Graphical abstract

Keywords: Longxuetongluo Capsule, Oxygen-glucose deprivation/reperfusion, Apoptosis, Endoplasmic reticulum stress
Abstract
Introduction
Longxuetongluo Capsule (LTC) is wildly applied to treat ischemic stroke in clinical practice in China. However, the pharmacological mechanism of LTC on ischemic stroke is still unstated.
Objective
Our research was designed to study the protective effect of LTC against cerebral ischemia–reperfusion (I/R) injury and reveal the underlying mechanism both in vivo and in vitro.
Methods
PC12 cells treated with glucose deprivation/reperfusion (OGD/R) were used to simulate in vitro ischemia/reperfusion (I/R) injury. The cell viability, apoptosis rate, and protein expressions of PC12 cells were evaluated. In vivo validation of the protective effect of LTC was carried out by middle cerebral artery occlusion (MCAO)/reperfusion treatment, and the underlying mechanism of its anti-apoptosis ability was further revealed by immunohistochemistry staining and Western blotting.
Results
In the current study, we observed that LTC effectively inhibited oxygen-glucose deprivation/reperfusion (OGD/R) induced apoptosis of PC12 cells through suppressing the cleavage of poly ADP-ribose polymerase (PARP), caspase-3, and caspase-9. Further investigation revealed that OGD/R insult remarkably triggered the endoplasmic reticulum stress responses (ER stress) to induce PC12 cell apoptosis. LTC treatment alleviated OGD/R induced ER stress by inhibiting the activation of protein kinase RNA (PKR)-like ER kinase (PERK)/eukaryotic translation initiation factor 2 (eIF2α) and inositol requiring enzyme 1 (IRE1)/tumor necrosis factor receptor-associated factor 2 (TRAF2) pathways. Additionally, LTC also restrained the OGD/R-induced PC12 cell apoptosis by reversing the activated mitogen-activated protein kinase (MAPK) through IRE1/TRAF2 pathway. Animal studies demonstrated LTC significantly restricted the infarct region induced by middle cerebral artery occlusion (MCAO)/reperfusion, the activation of ER stress and apoptosis of neuronal cells had also been suppressed by LTC in the penumbra region.
Conclusion
LTC protects the cerebral neuronal cell against ischemia/reperfusion injury through ER stress and MAPK-mediated mechanisms.
Introduction
Strokes are caused by inadequate blood supply, which consequently leads to the immediate oxygen and glucose depletion in brain tissue; ischemic stroke stands the proportion of approximately 70% among all strokes [1]. Timely restoration of blood flow and re-oxygenation is the only widely accepted approach although considerable advances have been made in the treatment of cerebral ischemia [2]. However, the ischemia/reperfusion (I/R) injury will possibly occur after the restoration of blood circulation to in ischemic brain which strongly affects neurons and leads to apoptosis of neurons eventually [3].
During I/R, increased hypoxia and free radicals exposure in the brain cells disrupted homeostasis in the endoplasmic reticulum (ER), resulting in ER stress [4]. Because ER stress reduces the protein folding capacity of the ER, unfolded proteins are accumulated and aggregated in the ER. In order to resist the destructive effects of ER stress, cells activate an adaptive cellular response – the unfolded protein response (UPR) to withstand the destructive effects of ER stress [5]. When the accumulation of unfolded proteins happens, the UPR is activated and triggered by the two ER receptors: inositol-requiring enzyme 1 (IRE1) and protein kinase RNA (PKR)-like ER kinase (PERK) [6]. However, when the adaptive response fails or the stress condition is prolonged, cell apoptosis will consequently emerge. That is to say, PERK and IRE1 in UPR signaling play the central role in ER stress-induced apoptosis [7], [8]. During the process of apoptosis, the mitochondria-mediated intrinsic pathway activates caspase-9, which interacts with Bax in the mitochondrial membrane [9], [10]. Subsequently, effectors’ caspases are activated and cleaved in a cascade-like manner to dismantle the neurons [11], [12].
Chinese dragon’s blood is the red resin of Dracaena cochinchinensis (Lour.) S. C. Chen, which is known to stop bleeding, relieve pain, alleviate blood stasis, and improve perfusion [13]. Researches in recent years have revealed its pharmacological activities as the analgesic [14], wound healing [15], anti-bacterial [16], and anti-atherosclerosis [17]. In June 2013, Longxuetongluo Capsule (LTC) was authorized by China Food and Drug Administration to treat ischemic stroke. The total phenolic extract of the Chinese dragon's blood is its main active ingredient [18]. The protective activity of the Chinese dragon's blood against focal cerebral ischemia injury has been demonstrated in extensive clinical trials. The constituents and the effective components of LTC had also been comprehensively studied in our previous researches [19], [20]. However, the mechanism of its protective effect against cerebral I/R injury is still unclear. Here, we investigate whether LTC confers resistance to reperfusion injury after cerebral ischemia. Our observations indicate that LTC exerts the protective effect in the context of I/R injury through ER stress and activated mitogen-activated protein kinase (MAPK)-mediated apoptotic actions on the neuron.
Materials and methods
Medicinal materials
LTC provided by Jiangsu Kanion Pharmaceutical Co. Ltd. (Jiangsu, China), in which the quantitative and qualitative characterization of chemical components in LTC has been studied in our previous research [19], [21].
Oxygen-glucose deprivation/reperfusion (OGD/R) treatment, MTT assay, and lactate dehydrogenase (LDH) assay
The PC12 cell was gifted by Prof. K.W. Zeng (Peking University Health Science Center, Beijing, China). Cells were cultured in DMEM containing 10% fetal bovine serum in an atmosphere of 5% CO2 at 37 °C. Before OGD insult, PC12 cells were seeded into the 96-well plate and kept on culturing for 24 h. The culture medium of PC12 cells was replaced with Earle’s balanced salt solution (Solarbio, Beijing, China), which was applied to initiate glucose deprivation before the treatment. Then, the plates were incubated anaerobically for 2–6 h with the AnaeroPack system (Mitsubishi GAS CHEMICAL, Tokyo, Japan) to initiate the OGD insult. Fresh complete medium with or without LTC was added after OGD insult was terminated. Cells were then kept on culturing for an additional period of time under the normoxic condition. After OGD treatment as described above, the OGD insult was terminated and PC12 cells were treated with different concentrations of LTC or curcumin (2 μm/ml) under the normoxic condition for an additional 24 h. MTT and LDH kit were applied to measure cell viability and LDH release, respectively [22].
Annexin V-FITC/PI flow cytometry and AO/EB staining
PC12 cells were treated with the combined reagent of AO/EB (Solarbio, Beijing, China) following the manufacturer’s instruction to perform AO/EB staining after OGD/R and LTC treatment (2.5 and 5 μg/ml). The confocal laser scanning microscope was applied to visualize and capture images of the cells. Furthermore, flow cytometry was adopted for the detection of PC12 cell apoptosis after the cells were labeled with Fluorescein Isothiocyanate (FITC) Annexin V and PI staining reagent (BD Bioscience, San Jose, USA). As described previously, PC12 cells were treated with LTC for 12 h after OGD/R insult. After 15 min incubation with FITC Annexin V and PI in the dark, the apoptosis of PC12 cells was detected by flow cytometry.
Western blot assay
PC12 cells were subjected to OGD/R insult and LTC treatment as described previously or subjected to thapsigargin (TG, MedChemexpress, New Jersey, USA) treatment with the presence of LTC. The same amount of total protein in cells or rat brain tissue was resolved by 4–15% SDS-PAGE. The protein in the PAGE gel was then transferred to PVDF membranes. After blocked in 5% non-fat milk, the membranes were incubated with primary antibodies (1:1000) at 4 °C overnight. Then further incubation with the secondary antibodies (1:2000) proceeded for 1 h at room temperature. The targeted proteins were visualized using the ECL chemiluminescence detection kit. Primary antibodies of P-IRE1, IRE1, tumor necrosis factor type 2 receptor associated protein 2 (TRAF2), and heavy chain binding protein (Bip) were obtained from abcam (Cambridge, UK), the others were purchased from Cell Signaling Technology (Danvers, MA, USA). Secondary antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA).
Ischemia-reperfusion brain injury model and LTC treatment
Seventy male Sprague-Dawley (SD) rats weighing around 300 g, age from 7 to 8 weeks, were obtained from SPF Biotechnology Co (Beijing, China). The protocol adopted in this study had been approved by the medical animal experiment ethics committee of the Beijing University of Chinese Medicine (Approval Number: BUCM-4-2018050601-2072). Rats were kept at 24 ± 2 °C and relative humidity of 50–60% with a light/dark cycle of 12/12 h. The middle cerebral artery occlusion (MCAO)/reperfusion model was performed following the previous protocol with modification [23]. After a midline ventral neck incision was made, the internal carotid artery and the pterygopalatine artery were divided and ligated firstly. The external carotid artery was inserted with a 0.25-mm nylon thread and the origin of the middle cerebral artery was blocked consequently to initiate MCAO. Nylon thread was then withdrawn to restore cerebral blood flow after 1.5 h of transient focal cerebral ischemia. After the time of reperfusion, rats were treated 300 mg/kg LTC (n = 14) or 10 mg/kg Nimodipine (as a positive control, n = 14) by intragastric administration in 5 consecutive days. A sham-operated group (n = 16) without inserting nylon thread after subjected to the same surgical procedure was treated with phosphate buffer saline (PBS) and sacrificed at corresponding time points. The LTC treated sham group (n = 14) was also administrated the 300 mg/kg LTC after sham-operation. The dosages selected were based on the clinical dosage of LTC. After treated with Nimodipine or LTC for 5 days, the rats were sacrificed. Immediately the brains were dissected out. Part of brain tissues was used as the following staining. The others were frozen in −80 °C for western blot analysis.
Infarct volume analysis, HE staining, and TUNEL assay of rat brain
Part of rat brain tissue was collected after rats were sacrificed, and the hind region was removed. The coronal brain section was cut into 3 mm slices and stained with 2,3,5-Triphenyltetrazolium chloride (TTC) solution [24]. Part of rat brain was immediately dissected out and embedded with paraffin after fixation. The 5 μm slices of the coronal section with cortex region were stained with HE. Histological aspects of apoptosis were studied by TUNEL histology [24].
Immunohistochemistry (IHC) study with CHOP and NeuN
The immunohistochemistry was preceded following the method described by the previous study [25]. Different primary antibodies (1:100) were used to reveal the expression of C/EBP-homologous protein (CHOP, Cell Signaling Technology, MA, USA) and Neuronal nuclei (NeuN, Santa Cruz Biotechnology, TX, USA) in the coronal brain section of rats in each group.
Statistical analysis
All data were statistically analyzed using Prism 5.0 software. Differences were analyzed by One way ANOVA and Student's t-test, with the statistically significant values set to p < 0.05.
Results
LTC increased OGD/R-induced cell viability of PC12 by inhibiting apoptosis
PC12 cells were injured by OGD/R insult followed by 24 h of LTC treatment. Cell viability and LDH release of PC12 cells were tested subsequently. The cell viability showed the 7.61% and 10.69% increase in cells treated with 5 μg/ml LTC and 5 μM curcumin (positive control), respectively. The dose-dependent decreases in LDH release were further confirmed by LTC treatment (Fig. 1A). These results suggest that LTC conserve neuron viability and integrity after OGD/R insult. Subsequent experiments confirmed that even 400 μg/ml of LTC did not show cytotoxicity after 48 h incubation alone (Fig. 1B). To elucidate the mechanism by how LTC increases cell viability, we analyzed the effect of LTC on cell apoptosis by FITC Annexin V/PI flow cytometry. We demonstrated observed the percentage of late apoptotic cells (PI+/Annexin-V+, Q2) and early apoptotic cells (PI−/Annexin-V+, Q4) enhanced in response to OGD/R insult (4- and 36-fold, respectively, p < 0.05, Fig. 1C). The percentage of both late apoptotic and early apoptotic cells was dramatically decreased after LTC treatment (Fig. 1C). The results of the AO/EB staining analysis further supported the anti-apoptosis ability of LTC. Apoptotic and living cells emit green fluorescence by AO staining, whereas only apoptotic cells emit red fluorescence by EB staining [26]. The proportion of apoptotic cells in AO/EB double staining dramatically increased after the OGD/R insult; these effects were significantly reversed by LTC treatment (Fig. 1D). The above results indicated that LTC significantly reduced the OGD/R-induced PC12 cell apoptosis.
Fig. 1.
LTC protected PC12 cells against oxygen-glucose deprivation/reperfusion (OGD/R)-induced cell apoptosis. (A) LTC improved the cell viability and reduced the LDH release of OGD/R-insulted PC12 cells. Cur: curcumin. (B) The effect of LTC alone on PC12 cell viability. (C) LTC suppressed the apoptosis rate of PC12 cell as measured by Annexin V staining and flow cytometry analysis. Q4: early apoptosis, Q2: late apoptosis. (D) Apoptotic PC12 cells were identified using AO/EB staining. Apoptotic cells were identified by double staining with acridine orange (AO) and ethidium bromide (EB). Cells that took up both dyes were classified as apoptotic (indicated by arrows in the EB and merged panels). Scale bar = 50 μm. All data are presented as Mean ± SEM, from independent experiments performed in triplicate, and statistical comparisons between the different groups were performed using one-way analysis of variance (ANOVA), followed by Student's t-test. ##P < 0.01, relative to untreated group; *P < 0.05, **P < 0.01, relative to OGD/R treated group.
LTC inhibited PC12 cells apoptosis via alleviating PERK/eIF2α and IRE1/TRAF2 pathways mediated ER stress
To analyze the anti-apoptotic actions of LTC, the expressions of apoptosis-related protein were examined under the condition of OGD/R insult in the presence or absence of LTC (Fig. 2A). Western blot results revealed that LTC markedly reversed the enhanced cleaved forms levels of the caspase-3, 9, and PARP in PC12 cells after OGD/R insult (Fig. 2A). GAPDH and β-actin were presented as the loading control. This result suggested that LTC effectively protected PC12 cells against apoptosis by regulation of the caspase-3/9 signaling pathway.
Fig. 2.
LTC protected PC12 cells against OGD/R-mediated apoptosis by alleviating PERK- eIF2α mediated ER stress. (A) LTC reversed OGD/R-induced expression of cleaved PARP, caspase-3, and caspase-9. GAPDH was presented as the loading control. (B) OGD/R insult increased the expression of CHOP and the phosphorylation of PERK and eIF2αin PC12 cells at different time points. β-actin was presented as the loading control. (C) LTC markedly reversed the phosphorylation of PERK and eIF2α induced by OGD/R in a dose-dependent manner. (D) LTC reduced the expression of CHOP induced by OGD/R insult in PC12 cells. (E) LTC markedly reversed the phosphorylation of IRE1, Bip, and TRAF2 overexpression induced by OGD/R. Data are presented as Mean ± SEM, from independent experiments performed in triplicate ##P < 0.01, relative to untreated group; *P < 0.05, **P < 0.01, relative to OGD/R-treated group.
Recently, ER dysfunction has been implicated in neuronal apoptosis after OGD/R insult [27], which can eventually activate the caspase-3/9 pathway [28]. The PERK-eIF2α pathway is one of the essential regulators in ER stress, which is touches upon ER stress-induced apoptosis [29]. Therefore, the potential mechanism of LTC on the PERK-eIF2α pathway following OGD/R induced ER stress was further investigated. We first studied the activation of PERK, eIF2a, and CHOP in response to OGD/R insult (Fig. 2B). According to the results of western blot, after OGD treatment, both PERK phosphorylation and eIF2α phosphorylation were activated at 1 h post-treatment and declined after 2 h. The expression of CHOP was activated after prolonged reperfusion treatment at 12 h post-treatment and persisted to 24 h. Based on the findings above, we examined the related protein expressions with different time spots of LTC treatment. Immunoblotting revealed that after LTC treatment for 1 h (Fig. 2C), the phosphorylation of PERK and eIF2α induced by OGD/R injury was markedly suppressed. Then we found the expressions of increased CHOP and Bip were also significantly reduced after prolonged LTC treatment for 24 h (Fig. 2D and E). In addition to the PERK/eIF2α pathway, another ER stress-induced cell apoptosis depends on the IRE1/TRAF2 pathway [30]. As depicted in Fig. 2E, the OGD/R injury induced-overexpression of phosphorylated IRE1 and the TRAF2 were down-regulated by LTC treatment in a dose-dependent manner. The above results indicated the PERK/eIF2α and IRE1/TRAF2 pathways are related to the protection of LTC against OGD/R-induced ER stress in neurons.
LTC regulated MAPK pathway in PC12 cells after OGD/R insult
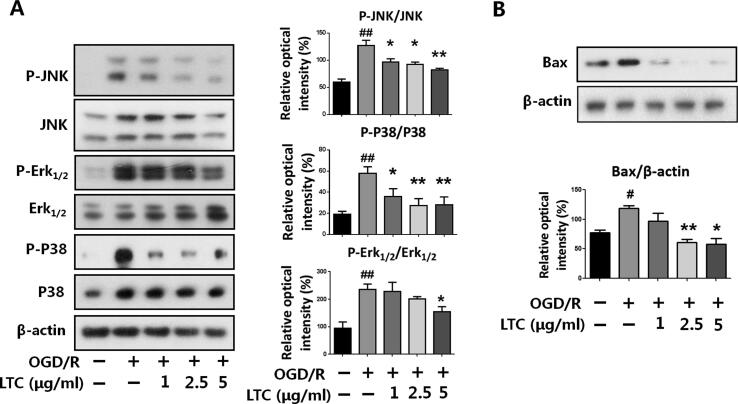
MAPK signaling network is also known to regulate cell apoptosis following the ER stress [31]. As ER stress-triggered IRE1 phosphorylation, the downstream TRAF2 was recruited to phosphorylate JNK and P38 MAPK, which leads to the translocation of pro-apoptotic Bax and phosphorylation of Bcl-2 [32], [33], and eventually induces cell apoptosis [34]. Our results revealed that the phosphorylation of JNK, P38, and ErK1/2 was promoted after OGD/R insult in PC12 cells; and these activated proteins were dose-dependently reversed by LTC treatment (Fig. 3A). Consistent with the changes of the MAPK pathway, the expression of pro-apoptotic Bax appeared to be suppressed by LTC treatment (Fig. 3B). These results showed that LTC also improved OGD/R-induced apoptosis by inhibiting MAPK signaling pathway activation.
Fig. 3.
LTC down-regulated the activation of MAPK signaling pathway and Bax expression in PC12 cells. (A) LTC reversed OGD/R-induced phosphorylation of JNK, Erk1/2, and P38 dose-dependently. (B) LTC markedly reduced the expression of Bax induced by OGD/R insult. Data are presented as Mean ± SEM, from independent experiments performed in triplicate. ##P < 0.01, relative to untreated group; *P < 0.05, **P < 0.01, relative to OGD/R-treated group.
LTC alleviated thapsigargin-induced ER stress and apoptosis
To validate the protective effect of LTC against OGD/R injury through alleviating ER stress-mediated apoptosis in PC12 cells, TG was used to stimulate ER stress [35]. Exposure to TG at a concentration of 10 μM dramatically enhanced the levels of P-PERK, P-eIF2α and P-IRE1. After LTC treatment, the elevated expression of P-PERK, P-eIF2α and P-IRE1 induced by TG was significantly suppressed (Fig. 4A). The subsequent studies revealed that TG treatment also triggered the expression of CHOP and phosphorylation of P38 (P-P38). The dose-dependent reduction of CHOP expression and phosphorylation of P38 in LTC-treated PC12 cells after TG-induction were also observed (Fig. 4B and C). Furthermore, we investigated the effect of LTC on TG-induced cell apoptosis. Our results revealed that LTC markedly reversed the enhanced levels of cleaved forms of PARP and caspase-3 in PC12 cells after TG treatment (Fig. 4D). The above results verified the effect that LTC protect PC12 cell against OGD/R insult by inhibiting PERK/eIF2α and IRE1 mediated ER stress-induced apoptosis.
Fig. 4.
LTC inhibited thapsigargin-induced activation of ER stress and phosphorylation of P38 in vitro. (A) LTC inhibited PERK, eIF2α, and IRE1 phosphorylation induced by thapsigargin (TG) in PC12 cells. (B) LTC suppressed TG-induced CHOP expression in PC12 cells. (C) LTC reversed the TG-induced phosphorylation of P38 in a dose-dependent manner. (D) LTC suppressed TG-induced PC12 apoptosis. Data are presented as Mean ± SEM, from independent experiments performed in triplicate. ##P < 0.01, relative to untreated group; *P < 0.05, **P < 0.01, relative to OGD/R treated group.
LTC inhibited cerebral I/R injury in vivo through alleviating apoptosis and ER stress
As the protective effect of LTC against OGD/R injury had been proved in vitro, the cerebral I/R injury experiments in vivo were designed to confirm the therapeutic effect of LTC. Rats were initially subjected to MCAO for 1.5 h and then followed by reperfusion. LTC (300 mg/kg) and nimodipine (10 mg/kg) were administered intraperitoneally (i.p.) immediately to animals after reperfusion and repeated daily for consecutive 5 days. Evaluation of infarct size by TTC staining revealed LTC and nimodipine displaying the same significant cytoprotection (Fig. 5A). HE staining revealed that obvious structure changes such as karyopyknosis and vacuolation were caused by MCAO/reperfusion in cerebral neuronal; these pathological changes are restricted by LTC treatment (Fig. 5B). To investigate the extent of apoptosis of neuron in the brain infarcted regions, we performed NeuN (marker of neuronal cells) and TUNEL staining on the different experimental groups [36]. The apoptosis of neuronal cells in the coronal section of rat brains caused by MCAO/reperfusion was alleviated by LTC treatment (Fig. 5B).
Fig. 5.
LTC protected cerebral I/R injury in vivo. (A) LTC remarkably reduced the infarct size in the coronal section of the rat brain. SD rats were subjected to MCAO (1.5 h) with the treatment of LTC (300 mg/kg) or nimodipine (10 mg/kg) for 5 days. Then, the coronal brain sections were cut and stained with TTC. The red regions indicated viable brain tissue, whereas non-stained pale regions indicated infarct brain tissue (n = 8). Scale bar = 10 mm. Nim: nimodipine. (B) LTC significantly reversed the neuronal structure changes, CHOP expression, and neuronal cell apoptosis in the coronal section of the rat brain caused by MCAO. The coronal brain sections were cut and stained with HE, TUNEL, or the antibodies of CHOP and NeuN in IHC assay. Black arrows indicate vacuolation and karyopyknosis of the neuronal cell in the representative photomicrographs of HE staining. Red arrows indicate apoptotic vascular cells in the representative photomicrographs of TUNEL staining. Yellow arrows show the positive staining in the representative photomicrographs of the CHOP IHC assay. Green arrows show the positive staining of the neuronal cell in the representative photomicrographs of the NeuN IHC assay. Scale bar = 50 μm. (C) LTC reversed the expressions of CHOP, cleaved caspase-3 expression, and P-P38 in rat brain caused by MCAO (n = 6). Data are presented as Mean ± SEM. ##P < 0.01, relative to sham group; *P < 0.05, **P < 0.01, relative to middle cerebral artery occlusion group.
Further investigation of IHC for CHOP revealed that there is a noticeable increase in CHOP expression of the infarcted regions, LTC treatment also significantly suppressed CHOP expression (Fig. 5B). These results above revealed that LTC exerted the protective effect in cerebral I/R injury through inhibited ER stress-induced apoptosis of the neuronal cell. To confirm the inhibitory effect of LTC on CHOP and caspase-induced cell apoptosis, the expression levels of CHOP and cleaved caspase-3 were examined in the infarcted regions by western bolt. Our findings revealed that LTC remarkably inhibited the expression of the CHOP and cleaved caspase-3, which indicated that LTC inhibited cell apoptosis in brain tissue by alleviating ER-stress. Moreover, we discovered that the increased level of phosphorylated P38 induced by MCAO/reperfusion was inhibited by LTC treatment (Fig. 5C).
Discussions
During cerebral ischemia injury, numerous researches have stated the fact that inflammation is constantly occurred after reperfusion [37], and apoptosis is one of the most critical forms of cell death in cerebral I/R injury [38]. It has been reported recently that LTC and its component exhibited a protective effect against I/R injury and the ability to alleviate neuroinflammation [39], [40]. Further investigation to the pathology mechanism, our findings indicated LTC improved PC12 cell variability by inhibiting cell apoptosis after OGD/R injury in vitro, which was verified by FITC-Annexin V/PI flow cytometry and AO/EB staining. The following study proved the activated (cleaved) PARP, caspase-3, and 9 were inhibited by LTC in a dose-dependent manner after OGD/R insult. Then the protection of LTC against the apoptosis of neuronal cells also had been found in vivo. LTC treatment dramatically decreased the infarct volume induced by MCAO/reperfusion and the rate of the apoptotic neuronal cell in the rat brain. These data proved that LTC had protected against cerebral I/R injury induced by MCAO/reperfusion.
ER stress caused by I/R injury is related to the higher-level expressions of CHOP and Bip. The PERK/eIF2α and IRE1/TRAF2 pathways are related to ER stress-induced cell apoptosis after I/R injury. After dissociation from Bip, PERK and IRE1 are activated by phosphorylating in the unfolded protein response (UPR), which aids cell survival by alleviating nascent proteins loading at the ER [41], [42]. When ER stress is prolonged, the translation of CHOP is promoted through PERK-eIF2α activation. Then this activation affects intracellular calcium metabolism and causes changes in gene expression, thereby promoting apoptosis [43]. At the same time, the expression of CHOP stimulates Bax expression and translocation to initiate mitochondrial-dependent apoptosis through changing mitochondrial permeability [44]. In addition to the PERK/eIF2α pathway, OGD/R injury increases the activation of phosphorylated IRE1 and induces IRE1-TRAF2 complex formation which triggers the phosphorylation of JNK and P38. The activated JNKs and p38-MAPKs were deemed to play an important role in apoptosis [30]. Therefore, neuronal cell apoptosis induced by the PERK/eIF2α and IRE1/TRAF2 pathways of ER stress has been reported to be an essential pathological process in I/R injury [45].
We discovered that LTC protected neuronal PC12 cells from OGD/R injury by regulation of the caspase-mediated apoptosis pathway. This protective effect of LTC was executed by inhibiting CHOP and Bip expression. Further mechanistic investigation revealed that LTC inhibited the Bip-induced phosphorylation of PERK, eIF2α, and IRE1 dramatically, resulting in the inhibition of CHOP expression and corresponding apoptosis. Our findings suggested that LTC inhibits the ER stress by the PERK/eIF2α and IRE1/TRAF2 pathways. The same protective effect of LTC was discovered in the inhibition of TG-induced ER stress, which verified the mechanism stated above.
In addition to PERK/eIF2α induced apoptosis, recent studies have shown that JNK and P38 MAPK pathways are involved in apoptosis activation during cerebral I/R injury, and the activation of MAPK can be regulated by ER stress-induced IRE1 activation [46]. As LTC was reported to inhibit MAPK activation induced by neuroinflammation [40], our findings demonstrated that JNK, P38, and Erk1/2 phosphorylations in PC12 cells were suppressed by LTC treatment after OGD/R injury, which led to the decrease of CHOP and pro-apoptotic Bax as the previous report [44]. These results indicated that LTC might inhibit the activation of the MAPK signal pathway by alleviating the activation of the IRE1/TRAF2 pathway in PC12 cells during OGD/R injury. In summary, our study had demonstrated that LTC exerted its protective effect against I/R injury in vitro by the ER stress and MAPK-mediated mechanisms. Based on the studies in PC12 cells, we further observed the effect of LTC on I/R rats. Consistent with our conclusion in vitro, the ER-stress, apoptosis, and MAPK pathway in the cerebral infarcted regions was significantly suppressed. Rats treated with a 300 mg/kg dose of LTC exhibited abrogation of apoptosis, ER-stress, and activation of P38, as demonstrated by IHC staining and protein expression quantification.
Conclusions
LTC has been used to treat stroke in clinical for a long time. However, the problem of the unclear mechanism of action has severely restricted its clinical application. Here, our findings strongly suggest that LTC protected the cerebral neuronal cell against I/R injury through alleviating ER stress and MAPK-mediated mechanisms, eventually inhibited caspase-dependent cell apoptosis (Fig. 6).
Fig. 6.
Proposed mechanism of the protective effect of LTC against cerebral I/R injury in neuronal cells.
Compliance with Ethics Requirements
The research was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). The experimental protocol was approved by the medical animal experiment ethics committee of the Beijing University of Chinese Medicine (Approval Number: BUCM-4-2018050601-2072).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China, China (Grant No. 81573572); National Key Research and Development Project (2018YFC1706403); the Foundation Research Funds of the Central Universities (No. 2019-JYB-JS-013).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.01.016.
Contributor Information
Jiao Zheng, Email: zj98v2@163.com.
Jun Li, Email: drlj666@163.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Marciniec M., Sapko K., Kulczynski M., Popek-Marciniec S., Szczepanska-Szerej A., Rejdak K. Non-traumatic cervical artery dissection and ischemic stroke: a narrative review of recent research. Clin Neurol Neurosur. 2019;187 doi: 10.1016/j.clineuro.2019.105561. [DOI] [PubMed] [Google Scholar]
- 2.Perez-de-Puig I., Miro-Mur F., Ferrer-Ferrer M., Gelpi E., Pedragosa J., Justicia C. Neutrophil recruitment to the brain in mouse and human ischemic stroke. ACTA Neuropathol. 2015;129:239–257. doi: 10.1007/s00401-014-1381-0. [DOI] [PubMed] [Google Scholar]
- 3.Wei K., Wan L., Liu J., Zhang B., Li X., Zhang Y. Downregulation of Trb3 protects neurons against apoptosis induced by global cerebral ischemia and reperfusion Injury in rats. Neuroscience. 2017;360:118–127. doi: 10.1016/j.neuroscience.2017.07.062. [DOI] [PubMed] [Google Scholar]
- 4.Shu Q., Fan H., Li S.J., Zhou D., Ma W., Zhao X.Y. Protective effects of Progranulin against focal cerebral ischemia-reperfusion injury in rats by suppressing endoplasmic reticulum stress and NF-kappa B activation in reactive astrocytes. J Cell Biochem. 2018;119:6584–6597. doi: 10.1002/jcb.26790. [DOI] [PubMed] [Google Scholar]
- 5.Pluquet O., Pourtier A., Abbadie C. The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am J Physiol-Cell Ph. 2015;308:415–425. doi: 10.1152/ajpcell.00334.2014. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto S., Saido T.C. Critical review: involvement of endoplasmic reticulum stress in the aetiology of Alzheimer's disease. Open Biol. 2018;8(4) doi: 10.1098/rsob.180024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan H.D., Wang Q., Chen X.T., Zeng Q.F., Shao Y.J., Fang H.Q. WDR45 contributes to neurodegeneration through regulation of ER homeostasis and neuronal death. Autophagy. 2019;23:1–17. doi: 10.1080/15548627.2019.1630224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollville E., Romero S.E., Deshmukh M. Apoptotic cell death regulation in neurons. FEBS J. 2019;286(17):3276–3298. doi: 10.1111/febs.14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrido C., Galluzzi L., Brunet M., Puig P.E., Didelot C., Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y., Fan J.T., Gu X.L., Zhang L.Y., Han J., Du S.H. Neuroprotective activity of cerebrosides from typhonium giganteum by regulating caspase-3 and Bax/Bcl-2 signaling pathways in PC12 cells. J Nat Prod. 2017;80:1734–1741. doi: 10.1021/acs.jnatprod.6b00954. [DOI] [PubMed] [Google Scholar]
- 11.Radak D., Katsiki N., Resanovic I., Jovanovic A., Sudar-Milovanovic E., Zafirovic S. Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc Pharmacol. 2017;15:115–122. doi: 10.2174/1570161115666161104095522. [DOI] [PubMed] [Google Scholar]
- 12.Broughton B.R.S., Reutens D.C., Sobey C.G. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 13.Chen H.-H., Sun C.-C., Tseng M.-P., Hsu C.-J. A patch test study of 27 crude drugs commonly used in Chinese topical medicaments. Contact Dermatitis. 2003;49:8–14. doi: 10.1111/j.0105-1873.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 14.Li Y.S., Wang J.X., Jia M.M., Liu M., Li X.J., Tang H.B. Dragon's Blood inhibits chronic inflammatory and neuropathic pain responses by blocking the synthesis and release of substance P in rats. J Pharmacol Sci. 2012;118:43–54. doi: 10.1254/jphs.11160fp. [DOI] [PubMed] [Google Scholar]
- 15.Liu H.H., Lin S.H., Xiao D., Zheng X., Gu Y., Guo S.Y. Evaluation of the wound healing potential of resina Draconis (Dracaena cochinchinensis) in animal models. Evid-Based Compl Alt. 2013;2013 doi: 10.1155/2013/709865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y.D., Zhang P., Yu H.P., Li H., Wang M.W., Zhao W.M. Anti-Helicobacter pylori and thrombin inhibitory components from Chinese Dragon's blood, Dracaena cochinchinensis. J Nat Prod. 2007;70:1570–1577. doi: 10.1021/np070260v. [DOI] [PubMed] [Google Scholar]
- 17.Zheng J., Liu B.L., Lun Q.X., Gu X.P., Pan B., Zhao Y.F. Longxuetongluo capsule inhibits atherosclerosis progression in high-fat diet-induced ApoE(-/-) mice by improving endothelial dysfunction. Atherosclerosis. 2016;255:156–163. doi: 10.1016/j.atherosclerosis.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Su X.Q., Song Y.L., Zhang J., Huo H.X., Huang Z., Zheng J. Dihydrochalcones and homoisoflavanes from the red resin of Dracaena cochinchinensis (Chinese dragon's blood) Fitoterapia. 2014;99:64–71. doi: 10.1016/j.fitote.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Sun J., Song Y., Sun H., Liu W., Zhang Y., Zheng J. Characterization and quantitative analysis of phenolic derivatives in Longxuetongluo Capsule by HPLC-DAD-IT-TOF-MS. J Pharm Biomed Anal. 2017;145:462–472. doi: 10.1016/j.jpba.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Sun J., Huo H., Song Y., Zheng J., Zhao Y., Huang W. Method development and application for multi-component quantification in rats after oral administration of Longxuetongluo Capsule by UHPLC-MS/MS. J Pharm Biomed Anal. 2018;156:252–262. doi: 10.1016/j.jpba.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Zheng J., Liu B., Lun Q., Yao W., Zhao Y., Xiao W. Longxuetongluo Capsule improves erythrocyte function against lipid peroxidation and abnormal hemorheological parameters in high fat diet-Induced ApoE-/- mice. Oxid Med Cell Longev. 2016;2016:2603219. doi: 10.1155/2016/2603219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerlier D., Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods. 1986;94:57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- 23.Longa E.Z., Weinstein P.R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Koike S., Yano S., Tanaka S., Sheikh A.M., Nagai A., Sugimoto T. Advanced glycation end-products induce apoptosis of vascular smooth muscle cells: a mechanism for vascular calcification. Int J Mol Sci. 2016;17:1567. doi: 10.3390/ijms17091567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F., Hayashi T., Jin G., Deguchi K., Nagotani S., Nagano I. The protective effect of dantrolene on ischemic neuronal cell death is associated with reduced expression of endoplasmic reticulum stress markers. Brain Res. 2005;1048:59–68. doi: 10.1016/j.brainres.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 26.Kasibhatla S., Amarante-Mendes G.P., Finucane D., Brunner T., Bossy-Wetzel E., Green D.R. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. CSH Protoc. 2006;2006(3):4493. doi: 10.1101/pdb.prot4493. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Tu L., Li Y., Chen D., Wang S. Notoginsenoside R1 protects against neonatal cerebral hypoxic-ischemic injury through estrogen receptor-dependent activation of endoplasmic reticulum stress pathways. J Pharmacol Exp Ther. 2016;357:591–605. doi: 10.1124/jpet.115.230359. [DOI] [PubMed] [Google Scholar]
- 28.Cao G., Luo Y., Nagayama T., Pei W., Stetler R.A., Graham S.H. Cloning and characterization of rat caspase-9: implications for a role in mediating caspase-3 activation and hippocampal cell death after transient cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:534–546. doi: 10.1097/00004647-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Zhang K., Kaufman R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabas I., Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darling N.J., Cook S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim Biophys Acta. 2014;1843:2150–2163. doi: 10.1016/j.bbamcr.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 33.Davis R.J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 34.Wei Q., Dong G., Chen J.-K., Ramesh G., Dong Z. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int. 2013;84:138–148. doi: 10.1038/ki.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou H., Limpert A.S., Zou J., Dembo A., Lee P.-S., Grant D. Benzodiazepinone derivatives protect against endoplasmic reticulum stress-mediated cell death in human neuronal cell lines. ACS Chem Neurosci. 2015;6:464–475. doi: 10.1021/cn500297v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka M., Ogaeri T., Samsonov M., Sokabe M. The 5-reductase inhibitor finasteride exerts neuroprotection against ischemic brain injury in aged male rats. Transl Stroke Res. 2019;10:67–77. doi: 10.1007/s12975-018-0624-0. [DOI] [PubMed] [Google Scholar]
- 37.Eltzschig H.K., Eckle T. Ischemia and reperfusion-from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harding H.P., Zhang Y., Bertolotti A., Zeng H., Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 39.Xin N., Yang F.J., Li Y., Li Y.J., Dai R.J., Meng W.W. Dragon's blood dropping pills have protective effects on focal cerebral ischemia rats model. Phytomedicine. 2013;21:68–74. doi: 10.1016/j.phymed.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Hong Q., Yang Y., Wang Z., Xu L., Yan Z. Longxuetongluo capsule alleviates lipopolysaccharide-induced neuroinflammation by regulating multiple signaling pathways in BV2 microglia cells. J Chin Med Assoc. 2020;83:255–265. doi: 10.1097/JCMA.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 41.Bertolotti A., Zhang Y., Hendershot L.M., Harding H.P., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 42.Malhotra J.D., Kaufman R.J. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18(6):716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puthalakath H., O'Reilly L.A., Gunn P., Lee L., Kelly P.N., Huntington N.D. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 44.Kim B.J., Ryu S.W., Song B.J. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 45.Yu L., Li B., Zhang M., Jin Z., Duan W., Zhao G. Melatonin reduces PERK-eIF2alpha-ATF4-mediated endoplasmic reticulum stress during myocardial ischemia-reperfusion injury: role of RISK and SAFE pathways interaction. Apoptosis. 2016;21:809–824. doi: 10.1007/s10495-016-1246-1. [DOI] [PubMed] [Google Scholar]
- 46.Wei S.G., Yu Y., Weiss R.M., Felder R.B. Endoplasmic reticulum stress increases brain MAPK signaling, inflammation and renin-angiotensin system activity and sympathetic nerve activity in heart failure. Am J Physiol Heart Circ Physiol. 2016;311:871–880. doi: 10.1152/ajpheart.00362.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.