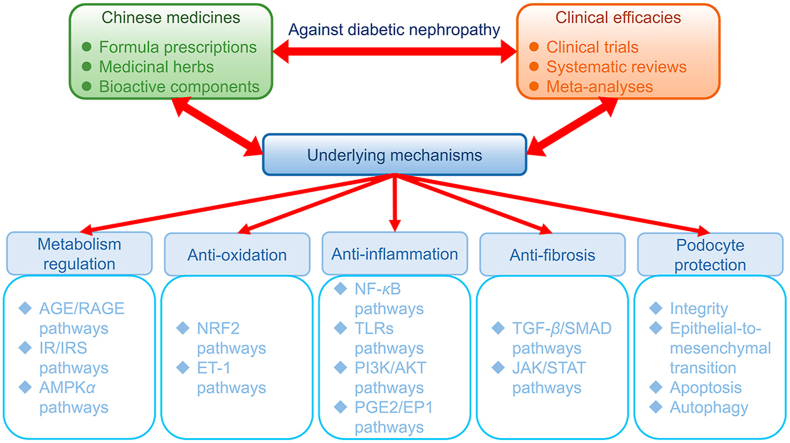
Abstract
Diabetic nephropathy (DN) has been recognized as a severe complication of diabetes mellitus and a dominant pathogeny of end-stage kidney disease, which causes serious health problems and great financial burden to human society worldwide. Conventional strategies, such as renin-angiotensin-aldosterone system blockade, blood glucose level control, and bodyweight reduction, may not achieve satisfactory outcomes in many clinical practices for DN management. Notably, due to the multi-target function, Chinese medicine possesses promising clinical benefits as primary or alternative therapies for DN treatment. Increasing studies have emphasized identifying bioactive compounds and molecular mechanisms of reno-protective effects of Chinese medicines. Signaling pathways involved in glucose/lipid metabolism regulation, antioxidation, anti-inflammation, anti-fibrosis, and podocyte protection have been identified as crucial mechanisms of action. Herein, we summarize the clinical efficacies of Chinese medicines and their bioactive components in treating and managing DN after reviewing the results demonstrated in clinical trials, systematic reviews, and meta-analyses, with a thorough discussion on the relative underlying mechanisms and molecular targets reported in animal and cellular experiments. We aim to provide comprehensive insights into the protective effects of Chinese medicines against DN.
Key words: Chinese medicine, Herbal medicine, Diabetic nephropathy, Diabetic kidney disease, Signaling pathway, Molecular target
Abbreviations: α-SMA, α smooth muscle actin; ACEI, angiotensin-converting enzyme inhibitor; ADE, adverse event; AGEs, advanced glycation end-products; ATK, protein kinase B; AM, mesangial area; AMPKα, adenosine monophosphate-activated protein kinase α; ARB, angiotensin receptor blocker; AREs, antioxidant response elements; BAX, BCL-2-associated X protein; BCL-2, B-cell lymphoma 2; BCL-XL, B-cell lymphoma-extra large; BMP-7, bone morphogenetic protein-7; BUN, blood urea nitrogen; BW, body weight; C, control group; cAMP, cyclic adenosine monophosphate; CCR, creatinine clearance rate; CD2AP, CD2-associated protein; CHOP, C/EBP homologous protein; CI, confidence interval; COL-I/IV, collagen I/IV; CRP, C-reactive protein; CTGF, connective tissue growth factor; D, duration; DAG, diacylglycerol; DG, glomerular diameter; DKD, diabetic kidney disease; DM, diabetes mellitus; DN, diabetic nephropathy; eGFR, estimated GFR; eIF2α, eukaryotic initiation factor 2α; EMT, epithelial-to-mesenchymal transition; EP, E-prostanoid receptor; ER, endoplasmic reticulum; ESRD, end-stage renal disease; ET-1, endothelin-1; ETAR, endothelium A receptor; FBG, fasting blood glucose; FN, fibronectin; Gαq, Gq protein alpha subunit; GCK, glucokinase; GCLC, glutamate-cysteine ligase catalytic subunit; GFR, glomerular filtration rate; GLUT4, glucose transporter type 4; GPX, glutathione peroxidase; GRB 10, growth factor receptor-bound protein 10; GRP78, glucose-regulated protein 78; GSK-3, glycogen synthase kinase 3; HbA1c, glycosylated hemoglobin; HDL-C, high density lipoprotein-cholesterol; HO-1, heme oxygenase-1; ICAM-1, intercellular adhesion molecule-1; IGF-1, insulin-like growth factor 1; IGF-1R, insulin-like growth factor 1 receptor; IκB-α, inhibitory protein α; IKK-β, IκB kinase β; IL-1β/6, interleukin 1β/6; IR, insulin receptor; IRE-1α, inositol-requiring enzyme-1α; IRS, insulin receptor substrate; JAK, Janus kinase; JNK, c-Jun N-terminal kinase; LC3, microtubule-associated protein light chain 3; LDL, low-density lipoprotein; LDL-C, low density lipoprotein-cholesterol; LOX1, lectin-like oxidized LDL receptor 1; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemotactic protein-1; MD, mean difference; MDA, malondialdehyde; MMP-2, matrix metallopeptidase 2; mTOR, mammalian target of rapamycin; MYD88, myeloid differentiation primary response 88; N/A, not applicable; N/O, not observed; NOX-4, nicotinamide adenine dinucleotide phosphate-oxidase-4; NQO1, NAD(P)H:quinone oxidoreductase 1; N/R, not reported; NRF2, nuclear factor erythroid 2-related factor 2; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; OCP, oxidative carbonyl protein; ORP150, 150-kDa oxygen-regulated protein; p62, sequestosome 1 protein; P70S6K, 70-kDa ribosomal protein S6 kinase; PAI-1, plasminogen activator inhibitor-1; PARP, poly(ADP-Ribose) polymerase; PBG, postprandial blood glucose; PERK, protein kinase RNA-like eukaryotic initiation factor 2A kinase; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1α; PGE2, prostaglandin E2; PI3K, phosphatidylinositol 3 kinases; PINK1, PTEN-induced putative kinase 1; p-IRS1, phospho-IRS1; PKC, protein kinase C; PTEN, phosphatase and tensin homolog; RAGE, receptors of AGE; RASI, renin-angiotensin system inhibitor; RCT, randomized clinical trial; ROS, reactive oxygen species; SCr, serum creatinine; SD, standard deviation; SD-rat, Sprague–Dawley rat; SIRT1, sirtuin 1; SMAD, small mothers against decapentaplegic; SMD, standard mean difference; SMURF-2, SMAD ubiquitination regulatory factor 2; SOCS, suppressor of cytokine signaling proteins; SOD, superoxide dismutase; STAT, signal transducers and activators of transcription; STZ, streptozotocin; T, treatment group; TBARS, thiobarbituric acid-reactive substance; TC, total cholesterol; TCM, traditional Chinese medicine; TFEB, transcription factor EB; TG, triglyceride; TGBM, thickness of glomerular basement membrane; TGF-β, tumor growth factor β; TGFβR-I/II, TGF-β receptor I/II; TII, tubulointerstitial injury index; TLR-2/4, toll-like receptor 2/4; TNF-α, tumor necrosis factor α; TRAF5, tumor-necrosis factor receptor-associated factor 5; UACR, urinary albumin to creatinine ratio; UAER, urinary albumin excretion rate; UMA, urinary microalbumin; UP, urinary protein; VCAM-1, vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor; WMD, weight mean difference; XBP-1, spliced X box-binding protein 1
Graphical abstract
Diabetic nephropathy is one of the most severe complications of diabetes mellitus. Chinese medicines have been intensively studied in treating diabetic nephropathy. This review comprehensively summarizes and discusses the clinical efficacies and underlying mechanisms of Chinese medicines for diabetic nephropathy, providing deep insights and profound perspectives into this field.
1. Introduction
Diabetic nephropathy (DN), or diabetic kidney disease (DKD), is a severe microvascular complication of diabetes mellitus (DM)1,2. DN progressively develop upon onset of hyperglycaemia from low grade renal inflammation to renal fibrosis, renal sclerosis, and ultimately end-stage renal disease (ESRD)3. It is indispensable for the patients to receive dialysis or kidney transplantation when DN develops into ESRD4. DN induces a great burden to public health because of the worldwide prevalence and serious health lesion5.
According to the classic descriptions of DN, several histopathological alterations may exist in the kidney of patients. In the glomerulus, capillary lumens extension, basement membrane thickness, extracellular matrix expansion, podocyte injury, and fibrosis are generally observed; while in the tubulointerstitium, vacuolar degeneration, loose arrangement, and fibrosis can be detected6, 7, 8. Patients are usually determined with glomerular hyperfiltration, microproteinuria, macroproteinuria, and then decreased glomerular filtration rate (GFR) with the progression of DN9. The urinary protein (UP) levels including urinary albumin excretion rate (UAER) for patients with microproteinuria or 24-h UP for patients with macroproteinuria are often regarded as the primary indices for DN detection in clinical practice10. Other indices may involve measurements regarding renal functions, blood glucose/lipid levels, and overall symptoms10.
So far, strategies for DN treatment and management mainly concern the reduction in body weight, blood glucose, and blood pressure, with renin-angiotensin system inhibitors (RASIs) including angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) as commonly used first-line therapies11,12. However, satisfactory outcomes did not always occur with the use of these conventional approaches in treating DN. For example, ARB was reported not sufficient for the prevention of albuminuria progression in DM patients with normal blood pressure13. In a multicenter, controlled trial involving 285 normotensive patients with type 1 diabetes and normoalbuminuria, early blockade of the renin–angiotensin system with the use of losartan (100 mg daily), enalapril (20 mg daily) did not slow nephropathy progression but retinopathy progression14. Chinese medicine has a long history as a commonly used therapy for DM and relative complications like DN with promising effectiveness in clinical practice15,16. Some bioactive compounds in Chinese medicines that benefit DN patients have been identified and purified, and the mechanisms of action have also been widely investigated. In this review, we aim to provide updated and comprehensive insights into the clinical efficacies of Chinese medicines and bioactive compounds for DN treatment and management and emphasize the underlying mechanisms and molecular targets, especially those signaling pathways involved in metabolism regulation, antioxidant, anti-inflammatory, anti-fibrosis, and podocyte-protective actions.
2. Clinical efficacies of Chinese medicines in DN treatment
DN is a common complication that severely lower the overall life quality of patients with DM, which remains an unsettled public issue regarding human health. Traditional Chinese medicines (TCMs) are extensively utilized as either independent or adjuvant therapy for DM and DN, and exhibit promising efficacy in clinical practice17, 18, 19, 20. As revealed by a meta-analysis referring to 29 randomized clinical trials (RCTs) involving 2440 DN patients, Chinese herbal medicine was more efficacious in decreasing UAER and proteinuria compared with placebo and even some RASI19. Similarly, another meta-analysis with 20 selective RCTs involving 2719 DN patients reported that Chinese herbal medicine was observed to significantly reduce albuminuria when compared with placebo, regardless of the concurrent administration of RASI or not20. In addition, combined therapy of Chinese herbal medicine and ACEI/ARB could exert a greater beneficial action on proteinuria, UAER, urinary albumin to creatinine ratio (UACR) and urinary protein to creatinine ratio than ACEI/ARB alone19. Besides the details demonstrated below, the results from those studies investigating the clinical efficacies of Chinese medicine on DN are also displayed in the Supporting Information Table S1 13,21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57.
2.1. Chinese medicine prescriptions for DN treatment
Some Chinese medicine formulae comprised of several medicinal herbs have been developed to treat DN, such as Buyang-Huanwu Decoction, Danshao Decoction, Didang Decoction, Jiawei-Zhuling Decoction, Liuwei-Dihuang Pill, Qidan-Dihuang Grain, Qidi-Yiqi-Yangyin-Huoxue Recipe, Shenqi-Dihuang Decoction, Shenshuaining Granule, Tangshen Formula, Tongluo Capsule, Xiaoke-Shen'an Capsule, Yiqi-Huayu-Jiangzhuo Formula, and Zishen-Tongluo Granule13,21,22,36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48. It has been shown that Chinese medicines exert promising efficacies and are even superior to some conventional therapies owing to the multiple bioactive phytochemicals and molecular targets of action, with rarely observed adverse effects in clinical practice43,45,47,48. For example, Tongluo Capsule and Zishen-Tongluo Granule were reported to provide more protections against DN when compared with ACEI45,48. In a RCT with 97 DN patients, Tongluo Capsule (4 capsules per time, 3 times per day, 24 weeks) lowered 24-h UP [mean difference (MD)±standard deviation (SD): −1.12 ± 0.22 g/24 h)] compared with baseline, and was more effective in improving serum creatinine (SCr), blood urea nitrogen (BUN), total cholesterol (TC), triglyceride (TG), low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), and tumor growth factor-β1 (TGF-β1) compared with perindopril (4 mg per time, twice daily, 24 weeks)45. In another RCT enrolling 45 patients suffering from inchoate DN, Zishen-Tongluo Granule (150 mL with half package of the granule per time, twice daily, 36 weeks) was more effectual in comparison with benazepril (10 mg per time, once daily, 36 weeks) at ameliorating renal function and metabolism as revealed by improved biomarkers including UAER [MD (95% CI): −106.99 (−121.29, −85.55) vs. −69.38 (−86.89, −51.86) μg/min], SCr, endogenous creatinine clearance rate (CCR), fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), TC and TG levels; the mechanisms of action may involve regulating endothelin-1 (ET-1), atrial natriuretic peptide, and vascular endothelial growth factor (VEGF)48. In addition, in a RCT containing 204 DN patients, Shenshuaining Granule (5 g per time, 3 times per day, 12 weeks) could significantly decreased 24-h urinary microalbumin (UMA, MD±SD: −6.2 ± 2.2 mg/24 h) compared with baseline, and also reduced SCr and BUN compared with telmisartan (80 mg per time, once daily, 12 weeks), a commonly used ARB for DN patients43. While in a RCT recruiting 72 patients with DN, treatment with Yiqi-Huayu-Jiangzhuo Formula (3 times per day, 4 weeks) as compared with piperazine (3 times per day, 4 weeks) significantly decreased UAER and blood levels of 2-h postprandial blood glucose (PBG), FBG, HbA1c, TC, TG, C-reactive protein (CRP), and fibrinogen, with no observed allergic reactions or other adverse effects on liver, kidney, and gut-intestine47. These results together indicated that Yiqi-Huayu-Jiangzhuo Formula could serve as an efficacious and safe therapeutics for DN treatment.
Some Chinese medicine prescriptions, including Jiawei-Zhuling Decoction, Qidan-Dihuang Grain, and Shenqi-Dihuang Decoction, were reported to provide additional effects to ACEI or ARB in treating DN13,38,39,42. In a RCT involving 205 DN patients, treatment with Shenqi-Dihuang Decoction (1 decoction per day, twice daily) plus metformin (500 mg per day) and captopril (12.5 g per time, twice daily) for 12 weeks significantly decreased UMA [MD±SD: (−13.96 ± 4.29) vs. (−10.71 ± 2.62) mg/L], β2-microglobuli, and serum interleukin 6 (IL-6), IL-8, tumor necrosis factor α (TNF-α), CRP, and ET-1, while improving endothelial function when compared with metformin and captopril, an ACEI for DN patients42. In another RCT with 102 normotensive DN patients, treatment of Qidan-Dihuang Grain (1 package per time, twice daily, 12 weeks) plus ARB at minimum recommended dosage significantly ameliorated 24-h urinary albuminuria, total proteinuria, and UACR on the 4th, 8th, and 12th week compared to baseline, while lowering 24-h urinary albuminuria at the 8th [mean (95% CI): 51.00 (37.00, 90.00) vs. 70.00 (53.00, 100.93) mg/24 h] and 12th [mean (95% CI): 41.40 (29.00, 68.00) vs. 47.65 (36.30, 100.53) mg/24 h] week compared with ARB (at the minimum recommended dosage)13. Besides Qidan-Dihuang Grain, Jiawei-Zhuling Decoction was also demonstrated to improve clinical efficacy when combined with ARB, losartan38,39. It was reported in a RCT with 60 DN patients that Jiawei-Zhuling Decoction (1 decoction per day, 3 times per day) plus losartan (100 mg per time, once daily) improved 24-UP [MD±SD: (−2.08 ± 1.09) vs. (−1.83 ± 0.88) g/24 h], UMA [MD±SD: (−10.52 ± 7.28) vs. (−5.13 ± 4.65) mg/L], FBG, 2-h PBG, BUN, and SCr compared with losartan alone after 8-week treatment39. Similarly, in another RCT with 88 DN patients, at the same dose and duration, Jiawei-Zhuling Decoction plus losartan was also observed to improve 24-h UP [mean ± SD: (1.1 ± 0.4) vs. (1.6 ± 0.6) g/24 h], SCr, BUN, and serum microalbumin significantly38.
Notably, though intensive blood glucose control can prevent the development of microvascular complications, especially at the early stage of DM, it seems that hypoglycemic action is not always necessary for the protective effect against DN7,11. As shown in a meta-analysis including 1275 patients from 18 RCTs, Liuwei-Dihuang Pill plus Western drugs versus Western drugs lowered 24-h UP [standard mean difference (SMD) (95% CI): −0.67 (−0.95, −0.38)], UAER [MD (95% CI): −43.65 (−45.73, −41.58)] and UMA [SMD (95% CI): −1.37 (−1.68, −1.06)] as well as BUN, SCr, FBG, PBG, and HbA1c22. However, in another meta-analysis with 26 RCTs including 2198 early-stage DN participants, Jinshuibao Capsule combined with ARB compared with ARB alone remarkably decreased systolic/diastolic blood pressure, 24-h UP [MD (95% CI): −93.32 (−128.60, −58.04)], UAER [MD (95% CI): −24.02 (−30.93, −17.11)], UACR [MD (95% CI): −17.55 (−22.81, −12.29)], SCr, BUN, and TG, but not serum TC, FBG, HbA1c, and β2-microglobulin23. In addition, there is a phenomenon called metabolic memory that presents as the persistent long-standing expressions of DKD-related genes and phenotypes induced by previous hyperglycaemia despite subsequent glycaemic control58. In this regard, simply blood glucose control is not sufficient enough for DKD management, at least at the late stage of DM. Furthermore, tight blood glucose control in the physiological range (FBG: 4–6 mmol/L, PBG: <7.8 mmol/L) is difficult to achieve, and it is even reported to exert a negative influence on mortality7. Thus, tight blood glucose control is no longer recommended to patients with DM for preventing the progression of DN.
2.2. Medicinal herbs for DN treatment
Herbs with potent pharmaceutical properties are perfect natural sources of ingredients to develop a novel therapy for clinical use. Some medicinal herbs that are commonly applied in TCM for DN treatment, such as Cordyceps (described previously as Jinshuibao Capsule), Flos Abelmoschi, Folium Ginkgo, Folium Mori, Radix Astragali, and Radix et Rhizoma Tripterygii, have been demonstrated with reno-protective effects against DN as proved by RCTs and meta-analyses23, 24, 25, 26, 27, 28,49, 50, 51.
Radix Astragali, the root of Astragalus membranaceus (Fisch.) Bge., and its extracts exhibit a profound beneficial effect on DN with satisfactory safety profile, which may be attributed to the major bioactive constituents including flavonoids and saponins24,25. It has been used clinically as Astragalus Injection or Astragalus Oral Liquid. As analyzed and summarized by a meta-analysis containing 21 RCTs and 4 semi-randomized control trials with in total 1804 patients, Astragalus Injection with/without ACEI/ARB showed a significant reno-protective effect on DN patients, in terms of improving 24-h UP [SMD (95% CI): −1.78 (−2.54, −1.02)], UMA [WMD (95% CI): −53.37 (−79.81, 26.93)], BUN, SCr, CCR, and serum albumin levels when compared to the counterpart25. In addition, another meta-analysis comprising 66 RCTs with in total 4785 DN patients demonstrated that adjunctive use of Astragalus Injection could markedly reduce more albuminuria [SMD (95% CI): −2.05 (−2.49, −1.61)], proteinuria [SMD (95% CI): −1.85 (−2.34, −1.37)], and SCr levels than conventional therapies alone did, while Astragalus Oral Liquid was also observed to decrease more albuminuria [SMD (95% CI): −1.27 (−1.82, −0.73)]24. Adverse effects were not observed/reported by original studies in the above meta-analysis24,25. These results suggested that Radix Astragali may be a good approach to treat DN with satisfying efficacy and safety, no matter used as a dominant therapy or an adjunctive medicine.
Flos Abelmoschi, the flower of Abelmoschus manihot (L.) Medic., has a long history as an herbal medicine for chronic glomerulonephritis treatment. Huangkui Capsule or Huangshukuihua Capsule prepared from its extract has recently been adopted as important adjunctive therapeutics to patients with acute/chronic kidney diseases like DN, alleviating proteinuria and hematuria and improving kidney function partially via inhibiting immune reaction, inflammatory injury, and interstitial fibrosis26,27. In a meta-analysis consisting of 7 RCTs within a total of 531 DN patients, Huangkui Capsule or Flos Abelmoschi Extract was found to significantly reduce proteinuria [MD (95% CI): −317.32 (−470.48, −164.17) mg/24 h] and SCr26. Moreover, a meta-analysis for 27 RCTs involving 2239 DN patients reported that Huangshukuihua Capsule could significantly improve renal function, as revealed by the improvement in BUN and SCr27. Serious adverse events caused by Flos Abelmoschi treatment were rarely reported, though mild to moderate gastrointestinal discomfort occasionally occurred with no statistical significance between treatment and control groups, which is tolerable to the patients26,27. Thus, Flos Abelmoschi can serve as a promising indispensable therapeutic strategy or a conjugant medicine in addition to first-line therapies for DN.
Radix et Rhizoma Tripterygii, the root and rhizome of Tripterygium wilfordii Hook, has been widely used as a Chinese medicine for many years and in many ways, especially in treating glomerulonephritis and DN, showing beneficial effects against kidney inflammation, podocyte injury, and albuminuria/proteinuria28,49. As reported in a RCT with 124 DN patients, 24-week treatment with Radix et Rhizoma Tripterygii Extract Tablet (60 mg per day) plus valsartan (160 mg per day) compared to valsartan monotherapy significantly resulted in more reduction of proteinuria (MD±SD: −1.59 ± 0.44 g/24 h) (MD±SD: 0.69 ± 0.53 g/24 h), with significant increase in serum albumin level49. A meta-analysis involving 1414 DN patients from 22 RCTs systematically evaluated the clinical efficacy of Radix et Rhizoma Tripterygii in the treatment of stage IV DN, and the results suggested that compared with ACEI/ARB alone, Radix et Rhizoma Tripterygii plus ACEI/ARB showed significant effects in reducing 24-h UP [MD (95% CI): −0.87 (−1.03, −0.71)] and raising serum albumin28. However, it is of note that Radix et Rhizoma Tripterygii may increase the risk of adverse event (ADE), including liver function damage, gastrointestinal reaction, menstrual disorder, leukopenia, hyperkalemia, itchy skin, rash, leucopenia, and joint pain28,49. Thus, appropriate dose and duration should be taken into consideration when applying Radix et Rhizoma Tripterygii in DN patients to avoid ADE.
2.3. Isolated phytochemicals for DN treatment
Some bioactive phytochemicals contained in Chinese medicines have been extracted and isolated using modern technologies, then developed as principal or adjuvant therapies and widely used for the purpose of treating DN. Modern pharmacological researches and clinical trials have demonstrated the therapeutic effects of these phytochemicals, mainly belonging to the classic of polyphenols (breviscapine, puerarin, resveratrol, safflower yellow, silymarin), alkaloids (berberine, ligustrazine, paeony glucosides, tripterygium glycosides) and hydroxyanthraquinones (emodin) that are discussed below in order30, 31, 32, 33, 34, 35,54, 55, 56, 57.
Breviscapine, purified flavonoids isolated from Herba Erigerontis [Erigeron breviscapus (Vant.) Hand.-Mazz], mainly contains scutellarein-7-glucuronide (scutellarin, 90%) and apigenin-7-O-glucoside (4%). Breviscapine has been established as principal therapy and used in injection form for the treatment of ischemic cardiovascular/cerebrovascular diseases and chronic renal diseases30,31. As shown in a meta-analysis involving 35 RCTs with 2320 DN patients, Breviscapine Injections with/without ACEI/ARB exerted greater curative properties if compared with ACEI/ARB/placebo, in protecting renal injury by decreasing 24-h UP [SMD (95% CI): −1.42 (−1.83, −1.02)], SCr, and BUN, as well as attenuating dyslipidemia by improving levels of TC, TG, and high-density lipoproteins31. In another meta-analysis including 2097 DN patients from 37 RCTs, Breviscapine Injection plus basic therapy improved 24-h UP [SMD (95% CI): −1.21 (−1.56, −0.87)], UAER [MD (95% CI): −20.30 (−28.14, −12.46)], UMA [MD (95% CI): −10.03 (−10.62, −9.46)], SCr, BUN, and serum albumin in stage Ⅲ DN; 24-h UP [SMD (95% CI): −0.52 (−0.71, −0.33)], SCr, and BUN in stage IV DN; and SCr in stage V DN30. Overall, the safety of Breviscapine Injection is satisfactory in patients, except for the reported dry cough and dry allergy, which is not surely resulted from Breviscapine Injection or enalapril during intervention30.
Puerarin, also known as kakonein, is the main isoflavonoid c-glycoside found in Radix Puerariae [Pueraria lobata (wild)]. Modern pharmacological research has illustrated that puerarin exerts a protective effect against myocardial injury, retinopathy, DM, and its complication DN, by improving insulin sensitivity, increasing glucose utilization, and promoting blood circulation33. In a meta-analysis with 10 RCTs and 669 DN participants, combined treatment of puerarin and ACEI led to a significant reduction in UAER [MD (95% CI): −23.43 (−33.95, −12.91)], though showing no action in 24-h UP [MD (95% CI): −56.76 (−122.65, 9.12)], BUN, and SCr, with ventral indisposition and sicchasia (2 participants) reported during intervention within one trial33.
Resveratrol, namely 3,5,4′-trihydroxystilbene, is a natural plant polyphenol (more specifically classified as stilbenes) firstly isolated from Radix Platycodonis [Platycodon grandiflorum (Jacq.) A.DC.], which has been proposed for preventing and treating hyperlipidemia, atherosclerosis, fatty liver, diabetes, and DN55. As evaluated in a randomized, double-blind placebo-controlled trial recruiting 60 DN patients, resveratrol (500 mg per day) plus losartan (12.5 mg per day) compared to placebo (500 mg per day) plus losartan (12.5 mg per day) exerted more protective effects after 90-day intervention in patients, showing improvements in UACR [MD (95% CI): −46.4 (−64.5, −28.3) vs. 29.9 (4.9, 54.9) mg/g] and serum antioxidant enzymes, though no alteration was observed in estimated GFR (eGFR) [MD (95% CI): 1.7 (−3.4, 6.8) vs. −4.0 (−8.2, 0.2) mL/min/1.73 m2] and SCr55. Two participants (one in the treatment group, the other in the placebo group) complained of gastrointestinal ADE like mild dyspepsia. Resveratrol is considered relatively safe for human without hazard of liver injury.
Safflower yellow, earlier known as carthamine (classified as a flavone) extracted from Flos Carthami (Carthamus tinctorius L.), has been shown with properties in inhibiting platelet/neutrophil adhesion, vascular endothelium damage, and vascular smooth muscle hyperplasia as well as ameliorating DN-related diseases by modulating hemodynamics, oxidative stress, fibrosis, hypolipidemia, and apoptosis34. Safflower Yellow Injection used alone or in combination with Western medicine was better than Western medicine/placebo alone in treating DN, showing significant benefits to lessen UAER [MD (95% CI): −39.70 (−52.05, −27.35)], BUN, FBG, and high-sensitivity CRP, as assessed in a meta-analysis including 1289 DN participants from 18 RCTs34. Of note, 4 trials reported ADE during intervention, including headache, nausea, fatigue, and orthostatic hypotension after pausing/slowing the drip, but the safety of Safflower Yellow Injection was still more appropriate than that of Western medicine alone.
Silymarin is the main bioactive components extracted from Fructus Silybi (Silybum marianum), which approximately (70%–80%) consists of a mixture of three flavonolignans (silybin, silydianin, and silychristin). Recent studies have suggested that silymarin possesses functions of antioxidation, hepato-protection, and reno-protection56. A randomized placebo-controlled trial has assessed the reno-protective effect of silymarin against DN in 102 patients, but there were no significant differences in the primary and renal outcomes between the two groups treated with silymarin (150 mg per time, 3 times per day) and placebo after 2-year intervention, though with a significant reduction in the hospitalization rate56.
Berberine, benzylisoquinoline alkaloid with heteropentacyclic group, is the main bioactive alkaloid extracted from Rhizoma Coptidis (Coptis chinensis Franch.)59. Berberine has been reported with various health functions including antioxidant, anti-inflammatory hpyerglycemic, hypolipidemic, and reno-protective effects29,52. As observed in a RCT with 67 DN patients, patients treated with berberine (0.1 g per time, 3 times per day, 24 weeks) were detected with lowered UAER, FBG, HbA1c, and increased glutathione peroxidase (GPX) levels compared with those treated with only basic therapies52. A meta-analysis involving 437 DN patients from 7 RCTs also demonstrated that treatment with berberine with/without ACEI/ARB reduced 24-h UP [MD (95% CI): −0.52 (−0.83, −0.22)], FBG, TC, and CRP levels compared with ACEI/ARB/placebo, but it may also increase the risk of ADE, which mainly presented as gastrointestinal reactions like coprostasis that reported in 3 trials29.
Ligustrazine, also named as tetramethylpyrazine and classified as an alkylpyrazine, is a bioactive phytochemical derived from Rhizoma Chuanxiong (Ligusticum chuanxiong Hort)32. Ligustrazine is widely used to treat ischemia/reperfusion-induced myocardial, cerebral, and renal injury. In addition, ligustrazine could reduce 24-h UP, inhibit blood glucose level and BUN elevation, and decrease the degree of lipoperoxidation32. Ligustrazine has also been developed as Ligustrazine Injection for postponing DN progression. A meta-analysis containing 25 RCTs with 1645 DN patients reported Ligustrazine Injection with/without ACEI/ARB compared with ACEI/ARB/placebo exhibited significant beneficial effects on the clinical outcomes regarding 24-h UP [SMD (95% CI): −0.36 (−0.56, −0.17)], UAER [SMD (95% CI): −21.42 (−29.01, −13.83)], UMA [SMD (95% CI): −50.78 (−72.20, −29.36)], BUN, and SCr32.
Paeony glucosides are the active compounds extracted from Raidx Paeoniae Alba or Radix Paeoniae Rubra (Paeonia lactiflora Pall.), and mainly contain paeoniflorin, albiflorin, hydroxy paeoniflorin, benzoyloxypaeoniflorin, and other monoterpene glycosides54. In clinical practice, paeony glucosides have been applied to treat systemic lupus erythematosus, rheumatoid arthritis, chronic glomerulonephritis, and lupus nephritis, and these therapeutic actions are considered to be correlated with antioxidant, anti-inflammatory, and immunosuppressive effects54. In an open-label, randomized, parallel-grouped, single-site trial with 76 DN patients, paeony glucosides (1800 mg per day) plus losartan (100 mg per day) compared to losartan alone (100 mg per day) lowered UAER [mean ± SD: (132.58 ± 32.42)/(93.54 ± 30.16)/(56.87 ± 11.71) vs. (138.4 ± 38.64)/(112.23 ± 28.57)/(104.22 ± 34.24) mg/24 h, at baseline/12 weeks/24 weeks], high sensitivity-CRP, monocyte chemotactic protein-1 (MCP-1), and TNF-α in patients after 24-week treatment54. Of note, in the group treated with paeony glucosides, 3 cases of drug-related ADE occurred with tolerable gastrointestinal symptoms including changes in stool properties (2 cases) and abdominal pain (1 case) to a lesser extent.
Tripterygium glycosides are a series of phytochemicals of Radix et Rhizoma Tripterygii, a Chinese herbal medicine used in rheumatoid arthritis treatment for its immunosuppressive and anti-inflammatory effects35. In addition, tripterygium glycosides can serve as promising reno-protective therapies for the treatment of DN regarding attenuating albuminuria. In a meta-analysis performed among 12 RCTs involving 829 DN patients, the combined utilization of tripterygium glycosides/valsartan was observed to increase serum albumin level while decreasing 24-h UP [MD (95% CI): −0.97 (−1.19, −0.76)], UAER [MD (95% CI): −145.53 (−227.95, −63.11)], and urinary β2-microglobuli level, though no significant effect was detected on the levels of BUN, SCr, and CCR35. However, it seemed that tripterygium glycosides/valsartan combination exerted more side-effects compared to valsartan alone35. In a RCT with 67 DN patients, treatment (last for 24 weeks, and after 4 weeks, treatment continues for 2 weeks following with a pause for 2 weeks) with triptolide (1–2 mg per kg body weight per day) plus benazepril (5–20 mg per day) compared with benazepril decreased UAER, CCR, serum albumin, urinary β2-microglobuli, N-acetyl-β-d-glucosaminidase, and MCP-1 levels in patients57. These intervention methods with paused period may reduce the risk of ADE while maintaining the satisfactory clinical efficacy, which offers a reference for the purpose of minimizing side effects of Chinese medicine, though more trials are warranted for confirmation.
Emodin, also known as schuttgelb or archin, belongs to the class of organic compounds recognized as hydroxyanthraquinones. Emodin is derived from Radix Et Rhizoma Rhei (Rheum palmatum L.), a Chinese herb with a variety of bioactivities including immunosuppression, anti-inflammation, and anti-proliferation60,61. In addition, previous studies have revealed that emodin was effective to suppress cell proliferation and fibronectin expression in rat mesangial cells cultured under high glucose, thus possess beneficial effect on DN patients61. A small RCT investigated the effect of emodin in 32 DN patients, and the results showed that treatment group with emodin (200 mg per day, 8 weeks) alone, fluvastatin (40 mg per day, 8 weeks) alone, and their combination exhibited reduced 24-h UP [MD±SD: (2.63 ± 0.18), (2.53 ± 0.12), (2.49 ± 0.14), vs. (3.32 ± 0.18) g/24 h, respectively] and serum TGF-β1 as compared to control group with basic therapy, while no significant difference was observed in the three treatment group53.
Based on these results from RCTs, systematic reviews and meta-analyses, it can be summarized that Chinese medicines possess relatively potent clinical efficacies in remedying DN, in terms of ameliorating proteinuria and improving renal functions. The safety of Chinese medicine is acceptable in many practices, whereas some adverse effects like abdominal discomfort and nausea, though not severe or prevalent, could be observed in a few studies. Thus, Chinese medicines may be promising candidates for DN management, but it is still of great importance to ensure the safety of Chinese medicine to acquire a more satisfactory curative effect.
3. Mechanisms involved in the protective effects of Chinese medicines against DN
Many studies have focused on identifying and verifying the underlying mechanisms involved in the protective effects of Chinese medicines against DN. Generally, those mechanisms may include metabolism regulation, antioxidation, anti-inflammation, anti-fibrosis, and podocyte protection, which are thoroughly discussed below. In addition, a table (Supporting Information Table S2 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136) is also provided for quick check.
3.1. Metabolism regulation
DN has been deemed as one of the serious long-term complications of DM influencing microvasculature, while hyperglycaemia is a crucial factor for DN initiation as revealed by the data from various animal and human studies137,138. At the early stage, hyperglycemia and disturbed glucose/lipid metabolic homeostasis driven by hyperglycaemia gradually promote the structural and functional alterations in the kidney, such as hypertension, hyperfiltration, basement membrane thickness, and mesangial matrix expansion in glomerulus as well as hypertrophy in both glomerulus and tubule, along with microalbuminuria139, 140, 141. Blocking metabolism dysfunction, maintaining glucose homeostasis and ameliorating hyperglycaemia can serve as potential approaches to slow down and even prevent the pathogenesis of DN in patients with DM67,142. Some Chinese medicines and herb-derived constituents have been shown with anti-hyperglycaemic property, showing reno-protective effects against DN via glucose/lipid metabolism-related signaling pathways.
3.1.1. AGE/RAGE related pathways
Advanced glycation end-products (AGEs) refer to series substances that are generated during the non-enzymatic bio-reactions between the aldehyde group of saccharide and amino group of protein, lipid, or nucleic acid111. AGEs may lead to the irreversible transformation of tissue proteins (due to protein glycation) and consequently several pathological conditions including vascular permeability augment, cytokine release induction, and nitric oxide inactivation62. AGEs accumulation in the kidney has been implicated in hypotrophy of glomerulus and tubule as well as progressive proteinuria62. In addition, expression of the receptors of AGEs (RAGEs) is also crucial in the pathogenesis of DN66.
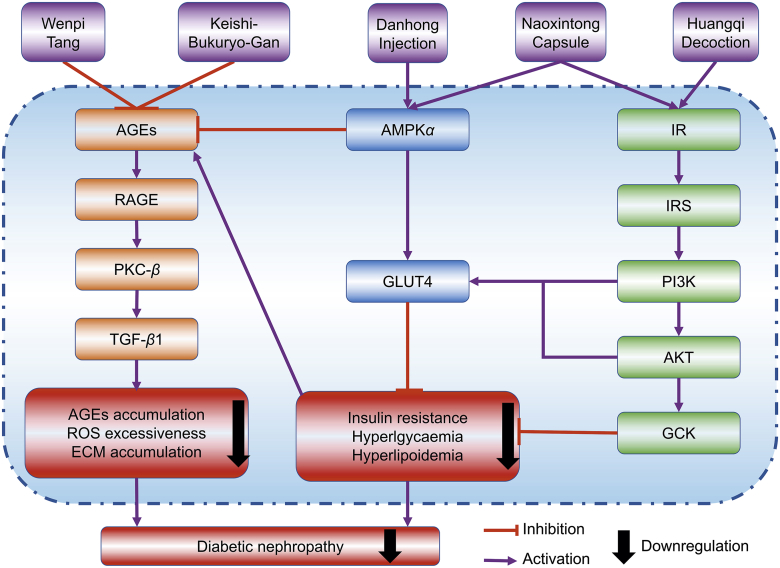
Wenpi Tang is a TCM prescription for the treatment of patients with moderate renal failure. It was reported that Wenpi Tang treatment [50, 100, and 200 mg/kg body weight (BW), daily, 15 weeks] to male Wistar rats with DN (induced by streptozotocin, STZ) resulted in significant reductions in FBG, BUN, serum TG, and thiobarbituric acid-reactive substance (TBARS) levels and ameliorations in mesangial matrix expansion, glomerular sclerosis, and renal lesions, partially by attenuating the disorders of the glucose-dependent metabolic pathway and decreasing AGEs accumulation in the kidney62. In another study, the researchers reported that Keishi-Bukuryo-Gan (50, 100, and 200 mg/kg BW, daily, 15 weeks) significantly improved the increased BUN and SCr levels, decreased CCR and depraved proteinuria with consistent improvement in pathological alterations of the kidney, including diffuse, nodular and exudative lesions, and vacuolization in arteriole, which may be attributed to the inhibition in hyperglycemia-mediated metabolic abnormalities with regard to glycation reaction, polyol pathway, and lipid metabolism that induce AGEs accumulation and lipid peroxidation63. In addition, extract of Radix et Rhizoma Polygoni Cuspidati (Polygonum cuspidatum Sieb. et Zucc.) could reduce early renal podocyte injury in streptozotocin-induced diabetic rats, which may be correlated with inhibition of methylglyoxal-mediated protein glycation by its active compound emodin111. Of note, berberine exhibited reno-protective effects in diabetic nephropathy rats, and the molecular mechanism was associated with changes in the levels and regulation of the AGE/RAGE/protein kinase C (PKC)-β/TGF-β1 signaling pathway69. In this context, abnormal glucose metabolism can directly activate the diacylglycerol (DAG)/PKC pathway and promote the expression of PKC, while PKC may serve as the link between AGE/RAGE signaling and TGF-β1-induced renal fibrosis in DN, since activated PKC may stimulate the expression of TGF-β1, which promotes the increased expression of collagen and fibronectin, the accumulation of extracellular matrix and finally renal fibrosis69,143. More detailed mechanisms of renal fibrosis in DN will be discussed later.
3.1.2. IR/IRS signaling pathways
Hyperglycemia in DM can be mainly attributed to insulin resistance in the liver in which excessive glucose is generated due to the enhancement of gluconeogenesis and glycogen degradation66. Intracellular glucose metabolism disorders have been recognized as important pathogenesis of DN64. Insulin receptor/insulin receptor substrate/phosphatidylinositol 3 kinases (IR/IRS/PI3K) signaling pathways that can be activated by the binding of insulin to IR are the most essential mechanism in monitoring blood glucose level64,66. Glucose transporter type 4 (GLUT4), which is the downstream signal of IR/IRS/PI3K/GLUT signaling pathway, is mainly located in intracellular vesicles in the condition without insulin stimulation, but activation of IR can trigger the cell cascade reactions and the translocation of GLUT4 to the extracellular membrane, leading to enhanced activity of GLUT4 and subsequently increased glucose uptake64,66. In contrast, overexpression of GLUT1 can result in increased extracellular glucose level, decreased peripheral tissue glucose intake, glucose metabolic disturbance, and high blood glucose level66.
In a study, Huangqi Decoction treatment (1.08, 0.36, and 0.12 g/kg BW, daily, 14 weeks) alleviated renal dysfunction in db/db mice with DN, with lowered SCr, BUN, and urinary albumin, improved GFR and inhibited degradation of capillary basement membrane in glomeruli, expansion of mesangial matrix, and disturbance of tubular lumen64. Mechanism study showed that Huangqi Decoction functioned via regulating the IR/IRS1/PI3K/GLUT4 signaling pathway, as revealed by the significantly increased phosphorylation of IRY1361, IRS1Y896, and PI3K, enhanced expression of GLUT4 and reduced expression of GLUT1 in the kidney64. Similarly, Naoxintong Capsule (620 mg/kg BW, daily, 14 weeks) restrained the development of DN in db/db mice via regulating IR/IRS/PI3K/protein kinase B (AKT)/GLUT4 signaling pathway, as indicated by the raised levels of IR, IRS1/2, phospho-IRS1 (p-IRS1), PI3K, AKT, GLUT4, and glucokinase in liver and kidney66.
3.1.3. AMPKα signaling pathways
Adenosine monophosphate-activated protein kinase α (AMPKα) can be activated by the rising ratio of adenosine monophosphate to adenosine triphosphate, which is of great importance in regulating energy metabolism. After activation, AMPKα can inhibit lipid/cholesterol synthesis and gluconeogenesis, while promoting lipid/glucose uptake and metabolism as well as mitochondrial function65,66. Additionally, GLUT4 is deemed as another modulator specific to glucose uptake and energy metabolism66.
Naoxintong Capsule was demonstrated to have a reno-protective effect against DN in db/db mice66. The hepatic levels of both AMPKα and p-AMPKα were increased by Naoxintong Capsule, which correspondingly increased GLUT4 expression in skeletal muscle and enhanced glucose uptake/metabolism and subsequently ameliorated hyperglycaemia induced kidney injury66. Danhong Injection (5 mL/kg BW, daily, 14 weeks) improved renal functions in db/db mice by decreasing mesangial matrix expansion, renal levels of AGEs, VEGF-A, and fibronectin. Mechanistically, the reno-protective effect of Danhong Injection was mediated by inducing AMPKα/p-AMPKα expressions, which are responsible for glucose metabolism and energy expenditure65.
Taken together, Chinese medicines exert therapeutic effects against DN through restoring metabolism dysfunction upon hyperglycaemia, which leads to insulin resistance and lipid/glucose metabolism abnormality and ultimately triggers renal injury. As illustrated in Fig. 1, AGEs/RAGE, IR/IRS, and AMPKα may be the molecular targets of Chinese medicines with regard to DN treatment. Of note, hypoglycemic action is not always a necessary process for the reno-protective effects of Chinese medicines.
Figure 1.
Chinese medicines exert reno-protective effects against diabetic nephropathy by ameliorating metabolism dysfunction via inhibiting AGEs/RAGE signaling pathways and activating IR/IRS1/PI3K/AKT/GLUT4 and AMPKα/GLUT4 signaling pathways.
3.2. Antioxidation
In the literature, many studies indicated that hyperglycemia may increase oxidative stress in the kidney, with regard to the reduction of antioxidant enzyme activity, the excessiveness of reactive oxygen species (ROS), as well as the oxidation of macromolecules75,76,144. The activity of antioxidant enzymes like superoxide dismutase (SOD) and GPX are usually decreased in diabetic conditions. In addition, hyperlycaemia can promote ROS generation by activating several pathways, including protein kinase C activation, non-enzymatic glycation, and polyol and hexosamine pathways74. Subsequently, the levels of macromolecule oxidative products, such as AGEs, malondialdehyde (MDA), 8-hydroxy-2ʹ-deoxyguanosine, and oxidative carbonyl proteins, are increased in the kidney75,76. Excessive oxidative stress has been highly implicated in DN pathogenesis, in particular extracellular matrix expansion and glomerular hyperfiltration74,96. Hence, the cleavage of peroxides is a promising approach for DN management.
3.2.1. NRF2 signaling pathways
Nuclear factor erythroid 2-related factor 2 (NRF2) is one of the dominant regulators in the antioxidant defense system. Downstream signaling molecules of NRF2 comprise phase II detoxifying enzymes heme oxygenase-1 (HO-1), NAD(P)H:quinone oxidoreductase 1 (NQO1), and glutamate-cysteine ligase catalytic subunit (GCLC). Antioxidant response elements (AREs), encoded by the cis-regulatory DNA sequences located in the promoter and enhancer regions, mediates the regulation of NRF2 on downstream molecules. The significance of NRF2/ARE signaling pathway in DN development has been illustrated by accumulative evidence, hence makes NRF2 activation a potential strategy for DN prevention70,72,145.
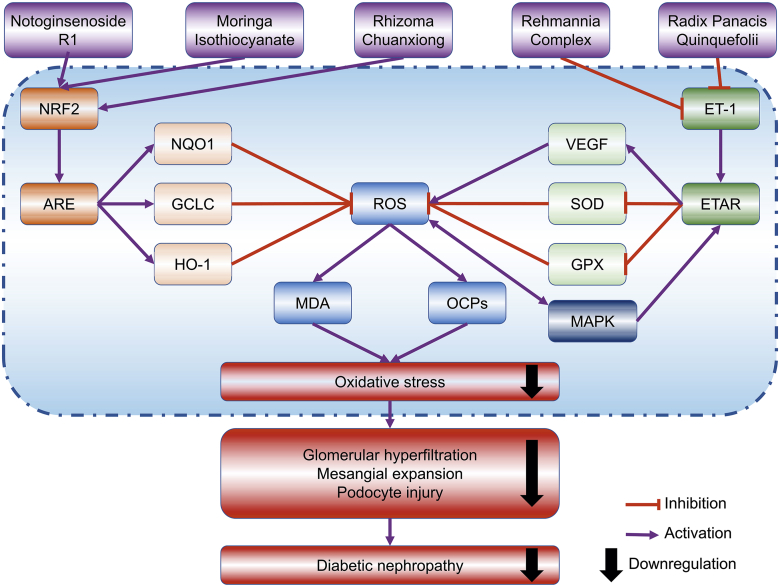
As the major bioactive isothiocyanate derived from Moringa oleifera Lam., moringa isothiocyanate (1.25, 2.5, and 5 μmol/L) was observed to activate NRF2/ARE signaling pathway and increase the gene expressions of GCLC, NQO1, HO-1, as well as protein expressions of GCLC and HO-1, consequently decreasing the levels of ROS in a DN model induced in high glucose-treated HK-2 cells70. These results indicated that moringa isothiocyanate may serve as a potential therapy for DN, whereas further studies are warranted in the future. Similarly, notoginsenoside R1 was found to protect db/db mice against DN via upregulating NRF2-mediated HO-1 expression72. In another study, it was described that administration with ethanol extract of Rhizoma Chuanxiong (25 and 50 mg/kg BW, 3 times per week, 16 weeks) to mice with STZ-induced DN significantly improved urine production, UAER, UACR, and renal morphological damages regarding glomerulosclerosis and fibrosis145. In mechanism study, it was found that these effects were mediated by the inhibition of oxidative stress via stimulating the NRF2 signaling pathway and its downstream targets including NQO1 and GCLC145.
3.2.2. ET-1 signaling pathways
ET-1 exhibits a markable role in monitoring vascular function, and overexpression of ET-1 impairs endothelium-dependent moderation in DM. Damaged endothelium function (e.g., hyperfiltration) in the renal vascular system as a result of ET-1 pathway activation73,74. Notably, the activation of ET-1 receptors (ETARs) results in oxidative stress with regard to the weakened free radical scavenging ability and enhanced ROS formation, which in turn stimulates the mitogen-activated protein kinase (MAPK) signaling pathway and causes ETARs upregulation74. Thus, inhibiting ROS generation and ET-1 activation has the potential as a strategy for DN management.
In a study, a modified TCM prescription Liuwei-Dihuang Decoction (56.8, 107.8, and 154.7 mg/kg BW, daily, 4 weeks), namely Rehmannia Complex without Fructus Corni, improved STZ-induced early-stage DN in Sprague–Dawley rats (SD-rats) with reductions in 24-h urinary albumin, SCr, and BUN73. In the renal cortex, reduced activities of SOD and GPX, enlarged contents of MDA and ROS, activated activity of ET-1 and augmented expression of ETAR were reversed by ethanol extract of Rehmannia Complex, indicating the potential of Rehmannia Complex to attenuate DN by reducing oxidative stress in the kidney targeting ET-1/ROS signaling pathway73. Similarly, ethanol extract of Radix Panacis Quinquefolii (Panax quinquefolium L.) (200 mg/kg BW, daily, 8–16 weeks) was found to prevent albuminuria, mesangial expansion, and extracellular matrix deposition in STZ-treated C57BL/6 mice, mainly through reducing increased mRNA and protein expressions of ET-1 and VEGF with the corresponding effect of ameliorating renal oxidative stress74.
Collectively, Chinese medicines have the potential to prevent renal injury caused by hyperglycaemia-induced AGEs accumulation and excessive ROS, by targeting the NRF2/ARE and ET-1/ETAR signaling pathways (Fig. 2). For DN management, it is crucial to alleviate oxidative kidney injury, since oxidative stress-related damage is dominant in the early stage of DN but later may lead to some severer pathogenesis like inflammation and podocyte injury.
Figure 2.
Chinese medicines may protect against diabetic nephropathy by antioxidant actions via blocking the ET-1 signaling pathway and stimulating NRF2 signaling pathway.
3.3. Anti-inflammation
The AGEs, generated from nonenzymatic glycation reaction following the onset of hyperglycaemia, accumulate in glomerular basement membrane and mesangial cells and promote the progression of DN. Mechanically, it was also reported that the augmented AGEs deposition in vasculum and the interaction between RAGE and AGEs may boost the filtration of inflammatory cells (like macrophages and monocytes) and the release of proinflammatory molecules, such as Toll-like receptors (TLRs), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), IL-6, TNF-α, MCP-1, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1)71,80,134,136,146. Notably, besides the AGEs-promoted oxidative stress, the endoplasmic reticulum stress-mediated by the activation of inositol-requiring enzyme-1α (IRE-1α) along with the upregulation of spliced X box-binding protein 1 (XBP-1) and glucose-regulated protein 78 (GRP78) can also accelerate inflammatory response in the kidney, thereby promoting DN pathogenesis80,147. It is reasonable to handle DN by targeting inflammation-related signaling pathways.
3.3.1. NF-κB signaling pathways
NF-κB, interacting tightly with inhibitory proteins (IκB) and IκB kinase (IKK), is a key intracellular molecule monitoring the inflammatory response. IκB and IKK are two essential upstream modulative elements for the transduction cascade of NF-κB signals88. In detail, IKK-β is a protein subunit of IκB-α that can bound to cytoplasmic NF-κB to make it remain inactive. After transitions into the nucleus, NF-κB is activated and herein promotes the transcriptions of pro-inflammatory factors like TNF-α, IL-1β, IL-6, and MCP-1, which highly correlate with the progression of DN77,79.
Tangshen Formula (1.67 g/kg BW, daily, 20 weeks) administration to Wistar rats with DN (caused by high-fat diet feeding with STZ injection) showed significant improvements regarding decreasing urinary excretion of albumin and attenuating renal histological injuries, with the mechanisms of inactivating NF-κB signaling and thereby restraining macrophage infiltration into the kidney and downregulating the expressions of TNF-α, IL-1β, and MCP-177. The same research group also illustrated that berberine (25 mg/kg BW, daily, 20 weeks), an isoquinoline alkaloid derived from Rhizoma Coptidis or Cortex Phellodendri Chinensis (Phellodendron chinense Schneid.), exerted similar effect with Tangshen Formula78. In another study, Chaihuang-Yishen Granule (0.56 g/kg BW, daily, 20 weeks) was shown to protect against DN in Wistar rats (developed by a right uninephrectomy plus STZ injection), in terms of alleviating 24-h UP excretion and glomerular mesangial expansion by markedly reducing both mRNA and protein levels of NF-κB followed by decreased levels of renal TNF-α and MCP-179. Moreover, Cortex Moutan (Paeonia suffruticosa Andr.) bioactive component terpene glycoside (0.404 and 0.808 g/kg BW, daily, 4 weeks) could down-regulate the endoplasmic reticulum stress-associated molecules IRE-1α, XBP-1, and GRP78, as well as the pro-inflammatory factors IL-6, ICAM-1, and MCP-1, presenting protective effect against DN, established with a high-fat diet and STZ treatment in SD-rats80.
3.3.2. TLRs signaling pathways
TLRs represent a family of germline-encoded receptors that are responsible for the development of inflammatory and immune response82,83. TLRs express on various cells including antigen-presenting cells and kidney intrinsic cells83. The recognition of TLRs ligands prompts the innate immune response to stimulate TLRs signaling, which initiates M1 macrophage polarization and infiltration, mediates the transcription of NF-κB followed by the inflammatory cascade with pro-inflammatory cytokine and chemokine release83,134. Of note, almost all TLRs (except for TLR-3) use myeloid differentiation primary response 88 (MYD88) as a general adapter protein when activating NF-κB84. Activation of TLRs signaling pathway has been reported to exacerbate inflammation and ultimately aggravate DN progression83.
Treatment with Caulis Dendrobii Officinalis (Dendrobium officinale Kimura et Migo) extract (10 and 20 g/kg, daily, 4 weeks) to SD-rats with DN (induced by high-fat/sucrose diet plus STZ injection) decreased the levels of albuminuria, SCr, UACR, and BUN, as well as the expressions of TLR-2, TLR-4, MYD88, TNF-α, IL-6, and high-sensitivity CRP, all of which suggested that Caulis Dendrobii Officinalis could alleviate DN by reducing inflammatory response targeting TLRs signaling pathway82. Similarly, paeoniflorin (15, 30, and 60 mg/kg BW, daily, 2 weeks) was demonstrated to block the AGEs-mediated TLR2/4 stimulation and inflammatory responses and ultimately attenuate DN in db/db mice, as revealed by downregulated TLR-2, TLR-4, MYD88, CD68, NF-κB, TNF-α, IL-1β, and MCP-183. Consistent results could be observed in db/db mice that were treated with TLR-2/4 inhibitor, indicating that paeoniflorin may benefit DN by preventing macrophage activation and following inflammatory response via inhibition of TLR-2/4 signaling pathway. The same research group also examined the reno-protective potential role of paeoniflorin in DN progression using STZ-treated TLR-2-knockout mice and wild-type littermates. The results showed that intraperitoneal injection of paeoniflorin (25, 50, and 100 mg/kg BW, daily, 12 weeks) significantly decreased proteinuria and lessened renal histopathological alterations, which were connected with noticeably reduced macrophage infiltration and the expressions of TLR-2 and downstream inflammatory factors84. These data further supported paeoniflorin may ameliorate DN by targeting TLRs signaling pathways to inhibit inflammation in the kidney. Moreover, it was reported that berberine treatment (50, 100, or 200 mg/kg BW, daily, 8 weeks) could decrease 24-h UP, SCr, BUN, FBG, and the ratio of kidney weight to body weight by inhibiting TLR-4/NF-κB pathway and downstream inflammation response that promotes kidney injury85.
3.3.3. PI3K/AKT signaling pathways
The PI3K/AKT signaling pathway is vital for cell proliferation, growth and viability89. As an upstream-modulator of NF-κB, the PI3K/AKT signaling pathway activation has been demonstrated to ameliorate inflammation in DN88. Alternative strategies including activating the PI3K/AKT signaling pathway and inhibiting NF-κB-mediated inflammatory response may have the possibility to protect against kidney damage after the onset of DM.
Jiangtang Decoction (2, 4, and 8 g/kg BW, daily, 12 weeks) restored the UAER, CCR, and kidney morphological alterations in KK-Ay DN mice88. These effects were correlated with the reductions of AGEs and RAGE, the up-regulations of IRS-1, IKK-β, and IκB-α, the activations of PI3K and AKT and the inhibitions of downstream pro-inflammatory mediators including both NF-κB and p-NF-B as well as TNF-α, IL-6, and ICAM-1, indicating that the protective effect of Jiangtang Decoction involved PI3K/AKT-influenced NF-κB signaling88. Differently, emodin (100 mg/kg BW, once every 3 days, 3 weeks) was reported to alleviate STZ-induced DN in Wistar rats with regard to normalizing adjusted kidney weight, UAER, SCr level, and tubulointerstitial injury index (TII) scores through regulating inflammation targeting the PI3K/AKT/GSK-3β signaling pathway89. Glycogen synthase kinase 3 (GSK-3) serves as a rate-limiting enzyme in glycogen biosynthesis and it can modulate several signals like insulin-dependent signaling pathway. It was observed that emodin could significantly increase p-AKT and p-GSK-3β expressions and suppress TNF-α, IL-6, and ICAM-1 expressions in the diabetic animals89.
3.3.4. PGE2/EP1 signaling pathway
Prostaglandin E2 (PGE2) is a dominant prostaglandin expressed in the kidney, which plays a crucial role in the pathophysiological process of DN regarding glomerular filtration alteration, renin release and tubular salt/water metabolism148. The effect of PGE2 is mediated by specific G protein-coupled receptors, namely E-prostanoid receptors (EPs) that are usually divided into four subtypes, including EP1, EP2, EP3, and EP4, while EP3 has multiple splicing isoforms of the subtype86,149. Each EP possesses different function in signal transduction. Above all, the EP1 receptor couples to Gq protein alpha subunit (Gαq) and raises intracellular Ca2+ concentration, the EP2 and EP4 receptors couple to Gs to stimulate adenylate cyclase that mediates the elevation of intracellular cyclic adenosine monophosphate (cAMP) concentrations, while the EP3 receptor is coupled with Gi and could inhibit the increase of cAMP via blocking adenylate cyclase86. The PGE2/EP1 axis has been documented in the development of renal inflammation in DN87. However, it should be noted that EPs do not couple exclusively to these pathways described but often to more than one G protein and signal transduction pathway, which needs to be further elucidated and clarified in the progression of DN.
Berberine has been reported to restore renal functional parameters and suppress histological/ultrastructural alterations of kidney tissues by increasing cAMP level, decreasing IL-6 and PGE2 levels, down-regulating total protein expression of EP1 and EP3 while up-regulating the expression of EP4 of renal cortex in DN rats (no significant difference on EP2 expression among all groups), demonstrating that berberine (50, 100, or 200 mg/kg BW, daily, 8 weeks) exerts reno-protective effect in high-fat diet plus STZ-induced DN rats by modulating the proteins expression of EPs in EP/G protein/cAMP signaling pathway86. In addition, berberine could protect against DN by regulating the PGE2–EP1–Gαq–Ca2+ signaling pathway87. Berberine (100 mg/kg BW, daily, 8 weeks) decreased the abnormal concentration of Ca2+, the increased level of PGE2, the high expression of EP1 and Gαq and suppressed the proliferation of mesangial cells, resulting in the improvement of renal biochemical and functional parameters as well as the histopathological alterations in DN rats (induced by high-fat and high-glucose diet plus STZ injection at the dose 35 mg/kg)87.
In short, Chinese medicines exhibit anti-inflammatory effect in DN mainly by inhibiting NF-κB, TLR-2/4, PI3K/AKT, and PGE2/EP1 signaling pathways (Fig. 3), which mainly suppress the expressions of inflammation-promoting cytokines, chemokines and adhesion factors including TNF-α, IL-1β, IL-6, MCP-1, and ICAM-1, and finally ameliorate kidney inflammation.
Figure 3.
Chinese medicines suppress inflammation in diabetic nephropathy by inhibiting PI3K/AKT, TLR-2/4, and NF-κB signaling pathways.
3.4. Anti-fibrosis
Renal fibrosis has been recognized as one of the most crucial processes for the development of DN from metabolism dysfunction, oxidative stress, inflammation, fibrosis, sclerosis, and ultimately ESRD. Accumulative studies revealed that renal fibrosis is highly correlated with enhanced synthesis, weakened degradation, and excessive deposition of the extracellular matrix, partially including collagen I/IV and fibronectin93,96,150. The TGF-β/small mothers against decapentaplegic (SMAD) and Janus kinase/signal transducers and activators of transcription/suppressor of cytokine signaling proteins (JAK/STAT/SOCS) signaling pathways, accompanied with angiotensin II, connective tissue growth factor (CTGF), matrix metallopeptidase 2 (MMP-2), bone morphogenetic protein-7 (BMP-7), and α smooth muscle actin (α-SMA), are involved in regulative action of glomerular and tubulointerstitial fibrosis in DN90,91,94,96, 97, 98.
3.4.1. TGF-β/SMAD signaling pathways
Hyperglycemia along with AGEs/ROS-mediated oxidative stress stimulates the expression of TGF-β, which forms by binding with its two receptors (two transmembrane serine/threonine kinases) into a complex101. TGF-β is widely expressed in all kinds of kidney cells and acts as the most dominant cytokine for regulating extracellular matrix formation/degradation, mesangial expansion, and glomerular basement membrane thickening93,96,151. Traditionally, resident fibroblasts are considered as the key mediators of renal fibrosis, however, emerging evidence has suggested that the appearance of interstitial myofibroblasts generated by TGF-β-mediated epithelial-to-mesenchymal transition (EMT) is also a crucial contributor, since about 30% fibroblasts are derived from the tubular epithelial cells via EMT95. In addition, it is well established that after binding to its receptors, TGF-β activates downstream signaling molecules (SMAD2 and SMAD3) to mediate fibrosis77. Experimental studies have confirmed the positive role of inhibiting TGF-β signaling pathways and downstream extracellular matrix protein production in preventing renal fibrosis95,101.
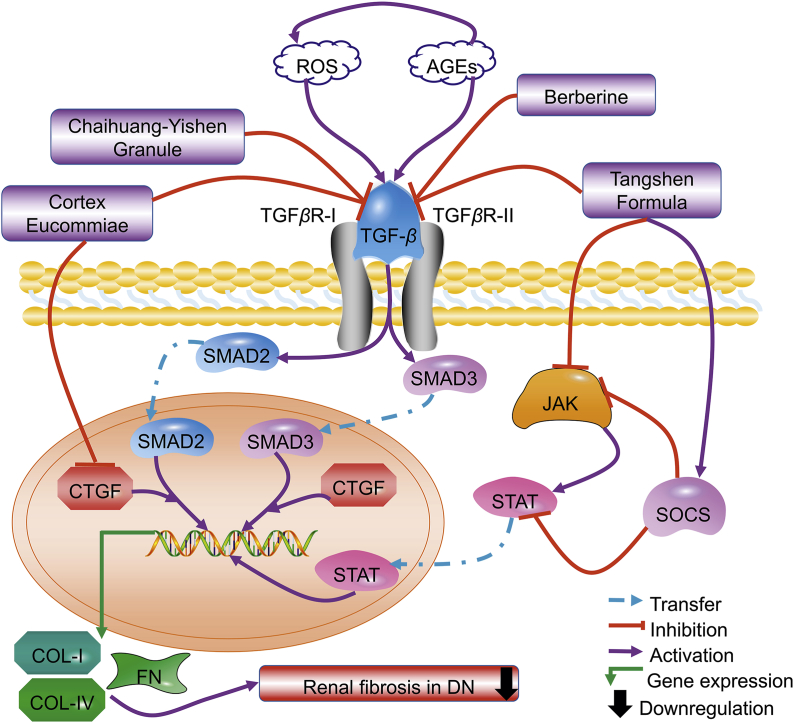
As reported by the same research group94,95, Tangshen Formula (1.67 g/kg, daily, 20 weeks) or berberine (25 mg/kg BW, daily, 20 weeks) attenuated high-fat diet plus STZ injection-induced DN in Wistar rats by significantly inhibiting UAER and renal fibrosis, which were believed to associate with the SMAD ubiquitination regulatory factor 2 (SMURF-2)-dependent ubiquitin degradation of SMAD7 and the inactivation of TGF-β1/SMAD3 signaling pathway followed by the suppressed expressions of fibronectin and collagen I/IV77,78. Another study has confirmed the reno-protective effects of berberine (200 mg/kg BW, daily, 12 weeks) in STZ-induced mice via inactivating TGF-β/SMAD/EMT signaling pathway92. Similarly, treatment with Chaihuang-Yishen Granule (0.56 g/kg BW, daily, 20 weeks) to Wistar rats with DN (induced by uninephrectomy plus STZ injection) significantly inhibited 24-h proteinuria and progressive renal fibrosis as measured by glomerulosclerosis index, tubulointerstitial fibrosis index, and up-expression of collagen I/IV and fibronectin, through inhibiting TGF-β1/SMAD3 signaling as revealed by upregulation of SMAD7 and downregulation of TGF-β1, TGF-β1 receptors, and SMAD394. Besides inhibiting TGF-β1/SMAD3 signaling pathway with reduced phosphorylation of SMAD2/3, oral administration of Cortex Eucommiae (Eucommia ulmoides Oliv.) (1 g/kg BW, daily, 3 weeks) also suppressed the expressions of TGF-β and CTGF (a key mediator of renal fibrosis) in Wistar rats with STZ-induced DN, resulting in decreased levels of BUN and SCr and improved renal fibrosis99.
3.4.2. JAK/STAT/SCOS signaling pathways
The JAK/STAT system comprises of 4 JAK and 7 STAT family members. JAK/STAT is an essential intracellular signaling pathway of cytokines and other stimulators that regulate gene expression, cell activation, proliferation, and differentiation as well as EMT and fibrosis in DN90. In the early stage of DN, mRNA expressions of JAK members are upregulated, whereas they are downregulated in progressive DN. Meanwhile, SOCS proteins have been regarded as important elements for the negative regulation of the JAK/STAT signaling pathway90. Administration with Tangshen Formula (2.08 g/kg, daily, 12 weeks) reduced urinary albumin level and ameliorated renal fibrosis in db/db mice, with the involvement of JAK/STAT/SOCS signaling pathway90. In detail, following Tangshen Formula treatment, the expressions of JAK1, JAK2, and STAT3 were upregulated while STAT4 was downregulated, with SOCS1/3/7 were all activated to provide negative feedback regulation to other related genes in the JAK/STAT/SOCS pathway.
According to the above demonstration, TGF-β/SMAD and JAK/STAT/SOCS signaling pathways are highly implicated in renal fibrosis upon diabetic nephropathy (Fig. 4). By targeting these signaling pathways, Chinese medicines are capable to block EMT of kidney cells and decrease the expression of collagen I/IV and fibronectin, subsequently reducing extracellular deposition and preventing renal fibrosis.
Figure 4.
Chinese medicines may attenuate renal fibrosis in diabetic nephropathy by restraining TGF-β/Smad signaling pathways and regulating JAK/STAT/SOCS signaling pathway.
3.5. Podocyte protection
Glomerular filtration barrier mainly comprises of fenestrated endothelial cells, basement membrane, mesangial cells, and podocytes, morphological and functional damage of which has been cognized as causation to the dysfunction of glomerular filtration and the development of proteinuria103. Podocyte is a kind of highly specialized cell of glomerulus that wraps around capillaries and neighbor cells of the Bowman's capsule104. With the progression of DN, metabolism dysfunction, oxidative stress, endoplasmic reticulum stress, and inflammation may lead to podocyte injury and even abnormal apoptosis, autophagy, and disappearance, which tend to be irreversible66,116. Loss of podocyte integrity regarding cytoskeleton and slit diaphragm, or even worse, podocyte disappearance, is the most important contributor to proteinuria, progressively presenting as microproteinuria to macroproteinuria102, 103, 104,116. Therefore, it is commonly agreed that podocyte protection is of great importance to manage DN.
3.5.1. Maintaining podocyte integrity
Foot process fusion is one of the most common types of podocyte injury, in which rearrangement of cytoskeleton acts as the intrinsic molecular mechanism. Cytoskeleton proteins of podocyte mainly comprise of associated regulatory proteins and actin. In addition, it has been established that synaptopodin is the principal regulatory protein that binds to actin and interacts with α-actin-4 proteins to maintain the stability of cytoskeleton, while F-actin is the major element in cytoskeleton fibers, with α-actin-4 as the crucial actin cross-linking proteins to assemble actin fibers102. Tongluo-Yishen Formula (13.6 g/kg BW, daily, 6 weeks) was observed to significantly lower the levels of 24-h UP, urinary albumin, serum creatine, and BUN in SD-rats with DN (induced by removal of right kidney plus STZ injection), while improving podocyte injury as eliminating podocyte fusion and alleviating irregular cytoskeleton fibers, which might associate with the elevated mRNA expressions of synaptopodin, F-actin, and α-actinin-4102.
3.5.2. Improving slit diaphragm dysfunction
Dysfunction of slit diaphragm is another type of podocyte injury. Located on podocyte membrane, transmembrane protein podocin with ion channels and signal transduction functions is the key functional unit of the slit diaphragm and oligomerizes with other transmembrane proteins nephrin and CD2-associated protein (CD2AP) to form the lipid rafts-like nephrin/CD2AP/podocin functional complex, which is essential to maintain the glomerular filtration function103, 104, 105, 106. In a study, it was found that administrating Wenshen-Jianpi Recipe (7.5, 15, and 30 g/kg BW, daily, 8 weeks) to SD-rats with STZ-induced DN improved the serum total protein and albumin, reduced the excretion rates of UP, albumin, and BUN, ameliorated glomerular hypertrophy and mesangial expansion, along with the up-regulated nephrin and podocin expression at mRNA levels103. In another study, Erzhi Formula (5, 10, and 15 g/kg BW, daily, 16 weeks) significantly lowered the blood glucose and the 24-h UP and alleviated the deterioration of glomerular morphology in SD-rats with DN (induced by high-glucose and high-fat diet plus STZ-injection)104. These effects were deemed to driven by Erzhi Formula that attenuated oxidative stress (increasing SOD activity and decreasing MDA content) and inflammation (reducing TNF-α, IL-1β, and IL-6) and protect podocyte (increasing the expression of podocin and CD2AP protein/mRNA)104.
3.5.3. Restoring podocyte EMT
The mechanism mediating podocyte injury remains controversial, whereas EMT has been considered to be involved in this pathogenesis. Upon EMT, which can be induced by hyperglycaemia and oxidative stress, podocytes lose the original phenotype as epithelial cells, and eventually acquire some characteristics specific to mesenchymal cells106. In C57BL/6 mice with STZ-induced DN, Fructus Schisandrae Chinensis [Schisandra chinensis (Turcz.) Baill.] extract (5 g/kg BW, daily, 7 weeks) could effectively protect against podocyte loss and integrity of the slit diaphragm by rescuing nephrin distribution and Wilms’ tumor 1 expression, and prevent podocytes EMT by inhibiting the expressions of α-SMA and plasminogen activator inhibitor-1 while inducing the expression of E-cadherin, ultimately decreasing the UAER and UACR106.
3.5.4. Inhibiting podocyte apoptosis
Increasing studies have demonstrated that podocyte apoptosis is one of the leading causes of podocyte loss in DN107,109,112. Generally, cell apoptosis can be detected by B-cell lymphoma 2 (BCL-2) and BCL-2-associated X protein (BAX), as BAX is a kind of pro-apoptotic protein, while BCL-2 is a kind of anti-apoptotic protein107. The activated p38 MAPK pathway by AGE, ROS, or other factors can increase BAX expression and decrease BCL-2 expression, thus severs as an important mechanism of apoptosis107,112. A study has reported that Baoshenfang Formula (0.75 g/kg BW, daily, 12 weeks) can decrease proteinuria and protect podocytes from apoptosis in DN partially through inhibiting the nicotinamide adenine dinucleotide phosphate-oxidase-4 (NOX-4)/ROS/p38 MAPK pathway, given that p38 MAPK phosphorylation-induced podocyte apoptosis was dependent to NOX-4, an important number of nicotinamide adenine dinucleotide phosphate oxidase contributing to ROS production in podocyte107. In another study, Radix Astragali (0.8 g/kg BW, daily, 12 weeks) and Radix et Rhizoma Notoginseng [Panax notoginseng (Burk.) F. H. Chen] (0.4 g/kg BW, daily, 12 weeks) could synergistically ameliorate renal histopathological alteration and albuminuria in STZ-induced DN rats by inhibiting podocyte apoptosis via down-regulating BAX and NOX-4 as well as up-regulating B-cell lymphoma-extra large (BCL-XL), accompanied with upregulation of nephrin and α-dystroglycan109. Besides p38 MAPK, p53 and p65 NF-κB are also involved in the regulation of podocyte apoptosis by NOX-4, in which p53 plays a compelling role in the mitochondrial apoptotic pathway through integrating mitochondrial dysfunction induced by BAX, while p65 NF-κB stimulates inflammation following with pro-apoptotic action108,112. Huangqi Decoction (1.08 g/kg BW, daily, 8 weeks) has been shown to inhibit hyperglycemia-induced podocyte apoptosis by the down-regulation of NOX-4/p53/BAX signaling pathway in vitro and in vivo108, while loganin (50 mg/kg BW, daily, 8 weeks) and catalpol (50 mg/kg BW, daily, 8 weeks) could cooperatively restore podocyte apoptosis in DN by inhibiting AGE/RAGE/p38 MAPK/p65 NF-κB and AGE/RAGE/NOX-4/p65 NF-κB pathways112.
In addition to the NOX-4-related pathways, some other anti-apoptotic mechanisms have been implicated in the protective effects against podocyte injury in DN by Chinese medicines, such as the regulation of miR-378/tumor-necrosis factor receptor-associated factor 5 (TRAF5) and lncRNA-TUG1/TRAF5 pathways by astragaloside114,115, the inhibition of protein kinase RNA-like endoplasmic reticulum kinase/eukaryotic initiation factor 2α (PERK/eIF2α) pathway by emodin119, and the suppression of NF-κB/podoplanin pathway by berberine120.
Meanwhile, Chinese medicines also exert anti-apoptotic effects in other cell types (like mesangial and proximal tubular cells) of kidney besides podocyte, or at least not specific to podocyte128. For example, amelioration of DN in mice by catalpol was correlated with regulation of growth factor receptor-bound protein 10/insulin-like growth factor 1/insulin-like growth factor 1 receptor (GRB10/IGF-1/IGF-1R) signaling pathway128, Radix Trichosanthis (Trichosanthes kirilowii Maxim.) lectin could alleviate DN in rats by inhibiting the lectin-like oxidized LDL receptor 1 (LOX1)/NF-κB/caspase-9 signaling pathway129, and probucol was shown to protect against contrast-induced acute kidney injury via the extracellular signal-regulated kinases 1 and 2/c-Jun N-terminal kinase/caspase-3 signaling pathway in diabetic rats152.
3.5.5. Enhancing podocyte autophagy
Autophagy has been reported with dominant influence on the cellular homeostasis of all major cells in kidney, including podocytes, mesangial cells, and endothelial cells121,126,133. Autophagy alteration can be determined by measuring sequestosome 1 protein (p62) and microtubule-associated protein light chain 3 (LC3), which locates on the membrane of autophagosome and can be classified into cytosolic LC3 I and membrane-bound LC3 II121,126. Generally, autophagy is relatively enhanced in the early stage of DN, but it is deficient subsequently, and autophagy dysfunction has been implicated in the progression of DN126.
Mammalian target of rapamycin (mTOR) is one of the vital kinases to modulate autophagy, while PI3K/AKT is one of the pathways to regulate the activity of mTOR110. PI3K/AKT/mTOR has been recognized as a negative regulatory pathway of autophagy to maintain podocyte homeostasis, through which Paecilomyces cicadae-fermented Radix Astragali (4.5 g/kg BW, daily, 6 weeks) and curcumin (300 mg/kg BW, daily, 8 weeks) could activate podocyte autophagy and subsequently protect against DN110,122. Recent studies have also shown the crucial role of Pim1, a proto-oncogene serine/threonine-protein kinase, in autophagy via regulating the phosphorylation and degradation of an autophagy-related protein p21123. Hispidulin could induce autophagy in podocyte through the inhibition of Pim1 and the regulation of Pim1–p21–mTOR signaling axis123. AMPK is another upstream regulator of mTOR, and it has been revealed that astragaloside IV (3, 6, and 12 mg/kg BW, daily, 8 weeks) could protect against albuminuria, glomerulosclerosis, and podocyte injury via AMPKα-mediated autophagy enhancement in STZ-induced DN mice117. Moreover, growing evidence has revealed that Sirtuin 1 (SIRT1) and HO-1 can promote autophagy through the activation of AMPK118,124,153. In a study, puerarin (5, 10, 20, and 40 mg/kg BW, daily, 12 weeks) was reported to protect podocytes from hyperglycaemia-induced injury through HO-1 and SIRT1-mediated upregulation of autophagy124. In another study, astragaloside IV (40 mg/kg BW, daily, 12 weeks) exhibited protective effects against glucose-induced EMT of podocyte through autophagy enhancement via the SIRT/NF-κB p65 axis, with increase in the SIRT1 expression and decrease in the NF-κB subunit p65 acetylation118.
As for downstream signals of mTOR, inhibition of mTOR has been documented to protect podocyte injury in DN mice by promoting nuclear translocation of transcription factor EB (TFEB), which was identified to regulated the transcription of various genes involved in autophagy and lysosomal biogenesis113. It was found that catalpol (30, 60, and 120 mg/kg BW, daily, 8 weeks) could ameliorate podocyte injury in DN by enhancing autophagy via inhibiting mTOR activity followed with promotion of the TFEB nuclear translocation. Differently, berberine was observed to mitigate high glucose-induced podocyte apoptosis by modulating autophagy via the mTOR/p70 ribosomal S6 kinase/4E-binding protein 1 (mTOR/P70S6K/4EBP1) pathway, in which the phosphorylation of P70S6K/S6 ribosomal protein and eukaryotic translation initiation factor 4EBP1 stimulated ribosome biogenesis and protein synthesis following the activation of mTOR complex 1121.
Like apoptosis inhibition, autophagy promotion in other cell types (like mesangial and tubular cells) of kidney is also involved in the reno-protective effects against DN by Chinese medicines126,127,133,135. The mechanisms included the regulations of miR-141-3p/phosphatase and tensin homolog (PTEN)/AKT/mTOR signaling pathway by triptolide126, mTOR/PTEN-induced putative kinase 1 (PINK1)/Parkin signaling pathway by Radix Astragali-Radix et Rhizoma Notoginseng Formula135, AKT/mTOR/P70S6K signaling pathway by Huangkui Capsule133, and AMPK/mTOR signaling pathway by triterpenic acids fraction of Folium Cyclocaryae Paliuri [Cyclocarya paliurus (Batal.) Iljinsk.]127.
So far, it has been well established that podocyte injury plays a principal role in proteinuria initiation and development with the progression of DN. Chinese medicines possess protective effects in terms of maintaining podocyte integrity, improving slit diaphragm dysfunction, restoring podocyte EMT, inhibiting podocyte apoptosis, enhancing podocyte autophagy, and preventing podocyte loss, and consequently help to reduce proteinuria.
As discussed in this section, the underlying mechanisms involved in the reno-protective effects of Chinese medicines mainly include those actions that are closely related to metabolism regulation, antioxidation, anti-inflammation, anti-fibrosis, and podocyte protection.
4. Discussion and perspective
In view of the evidence in RCTs, systematic reviews and meta-analysis retrieved from Web of Science Core Collection, PubMed, CNKI, and ClinicalTrials.gov, Chinese medicine exerted promising protective effects against DN. However, much more clinical trials, which are well-designed and properly conducted, are still warranted before Chinese medicine can be applied as primary therapy for DN treatment and management. Besides the general principles that guarantee the good quality of clinical trials, such as multi-center cooperation, enough sample size, randomized grouping, blinding method, good clinical practice, and human subject research ethics, there are still many aspects that we should specifically take into consideration when investigating the clinical efficiency of Chinese medicine in treating DN. As for Chinese medicine, it may be more reliable to use purified single bioactive compound or clarified blended bioactive components considering the advantages of multi-constituents and multi-targets of Chinese medicine. Quality control of Chinese medicine regarding composition and stability should always be implemented during the trials. Proper dosage and duration should be adopted to subjects based on preclinical studies. The safety of Chinese medicine is also of great importance so that we should closely monitor it before/during clinical trials to ensure not only potent clinical efficacy but also satisfactory tolerance and safety. As for DN, subjects should only be recruited with definite diagnoses of DN, which should be distinguished from other kinds of kidney diseases like acute glomerular nephritis, hypertensive nephropathy, and nephrotic syndrome. Some emerging biomarkers regarding epigenetic alterations (e.g., DNA methylation, histone modification, and micro-RNA) may be employed to promote accurate diagnoses and prognosis of DN. In addition, it is important to classify DN into different grades/stages and to apply grade/stage-specific strategies to guide precise medicine and individual therapy, so as to optimize the clinical efficacy of Chinese medicine in treating DN.
On the other hand, the mechanisms involved in the initiation and progression of DN as well as the effect of Chinese medicine against DN have not been fully elucidated, though accumulating studies have recognized some signaling pathways related to metabolism regulation, antioxidation, anti-inflammation, anti-fibrosis, and podocyte protection as important mechanisms of action. More animal and cellular studies should be conducted to address this research gap. Future directions may include but not limited to epigenetic alterations, such as DNA methylation, histone modification, RNA editing, MircoRNA, siRNA, and long non-coding RNA, as well as the cross talk between these alterations and conventional signaling pathways, which have not been fully explored and elucidated currently in the reno-protective effects of Chinese medicine against DN. Also, special attention should be paid to the safety of Chinese medicine in preclinical experimental periods. In this regard, it is crucial to classify the discrepancy between herb-induced kidney injury and beneficial function of Chinese medicine on DN, while observing whether Chinese medicine can induce adverse effects to animals at the whole level, organ level, cellular level, and molecular level. At least, acute, sub-acute, and sub-chronic toxicity studies are needed to confirm that certain Chinese medicines possess reno-protective functions against DN with tolerable and limited side effects to animals and human beings with/without health impairment.
5. Conclusions
It is a good strategy to apply Chinese medicines to treat and manage diabetic nephropathy since the clinical efficacies are considered satisfactory and the safety is acceptable. Metabolism regulative, antioxidant, anti-inflammatory, anti-fibrotic, and podocyte protective actions are closely correlated to the effects of Chinese medicines against DN. Mechanically, signaling pathways and molecular targets involved in the reno-protective functions of Chinese medicines may include, but not limited to, IR/IRS/PI3K/AKT/GLUT4, NRF2/ARE, NF-κB, and TGF-β/SMAD3. In the future, further studies may pay attention to the effects of Chinese medicines on the initiation and progression of DN via regulating epigenetic alterations and their cross talk with conventional signaling pathways.
Acknowledgments
The study was financially supported by General Research Fund from the Research Grant Council, Hong Kong Special Administrative Region (Project code: 17152116, China), Health and Medical Research Fund from the Food and Health Bureau, Hong Kong Special Administrative Region (Project codes: 15162961, 16172751, and 17181101, China), Wong's Donation (Project code: 200006276, China), a donation from the Gaia Family Trust of New Zealand (Project code: 200007008, New Zealand), and a contract research (Project code: CR-BL03, New Zealand).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2020.12.020.
Author contributions
Yibin Feng conceived and designed the study and drafted the manuscript. Guoyi Tang collected the data and drafted the manuscript. Sha Li, Haiyong Chen, Cheng Zhang, and Ning Wang revised the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhong Y.F., Lee K., He J.C. SIRT1 is a potential drug target for treatment of diabetic kidney disease. Front Endocrinol. 2018;9:624. doi: 10.3389/fendo.2018.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piao Y.L., Yin D.H. Mechanism underlying treatment of diabetic kidney disease using traditional Chinese medicine based on theory of Yin and Yang balance. J Tradit Chin Med. 2018;38:797–802. [PubMed] [Google Scholar]
- 3.Xue R., Gui D.K., Zheng L.Y., Zhai R.N., Wang F., Wang N.S. Mechanistic insight and management of diabetic nephropathy: Recent progress and future perspective. J Diabetes Res. 2017;2017:1839809. doi: 10.1155/2017/1839809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun G.D., Li C.Y., Cui W.P., Guo Q.Y., Dong C.Q., Zou H.B. Review of herbal traditional Chinese medicine for the treatment of diabetic nephropathy. J Diabetes Res. 2016;2016:5749857. doi: 10.1155/2016/5749857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Boer I.H., Rue T.C., Hall Y.N., Heagerty P.J., Weiss N.S., Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. J Am Med Assoc. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang G.Z., Luk A.O.Y., Tam C.H.T., Xie F.Y., Carstensen B., Lau E.S.H. Progression of diabetic kidney disease and trajectory of kidney function decline in Chinese patients with type 2 diabetes. Kidney Int. 2019;95:178–187. doi: 10.1016/j.kint.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Anders H.J., Huber T.B., Isermann B., Schiffer M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14:361–377. doi: 10.1038/s41581-018-0001-y. [DOI] [PubMed] [Google Scholar]
- 8.Thomas M.C., Brownlee M., Susztak K., Sharma K., Jandeleit-Dahm K.A.M., Zoungas S. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. doi: 10.1038/nrdp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jefferson J.A., Shankland S.J., Pichler R.H. Proteinuria in diabetic kidney disease: A mechanistic viewpoint. Kidney Int. 2008;74:22–36. doi: 10.1038/ki.2008.128. [DOI] [PubMed] [Google Scholar]
- 10.de Zeeuw D., Renfurm R.W., Bakris G., Rossing P., Perkovic V., Hou F.F. Efficacy of a novel inhibitor of vascular adhesion protein-1 in reducing albuminuria in patients with diabetic kidney disease (ALBUM): A randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2018;6:925–933. doi: 10.1016/S2213-8587(18)30289-4. [DOI] [PubMed] [Google Scholar]
- 11.Doshi S.M., Friedman A.N. Diagnosis and management of type 2 diabetic kidney disease. Clin J Am Soc Nephrol. 2017;12:1366–1373. doi: 10.2215/CJN.11111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathi Y.B., Yadav D. Diabetic nephropathy: Causes and managements. Recent Pat Endocr Metab Immune Drug Discov. 2013;7:57–64. [PubMed] [Google Scholar]
- 13.Xiang L., Jiang P.P., Zhou L., Sun X.M., Bi J.L., Cui L.J. Additive effect of Qidan Dihuang Grain, a traditional Chinese medicine, and angiotensin receptor blockers on albuminuria levels in patients with diabetic nephropathy: A randomized, parallel-controlled trial. Evid Based Complement Alternat Med. 2016;2016:1064924. doi: 10.1155/2016/1064924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katavetin P., Katavetin P. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. [PubMed] [Google Scholar]
- 15.Liu J.Y., Chen X.X., Tang S.C.W., Lao L.X., Sze S.C.W., Lee K.F. Edible plants from traditional Chinese medicine is a promising alternative for the management of diabetic nephropathy. J Funct Foods. 2015;14:12–22. [Google Scholar]
- 16.Wang B., Lin L., Ni Q., Su C.L. Chinese medicine for treating diabetic nephropathy. Chin J Integr Med. 2011;17:794–800. doi: 10.1007/s11655-011-0880-2. [DOI] [PubMed] [Google Scholar]
- 17.Wen Y.M., Yan M.H., Zhang B.X., Li P. Chinese medicine for diabetic kidney disease in China. Nephrology. 2017;22:50–55. doi: 10.1111/nep.13149. [DOI] [PubMed] [Google Scholar]
- 18.Tu X., Ye X.F., Xie C.G., Chen J., Wang F., Zhong S. Combination therapy with Chinese medicine and ACEI/ARB for the management of diabetic nephropathy: The promise in research fragments. Curr Vasc Pharmacol. 2015;13:526–539. doi: 10.2174/1570161112666141014153410. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Y., Liu Y.Y., Yu K.Q., Zhou L., Bi J.L., Cheng J.R. The effect of Chinese herbal medicine on albuminuria levels in patients with diabetic nephropathy: A systematic review and meta-analysis. Evid Based Complement Alternat Med. 2013;2013:937549. doi: 10.1155/2013/937549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L., Yang L.H., Shergis J., Zhang L., Zhang A.L., Guo X.F. Chinese herbal medicine for diabetic kidney disease: A systematic review and meta-analysis of randomised placebo-controlled trials. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-025653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J., Mo C., Meng L.F., Liang C.Q., Cao X., Shi W. Efficacy and safety of Buyang Huanwu Decoction for early-stage diabetic nephropathy: A meta-analysis. China J Chin Mater Med. 2019;44:1660–1667. doi: 10.19540/j.cnki.cjcmm.20190109.001. [DOI] [PubMed] [Google Scholar]
- 22.Lin L., Wang Q.H., Yi Y.X., Wang S.H., Qiu Z.L. Liuwei Dihuang Pills enhance the effect of Western medicine in treating diabetic nephropathy: A meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2016;2016:1509063. doi: 10.1155/2016/1509063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Q., Li C.L., Chen W.W., Shi Z.F., Zhan R.T., He R. Clinical efficacy of Jinshuibao Capsules combined with angiotensin receptor blockers in patients with early diabetic nephropathy: A meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2018;2018:6806943. doi: 10.1155/2018/6806943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L., Shergis J.L., Yang L.H., Zhang A.L., Guo X.F., Zhang L. Astragalus membranaceus (Huang Qi) as adjunctive therapy for diabetic kidney disease: An updated systematic review and meta-analysis. J Ethnopharmacol. 2019;239:111921. doi: 10.1016/j.jep.2019.111921. [DOI] [PubMed] [Google Scholar]
- 25.Li M.X., Wang W.X., Xue J., Gu Y., Lin S.Y. Meta-analysis of the clinical value of Astragalus membranaceus in diabetic nephropathy. J Ethnopharmacol. 2011;133:412–419. doi: 10.1016/j.jep.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y.Z., Gong Z.X., Cai G.Y., Gao Q., Chen X.M., Tang L. Efficacy and safety of Flos Abelmoschus manihot (Malvaceae) on type 2 diabetic nephropathy: A systematic review. Chin J Integr Med. 2015;21:464–472. doi: 10.1007/s11655-014-1891-6. [DOI] [PubMed] [Google Scholar]
- 27.Sun Q., Yang G.Y., Zhang M., Zhang M., Chen S., Chen P. Effect of Huangshukuihua (Flos Abelmoschi manihot) on diabetic nephropathy: A meta-analysis. J Tradit Chin Med. 2015;35:15–20. doi: 10.1016/s0254-6272(15)30003-0. [DOI] [PubMed] [Google Scholar]
- 28.Ren D., Zuo C., Xu G. Clinical efficacy and safety of Tripterygium wilfordii Hook in the treatment of diabetic kidney disease stage IV: A meta-analysis of randomized controlled trials. Medicine (Baltim) 2019;98 doi: 10.1097/MD.0000000000014604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao J.M., Song X.L., Wang H.J., Zhao J.Y., Shang H.X., Leng L. Efficacy and safety of berberine in treatment of diabetic nephropathy: A meta analysis. Chin J Clin. 2015;9:4396–4402. [Google Scholar]
- 30.Zhao J., Zhi Y.J., Zhao H., Yu D.D. Efficacy and safety of Breviscapine Injection in treatment of diabetic nephropathy: Systematic review and meta-analysis of randomized controlled trials. China J Chin Mater Med. 2019;44:833–844. doi: 10.19540/j.cnki.cjcmm.20181128.007. [DOI] [PubMed] [Google Scholar]
- 31.Liu X.D., Yao L., Sun D., Zhu X.W., Liu Q., Xu T.H. Effect of Breviscapine Injection on clinical parameters in diabetic nephropathy: A meta-analysis of randomized controlled trials. Exp Ther Med. 2016;12:1383–1397. doi: 10.3892/etm.2016.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang B., Ni Q., Wang X., Lin L. Meta-analysis of the clinical effect of ligustrazine on diabetic nephropathy. Am J Chin Med. 2012;40:25–37. doi: 10.1142/S0192415X12500036. [DOI] [PubMed] [Google Scholar]
- 33.Wang B., Chen S.B., Yan X.F., Li M.D., Li D.Q., Ti G.X. The therapeutic effect and possible harm of puerarin for treatment of stage III diabetic nephropathy: A meta-analysis. Alternative Ther Health Med. 2015;21:36–44. [PubMed] [Google Scholar]
- 34.Wang X.C., Xu Y.M., Chu C.L., Li H.Y., Mi J., Wen Z.H. Effect of safflower yellow on early type II diabetic nephropathy: A systematic review and meta-analysis of randomized controlled trials. J Pediatr Endocrinol Metab. 2019;32:653–665. doi: 10.1515/jpem-2018-0425. [DOI] [PubMed] [Google Scholar]
- 35.Ye W.C., Ye J.Z., Zheng C., He X.W., Huang J.J., Ye R. Combination therapy of tripterygium glycosides plus valsartan in diabetic nephropathy treatment: A systematic review and meta-analysis. Chin Herb Med. 2019;11:222–230. [Google Scholar]
- 36.Wu X.L., Li J.B., Ao Q.X., Zhang W.J., Liu B.L., Shen W.Z. Clinical study on Dan Shao Tang in treating diabetic nephropathy of deficiency of Yin with damp-heat symptom. J Chin Med Mater. 2006;29:411–414. [PubMed] [Google Scholar]
- 37.Zhang Y.Y., Huang Q.H. Study of improving prescription of Didang Decoction on diabetic nephropathy in earlier period. Modern J Integr Tradit Chin West Med. 2002;2002:2091–2092. [Google Scholar]
- 38.Zhuang J. Clinical efficacy analysis of Jiawei Zhuling Tang combined with conventional therapy for the treatment of diabetic nephropathy. Guide China Med. 2019;17:151. [Google Scholar]
- 39.Ma Y.E. Combination of Jiawei Zhuling Tang and conventional therapy for the treatment of diabetic nephropathy: 30 clinical cases. J Gansu Univ Chin Med. 2017;34:42–45. [Google Scholar]
- 40.Song X.Y., Chen Q., Qi X.Y. Effect of Liuwei Dihuang Pill on erythrocyte aldose reductase activity in early diabetic nephropathy patients. Chin J Integr Tradit West Med. 2004;24:1087–1090. [PubMed] [Google Scholar]
- 41.Zou L.H., Zhang J.H., Liu P.F. Clinical observation on Qidi Yiqi yangyin huoxue recipe in treating diabetic nephropathy at stage III and IV. Chin J Integr Tradit West Med. 2006;26:1023–1026. [PubMed] [Google Scholar]
- 42.Wang M.R., Yu L.H., Wang T.T., Wang Y.M., Han M.X. Effect of Shenqi Dihuang Decoction on inflammatory factor, renal function and microcirculation in patients with early diabetic nephropathy. China J Chin Mater Med. 2018;43:1276–1281. doi: 10.19540/j.cnki.cjcmm.2018.0050. [DOI] [PubMed] [Google Scholar]
- 43.Li B.Y., Peng H., Xiong D.L., Yi J., Chen H. Efficacy observation of treating diabetic nephropathy by Shenshuaining Granule combined telmisartan tablet. Chin J Integr Tradit West Med. 2015;35:142–146. [PubMed] [Google Scholar]
- 44.Li P., Chen Y., Liu J., Hong J., Deng Y., Yang F. Efficacy and safety of tangshen formula on patients with type 2 diabetic kidney disease: A multicenter double-blinded randomized placebo-controlled trial. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun W.S., Wu X.L., Qiao C.L. Clinical study on effect of tongluo capsule in treating diabetic nephropathy caused chronic renal failure. Chin J Integr Tradit West Med. 2004;24:704–706. [PubMed] [Google Scholar]
- 46.Piao C.L., Nan H.M., Jiang Z., Nan Z. Effect of combined therapy of Xiaoke Shen'an Capsule and western medicine in diabetic nephropathy. Chin J Integr Tradit West Med. 2005;25:650–652. [PubMed] [Google Scholar]
- 47.Liu H., Zheng J., Li R.H. Clinical efficacy of 'Spleen-kidney-care' Yiqi Huayu and Jiangzhuo traditional Chinese medicine for the treatment of patients with diabetic nephropathy. Exp Ther Med. 2015;10:1096–1102. doi: 10.3892/etm.2015.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma J.W., Xu L.M., Dong J., Wei H.L., Zhi Y., Ma X.Y. Effects of Zishentongluo in patients with early-stage diabetic nephropathy. Am J Chin Med. 2013;41:333–340. doi: 10.1142/S0192415X13500249. [DOI] [PubMed] [Google Scholar]
- 49.Xiong C., Li L., Bo W., Chen H., XiaoWei L., Hongbao L. Evaluation of the efficacy and safety of TWHF in diabetic nephropathy patients with overt proteinuria and normal eGFR. J Formos Med Assoc. 2020;119:685–692. doi: 10.1016/j.jfma.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Taghizadeh M., Soleimani A., Bahmani F., Moravveji A., Asadi A., Amirani E. Metabolic response to mulberry extract supplementation in patients with diabetic nephropathy: A randomized controlled trial. Iran J Kidney Dis. 2017;11:438–446. [PubMed] [Google Scholar]
- 51.Zhu H.W., Shi Z.F., Chen Y.Y. Effect of extract of Ginkgo bilboa leaf on early diabetic nephropathy. Chin J Integr Tradit West Med. 2005;25:889–891. [PubMed] [Google Scholar]
- 52.Wang Y. Clinical efficacy of berberine on early-stage diabetic nephtopathy. Chin J Modern Drug Appl. 2016;10:86–87. [Google Scholar]
- 53.Zhu L.B. Effect of fluvastatin and emodin on TGF-β1 level in patients with eraly-stage diabetic nephropathy. Heilongjiang J Tradit Chin Med. 2009;38:52–53. [Google Scholar]
- 54.Zhu Q., Qi X., Wu Y., Wang K. Clinical study of total glucosides of paeony for the treatment of diabetic kidney disease in patients with diabetes mellitus. Int Urol Nephrol. 2016;48:1873–1880. doi: 10.1007/s11255-016-1345-5. [DOI] [PubMed] [Google Scholar]
- 55.Sattarinezhad A., Roozbeh J., Shirazi Yeganeh B., Omrani G.R., Shams M. Resveratrol reduces albuminuria in diabetic nephropathy: A randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019;45:53–59. doi: 10.1016/j.diabet.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 56.Voroneanu L., Siriopol D., Dumea R., Badarau S., Kanbay M., Afsar B. Addition of silymarin to renin-angiotensin system blockers in normotensive patients with type 2 diabetes mellitus and proteinuria: A prospective randomized trial. Int Urol Nephrol. 2017;49:2195–2204. doi: 10.1007/s11255-017-1697-5. [DOI] [PubMed] [Google Scholar]
- 57.Song H.X., Gong J., Chen W. Effect of triptolide on urinary monocyte chemottractant protein-1 in patients with diabetic nephropathy. Chin J Integr Tradit West Med. 2005;25:416–418. [PubMed] [Google Scholar]
- 58.Kato M., Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol. 2019;15:327–345. doi: 10.1038/s41581-019-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan X.L., Han X.J., Su R.T. Impacts of berberine on vascular endothelial function in the patients of early diabetic nephropathy. World J Integr Tradit West Med. 2017;12:1707–10+14. [Google Scholar]
- 60.Wang J., Huang H., Liu P., Tang F., Qin J., Huang W. Inhibition of phosphorylation of p38 MAPK involved in the protection of nephropathy by emodin in diabetic rats. Eur J Pharmacol. 2006;553:297–303. doi: 10.1016/j.ejphar.2006.08.087. [DOI] [PubMed] [Google Scholar]
- 61.Gao J., Wang F., Wang W., Su Z., Guo C., Cao S. Emodin suppresses hyperglycemia-induced proliferation and fibronectin expression in mesangial cells via inhibiting cFLIP. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yokozawa T., Satoh A., Nakagawa T., Yamabe N. Attenuating effects of Wen-Pi-Tang treatment in rats with diabetic nephropathy. Am J Chin Med. 2006;34:307–321. doi: 10.1142/S0192415X06003850. [DOI] [PubMed] [Google Scholar]
- 63.Nakagawa T., Yokozawa T., Terasawa K., Nakanishi K. Therapeutic usefulness of Keishi-bukuryo-gan for diabetic nephropathy. J Pharm Pharmacol. 2003;55:219–227. doi: 10.1211/002235702450. [DOI] [PubMed] [Google Scholar]
- 64.Chen X., Wang H., Jiang M.Q., Zhao J., Fan C.L., Wang Y.M. Huangqi (Astragalus) decoction ameliorates diabetic nephropathy via IRS1–PI3K–GLUT signaling pathway. Am J Transl Res. 2018;10:2491–2501. [PMC free article] [PubMed] [Google Scholar]
- 65.Liu M.Y., Pan Q., Chen Y.L., Yang X.X., Zhao B.C., Jia L.F. Administration of Danhong Injection to diabetic db/db mice inhibits the development of diabetic retinopathy and nephropathy. Sci Rep. 2015;5:11219. doi: 10.1038/srep11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang S., Liu M.Y., Chen Y.L., Ma C.R., Liu L.P., Zhao B.C. NaoXinTong Capsules inhibit the development of diabetic nephropathy in db/db mice. Sci Rep. 2018;8:9158. doi: 10.1038/s41598-018-26746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao L.C., Gao H.C., Zhao Y.X., Lin D.H. Metabonomic analysis of the therapeutic effect of Zhibai Dihuang Pill in treatment of streptozotocin-induced diabetic nephropathy. J Ethnopharmacol. 2012;142:647–656. doi: 10.1016/j.jep.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 68.Hu S.J., Shu B., Jin H.E., Li X.F., Mao J.R., Ren K.J. Therapeutic role of Tangshenkang Granule in rat model with diabetic nephropathy. Chin J Integr Med. 2018;24:600–605. doi: 10.1007/s11655-016-2607-x. [DOI] [PubMed] [Google Scholar]
- 69.Qiu Y.Y., Tang L.Q., Wei W. Berberine exerts renoprotective effects by regulating the AGEs-RAGE signaling pathway in mesangial cells during diabetic nephropathy. Mol Cell Endocrinol. 2017;443:89–105. doi: 10.1016/j.mce.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 70.Cheng D., Gao L.B., Su S., Sargsyan D., Wu R.Y., Raskin I. Moringa Isothiocyanate activates Nrf2: Potential role in diabetic nephropathy. AAPS J. 2019;21:31. doi: 10.1208/s12248-019-0301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang W.J., Li Y.R., Gao H., Wu X.Y., Wang X.L., Wang X.N. Protective effect of the ethanol extract from Ligusticum chuanxiong rhizome against streptozotocin-induced diabetic nephropathy in mice. J Ethnopharmacol. 2018;227:166–175. doi: 10.1016/j.jep.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 72.Zhang B., Zhang X., Zhang C., Shen Q., Sun G., Sun X. Notoginsenoside R1 protects db/db mice against diabetic nephropathy via upregulation of Nrf2-mediated HO-1 expression. Molecules. 2019;24:247. doi: 10.3390/molecules24020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu H.R., Tang X.Y., Dai D.Z., Dai Y. Ethanol extracts of Rehmannia complex (Di Huang) containing no Corni fructus improve early diabetic nephropathy by combining suppression on the ET-ROS axis with modulate hypoglycemic effect in rats. J Ethnopharmacol. 2008;118:466–472. doi: 10.1016/j.jep.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 74.Sen S., Chen S.L., Feng B.A., Wu Y.X., Lui E., Chakrabarti S. Preventive effects of North American ginseng (Panax quinquefolium) on diabetic nephropathy. Phytomedicine. 2012;19:494–505. doi: 10.1016/j.phymed.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Fang D.H., Wan X.S., Deng W.P., Guan H.Y., Ke W.J., Xiao H.P. Fufang Xue Shuan Tong Capsules inhibit renal oxidative stress markers and indices of nephropathy in diabetic rats. Exp Ther Med. 2012;4:871–876. doi: 10.3892/etm.2012.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong X.G., An Z.M., Guo Y., Zhou J.L., Qin T. Effect of triptolide on expression of oxidative carbonyl protein in renal cortex of rats with diabetic nephropathy. J Huazhong Univ Sci Technolog Med Sci. 2017;37:25–29. doi: 10.1007/s11596-017-1689-9. [DOI] [PubMed] [Google Scholar]
- 77.Zhao T.T., Sun S.F., Zhang H.J., Huang X.R., Yan M.H., Dong X. Therapeutic effects of Tangshen Formula on diabetic nephropathy in rats. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun S.F., Zhao T.T., Zhang H.J., Huang X.R., Zhang W.K., Zhang L. Renoprotective effect of berberine on type 2 diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2015;42:662–670. doi: 10.1111/1440-1681.12402. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H.J., Zhao T.T., Gong Y.W., Dong X., Zhang W.K., Sun S.F. Attenuation of diabetic nephropathy by Chaihuang-Yishen Granule through anti-inflammatory mechanism in streptozotocin-induced rat model of diabetics. J Ethnopharmacol. 2014;151:556–564. doi: 10.1016/j.jep.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 80.Chen J., Hou X.F., Wang G., Zhong Q.X., Liu Y., Qiu H.H. Terpene glycoside component from Moutan Cortex ameliorates diabetic nephropathy by regulating endoplasmic reticulum stress-related inflammatory responses. J Ethnopharmacol. 2016;193:433–444. doi: 10.1016/j.jep.2016.09.043. [DOI] [PubMed] [Google Scholar]
- 81.Zhang M., Chen Y., Yang M.J., Fan X.R., Xie H., Zhang L. Celastrol attenuates renal injury in diabetic rats via MAPK/NF-κB pathway. Phytother Res. 2019;33:1191–1198. doi: 10.1002/ptr.6314. [DOI] [PubMed] [Google Scholar]
- 82.Zhao M., Han J.G. Dendrobium Officinale Kimura et Migo ameliorates insulin resistance in rats with diabetic nephropathy. Med Sci Monit Basic Res. 2018;24:84–92. doi: 10.12659/MSMBR.909242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang T.M., Zhu Q.J., Shao Y.X., Wang K., Wu Y.G. Paeoniflorin prevents TLR2/4-mediated inflammation in type 2 diabetic nephropathy. Biosci Trends. 2017;11:308–318. doi: 10.5582/bst.2017.01104. [DOI] [PubMed] [Google Scholar]
- 84.Shao Y.X., Xu X.X., Wang K., Qi X.M., Wu Y.G. Paeoniflorin attenuates incipient diabetic nephropathy in streptozotocin-induced mice by the suppression of the Toll-like receptor-2 signaling pathway. Drug Des Dev Ther. 2017;11:3221–3233. doi: 10.2147/DDDT.S149504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu L., Han J., Yuan R., Xue L., Pang W. Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway. Biol Res. 2018;51:9. doi: 10.1186/s40659-018-0157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang L.Q., Liu S., Zhang S.T., Zhu L.N., Wang F.L. Berberine regulates the expression of E-prostanoid receptors in diabetic rats with nephropathy. Mol Biol Rep. 2014;41:3339–3347. doi: 10.1007/s11033-014-3196-4. [DOI] [PubMed] [Google Scholar]
- 87.Ni W.J., Tang L.Q., Zhou H., Ding H.H., Qiu Y.Y. Renoprotective effect of berberine via regulating the PGE2–EP1–Gαq–Ca2+ signalling pathway in glomerular mesangial cells of diabetic rats. J Cell Mol Med. 2016;20:1491–1502. doi: 10.1111/jcmm.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hong J.N., Li W.W., Wang L.L., Guo H., Jiang Y., Gao Y.J. Jiangtang Decoction ameliorate diabetic nephropathy through the regulation of PI3K/Akt-mediated NF-κB pathways in KK-Ay mice. Chin Med. 2017;12:13. doi: 10.1186/s13020-017-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jing D.Q., Bai H., Yin S.N. Renoprotective effects of emodin against diabetic nephropathy in rat models are mediated via PI3K/Akt/GSK-3β and Bax/caspase-3 signaling pathways. Exp Ther Med. 2017;14:5163–5169. doi: 10.3892/etm.2017.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu J.J., Fan X.M., Meng X.S., Wang Y.M., Liang Q.L., Luo G.A. Evidence for the involvement of JAK/STAT/SOCS pathway in the mechanism of Tangshen Formula-treated diabetic nephropathy. Planta Med. 2014;80:614–621. doi: 10.1055/s-0034-1368454. [DOI] [PubMed] [Google Scholar]
- 91.Li Z., Zhang W. Protective effect of berberine on renal fibrosis caused by diabetic nephropathy. Mol Med Rep. 2017;16:1055–1062. doi: 10.3892/mmr.2017.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X., He H., Liang D., Jiang Y., Liang W., Chi Z.H. Protective effects of berberine on renal injury in streptozotocin (STZ)-induced diabetic mice. Int J Mol Sci. 2016;17:1327. doi: 10.3390/ijms17081327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qi M.Y., Kai C., Liu H.R., Su Y.H., Yu S.Q. Protective effect of icariin on the early stage of experimental diabetic nephropathy induced by streptozotocin via modulating transforming growth factor β1 and type IV collagen expression in rats. J Ethnopharmacol. 2011;138:731–736. doi: 10.1016/j.jep.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 94.Zhao T.T., Zhang H.J., Lu X.G., Huang X.R., Zhang W.K., Wang H. Chaihuang-Yishen Granule inhibits diabetic kidney disease in rats through blocking TGF-β/Smad3 signaling. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang N., Gao Y.B., Zou D.W., Wang J.Y., Li J.Y., Zhou S.N. Effects of Chinese medicine Tong xinluo on diabetic nephropathy via inhibiting TGF-β1-induced epithelial-to-mesenchymal transition. Evid Based Complement Alternat Med. 2014;2014:123497. doi: 10.1155/2014/123497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin C.C., Lin L.T., Yen M.H., Cheng J.T., Hsing C.H., Yeh C.H. Renal protective effect of Xiao-Chai-Hu-Tang on diabetic nephropathy of type 1-diabetic mice. Evid Based Complement Alternat Med. 2012;2012:984024. doi: 10.1155/2012/984024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y.W., Xie D., Chen Y.X., Zhang H.Y., Xia Z.X. Protective effect of Gui Qi mixture on the progression of diabetic nephropathy in rats. Exp Clin Endocrinol Diabetes. 2006;114:563–568. doi: 10.1055/s-2006-948307. [DOI] [PubMed] [Google Scholar]
- 98.Jin O., Gao L.P., Chen X., Zhu Y., Zhou Y.F., Wu W.M. Protective effects of She Jing Xiao Bai Capsule on diabetic nephropathy in a diabetic rat model. Int J Clin Exp Med. 2016;9:2873–2880. [Google Scholar]
- 99.Niu H.S., Liu I.M., Niu C.S., Ku P.M., Hsu C.T., Cheng J.T. Eucommia bark (Du-Zhong) improves diabetic nephropathy without altering blood glucose in type 1-like diabetic rats. Drug Des Dev Ther. 2016;10:971–978. doi: 10.2147/DDDT.S98558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y., Shi L.L., Wang L.Y., Xu J.W., Feng Y. Protective effects of MDG-1, a polysaccharide from Ophiopogon japonicus on diabetic nephropathy in diabetic KKA(y) mice. Int J Mol Sci. 2015;16:22473–22484. doi: 10.3390/ijms160922473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang S., Yang J.Z., Li H.Y., Li Y., Liu Y., Zhang D.M. Skimmin, a coumarin, suppresses the streptozotocin-induced diabetic nephropathy in wistar rats. Eur J Pharmacol. 2012;692:78–83. doi: 10.1016/j.ejphar.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 102.Li X.H., Lei G.P., Li J.T., Wang J., Gu H.R., Gao B.F. Effect of TongluoYishen Formula on rearrangement of cytoskeleton of glomerular podocyte in diabetic nephropathy rats. Int J Clin Exp Med. 2017;10:8458–8463. [Google Scholar]
- 103.Cao X.D., Wei R.X., Zhou J., Zhang X.X., Gong W.B., Jin T.L. Wenshen Jianpi recipe, a blended traditional Chinese medicine, ameliorates proteinuria and renal injury in a rat model of diabetic nephropathy. BMC Compl Alternative Med. 2019;19:193. doi: 10.1186/s12906-019-2598-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiang J., Yin J.N., Liu X., Wang H.J., Lu G.Y. Erzhi Formula extracts reverse renal injury in diabetic nephropathy rats by protecting the renal podocytes. Evid Based Complement Alternat Med. 2018;2018:1741924. doi: 10.1155/2018/1741924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai Y., Chen J.W., Jiang J.M., Cao W.W., He L. Zhen-Wu-tang, a blended traditional Chinese herbal medicine, ameliorates proteinuria and renal damage of streptozotocin-induced diabetic nephropathy in rats. J Ethnopharmacol. 2010;131:88–94. doi: 10.1016/j.jep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 106.Zhang M.Z., Liu M., Xiong M., Gong J.B., Tan X.Y. Schisandra chinensis fruit extract attenuates albuminuria and protects podocyte integrity in a mouse model of streptozotocin-induced diabetic nephropathy. J Ethnopharmacol. 2012;141:111–118. doi: 10.1016/j.jep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 107.Cui F.Q., Tang L., Gao Y.B., Wang Y.F., Meng Y., Shen C. Effect of Baoshenfang Formula on podocyte injury via inhibiting the NOX-4/ROS/p38 pathway in diabetic nephropathy. J Diabetes Res. 2019;2019:2981705. doi: 10.1155/2019/2981705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Z., Deng W., Cao A., Zang Y., Wang Y., Wang H. Huangqi Decoction inhibits hyperglycemia-induced podocyte apoptosis by down-regulated Nox4/p53/Bax signaling in vitro and in vivo. Am J Transl Res. 2019;11:3195–3212. [PMC free article] [PubMed] [Google Scholar]
- 109.Zhai R., Jian G., Chen T., Xie L., Xue R., Gao C. Astragalus membranaceus and Panax notoginseng, the novel renoprotective compound, synergistically protect against podocyte injury in streptozotocin-induced diabetic rats. J Diabetes Res. 2019;2019:1602892. doi: 10.1155/2019/1602892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang F., Qu Q., Zhao C., Liu X., Yang P., Li Z. Paecilomyces cicadae-fermented Radix astragali activates podocyte autophagy by attenuating PI3K/AKT/mTOR pathways to protect against diabetic nephropathy in mice. Biomed Pharmacother. 2020;129:110479. doi: 10.1016/j.biopha.2020.110479. [DOI] [PubMed] [Google Scholar]
- 111.Sohn E., Kim J., Kim C.S., Jo K., Kim J.S. Extract of Rhizoma Polygonum cuspidatum reduces early renal podocyte injury in streptozotocin-induced diabetic rats and its active compound emodin inhibits methylglyoxal-mediated glycation of proteins. Mol Med Rep. 2015;12:5837–5845. doi: 10.3892/mmr.2015.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Y., Chen J., Jiang M., Fu Y., Zhu Y., Jiao N. Loganin and catalpol exert cooperative ameliorating effects on podocyte apoptosis upon diabetic nephropathy by targeting AGEs-RAGE signaling. Life Sci. 2020;252:117653. doi: 10.1016/j.lfs.2020.117653. [DOI] [PubMed] [Google Scholar]
- 113.Chen Y., Liu Q., Shan Z., Mi W., Zhao Y., Li M. Catalpol ameliorates podocyte injury by stabilizing cytoskeleton and enhancing autophagy in diabetic nephropathy. Front Pharmacol. 2019;10:1477. doi: 10.3389/fphar.2019.01477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lei X., Zhang B.D., Ren J.G., Luo F.L. Astragaloside suppresses apoptosis of the podocytes in rats with diabetic nephropathy via miR-378/TRAF5 signaling pathway. Life Sci. 2018;206:77–83. doi: 10.1016/j.lfs.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 115.Lei X., Zhang L., Li Z., Ren J. Astragaloside IV/lncRNA-TUG1/TRAF5 signaling pathway participates in podocyte apoptosis of diabetic nephropathy rats. Drug Des Dev Ther. 2018;12:2785–2793. doi: 10.2147/DDDT.S166525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Z.S., Xiong F., Xie X.H., Chen D., Pan J.H., Cheng L. Astragaloside IV attenuates proteinuria in streptozotocin-induced diabetic nephropathy via the inhibition of endoplasmic reticulum stress. BMC Nephrol. 2015;16:44. doi: 10.1186/s12882-015-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guo H., Wang Y., Zhang X., Zang Y., Zhang Y., Wang L. Astragaloside IV protects against podocyte injury via SERCA2-dependent ER stress reduction and AMPKα-regulated autophagy induction in streptozotocin-induced diabetic nephropathy. Sci Rep. 2017;7:6852. doi: 10.1038/s41598-017-07061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang X., Gao Y., Tian N., Wang T., Shi Y., Xu J. Astragaloside IV inhibits glucose-induced epithelial-mesenchymal transition of podocytes through autophagy enhancement via the SIRT–NF-κB p65 axis. Sci Rep. 2019;9:323. doi: 10.1038/s41598-018-36911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tian N., Gao Y., Wang X., Wu X., Zou D., Zhu Z. Emodin mitigates podocytes apoptosis induced by endoplasmic reticulum stress through the inhibition of the PERK pathway in diabetic nephropathy. Drug Des Dev Ther. 2018;12:2195–2211. doi: 10.2147/DDDT.S167405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu J., Zong G.N., Wu H., Zhang K.Q. Podoplanin mediates the renoprotective effect of berberine on diabetic kidney disease in mice. Acta Pharmacol Sin. 2019;40:1544–1554. doi: 10.1038/s41401-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li C., Guan X.M., Wang R.Y., Xie Y.S., Zhou H., Ni W.J. Berberine mitigates high glucose-induced podocyte apoptosis by modulating autophagy via the mTOR/P70S6K/4EBP1 pathway. Life Sci. 2020;243:117277. doi: 10.1016/j.lfs.2020.117277. [DOI] [PubMed] [Google Scholar]
- 122.Tu Q., Li Y., Jin J., Jiang X., Ren Y., He Q. Curcumin alleviates diabetic nephropathy via inhibiting podocyte mesenchymal transdifferentiation and inducing autophagy in rats and MPC5 cells. Pharm Biol. 2019;57:778–786. doi: 10.1080/13880209.2019.1688843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu F., Li S., Zhang N., Huang W., Li X., Wang M. Hispidulin alleviates high-glucose-induced podocyte injury by regulating protective autophagy. Biomed Pharmacother. 2018;104:307–314. doi: 10.1016/j.biopha.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 124.Li X., Zhu Q., Zheng R., Yan J., Wei M., Fan Y. Puerarin attenuates diabetic nephropathy by promoting autophagy in podocytes. Front Physiol. 2020;11:73. doi: 10.3389/fphys.2020.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao Q., Shen W.W., Qin W.S., Zheng C.X., Zhang M.C., Zeng C.H. Treatment of db/db diabetic mice with triptolide: A novel therapy for diabetic nephropathy. Nephrol Dial Transplant. 2010;25:3539–3547. doi: 10.1093/ndt/gfq245. [DOI] [PubMed] [Google Scholar]
- 126.Li X.Y., Wang S.S., Han Z., Han F., Chang Y.P., Yang Y. Triptolide restores autophagy to alleviate diabetic renal fibrosis through the miR-141-3p/PTEN/Akt/mTOR pathway. Mol Ther Nucleic Acids. 2017;9:48–56. doi: 10.1016/j.omtn.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang X.X., Jiang C.H., Liu Y., Lou D.X., Huang Y.P., Gao M. Cyclocarya paliurus triterpenic acids fraction attenuates kidney injury via AMPK-mTOR-regulated autophagy pathway in diabetic rats. Phytomedicine. 2019;64:153060. doi: 10.1016/j.phymed.2019.153060. [DOI] [PubMed] [Google Scholar]
- 128.Yang S.S., Deng H.C., Zhang Q.Z., Xie J., Zeng H., Jin X.L. Amelioration of diabetic mouse nephropathy by catalpol correlates with down-regulation of Grb10 expression and activation of insulin-like growth factor 1/insulin-like growth factor 1 receptor signaling. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lu J., Peng J., Xiang M., He L., Wang D., Xiong G. Trichosanthes kirilowii lectin alleviates diabetic nephropathy by inhibiting the LOX1/NF-κB/caspase-9 signaling pathway. Biosci Rep. 2018;38 doi: 10.1042/BSR20180071. BSR20180071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xue H.Y., Li P.P., Luo Y.S., Wu C.W., Liu Y., Qin X.G. Salidroside stimulates the Sirt1/PGC-1α axis and ameliorates diabetic nephropathy in mice. Phytomedicine. 2019;54:240–247. doi: 10.1016/j.phymed.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 131.Wang F.L., Wang Y.H., Han L., An H.Y., Zhang J.H., Zhang X.Y. Renoprotective effect of Yiqi yangyin huayu tongluo formula against diabetic nephropathy in diabetic rats. Evid Based Complement Alternat Med. 2018;2018:4276052. doi: 10.1155/2018/4276052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Seo E., Kang H., Oh Y.S., Jun H.S. Psoralea corylifolia L. seed extract attenuates diabetic nephropathy by inhibiting renal fibrosis and apoptosis in streptozotocin-induced diabetic mice. Nutrients. 2017;9:828. doi: 10.3390/nu9080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu W., Hu W., Han W.B., Liu Y.L., Tu Y., Yang H.M. Inhibition of Akt/mTOR/p70S6K signaling activity with Huangkui Capsule alleviates the early glomerular pathological changes in diabetic nephropathy. Front Pharmacol. 2018;9:443. doi: 10.3389/fphar.2018.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Han W.B., Ma Q., Liu Y.L., Wu W., Tu Y., Huang L. Huangkui Capsule alleviates renal tubular epithelial-mesenchymal transition in diabetic nephropathy via inhibiting NLRP3 inflammasome activation and TLR4/NF-κB signaling. Phytomedicine. 2019;57:203–214. doi: 10.1016/j.phymed.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 135.Wen D., Tan R.Z., Zhao C.Y., Li J.C., Zhong X., Diao H. Astragalus mongholicus Bunge and Panax notoginseng (Burkill) F.H. Chen Formula for renal injury in diabetic nephropathy-in vivo and in vitro evidence for autophagy regulation. Front Pharmacol. 2020;11:732. doi: 10.3389/fphar.2020.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.An X.F., Zhang M.X., Zhou S.S., Lu T., Chen Y.J., Yao L. Xiao-Shen-Formula, a traditional Chinese medicine, improves glomerular hyper-filtration in diabetic nephropathy via inhibiting arginase activation and heparanase expression. Front Physiol. 2018;9:1195. doi: 10.3389/fphys.2018.01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Molitch M.E., Adler A.I., Flyvbjerg A., Nelson R.G., So W.Y., Wanner C. Diabetic kidney disease: A clinical update from kidney disease: improving global outcomes. Kidney Int. 2015;87:20–30. doi: 10.1038/ki.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alicic R.Z., Johnson E.J., Tuttle K.R. SGLT2 inhibition for the prevention and treatment of diabetic kidney disease: A review. Am J Kidney Dis. 2018;72:267–277. doi: 10.1053/j.ajkd.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 139.Neumiller J.J., Alicic R.Z., Tuttle K.R. Therapeutic considerations for antihyperglycemic agents in diabetic kidney disease. J Am Soc Nephrol. 2017;28:2263–2274. doi: 10.1681/ASN.2016121372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gnudi L., Thomas S.M., Viberti G. Mechanical forces in diabetic kidney disease: A trigger for impaired glucose metabolism. J Am Soc Nephrol. 2007;18:2226–2232. doi: 10.1681/ASN.2006121362. [DOI] [PubMed] [Google Scholar]
- 141.Tonna S., El-Osta A., Cooper M.E., Tikellis C. Metabolic memory and diabetic nephropathy: Potential role for epigenetic mechanisms. Nat Rev Nephrol. 2010;6:332–341. doi: 10.1038/nrneph.2010.55. [DOI] [PubMed] [Google Scholar]
- 142.Ankita P., Deepti B., Nilam M. Flavonoid rich fraction of Punica granatum improves early diabetic nephropathy by ameliorating proteinuria and disturbed glucose homeostasis in experimental animals. Pharm Biol. 2015;53:61–71. doi: 10.3109/13880209.2014.910533. [DOI] [PubMed] [Google Scholar]
- 143.Gould C.M., Newton A.C. The life and death of protein kinase C. Curr Drug Targets. 2008;9:614–625. doi: 10.2174/138945008785132411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen Y., Liu Z.Y., Zhou F.X., Zhao H., Yang Q., Li H. Evaluating pharmacological effects of two major components of Shuangdan Oral Liquid: Role of danshensu and paeonol in diabetic nephropathy rat. Biomol Ther. 2016;24:536–542. doi: 10.4062/biomolther.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zou T., Zhu M., Ma Y.C., Xiao F., Yu X., Xu L. MicroRNA-410-5p exacerbates high-fat diet-induced cardiac remodeling in mice in an endocrine fashion. Sci Rep. 2018;8:8780. doi: 10.1038/s41598-018-26646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang M.H., Feng L., Zhu M.M., Gu J.F., Jiang J., Cheng X.D. The anti-inflammation effect of Moutan Cortex on advanced glycation end products-induced rat mesangial cells dysfunction and high-glucose-fat diet and streptozotocin-induced diabetic nephropathy rats. J Ethnopharmacol. 2014;151:591–600. doi: 10.1016/j.jep.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 147.Wu J.S., Liu Y., Shi R., Lu X., Ma Y.M., Cheng N.N. Effects of combinations of Xiexin Decoction constituents on diabetic nephropathy in rats. J Ethnopharmacol. 2014;157:126–133. doi: 10.1016/j.jep.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 148.Hao C.M., Breyer M.D. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 149.Makino H., Tanaka I., Mukoyama M., Sugawara A., Mori K., Muro S. Prevention of diabetic nephropathy in rats by prostaglandin E receptor EP1-selective antagonist. J Am Soc Nephrol. 2002;13:1757–1765. doi: 10.1097/01.asn.0000019782.37851.bf. [DOI] [PubMed] [Google Scholar]
- 150.Wu X.M., Gao Y.B., Xu L.P., Zou D.W., Zhu Z.Y., Wang X.L. Tongxinluo inhibits renal fibrosis in diabetic nephropathy: Involvement of the suppression of intercellular transfer of TGF-β1-containing exosomes from GECs to GMCs. Am J Chin Med. 2017;45:1075–1092. doi: 10.1142/S0192415X17500586. [DOI] [PubMed] [Google Scholar]
- 151.Mao Z.M., Shen S.M., Wan Y.G., Sun W., Chen H.L., Huang M.M. Huangkui Capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38MAPK/Akt pathways, compared to alpha-lipoic acid. J Ethnopharmacol. 2015;173:256–265. doi: 10.1016/j.jep.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 152.Ma X., Jiao Z., Liu Y., Chen J., Li G., Liu T. Probucol protects against contrast-induced acute kidney injury via the extracellular signal-regulated kinases 1 and 2 (ERK1/2)/JNK-caspase 3 pathway in diabetic rats. Med Sci Monit. 2019;25:1038–1045. doi: 10.12659/MSM.913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang X., Zhao L., Ajay A.K., Jiao B., Zhang X., Wang C. QiDiTangShen Granules activate renal nutrient-sensing associated autophagy in db/db mice. Front Physiol. 2019;10:1224. doi: 10.3389/fphys.2019.01224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.