Abstract
Non-communicable diseases (NCDs) are fatal for more than 38 million people each year and are thus the main contributors to the global burden of disease accounting for 70% of mortality. The majority of these deaths are caused by cardiovascular disease (CVD). The risk of NCDs is strongly associated with exposure to environmental stressors such as pollutants in the air, noise exposure, artificial light at night, and climate change, including heat extremes, desert storms, and wildfires. In addition to the traditional risk factors for CVD such as diabetes, arterial hypertension, smoking, hypercholesterolaemia, and genetic predisposition, there is a growing body of evidence showing that physicochemical factors in the environment contribute significantly to the high NCD numbers. Furthermore, urbanization is associated with accumulation and intensification of these stressors. This comprehensive expert review will summarize the epidemiology and pathophysiology of environmental stressors with a focus on cardiovascular NCDs. We will also discuss solutions and mitigation measures to lower the impact of environmental risk factors with focus on CVD.
Keywords: Environmental stressors, Cardiovascular risk factors, Oxidative stress, Cardiovascular disease, Noise, Heat, Air pollution, Light pollution
Graphical Abstract
Graphical Abstract.
1. Introduction
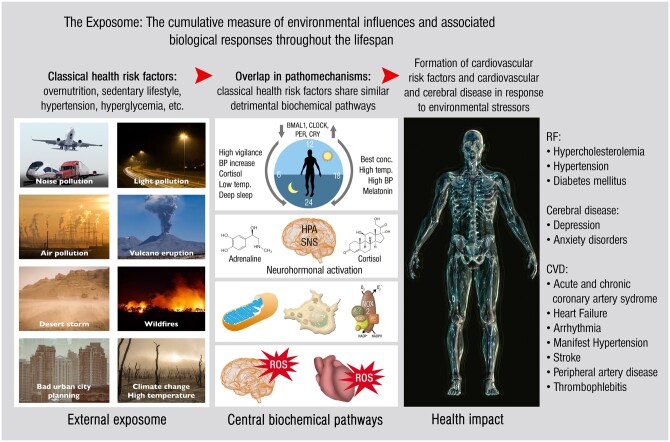
Cardiovascular diseases (CVDs), besides chronic respiratory and metabolic diseases, constitute a large part of non-communicable diseases (NCDs), including acute and chronic coronary artery disease, heart failure and arrhythmia, stroke and arterial hypertension. Importantly, 70% of annual global deaths (around 40 million people) can be attributed to NCDs and this share will further increase by 10% according to the World Health Organization (WHO) projections for the year 2030.1 NCDs account for 80.6% [95% confidence interval (CI) 78.2–82.5] of age-standardized years lived with disability in 2016, as indicated by data of the global burden of disease (GBD) study.2 CVDs are responsible for the majority of deaths that are caused by NCDs.3 In the GBD study (2019 update), the contribution of CVDs to overall global mortality continuously increased from 12.1 million in 1990 to 18.6 million in 2019.4 Interestingly, low- and middle-income countries have the highest share (86%) of premature deaths triggered by NCDs.5,6 The economic burden caused by NCDs are severe, and may amount to global economic costs of $47 trillion within the coming 20 years.7 Risk factors for NCDs are mostly originating from the environment, which is supported by observations that up to 25% of all ischaemic heart disease (IHD) are related to an unhealthy environment, especially to air pollution.8 Nevertheless, the environmental share to NCDs is notoriously ignored as reflected by the failure to mention environmental risk factors in the 2013 WHO NCD Global Action Plan.6 In addition research on, prevention of, and treatment of environmentally triggered NCDs are severely underfunded, relative to their disease burden in the general population.9 This dramatic gap is now paid more attention by the emerging ‘exposome’ research field, investigating the life-long effects of all environmental exposures on biochemical pathways and health effects (Figure 1)12,13 as well as ‘healthy cities’ campaigns.14,15
Figure 1.
The exposome concept. Exposure to environmental risk factors (=external exposome) leads to changes of central biochemical pathways with associated health impact. The central biochemical pathways comprise changes in circadian clock genes leading to impaired rhythmicity and phase-shifts, stress hormone release (cortisol and catecholamines), production of reactive oxygen species by mitochondria and NADPH oxidase in activated immune cells, inflammation with tissue infiltration of activated immune cells, and oxidative damage in different organs. Because classical health risk factors share similar pathomechanisms, people with existing classical health risk factors or disease (e.g. diabetes or hypertension) may experience additive adverse health effects upon exposure to environmental risk factors. HPA, hypothalamic–pituitary–adrenal axis; NOX-2, phagocytic NADPH oxidase (isoform 2); ROS, reactive oxygen species; SNS, sympathetic nervous system. Merged and redrawn from previous reports refs10,11 with permission; Copyright © 2020, The authors; Published by Elsevier B.V.
The exposome concept comprises a multiexposure perspective.16 Besides external environmental risk factors (e.g. traffic noise and air pollution), our lifestyle and environmental factors on the whole (e.g. socioeconomic status and climate) also characterize the exposome of an individual,10,17 the assessment of which requires a multidisciplinary approach using smart sensor devices, multi-OMICs techniques, and big data handling using bioinformatics and systems biology approaches.18 In order to better address these multiexposure conditions, the refined ‘envirome’ concept was developed, which is defined by three consecutively nested domains, consisting of natural, social, and personal environments that are monitored in parallel and connected to biochemical changes and health effects using ‘enviromics’.19 Based on the increasing awareness of the major impact of environmental risk factors, the term was coined ‘Genetics load the gun, but the environment pulls the trigger’.20
This comprehensive expert review will summarize the epidemiology and pathophysiology of environmental stressors on NCDs, however without considering the contribution of other important environmental health risk factors, e.g. mental stress21 and ionizing radiation (either by anticancer therapy22 or ionospheric and geomagnetic exposures23). We will also discuss solutions and mitigation measures to lower the adverse health effects by environmental stressors with focus on CVDs.
2. Noise and cardiovascular risk
2.1 Epidemiological evidence for adverse effects of noise on our health
Noise pollution from traffic is an increasing public health problem. Road traffic noise is the dominant source of transportation noise-associated health effects, and mapping of the European Union (EU) in 2019 showed that 113 million Europeans (20%) are subjected to a burden of road traffic noise that exceeds the limit of 55 dB(A) (LDEN: day-evening-night average) as suggested by the EU guideline.24 This estimate is most likely underestimated, as the Environmental Noise Directive is not ubiquitously applied in all urban areas and roads in entire Europe.24
In 2018, a WHO expert panel stated that there was high quality of evidence to conclude that road noise was associated with IHD.25 Based on a meta-analysis, the group of experts calculated that per 10 dB increase in road noise the relative risk (RR) for IHD was 1.08 (95% CI 1.01–1.15), starting at chronic exposure levels of 53 dB where significant health effects were observed. For noise from trains and aircrafts in relation to IHD, the expert panel ranked the quality of evidence as very low and low, respectively, due to few high-quality studies. However, recent studies covering Switzerland, the Rhine-Main region, and the island of Montreal have suggested that these noise sources may also be risk factors for myocardial infarction (MI), although results are not consistent and more evidence is needed.26–28
For all other cardiovascular health effects excluding IHD, the WHO group of experts found very low, low, or moderate evidence due to lack of high-quality studies.25 However, high-quality studies have subsequently emerged together with studies on new CVD outcomes and risk factors that were not studied in a noise context in the past, which we have summarized in the following Supplementary material online, Table S1.
Numerous studies addressed whether traffic noise is a risk factor for hypertension, but unfortunately using a cross-sectional design in most cases.25 The WHO group of experts found >35 cross-sectional studies on traffic noise and hypertension, with a joined RR for prevalent hypertension of 1.05 (1.02–1.08) for road noise, but the quality level was judged as ‘very low’ due to the inherent problem of the cross-sectional design.25 Later studies on noise and hypertension incidence have reported inconsistent results.29–31 However, there is a large variation between the different studies with regard to the way hypertension was defined, which complicates reliable conclusions and warrants for more studies.
The quality of evidence for stroke incidence was by the WHO judged as moderate based on a single study that reported road noise to increase risk of stroke.25 Subsequently, five studies on road traffic noise and incident stroke have been published: three large population-based studies that cover an entire region or country (London, Frankfurt, and Denmark) found road noise to aggravate stroke risk,32–34 whereas smaller classical cohort studies from Sweden, Norway, and UK with a limited number of cases (900–1900) found no association.35,36 Effects of noise on incident heart failure were not evaluated by WHO, but the few recent studies conducted have consistently showed transportation noise to increase the risk.26,27,37–39 In contrast, the few studies investigating the impact of noise on atrial fibrillation have reported inconsistent results.38,40
Studies investigating transportation noise as a risk factor for cardiovascular death have been summarized in a meta-analysis from 2021.41 This study reported a pooled RR for road traffic noise per 10 dB of 1.02 (0.97–1.08) for IHD mortality and 1.06 (0.94–1.20) for stroke mortality (based on cohort and case–control studies) suggesting that road noise is associated with a slightly increased risk of cardiovascular mortality. However, the quality level of evidence was judged as moderate and more longitudinal high-quality studies are required. Importantly, a study from 2021 investigating acute effects of aircraft noise led further support to noise from all sources of transportation as a risk factor of cardiovascular mortality.42 The authors report that high aircraft noise exposure 2 h preceding death was found to trigger nighttime cardiovascular deaths, with an odds ratio of 1.44 (1.03–2.04) when comparing exposures >50 dB with <20 dB. As the first of its kind, this novel study needs to be reproduced.
Epidemiological studies suggest associations of transportation noise, mainly from road traffic, with several cardiovascular risk factors (Supplementary material online, Table S2). One of these is disturbance of sleep, which is hypothesized to be a key pathway through which noise is thought to impair the cardiovascular system.43,44 A pooled analysis of polysomnographic studies on the adverse health effects of acute noise, found that the awakening probability was increased with greater exposure to road, rail, and aircraft noise.45 The study also found an association of nighttime noise with severe sleep disturbance (self-reported questionnaires).
A cardiovascular risk factor consistently found associated with road noise is metabolic disease. A 2019 meta-analysis found a RR of 1.11 (1.08–1.15) per 10 dB higher road noise for incident diabetes based on five high-quality longitudinal studies.46 In support of noise as an important metabolic risk factor, several studies have found road noise associated with adiposity markers and obesity.47–50 Of note, results demonstrating that central obesity and waist circumference are associated with noise are more consistent than results on body mass index, which perfectly agrees with the concept that noise increases cortisol (stress hormone), which is known to cause mainly central obesity.
Some studies have reported on noise from all forms of transportation as a risk factor for an unhealthy lifestyle. According to two studies road noise exposure was associated with reduced physical activity, mainly with any leisure time sport and not intensity, implying that noise may influence whether people exercise at all.51,52 Furthermore, a study suggested that road noise may potentially be associated with alcohol consumption and smoking.53 More studies investigating noise-induced changes in health behaviour are important as these may represent an important link between noise and CVDs.
Lastly, studies have suggested that road noise may cause higher risk of depression.26,54,55 However, a complicating factor in these studies is that they use different definitions of depression, e.g. interviews, self-reports, use of antidepressants, and hospital admissions, making between-study comparisons difficult, and a 2020 review judged that the evidence for an association may be insufficient for an overall conclusion.56
2.2 Mechanistic insights into noise-induced pathophysiology by clinical studies
The cognition of noise and the resulting cortical and sympathetic activation causes the generation of stress hormones (e.g. cortisol and catecholamines), with subsequent activation of the renin–angiotensin–aldosterone system. If chronically present, this pathway may first lead to development of cardiovascular risk factors (e.g. hyperglycaemia and hypercholesterolaemia), blood clotting factor activation, and high blood pressure, ultimately leading to MI, heart failure, arterial hypertension, arrhythmia, and stroke (Figure 2A).62–64 Moreover, noise causes sleep disturbance, interferes with activities, and impairs communication, all of which can trigger annoyance and increased CVD risk. Recently, it was established that the limbic system, more precise the amygdala nuclei, becomes activated in response to transportation noise caused by cars and aircraft.59 In this study, around 500 patients underwent a 18F-fluorodeoxyglucose positron emission tomography/computed tomography imaging investigation and the authors demonstrated that noise ‘dose-dependently’ increased amygdala activity, with coronary inflammation and major adverse cardiovascular events (e.g. CVD death, MI, stroke and coronary/peripheral revascularization) (Figure 2B).59 In a subsequent investigation, the authors found that more pronounced resilience to chronic socioeconomic or environmental stressors such as transportation noise was clearly associated with lower risk for CVD events.65
Figure 2.
Noise–stress concept and the adverse health consequences in humans. (A) Noise reaction model for the direct (auditory) and indirect (non-auditory) effects of noise exposure. Adapted from ref.57 with permission; Copyright © 2014, Oxford University Press. (B) Neuronal activation (arousals), e.g. by noise exposure, causes signalling via the hypothalamic–pituitary–adrenocortical (HPA) axis and sympathetic nervous system (SNS) via corticotrophin-releasing factor (CRF) in the pituitary gland and adrenocorticotropic hormone (ACTH) in the adrenal gland leading to activation of other neurohormones (e.g. the renin–angiotensin–aldosterone system), inflammation and oxidative stress. The adverse effects of cortisol (or corticosterone) and catecholamines on cardiovascular function and molecular targets are well characterized. Adapted from ref.58 with permission; Copyright © 2013, Campos-Rodríguez et al.; Creative Commons Attribution License (CC BY). (C) Neuronal activation (arousals) and subsequent atherosclerosis with a higher cardiovascular risk by noise exposure was proven in subjects by 18F-PET scans indicating an association of amygdala activation, coronary inflammation, and increased incidence of major adverse cardiovascular events (MACE). Adapted from refs59,60 with permission; Copyright © 2019, Oxford University Press. (D) Flow-mediated dilation (FMD) is measured by high-resolution B-mode ultrasound. Schematic presentation of adverse effects of simulated nighttime aircraft or train noise (either 30 or 60 events for one night) vs. unexposed control group (CTR) on FMD of the brachial artery in response to post-ischaemic hyperaemia and the beneficial acute effects of the antioxidant vitamin C. Results of own studies refs.44,61
Translational field studies found adverse effects of simulated noise from aircrafts and trains on vascular function, stress hormone release, sleeping quality, and inflammation markers in healthy subjects and coronary artery disease patients.44,61,66 Furthermore, flow-mediated dilation (FMD) was found impaired by noise in an exposure dose-dependent manner, and the antioxidant vitamin C (2 g p.o.) significantly improved FMD, pointing to an important role of reactive oxygen species in this phenomenon (Figure 2C).44,61 Proteomic analysis of plasma proteins revealed that redox, pro-thrombotic and pro-inflammatory pathways were significantly affected in noise-exposed subjects as compared with unexposed controls,61 The impairment of cardiac function seemed to be aggravated by the number of noise events despite preserved average sound pressure level,67 which may provide an explanation for the heart failure risk by transportation noise.68
It has been found that these noise-induced adverse health effects correlate with higher circulating cortisol levels and more pronounced noise sensitivity.69,70 A Swiss cohort study (SAPALDIA) demonstrated that traffic noise and air pollution were associated with alterations of epigenetic DNA changes priming the tissues for altered inflammatory cascades and changes of immune responses.71 The SAPALDIA consortium also found that intermittent nighttime railway and road noise may affect arterial stiffness as shown by measurement of pulse wave velocity.72 These data were supported by results of a German cohort study, which found an association between nighttime traffic noise and subclinical atherosclerosis.73,74 Altogether these studies support the concept that psychological stress in general and noise exposure in particular promotes the release of stress hormones, the activation and recruitment of immune cells and impairs cardiovascular function in men. This concept is also in accordance with the observation that the severity of immunological changes in response to psychological stressors correlates with the number of cardiovascular events.75,76
2.3 Cardiovascular effects of transportation noise exposure: mechanistic insights from animal studies
Early animal studies demonstrated that chronic noise exposure (85 dB(A) for 4 weeks to 9 months) caused a persistent increase in blood pressure in monkeys77 or rats.78 When rats were exposed to white noise (100 dB(A) for 1–4 weeks) an impaired endothelium-dependent vasodilation of the thoracic aorta79 and the mesenteric artery80 could be observed. These previous landmark studies are in accordance with strong evidence suggesting that background noise levels ≥42 dB(A) in animal housing buildings may induce a significant pathophysiology based on hypertension, impaired vascular function, endocrine stress responses, but also modulation of the immune system, slower wound healing, impaired fertility, and reproduction.81 More animal studies on noise effects (≤100 dB(A)) can be found in Supplementary material online, Table S3.
Mouse studies conducted by Münzel et al.82 showed dysregulation of vascular gene networks by noise (revealed by RNAseq) and downstream impairment of endothelial/vascular signalling. Their data also clearly showed that noise exposure of sleeping mice but not during their activity phase causes more pronounced cardiovascular complications via major pathomechanisms comprising endothelial dysfunction, oxidative stress, and inflammation in the vasculature as well as in the brain and by dysregulated Foxo3/circadian clock signalling (identified by RNAseq).83 These adverse effects of noise were mostly normalized by Nox2 knockout, supporting a major role of phagocytic cells. They also reported normalization of noise-induced microvascular dysfunction (in dorsal and cerebral arterioles), proinflammatory changes of the plasma proteome, and endothelial adhesion of leucocytes in Nox2 deficient mice.84 This proposed concept was confirmed using a mouse model with lysozyme M (LysM)-specific overexpression of an inducible diphtheria toxin receptor (LysMiDTR mice) allowing specific removal of LysM-positive myelomonocytic cells by diphtheria toxin treatment.85 Detailed flow cytometric analysis demonstrated that genetic ablation of LysM-positive monocytes/macrophages prevented vascular inflammation and oxidative stress but also impaired endothelium-dependent relaxation and increased blood pressure in the peripheral circulation but failed to prevent neuroinflammation and stress hormone release in the brain as activation of microglia by noise was not suppressed in LysMiDTR mice. Aircraft noise also caused lower expression and uncoupling of the neuronal nitric oxide synthase, which may explain at least in part the impaired cognitive development of noise-exposed children.83 Of note, noise-dependent development of inflammation and oxidative stress, impairment of endothelial function and onset of hypertension were all improved by heme oxygenase-1 induction (using hemin) and NRF2 activation (using dimethyl fumarate).86
As the pathomechanisms of noise-induced cardiovascular damage show large overlap with traditional risk factors for cardiovascular events, such as diabetes,87 hypertension,88 and hypercholesterolaemia,89 it may be speculated that noise exposure on top of an established CVD or risk factor contributes to accelerated vascular/cerebral atherosclerosis and neurodegenerative disease and adds to the severity of these disease in an additive manner. In line with this concept noise exposure has been found to aggravate arterial hypertension and all associated cardiovascular as well as cerebral complications in a mouse model of angiotensin-II infusion.90 A similar observation was made regarding the more pronounced impairment of endothelial dysfunction by nighttime aircraft noise in coronary artery disease patients in comparison with healthy controls.44,66
3. Air pollution
3.1 Air pollution components
Air pollutants have been known since antiquity but their sources and composition have largely evolved with industrialization and urbanization and the generation of anthropogenic (combustion-derived) air pollutants that are now a major public health concern.91 Air pollution is the result of complex chemical reactions of components from various emissions requiring new classification criteria of fine particles that are not solely based on size or mass of these particles but on the surface reactivity, loading with toxic contaminants such as transition metals or bacterial/fungal pyrogens.92 Over 90% of the urban pollutant mass comes from gases or vapour-phase compounds such as O3, •NO2, volatile organic compounds (e.g. benzene), CO, and SO2. Combustion emissions that contain ultrafine particles (UFPs) or PM0.1 (PM < 0.1 μm in diameter) display the most potent toxic cardiovascular capacity due to the high particle number, reactive surface (e.g. pro-oxidative) and high surface/mass ratio that together with high solubility and charge, facilitate the alveolar penetration, systemic circulation, and damage of various end organs by these UFPs.92 Importantly, CO is toxic at excessive levels that normally do not occur in ambient air. Its toxicity differs from that of other air pollutants, which e.g. exert oxidative stress. CO displaces O2 in haemoglobin, depriving organs from oxygen. Additive effects of CO with •NO2, O3, and PM2.5 are not expected. This may be different for oxidants such as •NO2, O3, and PM2.5 components that generate reactive oxygen species. There is a need to address this issue through toxicological, modelling and epidemiological studies.93–95 Importantly, the degree of air pollution is significantly modified by climate changes (highly reactive pollutants are formed by hot weather and high UV radiation)96 but vice versa may contribute to global warming that may adversely affect cardiovascular health.97
3.2 Air pollution, global burden of disease, and mortality models
Air pollution is a main health hazard contributing to morbidity and excess mortality.4,8,98 The WHO has identified gaseous and particulate pollutants as significant risk factors of infections of the respiratory tract, chronic obstructive pulmonary disease (COPD), lung cancer, and CVDs, leading to heart attacks and strokes. Of specific interest is the contribution of chronic exposure to low level air pollution to NCDs such as COPD that is currently studied within the ELAPSE project in pooled European cohorts99 or all-cause mortality that is currently studied within the MAPLE project in pooled Canadian cohorts.100 Of note, positive associations were also found at PM concentrations lower than the current European recommendations for the limits of annual PM2.5 and PM10 exposure.101 Worldwide, diseases due to air pollution cause greater loss of life than HIV/AIDS, tuberculosis, and malaria together and are responsible for trillions of US dollars in welfare losses each year.8 Of note, higher air pollution concentrations and specific characteristics of particles or gases (e.g. diesel exhaust) were found to be associated with higher COVID-19 prevalence and fatality rates.102 In contrast, COVID-19 pandemic induced lockdown decreased the air pollution and thereby cardiovascular events.103
We have to consider, however, that the drop in hospital admissions with respect to acute coronary syndromes (ACS), acute heart failure with decompensation, and arrhythmias, may have been not only due to the improved air quality during COVID-19-mediated lockdown but also due to the fear of the CVD patients to become infected.104 This in turn caused more acute cardiovascular deaths, with almost 50% in the community that were not related to manifest COVID-19 infection, all of which points to the anxiety of patients to visit the hospital during the pandemic or to a high share of undiagnosed COVID-19.105
Long-term exposure to PM2.5 can cause a chronic oxidant/antioxidant imbalance in the respiratory system, with inflammatory responses, and implications for the aetiology of respiratory and CVDs106,107 (see also Section 3.3). Oxidative stress can occur directly by the inhalation of reactive oxygen species in PM2.5, or indirectly from their catalytic generation within the epithelial lining fluid upon inhalation of toxic aerosol compounds, e.g. co-emitted by combustion sources.17,93 The long-term inflammatory impacts within the respiratory tract can have local consequences, e.g. asthma and emphysema, as well as chronic outcomes such as circulatory and cardiovascular disorders.92,108 Furthermore, ozone (O3) is a strong oxidant that leads to respiratory and circulatory diseases through oxidative stress, likewise with immune-inflammatory responses within and beyond the lungs.109,110
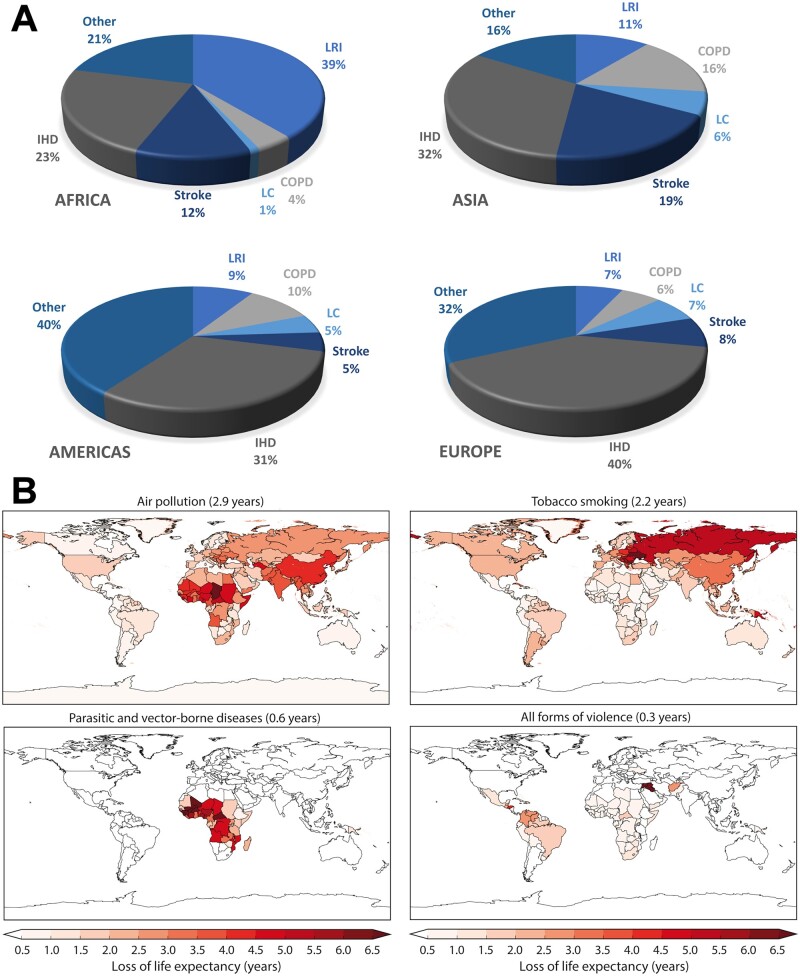
Exposure of the global population to PM2.5 and O3 can be estimated with satellite and ground-based measurements and data-informed modelling.4,111 To assess health outcomes, the Global Excess Mortality Model (GEMM) was developed, which utilizes hazard ratio functions based on 41 cohort studies performed in 16 countries.112 Results include excess mortality rates and years of life lost from five disease categories: lower respiratory tract infections, COPD, IHD, cerebrovascular diseases (strokes), and lung cancer; and one general category that accounts for all NCDs, from which impacts by ‘other’ diseases are estimated through subtraction. The latter include neurological disorders, hypertension, and diabetes, for example.113Figure 3A shows percentages of excess mortality from exposure to PM2.5 and O3 by different disease categories. In middle- and high-income countries CVDs are predominant (IHD, strokes), while in low-income countries lower respiratory infections, in particular under children, are significant. In Africa, many children die from pneumonia, whereas in Europe, this is a minor cause of mortality. Globally, air pollution-induced CVDs contribute 45–50% to excess deaths. Because the ‘other’ category includes hypertension and diabetes, which contribute to cardiovascular disorders, it follows that these diseases make up the leading health outcome of air pollution. In the European Union (EU-27), PM2.5 and O3 together cause about 592 000 (483 000–701 000) excess deaths per year.113 About 247 000 (206 000–285 000) per year are directly attributed to IHDs and strokes.
Figure 3.
Global burden of disease of air pollution. (A) Disease categories that contribute to excess mortality from the long-term exposure to ambient PM2.5 and O3. COPD, chronic obstructive pulmonary disease; IHD, ischaemic heart diseases; LRI, lower respiratory infections. (B) Mean global and country-level loss of life expectancy from air pollution, tobacco smoking (active and passive), parasitic and vector-borne diseases (e.g. malaria), and all forms of violence (interpersonal, collective conflict, and armed intervention). Adapted from ref.111 with permission; Copyright © 2020, Oxford University Press.
It was estimated that global excess mortality from the chronic impacts of PM2.5 and O3 amounts to 8.8 (7.11–10.3) million per year,111 in accordance with Burnett et al.,112 but significantly higher than estimated by the GBD,4 which accounts for PM2.5, not O3, and selected disease categories, not including the ‘other’ NCDs. However, it is lower than the recent global estimate by Vohra et al.,114 which exceeds 10 million per year for the part of PM2.5 from fossil fuels only. Global excess mortality estimates range from about 4.5 to more than 10 million per year, depending on the pollutant compounds, disease categories and exposure–response functions considered.4,98,111,112,114
The loss of life expectancy attributed to air pollution has been evaluated against other health risk factors (Figure 3B). Because about two-thirds of worldwide air pollution are anthropogenic and can be prevented, it follows that the global mean life expectancy loss from smoking and avoidable air pollution are similar. Figure 3B also shows the global life expectancy loss from all forms of violence, which is nearly an order of magnitude less than from air pollution. In Europe, the life expectancy loss by air pollution is about 2.2 years, of which 1.7 years count as avoidable if the emissions would be controlled. Therefore, the mitigation of air pollution is an effective health promotion intervention, like the banning of smoking115 and can make a major contribution to the prevention of CVDs.
3.3 Epidemiology: air pollution and CVDs
Increased levels of air pollution, mainly PM10 and/or PM2.5 show an association with higher risk of ACS, chronic coronary and peripheral artery disease, heart failure, and arrhythmia (reviewed in refs107,108,116). The classification of air pollution particles and the WHO interim target threshold concentrations for PM2.5 are shown in (Figure 4A). Clinical/epidemiological studies on association of air pollution with cardiovascular outcomes can be found in Supplementary material online, Table S4. Associations of air pollution with cardiovascular risk factors can be found in Supplementary material online, Table S2.
Figure 4.
Air pollution thresholds and guidelines as well as health effects. (A) Data for air pollution obtained from WHO air quality guidelines for particulate matter, ozone, nitrogen dioxide, and sulphur dioxide (update 2005 and summary of risk assessment) (http://apps.who.int/iris/bitstream/10665/69477/1/WHO_SDE_PHE_OEH_06.02_eng.pdf). Comparison of particle size with biochemical and biological entities. Reused from ref.117 with permission; Copyright © 2017, Oxford University Press. (B) PM2.5 exposure acutely triggers plaque rupture. Adopted from ref.118 (C) Effects of different legal thresholds for ambient particulate matter (PM2.5) concentrations in USA and Europe on cardiovascular health risk by development of high-risk plaques depicted as exponential fit for original data (left) and zoomed exponential fit with legal thresholds (right). Reused from ref.119 with permission; Copyright © 2019, Oxford University Press; generated from original data in ref.120
3.3.1 Ischaemic heart disease
In general, there is a higher incidence of fatal or non-fatal coronary artery disease in response to air pollution. The Women’s Health Initiative Study revealed a 21% (95% CI 4–42%) higher incidence of (non-)fatal coronary heart disease (CHD) with increment of 10 µg/m3 in long-term PM2.5 in 65 000 women studied.121 The European Study of Cohorts for Air Pollution Effects (ESCAPE) trial (100 000 participants, 11 EU cohorts) established a 12% higher risk with increment of 10 µg/m3 in long-term PM10 and a 13% higher incidence of coronary events with increment of 5 µg/m3 in long-term PM2.5.101 A meta-analysis addressing the effects of short-term air pollution exposures and incident ACS revealed that PM2.5, along with nitrogen dioxide (NO2), and sulfur dioxide and carbon monoxide were linked to a higher incidence of MI.122 Importantly, patients with already established diagnosis of coronary artery disease are at higher risk than healthy individuals for developing an acute coronary syndrome upon short-term exposure to PM2.5. In coronary artery disease patients (N = 16 314, diagnosed by angiography), odds ratios of 1.06 (95% CI 1.02–1.11) for ACS, 1.15 (95% CI 1.03–1.29) for ST-elevation MI, 1.02 (95% CI 0.97–1.08) for non-ST-elevation MI, 1.09 (95% CI 1.02–1.17) for unstable angina, and 1.05 (95% CI 1.00–1.10) for incident non-ST-segment elevation ACS were found with increment of 10 μg/m3 in short-term PM2.5 (on the same day, exceeding 25 μg/m3).123 Of note, higher odds ratios by air pollution were only observed in patients with coronary artery disease that was diagnosed by angiography.123 Also prognosis after an acute coronary syndrome is worse in response to chronic PM2.5 exposure.124,125 It should be noted that an appreciable part of the cited literature deals with acute effects of air pollution on IHD as the evidence for these short-term effects are really substantial. In contrast, chronic effects of air pollution on MI incidence may be less conclusive as reported by a meta-analysis.126
In general, it is believed that long-term PM2.5 exposure enhances cardiovascular risk through a continuous plaque progression, whereas short-term PM2.5 seems to acutely trigger plaque rupture, and short- and long-term exposure in concert increases the risk for cardiovascular events (Figure 4B) (reviewed in ref.118).
Overall, the higher burden of IHD by air pollutants is also paralleled by higher plaque vulnerability at PM2.5 >20 µg/m3 indicating that even short-term high concentrations of PM2.5 µg/m3 may cause acute plaque rupture and that the European threshold (PM2.5 ≤ 25 µg/m3) is clearly too high (Figure 4B and C) to protect exposed people from acute and chronic cardiovascular adverse events.119,120
3.3.2 Heart failure
Heart failure is an established major and escalating health problem in the population of Western societies with ageing populations and heart failure has a very high prevalence (64 million individuals at the global level).127 Many cardiac diseases lead to heart failure as a final outcome, leading to high hospitalization numbers (3–5% of all affected patients) and high mortality numbers (30% within 1 year after diagnosis).127 (Re)hospitalization of the elderly (age above 65 years) is most often due to heart failure (5% of all-cause hospitalization).127 The sources of acute decompensated heart are therefore a major concern of public health systems. A cohort study from UK revealed that chronic exposure to PM and NO2 was linked to a higher incidence of heart failure.128 A meta-analysis of 35 studies, reported an association of short-term increase in reactive gases and PM10 as well as PM2.5 with higher incidence of heart failure hospitalization or death.129 As also described in subjects with pre-existing coronary artery disease, heart failure, hypertension, and arrhythmia are the major cardiovascular risk factors for air pollution associated major cardiovascular events. According to the Air Quality Health Index, an increase of 10 µg/m3 in PM2.5 caused a higher hospitalization rate and higher number of deaths from heart failure with an increase in RR of 2.1% (95% CI 1.014–1.028).130 A Chinese study in 26 cities with highly polluted air, higher long-term PM2.5 exposure caused a 1.3% higher incidence of hospitalization for heart failure.131 However, high-quality studies on the effects of chronic air pollution exposure on the risk of chronic heart failure are highly needed. Recent large population data show that risk for incident heart failure is increased by chronic exposure to PM at different sizes in an additive manner.132
3.3.3 Heart rhythm disturbances
Controlled exposure studies in general (healthy) populations failed to provide convincing evidence that air pollution directly affects arrhythmias or the frequency of ventricular ectopic beats,133 which is also supported by studies in subjects with high cardiovascular risk as indicated by an implanted defibrillator.134 On the other hand, it was found that out-of-hospital cardiac arrest is associated with short-term air pollution such as ozone135 and particulate matter (especially PM2.5).136
3.3.4 Cerebrovascular disease
Korean studies showed that long-term air pollution is associated with stroke mortality.137 In support of this, large scale studies showed an association of hospital admissions for stroke with PM in the USA (intermediate to long-term effects)138 and Denmark (short-term effects).139 According to the Women’s Health Initiative study, the risk of stroke and death from cerebrovascular disease was 35% higher and the risk of death from cerebrovascular disease was 83% higher with increment of 10 µg/m3 in chronic PM2.5 exposure.121 In line with this, additive effect of PM10 and NO2 exposure for 12 years contributed to higher cerebrovascular mortality in China.140 The ESCAPE study reported a 19% (95% CI 12–62%) higher risk of stroke with increment of 5 µg/m3 in long-term PM2.5 (almost 100 000 participants, 11 EU cohorts).141 An increased risk was observed specifically in the elderly (>60 years) and the non-smoking population and effects were already observed at low PM2.5 concentrations (<25 µg/m3).141 A meta-analysis (94 studies from 28 countries), an increment of 10 µg/m3 in short-term PM2.5 and PM10 was linked to a 1% higher risk of hospitalization for stroke and stroke mortality.142 The proximity of the home address to main roads and low socioeconomic status showed an association with ischaemic stroke and stroke severity.143,144 Two independent meta-analysis have shown that long-term exposure to air pollution in the form of PM2.5 or PM10 were associated with a higher risk for incident stroke by 13%126 or 6.4%, respectively.145 In summary, a systematic study and analysis of the impact of air pollution on cerebrovascular disease is urgently required.
3.3.5 Cardiovascular mortality
An appreciable number of single-city and multicentre studies as well as meta-analyses demonstrated a higher mortality rate in relation to short-term exposure to PM, NO2, and ozone (reviewed in ref.108). For a short-term exposure scenario pooled RRs of 1.0060 (95% CI 1.0044–1.0077) for PM10 and 1.0092 (95% CI 1.0061–1.0123) for PM2.5 were reported for the cardiovascular mortality by a recent meta-analysis.146 Another meta-analysis reported that increment of 10 μg/m3 in NO2 concentration on the previous day was linked to 0.37% (95% CI 0.22–0.51%) higher numbers of cardiovascular mortality.147 A position paper by the American Heart Association reported a higher all-cause mortality rate in association with chronic as compared with acute PM2.5 exposure.148 A 11% (95% CI 6–16%) higher cardiovascular mortality rate with increment of 10 µg/m3 in PM2.5 was demonstrated.149 The pooled hazard ratio for cardiovascular mortality per 10 ppb NO2 increase was 1.11 (95% CI 1.07–1.16).150 However, in the ESCAPE study (22 EU cohort, >300 000 subjects), no statistically significant association of long-term PM2.5 exposure with the number of cardiovascular deaths was found.151 In contrast, the effect of PM2.5 on all-cause mortality is well accepted and population-based studies estimate a gain of life expectancy (>22 months at age 30) when strictly following the recommended WHO threshold for PM2.5 of a mean exposure of 10 µg/m3 per year.151,152
3.4 Pathophysiology of air pollution induced CVDs
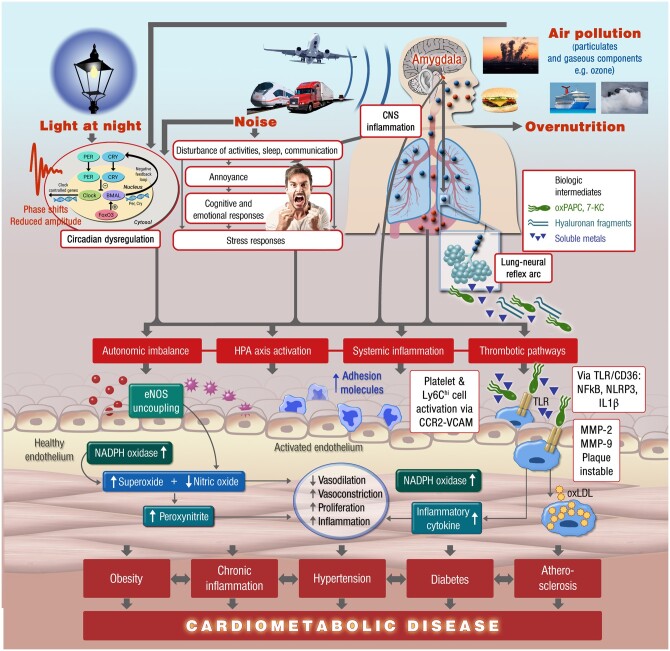
Animal studies show appreciable variation of results, which is mainly due to the exposure duration, strain/susceptibility of animals, and the particle characteristics (mostly size and chemical composition). Air pollution-induced oxidative stress mechanisms are responsible for cardiovascular and cerebral damage, also triggering subsequent inflammation and gene activation, which is largely consistent over a wide range of different particles (and reactive gases) such as diesel exhaust, wood smoke, PM2.5, or UFPs (Figure 5).154–157 Inflammatory responses to PM exposure are repeatedly demonstrated in experimental animal models starting with adhesion and infiltration of Ly6Chigh immune cells via CCR2/VCAM interaction, TLR/CD36-mediated activation of NFkB, NLRP3, IL1β, which is associated with foam cell formation and plaque instability due to MMP-2/9 up-regulation.158–160 A direct activation of the lung-neural reflex arcs facilitates systemic inflammation as well as neuronal activation/neuroinflammation linking pulmonary exposures to cerebral as well as systemic health effects of air pollution.161–163 The activation of central sympathetic mechanisms is also leading to arterial hypertension.164 Importantly, also other environmental pollutants such as traffic noise and artificial light at night share an appreciable part of these pathomechanisms that likely curdle at the level of oxidative stress and inflammation (Figure 5).10,15,165
Figure 5.
Proposed pathophysiological mechanisms of cardiovascular disease induced by environmental air, light, and noise pollution. Major pathomechanisms comprise neuronal activation and stress response, disruption of circadian rhythms, all of which initiates cerebral and systemic inflammation as well as oxidative stress leading to endothelial dysfunction, atherothrombotic changes, dysregulated metabolism, and manifest cardiometabolic diseases. Modified from ref.153 with permission; Copyright © 2016, Oxford University Press.
Nanoscale (ultrafine) particles and toxic contaminations on the particle surface (e.g. heavy metals, polycyclic aromatic hydrocarbons or fungal pyrogens/bacterial endotoxins) can directly penetrate the lung tissue into the systemic circulation and lead to additional activation of immune cells or inflict direct (oxidative) damage to endothelial cells.159,166,167 Other toxic mediators originate from air pollution and inflammation induced formation of oxidized biomolecules such as 7-ketocholesterol (7-KC) or oxidized phospholipid derivatives of 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine (oxPAPC) that have their own biochemical toxicity (Figure 5)168,169 promoting the infiltration of bone-marrow derived CD11b+Ly6Chigh cells into atherosclerotic plaques.170 PM2.5, UFPs and in particular diesel exhaust can cause endothelial dysfunction due to diminished vascular nitric oxide availability in response to augmented production of reactive oxygen species as a consequence of a higher vascular and/or phagocytic NADPH oxidase activity and/or endothelial nitric oxide synthase uncoupling.171–174 In animals, PM2.5 activates pathways that are analogous to those of angiotensin II such as induction of Rho/ROCK signalling and more pronounced calcium sensitivity.174,175
A more recent identified pathomechanism of air pollution-mediated cardiovascular damage and disease is the dysregulation of the circadian clock, which is also the major pathophysiological target of nocturnal light or noise at night (Figure 5).165 It was shown that PM2.5 exposure also impairs circadian rhythms as observed by phase shift and altered amplitudes of circadian gene expression including BMAL1, CLOCK, periods, and cryptochromes that was quite similar to circadian dysregulation observed in response to light at night.176 PM2.5 exposure impairs oscillations of circadian genes and thereby alters the lipid metabolism in white and brown adipose tissues providing a direct link to PM2.5-induced obesity and diabetes.177 Importantly, impaired circadian rhythm (e.g. as observed in shift workers or people with chronic sleep fragmentation/deprivation) is acknowledged as a potent trigger of CVDs.11,178
Of note, the results established in animal studies have mostly been translated to the human setting. PM2.5 and transportation-based pollutants such as diesel exhaust cause acute peripheral endothelial dysfunction, ischaemia and prothrombotic changes of the vasculature of humans within a few hours after exposure.155,179–182 Diesel exhaust acutely impairs resistance vessel responses to endothelium dependent dilators such as acetylcholine and bradykinin and the direct NO-donor sodium nitroprusside coupled with increase in vasoconstrictor potency of ET-1 in mice but also humans.179–181,183 Short-term exposure to diesel causes ischaemia in patients with already established coronary artery disease.182 Diastolic dysfunction and impaired contractile reserve may be explained by PM2.5-induced up-regulation of the β-myosin heavy chain and lower expression levels of SERCA2a, indicating abnormal calcium signalling.184 PM also impairs the anti-inflammatory and antioxidant properties of HDL, and thereby promotes the adverse health effects of upregulated LDL.185 More animal studies on air pollution-mediated cardiovascular damage can be found in Supplementary material online, Table S5.
The above described pathomechanisms may explain at least in part the accelerated development of atherosclerosis and inflammatory processes in the plaques observed in response to exposure of animals to PM2.5, diesel exhaust, reactive gases, and UFPs.186–188 Diesel exhaust particles were demonstrated to cause activation of platelets in murine models of arterial thrombosis.189 These animal data were in accordance with enhanced fibrinolytic function in healthy individuals and in CHD patients in response to exposure to diesel exhaust particles.180,182 Previous reviews provided an excellent summary of the experimental evidence and pathomechanisms, underlying the contribution of air pollution to cardiometabolic disease such as diabetes.161,162 Also the mechanisms leading to a higher risk of CVD by air pollution, as indicated by enhanced carotid intima media thickness and impaired ankle brachial indices, was reviewed in the past.108 This also includes PM2.5 triggered mechanisms of inflammation and impairment of insulin response pathways, induction of brown adipose tissue dysfunction, and adverse central nervous system activation involved in glycaemic control, regulation of satiation, and metabolic pathways.92
4. Outdoor light pollution and cardiovascular disease (epidemiology and pathophysiology)
A rather novel environmental risk factor of concern is light pollution and its potential large impact on NCDs.190 Anthropogenic sources of natural nighttime sky brightness represent a major challenge in huge cities and metropolitan areas but can additionally cause effects in distant rural places such as national parks.191,192 Up to 83% of the population on earth and >99% of the people in the USA and EU may live under light-polluted skies (>14 µcd/m2 artificial nighttime sky illumination).191 This unfortunate situation was nicely summarized with the title of the review article ‘Missing the Dark—Health Effects of Light Pollution’, where satellite images document the progression of artificial light at night in the USA over a period of seven decades.190 Light pollution induces premature mortality and loss of biodiversity of insects, animals, and birds by impairment of their circadian rhythm.190 In humans, the dysregulation of circadian genes is a major contributor to NCDs, also due to the circadian control of inflammatory and metabolic pathways (Figure 5).178,193 Mutations and expression changes of key circadian genes can contribute to obesity and hyperglycaemia194–196 and the ‘chronotype’ (morningness or eveningness person) has a significant health impact in humans, specifically with respect to metabolic diseases197,198 and is based on genetic profiles as revealed by genome-wide association studies.199,200
In elderly subjects, blood pressure was increased by 3–4 mmHg per 5 lux (=1 lumen/m2) increase in outdoor nighttime light pollution.201 In addition, artificial light at night is associated with a higher risk of CHD and mortality in the older population.202 An increment of hazard ratios for CHD hospitalizations of 1.11 (95% CI 1.03–1.18) and for CHD mortality of 1.10 (95% CI 1.00–1.22) was reported per change of 60 Units of radiance (nW/cm2/steridian). Of note, there was an additive increase of hazard ratio for CHD mortality in the upper light pollution quintiles in combination with highly polluted air based on the measured PM2.5 concentrations (1.32 and 1.39, respectively),202 which goes hand in hand with data on combined PM2.5 and light at night exposure-induced circadian phase shifts and reduced amplitudes that, however, induced distinct epigenetic changes along with a specific pattern of circadian gene disruption.176 As all of these studies were conducted in East-Asia and often in aged participants, replications of these studies in a wider context (e.g. different age groups and geographical locations) are urgently needed.
5. Climate change, increases in temperature, desert storms, and wildfires
A growing body of evidence also supports an association of particles from natural sources (e.g. desert dust, wildfires, and volcano eruptions) with adverse effects on public health. Based on estimations an annual number of 400 000–500 000 global deaths from cardiopulmonary causes (approximately 18% of all premature deaths), can be attributed to air pollution.203 In line with this, Asian desert dust was found to potentially contribute to the risk of CVD hospital admissions,204 also supported by a significant association of Asian dust storms up to 4 days before hospitalization and the risk of incident acute MI.205 Also a recent meta-analysis reported higher mortality rates and more hospital admissions for major adverse events of cardiopulmonary origin in association with desert dust.206 Forest fires in Southeast Asia create extremely high levels of PM10 that are linked to higher all-cause mortality and hospitalization for IHD in Malaysia.207 Also the generation of PM2.5 and other air pollutants from wildfires in California,208 Brazil,209 Australia,210 Southern Europe,211 and at a global scale212 was reported to affect respiratory and cardiovascular health, whereas forest fires in Siberia were so far rather mentioned in the context of global warming. Although it is unclear to what extent wildfires and dust events are anthropogenic, the rapidly growing populations in Africa and Southeast Asia foster projections that more and more people will suffer from mixed exposure to natural and human-made air pollution in the future, including the exacerbated number of wildfires in California and Southern Europe with high population density. This warrants more clinical studies on the potential health harms of natural air pollution sources.
In addition, with increases in wildfire frequency, volcano eruptions, and desert storms, there will be also a substantial increase in the temperature further aggravating the heat wave in a positive feedback fashion (global warming by greenhouse gases). Wildfires release smoke that mainly consists of particulate matter. Importantly, PM from wildfires causes more pronounced effects on mortality than urban PM, which is mostly due to smaller particle size213,214 and contamination with oxidative and proinflammatory compounds213 causing an amplification of the adverse health effects of the increasing global warming215 and O3.216
The healthcare industry is a huge and socioeconomically powerful branch of trade, and by itself contributes significantly to global CO2 emissions. Interestingly, healthcare in world’s largest economies account for around 5% of all CO2 emissions making this sector comparable to the importance of the food sector.217 Importantly, the Lancet Commission on Health and Climate Change recommended that greenhouse gases emissions from the healthcare sector should be also considered as an indicator in evaluations of health and climate.218
Concerning the influence of climate change, changes in temperature and specifically subsequent CVDs such as acute coronary syndrome, epidemiological studies have postulated that high (‘heat waves’ and ‘heat islands’) but also low (‘cold effect’) temperatures may cause an increase in cardiovascular mortality and morbidity.219–221
There is evidence for an association between air temperature and acute MI. Many studies established a significant cold effect on MI occurrence,222–224 while other studies suggested a higher risk of MI as a consequence of heat exposure.225 Chen et al.226 reported in a study over 28 years, which was based on a registry with time-stratification and cross-over design, that the risk of MI in relation to higher temperature increased over time, when comparing the period from 2001 to 2014 with the one from 1987 to 2000. Importantly, the risk of MI in relation to cold decreased during the study. In the late study period, the authors established that heat-induced MI was more pronounced in rural populations.
Associations between (climate change-related) high temperatures and cardiovascular events may occur through several direct and indirect mechanisms:
Higher surface blood circulation and sweating is associated with high temperature, all of which contributes to higher cardiac strain, blood viscosity, plasma cholesterol, and interleukin-6 levels.227
Warmer temperature causes sleep disturbance228 such as too short sleep (<6 h) or fragmentation of sleep, which conversely increases the risk of CVD.229
Very high temperature reduces physical activity,230 which is conversely associated with higher cardiovascular risk.231 Vice versa, physical activity at very high temperature may represent a risk factor of its own.
In summary, there is emerging evidence that the rising temperature in part triggered by wildfires and substantial greenhouse gas emissions due to biomass burning may in addition to inner cities heat islands (see below heart healthy cities) increase the susceptibility to heat related-MIs, which is further exacerbated by co-exposure to high PM2.5 concentrations.221 This indicates that, similar to air pollution exposure, heat exposure should be considered as an acute environmental stimulus of acute coronary syndrome especially in light of global warming.
6. Gaps in knowledge
There are only few high-quality animal or human studies that consider potential additive effects of combined noise, air, and light pollution. Although it is known that also air pollution from natural sources are associated with higher morbidity and death rates there are numerous gaps in knowledge with respect to their adverse health effect.
Mechanistic studies in animals may help to provide a direction for future human studies. The questions that should be answered comprise: (i) the of the additive effects and time-dependent biological/functional responses of different co-exposures; (ii) are the induced effects reversible; (iii) impact on circadian rhythm; and (iv) the effect of lifestyle modifications (e.g. diet, stress, and exercise). Finally, the development of novel technologies that enable personal measures of health together with public data on environmental pollutants would foster an advanced understanding of the interactions between environmental and non-environmental risk factors.
7. Mitigation measures
7.1 Societal/political noise exposure mitigation strategies
People in industrialized and urbanized societies are largely exposed to traffic noise, as reflected by >30% of the people in Europe being exposed to residential noise levels above 55 dB(A) Lden.232 As this contributes to a higher incidence and mortality of major CVDs,232 the implementation of new and effective mitigation measures is urgently required. Several noise interventional approaches are already in use, as propagated by the European Commission (Table 1).233
Table 1.
Noise-abatement approaches
| Abatement procedures | Reduction in noise (dB) | Cost-effectiveness score (1–5)a |
|---|---|---|
| Noise barriers | 3–20 | 2 |
| Brake blocks for trains | 8–10 | 4 |
| Building insulation | 5–10 | 1 |
| Building design | 2–15 | 3 |
| Changing driving styles | 5–7 | 3 |
| Quiet road surfaces | 3–7 | 5 |
| Low-noise tires | 3–4 | 3 |
| Land-use planning and design | Unknown | 4 |
| Electric cars | 1 | 1 |
| Traffic management | 3 | 3 |
dB, decibel.
Evaluated by the European Commission in ‘10 ways to combat noise pollution’ 231 lowest score = 1; highest score = 5.
Buildings can be insulated against noise, which efficiently reduces exposure to all outdoor noise sources. However, this intervention has a low cost-effectiveness ratio due to the very high costs, especially when retrofit is required. Novel technologies and advances such as less noisy engines and tires for vehicles as well as silent brake blocks for trains are key to reduction of noise levels from all traffic-related sources.
Traffic noise pollution is significantly determined by road noise, which can be effectively mitigated by lower speed limits, silent road surfaces, and construction of noise barriers at main streets. However, in light of the continuously increasing traffic volume and accordingly constantly rising noise exposure levels, superior traffic management and regulation may represent key concepts for the future (see Table 1).
Aircraft noise exposure levels continuously increased over the last years, which has led to the ban of nocturnal air traffic at many airports because noise during nighttime is associated with the most pronounced adverse health effects.66,73,234 However, noise exposure, in particular during the night has severe adverse effects on health,235 which may be prevented by new engine technologies (fleet evolution), lower noise thresholds, longer night bans, and better air traffic management. Further measures include zoning, which means the restricted land-uses in areas of highest noise sensitivity, e.g. when housing complexes, schools, hospitals are in close proximity to airports or flight paths. Other mitigation measures also include facade insulation of residential buildings, tax incentives and fines for noise initiators (polluter liability), movement limitations, and noise quotas. Also new air traffic protocols such as the continuous descending approach that is based on high altitude of the aircraft until shortly before landing or GPS-assisted starting/landing procedures, which both may significantly reduce the noise exposure levels of residents nearby airports.164
In summary, new noise reducing technological advances and legislative mitigation approaches are important to protect the general population from adverse effects of noise on health. These preventive measures are urgently needed in light of a growing global traffic load.
7.2 Air pollution
7.2.1 Personal exposure mitigation strategies
It is important to note that so far no personalized intervention for reduction of air pollution exposure has been demonstrated to improve life expectancy or to reduce cardiovascular events. Recently, the topic of personal exposure mitigation strategies has been extensively summarized (Figure 6). Here are some important notices. Portable air cleaners are inexpensive and can be employed in nearly all homes and apartments in locations with electricity. High Efficiency Home Air Filtration Systems: Central HVAC units with inbuilt filters can be an effective means for particle removal in residential indoor environments. There are, however, currently no clear studies demonstrating health benefits of filters in forced air systems in residences. Fisk and Chan237 have estimated their potential benefits during wildfires in a modelling analysis and found that they are likely to be less effective than using other methods such as portable air cleaners. Personal air purifying respirators is a personal protective device that covers the nose and mouth and is used to reduce inhalation of PM2.5 and other particles depending on their rating efficiency (removal of >95% or 99% of inhaled particles at 0.3 µm in size by N95 or N99). Some studies suggest that at least under conditions of high ambient exposures, there could be meaningful reductions in blood pressure in response to an N95 respirator intervention.238 Some concerns over potential adverse cardio-pulmonary stress induced by wearing a respirator thereby mitigating health benefits, have been raised. However, there is no evidence that the short-term use of a respirator adversely affects health parameters such as blood pressure, heart rate, or aortic haemodynamics.239
Figure 6.
Personal mitigation manoeuvres and air pollution (significantly modified from ref.236)
Face masks (typically made of gauze, cotton, or cloth) and surgical masks are commercially available, but show large differences in filtering PM2.5. FFP2 face masks provide a certain protection from solid air pollution components. While not directly relevant here, it was shown that face masks (all types and especially FFP2) effectively prevent SARS-CoV-2 infections through the spreading of droplets and aerosols.240
In summary, the current level of evidence demonstrates that wearing validated N95 respirators over a few hours to days in supervised experiments may improve surrogate markers of cardiovascular risk in environments with high PM2.5 levels, although the data are inconsistent across studies and no evidence exists regarding their impact on cardiovascular events. Nonetheless, their brief use during extremely poor PM2.5 air quality events might be beneficial. It is logical to question if the use of surgical masks should be advocated if N95 respirators are not available. On the one hand, some degree of protection against PM2.5 exposure, even if only incomplete (e.g. 25–75%), among many millions of people facing high levels of exposure might translate into significant public health benefits considering the well described linear dose-risk response. Unlike a viral contagion in which an unknown threshold of exposure reduction is required to prevent the spread of infection to an individual, any decrease in exposure to air pollution should reduce health risks in a population. Conversely, arguments have been made that these masks might engender a false sense of security, thereby worsening overall exposure. While they can reduce the inhalation of PM2.5 by a variable degree, any health benefits of wearing less cumbersome facemasks (e.g. surgical style) have yet to be shown. At this point, there is insufficient evidence to support or proscribe against the use of simple face masks.
Automobile air filters and air-conditioning are approaches to reduce PM2.5 and UFPs exposure during travel that may be of use for highly susceptible individuals, but also for those staying for significant parts of their daily life in transportation microenvironments.
Simple strategies implemented into one’s lifestyle can help to reduce air pollution exposure. Some of these practical strategies are general and may not necessarily reduce exposures to PM2.5, which is considered a regional pollutant. However, they may exert health benefits through their impact on UFPs and/or gaseous co-pollutants (in particular ozone), which have also been associated with health risk.
Other more simple recommendations include air pollution avoidance, staying indoors, and closing windows. An important question is at which level of air pollution (PM2.5), exercise may be allowed without adverse health effects.
7.2.2 Personal mitigation strategies by physical activity
There is an ongoing discussion on the health benefits of physical exercise and the potential adverse health effects of higher exposure to air pollution during outdoor physical activity. In order to answer this question, Kim et al.241 conducted a nationwide cohort study (1 469 972 young adults with an age of 20–39 years). Air pollution exposure was calculated by the average cumulative level of PM2.5 and PM10 per year at the residential addresses and physical exercise was determined by the minutes of metabolic equivalent tasks per week (MET-min/week) for each participant for the years 2009 to 2012. As a major outcome of the study, there was a clear benefit of outdoor physical activity, even when exposed to low or moderate PM concentrations. Those participants with a sedentary lifestyle had a clearly increased cardiovascular risk. Of note, extremely high levels of outdoor physical exercise (≥1000 MET-min/week) in highly polluted air also caused a higher cardiovascular risk. These observations are also in accordance with the reported ‘break-even point’ for lowering of air pollution-associated RR of all-cause mortality by physical activity, which was calculated by the authors at 100 µg/m3 of PM2.5 making 1.5 h cycling or 10 h walking per day in more polluted air detrimental.242
7.2.3 Societal/political exposure mitigation strategies by improved air quality
The most promising manoeuvres to protect people from air pollution induced CVDs is the lowering of the allowed emission levels. Since 2015, the EU recommends an annual mean air quality limit of 25 µg/m3 for PM2.5, which is 2.5-fold higher than the WHO recommendation of 10 µg/m3. Even at PM concentrations of 10 µg/m3, hazard ratios are greater than 1.0 as based on calculation using the GEMM or the GBD model from the year 2015. A hazard ratio of approximately 1.5 for the risk of IHD was found at PM levels of 25 µg/m3, which clearly indicates that the EU-28 air quality standard is not effective. This becomes even clearer, when comparing the annual mean limits in the USA of currently 12 µg/m3 (since 2012), and in Canada of 10 µg/m3 since 2015, with a further intended reduction to 8.8 µg/m3. The Australian annual PM2.5 limit is 8 µg/m3 with another intended reduction to 7 µg/m3 within the next years. The EU had the intention to reduce PM exposure limits until 2020 to a target concentration for PM2.5 of 20 µg/m3. However, even the current recommended PM2.5 limit is exceeded in several European countries113 and the targets for 2020 have not yet been ratified by the EU member states and it seems that this will not happen soon. Therefore, additional efforts are urgently warranted to improve air quality in Europe.
7.3 Mitigation strategies for light pollution exposure and of adverse health effects
The easiest mitigation approach to decrease light pollution is to switch-off lights, especially when light is not absolutely necessary.190,191 Also technical advances may help to reduce light pollution: light shielding helps to send light rather to the base than to the sky, energy-efficient lights emit yellow light within a nanometer range where the human eye is most sensitive and smart city techniques control light dimming when nobody is around or when the weather/time of the day allows light reduction.190,243 Reduction in ‘blue’ light emissions (LEDs display peak light spectrum at 400–490 nm) is highly effective as this kind of light is most detrimental for dysregulation of circadian rhythms.243 An impaired circadian clock can also be ‘reset’ by chronotherapy (e.g. melatonin), which can especially help to prevent sleep disorders,11 not only for those caused by light pollution but also ones triggered by nocturnal traffic noise235 and air pollution.244,245 Circadian dysregulation by these environmental factors resembles the desynchronized profile observed in shift workers who have an increased risk for CVD and events.246,247
7.4 Strategies for mitigation of climate change including greenhouse gas emission, temperature, desert storms, and wildfires
Human use of resources and energy generation has caused a 1.0°C global warming above preindustrial level, which will probably reach 2.0–2.5 or even higher in 2030–2052 if the emissions are not dramatically reduced. The natural disasters in 2018 were mainly climate related and involved almost 70 million people with wild fires, storms, extreme temperature, flooding, and landslides.248 With respect to economic losses this summed up to 131.7 billion dollars. The economic loss by wildfires in 2018 reached almost the similar amount as all losses from wildfires during the last decade combined.249
Conventional mitigation strategies may focus on the reduction of CO2 emissions from fossil sources. Negative emission technologies have the capacity to trap and bind atmospheric carbon to reduce CO2 concentrations. Geoengineering techniques of radiative forcing may positively affect the radiative energy budget of our planet to stabilize or even decrease the global temperature. In light of global warming and climate disaster respective mitigation measures are urgently needed. Carbon emission fines that foster carbon removal should be introduced now.250
What can a cardiologist do to reduce greenhouse gases emissions? Should it be important also in decision-making of clinicians in cardiology? More recent studies compared the environmental impact (with respect to emissions and energy consumption) of magnetic resonance imaging (MRI), single photon emission tomography and cardiac ultrasound (echo) that are used for the diagnosis of cardiovascular complications. The data indicate that ultrasound is the most eco-friendly method and only caused 1–20% of the effects on human health, ecosystem effects, and resource use compared to MRI and tomography.251
7.5 Heart healthy city design: mitigation of the cardiovascular risk
Urban environments are still hotspots of all environmental stressors together including climate change, air pollution, noise and light and heat in form from heat island effects.252 Further risk exposures that may affect the risk of NCD’s include safety from crime, social isolation, prolonged sitting, sedentary lifestyle, and unhealthy nutrition with, just concerning physical inactivity, disabilities life years lost up to 70 million DALYS including 3.2 million deaths annually.252 More recently, compacts cities are being promoted as they are considered more sustainable and healthier.252,253 Compact cities are cities with higher density, shorter travel distances, and higher diversity that are healthier because of increased land use mix and the healthier mobility opportunities, all of which leads to lower CO2 emissions.254
Boston and Melbourne are low-density sprawling communities with a high share of 80.1% and 85.1% attributable to transportation by cars and the health of these cities could be significantly improved by altered land use and transport mode.254 However, compact cities are not without their problems if the existing (public) space is not used well. Many compact cites like Barcelona or Paris still suffer considerable adverse health impacts due to their air pollution levels and other exposures related to urban and transport planning.255,256 Therefore, we need a better use of the new and existing public spaces.
Several novel urban concepts are currently implemented in various cities that partly solved these problems, such as the Superblocks, the low traffic neighbourhood, 15-min city (Figure 7), car free city, or mixed models. All of these models are aiming at reduction of private car use and promoting public and active transportation (walking and cycling) as well as reducing CO2 emissions. The benefits are clear and comprise reduction of air pollution, noise, and heat island effects, whereas physical activity is increased, all of which has beneficial health effects.253 Cars use a lot of public space (road) network and parking, which could be used much better for the creation of green spaces and infrastructure. Barcelona uses 60% of public space for cars, while their share in overall transportation is only 25%.253
Figure 7.
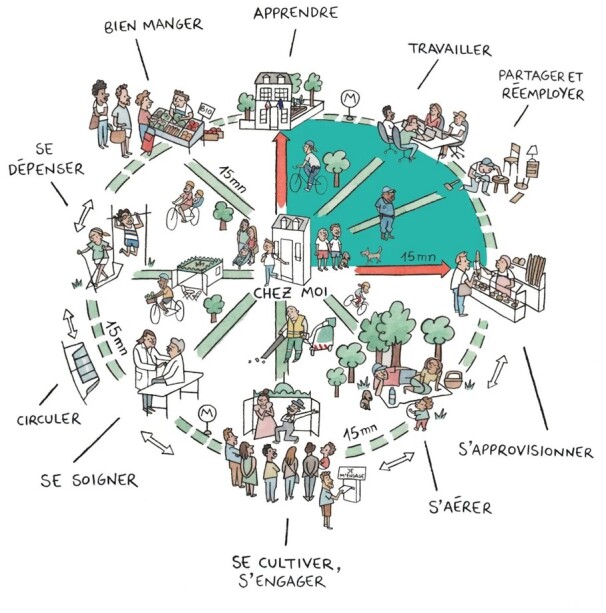
The present figure depicts the 15-min city, the brain child of Carlos Moreno, where work, school, entertainment, and other activities are reachable within a 15-min walk of the home.257,258 The 15-min city involves the creation of a city of villages and a return to more traditional city design. The 15-min city will encourage more physical activity through more active transport, and is likely to reduce urban inequalities and health inequities.259 Critically, it will also reduce the need for long distance travel and thereby reduce CO2 emissions, and air pollution and noise levels (for review see ref.15) Translation of French words: apprendre = to learn; travailler = to work; partager et réemployer = to share and reuse; s’approvisionner = to supply oneself; s’aérer = to get some fresh air; se cultiver, s’engager = to subdue, to be committed; se soigner = to nurse oneself; circuler = to be on the move; se dépenser = to wear oneself out; bien manger = to eat well; chez moi = at home. Reused from ref.257 with permission of the copyright owner Micael queiroz, www.micaeldessin.com.
The construction of over 500 superblocks are intended in Barcelona, which will decrease motorized traffic in a part of the roads of a block providing more room for people, active travel, and green space. The aim is to generate a healthy, greener, fairer and safer public environment promoting social relations and local economy. Air pollution lowering and noise mitigation, heat island prevention and better green space implementation for physical activity are the other beneficial side effects of this renewing city design, which can easily prevent up to 700 annual premature deaths in Barcelona (effects on premature deaths are by air quality > noise > heat island > green space).260
Similar concepts provide the basis of low traffic neighbourhoods that can be implemented by cheap and quick streetscape changes.261,262 These measures are implemented by some governments already to create safer walking and cycling environments (lower COVID-19 and traffic injury risk).262
Paris will implement the 15-min city, where work, school, shops, entertainment, culture, leisure, and other social activities can be reached within a 15-min walk or bicycle ride from the residence.263,264 The 15-min city comprises formation of a city of villages and retro city design. Ecology (green space), proximity, solidarity among citizens, and participation of citizens are some of the key aspects.265 The envisaged new trees and cycle ways, community facilities and social housing, homes and workplaces all go hand-in-hand with a new vision for urban planning, and will result in better health.
Even further go car free cities. For example, Hamburg intends to become a car-free city by 2034, mostly in response to the climate crisis. Car free cities or neighbourhoods decrease private motorized traffic and promote active and public transportation. A successful example is Vauban and to a lesser extent Rieselfeld in Freiburg, Germany, that are neighbourhoods without cars and with housing with higher sustainability.266 Pontevedra is a small car-free city in Spain. Cars are prohibited in Pontevedra’s city centre, representing an excellent example of a pedestrian-friendly living with low CO2 emissions.267 The transition to a city without cars will result in a significant improvement of the livability of neighbourhoods also preventing disproportional burdens of pollution, social disadvantage, crashes, and public transport disinvestment. Car free cities or neighbourhoods will reduce air and noise pollution, promote physical activity, and foster generation of green space, and thereby reduce heat island effects and improve public health.268
Some of these changes take longer to implement, while we need to have faster action. New and easier to implement policies such as introducing 30 km/h speed limits on all roads in urban areas could have a significant impact on accidents and health. Further temporary tactical urbanism could help transform public urban space fairly quickly. Tactical urbanism refers to low-cost, temporary, and scalable interventions and policies intended to improve urban environments.269 Although these tactical urbanism interventions are designed to be implemented in a temporary and low-cost approach, these interventions can be considered as pilot programs that could involve the community in selecting future permanent infrastructure. Except for the compact city concept,254 these new urban models have not specifically evaluated CVD effects, but there are likely beneficial effects on the heart as an increase in active transportation and green space and a reduction in air pollution, noise and heat island effects have all been associated with better cardiovascular health.14,15
8. Major conclusion and resulting political/societal needs for action
The exposure to almost all environmental risk factors triggers a specific set of pathophysiological mechanisms that are centred on stress hormone signalling, oxidative stress, and inflammation.10,16,17,107 As a result, exposome studies will face the problem of identifying specific biochemical signatures (footprints) of different environmental risk factors.270 In addition, oxidative stress and inflammation also represent major pathomechanisms of cardiovascular, neurodegenerative, and metabolic diseases, which further complicates exposome research. Considering that environmental stressors, unhealthy behaviour (e.g. smoking, sedentary lifestyle), and classical risk factors (e.g. hypertension, diabetes, obesity) all trigger similar pathomechanisms, additive/synergistic effects should be present, leading to more pronounced development and faster progression of NCDs (Figure 1).10,17 Smart city planning may be a key mitigation strategy of unhealthy environmental exposures because environmental stressors such as noise, air pollution or heat islands show an accumulation in big cities and large urbanized areas and their combination aggravates health problems and disease burden that may exceed even the most pessimistic projections.16
Concerning all environmental stressors societal action is highly needed. Without fast actions to decrease the environmental stressors at all levels (chemical, physical, and mental sources), the sum of all ‘pollution’ and climate change will likely form a reinforcing feedback loop. Currently, around 96% of public health funding is spent for treatment and only 4% for prevention.271 Additionally, the funding for prevention is mostly spent at the individual level with only limited effort or money dedicated to the collective social and physical environment for prevention of environmental exposures. What we need are intersectoral actions for better city design, which has to be done by urban, transport and health planners, all of which has the largest impact on health. Unfortunately, these experts often do not realize the impact of their work on human health and how they can improve health.
We as health professionals have to play a leading role in addressing and in acknowledging environmental stressors such as noise and air pollution as cardiovascular risk factors that are as important as e.g. smoking, diabetes and hypercholesterolaemia. We also have to take care that environmental researchers are getting integrated into these guidelines writing teams to ensure that in the very near future environmental stressors (e.g. climate change, air pollution) rather than classical risk factors (e.g. high cholesterol or diabetes) are defined as the cardiovascular risk factors of the future. Funding for environmental research, teaching and education of the consequences of climate change has to be intensified dramatically, especially medical/health care field, in order to protect the health of our current and future generations.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgements
The authors also acknowledge the continuous support by the Foundation Heart of Mainz and the DZHK (German Center for Cardiovascular Research), Partner Site Rhine-Main, Mainz, Germany.
Funding
The present work was supported by a vascular biology research grant from the Boehringer Ingelheim Foundation for the collaborative research group ‘Novel and neglected cardiovascular risk factors: molecular mechanisms and therapeutic implications’ to study the effects of environmental risk factors on vascular function and oxidative stress (A.D. and T.M.).
Contributor Information
Thomas Münzel, Department of Cardiology, University Medical Center Mainz, Johannes Gutenberg University, Langenbeckstrasse 1, 55131 Mainz, Germany.
Omar Hahad, Department of Cardiology, University Medical Center Mainz, Johannes Gutenberg University, Langenbeckstrasse 1, 55131 Mainz, Germany.
Mette Sørensen, Work, Environment and Cancer, Danish Cancer Society Research Center, Copenhagen, Denmark; Department of Natural Science and Environment, Roskilde University, Roskilde, Denmark.
Jos Lelieveld, Atmospheric Chemistry Department, Max Planck Institute for Chemistry, Mainz, Germany.
Georg Daniel Duerr, Department of Cardiovascular Surgery, University Medical Center Mainz, Johannes Gutenberg University, Mainz, Germany.
Mark Nieuwenhuijsen, Institute for Global Health (ISGlobal), Barcelona, Spain; Department of Experimental and Health Sciences, Universitat Pompeu Fabra (UPF), Barcelona, Spain; CIBER Epidemiología y Salud Pública (CIBERESP), Madrid, Spain.
Andreas Daiber, Department of Cardiology, University Medical Center Mainz, Johannes Gutenberg University, Langenbeckstrasse 1, 55131 Mainz, Germany.
References
- 1. Hughes BB, Kuhn R, Peterson CM, Rothman DS, Solorzano JR, Mathers CD, Dickson JR. Projections of global health outcomes from 2005 to 2060 using the International Futures integrated forecasting model. Bull World Health Organ 2011;89:478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Noncommunicable diseases: Mortality. https://www.who.int/gho/ncd/mortality_morbidity/en/ (13 October 2021, date last accessed).
- 4. GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396:1223–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bygbjerg IC. Double burden of noncommunicable and infectious diseases in developing countries. Science 2012;337:1499–1501. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization . Global action plan for the prevention and control of noncommunicable diseases 2013–2020. https://apps.who.int/iris/bitstream/handle/10665/94384/9789241506236_eng.pdf;jsessionid=71BCEA94B3F85737AB42F3C84216E54A? (13 October 2021, date last accessed).
- 7. Bloom DE, Cafiero ET, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya A, Prettner K, Rosenberg L, Seligman B, Stein AZ, Weinstein C. The Global Economic Burden of Non-Communicale Diseases. Geneva: World Economic Forum; 2011. https://www3.weforum.org/docs/WEF_Harvard_HE_GlobalEconomicBurdenNonCommunicableDiseases_2011.pdf (13 October 2021, date last accessed). [Google Scholar]
- 8. Landrigan PJ, Fuller R, Acosta NJR, Adeyi O, Arnold R, Basu NN, Balde AB, Bertollini R, Bose-O'Reilly S, Boufford JI, Breysse PN, Chiles T, Mahidol C, Coll-Seck AM, Cropper ML, Fobil J, Fuster V, Greenstone M, Haines A, Hanrahan D, Hunter D, Khare M, Krupnick A, Lanphear B, Lohani B, Martin K, Mathiasen KV, McTeer MA, Murray CJL, Ndahimananjara JD, Perera F, Potocnik J, Preker AS, Ramesh J, Rockstrom J, Salinas C, Samson LD, Sandilya K, Sly PD, Smith KR, Steiner A, Stewart RB, Suk WA, van Schayck OCP, Yadama GN, Yumkella K, Zhong M. The Lancet Commission on pollution and health. Lancet 2018;391:462–512. [DOI] [PubMed] [Google Scholar]
- 9. Allen L. Non-communicable disease funding. Lancet Diabetes Endocrinol 2017;5:92. [DOI] [PubMed] [Google Scholar]
- 10. Daiber A, Munzel T. Special Issue "Impact of environmental pollution and stress on redox signaling and oxidative stress pathways". Redox Biol 2020;37:101621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li H, Kilgallen AB, Munzel T, Wolf E, Lecour S, Schulz R, Daiber A, Van Laake LW. Influence of mental stress and environmental toxins on circadian clocks: implications for redox regulation of the heart and cardioprotection. Br J Pharmacol 2020;177:5393–5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev 2005;14:1847–1850. [DOI] [PubMed] [Google Scholar]
- 13. Vrijheid M. The exposome: a new paradigm to study the impact of environment on health. Thorax 2014;69:876–878. [DOI] [PubMed] [Google Scholar]
- 14. Nieuwenhuijsen MJ. Influence of urban and transport planning and the city environment on cardiovascular disease. Nat Rev Cardiol 2018;15:432–438. [DOI] [PubMed] [Google Scholar]
- 15. Munzel T, Sorensen M, Lelieveld J, Hahad O, Al-Kindi S, Nieuwenhuijsen M, Giles-Corti B, Daiber A, Rajagopalan S. Heart healthy cities: genetics loads the gun but the environment pulls the trigger. Eur Heart J 2021;42:2422–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Munzel T, Sorensen M, Daiber A. Transportation noise pollution and cardiovascular disease. Nat Rev Cardiol 2021;18:619–636. [DOI] [PubMed] [Google Scholar]
- 17. Munzel T, Daiber A. Environmental stressors and their impact on health and disease with focus on oxidative stress. Antioxid Redox Signal 2018;28:735–740. [DOI] [PubMed] [Google Scholar]
- 18. Sainani K. Taking on the exposome—bringing bioinformatics tools to the environmental side of the health equation. Biomed Comput Rev 2016;Fall 2016:14–21. [Google Scholar]
- 19. Riggs DW, Yeager RA, Bhatnagar A. Defining the human envirome: an omics approach for assessing the environmental risk of cardiovascular disease. Circ Res 2018;122:1259–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bray GA, Paeratakul S, Popkin BM. Dietary fat and obesity: a review of animal, clinical and epidemiological studies. Physiol Behav 2004;83:549–555. [DOI] [PubMed] [Google Scholar]
- 21. Booth J, Connelly L, Lawrence M, Chalmers C, Joice S, Becker C, Dougall N. Evidence of perceived psychosocial stress as a risk factor for stroke in adults: a meta-analysis. BMC Neurol 2015;15:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Little MP, Azizova TV, Bazyka D, Bouffler SD, Cardis E, Chekin S, Chumak VV, Cucinotta FA, de Vathaire F, Hall P, Harrison JD, Hildebrandt G, Ivanov V, Kashcheev VV, Klymenko SV, Kreuzer M, Laurent O, Ozasa K, Schneider T, Tapio S, Taylor AM, Tzoulaki I, Vandoolaeghe WL, Wakeford R, Zablotska LB, Zhang W, Lipshultz SE. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect 2012;120:1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Podolska K. The impact of ionospheric and geomagnetic changes on mortality from diseases of the circulatory system. J Stroke Cerebrovasc Dis 2018;27:404–417. [DOI] [PubMed] [Google Scholar]
- 24. European Environment Agency . Environmental noise in Europe—2020. https://www.eea.europa.eu/publications/environmental-noise-in-europe (13 October 2021, date last accessed).
- 25. Kempen EV, Casas M, Pershagen G, Foraster M. WHO environmental noise guidelines for the European region: a systematic review on environmental noise and cardiovascular and metabolic effects: a summary. Int J Environ Res Public Health 2018;15:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seidler A, Hegewald J, Seidler AL, Schubert M, Wagner M, Droge P, Haufe E, Schmitt J, Swart E, Zeeb H. Association between aircraft, road and railway traffic noise and depression in a large case-control study based on secondary data. Environ Res 2017;152:263–271. [DOI] [PubMed] [Google Scholar]
- 27. Heritier H, Vienneau D, Foraster M, Eze IC, Schaffner E, Thiesse L, Rudzik F, Habermacher M, Kopfli M, Pieren R, Brink M, Cajochen C, Wunderli JM, Probst-Hensch N, Roosli M; SNC study group . Transportation noise exposure and cardiovascular mortality: a nationwide cohort study from Switzerland. Eur J Epidemiol 2017;32:307–315. [DOI] [PubMed] [Google Scholar]
- 28. Yankoty LI, Gamache P, Plante C, Goudreau S, Blais C, Perron S, Fournier M, Ragettli MS, Fallah-Shorshani M, Hatzopoulou M, Liu Y, Smargiassi A. Manuscript title: long horizontal line term residential exposure to environmental/transportation noise and the incidence of myocardial infarction. Int J Hyg Environ Health 2021;232:113666. [DOI] [PubMed] [Google Scholar]
- 29. Fuks KB, Weinmayr G, Basagana X, Gruzieva O, Hampel R, Oftedal B, Sorensen M, Wolf K, Aamodt G, Aasvang GM, Aguilera I, Becker T, Beelen R, Brunekreef B, Caracciolo B, Cyrys J, Elosua R, Eriksen KT, Foraster M, Fratiglioni L, Hilding A, Houthuijs D, Korek M, Kunzli N, Marrugat J, Nieuwenhuijsen M, Ostenson CG, Penell J, Pershagen G, Raaschou-Nielsen O, Swart WJR, Peters A, Hoffmann B. Long-term exposure to ambient air pollution and traffic noise and incident hypertension in seven cohorts of the European study of cohorts for air pollution effects (ESCAPE). Eur Heart J 2017;38:983–990. [DOI] [PubMed] [Google Scholar]
- 30. Zeeb H, Hegewald J, Schubert M, Wagner M, Droge P, Swart E, Seidler A. Traffic noise and hypertension - results from a large case-control study. Environ Res 2017;157:110–117. [DOI] [PubMed] [Google Scholar]
- 31. Pyko A, Lind T, Mitkovskaya N, Ogren M, Ostenson CG, Wallas A, Pershagen G, Eriksson C. Transportation noise and incidence of hypertension. Int J Hyg Environ Health 2018;221:1133–1141. [DOI] [PubMed] [Google Scholar]
- 32. Seidler AL, Hegewald J, Schubert M, Weihofen VM, Wagner M, Droge P, Swart E, Zeeb H, Seidler A. The effect of aircraft, road, and railway traffic noise on stroke—results of a case-control study based on secondary data. Noise Health 2018;20:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Halonen JI, Hansell AL, Gulliver J, Morley D, Blangiardo M, Fecht D, Toledano MB, Beevers SD, Anderson HR, Kelly FJ, Tonne C. Road traffic noise is associated with increased cardiovascular morbidity and mortality and all-cause mortality in London. Eur Heart J 2015;36:2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sorensen M, Poulsen AH, Hvidtfeldt UA, Munzel T, Thacher JD, Ketzel M, Brandt J, Christensen JH, Levin G, Raaschou-Nielsen O. Transportation noise and risk of stroke: a nationwide prospective cohort study covering Denmark. Int J Epidemiol 2021;50:1147–1156. [DOI] [PubMed] [Google Scholar]
- 35. Pyko A, Andersson N, Eriksson C, de Faire U, Lind T, Mitkovskaya N, Ogren M, Ostenson CG, Pedersen NL, Rizzuto D, Wallas AK, Pershagen G. Long-term transportation noise exposure and incidence of ischaemic heart disease and stroke: a cohort study. Occup Environ Med 2019;76:201–207. [DOI] [PubMed] [Google Scholar]
- 36. Cai Y, Hodgson S, Blangiardo M, Gulliver J, Morley D, Fecht D, Vienneau D, de Hoogh K, Key T, Hveem K, Elliott P, Hansell AL. Road traffic noise, air pollution and incident cardiovascular disease: a joint analysis of the HUNT, EPIC-Oxford and UK Biobank cohorts. Environ Int 2018;114:191–201. [DOI] [PubMed] [Google Scholar]
- 37. Sorensen M, Wendelboe Nielsen O, Sajadieh A, Ketzel M, Tjonneland A, Overvad K, Raaschou-Nielsen O. Long-term exposure to road traffic noise and nitrogen dioxide and risk of heart failure: a cohort study. Environ Health Perspect 2017;125:097021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carey IM, Anderson HR, Atkinson RW, Beevers S, Cook DG, Dajnak D, Gulliver J, Kelly FJ. Traffic pollution and the incidence of cardiorespiratory outcomes in an adult cohort in London. Occup Environ Med 2016;73:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bai L, Shin S, Oiamo TH, Burnett RT, Weichenthal S, Jerrett M, Kwong JC, Copes R, Kopp A, Chen H. Exposure to road traffic noise and incidence of acute myocardial infarction and congestive heart failure: a population-based cohort study in Toronto, Canada. Environ Health Perspect 2020;128:87001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Monrad M, Sajadieh A, Christensen JS, Ketzel M, Raaschou-Nielsen O, Tjønneland A, Overvad K, Loft S, Sørensen M. Residential exposure to traffic noise and risk of incident atrial fibrillation: a cohort study. Environ Int 2016;92-93:457–463. [DOI] [PubMed] [Google Scholar]
- 41. Cai Y, Ramakrishnan R, Rahimi K. Long-term exposure to traffic noise and mortality: a systematic review and meta-analysis of epidemiological evidence between 2000 and 2020. Environ Pollut 2021;269:116222. [DOI] [PubMed] [Google Scholar]
- 42. Saucy A, Schaffer B, Tangermann L, Vienneau D, Wunderli JM, Roosli M. Does night-time aircraft noise trigger mortality? A case-crossover study on 24 886 cardiovascular deaths. Eur Heart J 2021;42:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Basner M, Babisch W, Davis A, Brink M, Clark C, Janssen S, Stansfeld S. Auditory and non-auditory effects of noise on health. Lancet 2014;383:1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmidt FP, Basner M, Kroger G, Weck S, Schnorbus B, Muttray A, Sariyar M, Binder H, Gori T, Warnholtz A, Munzel T. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J 2013;34:3508–3514a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Basner M, McGuire S. WHO environmental noise guidelines for the european region: a systematic review on environmental noise and effects on sleep. Int J Environ Res Public Health 2018;15:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vienneau D, Eze IC, Probst-Hensch N, Roosli M. Association between transportation noise and cardio-metabolic diseases: an update of the WHO meta-analysis. In Proceedings of the 23rd International Conference on Acoustics. 2019. pp. 1543–1550. https://edoc.unibas.ch/70857/1/ICA_2019_manuscript%20Vienneau%20final.pdf (13 October 2021, date last accessed)
- 47. Pyko A, Eriksson C, Lind T, Mitkovskaya N, Wallas A, Ogren M, Ostenson CG, Pershagen G. Long-term exposure to transportation noise in relation to development of obesity—a cohort study. Environ Health Perspect 2017;125:117005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Foraster M, Eze IC, Vienneau D, Schaffner E, Jeong A, Heritier H, Rudzik F, Thiesse L, Pieren R, Brink M, Cajochen C, Wunderli JM, Roosli M, Probst-Hensch N. Long-term exposure to transportation noise and its association with adiposity markers and development of obesity. Environ Int 2018;121:879–889. [DOI] [PubMed] [Google Scholar]
- 49. Christensen JS, Raaschou-Nielsen O, Tjønneland A, Nordsborg RB, Jensen SS, Sørensen TIA, Sørensen M. Long-term exposure to residential traffic noise and changes in body weight and waist circumference: a cohort study. Environ Res 2015;143:154–161. [DOI] [PubMed] [Google Scholar]
- 50. Cai Y, Zijlema WL, Sørgjerd EP, Doiron D, de Hoogh K, Hodgson S, Wolffenbuttel B, Gulliver J, Hansell AL, Nieuwenhuijsen M, Rahimi K, Kvaløy K. Impact of road traffic noise on obesity measures: observational study of three European cohorts. Environ Res 2020;191:110013. [DOI] [PubMed] [Google Scholar]
- 51. Foraster M, Eze IC, Vienneau D, Brink M, Cajochen C, Caviezel S, Heritier H, Schaffner E, Schindler C, Wanner M, Wunderli JM, Roosli M, Probst-Hensch N. Long-term transportation noise annoyance is associated with subsequent lower levels of physical activity. Environ Int 2016;91:341–349. [DOI] [PubMed] [Google Scholar]
- 52. Roswall N, Ammitzbøll G, Christensen JS, Raaschou-Nielsen O, Jensen SS, Tjønneland A, Sørensen M. Residential exposure to traffic noise and leisure-time sports—a population-based study. Int J Hyg Environ Health 2017;220:1006–1013. [DOI] [PubMed] [Google Scholar]
- 53. Roswall N, Christensen JS, Bidstrup PE, Raaschou-Nielsen O, Jensen SS, Tjønneland A, Sørensen M. Associations between residential traffic noise exposure and smoking habits and alcohol consumption—a population-based study. Environ Pollut 2018;236:983–991. [DOI] [PubMed] [Google Scholar]
- 54. Beutel ME, Brahler E, Ernst M, Klein E, Reiner I, Wiltink J, Michal M, Wild PS, Schulz A, Munzel T, Hahad O, Konig J, Lackner KJ, Pfeiffer N, Tibubos AN. Noise annoyance predicts symptoms of depression, anxiety and sleep disturbance 5 years later. Findings from the Gutenberg Health Study. Eur J Public Health 2020;30:516–521. [DOI] [PubMed] [Google Scholar]
- 55. Orban E, McDonald K, Sutcliffe R, Hoffmann B, Fuks KB, Dragano N, Viehmann A, Erbel R, Jockel KH, Pundt N, Moebus S. Residential road traffic noise and high depressive symptoms after five years of follow-up: results from the Heinz Nixdorf Recall Study. Environ Health Perspect 2016;124:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Clark C, Crumpler C, Notley AH. Evidence for environmental noise effects on health for the United Kingdom policy context: a systematic review of the effects of environmental noise on mental health, wellbeing, quality of life, cancer, dementia, birth, reproductive outcomes, and cognition. Int J Environ Res Public Health 2020;17:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Munzel T, Gori T, Babisch W, Basner M. Cardiovascular effects of environmental noise exposure. Eur Heart J 2014;35:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Campos-Rodriguez R, Godinez-Victoria M, Abarca-Rojano E, Pacheco-Yepez J, Reyna-Garfias H, Barbosa-Cabrera RE, Drago-Serrano ME. Stress modulates intestinal secretory immunoglobulin A. Front Integr Neurosci 2013;7:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Osborne MT, Radfar A, Hassan MZO, Abohashem S, Oberfeld B, Patrich T, Tung B, Wang Y, Ishai A, Scott JA, Shin LM, Fayad ZA, Koenen KC, Rajagopalan S, Pitman RK, Tawakol A. A neurobiological mechanism linking transportation noise to cardiovascular disease in humans. Eur Heart J 2020;41:772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Munzel T, Steven S, Hahad O, Daiber A. The sixth sense is involved in noise-induced stress responses and vascular inflammation: evidence for heightened amygdalar activity in response to transport noise in man. Eur Heart J 2020;41:783–785. [DOI] [PubMed] [Google Scholar]
- 61. Herzog J, Schmidt FP, Hahad O, Mahmoudpour SH, Mangold AK, Garcia Andreo P, Prochaska J, Koeck T, Wild PS, Sørensen M, Daiber A, Münzel T. Acute exposure to nocturnal train noise induces endothelial dysfunction and pro-thromboinflammatory changes of the plasma proteome in healthy subjects. Basic Res Cardiol 2019;114:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Babisch W. Updated exposure-response relationship between road traffic noise and coronary heart diseases: a meta-analysis. Noise Health 2014;16:1–9. [DOI] [PubMed] [Google Scholar]
- 63. Munzel T, Schmidt FP, Steven S, Herzog J, Daiber A, Sorensen M. Environmental noise and the cardiovascular system. J Am Coll Cardiol 2018;71:688–697. [DOI] [PubMed] [Google Scholar]
- 64. Babisch W. The noise/stress concept, risk assessment and research needs. Noise Health 2002;4:1–11. [PubMed] [Google Scholar]
- 65. Dar T, Osborne MT, Abohashem S, Abbasi T, Choi KW, Ghoneem A, Naddaf N, Smoller JW, Pitman RK, Denninger JW, Shin LM, Fricchione G, Tawakol A. Greater neurobiological resilience to chronic socioeconomic or environmental stressors associates with lower risk for cardiovascular disease events. Circ Cardiovasc Imaging 2020;13:e010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schmidt F, Kolle K, Kreuder K, Schnorbus B, Wild P, Hechtner M, Binder H, Gori T, Munzel T. Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clin Res Cardiol 2015;104:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schmidt FP, Herzog J, Schnorbus B, Ostad MA, Lasetzki L, Hahad O, Schafers G, Gori T, Sorensen M, Daiber A, Munzel T. The impact of aircraft noise on vascular and cardiac function in relation to noise event number: a randomized trial. Cardiovasc Res 2021;117:1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Seidler A, Wagner M, Schubert M, Droge P, Romer K, Pons-Kuhnemann J, Swart E, Zeeb H, Hegewald J. Aircraft, road and railway traffic noise as risk factors for heart failure and hypertensive heart disease—a case-control study based on secondary data. Int J Hyg Environ Health 2016;219:749–758. [DOI] [PubMed] [Google Scholar]
- 69. Kim A, Sung JH, Bang JH, Cho SW, Lee J, Sim CS. Effects of self-reported sensitivity and road-traffic noise levels on the immune system. PLoS One 2017;12:e0187084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cai Y, Hansell AL, Blangiardo M, Burton PR, de Hoogh K, Doiron D, Fortier I, Gulliver J, Hveem K, Mbatchou S, Morley DW, Stolk RP, Zijlema WL, Elliott P, Hodgson S. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the HUNT and lifelines cohorts. Eur Heart J 2017;38:2290–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Eze IC, Jeong A, Schaffner E, Rezwan FI, Ghantous A, Foraster M, Vienneau D, Kronenberg F, Herceg Z, Vineis P, Brink M, Wunderli JM, Schindler C, Cajochen C, Roosli M, Holloway JW, Imboden M, Probst-Hensch N. Genome-wide DNA methylation in peripheral blood and long-term exposure to source-specific transportation noise and air pollution: the SAPALDIA Study. Environ Health Perspect 2020;128:067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Foraster M, Eze IC, Schaffner E, Vienneau D, Héritier H, Endes S, Rudzik F, Thiesse L, Pieren R, Schindler C, Schmidt-Trucksäss A, Brink M, Cajochen C, Marc Wunderli J, Röösli M, Probst-Hensch N. Exposure to road, railway, and aircraft noise and arterial stiffness in the SAPALDIA study: annual average noise levels and temporal noise characteristics. Environ Health Perspect 2017;125:097004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kalsch H, Hennig F, Moebus S, Mohlenkamp S, Dragano N, Jakobs H, Memmesheimer M, Erbel R, Jockel KH, Hoffmann B; Heinz Nixdorf Recall Study Investigative Group . Are air pollution and traffic noise independently associated with atherosclerosis: the Heinz Nixdorf Recall Study. Eur Heart J 2014;35:853–860. [DOI] [PubMed] [Google Scholar]
- 74. Hennig F, Moebus S, Reinsch N, Budde T, Erbel R, Jockel KH, Lehmann N, Hoffmann B, Kalsch H; Heinz Nixdorf Recall Study Investigative Group . Investigation of air pollution and noise on progression of thoracic aortic calcification: results of the Heinz Nixdorf Recall Study. Eur J Prev Cardiol 2020;27:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Atanackovic D, Atanackovic D, Brunner-Weinzierl MC, Kröger H, Serke S, Deter HC. Acute psychological stress simultaneously alters hormone levels, recruitment of lymphocyte subsets, and production of reactive oxygen species. Immunol Invest 2002;31:73–91. [DOI] [PubMed] [Google Scholar]
- 76. Herbert TB, Cohen S, Marsland AL, Bachen EA, Rabin BS, Muldoon MF, Manuck SB. Cardiovascular reactivity and the course of immune response to an acute psychological stressor. Psychosom Med 1994;56:337–344. [DOI] [PubMed] [Google Scholar]
- 77. Peterson EA, Augenstein JS, Tanis DC, Augenstein DG. Noise raises blood pressure without impairing auditory sensitivity. Science 1981;211:1450–1452. [DOI] [PubMed] [Google Scholar]
- 78. Altura BM, Altura BT, Gebrewold A, Ising H, Gunther T. Noise-induced hypertension and magnesium in rats: relationship to microcirculation and calcium. J Appl Physiol (1985) 1992;72:194–202. [DOI] [PubMed] [Google Scholar]
- 79. Wu CC, Chen SJ, Yen MH. Effects of noise on blood pressure and vascular reactivities. Clin Exp Pharmacol Physiol 1992;19:833–838. [DOI] [PubMed] [Google Scholar]
- 80. Wu CC, Chen SJ, Yen MH. Attenuation of endothelium-dependent relaxation in mesenteric artery during noise-induced hypertension. J Biomed Sci 1994;1:49–53. [DOI] [PubMed] [Google Scholar]
- 81. Turner JG, Parrish JL, Hughes LF, Toth LA, Caspary DM. Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comp Med 2005;55:12–23. [PMC free article] [PubMed] [Google Scholar]
- 82. Münzel T, Daiber A, Steven S, Tran LP, Ullmann E, Kossmann S, Schmidt FP, Oelze M, Xia N, Li H, Pinto A, Wild P, Pies K, Schmidt ER, Rapp S, Kröller-Schön S. Effects of noise on vascular function, oxidative stress, and inflammation: mechanistic insight from studies in mice. Eur Heart J 2017;38:2838–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kröller-Schön S, Daiber A, Steven S, Oelze M, Frenis K, Kalinovic S, Heimann A, Schmidt FP, Pinto A, Kvandova M, Vujacic-Mirski K, Filippou K, Dudek M, Bosmann M, Klein M, Bopp T, Hahad O, Wild PS, Frauenknecht K, Methner A, Schmidt ER, Rapp S, Mollnau H, Münzel T. Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation. Eur Heart J 2018;39:3528–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Eckrich J, Frenis K, Rodriguez-Blanco G, Ruan Y, Jiang S, Bayo Jimenez MT, Kuntic M, Oelze M, Hahad O, Li H, Gericke A, Steven S, Strieth S, von Kriegsheim A, Münzel T, Ernst BP, Daiber A. Aircraft noise exposure drives the activation of white blood cells and induces microvascular dysfunction in mice. Redox Biol 2021;46:102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Frenis K, Helmstädter J, Ruan Y, Schramm E, Kalinovic S, Kröller-Schön S, Bayo Jimenez MT, Hahad O, Oelze M, Jiang S, Wenzel P, Sommer CJ, Frauenknecht KBM, Waisman A, Gericke A, Daiber A, Münzel T, Steven S. Ablation of lysozyme M-positive cells prevents aircraft noise-induced vascular damage without improving cerebral side effects. Basic Res Cardiol 2021;116:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bayo Jimenez MT, Frenis K, Kröller-Schön S, Kuntic M, Stamm P, Kvandová M, Oelze M, Li H, Steven S, Münzel T, Daiber A. Noise-induced vascular dysfunction, oxidative stress, and inflammation are improved by pharmacological modulation of the NRF2/HO-1 axis. Antioxidants (Basel) 2021;10:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 2001;88:E14–E22. [DOI] [PubMed] [Google Scholar]
- 88. Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 2002;90:E58–E65. [DOI] [PubMed] [Google Scholar]
- 89. Oelze M, Mollnau H, Hoffmann N, Warnholtz A, Bodenschatz M, Smolenski A, Walter U, Skatchkov M, Meinertz T, Munzel T. Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric Oxide/cGMP signaling and endothelial dysfunction. Circ Res 2000;87:999–1005. [DOI] [PubMed] [Google Scholar]
- 90. Steven S, Frenis K, Kalinovic S, Kvandova M, Oelze M, Helmstädter J, Hahad O, Filippou K, Kus K, Trevisan C, Schlüter K-D, Boengler K, Chlopicki S, Frauenknecht K, Schulz R, Sorensen M, Daiber A, Kröller-Schön S, Münzel T. Exacerbation of adverse cardiovascular effects of aircraft noise in an animal model of arterial hypertension. Redox Biol 2020;34:101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Smith KR, Jerrett M, Anderson HR, Burnett RT, Stone V, Derwent R, Atkinson RW, Cohen A, Shonkoff SB, Krewski D, Pope CA 3rd, Thun MJ, Thurston G. Public health benefits of strategies to reduce greenhouse-gas emissions: health implications of short-lived greenhouse pollutants. Lancet 2009;374:2091–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2054–2070. [DOI] [PubMed] [Google Scholar]
- 93. Lakey PS, Berkemeier T, Tong H, Arangio AM, Lucas K, Poschl U, Shiraiwa M. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci Rep 2016;6:32916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lelieveld J, Poschl U. Chemists can help to solve the air-pollution health crisis. Nature 2017;551:291–293. [DOI] [PubMed] [Google Scholar]
- 95. Daellenbach KR, Uzu G, Jiang J, Cassagnes L-E, Leni Z, Vlachou A, Stefenelli G, Canonaco F, Weber S, Segers A, Kuenen JJP, Schaap M, Favez O, Albinet A, Aksoyoglu S, Dommen J, Baltensperger U, Geiser M, El Haddad I, Jaffrezo J-L, Prévôt ASH. Sources of particulate-matter air pollution and its oxidative potential in Europe. Nature 2020;587:414–419. [DOI] [PubMed] [Google Scholar]
- 96. Poschl U, Shiraiwa M. Multiphase chemistry at the atmosphere-biosphere interface influencing climate and public health in the anthropocene. Chem Rev 2015;115:4440–4475. [DOI] [PubMed] [Google Scholar]
- 97. Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. Impact of regional climate change on human health. Nature 2005;438:310–317. [DOI] [PubMed] [Google Scholar]
- 98. Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA 3rd, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL, Forouzanfar MH. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017;389:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu S, Jorgensen JT, Ljungman P, Pershagen G, Bellander T, Leander K, Magnusson PKE, Rizzuto D, Hvidtfeldt UA, Raaschou-Nielsen O, Wolf K, Hoffmann B, Brunekreef B, Strak M, Chen J, Mehta A, Atkinson RW, Bauwelinck M, Varraso R, Boutron-Ruault MC, Brandt J, Cesaroni G, Forastiere F, Fecht D, Gulliver J, Hertel O, de Hoogh K, Janssen NAH, Katsouyanni K, Ketzel M, Klompmaker JO, Nagel G, Oftedal B, Peters A, Tjonneland A, Rodopoulou SP, Samoli E, Bekkevold T, Sigsgaard T, Stafoggia M, Vienneau D, Weinmayr G, Hoek G, Andersen ZJ. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: the ELAPSE project. Environ Int 2021;146:106267. [DOI] [PubMed] [Google Scholar]
- 100. Brauer M, Brook JR, Christidis T, Chu Y, Crouse DL, Erickson A, Hystad P, Li C, Martin RV, Meng J, Pappin AJ, Pinault LL, Tjepkema M, van Donkelaar A, Weichenthal S, Burnett RT. Mortality-air pollution associations in low-exposure environments (MAPLE): phase 1. Res Rep Health Eff Inst 2019;203:1–87. [PMC free article] [PubMed] [Google Scholar]
- 101. Cesaroni G, Forastiere F, Stafoggia M, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, de Faire U, Erbel R, Eriksen KT, Fratiglioni L, Galassi C, Hampel R, Heier M, Hennig F, Hilding A, Hoffmann B, Houthuijs D, Jockel K-H, Korek M, Lanki T, Leander K, Magnusson PKE, Migliore E, Ostenson C-G, Overvad K, Pedersen NL, J JP, Penell J, Pershagen G, Pyko A, Raaschou-Nielsen O, Ranzi A, Ricceri F, Sacerdote C, Salomaa V, Swart W, Turunen AW, Vineis P, Weinmayr G, Wolf K, de Hoogh K, Hoek G, Brunekreef B, Peters A. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 2014;348:f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hendryx M, Luo J. COVID-19 prevalence and fatality rates in association with air pollution emission concentrations and emission sources. Environ Pollut 2020;265:115126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Giani P, Castruccio S, Anav A, Howard D, Hu W, Crippa P. Short-term and long-term health impacts of air pollution reductions from COVID-19 lockdowns in China and Europe: a modelling study. Lancet Planet Health 2020;4:e474–e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wu J, Mamas MA, Mohamed MO, Kwok CS, Roebuck C, Humberstone B, Denwood T, Luescher T, de Belder MA, Deanfield JE, Gale CP. Place and causes of acute cardiovascular mortality during the COVID-19 pandemic. Heart 2021;107:113–119. [DOI] [PubMed] [Google Scholar]
- 105. Niccoli G, Luescher TF, Crea F. Decreased myocardial infarction admissions during COVID times: what can we learn? Cardiovasc Res 2020;116:e126–e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Xing YF, Xu YH, Shi MH, Lian YX. The impact of PM2.5 on the human respiratory system. J Thorac Dis 2016;8:E69–E74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Munzel T, Gori T, Al-Kindi S, Deanfield J, Lelieveld J, Daiber A, Rajagopalan S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur Heart J 2018;39:3543–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, Hoek G, Hoffmann B, Hoylaerts MF, Kunzli N, Mills N, Pekkanen J, Peters A, Piepoli MF, Rajagopalan S, Storey RF; ESC Working Group on Thrombosis, European Association for Cardiovascular Prevention and Rehabilitation and ESC Heart Failure Association . Expert position paper on air pollution and cardiovascular disease. Eur Heart J 2015;36:83–93b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Turner MC, Jerrett M, Pope CA 3rd, Krewski D, Gapstur SM, Diver WR, Beckerman BS, Marshall JD, Su J, Crouse DL, Burnett RT. Long-term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med 2016;193:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhang JJ, Wei Y, Fang Z. Ozone pollution: a major health hazard worldwide. Front Immunol 2019;10:2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lelieveld J, Pozzer A, Poschl U, Fnais M, Haines A, Munzel T. Loss of life expectancy from air pollution compared to other risk factors: a worldwide perspective. Cardiovasc Res 2020;116:1910–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Burnett R, Chen H, Szyszkowicz M, Fann N, Hubbell B, Pope CA 3rd, Apte JS, Brauer M, Cohen A, Weichenthal S, Coggins J, Di Q, Brunekreef B, Frostad J, Lim SS, Kan H, Walker KD, Thurston GD, Hayes RB, Lim CC, Turner MC, Jerrett M, Krewski D, Gapstur SM, Diver WR, Ostro B, Goldberg D, Crouse DL, Martin RV, Peters P, Pinault L, Tjepkema M, van Donkelaar A, Villeneuve PJ, Miller AB, Yin P, Zhou M, Wang L, Janssen NAH, Marra M, Atkinson RW, Tsang H, Quoc Thach T, Cannon JB, Allen RT, Hart JE, Laden F, Cesaroni G, Forastiere F, Weinmayr G, Jaensch A, Nagel G, Concin H, Spadaro JV. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc Natl Acad Sci USA 2018;115:9592–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lelieveld J, Klingmuller K, Pozzer A, Poschl U, Fnais M, Daiber A, Munzel T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J 2019;40:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Vohra K, Vodonos A, Schwartz J, Marais EA, Sulprizio MP, Mickley LJ. Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: results from GEOS-Chem. Environ Res 2021;195:110754. [DOI] [PubMed] [Google Scholar]
- 115. Munzel T, Hahad O, Kuntic M, Keaney JF, Deanfield JE, Daiber A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J 2020;41:4057–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Al-Kindi SG, Brook RD, Biswal S, Rajagopalan S. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol 2020;17:656–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook J, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J 2017;38:550–556. [DOI] [PubMed] [Google Scholar]
- 118. Bevan GH, Al-Kindi SG, Brook RD, Münzel T, Rajagopalan S. Ambient air pollution and atherosclerosis: insights into dose, time, and mechanisms. Arterioscler Thromb Vasc Biol 2021;41:628–637. [DOI] [PubMed] [Google Scholar]
- 119. Munzel T, Daiber A. The air pollution constituent particulate matter (PM2.5) destabilizes coronary artery plaques. Eur Heart J Cardiovasc Imaging 2019;20:1365–1367. [DOI] [PubMed] [Google Scholar]
- 120. Yang S, Lee SP, Park JB, Lee H, Kang SH, Lee SE, Kim JB, Choi SY, Kim YJ, Chang HJ. PM2.5 concentration in the ambient air is a risk factor for the development of high-risk coronary plaques. Eur Heart J Cardiovasc Imaging 2019;20:1355–1364. [DOI] [PubMed] [Google Scholar]
- 121. Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 2007;356:447–458. [DOI] [PubMed] [Google Scholar]
- 122. Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Perier MC, Marijon E, Vernerey D, Empana JP, Jouven X. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA 2012;307:713–721. [DOI] [PubMed] [Google Scholar]
- 123. Pope CA, Muhlestein JB, Anderson JL, Cannon JB, Hales NM, Meredith KG, Le V, Horne BD. Short-term exposure to fine particulate matter air pollution is preferentially associated with the risk of ST-segment elevation acute coronary events. J Am Heart Assoc 2015;4:e002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen H, Burnett RT, Copes R, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Brook JR, Kopp A, Tu JV. Ambient fine particulate matter and mortality among survivors of myocardial infarction: population-based cohort study. Environ Health Perspect 2016;124:1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tonne C, Wilkinson P. Long-term exposure to air pollution is associated with survival following acute coronary syndrome. Eur Heart J 2013;34:1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Alexeeff SE, Liao NS, Liu X, Van Den Eeden SK, Sidney S. Long-term PM2.5 exposure and risks of ischemic heart disease and stroke events: review and meta-analysis. J Am Heart Assoc 2021;10:e016890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail 2020;22:1342–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG. Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology 2013;24:44–53. [DOI] [PubMed] [Google Scholar]
- 129. Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, Newby DE, Mills NL. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet 2013;382:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. To T, Shen S, Atenafu EG, Guan J, McLimont S, Stocks B, Licskai C. The air quality health index and asthma morbidity: a population-based study. Environ Health Perspect 2013;121:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Liu H, Tian Y, Song J, Cao Y, Xiang X, Huang C, Li M, Hu Y. Effect of ambient air pollution on hospitalization for heart failure in 26 of China's largest cities. Am J Cardiol 2018;121:628–633. [DOI] [PubMed] [Google Scholar]
- 132. Wang M, Zhou T, Song Y, Li X, Ma H, Hu Y, Heianza Y, Qi L. Joint exposure to various ambient air pollutants and incident heart failure: a prospective analysis in UK Biobank. Eur Heart J 2021;42:1582–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Watkins A, Danilewitz M, Kusha M, Masse S, Urch B, Quadros K, Spears D, Farid T, Nanthakumar K. Air pollution and arrhythmic risk: the smog is yet to clear. Can J Cardiol 2013;29:734–741. [DOI] [PubMed] [Google Scholar]
- 134. Anderson HR, Armstrong B, Hajat S, Harrison R, Monk V, Poloniecki J, Timmis A, Wilkinson P. Air pollution and activation of implantable cardioverter defibrillators in London. Epidemiology 2010;21:405–413. [DOI] [PubMed] [Google Scholar]
- 135. Raza A, Bellander T, Bero-Bedada G, Dahlquist M, Hollenberg J, Jonsson M, Lind T, Rosenqvist M, Svensson L, Ljungman PLS. Short-term effects of air pollution on out-of-hospital cardiac arrest in Stockholm. Eur Heart J 2014;35:861–868. [DOI] [PubMed] [Google Scholar]
- 136. Teng T-HK, Williams TA, Bremner A, Tohira H, Franklin P, Tonkin A, Jacobs I, Finn J. A systematic review of air pollution and incidence of out-of-hospital cardiac arrest. J Epidemiol Community Health 2014;68:37–43. [DOI] [PubMed] [Google Scholar]
- 137. Hong YC, Lee JT, Kim H, Ha EH, Schwartz J, Christiani DC. Effects of air pollutants on acute stroke mortality. Environ Health Perspect 2002;110:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Low RB, Bielory L, Qureshi AI, Dunn V, Stuhlmiller DF, Dickey DA. The relation of stroke admissions to recent weather, airborne allergens, air pollution, seasons, upper respiratory infections, and asthma incidence, September 11, 2001, and day of the week. Stroke 2006;37:951–957. [DOI] [PubMed] [Google Scholar]
- 139. Andersen ZJ, Olsen TS, Andersen KK, Loft S, Ketzel M, Raaschou-Nielsen O. Association between short-term exposure to ultrafine particles and hospital admissions for stroke in Copenhagen, Denmark. Eur Heart J 2010;31:2034–2040. [DOI] [PubMed] [Google Scholar]
- 140. Zhang P, Dong G, Sun B, Zhang L, Chen X, Ma N, Yu F, Guo H, Huang H, Lee YL, Tang N, Chen J. Long-term exposure to ambient air pollution and mortality due to cardiovascular disease and cerebrovascular disease in Shenyang, China. PLoS One 2011;6:e20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Stafoggia M, Cesaroni G, Peters A, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, Cyrys J, de Faire U, de Hoogh K, Eriksen KT, Fratiglioni L, Galassi C, Gigante B, Havulinna AS, Hennig F, Hilding A, Hoek G, Hoffmann B, Houthuijs D, Korek M, Lanki T, Leander K, Magnusson PKE, Meisinger C, Migliore E, Overvad K, Ostenson C-G, Pedersen NL, Pekkanen J, Penell J, Pershagen G, Pundt N, Pyko A, Raaschou-Nielsen O, Ranzi A, Ricceri R, Sacerdote C, Swart W, Turunen A, Vineis P, Weimar C, Weinmayr G, Wolf K, Brunekreef B, Forastiere F. Long-term exposure to ambient air pollution and incidence of cerebrovascular 1 events—results from 11 european cohorts within the ESCAPE project. Environ Health Perspect 2014;122:919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, Langrish JP, Newby DE, Mills NL. Short term exposure to air pollution and stroke: systematic review and meta-analysis. BMJ 2015;350:h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kulick ER, Wellenius GA, Boehme AK, Sacco RL, Elkind MS. Residential proximity to major roadways and risk of incident ischemic stroke in NOMAS (The Northern Manhattan Study). Stroke 2018;49:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wing JJ, Sanchez BN, Adar SD, Meurer WJ, Morgenstern LB, Smith MA, Lisabeth LD. Synergism of short-term air pollution exposures and neighborhood disadvantage on initial stroke severity. Stroke 2017;48:3126–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Scheers H, Jacobs L, Casas L, Nemery B, Nawrot TS. Long-term exposure to particulate matter air pollution is a risk factor for stroke: meta-analytical evidence. Stroke 2015;46:3058–3066. [DOI] [PubMed] [Google Scholar]
- 146. Orellano P, Reynoso J, Quaranta N, Bardach A, Ciapponi A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: systematic review and meta-analysis. Environ Int 2020;142:105876. [DOI] [PubMed] [Google Scholar]
- 147. Meng X, Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Milojevic A, Guo Y, Tong S, Coelho M, Saldiva PHN, Lavigne E, Correa PM, Ortega NV, Osorio S, Garcia Kysely J, Urban A, Orru H, Maasikmets M, Jaakkola JJK, Ryti N, Huber V, Schneider A, Katsouyanni K, Analitis A, Hashizume M, Honda Y, Ng CFS, Nunes B, Teixeira JP, Holobaca IH, Fratianni S, Kim H, Tobias A, Iniguez C, Forsberg B, Astrom C, Ragettli MS, Guo YL, Pan SC, Li S, Bell ML, Zanobetti A, Schwartz J, Wu T, Gasparrini A, Kan H. Short term associations of ambient nitrogen dioxide with daily total, cardiovascular, and respiratory mortality: multilocation analysis in 398 cities. BMJ 2021;372:n534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism . Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 149. Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD. Long-term air pollution exposure and cardiorespiratory mortality: a review. Environ Health 2013;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Huang S, Li H, Wang M, Qian Y, Steenland K, Caudle WM, Liu Y, Sarnat J, Papatheodorou S, Shi L. Long-term exposure to nitrogen dioxide and mortality: a systematic review and meta-analysis. Sci Total Environ 2021;776:145968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Fischer P, Nieuwenhuijsen M, Vineis P, Xun WW, Katsouyanni K, Dimakopoulou K, Oudin A, Forsberg B, Modig L, Havulinna AS, Lanki T, Turunen A, Oftedal B, Nystad W, Nafstad P, De Faire U, Pedersen NL, Ostenson CG, Fratiglioni L, Penell J, Korek M, Pershagen G, Eriksen KT, Overvad K, Ellermann T, Eeftens M, Peeters PH, Meliefste K, Wang M, Bueno-de-Mesquita B, Sugiri D, Kramer U, Heinrich J, de Hoogh K, Key T, Peters A, Hampel R, Concin H, Nagel G, Ineichen A, Schaffner E, Probst-Hensch N, Kunzli N, Schindler C, Schikowski T, Adam M, Phuleria H, Vilier A, Clavel-Chapelon F, Declercq C, Grioni S, Krogh V, Tsai MY, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Tamayo I, Amiano P, Dorronsoro M, Katsoulis M, Trichopoulou A, Brunekreef B, Hoek G. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 2014;383:785–795. [DOI] [PubMed] [Google Scholar]
- 152. Pascal M, Corso M, Chanel O, Declercq C, Badaloni C, Cesaroni G, Henschel S, Meister K, Haluza D, Martin-Olmedo P, Medina S, Aphekom G; Aphekom group . Assessing the public health impacts of urban air pollution in 25 European cities: results of the Aphekom project. Sci Total Environ 2013;449:390–400. [DOI] [PubMed] [Google Scholar]
- 153. Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J 2017;38:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Barregard L, Sallsten G, Gustafson P, Andersson L, Johansson L, Basu S, Stigendal L. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal Toxicol 2006;18:845–853. [DOI] [PubMed] [Google Scholar]
- 155. Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Soderberg S, Newby DE, Sandstrom T, Blomberg A. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med 2007;176:395–400. [DOI] [PubMed] [Google Scholar]
- 156. Peretz A, Peck EC, Bammler TK, Beyer RP, Sullivan JH, Trenga CA, Srinouanprachnah S, Farin FM, Kaufman JD. Diesel exhaust inhalation and assessment of peripheral blood mononuclear cell gene transcription effects: an exploratory study of healthy human volunteers. Inhal Toxicol 2007;19:1107–1119. [DOI] [PubMed] [Google Scholar]
- 157. Hiraiwa K, van Eeden SF. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators Inflamm 2013;2013:619523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Goto Y, Ishii H, Hogg JC, Shih CH, Yatera K, Vincent R, van Eeden SF. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am J Respir Crit Care Med 2004;170:891–897. [DOI] [PubMed] [Google Scholar]
- 159. Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, Kherada N, Brook RD, Reddy KM, Padture NP, Parthasarathy S, Chen LC, Moffatt-Bruce S, Sun Q, Morawietz H, Rajagopalan S. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res 2011;108:716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Deiuliis JA, Kampfrath T, Zhong J, Oghumu S, Maiseyeu A, Chen LC, Sun Q, Satoskar AR, Rajagopalan S. Pulmonary T cell activation in response to chronic particulate air pollution. Am J Physiol Lung Cell Mol Physiol 2012;302:L399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes 2012;61:3037–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Liu C, Ying Z, Harkema J, Sun Q, Rajagopalan S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol Pathol 2013;41:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Simon SA, Liedtke W. How irritating: the role of TRPA1 in sensing cigarette smoke and aerogenic oxidants in the airways. J Clin Invest 2008;118:2383–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, Zhao J, Liu D, Morishita M, Sun Q, Spino C, Brook RD, Harkema JR, Rajagopalan S. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect 2014;122:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Munzel T, Hahad O, Daiber A. The dark side of nocturnal light pollution. Outdoor light at night increases risk of coronary heart disease. Eur Heart J 2021;42:831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Dominici F, Peng RD, Ebisu K, Zeger SL, Samet JM, Bell ML. Does the effect of PM10 on mortality depend on PM nickel and vanadium content? A reanalysis of the NMMAPS data. Environ Health Perspect 2007;115:1701–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Liberda EN, Cuevas AK, Gillespie PA, Grunig G, Qu Q, Chen LC. Exposure to inhaled nickel nanoparticles causes a reduction in number and function of bone marrow endothelial progenitor cells. Inhal Toxicol 2010;22(Suppl 2):95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Campen MJ, Lund A, Rosenfeld M. Mechanisms linking traffic-related air pollution and atherosclerosis. Curr Opin Pulm Med 2012;18:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Rao X, Zhong J, Maiseyeu A, Gopalakrishnan B, Villamena FA, Chen LC, Harkema JR, Sun Q, Rajagopalan S. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ Res 2014;115:770–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 2007;117:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ying Z, Kampfrath T, Thurston G, Farrar B, Lippmann M, Wang A, Sun Q, Chen LC, Rajagopalan S. Ambient particulates alter vascular function through induction of reactive oxygen and nitrogen species. Toxicol Sci 2009;111:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Campen MJ, Babu NS, Helms GA, Pett S, Wernly J, Mehran R, McDonald JD. Nonparticulate components of diesel exhaust promote constriction in coronary arteries from ApoE-/- mice. Toxicol Sci 2005;88:95–102. [DOI] [PubMed] [Google Scholar]
- 173. Knuckles TL, Lund AK, Lucas SN, Campen MJ. Diesel exhaust exposure enhances venoconstriction via uncoupling of eNOS. Toxicol Appl Pharmacol 2008;230:346–351. [DOI] [PubMed] [Google Scholar]
- 174. Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang J-SS, Zweier JL, Chen LC, Rajagopalan S, Sun Yue P, Ying Z, Cardounal AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC, Rajagopalan SQ. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol 2008;28:1760–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ying Z, Yue P, Xu X, Zhong M, Sun Q, Mikolaj M, Wang A, Brook RD, Chen LC, Rajagopalan S. Air pollution and cardiac remodeling: a role for RhoA/Rho-kinase. Am J Physiol Heart Circ Physiol 2009;296:H1540–H1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Palanivel R, Vinayachandran V, Biswal S, Deiuliis JA, Padmanabhan R, Park B, Gangwar RS, Durieux JC, Ebreo Cara EA, Das L, Bevan G, Fayad ZA, Tawakol A, Jain MK, Rao S, Rajagopalan S. Exposure to air pollution disrupts circadian rhythm through alterations in chromatin dynamics. iScience 2020;23:101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Wang Y, Li R, Chen R, Gu W, Zhang L, Gu J, Wang Z, Liu Y, Sun Q, Zhang K, Liu C. Ambient fine particulate matter exposure perturbed circadian rhythm and oscillations of lipid metabolism in adipose tissues. Chemosphere 2020;251:126392. [DOI] [PubMed] [Google Scholar]
- 178. Crnko S, Du Pre BC, Sluijter JPG, Van Laake LW. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol 2019;16:437–447. [DOI] [PubMed] [Google Scholar]
- 179. Peretz A, Sullivan JH, Leotta DF, Trenga CA, Sands FN, Allen J, Carlsten C, Wilkinson CW, Gill EA, Kaufman JD. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect 2008;116:937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Mills NL, TöRnqvist HKAN, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 2005;112:3930–3936. [DOI] [PubMed] [Google Scholar]
- 181. Shah AP, Pietropaoli AP, Frasier LM, Speers DM, Chalupa DC, Delehanty JM, Huang LS, Utell MJ, Frampton MW. Effect of inhaled carbon ultrafine particles on reactive hyperemia in healthy human subjects. Environ Health Perspect 2008;116:375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, Boon NA, Donaldson K, Sandstrom T, Blomberg A, Newby DE. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med 2007;357:1075–1082. [DOI] [PubMed] [Google Scholar]
- 183. Lund AK, Lucero J, Lucas S, Madden MC, McDonald JD, Seagrave JC, Knuckles TL, Campen MJ. Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1-mediated pathways. Arterioscler Thromb Vasc Biol 2009;29:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wold LE, Ying Z, Hutchinson KR, Velten M, Gorr MW, Velten C, Youtz DJ, Wang A, Lucchesi PA, Sun Q, Rajagopalan S. Cardiovascular remodeling in response to long-term exposure to fine particulate matter air pollution. Circ Heart Fail 2012;5:452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Yin F, Lawal A, Ricks J, Fox JR, Larson T, Navab M, Fogelman AM, Rosenfeld ME, Araujo JA. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler Thromb Vasc Biol 2013;33:1153–1161. [DOI] [PubMed] [Google Scholar]
- 186. Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 2005;294:3003–3010. [DOI] [PubMed] [Google Scholar]
- 187. Campen MJ, Lund AK, Knuckles TL, Conklin DJ, Bishop B, Young D, Seilkop S, Seagrave J, Reed MD, McDonald JD. Inhaled diesel emissions alter atherosclerotic plaque composition in ApoE(-/-) mice. Toxicol Appl Pharmacol 2010;242:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Lund AK, Lucero J, Harman M, Madden MC, McDonald JD, Seagrave JC, Campen MJ. The oxidized low-density lipoprotein receptor mediates vascular effects of inhaled vehicle emissions. Am J Respir Crit Care Med 2011;184:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Nemmar A, Hoet PH, Dinsdale D, Vermylen J, Hoylaerts MF, Nemery B. Diesel exhaust particles in lung acutely enhance experimental peripheral thrombosis. Circulation 2003;107:1202–1208. [DOI] [PubMed] [Google Scholar]
- 190. Chepesiuk R. Missing the dark: health effects of light pollution. Environ Health Perspect 2009;117:A20–A27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Falchi F, Cinzano P, Duriscoe D, Kyba CC, Elvidge CD, Baugh K, Portnov BA, Rybnikova NA, Furgoni R. The new world atlas of artificial night sky brightness. Sci Adv 2016;2:e1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Falchi F, Furgoni R, Gallaway TA, Rybnikova NA, Portnov BA, Baugh K, Cinzano P, Elvidge CD. Light pollution in USA and Europe: the good, the bad and the ugly. J Environ Manage 2019;248:109227. [DOI] [PubMed] [Google Scholar]
- 193. Steffens S, Winter C, Schloss MJ, Hidalgo A, Weber C, Soehnlein O. Circadian control of inflammatory processes in atherosclerosis and its complications. Arterioscler Thromb Vasc Biol 2017;37:1022–1028. [DOI] [PubMed] [Google Scholar]
- 194. Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 2007;6:414–421. [DOI] [PubMed] [Google Scholar]
- 195. Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010;466:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005;308:1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol 2019;15:75–89. [DOI] [PubMed] [Google Scholar]
- 198. Merikanto I, Lahti T, Puolijoki H, Vanhala M, Peltonen M, Laatikainen T, Vartiainen E, Salomaa V, Kronholm E, Partonen T. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int 2013;30:470–477. [DOI] [PubMed] [Google Scholar]
- 199. Hu Y, Shmygelska A, Tran D, Eriksson N, Tung JY, Hinds DA. GWAS of 89,283 individuals identifies genetic variants associated with self-reporting of being a morning person. Nat Commun 2016;7:10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Lane JM, Vlasac I, Anderson SG, Kyle SD, Dixon WG, Bechtold DA, Gill S, Little MA, Luik A, Loudon A, Emsley R, Scheer FA, Lawlor DA, Redline S, Ray DW, Rutter MK, Saxena R. Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nat Commun 2016;7:10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Obayashi K, Saeki K, Iwamoto J, Ikada Y, Kurumatani N. Association between light exposure at night and nighttime blood pressure in the elderly independent of nocturnal urinary melatonin excretion. Chronobiol Int 2014;31:779–786. [DOI] [PubMed] [Google Scholar]
- 202. Sun S, Cao W, Ge Y, Ran J, Sun F, Zeng Q, Guo M, Huang J, Lee RS, Tian L, Wellenius GA. Outdoor light at night and risk of coronary heart disease among older adults: a prospective cohort study. Eur Heart J 2021;42:822–830. [DOI] [PubMed] [Google Scholar]
- 203. Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015;525:367–371. [DOI] [PubMed] [Google Scholar]
- 204. Chen YS, Yang CY. Effects of Asian dust storm events on daily hospital admissions for cardiovascular disease in Taipei, Taiwan. J Toxicol Environ Health A 2005;68:1457–1464. [DOI] [PubMed] [Google Scholar]
- 205. Matsukawa R, Michikawa T, Ueda K, Nitta H, Kawasaki T, Tashiro H, Mohri M, Yamamoto Y. Desert dust is a risk factor for the incidence of acute myocardial infarction in Western Japan. Circ Cardiovasc Qual Outcomes 2014;7:743–748. [DOI] [PubMed] [Google Scholar]
- 206. Hashizume M, Kim Y, Ng CFS, Chung Y, Madaniyazi L, Bell ML, Guo YL, Kan H, Honda Y, Yi SM, Kim H, Nishiwaki Y. Health effects of asian dust: a systematic review and meta-analysis. Environ Health Perspect 2020;128:66001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Ueda K, Shimizu A, Nitta H, Inoue K. Long-range transported Asian Dust and emergency ambulance dispatches. Inhal Toxicol 2012;24:858–867. [DOI] [PubMed] [Google Scholar]
- 208. Cleland SE, Serre ML, Rappold AG, West JJ. Estimating the acute health impacts of fire-originated PM2.5 exposure during the 2017 California Wildfires: sensitivity to choices of inputs. Geohealth 2021;5:e2021GH000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Ye T, Guo Y, Chen G, Yue X, Xu R, Coelho M, Saldiva PHN, Zhao Q, Li S. Risk and burden of hospital admissions associated with wildfire-related PM2.5 in Brazil, 2000-15: a nationwide time-series study. Lancet Planet Health 2021;5:e599–e607. [DOI] [PubMed] [Google Scholar]
- 210. Nguyen HD, Azzi M, White S, Salter D, Trieu T, Morgan G, Rahman M, Watt S, Riley M, Chang LT, Barthelemy X, Fuchs D, Lieschke K, Nguyen H. The Summer 2019-2020 Wildfires in East Coast Australia and their impacts on air quality and health in New South Wales, Australia. Int J Environ Res Public Health 2021;18:3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Faustini A, Alessandrini ER, Pey J, Perez N, Samoli E, Querol X, Cadum E, Perrino C, Ostro B, Ranzi A, Sunyer J, Stafoggia M, Forastiere F; MED-PARTICLES study group . Short-term effects of particulate matter on mortality during forest fires in Southern Europe: results of the MED-PARTICLES Project. Occup Environ Med 2015;72:323–329. [DOI] [PubMed] [Google Scholar]
- 212. Chen G, Guo Y, Yue X, Tong S, Gasparrini A, Bell ML, Armstrong B, Schwartz J, Jaakkola JJK, Zanobetti A, Lavigne E, Nascimento Saldiva PH, Kan H, Roye D, Milojevic A, Overcenco A, Urban A, Schneider A, Entezari A, Vicedo-Cabrera AM, Zeka A, Tobias A, Nunes B, Alahmad B, Forsberg B, Pan SC, Iniguez C, Ameling C, De la Cruz Valencia C, Astrom C, Houthuijs D, Van Dung D, Samoli E, Mayvaneh F, Sera F, Carrasco-Escobar G, Lei Y, Orru H, Kim H, Holobaca IH, Kysely J, Teixeira JP, Madureira J, Katsouyanni K, Hurtado-Diaz M, Maasikmets M, Ragettli MS, Hashizume M, Stafoggia M, Pascal M, Scortichini M, de Sousa Zanotti Stagliorio Coelho M, Valdes Ortega N, Ryti NRI, Scovronick N, Matus P, Goodman P, Garland RM, Abrutzky R, Garcia SO, Rao S, Fratianni S, Dang TN, Colistro V, Huber V, Lee W, Seposo X, Honda Y, Guo YL, Ye T, Yu W, Abramson MJ, Samet JM, Li S. Mortality risk attributable to wildfire-related PM2.5 pollution: a global time series study in 749 locations. Lancet Planet Health 2021;5:e579–e587. [DOI] [PubMed] [Google Scholar]
- 213. Verma V, Polidori A, Schauer JJ, Shafer MM, Cassee FR, Sioutas C. Physicochemical and toxicological profiles of particulate matter in Los Angeles during the October 2007 southern California wildfires. Environ Sci Technol 2009;43:954–960. [DOI] [PubMed] [Google Scholar]
- 214. Reid CE, Brauer M, Johnston FH, Jerrett M, Balmes JR, Elliott CT. Critical review of health impacts of wildfire smoke exposure. Environ Health Perspect 2016;124:1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Shaposhnikov D, Revich B, Bellander T, Bedada GB, Bottai M, Kharkova T, Kvasha E, Lezina E, Lind T, Semutnikova E, Pershagen G. Mortality related to air pollution with the moscow heat wave and wildfire of 2010. Epidemiology 2014;25:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Lavigne E, Burnett RT, Weichenthal S. Association of short-term exposure to fine particulate air pollution and mortality: effect modification by oxidant gases. Sci Rep 2018;8:16097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Pichler P-P, Jaccard IS, Weisz U, Weisz H. International comparison of health care carbon footprints. Environ Res Lett 2019;14:1–8.35340667 [Google Scholar]
- 218. Watts N, Adger WN, Agnolucci P, Blackstock J, Byass P, Cai W, Chaytor S, Colbourn T, Collins M, Cooper A, Cox PM, Depledge J, Drummond P, Ekins P, Galaz V, Grace D, Graham H, Grubb M, Haines A, Hamilton I, Hunter A, Jiang X, Li M, Kelman I, Liang L, Lott M, Lowe R, Luo Y, Mace G, Maslin M, Nilsson M, Oreszczyn T, Pye S, Quinn T, Svensdotter M, Venevsky S, Warner K, Xu B, Yang J, Yin Y, Yu C, Zhang Q, Gong P, Montgomery H, Costello A. Health and climate change: policy responses to protect public health. Lancet 2015;386:1861–1914. [DOI] [PubMed] [Google Scholar]
- 219. Basu R. High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ Health 2009;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Turner LR, Barnett AG, Connell D, Tong S. Ambient temperature and cardiorespiratory morbidity: a systematic review and meta-analysis. Epidemiology 2012;23:594–606. [DOI] [PubMed] [Google Scholar]
- 221. Peters A, Schneider A. Cardiovascular risks of climate change. Nat Rev Cardiol 2021;18:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Short term effects of temperature on risk of myocardial infarction in England and Wales: time series regression analysis of the Myocardial Ischaemia National Audit Project (MINAP) registry. BMJ 2010;341:c3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Danet S, Richard F, Montaye M, Beauchant S, Lemaire B, Graux C, Cottel D, Marécaux N, Amouyel P. Unhealthy effects of atmospheric temperature and pressure on the occurrence of myocardial infarction and coronary deaths. A 10-year survey: the Lille-World Health Organization MONICA project (Monitoring trends and determinants in cardiovascular disease). Circulation 1999;100:E1–E7. [DOI] [PubMed] [Google Scholar]
- 224. Wichmann J, Ketzel M, Ellermann T, Loft S. Apparent temperature and acute myocardial infarction hospital admissions in Copenhagen, Denmark: a case-crossover study. Environ Health 2012;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Bhaskaran K, Armstrong B, Hajat S, Haines A, Wilkinson P, Smeeth L. Heat and risk of myocardial infarction: hourly level case-crossover analysis of MINAP database. BMJ 2012;345:e8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Chen K, Breitner S, Wolf K, Hampel R, Meisinger C, Heier M, von Scheidt W, Kuch B, Peters A, Schneider A; KORA Study Group . Temporal variations in the triggering of myocardial infarction by air temperature in Augsburg, Germany, 1987-2014. Eur Heart J 2019;40:1600–1608. [DOI] [PubMed] [Google Scholar]
- 227. Schneider A, Ruckerl R, Breitner S, Wolf K, Peters A. Thermal control, weather, and aging. Curr Environ Health Rep 2017;4:21–29. [DOI] [PubMed] [Google Scholar]
- 228. Obradovich N, Migliorini R, Mednick SC, Fowler JH. Nighttime temperature and human sleep loss in a changing climate. Sci Adv 2017;3:e1601555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011;32:1484–1492. [DOI] [PubMed] [Google Scholar]
- 230. Edwards NM, Myer GD, Kalkwarf HJ, Woo JG, Khoury PR, Hewett TE, Daniels SR. Outdoor temperature, precipitation, and wind speed affect physical activity levels in children: a longitudinal cohort study. J Phys Act Health 2015;12:1074–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, Alter DA. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med 2015;162:123–132. [DOI] [PubMed] [Google Scholar]
- 232. European Commission . https://ec.europa.eu/info/events/launch-event-european-human-exposome-network-2020_en. 13 October 2021, date last accessed)
- 233. Produced for the European Commission DG . Environment by the Science Communication Unit U, Bristol. Noise abatement approaches. Future Brief 17. Science for Environment Policy 2017. pp. 1–18. https://ec.europa.eu/environment/integration/research/newsalert/pdf/noise_abatement_approaches_FB17_en.pdf (13 October 2021, date last accessed)
- 234. Dimakopoulou K, Koutentakis K, Papageorgiou I, Kasdagli MI, Haralabidis AS, Sourtzi P, Samoli E, Houthuijs D, Swart W, Hansell AL, Katsouyanni K. Is aircraft noise exposure associated with cardiovascular disease and hypertension? Results from a cohort study in Athens, Greece. Occup Environ Med 2017;74:830–837. [DOI] [PubMed] [Google Scholar]
- 235. Münzel T, Kröller-Schön S, Oelze M, Gori T, Schmidt FP, Steven S, Hahad O, Röösli M, Wunderli J-M, Daiber A, Sørensen M. Adverse cardiovascular effects of traffic noise with a focus on nighttime noise and the new WHO noise guidelines. Annu Rev Public Health 2020;41:309–328. [DOI] [PubMed] [Google Scholar]
- 236. Rajagopalan S, Brauer M, Bhatnagar A, Bhatt DL, Brook JR, Huang W, Munzel T, Newby D, Siegel J, Brook RD;American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Stroke Council . Personal-level protective actions against particulate matter air pollution exposure: a scientific statement from the American Heart Association. Circulation 2020;142:e411–e431. [DOI] [PubMed] [Google Scholar]
- 237. Fisk WJ, Chan WR. Health benefits and costs of filtration interventions that reduce indoor exposure to PM2.5 during wildfires. Indoor Air 2017;27:191–204. [DOI] [PubMed] [Google Scholar]
- 238. Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, Chen J, Hao K, Kinney PL, Chen H, Kan H. Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification. Circulation 2017;136:618–627. [DOI] [PubMed] [Google Scholar]
- 239. Morishita M, Wang L, Speth K, Zhou N, Bard RL, Li F, Brook JR, Rajagopalan S, Brook RD. Acute blood pressure and cardiovascular effects of near-roadway exposures with and without N95 respirators. Am J Hypertens 2019;32:1054–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Cheng Y, Ma N, Witt C, Rapp S, Wild PS, Andreae MO, Poschl U, Su H. Face masks effectively limit the probability of SARS-CoV-2 transmission. Science 2021;372:1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Kim SR, Choi S, Kim K, Chang J, Kim SM, Cho Y, Oh YH, Lee G, Son JS, Kim KH, Park SM. Association of the combined effects of air pollution and changes in physical activity with cardiovascular disease in young adults. Eur Heart J 2021;42:2487–2497. [DOI] [PubMed] [Google Scholar]
- 242. Tainio M, de Nazelle AJ, Götschi T, Kahlmeier S, Rojas-Rueda D, Nieuwenhuijsen MJ, de Sá TH, Kelly P, Woodcock J. Can air pollution negate the health benefits of cycling and walking? Prev Med 2016;87:233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Falchi F, Cinzano P, Elvidge CD, Keith DM, Haim A. Limiting the impact of light pollution on human health, environment and stellar visibility. J Environ Manage 2011;92:2714–2722. [DOI] [PubMed] [Google Scholar]
- 244. Nawrot TS, Saenen ND, Schenk J, Janssen BG, Motta V, Tarantini L, Cox B, Lefebvre W, Vanpoucke C, Maggioni C, Bollati V. Placental circadian pathway methylation and in utero exposure to fine particle air pollution. Environ Int 2018;114:231–241. [DOI] [PubMed] [Google Scholar]
- 245. Song P, Li Z, Li X, Yang L, Zhang L, Li N, Guo C, Lu S, Wei Y. Transcriptome profiling of the lungs reveals molecular clock genes expression changes after chronic exposure to ambient air particles. Int J Environ Res Public Health 2017;14:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Furlan R, Barbic F, Piazza S, Tinelli M, Seghizzi P, Malliani A. Modifications of cardiac autonomic profile associated with a shift schedule of work. Circulation 2000;102:1912–1916. [DOI] [PubMed] [Google Scholar]
- 247. Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci USA 2016;113:E1402–E1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. UNCCS . Climate action and support trends. United Nations Climate Change Secretariat2019. https://unfccc.int/sites/default/files/resource/Climate_Action_Support_Trends_2019.pdf. (13 October 2021, date last accessed)
- 249. CRED. Natural disaster 2018. 2019. https://www.cred.be/sites/default/files/CREDNaturalDisaster2018.pdf. (13 October 2021, date last accessed)
- 250. Fawzy S, Osman AI, Doran J, Rooney DW. Strategies for mitigation of climate change: a review. Environ Chem Lett 2020;18:2069–2094. [Google Scholar]
- 251. Marwick TH, Buonocore J. Environmental impact of cardiac imaging tests for the diagnosis of coronary artery disease. Heart 2011;97:1128–1131. [DOI] [PubMed] [Google Scholar]
- 252. Giles-Corti B, Vernez-Moudon A, Reis R, Turrell G, Dannenberg A, Badland H, Foster S, Lowe M, Sallis J, Stevenson M, Owen N. City planning and population health: a global challenge. Lancet 2016;388:2912–2924. [DOI] [PubMed] [Google Scholar]
- 253. Nieuwenhuijsen MJ. Urban and transport planning pathways to carbon neutral, liveable and healthy cities; a review of the current evidence. Environ Int 2020;140:105661. [DOI] [PubMed] [Google Scholar]
- 254. Stevenson M, Thompson J, de Sa TH, Ewing R, Mohan D, McClure R, Roberts I, Tiwari G, Giles-Corti B, Sun X, Wallace M, Woodcock J. Land use, transport, and population health: estimating the health benefits of compact cities. Lancet 2016;388:2925–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Mueller N, Rojas-Rueda D, Basagaña X, Cirach M, Cole-Hunter T, Dadvand P, Donaire-Gonzalez D, Foraster M, Gascon M, Martinez D, Tonne C, Triguero-Mas M, Valentín A, Nieuwenhuijsen M. Urban and transport planning related exposures and mortality: a health impact assessment for cities. Environ Health Perspect 2017;125:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Khomenko S, Cirach M, Pereira-Barboza E, Mueller N, Barrera-Gomez J, Rojas-Rueda D, de Hoogh K, Hoek G, Nieuwenhuijsen M. Premature mortality due to air pollution in European cities: a health impact assessment. Lancet Planet Health 2021;5:e121–e134. [DOI] [PubMed] [Google Scholar]
- 257. Moreno C. The 15 minutes-city: for a new chrono-urbanism! http://www.moreno-web.net/the-15-minutes-city-for-a-new-chrono-urbanism-pr-carlos-moreno/ (13 October 2021, date last accessed).
- 258. Sisson P. How the ‘15-minute city’ could help post-pandemic recovery. https://www.bloomberg.com/news/articles/2020-07-15/mayors-tout-the-15-minute-city-as-covid-recovery?cmpid=BBD071620_CITYLAB&utm_medium=email&utm_source=newsletter&utm_term=200716&utm_campaign=citylabdaily (13 October 2021, date last accessed).
- 259. Turrell G, Haynes M, Wilson LA, Giles-Corti B. Can the built environment reduce health inequalities? A study of neighbourhood socioeconomic disadvantage and walking for transport. Health Place 2013;19:89–98. [DOI] [PubMed] [Google Scholar]
- 260. Mueller N, Rojas-Rueda D, Khreis H, Cirach M, Andres D, Ballester J, Bartoll X, Daher C, Deluca A, Echave C, Mila C, Marquez S, Palou J, Perez K, Tonne C, Stevenson M, Rueda S, Nieuwenhuijsen M. Changing the urban design of cities for health: the superblock model. Environ Int 2020;134:105132. [DOI] [PubMed] [Google Scholar]
- 261. London Living Streets . Campaigning for safe and vibrant streets, where people want to walk. https://londonlivingstreets.com/low-traffic-liveable-neighbourhoods/ (13 October 2021, date last accessed).
- 262. Aldred R. Low Traffic Neighbourhoods: what is the evidence from the mini-Holland interventions? http://rachelaldred.org/research/low-traffic-neighbourhoods-evidence/ (13 October 2021, date last accessed).
- 263.this was a duplicate of reference 257.
- 264. Sisson P. How the ‘15-minute city’ could help post-pandemic recovery. https://www.bloomberg.com/news/articles/2020-07-15/mayors-tout-the-15-minute-city-as-covid-recovery?cmpid=BBD071620_CITYLAB&utm_medium=email&utm_source=newsletter&utm_term=200716&utm_campaign=citylabdaily (13 October 2021, date last accessed).
- 265. Moreno C. The 15-minute city. https://www.ted.com/talks/carlos_moreno_the_15_minute_city?language=en (13 October 2021, date last accessed).
- 266. Peters A. What can we learn from this thriving, car-free German neighborhood? Get rid of parking spaces. 2019. https://www.fastcompany.com/90327301/what-can-we-learn-from-this-thriving-car-free-german-neighborhood-get-rid-of-parking-spaces (13 October 2021, date last accessed).
- 267. Burgen S. ‘For me, this is paradise’: life in the Spanish city that banned cars. 2018. https://www.theguardian.com/cities/2018/sep/18/paradise-life-spanish-city-banned-cars-pontevedra (13 October 2021, date last accessed).
- 268. Nieuwenhuijsen MJ, Khreis H. Car free cities: pathway to healthy urban living. Environ Int 2016;94:251–262. [DOI] [PubMed] [Google Scholar]
- 269. Rojas-Rueda D, Morales-Zamora E. Built environment, transport, and COVID-19: a review. Curr Environ Health Rep 2021;8:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Daiber A, Lelieveld J, Steven S, Oelze M, Kröller-Schön S, Sørensen M, Münzel T. The "exposome" concept—how environmental risk factors influence cardiovascular health. Acta Biochim Pol 2019;66:269–283. [DOI] [PubMed] [Google Scholar]
- 271. Dye C. The Great Health Dilemma: Is Prevention Better than Cure? Oxford University Press; Published to Oxford Scholarship Online, 2021;DOI:10.1093/oso/9780198853824.001.0001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.