment (SOFA) scores, clinical outcomes and laboratory values for their association with retinopathy.
Results:
Thirty-seven patients with severe COVID-19 were reviewed, the majority of whom were female (n=23, 62%), Black (n=26, 69%), and admitted to the intensive care unit (n=35, 95%). Fourteen patients had retinopathy (38%) with retinal hemorrhage in 7 (19%), cotton wool spots in 8 (22%), and a branch retinal artery occlusion in 1 (3%) patient. Patients with retinopathy had higher SOFA scores than those without retinopathy (8.0 vs. 5.3, p=0.03), higher rates of respiratory failure requiring invasive mechanical ventilation and shock requiring vasopressors (p<0.01). Peak D-dimer levels were 28,971 ng/mL in patients with retinopathy compared to 12,575 ng/mL in those without retinopathy (p=0.03). Peak CRP was higher in patients with cotton wool spots versus those without cotton wool spots (354 mg/dL vs. 268 mg/dL, p=0.03). Multivariate logistic regression modeling showed an increased risk of retinopathy with higher peak D-dimers (aOR 1.32, 95% CI 1.01–1.73, p=0.04) and male sex (aOR 9.6, 95% CI 1.2–75.5, p=0.04).
Conclusion:
Retinopathy in severe COVID-19 was associated with greater systemic disease morbidity involving multiple organs. Given its association with coagulopathy and inflammation, retinopathy may offer insight into disease pathogenesis in patients with severe COVID-19.
Introduction
Coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has escalated from an emerging infectious disease to a global pandemic resulting in over 176 million cases and 3.8 million deaths worldwide.1 Following SARS-CoV-2 infection, patients may remain entirely asymptomatic or develop clinical disease ranging from mild flu-like symptoms to severe, life-threatening acute respiratory failure with multisystem organ failure.2 Multi-organ involvement stems from a variety of disease factors including direct viral effects, exuberant host immune response, and aberrant coagulation pathway activation, which result in pulmonary, cardiac, neurologic, renal, and hematologic pathology.3
Reports of ophthalmic manifestations in COVID-19 have varied widely with prevalence estimated between 1–32%.4,5 Ocular symptoms described have included epiphora, eye redness, ocular pain, and blurred vision.6,7 While conjunctivitis and viable SARS-CoV-2 in tear film have raised concerns about transmission,4,8–10 there have been few descriptions of retinal manifestations. Reports of retinal disease in the context of COVID-19 can vary from asymptomatic or mildly symptomatic retinopathy, including cotton wool spots and retinal hemorrhage, to more severe, vision-threatening complications including central retinal vein and artery occlusion.11–19 Proposed mechanisms of retinal microvascular findings in COVID-19 include hypoxia, inflammation and microthrombi, but the precise pathogenesis, risks factors for their development, and relationship of retinopathy with systemic morbidity in COVID-19 are unknown.20,21
Retinal microangiopathy has been correlated with clinical severity in diseases including diabetes, hypertension and human immunodeficiency virus (HIV).22 Acute ischemic changes from hypertensive urgency may lead to retinal hemorrhage and cotton wool spots23, while infectious and inflammatory sequelae of HIV can give rise to acute cotton wool spots.24 Although the initial insult may differ by underlying condition, the presence of retinopathy reflects a pathologic microvasculature injury, which might otherwise go undetected. An improved understanding of patients with retinopathy may provide unique insight into the pathogenic mechanisms that underlie COVID-19-associated microvascular disease. Herein, we report ophthalmic findings in a series of patients hospitalized with COVID-19 and report the association between retinopathy and severity of illness, coagulopathy, and systemic inflammation.
Methods
We conducted a retrospective study of patients with COVID-19 admitted between April 8th, 2020 and July 1st, 2020 to two hospitals in the Emory Healthcare System who underwent ophthalmic evaluation as part of their clinical work-up and care during the early months of the COVID-19 pandemic. This study was approved by the Institutional Review Board of Emory University and adhered to the Declaration of Helsinki and the Health Insurance Portability and Accountability Act of 1996.
Demographic, clinical and laboratory data
Clinical and laboratory information were collected from the electronic medical record for patients who underwent ophthalmic evaluation, which were classified by the following indications: 1) Retinopathy or retinal vascular assessment, often in the setting of coagulopathy or other hematologic abnormality; 2) Optic disc edema evaluation; 3) Acute ophthalmic symptoms or signs (e.g. eye pain, redness); and 4) Evaluation for chorioretinitis or endophthalmitis in the setting of fungemia or bacteremia. Demographic variables collected included the patient’s sex, age, and ethnicity at diagnosis. Baseline comorbidities including diabetes, hypertension, body mass index (BMI), hyperlipidemia, and systemic conditions or medications leading to an immunosuppressed status (i.e. HIV, history of transplantation, immune modulating medications) were reviewed.
Systemic disease complications and interventions during hospitalization were collected. These data included hypotension (mean arterial pressure < 65 mmHg) requiring any vasopressor use, cardiac disease, respiratory failure requiring mechanical ventilation, extracorporeal membrane oxygenation (ECMO) requirement, acute kidney injury (AKI) with or without need for renal replacement therapy (RRT), use of inhaled vasodilators, liver failure, and large vessel thromboses (i.e., deep venous thromboses [DVT], pulmonary embolus [PE]). Anticoagulation and antiplatelet therapies were also recorded (e.g., aspirin, low-molecular weight or unfractionated heparin, bivalirudin, argatroban). Clinical information collected also included date of COVID-19 symptom onset, length of hospital admission, length of intensive care unit (ICU) admission and mortality.
Laboratory data collected included values within 48 hours of ophthalmic examination and peak or nadir values during the patient’s hospitalization. These parameters included D-dimer, interleukin-6 (IL-6), fibrinogen, C-reactive protein (CRP), viscosity, hematocrit, platelets, white blood cell (WBC) count, and lymphocytes. Sequential organ failure assessment (SOFA) scores were calculated at the time of ICU admission and within 48 hours of the ophthalmic exam.
Ophthalmic examination data and portable fundus photography
Anterior segment examination was performed with a penlight or illumination from an indirect ophthalmoscope. A dilated funduscopic examination was performed by an ophthalmology resident or fellow with an attending uveitis and retinal physician. Fundus photography was performed to document optic nerve, macula, and peripheral retinal pathology with a portable fundus camera (Versacam DS-20, Nidek, Inc, San Jose, CA) when clinically indicated.
Retinopathy Case Definition
Retinopathy was defined as any of the following retinal findings in one or both eyes (without previous documentation in the medical record): 1) retinal hemorrhage (i.e. subretinal or intraretinal); 2) cotton-wool spots; 3) retinal arterial or venous occlusive disease. Retinal vascular tortuosity, arteriovenous sheathing, and vascular attenuation were also documented but not considered as acute retinopathy, given their potential to occur as a chronic disease finding.
Additional stratification of patients according to the presence of cotton wool spots or retinal hemorrhage was performed for laboratory parameters that could be mechanistically linked to retinal disease.
Statistical analysis
SAS software v9.4 (Cary, North Carolina) was used for descriptive and inferential statistical analysis. Demographic and treatment information were summarized as frequencies/percentages or means with standard deviations as appropriate.
Bivariate analyses (Chi-square tests or t-tests) were performed to determine clinical and laboratory parameters that were associated with patients having any retinopathy. Further, given the pathophysiologic differences between cotton wool spots and retinal hemorrhage, we stratified our analysis of inflammatory and coagulation markers by these distinct forms of retinopathy. Laboratory values assessed in relationship to the presence of cotton wool spots and retinal hemorrhage included D-dimer, fibrinogen, platelets, hematocrit, CRP, and IL-6.
A multivariate logistic regression model was constructed to examine the relationship between retinopathy and clinical and laboratory parameters of interest. Variables were included in the multivariate models on the basis of purposeful selection of clinical covariates, bivariate association, and directed acyclic graphs. These included peak D-dimer, peak CRP, SOFA scores within 24–48 hours of ophthalmic examination, and sex. Unadjusted and adjusted odds ratios with Wald 95% confidence intervals were calculated. A two-sided p-value < 0.05 was considered statistically significant for all analyses.
Results
Patient Characteristics
Thirty-seven COVID-19 patients were examined during their acute hospitalization. Twenty-three patients were female (62%) and mean age was 54 years (standard deviation [SD] 15.6, Table 1). Black race was most common (26 patients, 69%) followed by white (5, 14%). Ophthalmic evaluations were obtained for retinopathy evaluation in 12 (32%), optic disc edema assessment in 9 (24%), ocular symptoms and signs in 9 patients (24%) and fungemia or bacteremia in 7 (19%) of 37 patients. Common baseline comorbidities included hypertension (78%), diabetes mellitus (30%), and asthma (14%). Five (14%) patients were immunosuppressed including two patients with a history of solid organ transplantation.
Table 1:
Demographic and Baseline Characteristics
| Patient Characteristics | All (N=37 patients) |
Retinopathy (n=14 patients) |
No Retinopathy (n=23 patients) |
p |
|---|---|---|---|---|
| Age (Years) | 54.0 (15.6) | 50.1 (17.1) | 56.4 (14.4) | 0.24 |
| Sex | ||||
| Female | 23 (62%) | 5 (36%) | 18 (78%) | <0.01 |
| Male | 14 (39%) | 9 (64%) | 5 (22%) | |
| Race | 0.721 | |||
| White | 5 (14%) | 1 (7%) | 4 (17%) | |
| Black | 26 (69%) | 10 (71%) | 16 (69%) | |
| Asian | 2 (5%) | 1 (7%) | 1 (4%) | |
| Unknown / Other | 4 (11%) | 2 (14%) | 0 (0%) | |
| Ethnicity | ||||
| Hispanic | 3 (8%) | 1 (7%) | 2 (9%) | 0.87 |
| Non-Hispanic | 34 (92%) | 13 (93%) | 21 (91%) | |
| BMI | 34.6 (12.2) | 36.9 (15.4) | 33.2 (10.0) | 0.45 |
| Days of COVID-19 symptoms at eye exam 2 | 14.1 (11.3), (n=31) | 17.6 (10.6), (n=13) | 11.7 (11.4), (n=18) | 0.15 |
| Days of hospitalization at eye exam | 11.5 (10.4), (n=31) | 14.4 (9.1), (n=13) | 9.5 (10.9), (n=18) | 0.20 |
| Baseline comorbidities | ||||
| Hypertension | 29 (78%) | 10 (71%) | 19 (83%) | 0.44 |
| Diabetes | 11 (30%) | 3 (21%) | 8 (35%) | 0.48 |
| Coronary artery disease | 2 (5%) | 0 (0%) | 2 (9%) | 0.52 |
| Asthma | 5 (14%) | 3 (21%) | 2 (9%) | 0.34 |
| COPD | 3 (8%) | 1 (7%) | 2 (9%) | 1.00 |
| Chronic Kidney Disease | 3 (8%) | 0 (0%) | 3 (13%) | 0.27 |
| HIV | 0 (0%) | 0 (0%) | 0 (0%) | n/a |
| Other immunosuppression | 5 (14%) | 0 (0%) | 5 (22%) | 0.13 |
Abbreviations BMI Body mass index, COPD Chronic obstructive pulmonary disease, HIV Human immunodeficiency virus, ACE Angiotensin converting enzyme, COVID Coronavirus disease
Comparison of proportion of black v. non-black for presence or absence of retinopathy
Days of COVID illness based on patient self-reported symptoms by history and progress notes
Ocular Manifestations
Ophthalmic exams were performed at a mean of 11.5 days after hospital admission and 14.1 days after the onset of COVID-19 symptoms. Anterior segment findings included conjunctival injection in 8 patients (22%) and conjunctival chemosis in 4 patients (11%). Subconjunctival hemorrhage was observed in one patient (Table 2).
Table 2.
Ophthalmic findings: Anterior segment disease, retinopathy, and retinal vascular findings
| Ocular Finding | Patients (% of total, n = 37) |
|---|---|
| Anterior segment | |
| Conjunctival injection | 8 (22) |
| Conjunctival chemosis | 4 (11) |
| Subconjunctival hemorrhage | 1 (2.7) |
| Cataract | 4 (11) |
| Retinopathy | |
| Retinal hemorrhage | 7 (19) |
| Cotton-wool spot | 8 (22) |
| Branch retinal artery occlusion | 1 (2.7) |
| Other retinal vascular changes | |
| Vascular tortuosity | 3 (8.1) |
| Arteriovenous crossing changes | 5 (14) |
| Vascular attenuation | 8 (22) |
Footnote for Table: We assessed patients with anterior segment findings (conjunctival injection, chemosis, hemorrhage, and cataract) with respect to retinopathy with pairwise analyses and found no statistically significant difference between any of the anterior segment findings and observation of retinopathy (P>0.05 for all analyses). Unilateral findings (n, % of 37) included patients with conjunctival injection (2, 5%), subconjunctival hemorrhage (1, 3%), retinal hemorrhage (5, 14%), cotton wool spots (5, 14%), branch retinal artery occlusion (1, 3%), and arteriovenous crossing changes (1, 3%).
Retinopathy was diagnosed in 14 of 37 patients (38%), with bilateral disease observed in 6 of 37 patients (16%). Retinal hemorrhages and cotton wool spots were noted in 7 (19%) and 8 (22%) of 37 patients respectively (Figure 1). One patient (2.7%) had a branch retinal artery occlusion (Table 2). Two patients met more than one criterion for retinopathy.
Figure 1.
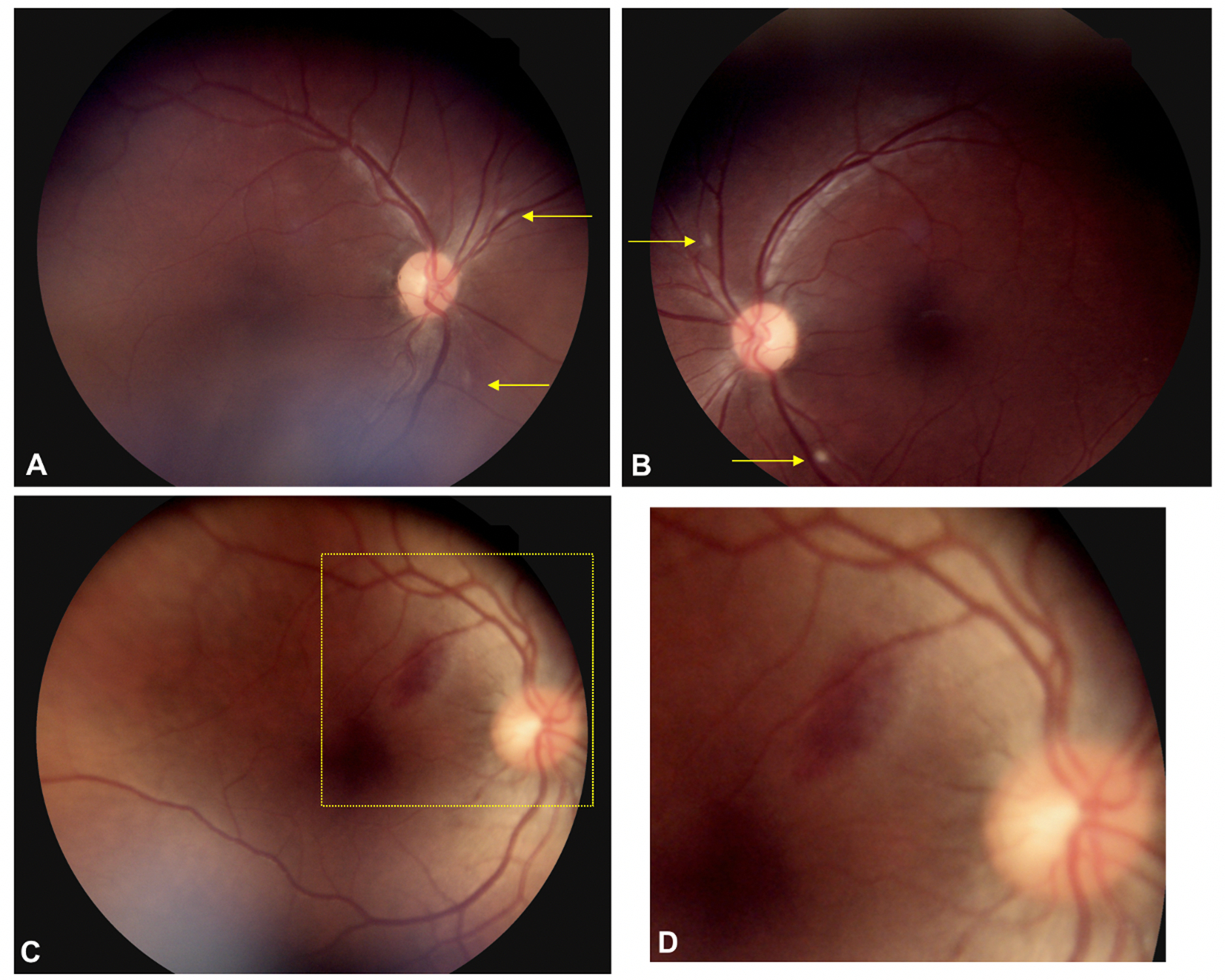
Fundus photos of COVID-19 patients. Fundus photos show the right and left eyes of a COVID-19 patient with acute hypoxic respiratory failure (A, B). There are focal cotton-wool spots in the peripapillary region surrounding the nerve (yellow arrows) of both eyes. Another COVID-19 patient with acute hypoxic respiratory failure shows an oval subretinal hemorrhage temporal to the optic nerve (C). A higher magnification view of the inset (C) shows the retinal vessel overlying the subretinal hemorrhage approximately 750 microns from the fovea (D).
Retinal vascular tortuosity was noted in 3 patients (8.1%) all of whom showed bilateral involvement. Arteriovenous crossing changes were observed in 5 patients (14%) and vascular attenuation was documented in 8 patients (22%).
Retinopathy was more prevalent in males (9 of 14, 64%) than females (5 of 23, 22%) (p=0.01). The prevalence of baseline comorbidities was similar in patients with and without retinopathy (Table 1). There was also no difference in the prevalence of anterior segment findings when comparing eyes with and without retinopathy (data not shown).
Major Clinical Outcomes, Interventions, and Retinopathy
Thirty-five patients (95%) were admitted to the ICU. The total hospital length of stay was approximately 8.5 days greater on average for patients with retinopathy as compared to those without retinopathy but was not statistically significant (34.5 ± 19.4 vs. 26.0 days ± 15.8, p=0.15). Patients with retinopathy also had an ICU length of stay that was 7.7 days longer than those without retinopathy (27.6 ± 18.7 vs 19.9 ± 15.4, p=0.19). The most common ICU interventions included mechanical ventilation (29 patients, 78%), vasopressor administration (26 patients, 70%), anticoagulation (33 patients, 89%) and corticosteroid administration (28 patients, 76%). All 14 patients with retinopathy required mechanical ventilation for respiratory failure, while 15 of 23 patients (65%) without retinopathy required mechanical ventilation (p=0.01). Furthermore, all 14 patients with retinopathy required vasopressors for hypotension while 12 of 23 (52%) patients without retinopathy required vasopressors (p=0.002). ECMO was required in 7 patients, 6 of whom had retinopathy (43% vs 4%, p<0.01, Table 3).
Table 3:
Systemic complications, treatment, and laboratory findings in COVID-19 patients*
| Patient Characteristics | All (n=37 patients) |
Retinopathy (n=14 patients) |
No Retinopathy (n=23 patients) |
P-value |
|---|---|---|---|---|
| SOFA Score | ||||
| Admission | 6.29 (3.83) | 6.57 (4.10) | 6.13 (3.74) | 0.74 |
| 24–48 hours of exam | 6.36 (3.61) | 8.00 (2.42) | 5.32 (3.89) | 0.03 |
| Change | 0.08 (4.66) | 1.43 (4.60) | −0.77 (4.59) | 0.17 |
| Laboratory Values | ||||
| D-dimer, ng/mL | 5634.3 (8512.9) | 9098.8 (10945.6) | 3224.7 (5596.7) | 0.08 |
| Peak D-dimer, ng/mL | 18946.5 (21678.6) | 28971.3 (16176.0) | 12575.0 (4452.4) | 0.03 |
| Fibrinogen, ng/mL | 550.2 (226.89) | 556.2 (237.4) | 545.3 (225.7) | 0.90 |
| Peak fibrinogen, ng/mL | 735.3 (197.5) | 773.4 (165.0) | 709.9 (216.7) | 0.36 |
| CRP, mg/dL | 143.7 (107.3) | 178.4 (135.3) | 120.6 (79.1) | 0.16 |
| Peak CRP, mg/dL | 289.5 (97.1) | 311.8 (100.8) | 275.3 (94.1) | 0.28 |
| WBC, 103 cells/mm3 | 12 (5.7) | 14.0 (6.40) | 11.3 (5.14) | 0.17 |
| Lymphocytes, 103 cells/mm3 | 11.6 (8.0) | 10.6 (7.91) | 12.3 (8.19) | 0.62 |
| Hematocrit (%) | 34.1 (6.8) | 31.7 (5.69) | 35.8 (7.19) | 0.09 |
| Platelets, 103 cells/mm3 | 284.4 (134.3) | 237.5 (101.5) | 318.3 (147.1) | 0.08 |
| Peak IL-6, pg/mL | 58.4 (112.1) | 102.5 (164.5) | 29.0 (46.2) | 0.25 |
| Viscosity, centipoise | 2.1 (0.5) | 2.1 (0.3) | 2.1 (0.7) | 0.93 |
| Interventions | ||||
| Mechanical ventilation | 29 (78%) | 14 (100%) | 14 (63%) | 0.01 |
| Vasopressors | 26 (70%) | 14 (100%) | 11 (50%) | <0.01 |
| RRT/Hemodialysis | 7 (19%) | 4 (29%) | 3 (13%) | 0.39 |
| ECMO | 7 (19%) | 6 (43%) | 1 (4%) | <0.01 |
| Anticoagulation therapy† | 33 (89%) | 13 (93%) | 20 (87%) | 1.00 |
| Corticosteroid | 28 (76%) | 11 (79%) | 17 (77%) | 1.00 |
| Complications | ||||
| Pulmonary embolism | 1 (3%) | 1 (7%) | 0 (0%) | 0.39 |
| Deep vein thrombosis | 2 (5%) | 1 (7%) | 1 (4%) | 1.00 |
| Seizure | 4 (11%) | 1 (7%) | 3 (13%) | 1.00 |
| Stroke | 2 (5%) | 1 (7%) | 1 (4%) | 1.00 |
| Outcomes | ||||
| Death | 11 (29%) | 4 (29%) | 7 (30%) | 1.00 |
| Hospital days | 29.0 (17.3) | 34.5 (19.4) | 26.0 (15.8) | 0.15 |
| ICU | 35 (95%) | 14 (100%) | 21 (91%) | 0.52 |
| ICU days | 22.6 (16.9) | 27.6 (18.7) | 19.9 (15.4) | 0.19 |
Means (Standard deviations) reported for laboratory values, SOFA scores, interventions, and outcomes.
Abbreviations SOFA Sequential Organ Failure Assessment score, SD Standard deviation, CRP C-reactive protein, WBC White blood cell count, RRT Renal replacement therapy, ECMO Extracorporeal membrane oxygenation, ACTT Adaptive COVID-19 Treatment Trial, ICU Intensive care unit
Missing data for the following variables: 24–48 hour SOFA score (1, 3%), Change in SOFA score (1, 3%), D-dimer (2, 5%), fibrinogen (8, 22%), CRP (2, 5%), Viscosity (15, 41%), Platelets (6, 16%), Hematocrit (6, 16%), WBC (2, 5%), Lymphocytes (11, 30%), Peak IL-6 (17, 46%), ICU days (2, 5%)
Patients (n) received the following anticoagulation medications: Heparin (11), enoxaparin (10), argatroban (6), apixaban (3), aspirin (2), and bivalirudin drip (1).
Systemic complications observed in the entire cohort during hospitalization included seizure (n=4), stroke (n=2), deep vein thrombosis (n=1), and pulmonary embolism (n=1, Table 3). There were no significant differences observed in systemic complications that developed during hospitalization between patients with and without retinopathy. Eleven patients (29%) died, and mortality did not differ according to the presence of retinopathy (p=1.00).
SOFA Score, Laboratory Investigations and Retinopathy
The mean SOFA score at ICU admission was 6.3 and did not significantly differ according to the presence or absence of retinopathy (6.6 vs 6.1, p=0.74, Table 3). However, the mean SOFA scores within 24–48 hours of the ophthalmic examination were significantly higher in patients with retinopathy at 8.0 compared to those without retinopathy at 5.3 (p=0.03, Figure 2).
Figure 2.
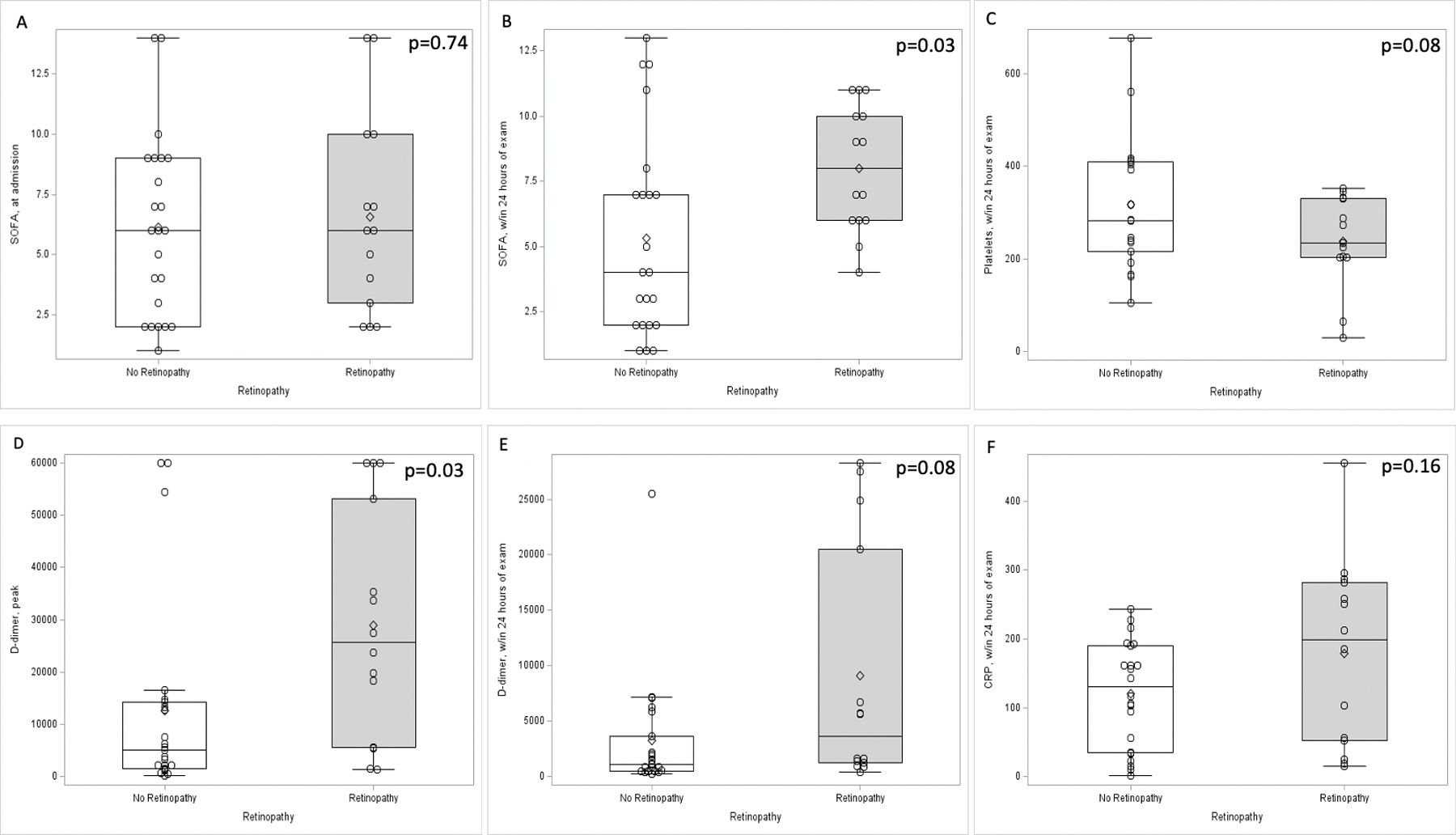
Relationship of retinopathy with mean clinical and laboratory indices. Box-and-whisker plots show that admission SOFA scores were similar between patients with and without retinopathy (p=0.74, A), but were greater for patients with retinopathy than those without within 24–48 hours of the ophthalmic exam (SOFA score 8.0 for retinopathy group compared to 5.3 for no retinopathy group, p=0.03, B). Platelets were lower in the retinopathy group compared to the no retinopathy group (p=0.08, C). D-dimers within 24 hours of ophthalmic examination were greater in the retinopathy group (9099 ng/mL) compared to the no retinopathy group (3225 ng/mL, p=0.08, D). The peak D-dimer during patient hospitalization was also greater in the retinopathy group (28971 ng/mL) compared to the no retinopathy group (12575 ng/mL, p=0.03, E). C-reactive protein also was greater in the retinopathy group (p=0.16, F).
Mean D-dimer at the time of ophthalmic exam was 5,634 ng/mL and the mean peak D-dimer throughout the hospital stay was 18,947 ng/mL for all patients. Patients with retinopathy had a significantly higher peak D-dimer than those without retinopathy (28,971 ng/mL vs 12,575 ng/mL, p=0.03, Figure 2). Mean fibrinogen within 48 hours of ophthalmic exam was elevated for all patients at 550 mg/dL, but there was no difference observed for those with retinopathy.
Peak CRP during hospitalization was also markedly elevated in the cohort, with an average of 290 mg/dL. While peak CRP values were higher in patients with retinopathy, this difference was not statistically significant (312 mg/dL vs 275 mg/dL, p=0.27).
Laboratory Values Associated with Cotton Wool Spots and Retinal Hemorrhage
In patients with cotton wool spots, both the CRP within 24 hours of ophthalmic exam and the peak CRP were significantly higher as compared to patients without cotton wool spots (within 24 hours 209.2 vs. 123.6 mg/dL, p=0.04; peak 353.7 vs. 271.2 mg/dL, p=0.02, Supplemental Table 1, Supplemental Figure 1). While patients with cotton wool spots trended towards a higher peak D-dimer and D-dimer within 48 hours of ophthalmic exam, neither was significantly different.
Conversely, peak D-dimer was higher in patients with retinal hemorrhage at 33,799 compared to 15,263 mg/mL in individuals without retinopathy (p=0.045). CRP trended towards higher levels in individuals with retinal hemorrhage with a mean of 228.0 mg/dL compared to 122.0 mg/dL in patients without hemorrhage, but this was not statistically significant (p=0.10). (Supplemental Table 2, Supplemental Figure 2).
Additional laboratory findings including white blood count, hematocrit, platelets, peak IL-6, and plasma viscosity are summarized in Table 3.
Multivariate analysis
In logistic regression analysis, every 5000-unit increase in peak D-dimer was associated with a 28% increase in the odds of retinopathy in unadjusted models (OR 1.28, 95% CI 1.05–1.57, p=0.02, Supplemental Table 3) and 32% in adjusted multivariate models (aOR 1.32, 95% CI 1.01–1.73, p=0.04). Male sex showed significantly increased odds of retinopathy in both the unadjusted (OR 8.1, 95% CI 1.7–37.8, p<0.01) and adjusted models (aOR 9.6, 95% CI 1.2–75.5, p=0.03). While unadjusted models showed that the SOFA score within 24–48 hours of the ophthalmic examination was associated with increased odds of retinopathy (OR 1.25, 95% CI 1.0–1.6, p=0.048), a significant relationship was not observed in the adjusted multivariate model (aOR 1.12, 95% CI 0.86–1.45, p=0.40). Peak CRP was not significantly associated with increased odds of retinopathy in either unadjusted (OR 1.29, 95% CI 0.90–1.86, p=0.17) or adjusted models (aOR 0.70, 95% CI 0.39–1.28, p=0.25).
Discussion
In this sample of 37 hospitalized COVID-19 patients, the majority of whom were critically ill, we observed retinopathy consisting of retinal hemorrhage, cotton wool spots or branch retinal artery occlusion in nearly 40% of patients. This markedly elevated prevalence of retinopathy is the highest yet reported in the literature for COVID-19 and was associated with greater disease severity, as reflected by higher SOFA scores at the time of ophthalmic examination, and the need for mechanical ventilation, ECMO, and vasopressor support. Moreover, the presence of retinopathy did not differ according to baseline comorbidities. Laboratory markers of inflammation and coagulopathy, including D-dimer and CRP, were also significantly associated with the presence of retinopathy. These findings highlight the potential for retinopathy to provide insight into disease pathogenesis and serve as a marker of clinical severity in COVID-19.
In comparison to our finding of retinopathy in nearly 40% of COVID-19 patients, published case series have reported rates ranging from 12 to 33%.12,20,21 In one early series of 12 patients who recovered from COVID-19, 4 patients showed cotton-wool spots.12 In another series of 25 severe or critically ill COVID-19 patients, 3 (12%) showed retinal changes including retinal hemorrhage and nerve fiber layer infarcts within the papillomacular bundle. One patient with retinopathy was critically ill with multiorgan involvement requiring mechanical ventilation and multiple supportive measures, while another showed particularly elevated D-dimers.21 Another recent report from Spain showed a 22% prevalence of retinal findings among 27 patients recently discharged from the hospital.20 While their study evaluated demographic, clinical and laboratory parameters, in contrast to our cohort, no clear differences were observed in individuals in whom retinopathy or cotton wool spots were present or absent. The higher prevalence of these ocular findings in our cohort — and their association with both clinical and laboratory markers of disease severity — strengthens the argument that retinopathy is a common and acute phenomenon in patients with severe COVID-19.
While there is limited insight into the mechanisms underlying retinopathy in COVID-19, one recent study of recovered COVID-19 patients showed a reduction in the retinal capillary density of the foveal region of the retina compared to healthy normal controls, suggesting a prior ischemic or inflammatory insult.26 In the context of recent COVID-19, these findings could represent sequelae from transient, undetected retinopathy during the acute phase of COVID-19. Similarly, cotton wool spots may arise from a variety of factors including ischemia due to hypoperfusion or thrombotic occlusion.27 Complement-mediated mechanisms may also contribute to cotton wool spot formation.28 While complement levels were not routinely measured in this hospitalized cohort, prominent complement activation has been implicated in severe COVID-19,29 as has cross-talk between complement, inflammation and coagulation pathways.29 Thus, the high rate of retinopathy in our patients may be related to their markedly elevated CRP and D-dimer levels, with resultant endothelial damage and microthrombi.
In one prior series of patients on ECMO who underwent ophthalmic examination, 11 (55%) of 20 patients showed acute retinal pathology including Purtscher-like retinopathy, intraocular hemorrhage, and septic chorioretinitis. We similarly observed a high rate of retinopathy in patients on ECMO although a minority of patients were on ECMO in our series.30 The combination of tissue hypoperfusion due to hypotension and hypoxic respiratory failure may also contribute to the retinopathy observed. A recent review of ocular complications associated with prone positioning also described orbital compartment syndrome and vascular occlusion as potential associated ocular complications.31
Branch retinal artery occlusion was observed in one patient from this series. Albeit a rare finding, arterial occlusive events can lead to severe vision impairment or blindness. While several recent case reports have identified temporal associations between retinal artery and venous occlusion with COVID-19,15–18 further studies are needed to define the precise mechanisms and risk factors for these potentially vision-compromising complications. Anterior segment findings were also observed in our cohort, although less commonly than retinopathy, with conjunctival injection and chemosis in 10–20% of patients. However, given the frequency of prone positioning and sedation with mechanical ventilation, these findings may be attributable to the ICU environment and care rather than direct effects of COVID-19.
Limitations of the study include the small sample size, retrospective design and the variable timing of ophthalmic exams. There is the potential for selection bias for patients with higher level of medical comorbidities that could lead to a higher rate of retinopathy observed. In addition, the assessment of retinopathy was unmasked and we acknowledge the potential for ascertainment bias. However, while the presence of prior retinopathy (i.e. due to diabetes or hypertension) was not documented in the medical records we reviewed, we observed that the prevalence of retinopathy observed did not differ significantly in patients with and without underlying comorbidities. Further prospective studies are needed to assess whether retinopathy increases with more severe COVID-19 compared to milder disease, as well as retinopathy in COVID-19 compared to other respiratory diseases requiring ICU care.
Nonetheless, in our evaluation of hospitalized, critically ill patients, the 38% prevalence of retinopathy that we observed exceeds previous reports of ophthalmic complications for patients with COVID-19. While retinopathy was not associated with higher mortality in this small sample, patients in whom retinopathy was observed also had received more ICU level interventions including mechanical ventilation for respiratory failure and vasopressor support for hypotension than individuals without clinically detectable retinopathy. In addition, retinopathy was significantly associated with elevated inflammatory and coagulation parameters. Although the findings from this study do not warrant change in the clinical management of patients with COVID-19, they do indicate that retinopathy may reflect a distinct clinical and immunologic state associated with increasing COVID-19 severity. Additional studies are needed to determine the immunopathogenesis of retinopathy in COVID-19 and whether patients with retinopathy may benefit from therapies targeting microvascular injury or microthromboses.
Supplementary Material
Supplemental Table 1. Relationship of cotton wool spots with laboratory values by patient
Supplemental Figure 2. Relationship of retinal hemorrhage with selected laboratory values. The mean peak D-dimer was greater for patients with retinal hemorrhage (33799 ng/mL) compared to those without retinal hemorrhage (15263 ng/mL, p=0.05, A). C-reactive protein within 24 hours was also greater for patients with retinal hemorrhage (228 mg/dL) compared to those without retinal hemorrhage (122 mg/dL, p=0.10, B).
Supplemental Figure 1. Relationship of cotton wool spots with selected laboratory values. Box-and-whisker plot shows that mean C-reactive protein within 24 hours of the ophthalmic exam was higher for patients with cotton wool spots (209 mg/dL) compared to those without cotton wool spots (124 mg/dL, p=0.04, A). In addition, peak C-reactive protein was higher for patients with cotton wool spots (354 mg/dL) compared to those without cotton wool spots (268 mg/dL, p=0.04, B).
Supplemental Table 2. Relationship of retinal hemorrhage with laboratory values by patient
Supplemental Table 3. Multivariate analysis for retinopathy
Funding:
This project was supported by unrestricted departmental grant from Research to Prevent Blindness, Inc. to the Emory Eye Center, Emory University School of Medicine, National Eye Institute/ National Institutes of Health core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine), National Eye Institute of the National Institutes of Health under award number K23 EY030158 (JGS) and R01 EY029594 (SY). This work is also supported by the NIH/NIAID K23 AI134182 (SCA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Funding support is also provided by the Woodruff Health Sciences Center Synergy Grant Award program, the Macula Society Retina Research Foundation Cox Family Grant, the Association for Research in Vision and Ophthalmology Mallinckrodt Foundation Young Investigator Award, the Sitaraman Family Foundation and the Stanley M. Truhlsen Family Foundation, Inc.
Footnotes
These data were presented, in part at the Association for Research in Vision and Ophthalmology Annual Meeting 2021 (Virtual).
References
- 1.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. June 16, 2021. at https://coronavirus.jhu.edu/map.html.)
- 2.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020;383:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perico L, Benigni A, Casiraghi F, et al. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol 2021;17:46–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol 2020; 92:589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangaputra SS, Patel SN. Ocular Symptoms among Nonhospitalized Patients Who Underwent COVID-19 Testing. Ophthalmology 2020;127:1425–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma N, Li P, Wang X, et al. Ocular Manifestations and Clinical Characteristics of Children With Laboratory-Confirmed COVID-19 in Wuhan, China. JAMA Ophthalmol 2020; 138:1079–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheema M, Aghazadeh H, Nazarali S, et al. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Can J Ophthalmol 2020; 55:e125–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling XC, Kang EY, Lin JY, et al. Ocular manifestation, comorbidities, and detection of severe acute respiratory syndrome-coronavirus 2 from conjunctiva in coronavirus disease 2019: A systematic review and meta-analysis. Taiwan J Ophthalmol 2020;10:153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khavandi S, Tabibzadeh E, Naderan M, Shoar S. Coronavirus disease-19 (COVID-19) presenting as conjunctivitis: atypically high-risk during a pandemic. Cont Lens Anterior Eye 2020; 43:211–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walinjkar JA, Makhija SC, Sharma HR, et al. Central retinal vein occlusion with COVID-19 infection as the presumptive etiology. Indian J Ophthalmol 2020;68:2572–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marinho PM, Marcos AAA, Romano AC, et al. Retinal findings in patients with COVID-19. Lancet 2020;395:1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Lopez JJ, Felix Espinar B, Ye-Zhu C. Symptomatic Retinal Microangiopathy in a Patient with Coronavirus Disease 2019 (COVID-19): Single Case Report. Ocul Immunol Inflamm 2020:1–3. [Online ahead of print] [DOI] [PubMed]
- 14.Invernizzi A, Torre A, Parrulli S, et al. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine 2020;27:100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acharya S, Diamond M, Anwar S, et al. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases 2020;21:e00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaba WH, Ahmed D, Al Nuaimi RK, et al. Bilateral Central Retinal Vein Occlusion in a 40-Year-Old Man with Severe Coronavirus Disease 2019 (COVID-19) Pneumonia. Am J Case Rep 2020;21:e927691. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yahalomi T, Pikkel J, Arnon R, Pessach Y. Central retinal vein occlusion in a young healthy COVID-19 patient: A case report. Am J Ophthalmol Case Rep. 2020. December; 20:100992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finn AP, Khurana RN, Chang LK. Hemi-retinal vein occlusion in a young patient with COVID-19. Am J Ophthalmol Case Rep. 2021. June;22:101046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Aloisio R, Nasillo V, Gironi M, Mastropasqua R. Bilateral macular hemorrhage in a patient with COVID-19. Am J Ophthalmol Case Rep. 2020. December; 20:100958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landecho MF, Yuste JR, Gándara E, et al. COVID-19 retinal microangiopathy as an in vivo biomarker of systemic vascular disease? J Intern Med 2021;289:116–20. [DOI] [PubMed] [Google Scholar]
- 21.Lani-Louzada R, Ramos C, Cordeiro RM, Sadun AA. Retinal changes in COVID-19 hospitalized cases. PLoS One 2020;15:e0243346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaworski C. Morphology of the HIV versus the diabetic cotton wool spot. Optom Vis Sci 2000;77:600–4. [DOI] [PubMed] [Google Scholar]
- 23.Walsh JB. Hypertensive retinopathy. Description, classificaton and prognosis. Ophthalmology 1982;89:1127–31. [PubMed] [Google Scholar]
- 24.Kim A, Dadgostar H, Holland GN, et al. Hemorheologic abnormalities associated with HIV infection: altered erythrocyte aggregation and deformability. Invest Ophthalmol Vis Sci 2006;47:3927–32. [DOI] [PubMed] [Google Scholar]
- 25.Metz CE. Basic principles of ROC analysis. Semin Nucl Med 1978; 8:283–98. [DOI] [PubMed] [Google Scholar]
- 26.Abrishami M, Emamverdian Z, Shoeibi N, et al. Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: a case-control study. Canadian journal of ophthalmology Journal canadien d’ophtalmologie 2021;56: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown GC, Brown MM, Hiller T, et al. Cotton-wool spots. Retina 1985; 5:206–14. [DOI] [PubMed] [Google Scholar]
- 28.Buckley SA, James B. Purtscher’s retinopathy. Postgrad Med J 1996;72: 409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo MW, Kemper C, Woodruff TM. COVID-19: Complement, Coagulation, and Collateral Damage. J Immunol 2020;205:1488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin HM, Blegen IV J, Plaster AL, et al. Posterior segment findings in patients on extracorporeal membrane oxygenation. J VitreoRetinal Dis 2020. November; 4(6): 490–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanghi P, Malik M, Hossain IT, Manzouri B. Ocular complications in the prone position in the critical care setting: the COVID-19 pandemic. J Intensive CAre Med 2021; 36(3): 361–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Relationship of cotton wool spots with laboratory values by patient
Supplemental Figure 2. Relationship of retinal hemorrhage with selected laboratory values. The mean peak D-dimer was greater for patients with retinal hemorrhage (33799 ng/mL) compared to those without retinal hemorrhage (15263 ng/mL, p=0.05, A). C-reactive protein within 24 hours was also greater for patients with retinal hemorrhage (228 mg/dL) compared to those without retinal hemorrhage (122 mg/dL, p=0.10, B).
Supplemental Figure 1. Relationship of cotton wool spots with selected laboratory values. Box-and-whisker plot shows that mean C-reactive protein within 24 hours of the ophthalmic exam was higher for patients with cotton wool spots (209 mg/dL) compared to those without cotton wool spots (124 mg/dL, p=0.04, A). In addition, peak C-reactive protein was higher for patients with cotton wool spots (354 mg/dL) compared to those without cotton wool spots (268 mg/dL, p=0.04, B).
Supplemental Table 2. Relationship of retinal hemorrhage with laboratory values by patient
Supplemental Table 3. Multivariate analysis for retinopathy


