Abstract
This study evaluated the effect of sterilizing harvesting knives with sodium hypochlorite (NaOCl) on soft rot in Kimchi cabbage. Knives were infected with Pectobacterium carotovorum subsp. carotovorum (Pcc), sterilized with NaOCl (100, 200, and 300 mg/L), and used to cut Kimchi cabbage slices, which were incubated for 70 h in a 28 °C incubator. In Kimchi cabbage slices cut with a Pcc-inoculated knife without NaOCl sterilization, symptoms began to appear after 20 h, and approximately 60% of the cabbage slices were infected after 70 h of incubation. In contrast, in cabbage cut with a sterilized knife, soft rot symptoms were delayed, and they began to appear after 40 h of incubation in the 200 mg/L-treated. The expression levels of PG10, PG12-1, PG12-3, WRKY 33, MPK3, ACO1, and ACO2 were increased in infected plants, and NaOCl treatment decreased these expression levels. Transmission of soft rot can be minimized by disinfecting harvesting knives with 200 mg/L NaOCl.
Keywords: Gene expression, Harvesting knife, NaOCl, Pectobacterium carotovorum subsp. carotovorum
Introduction
Brassica rapa var. pekinensis (Kimchi cabbage) is a major vegetable in South Korea. It is produced and supplied year-round and is classified according to the growing season as spring Kimchi cabbage, which is grown in the flatlands. Summer Kimchi cabbage, which is grown in the highlands, and winter Kimchi cabbage (Lee et al., 2016). Kimchi cabbage is a low-temperature vegetable as it grows best in a cool climate, and when it is exposed to high temperatures during the growing season, not only does the head not form but it can also become damaged by various diseases and pests (Eum et al., 2013). In particular, soft rot, which frequently occurs in Kimchi cabbages grown in the highlands in summer, causes enormous losses (Kim and Yeoung, 2004). Soft rot is a disease caused by Pectobacterium carotovorum subsp. carotovorum (Pcc), which causes severe damage to several major vegetables, including radish, potato, onion, and Kimchi cabbage (Smith and Bartz, 1990; Prithiviraj et al., 2004). Soft rot can occur throughout the growing period, and it enters the host plant through wounds caused by other diseases or pests.
In Kimchi cabbage, Pcc infection usually occurs at head formation, as the pathogen can enter the head where the outer leaves meet the soil, causing rot (Kim and Yeoung, 2004). After head formation, symptoms begin in the basal midribs and spread to the inner leaves and then the entire head. The infected area becomes soft and mushy, and it is accompanied by a severe odor (Tsuda et al., 2016). Chemical pesticides can be used to control soft rot; however, since they remain in the soil and destroy the ecosystem, these are used sparingly. Various biological methods to control soft rot have been studied, including Pseudomonas and Bacillus (Kyeremeh et al., 2000; Sharga and Lyon, 1998; Tsuda et al., 2016). Biological control is being discussed as an important sustainable and environmentally friendly way to manage plant diseases. Plant growth-promoting rhizobacteria, including Bacillus spp. and Pseudomonas spp., act to protect plants from infection by various pathogens (Tusda et al., 2016). In particular, cyclic lipopeptides and antifungal proteins produced by Bacillus spp. inhibit fungal growth and toxin production to resist plant diseases (Ongena and Jacques, 2008). However, these control agents are generally applied at the cultivation stage, and methods to control soft rot infection at harvest are limited.
At harvest, pathogens present in the cultivation field can be transmitted to uninfected Kimchi cabbages through harvesting tools. Therefore, harvesting knives must be sterilized to minimize infection during harvest. However, such management methods are often neglected in cultivation areas. Disinfection of harvesting knives is a basic requirement for food safety to allow the food to be safely consumed by minimizing the occurrence of microorganisms and the incidence of foodborne illness (Park et al., 2014; Salgado et al., 2014). Chlorine is widely used as a sanitizer for fresh crops because of its low cost, ease of use, and efficacy against various microorganisms (Gil et al., 2009). Sodium hypochlorite reacts with water to form hypochlorous acid and hypochlorite ions, of which hypochlorous acid has strong antimicrobial properties. The concentration of NaOCl, which is mainly used as a fungicide, is 50–200 ppm and is applied to most horticultural crops (Guo et al., 2020). However, since hypochlorite may react with nitrogen-containing substances to form carcinogenic compounds, excessive use should be cautioned (Allende et al., 2009).
The purpose of this study was to determine the effect of sterilizing harvesting knives with various concentrations of NaOCl on the occurrence of soft rot symptoms and changes in physiologically active substances in slices of Kimchi cabbage. We also examined the expression patterns of genes related to cell wall-degrading enzymes, plant hormones, and soft rot resistance with and without NaOCl treatment.
Materials and methods
Pectobacterium carotovorum subsp. carotovorum strain and growth conditions
An international standard strain of the bacterium causing Kimchi cabbage soft rot, P. carotovorum subsp. carotovorum ATCC 15,713, was used for the study. The strain was cultured in Lysogeny broth (LB) medium overnight at 28 °C, with continuous shaking (150 rpm). The bacteria were diluted with LB medium to 108 colony-forming unit (CFU)/mL (OD660 = 0.1) for inoculation of the harvesting knives. Inoculation was done by shaking a harvesting knife into the container.
Plant materials and treatment with Pcc and NaOCl
Kimchi cabbage harvested in July 2020 from Pyeongchang-gun (550 m above sea level), a highland in Gangwon-do, was used in this study. The harvested Kimchi cabbages were transferred to the laboratory, and the midrib was cut into 3 cm × 6 cm (width × length) pieces and used for the experiment. One side of each Kimchi cabbage slice was cut to approximately 5 mm using a knife treated with each test solution. The test solutions were as follows: sterilized water as a control, Pcc suspension (108 CFU/mL), and three NaOCl solutions (T1, 100 mg/L; T2, 200 mg/L; and T3, 400 mg/L). For the NaOCl treatments, the knife was first inoculated with the Pcc suspension by shaking it in a container, and then the knife was treated with NaOCl by shaking it in a container containing each NaOCl solution. The knives were then used to cut approximately 5 mm slices of Kimchi cabbage. Kimchi cabbage slices cut with knives treated with each test solution were placed in a plastic container and incubated in a temperature-controlled chamber at 28 °C for 70 h. The plastic container was placed on two layers of paper towels, and distilled water was poured into the container to maintain saturated humidity. During incubation, the length (mm) of the region on the cabbage slices showing symptoms of soft rot was measured.
Sample preparation
Kimchi cabbage slices from each treatment group were stored at − 80 °C until analysis of physiological activity in a portion of the cabbage without soft rot symptoms. The frozen cabbage slices were lyophilized and finely ground with a mortar and pestle, and a powdered form was prepared. The ground cabbage slices were extracted with 70% ethanol at 30 °C for 12 h. After centrifugation at 20,000 ×g, the supernatant was filtered through a 0.22 μm syringe filter and used for the analyses.
Determination of total phenolic content, total flavonoid content, DPPH, and ABTS
Total phenolic content (TPC) was analyzed using Folin-Ciocalteu reagent, and gallic acid was quantified as a standard (Anesini et al., 2008). Folin-Ciocalteu reagent (50 μL) was mixed with 100 μL of sample extract diluted to 10,000 μg mL−1 in 70% ethanol. After 3 min, 300 μL of 20% Na2CO3 solution was added, and the mixture was incubated at 23 °C for 15 min with intermittent shaking. The mixture was then cooled, and the absorbance was measured at 738 nm using a microplate photometer (Multiskan FC; Thermo Scientific, Waltham, MA, USA). Total phenolic content was expressed in milligrams of gallic acid equivalents per gram (mg GAE g−1) of dry weight (DW).
Total flavonoid content (TFC) was determined using the method reported by Re et al. (1999), with some modifications. Briefly, 100 μL of 10% aluminum nitrate and 100 μL of 1 M potassium acetate were mixed in 500 μL of sample extract (diluted to 10,000 μg mL−1 with 70% ethanol). After incubation for 40 min at room temperature, the absorbance was measured at 405 nm using a microplate photometer (Multiskan FC; Thermo Scientific). Total flavonoid content was expressed in milligrams of quercetin equivalents per gram DW (mg QE g−1 DW).
The radical scavenging activity of Kimchi cabbage was investigated using the method described by Re et al. (1999). Freeze-dried Kimchi cabbage samples were extracted with 70% ethanol, and an aliquot (100 μL) of the extraction solution was mixed with the same amount of a DPPH ethanol solution (0.15 mM). The mixture was incubated in the dark for 30 min, and then the absorbance of the mixture was measured at 517 nm using a microplate photometer (Multiskan FC; Thermo Scientific); ascorbic acid was used as a standard solution. The ABTS radical scavenging activity assay was performed as previously described by Ku et al. (2018). ABTS (7.4 mM) and potassium persulfate (2.45 mM) were mixed (1:1) to generate ABTS cation radical (ABTS+) at room temperature overnight in the dark. The ABTS solution was diluted in phosphate-buffered saline for analysis. The reaction solution was measured at 738 nm, and ascorbic acid was used as the standard.
cDNA synthesis and quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was isolated from the frozen cabbage leaves using the Ribospin Plant kit (GeneAll, Seoul, Korea) according to the manufacturer’s protocol. RNA quality was validated using a Colibri Microvolume Spectrometer (Berthold Titertek Instruments, Inc., Pforzheim, Germany). The PrimeScript RT reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Kyoto, Japan) was used to synthesize cDNA from the RNA isolated from each sample. The synthesized cDNA was diluted 1/20 and used as a template for PCR performed using TB Green Premix Ex Taq II (Tli RNaseH Plus) (TaKaRa). The Thermo Scientific PikoReal 96 RealTime PCR System (Thermo Scientific, Waltham, USA) was used for real-time PCR. Gene expression levels were determined using a standard curve for each gene, and BrUBC10 (Liang et al., 2015) was used as an internal reference gene.
Statistical analysis
The experiment was conducted using a completely randomized design. All measurements and analyses were performed in triplicate. Data are reported as the mean ± standard deviation. Gene expression data were reported as the mean ± standard deviation of the real-time PCR analyses and expressed in log scales. Statistical analyses were performed using analysis of variance in SAS (version 9.1). The significance of each measurement was determined using Duncan’s multiple range test at a significance level of p < 0.05. To investigate the relationships among groups, we visualized the data using R (version 4.0.3) (Performance Analytics, https://cran.rstudio.com/bin/windows/contrib/3.3/PerformanceAnalytics_1.5.2.zip).
Results and discussion
Effect of Pcc and NaOCl treatment of harvesting knives on infection length, total phenolic content, total flavonoid content, and the antioxidant activities of Kimchi cabbage slices
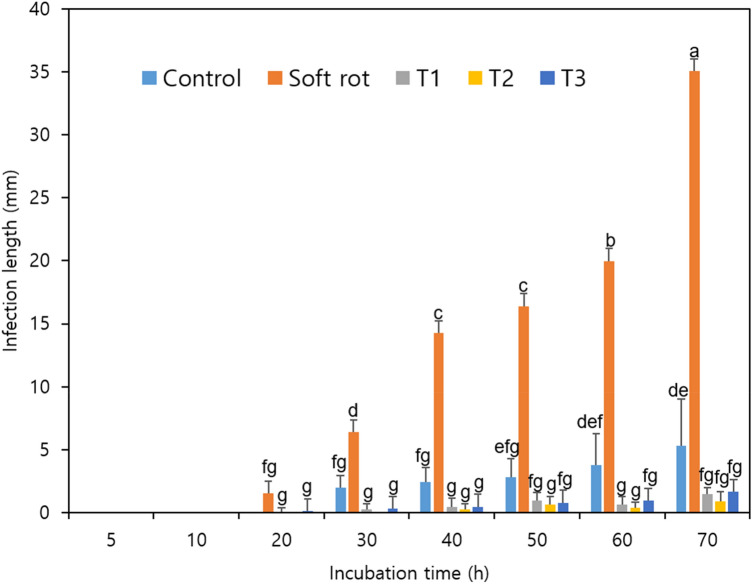
The lengths of the region with soft rot symptoms in the Kimchi cabbage slices were measured (Fig. 1). In the Pcc-treated group, which was cut with a knife infected with Pcc, a 3 mm infection began to appear after 20 h of storage, whereas in the control group, a 3 mm infection began to appear after 30 h. Symptoms were also delayed in the T1, T2, and T3 treatment groups, in which the knives were sterilized with NaOCl, and symptoms appeared at 30 h in the T1 and T3 treatment groups and after 40 h in culture in the T2 treatment group. The infection length in the Pcc treatment group continued to increase during the incubation period, and after 70 h, the infection progressed to approximately 60% of the Kimchi cabbage slices. In contrast, infection rates of 8.8%, 2.8%, 2.4%, and 1.5% occurred in the control, T3, T1, and T2 groups, respectively, with the lowest infection rate in the T2 treatment group. A significance test according to the length of infection in the Kimchi cabbage slices over the entire incubation period showed a highly significant difference between the Pcc treatment and control (p < 0.05). However, the T1, T2, and T3 treatment groups showed no significant differences after 40 h of incubation.
Fig. 1.
Length of the soft rot-infected region in Kimchi cabbage slices during incubation. Control, sterilized water; Soft rot, Pcc; T1, Pcc + 100 mg/L NaOCl; T2, Pcc + 200 mg/L NaOCl; and T3, Pcc + 400 mg/L NaOCl. Data are the means ± standard errors (n = 3). Significant differences (p < 0.05) between means are indicated by different letters
Treatment with NaOCl solution (20–200 mg/L) has been shown to be effective against pathogens for various crops; it was effective against Listeria innocua in Kimchi cabbage (Alenyorege et al., 2019) and against Cronobacter sakazakii in head lettuce (Park et al., 2016). It was also effective for inhibiting other microorganisms, such as aerobic mesophiles in arugula (Francisco et al., 2017) and Salmonella Typhimurium in purple cabbage (Duarte et al., 2018). These antibacterial effects are due to the generation of hypochlorous acid (HOCl) by NaOCl. Hypochlorous acid penetrates bacterial cells and dissociates into -OCl (hypochlorite ion) and H+, two substances that inhibit bacterial activity through oxidation (Duarte et al., 2018). Another study showed that the treatment effect of NaOCl was increased by combined treatment with sweeping frequency ultrasound (Alenyorege et al., 2019). Oxidative damage to pathogenic microorganisms is the reason that disinfectants like NaOCl are used in the food industry. This antibacterial effect of NaOCl seems to delay the symptoms of soft rot caused by Pcc in Kimchi cabbage slices.
The physiological activity of the Kimchi cabbage slices was assessed during incubation. TPC and TFC were significantly different among the control, Pcc, and T1, T2, and T3 treatment groups (p < 0.001) (Table 1). Interestingly, the Pcc treatment group, which showed increased infection-related lesions, also showed high TPC, which increased and decreased throughout the 70 h incubation. The increase in the number of lesions caused by soft rot did not appear to be directly related to the TPC and TFC of the Kimchi cabbage slices; thus, the incubation time was thought to be too short to observe changes in the TPC and TFC. A similar trend was observed for DPPH, which is a measurement of antioxidant activity, although significant differences were observed among the treatments (p < 0.01; p < 0.001). There was also a significant difference with incubation time, but there was not a clear trend, and there was no significant difference compared to the initial values (p < 0.05; Table 1). In contrast, ABTS antioxidant activity did not differ significantly among the treatments, although the trend in the change with incubation time was like those of TPC, TFC, and DPPH.
Table 1.
Changes in total phenolic content, total flavonoid content, and antioxidant activities in Kimchi cabbage slices cut with a Pcc-contaminated harvesting knife during 70 h of incubation
| Treatment | Culture time (hours) | |||||
|---|---|---|---|---|---|---|
| Total Phenolic Content (mg GAE/g DW) | ||||||
| 0 | 1 | 3 | 5 | 10 | 20 | |
| Control | 2.70 ± 0.03 aD | 3.50 ± 0.05 aA | 2.54 ± 0.00 bE | 1.94 ± 0.03 dF | 2.95 ± 0.02 bC | 3.08 ± 0.05 cB |
| Soft rot | 2.70 ± 0.03 aE | 2.67 ± 0.05 cE | 2.70 ± 0.15 aE | 3.03 ± 0.02 aD | 3.13 ± 0.02 aC | 2.23 ± 0.02 eG |
| T1 | 2.70 ± 0.03 aD | 2.40 ± 0.02 dF | 2.56 ± 0.03 bE | 2.75 ± 0.00 bD | 2.99 ± 0.05 bC | 3.17 ± 0.03 bB |
| T2 | 2.70 ± 0.03 aCD | 2.79 ± 0.03 bB | 2.08 ± 0.02 cH | 2.78 ± 0.05 bB | 2.59 ± 0.02 cE | 3.90 ± 0.00 aA |
| T3 | 2.70 ± 0.03 aCD | 2.74 ± 0.02 bC | 2.06 ± 0.03 cG | 2.40 ± 0.02 cF | 2.62 ± 0.02 cE | 2.95 ± 0.02 dB |
| Significance | ||||||
| Treatment | NS | *** | *** | *** | *** | *** |
| Total Flavonoids Content (mg QE/g DW) | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 10 | 20 | |
| Control | 0.036 ± 0.000 aF | 0.049 ± 0.004 bD | 0.046 ± 0.001 aD | 0.037 ± 0.003 dEF | 0.053 ± 0.002 bC | 0.066 ± 0.001 bA |
| Soft rot | 0.036 ± 0.000 aEF | 0.039 ± 0.001 dE | 0.034 ± 0.000 cF | 0.049 ± 0.000 bC | 0.064 ± 0.001 aA | 0.042 ± 0.000 dD |
| T1 | 0.036 ± 0.000 aH | 0.045 ± 0.000 cEF | 0.046 ± 0.001 aDE | 0.043 ± 0.001 cF | 0.053 ± 0.001 bB | 0.048 ± 0.001 cCD |
| T2 | 0.036 ± 0.000 aG | 0.053 ± 0.001 aA | 0.039 ± 0.001 bF | 0.044 ± 0.001 cDE | 0.052 ± 0.001 bA | 0.044 ± 0.001 dDE |
| T3 | 0.036 ± 0.000 aE | 0.043 ± 0.001 cD | 0.045 ± 0.000 aD | 0.054 ± 0.001 aB | 0.050 ± 0.005 bC | 0.068 ± 0.001 aA |
| Significance | ||||||
| Treatment | Culture time (hours) | *** | *** | *** | *** | *** |
| DPPH Inhibition rate (%) | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 10 | 20 | |
| Control | 22.37 ± 0.03 aB | 25.92 ± 0.03 aA | 21.94 ± 0.02 aB | 16.80 ± 0.01 cC | 22.02 ± 0.02 bB | 25.86 ± 0.01 aA |
| Soft rot | 22.37 ± 0.03 aABC | 22.45 ± 0.03 abABC | 18.02 ± 0.01 bDE | 25.18 ± 0.01 aA | 23.90 ± 0.01 aA | 19.14 ± 0.02 cD |
| T1 | 22.37 ± 0.03 aBC | 20.73 ± 0.02 bCD | 18.63 ± 0.00 bDE | 24.58 ± 0.01 aAB | 22.17 ± 0.01 bBC | 24.20 ± 0.01 abAB |
| T2 | 22.37 ± 0.03 aA | 19.91 ± 0.01 bABCD | 13.12 ± 0.00 cE | 22.02 ± 0.01 bAB | 21.42 ± 0.01 bABC | 22.32 ± 0.01 bA |
| T3 | 22.37 ± 0.03 aABCD | 22.09 ± 0.01 bBCD | 18.77 ± 0.01 bE | 20.44 ± 0.01 bCDE | 21.65 ± 0.01 bBCDE | 23.38 ± 0.01 bABC |
| Significance | ||||||
| Treatment | NS | ** | *** | *** | NS | *** |
| ABTS Inhibition rate (%) | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 5 | 10 | 20 | |
| Control | 12.26 ± 0.01 aABC | 13.16 ± 0.01 aAB | 11.75 ± 0.01 abABC | 10.91 ± 0.01 aC | 13.22 ± 0.01 aAB | 13.78 ± 0.01 abA |
| Soft rot | 12.26 ± 0.01 aCDE | 12.15 ± 0.02 aCDE | 10.69 ± 0.01 bE | 11.75 ± 0.01 aDE | 13.22 ± 0.01 aBCD | 12.37 ± 0.01 bBCDE |
| T1 | 12.26 ± 0.01 aBCD | 12.72 ± 0.01 aABCD | 11.02 ± 0.01 abCD | 11.25 ± 0.01 aCD | 12.32 ± 0.01 aBCD | 13.56 ± 0.01 abABC |
| T2 | 12.26 ± 0.01 aA | 13.28 ± 0.01 aA | 12.94 ± 0.01 aA | 11.42 ± 0.01 aA | 12.43 ± 0.01 aA | 13.78 ± 0.01 abA |
| T3 | 12.26 ± 0.01 aBCD | 12.04 ± 0.01 aCD | 10.80 ± 0.01 abD | 10.97 ± 0.01 aD | 13.89 ± 0.02 aABC | 15.75 ± 0.02 aA |
| Significance | ||||||
| Treatment | NS | NS | NS | NS | NS | NS |
| Treatment | Culture time (hours) | ||||
|---|---|---|---|---|---|
| Total Phenolic Content (mg GAE/g DW) | |||||
| 30 | 40 | 50 | 60 | 70 | |
| Control | 3.49 ± 0.05 bA | 2.65 ± 0.02 aD | 2.52 ± 0.03 bE | 2.95 ± 0.02 bC | 1.35 ± 0.03 dG |
| Soft rot | 3.85 ± 0.06 aA | 1.92 ± 0.02 cH | 2.40 ± 0.02 cF | 3.22 ± 0.02 aC | 3.42 ± 0.03 aB |
| T1 | 3.40 ± 0.05 bA | 1.75 ± 0.02 dI | 1.82 ± 0.03 dH | 2.08 ± 0.02 dG | 2.71 ± 0.05 bD |
| T2 | 2.41 ± 0.08 dF | 2.20 ± 0.05 bG | 2.65 ± 0.06 aDE | 2.73 ± 0.05 cBC | 2.42 ± 0.03 cF |
| T3 | 3.14 ± 0.05 cA | 2.68 ± 0.03 aCDE | 2.42 ± 0.05 cF | 3.20 ± 0.03 aA | 2.64 ± 0.09 bDE |
| Significance | |||||
| Treatment | *** | *** | *** | *** | *** |
| Total Flavonoids Content (mg QE/g DW) | |||||
|---|---|---|---|---|---|
| 30 | 40 | 50 | 60 | 70 | |
| Control | 0.056 ± 0.000 bBC | 0.039 ± 0.001 cEF | 0.041 ± 0.003 bE | 0.058 ± 0.002 aB | 0.032 ± 0.001 dG |
| Soft rot | 0.053 ± 0.000 cB | 0.036 ± 0.000 dEF | 0.062 ± 0.007 aA | 0.050 ± 0.001 bC | 0.056 ± 0.001 aB |
| T1 | 0.078 ± 0.001 aA | 0.028 ± 0.001 eJ | 0.039 ± 0.001 bG | 0.048 ± 0.001 bC | 0.031 ± 0.001 dI |
| T2 | 0.047 ± 0.000 dBC | 0.049 ± 0.002 aB | 0.045 ± 0.001 bCD | 0.39 ± 0.001 dF | 0.042 ± 0.002 bE |
| T3 | 0.055 ± 0.001 bB | 0.046 ± 0.001 bCD | 0.039 ± 0.005 bE | 0.045 ± 0.001 cD | 0.036 ± 0.000 cE |
| Significance | |||||
| Treatment | *** | *** | *** | *** | *** |
| DPPH Inhibition rate (%) | |||||
|---|---|---|---|---|---|
| 30 | 40 | 50 | 60 | 70 | |
| Control | 22.53 ± 0.01 bB | 20.73 ± 0.02 aB | 23.23 ± 0.01 aAB | 21.04 ± 0.01 bB | 13.50 ± 0.01 cD |
| Soft rot | 23.15 ± 0.00 bAB | 15.98 ± 0.01 bcE | 19.99 ± 0.01 bCD | 23.23 ± 0.01 aAB | 20.51 ± 0.00 abBCD |
| T1 | 26.25 ± 0.01 aA | 15.15 ± 0.01 cF | 16.89 ± 0.00 cEF | 15.76 ± 0.01 dF | 19.53 ± 0.01 bD |
| T2 | 18.85 ± 0.01 cD | 18.39 ± 0.02 abD | 19.76 ± 0.01 bBCD | 19.01 ± 0.01 cCD | 20.66 ± 0.01 abABCD |
| T3 | 23.82 ± 0.02 bAB | 20.58 ± 0.01 aCDE | 19.98 ± 0.01 bDE | 25.18 ± 0.02 aA | 21.95 ± 0.01 aBCD |
| Significance | |||||
| Treatment | *** | *** | *** | *** | *** |
| ABTS Inhibition rate (%) | |||||
|---|---|---|---|---|---|
| 30 | 40 | 50 | 60 | 70 | |
| Control | 12.88 ± 0.01 abABC | 13.22 ± 0.00 aAB | 13.10 ± 0.02 aAB | 13.33 ± 0.00 aAB | 11.19 ± 0.01 bBC |
| Soft rot | 14.45 ± 0.01 aABC | 12.60 ± 0.01 aBCDE | 12.94 ± 0.01 aBCDE | 14.74 ± 0.01 aAB | 15.63 ± 0.02 aA |
| T1 | 14.96 ± 0.01 aA | 10.06 ± 0.02 bD | 12.43 ± 0.01 aABCD | 12.10 ± 0.02 aBCD | 14.12 ± 0.02 abAB |
| T2 | 12.09 ± 0.01 bA | 11.81 ± 0.01 abA | 12.83 ± 0.01 aA | 12.26 ± 0.02 aA | 13.27 ± 0.01 abA |
| T3 | 14.96 ± 0.01 aAB | 13.16 ± 0.01 aABCD | 12.94 ± 0.01 aBCD | 14.68 ± 0.02 aABC | 13.78 ± 0.02 abABC |
| Significance | |||||
| Treatment | * | NS | NS | NS | NS |
Control, water; Soft rot, Pcc; T1, Pcc + 100 mg/L NaOCl; T2, Pcc + 200 mg/L NaOCl; T3, Pcc + 400 mg/L NaOCl
zDifferent lowercase and uppercase letters within a column and a row indicate significant differences among values by Duncan’s multiple range test at P < 0.05
NS, *, **, and ***: Nonsignificant or significant at p < 0.05, 0.01, and 0.001, respectively
In a previous study, strawberries, cucumbers, and rocket leaves treated with various sanitization solutions showed no significant differences in terms of pH, total titratable acidity, and total soluble solids compared to the controls, also NaOCl-treated plants maintained their vitamin C content (Alexandre et al., 2012; Rosário et al., 2018). Agricultural products are washed and disinfected to extend their shelf life by removing infecting microorganisms, and chlorine-based sanitizing reagents are the most easily used disinfection solutions (Francisco et al., 2017).
Effect of Pcc and NaOCl treatment on the expression of various genes in cut Kimchi cabbage
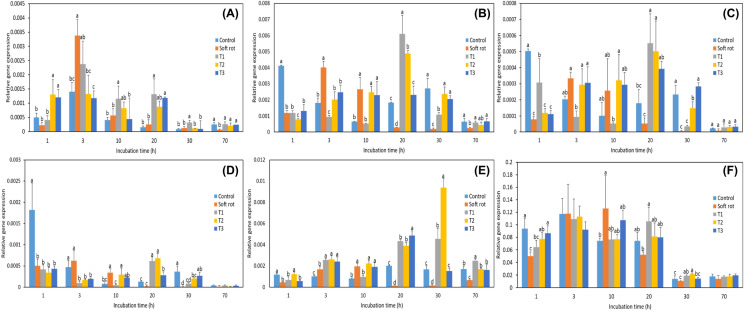
Plants infected with soft rot become soft and die. Therefore, the expression patterns of genes related to cell wall-degrading enzymes, plant hormones, and soft rot resistance were evaluated and plotted on heatmaps (Fig. 2). After 1 h of incubation, the expression levels of all evaluated genes in the control group were relatively high, and after 3 h of incubation, the expression levels of these genes showed clear differences between treatments. In particular, the expression levels of most genes related to cell wall-degrading enzymes, plant hormones, and soft rot resistance were higher in the Pcc treatment group; after 3 h of incubation, expression was relatively high compared to the levels in the control, and T1, T2, and T3 treatment groups. These patterns were maintained for up to 10 h of incubation. After 20 h of incubation, the expression of genes related to plant hormones and soft rot resistance were maintained, while the expression of genes related to cell wall-degrading enzymes decreased.
Fig. 2.
Heatmaps showing the expression profiles of differentially expressed genes examined during incubation for 1 h (A), 3 h (B), 10 h (C), 20 h (D), and 30 h (E). Columns and rows in the heatmaps represent different treatments and genes, C, control; S, soft rot, Pcc; T1, Pcc + 100 mg/L NaOCl; T2, Pcc + 200 mg/L NaOCl; T3, Pcc + 400 mg/L NaOCl
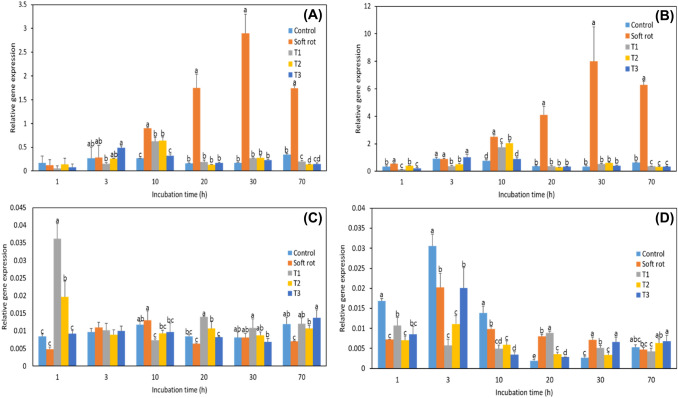
The expression of the polygalacturonase (PG) gene, which encodes a representative cell wall-degrading enzyme, started to increase after 1 h of incubation (Fig. 3), whereas the symptoms of soft rot did not begin to appear until after 20 h of incubation (Fig. 1). Thus, the expression of these genes increased well before symptoms became visible. The expression of most PG-related genes decreased after 30 h of incubation, at a time point when the infection length had increased significantly. The genes that showed the highest expression levels in the Pcc treatment group at the beginning of incubation were PG10, PG12-1, and PG12-3, which peaked at 3 h of incubation. In the NaOCl treatment groups, PG17 gene expression was highest after 30 h of incubation (Fig. 3E). In a study using a wrky33 mutant, the expression of the WRKY33 gene reached a maximum at 14 h after infection, at a time point when fungal growth and development were not observed (Birkenbihl et al., 2012). When invading the host plant, Pcc does not express symptoms but induces the expression of various genes that cause soft rot, such as the cell wall-degrading enzymes PG, pectate lyase, and cellulase, to aid in the generation of a Type II secretion system. This system allows the secretion of proteins from the bacterial cytoplasm into the intercellular space of plant tissues (Abbott and Boraston, 2008; Charkowski et al., 2012; Liu et al., 2019). The Type II secretion system effectively causes soft rot by transferring proteins synthesized by the bacteria into host cells (Charkowski et al., 2012).
Fig. 3.
Expression levels of polygalacturonase (PG) genes in Kimchi cabbage slices during 70 h of incubation. Control, sterilized water; Soft rot, Pcc; T1, Pcc + 100 mg/L NaOCl; T2, Pcc + 200 mg/L NaOCl; T3, Pcc + 400 mg/L NaOCl. A, PG10; B, PG12-1; C, PG12-2; D, PG12-3; E, PG17; F, PG59. Data are the mean ± standard error (n = 3). BrUBC was included as a reference gene. Significant differences (p < 0.05) between means at different timepoints are indicated by different letters
Lignin is a substance produced by plants that maintain the structural integrity of the cell wall and is part of the defense against pathogens, and caffeoyl-CoA O-methyltransferase (CCoAOMT) is involved in lignin biosynthesis (Liu et al., 2019). Kimchi cabbage has higher lignin content in the midrib than in the leaf blade. Expression of the CCoAOMT gene rapidly increased, decreased, and then increased again in all treatment groups, but especially in the Pcc treatment group. The expression levels of genes related to disease resistance were high at the beginning of incubation and low at the end of incubation. Cutting cabbage with a Pcc treated knife resulted in severe soft rot and increased gene expression, whereas the cutting cabbage with a NaOCl-treated knife (T1, T2, and T3) resulted in a lower incidence of soft rot, suggesting that the expression of disease resistance-related genes was low.
Another defense-related gene, mitogen-activated protein kinase (MPK), is present in the cytoplasm and nucleus and is involved in various cellular processes, including growth, development, and stress responses (Sinha et al., 2011). In many plants, MPK levels increase when wounds are inflicted by external stimuli. Even in Kimchi cabbage infected with soft rot, MPK gene expression was increased within 12 h of inoculation (Liu et al., 2019), and in this study, MPK3 levels were also relatively higher in the Pcc treatment group than in the other treatment groups. In the T1, T2, and T3 treatment groups, MPK3 expression was low, and it was concluded that the reaction to soft rot was minimized (Fig. 4B).
Fig. 4.
Expression levels of CCoAOMT (A), MPK3 (B), and WRKY33 (C) in Kimchi cabbage slices during 70 h of incubation. Control, sterilized water; Soft rot, Pcc; T1, Pcc + 100 mg/L NaOCl; T2, Pcc + 200 mg/L NaOCl; T3, Pcc + 400 mg/L NaOCl. Data are the means ± standard errors (n = 3). BrUBC was included as a reference gene. Significant differences (p < 0.05) between means at different timepoints are indicated by different letters
Plant hormones play important roles in regulating plant growth and development and mediate various defense mechanisms in response to the signals of pathogens, including viruses, bacteria, fungi, and insects (Kunkel and Brooks, 2002). Ethylene, jasmonic acid (JA), and salicylic acid activate and promote various immune responses in plants (Norman-Setterblad et al., 2000; Yang et al., 2015). Plants are constantly attacked by pathogens, and the pathogens that attack plant cells are largely divided into two types: biotrophic and necrotrophic. Biotrophic pathogens do not invade living plant cells and kill plant tissues or cells. Instead they take advantage of plants for the nutritional benefits over a long term. In contrast, necrotrophic pathogens kill plant cells to take their nutrients (Birkenbihl et al., 2012). According to previous studies, the defense of plants against biotrophic pathogens is regulated by salicylic acid, whereas the defense against necrotrophic pathogens is mediated by JA and ethylene (Pieterse et al., 2009).
Jasmonic acid is a plant hormone that regulates plant growth and development and mediates disease resistance as a signal to the presence of a pathogen. WRKY33 plays a role in regulating JA-dependent genes and is related to plant resistance to necrotrophic pathogens. In a study using Arabidopsis, wrky33 mutant plants were very susceptible to necrotrophic pathogens, whereas plants overexpressing WRKY33 showed increased resistance to Botrytis cinerea (Birkenbihl et al., 2012). Pathogens trigger JA biosynthesis in host plants, and when pathogens invade, plants biosynthesize high levels of JA (Peng et al., 2012). The Kimchi cabbage slices in the Pcc-treated group showed increased WRKY33 expression beginning at 5 h of incubation, and expression peaked at 20 h and then decreased. In contrast, in the disinfectant treatment groups, WRKY33 expression increased slightly until 5 h of incubation but did not show a sharp increase (Fig. 4C). It is believed that Pcc treatment induced disease resistance mechanisms in the Kimchi cabbage, and disinfectant treatment decreased the expression of the WRKY gene. WRKYs are among the largest families of transcription factors in plants that regulate the response to pathogens (Liu et al., 2019). WRKY70 encodes a critical factor involved in balancing SA-dependent and JA-dependent signaling for the defense against Pcc. In contrast, WRKY33 regulates JA-dependent signaling but inhibits the SA-dependent pathway (Liu et al., 2019; Zheng et al., 2006).
In addition to JA, genes related to ethylene biosynthesis, which plays an essential role in resistance to necrotrophic pathogens, were also examined (Fig. 5). In the Pcc treatment group, the expression levels of the genes encoding 1-aminocyclopropane carboxylic acid oxidase (ACO1 and ACO2) increased from 10 h and peaked at 30 h (Fig. 5A, B). These genes were expressed before the symptoms of soft rot were observed. The expression patterns of the ethylene receptor genes ER1 and ER2 did not show a clear trend in any of the groups, including the control. ER1 was expressed within the first 1–3 h of incubation in the sodium hypochlorite treatment groups. Both ER1 and ER2 were expressed within the first 10 h of incubation, and they tended to remain constant as incubation continued. Ethylene can function as a positive or negative regulator of plant disease resistance (Yang et al., 2015). In rice that is resistant and susceptible to blast disease, infection with aphids resulted in increased expression of ethylene and ethylene biosynthesis genes. However, in blast disease-resistant rice, ethylene generation increased more rapidly, which was caused by an increase in 1-aminocyclopropane carboxylic acid and an increase in ACO activity, which explains the importance of ethylene biosynthesis in disease resistance. Interestingly, when ethylene biosynthesis was inhibited, the lesion expanded (Iwai et al., 2006). As such, the expression levels of ACO1 and ACO2, the ethylene biosynthesis genes examined in this study, appeared to have increased sharply to resist infection by soft rot, whereas the NaOCl treatment group showed lower levels of ethylene-related gene expression due to less soft rot infection.
Fig. 5.
Expression levels of ethylene biosynthesis-related genes in Kimchi cabbage slices during 70 h of incubation. Control, sterilized water; Soft rot, Pcc; T1, Pcc + 100 mg/L NaOCl; T2, Pcc + 200 mg/L NaOCl; T3, Pcc + 400 mg/L NaOCl. A, ER1; B, ER2; C, ACO1; D, ACO2. Data are presented as mean ± standard error (n = 3). BrUBC was included as a reference gene. Significant differences (p < 0.05) between means at different timepoints are indicated by different letters.
NaOCl treatment has a good oxidation effect and is widely applied because of its low cost and ease of use. However, despite the effectiveness of chlorine as a disinfectant, its use is limited due to the generation of chlorinated by-products, such as trihalomethanes and haloacetic acids (Keskinen et al., 2009; Nguyen et al., 2019). Although some European countries may restrict or ban chlorinated compounds in the future, alternative methods are being introduced (Park et al., 2016; Salgado et al., 2014). Nonetheless, the amount of NaOCl used to sterilize the harvesting knife in this study was less than 200 mg/L, and it was only used for cutting the roots of the Kimchi cabbage, so it would not be on the leaves that are used for food. Also, the South Korean Ministry of Food and Drug Safety has approved chlorine-based sanitizer as a food additive for the purpose of sterilizing food.
In addition, since Kimchi cabbage is mainly used after washing, all the NaOCl remaining on the roots will be removed during the washing process, so it would not adversely affect the human body.
Taken together, these results suggest that the cutting knife used for harvesting Kimchi cabbage is a major contagion of soft rot. The application of a low concentration of NaOCl (200 mg/mL) to the harvesting tool can reduce the symptoms of soft rot and can be easily removed by washing and thus would not affect humans consuming the Kimchi cabbage.
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the Agri-Food Export Business Model Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (319088-3)
Declarations
Conflict of interest
The authors do not present any type of conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sae Jin Hong, Email: hongsj@gwnu.ac.kr.
Nam Il Park, Email: nipark@gwnu.ac.kr.
Yeri Park, Email: dyfl6854@hanmail.net.
Byung-Sup Kim, Email: bskim@gwnu.ac.kr.
Hyang Lan Eum, Email: eumhl@hanmail.net.
References
- Abbott DW, Boraston AB. Structural biology of pectin degradation by Enterobacteriaceae. Microbiology and Molecular Biology Reviews. 2008;72:301–316. doi: 10.1128/MMBR.00038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenyorege EA, Ma H, Ayim I, Aheto JH, Hong C, Zhou C. Reduction of Listeria innocua in fresh-cut Kimchi cabbage by a combined washing treatment of sweeping frequency ultrasound and sodium hypochlorite. LWT-Food Science and Technology. 2019;101:410–418. doi: 10.1016/j.lwt.2018.11.048. [DOI] [Google Scholar]
- Alexandre EMC, Brandão TRS, Silva CLM. Assessment of the impact of hydrogen peroxide solutions on microbial loads and quality factors of red bell peppers, strawberries and watercress. Food Control. 2012;27:362–368. doi: 10.1016/j.foodcont.2012.04.012. [DOI] [Google Scholar]
- Allende A, McEvoy J, Tao Y, Luo Y. Antimicrobial effect of acidified sodium chlorite, sodium chlorite, sodium hypochlorite, and citric acid on Escherichia coli O157:H7 and natural microflora of fresh-cut cilantro. Food Control. 2009;20:230–234. doi: 10.1016/j.foodcont.2008.05.009. [DOI] [Google Scholar]
- Anesini C, Ferraro GE, Filip R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. Journal of Agricultural and Food Chemistry. 2008;56:9225–9229. doi: 10.1021/jf8022782. [DOI] [PubMed] [Google Scholar]
- Birkenbihl RP, Diezel C, Somssich IE. Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiology. 2012;159:266–285. doi: 10.1104/pp.111.192641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkowski A, Blanco C, Condemine G, Expert D, Franza T, Hayes C, Hugouvieux-Cotte-Pattat N, Solanilla EL, Low D, Moleleki L, Pirhonen M, Pitman A, Perna N, Reverchon S, Palenzuela PR, Francisco MS, Toth I, Tsuyumu S, van der Waals J, van der Wolf J, Gijsegem FV, Yang CH, Yedidia I. The role of secretion systems and small molecules in soft rot Enterobacteriaceae pathogenicity. Annual Review of Phytopathology. 2012;50:425–449. doi: 10.1146/annurev-phyto-081211-173013. [DOI] [PubMed] [Google Scholar]
- Duarte ALA, do Rosário DKA, Oliveira SBS, de Souza HLS, de Carvalho RV, Carneiro JCS, Silva PI, Bernardes PC. Ultrasound improves antimicrobial effect of sodium dichloroisocyanurate to reduce Salmonella Typhimurium on purple cabbage. International Journal of Food Microbiology. 2018;269:12–18. doi: 10.1016/j.ijfoodmicro.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Eum HL, Kim BS, Yang YJ, Hong SJ. Quality evaluation and optimization of storage temperature with eight cultivars of Kimchi cabbage produced in summer at highland areas. Horticultural Science and Technology. 2013;31:211–218. doi: 10.7235/hort.2013.12170. [DOI] [Google Scholar]
- Francisco CA, Araujo Naves EA, Ferreira D, Rosario DK, Cunha MF, Bernardes P. Synergistic effect of sodium hypochlorite and ultrasound bath in the decontamination of fresh arugulas. Journal of Food Safety. 2017;38:1–7. [Google Scholar]
- Gil MI, Selma MV, López-Gálvez F, Allende A. Fresh-cut product sanitation and wash water disinfection: problems and solutions. International Journal of Food Microbiology. 2009;134:37–45. doi: 10.1016/j.ijfoodmicro.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Guo L, Sun Y, Zhu Y, Wang B, Xu L, Huang M, Li Y, Sun J. The antibacterial mechanism of ultranound in combination with sodium hypochlorite in the control of Escherichia coli. Food Research International. 2020;129:108887. doi: 10.1016/j.foodres.2019.108887. [DOI] [PubMed] [Google Scholar]
- Iwai T, Miyasaka A, Seo S, Ohashi Y. Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiology. 2006;142:1202–1215. doi: 10.1104/pp.106.085258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskinen LA, Burke A, Annous BA. Efficacy of chlorine, acidic electrolyzed water and aqueous chlorine dioxide solutions to decontaminate Escherichia coli O157:H7 from lettuce leaves. International Journal of Food Microbiology. 2009;132:134–140. doi: 10.1016/j.ijfoodmicro.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Kim BS, Yeoung YR. Suppression of bacterial soft rot on Kimchi cabbage by calcium fertilizer treatment. Research in Plant Disease. 2004;10:82–85. doi: 10.5423/RPD.2004.10.1.082. [DOI] [Google Scholar]
- Ku YG, Kang DH, Lee CK, Lee SY, Ryu CS, Kim DE, Polovka M, Namieśnik J, Gorinstein S. Influence of different cultivation systems on bioactivity of asparagus. Food Chemistry. 2018;244:349–358. doi: 10.1016/j.foodchem.2017.10.044. [DOI] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Current Opinion in Plant Biology. 2002;5:325–331. doi: 10.1016/S1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Kyeremeh AG, Kikumoto T, Chuang D, Gunji Y, Takahara Y, Ehara Y. Biological control of soft rot of Kimchi cabbage using single and mixed treatments of bacteriocin-producing avirulent mutants of Erwinia carotovora subsp. carotovora. Journal of General Plant Pathology. 2000;66:264–68. doi: 10.1007/PL00012957. [DOI] [Google Scholar]
- Lee SG, Kim SK, Lee HJ, Choi CS, Park ST. Impacts of climate change on the growth, morphological and physiological responses, and yield of Kimchi cabbage leaves. Horticulture, Environment, and Biotechnology. 2016;57:470–477. doi: 10.1007/s13580-016-1163-9. [DOI] [Google Scholar]
- Liang Y, Yu Y, Shen X, Dong H, Lyu M, Xu L, Ma Z, Liu T, Cao J. Dissecting the complex molecular evolution and expression of polygalacturonase gene family in Brassica rapa ssp. chinensis. Plant Molecular Biology 89: 629-646 (2015) [DOI] [PubMed]
- Liu M, Wu F, Wang S, Lu Y, Chen X, Wang Y, Gu A, Zhao J, Shen S. Comparative transcriptome analysis reveals defense responses against soft rot in Kimchi cabbage. Horticulture Research. 2019;6:68. doi: 10.1038/s41438-019-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Ross T, Chuyen HV. Evaluating the efficacy of three sanitizing agents for extending the shelf life of fresh-cut baby spinach: food safety and quality aspects. AIMS Agriculture and Food. 2019;4:320–339. doi: 10.3934/agrfood.2019.2.320. [DOI] [Google Scholar]
- Norman-Setterblad C, Vidal S, Palva ET. Interacting signal pathways control defense gene expression in Arabidopsis in response to cell wall degrading enzymes from Erwinia carotovora. Molecular Plant-Microbe Interactions. 2000;13:430–438. doi: 10.1094/MPMI.2000.13.4.430. [DOI] [PubMed] [Google Scholar]
- Ongena M, Jacques P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends in Microbiology. 2008;16:115–125. doi: 10.1016/j.tim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Park SY, Mizan FR, Ha S. Inactivation of Cronobacter sakazakii in head lettuce by using a combination of ultrasound and sodium hypochlorite. Food Control. 2016;60:582–587. doi: 10.1016/j.foodcont.2015.08.041. [DOI] [Google Scholar]
- Park SY, Song H, Ha S. Synergistic effects of NaOCl and ultrasound combination on the reduction of Escherichia coli and Bacillus cereus in raw laver. Foodborne Pathogens and Disease. 2014;11:373–378. doi: 10.1089/fpd.2013.1665. [DOI] [PubMed] [Google Scholar]
- Peng X, Hu Y, Tang X, Zhou P, Deng X, Wang H, Guo Z. Constitutive expression of rice WRKY30 gene increases the endogenous jasmonic acid accumulation, PR gene expression and resistance to fungal pathogens in rice. Planta. 2012;236:1485–1498. doi: 10.1007/s00425-012-1698-7. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nature Chemical Biology. 2009;5:308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- Prithiviraj B, Vikram A, Kushalappa AC, Yaylayan V. Volatile metabolite profiling for the discrimination of onion bulbs infected by Erwinia carotovora ssp. carotovora, Fusarium oxysporum and Botrytis allii. European Journal of Plant Pathology. 2004;110:371–377. doi: 10.1023/B:EJPP.0000021058.81491.f8. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rosário DK, Duarte ALA, Madalao M, Libardi MC, Teixeira LJ, Conte-Junior CA, Bernardes PC. Ultrasound improves antimicrobial effect of sodium hypochlorite and instrumental texture on fresh-cut yellow melon. Journal of Food Quality. 2018;2018:1–6. doi: 10.1155/2018/2936589. [DOI] [Google Scholar]
- Salgado PS, Pearlstein AJ, Luo Y, Feng H. Quality of Iceberg (Lactuca sativa L.) and Romaine (L. sativa L. var. longifolial) lettuce treated by combinations of sanitizer, surfactant, and ultrasound. LWT-Food Science and Technology. 2014;56:261–268. doi: 10.1016/j.lwt.2013.11.038. [DOI] [Google Scholar]
- Sharga BM, Lyon GD. Bacillus subtilis BS 107 as an antagonist of potato blackleg and soft rot bacteria. Canadian Journal of Microbiology. 1998;44:777–783. doi: 10.1139/w98-064. [DOI] [PubMed] [Google Scholar]
- Sinha AK, Jaggi M, Raghuram B, Tuteja N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signaling and Behavior. 2011;6:196–203. doi: 10.4161/psb.6.2.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Bartz JA. Variation in the pathogenicity and aggressiveness of strains of Erwinia carotovora subsp. carotovora isolated from different hosts. Plant Disease. 1990;74:505–509. doi: 10.1094/PD-74-0505. [DOI] [Google Scholar]
- Tsuda K, Tsuji G, Higashiyama M, Ogiyama H, Umemura K, Mitomi M, Kubo Y, Kosaka Y. Biological control of bacterial soft rot in Kimchi cabbage by Lactobacillus plantarum strain BY under field conditions. Biological Control. 2016;100:63–69. doi: 10.1016/j.biocontrol.2016.05.010. [DOI] [Google Scholar]
- Yang YX, Ahammed GJ, Wu C, Fan SY, Zhou YH. Crosstalk among jasmonate, salicylate and ethylene signaling pathways in plant disease and immune responses. Current Protein and Peptide Science. 2015;16:450–461. doi: 10.2174/1389203716666150330141638. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Qamar SA, Chen Z, Mengiste T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. The Plant Journal. 2006;48:592–605. doi: 10.1111/j.1365-313X.2006.02901.x. [DOI] [PubMed] [Google Scholar]