Abstract
Background and objective
Spinal cord injury (SCI) is a major disabling disorder for which no effective treatment has yet been found. Regenerative incapability of neuronal cells as well as the secondary mechanisms of injury are the major reasons behind this clinical frustration. Thus, here we fabricated an erythropoietin-chitosan/alginate (EPO-CH/AL) hydrogel and investigated its local therapeutic effects on the apoptotic and inflammatory indices of SCI secondary injury.
Methods
EPO-CH/AL hydrogels were fabricated by the ionic gelation method, and they were characterized using SEM and FTIR. In vitro drug release profile of EPO-CH/AL hydrogels was evaluated by UV–vis spectroscopy. Experimental SCI was inflicted in rats which were then treated with CH/AL hydrogels containing different doses of EPO (1000, 5000 and 10,000 IU/kg). The relative expression of Bax and Bcl2 (apoptosis index) and active and inactive forms of NF-κB (inflammation index) were assessed using western blot. Total serum levels of TNF-α were also assessed with ELISA, and histopathological and immunohistochemistry studies were carried out to check the overall changes in the injured tissues.
Results
In vitro drug release test indicated that the EPO-CH/AL hydrogels had a sustained- and controlled-release profile for EPO under these conditions. All the fabricated hydrogels dramatically reduced the elevated inflammation and apoptosis indices of the SCI-inflicted rats (p ≤ 0.05). Nevertheless, only EPO-CH/AL hydrogel (1000 IU/kg EPO) significantly improved the tissue repair and histopathological appearance of the spinal cord at the sites of injury.
Conclusion
Based on our findings, EPO-CH/AL hydrogel (1000 IU/kg EPO) can effectively improve experimental SCI in rats via inhibiting apoptosis and inflammation. Further studies are warranted to elucidate the contributing role of the scaffold in the observed effects.
Graphical abstract
Keywords: Alginate, Apoptosis, Chitosan, Erythropoietin, Inflammation, Spinal cord injury
Introduction
Spinal cord injury (SCI) has been among the major health issues for decades [1]. A variety of factors such as trauma, disturbances of local microcirculation, neurologic shock and edema can primarily cause SCI. It can lead to the complete paralysis of organs or partial locomotor and sensory dysfunction depending on the site and extent of the injury [2]. So far, extensive research has been performed with regard to the management of this debilitating condition. However, its successful treatment has remained a clinical frustration due to the limited regenerative capacity of neuronal cells [3]. Moreover, it is now clear that the primary insult to the spinal cord proceeds with a series of secondary mechanisms. This triggers a vicious environment, constantly exacerbating the condition [4]. Such secondary alterations include the activation of oxidative and peroxidation-inducing agents, electrolyte imbalances, mitochondrial disturbances, release of inflammatory cytokines and finally the apoptotic death of neuronal cells [5].
An effective therapeutic approach towards finding a definitive cure for SCI patients has remained a constant failure among researchers for years [6]. The extreme vulnerability of neuronal cells against external injury, their regenerative incapability and the self-deteriorating nature of SCI have made it quite challenging to reach a desirable clinical outcome [7]. Currently, the only clinically approved drug is Methylprednisolone MP. Although it has some beneficial effects in this regard, its use is limited since it is associated with a little functional improvements and serious side effects [8].
Prevention of the primary causes of SCI such as trauma is generally impossible and out of control; however, its successful therapy can be achieved in two ways: first, promoting the regenerative ability of the injured neurons; and second, inhibiting the underlying mechanisms of the secondary injury. In line with the first approach, a great deal of attempts have been carried out to promote nerve regeneration and repair using cell therapy [1] and surgical interventions [7]. In pursuit of effective agents to block the progression of the secondary injury, EPO has attracted the attentions of many researchers [9]. It is found that EPO not only inhibits the mechanisms of secondary injury [10], but also induces considerable locomotor functional improvements in experimental SCI models [11].
EPO is initially recognized as a hematopoietic molecule with well-known roles in erythropoiesis. It has also been found to possess significant neuroprotective effects [12]. It is proven that both EPO and its receptor are widely expressed in the nervous system. As well, their expression alters at early stages following SCI. Such features gave rise to the idea of using EPO as a therapeutic agent to treat SCI [13, 14]. So far, different studies have been published to investigate the possible therapeutic efficacy of EPO on SCI. The study of Kaptanoglu et al. (2003) was one of the pioneering research in this field. They evaluated the effectiveness of EPO (100 IU/kg, 1000 IU/kg and 5000 IU/kg) on the severity of experimental SCI. Their results showed that EPO (1000 IU/kg and 5000 IU/kg) protected the spinal cord from injury via inhibiting lipid peroxidation [2]. Other studies showed that EPO can improve neural regeneration and promote locomotor and sensory functions following SCI via different mechanisms. They included angiogenesis and blood flow promotion [5], anti-apoptotic [15] and anti-inflammatory [16] effects, induction of stem cell recruitment to the site of injury [17], etc. In addition to its direct effects, EPO has been shown to improve the outcomes of several experimental SCI treatment approaches such as hyperbaric oxygen therapy [11] and the use of neuronal stem cells [1, 18, 19].
Apoptosis inhibition is a critical mechanism through which EPO exerts its beneficial effects in SCI. Such an inhibition, not only prevents the death of neurons, but also averts other apoptosis-related harmful consequences such as inflammation and oxidative effect [4, 20]. There are a few reports in the literature that have investigated the anti-apoptotic effects of EPO after SCI. Also, the majority of them have administered EPO via the intraperitoneal route, which limits proper delivery of the drugs to the intended injury site.
Regarding the safety concerns on the use of EPO at doses effective for SCI, it seems crucial to use scaffolds supporting controlled release of EPO. Considering this gap and regenerative effect of EPO [21, 22], in the present work, we studied the efficacy of local administration of EPO, formulated within a chitosan alginate hydrogel, in an experimental SCI model of rats. These scaffolds can increase the efficacy of the treatment and subsequently reduce the required effective dose; hence, limiting its side effects.
Materials and methods
Materials
Most of the chemicals were bought from Sigma-Aldrich, including alginic acid sodium salt (Medium viscosity), chitosan (medium molecular weight, deacetylation degree ~ 75–85%) and carboxymethyl cellulose (CMC, Medium viscosity). Erythropoietin was purchased from CinnaGen (Tehran, Iran). Phosphate buffer saline (PBS), glycerol, MTT [3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide], penicillin–streptomycin, Dulbecco's Modified Eagle's medium (DMEM), and dimethyl sulfoxide (DMSO) were purchased from Merck chemicals Co. (Darmstadt, Germany) and fetal bovine serum (FBS) was obtained from (Gibco, Germany). Rat TNF-α ELISA kit was obtained from Diaclone (France).
Fabrication of erythropoietin-containing chitosan –alginate (EPO-CH/AL) hydrogel
Chitosan solution 4% (w/v) was prepared by dissolving chitosan powder in 1% (v/v) acetic acid aqueous solution for 48 h. Alginate and CMC powders were dissolved separately in Deionized water to make 4% and 1% (w/v) solutions, respectively. Erythropoietin (1000, 5000 and 1000 IU/kg) was added to the alginate solution and stirred for 30 min. Then, the alginate (with or without erythropoietin) and chitosan solution were mixed by volume ratio (1/1) using magnetic stirrer for 2 h to prepare a homogenous solution. Afterwards, for obtained hydrogel, the CMC solution wasadded to above-prepared solution by volume ratio (1/1) under vigorous stirring for 1 h. Weight percent of compounds used in the fabrication of hydrogel was represented in Table 1.
Table 1.
Weight percent of compounds used in the fabrication of hydrogels
| Sample | Weight percent of compounds in hydrogels | Drug | ||
|---|---|---|---|---|
| Chitosan | CMC | Alginate | EPO (IU) | |
| CH/AL | 1 | 0.5 | 1 | 0 |
| EPO1- CH/AL | 1 | 0.5 | 1 | 1000 |
| EPO5- CH/AL | 1 | 0.5 | 1 | 5000 |
| EPO10- CH/AL | 1 | 0.5 | 1 | 10,000 |
Electrostatic interaction study
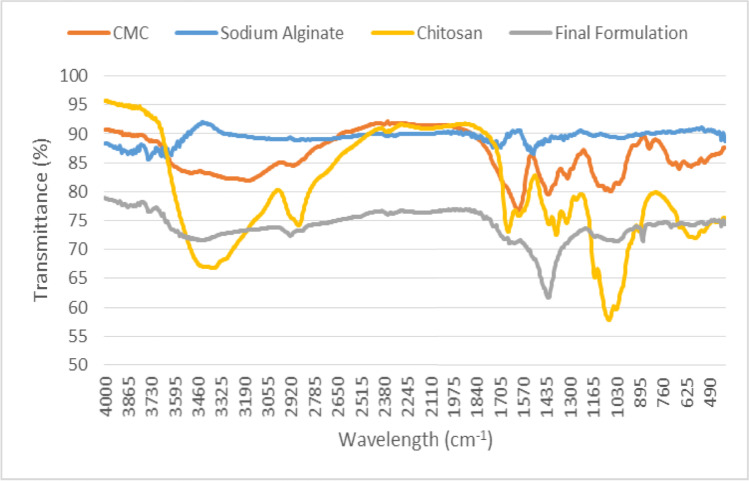
A Fourier-transform infrared (FTIR) spectroscopy (Nexus Por Euro, Bruker, Karlsruhe, Germany) was used to verify the electrostatic interactions between carboxymethyl cellulose (CMC), sodium alginate (AL), and chitosan (CH) in the fabricated formulation. The FTIR spectra of test samples was obtained by the average of 32 scans at the wave number range of 400 cm−1 and 4000 cm−1 at a resolution of 4 cm−1.
Morphological study
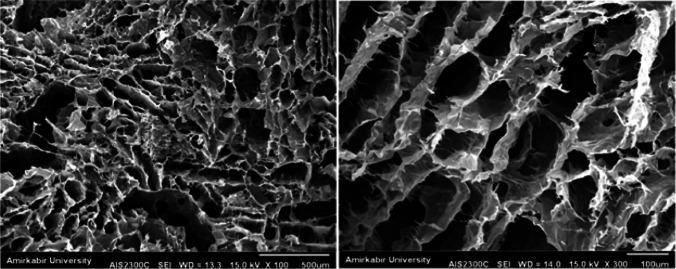
In order to study the morphology of the fabricated hydrogels, a scanning electron microscopy (SEM, KYKY-2800; Apparatus Factory, Chinese Academy of Sciences, Beijing, China) was applied. The voltage for accelerating the electrons was 20 kV. They were treated by sputter coating with a thin gold layer using an auto sputter fine coater. Then, SEM images of test samples were obtained by placing them into a scanning electron microscopy.
In vitro drug release study
In order to investigate the release profile of EPO from hydrogels (EPO1-CH/AL, EPO5-CH/AL and EPO10-CH/AL hydrogels), the hydrogels were immersed in 4 ml PBS (pH = 7.4, 10 mM) and shacked at 70 rpm and 37 °C using a shaker incubator. At predetermined time intervals, 500 µl of PBS was replaced with 500 µl fresh one. Then, the released samples of EPO were quantified with IMMULITE 2000 EPO assay (Siemens, Germany; range of normal values 4.3 – 29 mIU / ml).
Induction of SCI model and grouping
Forty male Wistar rats weighing 250–280 g were used. All animal-related procedures were carried out in complete accordance with the guidelines of the ethics committee of Islamic Azad University, Tehran, Iran (ethics code: IR.IAU.SRB.REC.1398.195). The animals were randomly allocated to 5 groups (n = 8 in each group) as follows:
Group 1: normal control (control group).
Group 2: SCI-induced group without any further intervention (SCI group).
Groups 3, 4 and 5: they were respectively treated by receiving EPO-CH/AL hydrogels (1000, 5000 and 10,000 IU/kg EPO), immediately following the induction of SCI.
To induce the experimental SCI models, the animals were first anesthetized with intraperitoneal injection of ketamine (80–100 mg/kg) and xylazine (5 mg/kg). An NYU impactor apparatus (New York University) was then used to induce the required contusive pressure [23]. Briefly, after determining the exact position of the 10th thoracic vertebral bone (T#10), a wider area of the overlying skin was shaved and scrubbed. The skin and connective tissue were then blunt dissected and the lamina of the T#10 was gently removed using a dentistry drill to expose the cord. The rats were then placed at prone position, fixed at T#9 and T#11 using the clips of the impactor and a 10 g weight was dropped at the exposed cord area from a height of 25 cm to induce the contusion. The observation of flutter reflexes of the tail and abrupt retraction of the hind limbs were deemed a sign of proper infliction of the model.
Cell viability assay
In order to evaluate the biocompatibility of EPO-CH/AL hydrogels, an indirect test was used based on ISO 10,993–5 standard. The MTT test was performed using U373-MG cells (Human glioblastoma-astrocytoma). For this purpose, the hydrogel with surface area 1 cm2 was immersed in 1 ml of culture medium and incubated at 37 °C for 1, 3, 7, 14 and 21 days. In each day, the culture medium was extracted and stored at 4 °C for further cell viability test. U373-MG cells (Human glioblastoma-astrocytoma) were used for MTT test. In this test, 1 × 105 cells were cultured in the solution extracted from the hydrogels in 12-well plates at 3, 7, 14 and 21 days in 37 °C with 5% CO2. At each time point, the culture medium was removed from wells. Then, the medium was replaced with 2% MTT solution for 4 h, and the precipitated formazan was solubilized with isopropanol for 15 min. The absorbance of supernatant was read at 570 nm.
Immunohistochemistry and histopathology
At the end of the experiment after 30 days, the rats were euthanized using CO2 and the whole spinal cords were dissected for further analysis. The dissected cord of each rat at the injury site was then divided into two separate segments: one of them was stored at -80 °C for later molecular studies, and the other segment was kept in 10% formaldehyde (pH = 7.26) for 48 h for histopathological and immunohistochemistry studies. Following fixation, the samples were embedded in paraffin. Then, 5 mm-thick transverse, and longitudinal sections were prepared in duplicate. Each replicate was used for either Hematoxylin or Eosin (E&E) staining or immunohistochemistry procedures. Finally, an expert pathologist reviewed and analyzed the slides using a light microscope (Olympus BX51; Olympus, Tokyo, Japan), and reported all the observed changes in a blind manner. Furthermore, the sections obtained for immunohistochemistry were stained using a rabbit polyclonal antibody against S100 (dilution 1:400, Dako). The H&E counterstaining was also performed to visualize the changes in the number of Shawn cells, etc.
Western blot analysis
Western blot analysis was performed to determine the changes in the expression of apoptosis and inflammation-related molecules. These included B cell lymphoma/leukemia2 (Bcl-2), Bcl-2 associated X (Bax), nuclear factor κB (NF-κB), and tumor necrosis factor (TNF-α). To this end, the frozen spinal cord specimens were homogenized using a freshly prepared lysis buffer containing a cocktail of different protease inhibitors (cOmplete; Roche Diagnostics). The obtained lysates were then applied to SDS-PAGE (10%) and transferred to polyvinylidene difluoride (PVDF) membranes (Roche, Mannheim, Germany). Blocking of the blocks was done for 1.5 h using skimmed dry milk in TBST (5%) at room temperature. Ultimately, the blocked membranes were incubated with the following antibodies: anti-Bcl-2 (1:200), anti-Bax (1:200), anti-t-NF-κB (1:200), anti-p-NF-κB (1:200) and an anti-β-actin antibody, as the loading control (1:200)(all from Santa Cruz Biotech, CA, USA). For visualization, the membranes were washed with TBST. Then, they were treated with a secondary antibody conjugated with horseradish peroxidase (1:5000; BioRad, Hercules, CA, USA) for 1 h at room temperature. Next step, developing the blots was performed using the BM chemiluminescence detection system (Roche). Finally, the quantification of the protein bands was done using the ImageJ software.
TNF-α assessment
To quantify the serum levels of TNF-α in the study groups, blood samples (3 ml) were collected after 30 days from the tail veins of the rats before they were euthanized. Each blood sample yielded to an approximate amount of 1 ml serum, which was used for the cytokine detection. A sandwich enzyme-linked immunosorbent (ELISA) assay was performed based on the instructions provided with the rat TNF-α ELISA kit protocol. Briefly, 100 µl of each sample was added in triplicate to the pre-coated plate. Then, 50 µl of diluted biotinylated anti-rat TNF-α secondary antibody was then added to each well, and the plate was sealed and incubated at 25 °C for 3 h. After multiple washing steps, the Streptavidin-HRP solution was added and incubated for further 30 min. Following a washing step, all wells were incubated with TMB substrate solution for 15 min. Next step, the reaction was stopped using 100 µl of the stop solution (H2SO4). Then, absorbance was measured at 450 nm using a spectrophotometer to obtain the optical densities. Finally, the precise concentration of TNF-α in each sample was calculated using a previously prepared standard curve and reported as pg/ml.
Statistical analysis
All the in vitro experiments were performed in triplicate and mean values were used for statistical analysis. One-way analysis of variance (ANOVA) was used to test the presence of any significant differences. The differences between groups were determined using the Tukey post-hoc test. P values below 0.05 were considered as statistically significant.
Results
FTIR analysis
The FTIR spectra of CH, AL, CMC and final formulation (EPO-CH/AL hydrogels) are shown in Fig. 1. The absorption band at 3178 cm−1 was due to the stretching frequency of the –OH group of CMC. Bands between 1427 cm−1 and 1315 cm−1 were assigned to C-H scissoring and –OH bending vibrations of CMC. The absorption bands at 1065 cm−1 and 2912 cm−1 were due to the C–O–C group and C-H stretching vibration of CMC, respectively [24]. Stretching vibrations of O–H bonds of alginate appeared in the range of 3000–3900 cm−1 [25]. Stretching vibrations of aliphatic C-H of alginate were observed at 2951–2812 cm−1. Observed bands at 1701 and 1512 cm−1 were attributed to the asymmetric and symmetric vibrations of carboxylate salt ion of alginate, respectively.
Fig. 1.
FTIR spectra of the carboxymethylcellulose, sodium alginate, chitosan, and the final formulation
The characteristic bands of chitosan were the overlapped bands at 1655 cm−1 and 1597 cm−1, which were attributed to the CONH2 and NH2 groups of chitosan, respectively [26, 27]. The double amide bands in the spectrum of chitosan corresponded to the partial N-deacetylation of the chitin [28]. The band at 3371 cm−1 was attributed to –NH2 and –OH stretching vibrations of chitosan [27]. In the spectrum of the final formulation (EPO-CH/AL hydrogels), the CONH2 bands of chitosan shifted from 1655 cm−1 to 1647 cm−1, while the NH2-bands were significantly reduced. Moreover, the amino band at 1076 cm−1 and the carboxyl band at 1655 cm−1 were reduced in the spectrum of the final formulation. These were due to the electrostatic interactions between carboxymethyl cellulose, sodium alginate and chitosan. Our results were similar to that of other previous studies [29, 30] in which scaffolds were prepared, taking advantage of electrostatic interactions between sodium alginate and chitosan.
SEM analysis
SEM technique was used to study the cross-section morphology of the freeze-dried EPO-CH/AL hydrogels. Figure 2 displayed SEM images of EPO-CH/AL hydrogels. As shown in Fig. 2, the freeze-dried EPO-CH/AL hydrogels exhibited highly porous and interconnected pore structure. Such structure was suitable for transportation nutrients and metabolites. Thus, EPO-CH/AL hydrogels provided an appropriate structure for cell attachment and proliferation.
Fig. 2.
Scanning electron microscopy (SEM) micrograph of EPO-CH/AL
In vitro release analysis
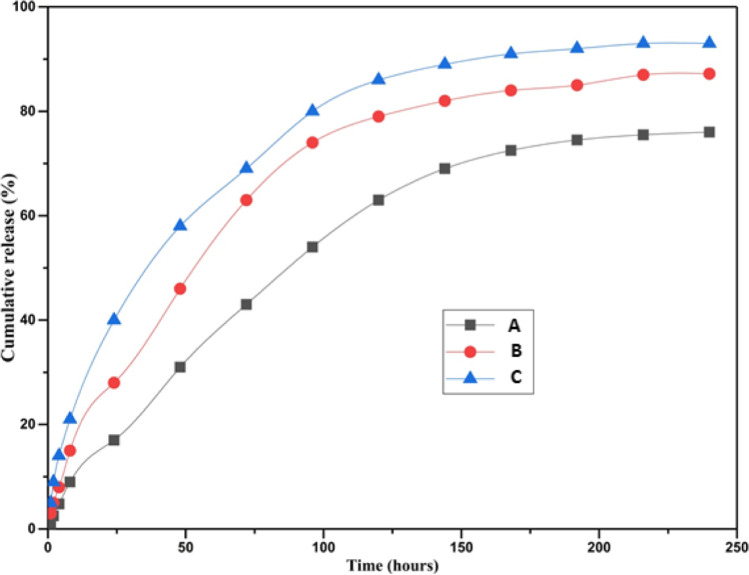
The cumulative release profiles of EPO from the fabricated hydrogels were represented in Fig. 3. According to the release profiles, the hydrogels with higher amount of EPO showed higher burst release in initial time (31, 46 and 58% drug released within 48 h for EPO1-CH/AL, EPO5-CH/AL and EPO10-CH/AL hydrogels, respectively). Then, EPO-CH/AL hydrogels showed a sustained release profile over a period of 240 h. The maximum cumulative release percent of EPO1-CH/AL, EPO5-CH/AL and EPO10-CH/AL hydrogels were 76, 87 and 93%, respectively.
Fig. 3.
Release profile of A EPO1-CH/AL, B EPO5-CH/AL and C EPO10-CH/AL hydrogels
Cell viability assay
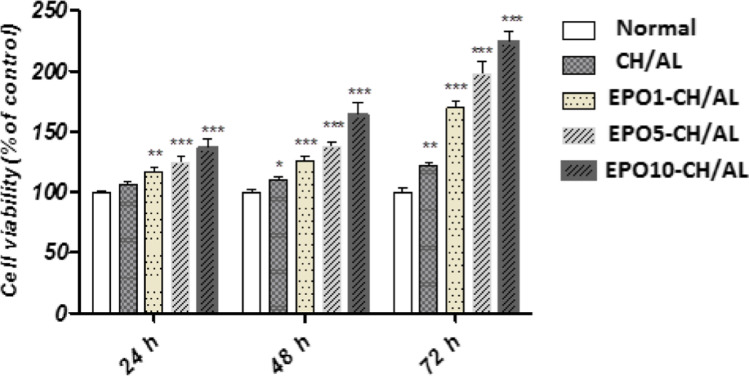
Figure 4 displayed the comparable viability of U373-MG cells on CH/AL and EPO-CH/AL hydrogels (1000, 5000 and 10,000 IU/kg EPO). Here, tissue culture polystyrene was used as a negative control group. According to the results, at day 3, all groups showed any inhibitory cell growth compared to the control group As well, EPO-CH/AL hydrogels (1000, 5000 and 10,000 IU/kg EPO) showed proliferation activity. 24 h after treatment, all groups showed proliferation activity compared to the control group. EPO-1000, -5000 and -10,000 had significant proliferation activity (p < 0.001). After 48 h, all groups exhibited significant difference in cell proliferation as compared to the control group. 72 h post treatment, best proliferation effect was seen with EPO10-CH/AL > EPO5-CH/AL > EPO1-CH/AL > CH/AL hydrogels, respectively. EPO10-CH/AL hydrogel had significant proliferation effect compared to other groups at all treated interval. Finally, the results indicated that the material and fabrication process had no negative effect on cell viability.
Fig. 4.
The viability of U373-MG cells after 24, 48 and 72 h
The effect of EPO-CH/AL hydrogels on apoptosis index
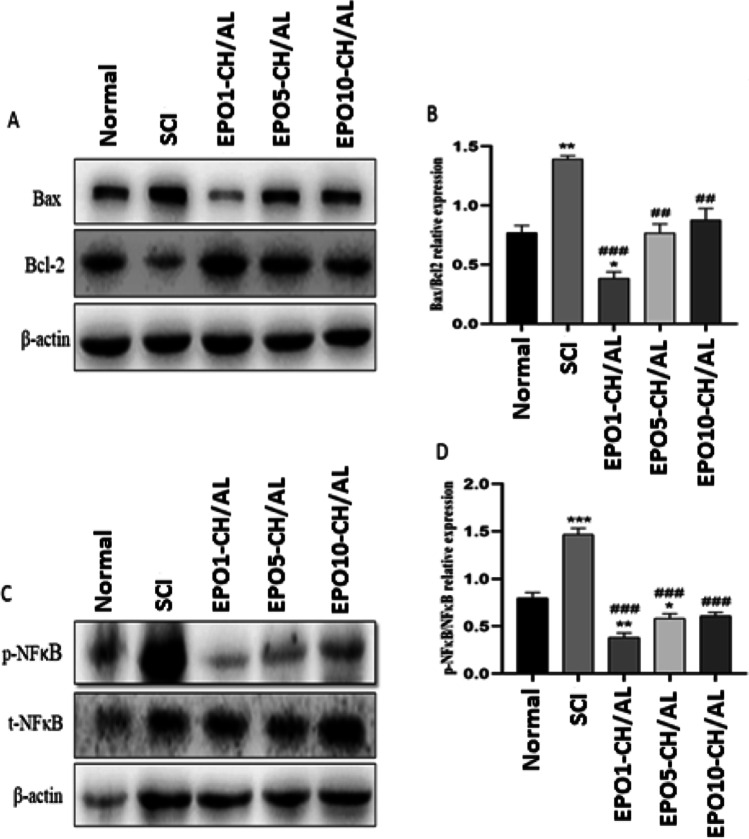
Importance of apoptotic death of neuronal cells in the prolongation and exacerbation of the secondary injury has been reported previously [31]. Here, we investigated effect of EPO-CH/AL hydrogels at different doses on the expression of apoptosis regulating molecules. Figure 5A displayed the western blot results of Bax and Bcl-2. Pairwise comparison of the normal control and the SCI group revealed that the expression of the pro-apoptotic molecule, Bax, had a notable increase in the SCI group while the expression of the anti-apoptotic Bcl-2 largely decreased. To perform a more precise comparison, the Bax/Bcl-2 ratio was calculated in each group to reach a more accurate index of apoptosis, wherein a higher ratio was indicative of a greater extent of apoptosis. Based on the results, the index significantly increased in the SCI group, compared to the normal control (p ≤ 0.01). EPO1-CH/AL hydrogel reduced the elevated apoptosis index by three folds, which was even lower than that of the control group (p ≤ 0.001). The other hydrogels (EPO5-CH/AL and EPO10-CH/AL) were also able to significantly reduce this index compared to the SCI group (p ≤ 0.01). However, the decline in apoptosis was less pronounced in these groups. These results suggested that treatment with EPO-CH/AL hydrogels at different doses can strongly reduce the expression of apoptotic markers in the SCI model based on an inverse dose-dependent manner.
Fig. 5.
The expression of NF-κB, Bax and Bcl-2 in different groups defined by western blot (β-actin was also used as an internal control). p- NF-κB and t- NF-κB represent the phosphorylated and the truncated forms of NF-κB, respectively. p-NF-κB /t-p-NF-κB ratio was used as the inflammation index. Bax/Bcl-2 was deemed as the apoptosis index. # and * represent significant differences compared to the control and SCI groups, respectively; #/* p ≤ 0.05; ##/** p ≤ 0.01; ###/*** p ≤ 0.001
The effect of EPO-CH/AL hydrogels on inflammation index
Inflammation and inflammatory cytokine production have been shown to largely contribute to the late pathology of SCI [32]. To assess the inflammatory status of each group, the expression of NF-κB was investigated using western blot (Fig. 5C and D). The ratio of phosphorylated NF-κB (p-NF-κB) to its truncated form (t-NF-κB) was used as a rough measure of inflammation status (inflammation index). Based on the results, the inflammation index was dramatically increased in the SCI group compared to the normal control (p ≤ 0.001). As well, all three doses of EPO-CH/AL hydrogels significantly reduced the elevated inflammation index, in which the strongest effect was related to the EPO1-CH/AL hydrogel (p ≤ 0.001). These results revealed a potent anti-inflammatory role of EPO-CH/AL hydrogels in the context of SCI.
To confirm the observed changes, the serum levels of TNF-α were then measured in each study groups (Fig. 6). The results were in line with those obtained in the western blot analysis of NF-κB expression. These results indicated that the serum TNF-α concentration was significantly elevated following the induction of SCI (p ≤ 0.001). Moreover, EPO1-CH/AL hydrogel reduced the TNF-α levels back to normal levels (p ≤ 0.001). Although the same effects were seen with EPO5-CH/AL and EPO10-CH/AL hydrogels, the extent of TNF-α reduction was lower when higher doses were used. These results were in parallel with the changes in NF-κB expression following SCI induction and the subsequent treatment with EPO-CH/AL hydrogels, revealing a potent anti-inflammatory role of them.
Fig. 6.
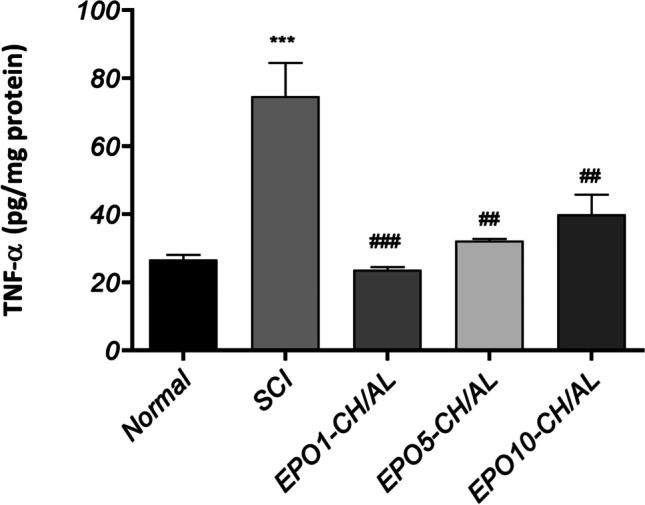
Total serum concentrations of TNF-α in different experimental groups measured by ELISA. The values are presented as pg/mg protein. # and * represent significant differences compared to the control and SCI groups, respectively; #/* p ≤ 0.05; ##/** p ≤ 0.01; ###/*** p ≤ 0.001
The effect of EPO-CH/AL hydrogels on the overall histopathological view
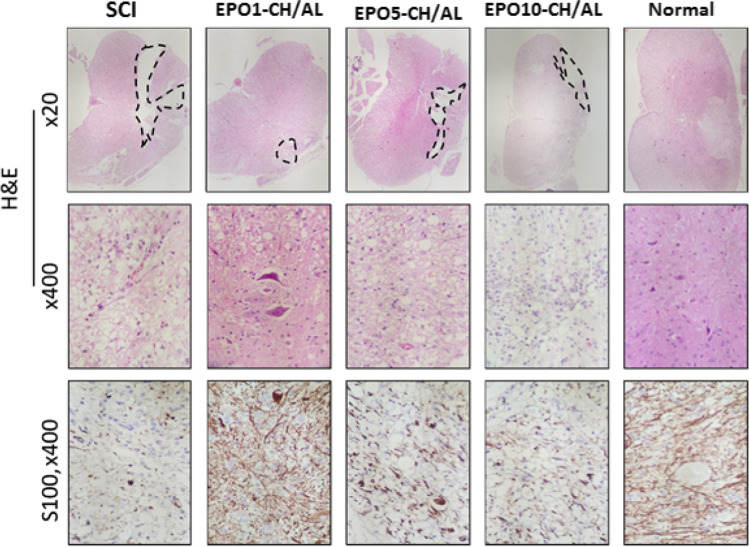
In the SCI group, H&E staining revealed an obvious cavity, liquefaction and inflammatory cell infiltration in the spinal cord lesion. Moreover, the spinal cord boundary between gray and white matter was unclear. The spinal cord tissue showed disorganized nerve fibers with a large number of necrotic cells and atrophy. Vacuolar degeneration was also evident in the cytoplasm of neuronal cells (Fig. 7). In the EPO1-CH/AL group, a few infiltrating inflammatory cells were observed in the injured spinal cord site. In addition, some neuronal cells exhibited vacuolar degeneration and inflammatory cell infiltration. The number of necrotic nerve cells and inflammatory cells in the EPO1-CH/AL group greatly decreased in comparison with the other treatments. The comparison of the relative lesion area (%) between groups was indicative of a significant decrease in the EPO1-CH/AL group (p ≤ 0.05) compared to the SCI. This is while the other hydrogels could not induce any significant changes (p > 0.05) (Table 2). The spinal cord boundary between gray and white matter was unclear, with inflammatory cell infiltration. As well, immunohistochemical analysis revealed that the number of Shawn cells was significantly higher in the EPO1-CH/AL and control groups in comparison with the other groups and the SCI. Taken together, our histopathological observations showed that EPO1-CH/AL hydrogel can significantly enhance spinal cord tissue repair in rats.
Fig. 7.
Light micrographs of the spinal cord in different experimental groups. Dotted lines represent lesion area. H&E (A-J) and S100 (K–O) staining. # and * represent significant differences compared to the control and SCI groups, respectively; #/* p ≤ 0.05; ##/** p ≤ 0.01; ###/*** p ≤ 0.001
Table 2.
The relative lesion are in (%) in the cord sections of the injured sites in each group
| Study group | Normal | SCI | EPO1-CH/AL | EPO5-CH/AL | EPO10-CH/AL |
|---|---|---|---|---|---|
| Mean ± SD | - | 91.25 ± 6.75* | 29.5 ± 6.75# | 69.5 ± 4.2 | 90 ± 4.16 |
*, # Represent significant differences compared to the control and SCI groups, respectively (p ≤ 0.05)
Discussion
A considerable majority, if not all, of the studies investigated the effects of EPO in SCI have taken advantage of the intraperitoneal route for administrating EPO [11, 23]. However, this route of administration has limited efficacy due to its transient effect and may also cause more serious side effects. On the other hand, despite the previous findings on the anti-apoptotic effects of EPO in the brain and other sites of the nervous system, no study has exclusively studied its apoptotic effects with regard to experimental SCI. Chitosan possesses many unique properties, including biocompatibility, biodegradability, antibacterial properties and hemostatic activities. Due to its unique properties, chitosan has been attracted more attention in the medical applications, especially tissue engineering scaffolds. Research has indicated that implantation of chitosan hydrogels after SCI can promote axonal regeneration. Furthermore, chitosan is considered as an ideal delivery system or carrier for the recovery of SCI [33–36]. Sodium alginate, anionic natural polysaccharide, possesses excellent stability, solubility, viscosity, and biocompatibility. Alginate chains can be crosslinked with Ca2+ in vivo and alginate gels; thus, it can create a three-dimensional scaffold to support cell growth. Research has indicated that implantation of sodium alginate scaffolds can promote axonal regeneration after SCI [37–39]. As well, the composite scaffold composed of chitosan and alginate has been applied for the recovery of SCI. Chitosan/alginate microcapsules have suitable biocompatibility. Francis et al. fabricated a specific type of chitosan/alginate scaffold, which could be used as tissue scaffolds [39]. Considering the previous findings on the beneficial effects of CH, AL and EPO for the recovery and treatment of SCI and the limitations of the previous studies, we made an effort to integrate EPO at different doses into a CH/AL hydrogel scaffold in order to: first, supporting the sustained release of the agent; second, reducing the risk of its systemic administration; and finally, combining the regenerative effects of chitosan with the ameliorative effects of EPO on secondary injury mechanisms, with a special focus on apoptosis. The results of our study showed that EPO-CH/AL hydrogels, containing different doses of EPO (100, 1000 and 5000 IU/kg), can significantly alter the expression of the apoptosis-related molecules Bax and Bcl-2 in view of inhibiting apoptosis (Fig. 4A and B). The strongest anti-apoptotic effect was seen in the EPO1000 group. These results are in line with previous studies in which EPO, at the same dose, had been shown to protect neuronal cells via inducing anti-apoptotic effects [40]. Considering the inflammatory nature of secondary events in SCI, we also checked to see if treatment with EPO-CH/AL hydrogel can reduce the harmful inflammation. Our results showed that EPO-CH/AL hydrogels could reduce the expression of the active NF-κB, a major transcription factor for inflammatory cytokines (Fig. 4C and D). Furthermore, significant declines in the total serum levels of TNF-α were observed in all treatment groups, providing more robust evidence on the anti-inflammatory role of EPO. The results of previous studies revealing the TNF-α-reducing effects of EPO in brain injury, hemodialysis patients, etc. supported our findings [40]. However, these results question the assumptions of previous studies in which the anti-inflammatory role of EPO was considered as a secondary consequence of its anti-apoptotic effects rather than a direct effect on inflammatory cytokines [4, 8]. Our results are also in contrary to the results of Zhao et al. In spite of reporting EPO`s nerve recovering effects, they did not observe any changes in the activity of NF-κB after treatment with EPO [32]. Nevertheless, it is important to note that these variations may be the result of our combination therapy using the chitosan alginate hydrogel scaffold as well as the differences in the route of administration.
Our histopathological and immunohistochemistry studies revealed a dramatic improvement in the overall histopathologic appearance of the spinal cord in the EPO1-CH/AL group. However, insignificant changes were observed in the other treatment groups. The reduction of the relative lesion area and the significant increase in the number of Schwan cells in the EPO1-CH/AL group, compared to the SCI group, indicated that this dose of EPO is the most effective concertation in this regard. Our findings also confirmed that the observed molecular changes were effective in improving the overall cellular composition and tissue repair of the injured sites in experimental SCI of rats.
Use of EPO to block the progression of SCI and improve functional status of the animal models of SCI has been a great promise over recent years. EPO has proven to be even more effective and safer than the currently used MP in this setting [41]. However, possessing other hematopoietic functions, EPO may also cause serious complications and adverse effects that may overweigh its benefits in its long-term use [20]. Therefore, it seems logical to reduce the required effective dose of this agent using strategies like its integration within scaffolds, taking the advantage of their synergistic effects and sustained release nature. Finally, based on the promising results obtained in this study, further studies on the local use of EPO as an anti-apoptotic and anti-inflammatory agent in combination with chitosan-containing scaffolds is recommended.
Conclusion
EPO-CH/AL hydrogels were successfully fabricated using a gelation technique. According to the SEM images, they exhibited highly porous and interconnected pore structure. As well, FTIR spectra indicated that hydrogels were fabricated by electrostatic interactions between sodium alginate and chitosan. Generally, our findings suggested potent anti-apoptotic and anti-inflammatory roles of EPO-CH/AL hydrogels. These factors have been shown to be the major culprits of the late stages of SCI pathology, which can be potentially reversed by EPO. In addition, the regenerative effects of chitosan particles may add to the efficacy of the treatment. Further studies are warranted to investigate the advantages of EPO combination with various scaffolds which can exert beneficial effects in boosting EPO efficacy for the treatment of SCI.
Funding
This research received no specific grant from any funder.
Declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhao Y, Zuo Y, Wang X, Huo H, Jiang J, Yan H, et al. Effect of neural stem cell transplantation combined with erythropoietin injection on axon regeneration in adult rats with transected spinal cord injury. Genet Mol Res. 2015;14:17799–17808. doi: 10.4238/2015.December.22.4. [DOI] [PubMed] [Google Scholar]
- 2.Kaptanoglu E, Solaroglu I, Okutan O, Surucu HS, Akbiyik F, Beskonakli E. Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: Effect on lipid peroxidation and early ultrastructural findings. Neurosurg Rev. 2004;27(2):113–120. doi: 10.1007/s10143-003-0300-y. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Guo W, Xiong M, Zhang S, Han H, Chen J, et al. Erythropoietin facilitates the recruitment of bone marrow mesenchymal stem cells to sites of spinal cord injury. Exp Ther Med. 2017;13(5):1806–1812. doi: 10.3892/etm.2017.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matis GK, Birbilis TA. Erythropoietin in spinal cord injury. Eur Spine J. 2009;18(3):314–323. doi: 10.1007/s00586-008-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carelli S, Marfia G, Di Giulio AM, Ghilardi G, Gorio A. Erythropoietin: Recent developments in the treatment of spinal cord injury. Neurol Rest In. 2011;2011. [DOI] [PMC free article] [PubMed]
- 6.Bustos ML, Huleihel L, Kapetanaki MG, Lino-Cardenas CL, Mroz L, Ellis BM, et al. Aging mesenchymal stem cells fail to protect because of impaired migration and antiinflammatory response. Am J Respir Crit Care Med. 2014;189(7):787–798. doi: 10.1164/rccm.201306-1043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehlings MG, Wilson JR, Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery. 2014;61(Suppl 1):36–42. doi: 10.1227/NEU.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 8.Ozkunt O, Sariyilmaz K, Gemalmaz HC, Gürgen SG, Yener U, Dikici F. Investigation of efficacy of treatment in spinal cord injury: Erythropoietin versus methylprednisolone. J Orthop Surg. 2017;25(3):2309499017739481. doi: 10.1177/2309499017739481. [DOI] [PubMed] [Google Scholar]
- 9.Boran BO, Colak A, Kutlay M. Erythropoietin enhances neurological recovery after experimental spinal cord injury. Restor Neurol Neurosci. 2005;23(5–6):341–345. [PubMed] [Google Scholar]
- 10.Vitellaro-Zuccarello L, Mazzetti S, Madaschi L, Bosisio P, Fontana E, Gorio A, et al. Chronic erythropoietin-mediated effects on the expression of astrocyte markers in a rat model of contusive spinal cord injury. Neuroscience. 2008;151(2):452–466. doi: 10.1016/j.neuroscience.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Su P, Pan Z, Liu D, Niu Y, Zhu W, et al. Combination Therapy With Hyperbaric Oxygen and Erythropoietin Inhibits Neuronal Apoptosis and Improves Recovery in Rats With Spinal Cord Injury. Phys Ther. 2019;99(12):1679–1689. doi: 10.1093/ptj/pzz125. [DOI] [PubMed] [Google Scholar]
- 12.King VR, Averill SA, Hewazy D, Priestley JV, Torup L, Michael-Titus AT. Erythropoietin and carbamylated erythropoietin are neuroprotective following spinal cord hemisection in the rat. Eur J Neurosci. 2007;26(1):90–100. doi: 10.1111/j.1460-9568.2007.05635.x. [DOI] [PubMed] [Google Scholar]
- 13.Hong Z, Hong H, Chen H, Wang Z, Hong D. Protective effects of erythropoietin in experimental spinal cord injury by reducing the C/EBP-homologous protein expression. Neurol Res. 2012;34(1):85–90. doi: 10.1179/1743132811Y.0000000026. [DOI] [PubMed] [Google Scholar]
- 14.Utada K, Ishida K, Tohyama S, Urushima Y, Mizukami Y, Yamashita A, et al. The combination of insulin-like growth factor 1 and erythropoietin protects against ischemic spinal cord injury in rabbits. J Anesth. 2015;29(5):741–748. doi: 10.1007/s00540-015-2031-y. [DOI] [PubMed] [Google Scholar]
- 15.Chen SQ, Xu YJ, Yu C, Hao YM, Zhang ZD, Gu YP. Protective effect of erythropoietin on neurocyte apoptosis following experimental acute spinal cord injury in rats. Journal of Clinical Rehabilitative Tissue Engineering Research. 2008;12(11):2034–2038. [Google Scholar]
- 16.Li J, Guo W, Xiong M, Han H, Chen J, Mao D, et al. Effect of SDF-1/CXCR4 axis on the migration of transplanted bone mesenchymal stem cells mobilized by erythropoietin toward lesion sites following spinal cord injury. Int J Mol Med. 2015;36(5):1205–1214. doi: 10.3892/ijmm.2015.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Xiao Y, Zuo Y, Wang X, Huo H, Jiang J, et al. Repair effect of neural stem cells transplantation combined with erythropoietin on spinal cord injury in rats. Journal of Jilin University Medicine Edition. 2015;41(2):333–337. [Google Scholar]
- 18.Zhang H, Fang X, Huang D, Luo Q, Zheng M, Wang K, et al. Erythropoietin signaling increases neurogenesis and oligodendrogenesis of endogenous neural stem cells following spinal cord injury both in vivo and in vitro. Mol Med Rep. 2018;17(1):264–272. doi: 10.3892/mmr.2017.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Zuo Y, Jiang J, Yan H, Wang X, Huo H, et al. Neural stem cell transplantation combined with erythropoietin for the treatment of spinal cord injury in rats. Exp Ther Med. 2016;12(4):2688–2694. doi: 10.3892/etm.2016.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nekoui A, Tresierra VDCE, Abdolmohammadi S, Shedid D, Blaise G. Neuroprotective effect of erythropoietin in postoperation cervical spinal cord injury: case report and review. Anesthesiology and pain medicine. 2015;5(6). [DOI] [PMC free article] [PubMed]
- 21.Hashimoto T, Suzuki Y, Suzuki K, Nakashima T, Tanihara M, Ide C. Review Peripheral nerve regeneration using non-tubular alginate gel crosslinked with covalent bonds. J Mater Sci - Mater Med. 2005;16(6):503–509. doi: 10.1007/s10856-005-0524-1. [DOI] [PubMed] [Google Scholar]
- 22.Deng B, Shen L, Wu Y, Shen Y, Ding X, Lu S, et al. Delivery of alginate-chitosan hydrogel promotes endogenous repair and preserves cardiac function in rats with myocardial infarction. J Biomed Mater Res - Part A. 2015;103(3):907–918. doi: 10.1002/jbm.a.35232. [DOI] [PubMed] [Google Scholar]
- 23.Barros AGCd, Cristante AF, Santos GBd, Natalino RJM, Ferreira RJR, Barros-Filho TEPd. Evaluation of the effects of erythropoietin and interleukin-6 in rats submitted to acute spinal cord injury. Clinics. 2019;74. [DOI] [PMC free article] [PubMed]
- 24.Tawfik S, Abd Elsalam S, El-Hennawi H, Abd El-Thalouth I, Adel E, editors. Synthesis of partially carboxymethyl cellulose derived from rice straw and its utilization as dye adsorbent. International Conference on Applied Life Sciences. 2012: IntechOpen.
- 25.Sadiq A, Choubey A, Bajpai A. Biosorption of chromium ions by calcium alginate nanoparticles. J Chil Chem Soc. 2018;63(3):4077–4081. doi: 10.4067/s0717-97072018000304077. [DOI] [Google Scholar]
- 26.Liu Q, Li Q, Xu S, Zheng Q, Cao X. Preparation and properties of 3D printed alginate–chitosan Polyion complex hydrogels for tissue engineering. Polymers. 2018;10(6):664. doi: 10.3390/polym10060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lustriane C, Dwivany FM, Suendo V, Reza M. Effect of chitosan and chitosan-nanoparticles on post harvest quality of banana fruits. J Plant Biotechnol. 2018;45(1):36–44. doi: 10.5010/JPB.2018.45.1.036. [DOI] [Google Scholar]
- 28.Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. biomaterials. 2005;26(18):3919–28. [DOI] [PubMed]
- 29.Baysal K, Aroguz AZ, Adiguzel Z, Baysal BM. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int J Biol Macromol. 2013;59:342–348. doi: 10.1016/j.ijbiomac.2013.04.073. [DOI] [PubMed] [Google Scholar]
- 30.Wang G, Wang X, Huang L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: a pilot study in vitro. Biotechnol Biotechnol Equip. 2017;31(4):766–773. [Google Scholar]
- 31.Özdemir B, Batçık E, Ayçiçek E, Canaz G, Akdemir O, Alataş İ, et al. Investigation of dose-dependent neuroprotective effect of human recombinant erythropoietin in acute spinal cord injury induced rats. Int J Clin Exp Med. 2016;9(3):6385–6393. [Google Scholar]
- 32.Zhao Y, Ding W, Zhang W. The study of the effect of erythropoietin on motor function of hind limbs and the expression of NF-κB after acute spinal cord injury in rats. Chinese J Rehabilitation Med. 2007;22(10):904–907. [Google Scholar]
- 33.Cheng M, Cao W, Gao Y, Gong Y, Zhao N, Zhang X. Studies on nerve cell affinity of biodegradable modified chitosan films. J Biomater Sci Polym Ed. 2003;14(10):1155–1167. doi: 10.1163/156856203769231628. [DOI] [PubMed] [Google Scholar]
- 34.Cho Y, Shi R, Borgens RB. Chitosan produces potent neuroprotection and physiological recovery following traumatic spinal cord injury. J Exp Biol. 2010;213(9):1513–1520. doi: 10.1242/jeb.035162. [DOI] [PubMed] [Google Scholar]
- 35.Jian R, Yixu Y, Sheyu L, Jianhong S, Yaohua Y, Xing S, et al. Repair of spinal cord injury by chitosan scaffold with glioma ECM and SB 216763 implantation in adult rats. J Biomed Mater Res, Part A. 2015;103(10):3259–3272. doi: 10.1002/jbm.a.35466. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Zhu X, Zhang D. Transplantation of bone marrow mesenchymal stem cells overexpressing Shootin1 for treatment of spinal cord injury. Chinese J Tissue Eng Res. 2016;20(50):7507–7517. [Google Scholar]
- 37.Zhang H, Zheng H, Zhang Q, Wang J, Konno M. The interaction of sodium alginate with univalent cations. Biopolymers: Original Research on Biomolecules. 1998;46(6):395–402.
- 38.Wang C-C, Yang K-C, Lin K-H, Liu H-C, Lin F-H. A highly organized three-dimensional alginate scaffold for cartilage tissue engineering prepared by microfluidic technology. Biomaterials. 2011;32(29):7118–7126. doi: 10.1016/j.biomaterials.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 39.Prang P, Müller R, Eljaouhari A, Heckmann K, Kunz W, Weber T, et al. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27(19):3560–3569. doi: 10.1016/j.biomaterials.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 40.Okutan O, Solaroglu I, Beskonakli E, Taskin Y. Recombinant human erythropoietin decreases myeloperoxidase and caspase-3 activity and improves early functional results after spinal cord injury in rats. J Clin Neurosci. 2007;14(4):364–368. doi: 10.1016/j.jocn.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 41.Costa DD, Beghi E, Carignano P, Pagliacci C, Faccioli F, Pupillo E, et al. Tolerability and efficacy of erythropoietin (EPO) treatment in traumatic spinal cord injury: a preliminary randomized comparative trial vs. methylprednisolone (MP). Neurol Sci. 2015;36(9):1567–74. [DOI] [PubMed]