Abstract
Background:
Glioblastoma multiforme (GBM) is an aggressive brain tumor. Patients commonly rely on family caregivers for physical and emotional support. We previously demonstrated that caregiver mastery measured shortly after diagnosis was predictive of GBM patient survival, corrected for known predictors of survival (N=88).
Objective:
To verify the contribution of caregiver mastery, and investigate the added value of mastery over other predictors to predict 15-month survival.
Methods:
Data collected for a longitudinal study (NCT02058745) were used. Multivariable Cox regression analyses were performed for models with known clinical predictors (patient age, Karnofsky Performance Status (KPS), type of surgery, O6-methylguanine-DNA methyltransferase (MGMT) promotor methylation status), with and without adding caregiver mastery to predict mortality. The added value of each model in discriminating between patients with the lowest and highest chance of survival at 15 months was investigated through Harrell’s C-statistics.
Results:
In total, 41 caregiver-patient dyads were included. When evaluating solely clinical predictors, KPS and patient age were significant predictors of mortality (Hazard Ratio [HR]=0.974 (95% Confidence Interval [CI]=0.949–1.000) and HR=1.045 (95%CI=1.002–1.091)), respectively. Adding caregiver mastery, these clinical predictors remained statistically significant and mastery showed a HR=0.843 (95%CI=0.755–0.940). The discriminative value improved from C=0.641 (model with known clinical predictors) to C=0.778 (model with mastery), indicating the latter is superior.
Conclusions:
We confirm that caregiver mastery is associated with GBM patient survival.
Implications for Practice:
Incorporating support and guidance for caregivers into standard care, could lead to benefits for caregiver wellbeing and patient outcomes.
Introduction
Glioblastoma (GBM, World Health Organization (WHO) grade IV) is the most common primary malignant brain tumor in adults and is followed by an extremely poor prognosis; with standard treatment, median survival is less than 15 months.1 Standard treatment, i.e. surgery followed by radiotherapy with concomitant and adjuvant temozolomide, is aimed at stabilizing disease and extending survival, while maintaining quality of life (QOL). In addition to treatment, longer survival following diagnosis is associated with younger age at diagnosis, better performance status, unifocal tumor location, O6-methylguanine-DNA-methyltransferase (MGMT) promotor methylation and IDH1 status.2–4
In addition to high mortality rates, morbidity following diagnosis often includes debilitating physical and neurological sequelae,5 creating a high demand for assistance from family caregivers (typically spouses).5,6 While providing care can be rewarding, neuro-oncology family caregivers report considerable burden and distress7 as well as effects on physical health.8,9 Caregiver mastery (the feeling of being in control of the care situation) has been shown to be a strong predictor of positive caregiver outcomes across multiple patient populations.10–12
Caregivers’ emotional health is often assumed to influence the quality of care delivered in the home and affecting patient outcomes, yet little evidence exists to support this hypothesis. In certain populations (e.g., dementia, end-of-life care), some evidence exists for an association between lower levels of caregiver wellbeing and worse patient outcomes (e.g., time to institutionalization, survival).13–17 However, these populations differ greatly from cancer, and brain cancer in particular as a brain tumor has both oncological and neurological sequelae. Like many cancers, GBM typically has a rapid onset and complex treatment regimen with often severe side-effects, but also presents with dementia-like sequelae including neuropsychological dysfunction.18 This presents unique challenges for neuro-oncology caregivers, with different disease-specific issues impacting on their emotional health and potentially, affecting patient outcomes differently.
We previously investigated whether key indicators of caregiver distress (i.e., anxiety, depressive symptoms, caregiver burden and mastery) were predictive of glioblastoma patient survival.19 In a sample of 88 dyads of patients newly diagnosed with GBM and their caregivers, higher levels of caregiver mastery were associated with a significant reduction in the probability of the patient dying sooner (Hazard Ratio (HR)=0.839 (95% confidence interval (CI)=0.771–0.913)), after correcting for other known predictors. This was the first study to investigate these associations, yet important molecular markers such as MGMT promotor methylation status were not routinely collected at the time, which could have biased results. Moreover, replicability of findings is a known difficulty in psychosocial and medical sciences.20 Therefore, we now aimed to verify the predictive value of caregiver mastery independent of other known predictors, and the added value of caregiver mastery over other predictors to predict 15-month survival within a new sample of GBM patients and caregivers. Further knowledge on links between caregivers’ emotional wellbeing and patient outcomes are key to intervention design aimed at improving both patient and caregiver wellbeing.
Materials and methods
Participants
Caregiver/patient dyads were recruited from a randomized controlled trial aimed at evaluating the effects of a computer mediated, nurse-facilitated representational (problem solving) intervention (SmartCare©)21 on family caregivers’ wellbeing (NCI R01-NR013170). Dyads were recruited within 3–6 months of the patient’s initial diagnosis from NCI designated cancer centers. Caregiver eligibility criteria were: 1) presence of depressive symptoms as measured with the shortened Center for Epidemiological Studies-Depression Scale; 2) ≥ 21 years of age; 3) able to read and speak English; 4) identified by patients as the primary non-professional person who provided the majority of emotional, financial and/or physical support; and 5) not currently a primary caregiver for anyone else other than children under age 21. Patient eligibility criteria were: 1) ≥21 years old, 2) diagnosed (verified via pathology) with a primary malignant brain tumor, and 3) able to read and speak English.
For the current analysis, only patients newly diagnosed with glioblastoma (≤4 months) and their caregivers were included. Only baseline data were used because: a) it eliminated bias from the intervention tested, and; b) we were able to test caregivers’ baseline level of mastery irrespective of the patient’s place in the disease trajectory.
Procedure and outcome measures
Sociodemographic and clinical data, patient functional status, and other covariates described in subsequent sections were obtained via interviews and medical record review. Caregiver survey data were obtained via online questionnaires completed by caregivers. We used only data collected at baseline (if done within 4 months of diagnosis) as potential predictors of patients’ time to death. The institutional review board approved the study protocol and all participants provided informed consent. The study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
Caregiver outcomes
Caregivers’ sociodemographic information (age, sex, education, relationship with patient) were self-report. Caregivers’ feelings of mastery (perception of control over the care situation) were assessed with the Caregiver Mastery Scale based on Pearlin & Schooler’s structure of coping.22 The 7-item Likert-type instrument was administered (α=0.65). Scales range from strongly disagree to strongly agree, with a neutral ‘no opinion’ answer. A total score is calculated with higher scores indicating a higher level of perceived control.
Patient outcomes
Patients’ date of diagnosis was defined as date of the surgery. Date of death was collected by medical record review and/or by checking obituaries and the Social Security Death Index through May 2019. Patients were classified as alive, deceased, or lost to follow up. Performance status was measured with the Karnofsky Performance Status (KPS) scale. Data on other factors known to influence survival e.g. extent of resection, postsurgical treatment, and molecular markers (MGMT; IDH) were obtained through medical record review.
Statistical analyses
All statistical analyses were performed with SPSS version 26.0. Descriptive statistics were performed to explore sociodemographic (age, sex, educational level, relationship), clinical variables (extent of resection, postsurgical treatment, tumor location, IDH mutation status, MGMT methylation status, KPS, symptom burden, survival at 15 months), and caregiver mastery. Multiple imputation techniques using fully conditional specification with five iterations23,24 were used if less than 10% of data for a given predictor were missing and missingness was determined to be at random through pattern analysis (caregiver mastery (2.4% missing); KPS (9.7% missing); MGMT methylation status (7.3% missing)). Multivariable Cox regression analyses were run for different models to predict GBM patient survival. First, model 1 was run to confirm the associations between known clinical predictors and patient survival: patients’ age, KPS, extent of resection, plus MGMT methylation status. Then, model 2 was run to investigate the added value of caregiver mastery: patients’ age, KPS, extent of resection, MGMT methylation status, plus caregiver mastery. For IDH mutation, data was too incomplete for imputation (14.6% missing), with only 2 cases of mutation recorded, so this could not be included in the models. Postsurgical treatment was removed as a predictor variable as all participants received postsurgical chemo- and radiotherapy, and due to multicollinearity concerns with KPS. All Cox regression models presented include the pooled estimate from five multiple imputation rounds, and P-values ≤.05 were considered statistically significant. Performance of the two multivariable models (original, non-imputed data only) was assessed with Harrell’s concordance-index (C-index),25 which estimates the probability of concordance between predicted and observed responses. For both models (original, non-imputed data only) Kaplan-Meier curves were generated for which the patient group was split into two groups based on the model-specific median prognostic index, representing patients with the lowest and highest chance of survival at 15 months.26 The further the survival curves lie from each other, the better the discrimination. These curves also give insight on how the discrimination capacity changes over time.
Results
Participant characteristics
In the main trial (recruitment period 2015–2017), 120 patient-caregiver dyads participated. Based on in- and exclusion criteria used for the present analyses, 34.2% of the study sample (N=41) could be included. Most caregivers were women (68.3%) and patients’ spouses (78%). The majority of patients were men (58.5%), and most received a surgical resection of the tumor (70.7%) as well as postoperative chemotherapy and radiotherapy (97.6%, data from 1 participant missing), see Table 1. Median overall survival was 15 months (range 2–61 months; 4 cases censored).
Table 1.
Participant Characteristics.
| Patient-Caregiver Dyads (N=41) | ||
|---|---|---|
| Age in years (caregiver) M (SD) | 55.51 (9.44) | |
| Sex (caregiver) N(%) | Male | N=12 (29.3%) |
| Female | N=28 (68.3%) | |
| Education (caregiver) N(%) | High school graduate | N=40 (97.6%) |
| Relationship with the patient N(%) | Spouse or partner | N=32 (78.0%) |
| Other | N=9 (22.0) | |
| Mastery (caregiver) M (SD)A | Total score | 18.58 (3.76) |
| Age in years (patient) M (SD) | 58.88 (8.86) | |
| Sex (patient) N(%) | Male | N=24 (58.5%) |
| Female | N=17 (41.5%) | |
| Karnofsky Performance Status Median, rangeB | 80 (40–100) | |
| Tumor location N(%) | Frontal | N=6 (14.6%) |
| Temporal | N=4 (9.8%) | |
| Parietal | N=5 (12.2%) | |
| Occipital | N=1 (2.4%) | |
| Middle | N=4 (9.8%) | |
| Mixed | N=8 (19.5%) | |
| Posterior | N=0 (0%) | |
| IDH mutation N(%) | Mutated | N=2 (4.9%) |
| Non-mutated | N=33 (80.5%) | |
| MGMT methylation status N(%) | Methylated | N=21 (51.2%) |
| Not methylated | N=17 (41.5%) | |
| Extent of resection N(%) | Biopsy | N=12 (29.3%) |
| Resection | N=29 (70.7%) | |
| Postoperative treatment N(%)A | Surgery only | N=0 (0%) |
| Surgery and radiotherapy | N=0 (0%) | |
| Surgery, radiotherapy | N=40 (97.6%) | |
| and chemotherapy | ||
| Further treatment N(%) | Re-resection | N=8 (19.5%) |
| Chemotherapy (change or additional) | N=16 (39.0%) | |
| Radiotherapy | N=13 (31.7%) | |
| Bevacizumab | N=30 (68.2%) | |
| Participation in any treatment trial N(%) | N=21 (51.2%) | |
| Deceased within 15 months N(%) | N=19 (46.3%) | |
Data from 1 participant missing;
Data from 4 participants missing.
Abbreviations: IDH, isocitrate dehydrogenase; KPS, Karnofsky Performance Status; MGMT, O6-methylguanine-DNA-methyltransferase.
Known clinical predictors and patient survival
A multivariable Cox regression model including patient age, KPS, extent of resection and MGMT methylation status (model 1, table 2), showed significant associations between patient survival and KPS (HR=0.974, 95%CI=0.949–1.000), and patient age (HR=1.045, 95%CI=1.002–1.091), based on 41 cases (C=0.659). Figure 1a shows Kaplan-Meier curves for patients with the lowest and highest chance of survival at 15 months.
Table 2.
Associations Between Known Clinical Predictors and Caregiver Mastery, and GBM Patients’ Probability of Dying Sooner (Hazard).
| Model | Variables | Hazard ratio (95% CI) | Likelihood ratio P |
|---|---|---|---|
| Model 1: | |||
| Patient age | 1.045 (1.002 – 1.091) | .041A | |
| KPS | 0.974 (0.949 – 1.000) | .050A | |
| Type of surgery | 0.761 (0.321 – 1.807) | .536 | |
| MGMT status | 0.560 (0.255 – 1.228) | .146 | |
| Model 2: | |||
| Patient age | 1.056 (1.004 – 1.111) | .034A | |
| KPS | 0.973 (0.947 – 1.000) | .052 | |
| Type of surgery | 0.580 (0.233 – 1.444) | .240 | |
| MGMT status | 0.483 (0.187 – 1.243) | .127 | |
| Caregiver mastery | 0.843 (0.755 – 0.940) | .002A |
P≤0.05
Abbreviations: CI, confidence interval; KPS, Karnofsky Performance Status; MGMT, O6-methylguanine-DNA-methyltransferase.
Figure 1a.
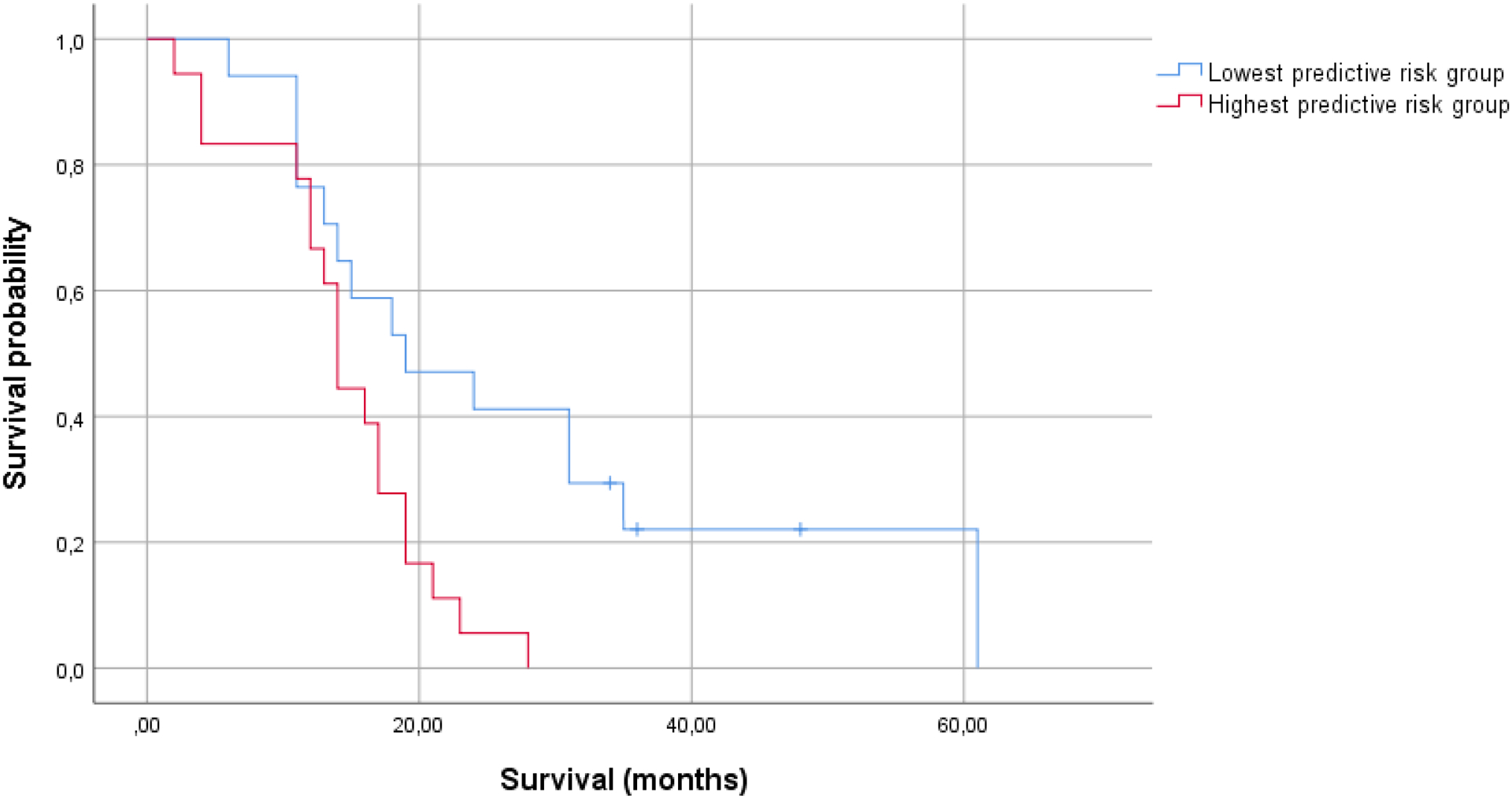
Kaplan-Meier curves for the group surviving longer than 15 months, and the group that does not (model 1: patient age, KPS, extent of resection, MGMT status; N=35).
Caregiver mastery, known clinical predictors and patient survival
A multivariable Cox regression model (model 2, table 2) where caregiver mastery was added to the known clinical predictors (patient age, KPS, extent of resection, MGMT status), showed significant associations between patient survival and patient age (HR=1.056, 95%CI=1.004–1.111), KPS (HR=0.973, 95%CI=0.947–1.000), and caregiver mastery (HR=0.843, 95%CI=0.755–0.940), based on 41 cases (C=0.737). Figure 1b shows Kaplan-Meier curves for patients with the lowest and highest chance of survival at 15 months.
Figure 1b.
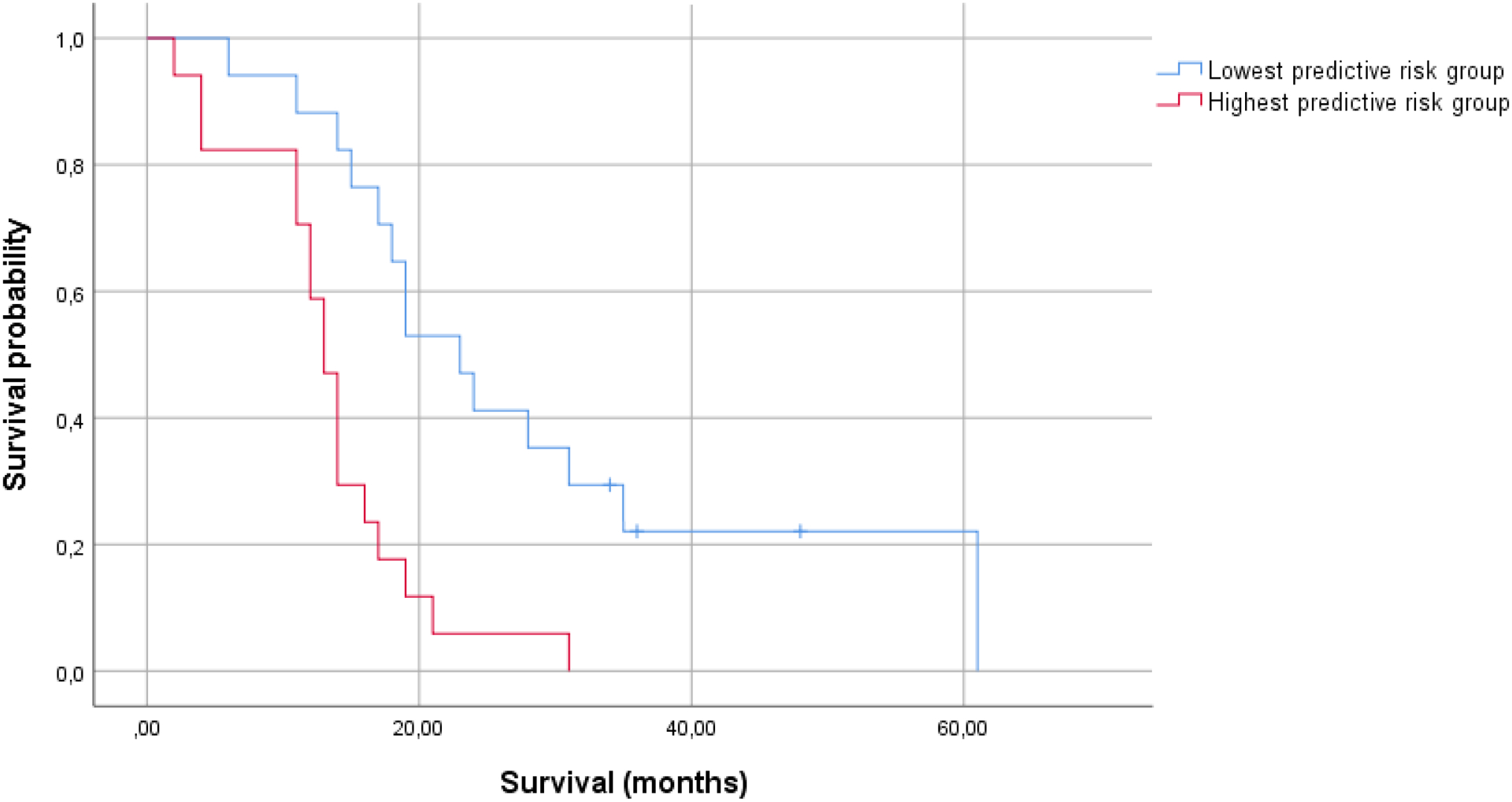
Kaplan-Meier curves for the group surviving longer than 15 months, and the group that does not (model 2: patient age, KPS, extent of resection, MGMT status, caregiver mastery; N=34).
Discussion
This is our second study examining relationships between caregiver mastery and GBM patient survival. In both reports, we focused only on GBM patients as these are the most common primary malignant brain tumors in adults and form a relatively homogenous group in terms of treatment and survival time. Data from the current study confirms previous findings.19 Corrected for other factors known to influence GBM patient survival, caregiver mastery measured shortly after diagnosis is associated with time to patient death. Indeed, a strong association (HR between 0.839 and 0.843) was consistently found and remained strong in models that included known predictors such as patient functional status (KPS), age, extent of resection, and MGMT methylation status. Adding caregiver mastery resulted in a higher C-statistic (0.737, compared to 0.659 without mastery) indicating the added value of caregiver mastery over known predictors at discriminating between patients who survive to 15 months and those who do not – which is also reflected in Kaplan-Meier curves (Figure1a&b).
Previous studies in other populations (e.g., elderly, dementia, non-central nervous system cancer), also show associations between aspects of caregiver wellbeing and patient outcomes. Most of these show a link between caregiver distress and patients’ physical functioning,27–30 although findings are not entirely consistent – one study found a relationship between patient self-efficacy and caregiver physical health instead.31 This does highlight the interdependence of caregiver and patient outcomes. Furthermore, unmet caregiver needs have been associated with poorer quality of care delivered,13 and higher burden as well as poorer caregiver training have been linked to time to patient institutionalization and/or death.15–17 The GBM patient-caregiver population is different from those investigated before, with its unique neurological and cognitive symptom pattern, rapid onset and decline in patient health. We have now confirmed our initial findings, highlighting that in this population, caregiver mastery is consistently linked to patient survival.
The next logical step in this research trajectory is to determine what constitutes mastery, and through which mechanisms mastery might influence patients’ physical health. In our previous report, mastery was not related to sociodemographic variables (age, sex, educational level, relationship with patient).19 Other studies link caregiver mastery to caregivers’ physical functioning32 and depressive symptoms, as well as patients’ problem behaviors.10 As mastery is conceptually closely related to resilience and coping, it is likely influenced by social (e.g., social support), biological (e.g., gene-environment), and personal factors (e.g., optimism, hope).33 While the precise contribution of these factors warrants further research, we have previously demonstrated that it is possible to improve mastery through psychosocial intervention during the disease trajectory.34 This underscores that caregiver mastery is a flexible state, rather than a fixed trait. Of note, our trial was the only out of eight randomized controlled trials aimed to improve neuro-oncology caregiver wellbeing in our recent Cochrane systematic review, that included a mastery outcome measure.35 Further work in this area is clearly warranted.
Study limitations included the following. First, the study sample from the intervention study was smaller than anticipated (N=41, as 34.2% of the sample met our criteria). This is reflected in larger confidence intervals than found in our first report. However, this investigation did not aim to build a new predictive model, but rather, aimed to verify a predictive model previously investigated within a larger sample. Second, there was too little variation in IDH status and postsurgical treatment for us to include these variables into the predictive models. Further validation studies of the association between caregiver mastery and GBM patient survival should aim for larger sample sizes so that all known clinical predictors can be incorporated. However, the added value of mastery to other known clinical predictors could still be demonstrated. Third, the Cox regression models performed are tests of association rather than causality. While we made efforts to focus on newly diagnosed patients, used baseline assessments of caregiver mastery to ensure minimal influence of each individual patient’s disease trajectory, and corrected for patient variables known to influence survival, causality cannot be ascertained until tested in a randomized, controlled setting. While investigating this as a primary aim may be unethical, further secondary analysis of caregiver intervention study data could help shed light on this.
This report confirms that in neuro-oncology, caregiver mastery is associated with GBM patient survival. This is vital as replicability of findings is a known difficulty in psychosocial and medical sciences. The need for support among neuro-oncology caregivers is well-defined and systematically high,5 and yet, caregiver support is often not part of standard care. Our work strengthens the argument that supporting family caregivers will not only improve their own wellbeing, but also has great potential to affect patient outcomes. Within the GBM population which is affected by high symptom burden, dismal prognosis, and limited treatment options, these data propose a new avenue through which both caregiver and patient outcomes may be improved.
Funding:
National Institutes of Health, National Cancer Institute (R01-NR013170; PIs PRS and HSD). Yorkshire Cancer Research Fellowship (FWB and LM). International Researcher Mobility Award (FWB).
Footnotes
Conflict of Interest: No conflict of interest exists for any author.
References
- 1.Ricard D, Idbaih A, ucray F, Lahutte M, Hoang-Xuan K, Delattre J. Primary brain tumours in adults. The lancet. 2012; 379(9830):1984–1996. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez de Eulate-Beramendi S, Alvarez-Vega M, Balbin M, Sanchez-Pitiot A, Vallina-Alvarez A, Martino-Gonzalez J. Prognostic factors and survival study in high-grade glioma in the elderly. British journal of neurosurgery. 2016; 30(3):330–336. [DOI] [PubMed] [Google Scholar]
- 3.Fekete B, Werlenius K, Orndal C, Rydenhag B. Prognostic factors for glioblastoma patients - a clinical population-based study. Acta Neurologica Scandinavica. 2015; 133(6):434–441. [DOI] [PubMed] [Google Scholar]
- 4.Wick W, Meisner C, Hentschel B, et al. Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology. 2013; 81(17):1515–1522. [DOI] [PubMed] [Google Scholar]
- 5.Sterckx W, Coolbrandt A, Dierckx de Casterle B, et al. The impact of a high-grade glioma on everyday life: A systematic review from the patients and caregivers perspective. European Journal of Oncology Nursing. 2013; 17(1):107–117. [DOI] [PubMed] [Google Scholar]
- 6.Piil K, Jakobsen J, Christensen KB, et al. Needs and preferences among patients with high‐grade glioma and their caregivers–A longitudinal mixed methods study. 2018; 27(2):e12806. [DOI] [PubMed] [Google Scholar]
- 7.Choi CW, Stone RA, Kim KH, et al. Group-based trajectory modeling of caregiver psychological distress over time. Annals of Behavioral Medicine. 2012; 44(1):73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherwood P, Price T, Weimer J, et al. Neuro-oncology family caregivers are at risk for systemic inflammation. Journal of neuro-oncology. 2016; 128(1):109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. Jama. 2014; 311(10):1052–1060. [DOI] [PubMed] [Google Scholar]
- 10.Sherwood PR, Given BA, Given CW, et al. The influence of caregiver mastery on depressive symptoms. Journal of Nursing scholarship. 2007; 39(3):249–255. [DOI] [PubMed] [Google Scholar]
- 11.Gaugler J, Hanna N, Linder J, et al. Cancer caregiving and subjective stress: a multi-site, multi-dimensional analysis. Psycho-Oncology. 2005; 14(9):771–785. [DOI] [PubMed] [Google Scholar]
- 12.Mausbach B, Patterson T, von Kanel R, et al. The attenuating effect of personal mastery on the relations between stress and Alzheimer caregiver health: a five-year longitudinal analysis. Aging & mental health. 2007; 11(6):637–644. [DOI] [PubMed] [Google Scholar]
- 13.Park S, Kim Y, Kim S, et al. Impact of caregivers’ unmet needs for supportive care on quality of terminal cancer care delivered and caregiver’s workforce performance. Supportive Care in Cancer. 2010; 18(6):699–706. [DOI] [PubMed] [Google Scholar]
- 14.Torti F, Gwyther L, Reed S, Friedman J, Schulman K. A multinational review of recent trends and reports in dementia caregiver burden. Alzheimer Disease & Associated Disorders. 2004; 18(2):99–109. [DOI] [PubMed] [Google Scholar]
- 15.Brodaty M, McGilchrist C, Harris L, Peters K. Time until institutionalization and death in patients with dementia: role of caregiver training and risk factors. Archives of neurology. 1993; 50(6):643–650. [DOI] [PubMed] [Google Scholar]
- 16.Kuzuya M, Enoki H, Hasegawa J, et al. Impact of Caregiver Burden on Adverse Health Outcomes in Community-Dwelling Dependent Older Care Recipients. The American Journal of Geriatric Psychiatry. 2011; 19(4):382–391. [DOI] [PubMed] [Google Scholar]
- 17.Dionne‐Odom JN, Hull JG, Martin MY, et al. Associations between advanced cancer patients’ survival and family caregiver presence and burden. Cancer medicine. 2016; 5(5):853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherwood PR, Cwiklik M, Donovan HS. Neuro-oncology family caregiving: review and directions for future research. CNS oncology. 2016; 5(1):41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boele F, Given C, Given B, et al. Family caregivers’ level of mastery predicts survival of glioblastoma patients: a preliminary report. Cancer. 2017; 123(5):832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pashler H, Wagenmakers EJ. Editors’ introduction to the special section on replicability in psychological science: A crisis of confidence? Perspectives on Psychological Science. 2012; 7(6):528–530. [DOI] [PubMed] [Google Scholar]
- 21.Donovan HS, Ward SE, Song MK, Heidrich SM, Gunnarsdottir S, Phillips CM. An update on the representational approach to patient education. Journal of Nursing Scholarship. 2007; 39(3):259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearlin LI, Schooler C. The structure of coping. Journal of health and social behavior. 1978:2–21. [PubMed] [Google Scholar]
- 23.Schafer JL. Multiple imputation: a primer. Statistical methods in medical research. 1999; 8(1):3–15. [DOI] [PubMed] [Google Scholar]
- 24.Van Buuren S Multiple imputation of discrete and continuous data by fully conditional specification. Statistical methods in medical research. 2007; 16(3):219–242. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in Medicine. 1996; 15(4):361–387. [DOI] [PubMed] [Google Scholar]
- 26.Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Medical Research Methodology. 2013; 13(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vrettos I, Kamposioras K, Kontodimopoulos N, et al. Comparing health-related quality of life of cancer patients under chemotherapy and of their caregivers. the Scientific World Journal. 2012:135283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grunfeld E, Coyle D, Whelan T, et al. Family caregiver burden: results of a longitudinal study of breast cancer patients and their principal caregivers. Canadian Medical Association Journal. 2004; 170(12):1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadhwa D, Burman D, Swami N, Rodin G, Lo C, Zimmermann C. Quality of life and mental health in caregivers of outpatients with advanced cancer. Psycho-Oncology. 2013; 22(2):403–410. [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Schuler T. Marital quality and survivorship: slowed recovery for breast cancer patients in distressed relationships. Cancer. 2009; 115:217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kershaw T, Ellis K, Yoon H, Schafenacker A, Katapodi M, Northouse L. The interdependence of advanced cancer patients’ and their family caregivers’ mental health, physical health, and self-efficacy over time. Annals of Behavioral Medicine. 2015; 49(6):901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantwell J, Muldoon O, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Research in developmental disabilities. 2014; 35:2215–2223. [DOI] [PubMed] [Google Scholar]
- 33.Seiler A, Jenewein J. Resilience in Cancer Patients. J Frontiers in psychiatry. 2019; 10:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boele FW, Hoeben W, Hilverda K, et al. Enhancing quality of life and mastery of informal caregivers of high-grade glioma patients: a randomized controlled trial. J. Neurooncol 2013; 111(3):303–311. [DOI] [PubMed] [Google Scholar]
- 35.Boele FW, Rooney AG, Bulbeck H, Sherwood PR. Interventions to help support caregivers of people with a brain or spinal cord tumour. Cochrane Database of Systematic Reviews. 2019(7): CD012582. [DOI] [PMC free article] [PubMed] [Google Scholar]


