Abstract
Background
An antiplatelet therapy with acetylsalicylic acid (ASA) is prescribed in the prevention of cardiovascular events, but around 24% of ASA takers are resistant to the treatment.
Aim
In this prospective, observational cohort study, we aimed to identify the prevalence and risk factors of ASA nonresponse in patients who underwent vascular surgery.
Methods
The study was conducted in the University hospital in Frankfurt am Main. In total, 70 patients were pre-treated with 100 mg of ASA per day and underwent either elective carotid thromboendarterectomy, femoral thromboendarterectomy or endovascular aneurysm repair of the abdominal aorta. The platelet function was measured on the first preoperative and the second or fourth postoperative day with the multiple electrode aggregometry by in-vitro stimulation with arachidonic acid (ASPItest) and thrombin receptor activating peptide 6 (TRAPtest). The primary end point was the in-vitro induced platelet aggregation in the ASPItest. If the ASPItest amounted ≥400 AU × min, the patients were categorized as ASA nonresponders.
Results
The total prevalence of ASA nonresponse in our study was 20% preoperatively and 35.7% postoperatively (p = 0.005). As significant predictors for ASA nonresponse, we demonstrated the area under the aggregation curve in the TRAPtest preoperatively (p = 0.04) and postoperatively (p = 0.02), and the two comorbidities arterial hypertension (P < .001; rho 0.44) and diabetes mellitus (p = 0.04; rho 0.39), which are already well known to be associated with ASA nonresponse.
Conclusion
In conclusion, data of the study indicate a high incidence of perioperative, laboratory ASA nonresponse in patients undergoing vascular surgery.
Keywords: ASA nonresponse, Multiplate Analyzer, platelet aggregation, vascular surgery
Introduction
In the primary prevention and secondary prophylaxis of cardiovascular events, an antiplatelet therapy with acetylsalicylic acid is decisive. Acetylsalicylic acid irreversibly inhibits the cyclooxygenase 1, and it reduces the synthesis of thromboxane A2 and thus platelet activity. 1
However, many studies have demonstrated that ASA does not always fulfill its antithrombotic effect.2–5 Nowadays, two established terms describe ASA nonresponse: clinical nonresponse and laboratory nonresponse. 2 Clinical nonresponse happens to be when cardiovascular events occur even though the patient is under regular treatment with aspirin. 3 Laboratory nonresponse is defined by tests with platelet function analyzers, for example, a multiple electrode aggregometry, resulting in ongoing platelet aggregation despite therapy with ASA.3,4 Depending on the method used, the aspirin nonresponse described in previous studies varied between <1% to 40 to 60%.1,5,6 Hovens et al. 7 exhibited in a large meta-analysis including 42 trials that approximately 24% of aspirin takers are laboratory ASA nonresponders. It is also a known fact that laboratory ASA nonresponders are at higher risk of thromboembolic events.4,5,8,9
The aim of our study was to investigate the pre- and postoperative prevalence and risk factors of ASA nonresponse in patients who undergo three different vascular surgical procedures (carotid thromboendarterectomy [group 1], femoral thromboendarterectomy [group 2] and endovascular aortic repair [group 3]) by using multiple electrode aggregometry. Our study was the first to compare these specific three types of vascular surgery towards ASA nonresponse.
Methods
Trial Design and Patients
Our study was undertaken at the University Hospital Frankfurt am Main, Germany and categorized as an observational, prospective, nonrandomized cohort study. The study was performed after approval by the ethics review board of the University Hospital Frankfurt am Main (approval numbers: 405/14) and obtaining written patient consent.
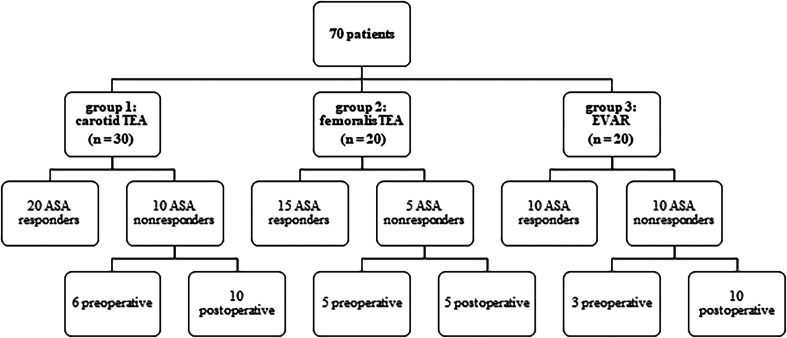
In total, 86 patients were initially enrolled from August 2016 to January 2019. Out of this initial cohort, 16 refused to participate postoperatively. Inclusion criteria were patients who underwent elective carotid thromboendarterectomy, femoral thromboendarterectomy and endovascular aneurysm repair of the abdominal aorta. They were pre-treated with 100 mg of ASA per day over a period of at least six weeks. If the patients did not take ASA over a period of at least 6 weeks before the trial, they were not concluded in our study. The perioperative adherence to treatment was also monitored as our proband were in-patient, so the treatment with ASA was given to them under controlled circumstances by the nurses. After taking the medicine, the taking was hacked down in the patients' electronic records data by the nurses. Exclusion criteria for the study were if the patients were under the age of 18 or allergic to ASA. According to the type of surgery they underwent, the patients were divided into three different groups. In the first group, we collected 30 patients who underwent a carotid thromboendarterectomy (CEA). The second group included 20 patients who underwent a femoral thromboendarterectomy, and the third group included 20 EVAR with abdominal aneurysm.
Our study was designed as all observational as we did not adjust the medication in ASA nonresponders. Demographic data, information concerning the surgical procedure, and pre-existing illness were collected from patients' electronic records at the hospital.
Sample Collection
To analyze the platelet function blood samples were taken on the first preoperative day and the second or fourth postoperative day. For this purpose, the blood was taken into a 2-mL heparin anticoagulated monovette (arterial blood gas monovette, Sarstedt AG & Co, Germany). Therefore, we used a central venous catheter (Vasofix Safety Braunüle, B. Braun Melsungen AG, Germany) which was placed preoperatively, or we punctured a vein one single time with a 21-G butterfly needle (Multifly; Sarstedt AG & Co, Germany). Additionally, blood samples were collected into a 4.7-mL EDTA tube (Sarstedt AG & Co, Germany) for hemoglobin and platelet count analyzes.
Multiple Electrode Aggregometry
Samples were evaluated within a maximum of 15 min with the help of the multiple electrode aggregometry (Multiplate® Analyzer, Roche Pharma AG, Grenzach-Wyhlen, Germany). This method works with impedance aggregometry, which results from platelet aggregation as described by Cardinal and Flower. 10 For the measurement, 300 µL of blood was diluted with 300 µL of 0.9% sodium chloride in a test cell. After 3 min of incubation, the in-vitro platelet aggregation was started by 20 µL of arachidonic acid (ASPItest) and 20 µL of thrombin receptor activating peptide 6 (TRAPtest). The platelet aggregation was measured for 6 min. 4 The area under the aggregation curve (AUC) indicated platelet aggregability. Therefore, the unit was named “aggregation units” (AU × min). In our study, ASA nonresponse was defined as when a result was ≥400 AU × min in the ASPItest whereas 790 to 1410 AU × min for the ASPItest and 923 to 1509 AU × min for the TRAPtest were considered as normal references by the manufacturer.
Primary End Point
The in-vitro induced platelet aggregation in the ASPItest was defined as primary end point (AU × min). All the patients with an ASPItest of ≥400 AU × min were classified as ASA nonresponders.
Secondary End Point
In-vitro induced platelet aggregation in the TRAPtest of MEA (AU × min) was defined as a secondary end point.
Statistical Analyses
Statistical analyses were conducted in collaboration with the Institute for Biostatistics and Mathematical Modelling Department of Medicine, Goethe University Frankfurt, by using BiAS for Windows (Edition 11.10). The statistical analyses were performed two-sided, and a P-value of <.05 predicted statistical significance. We used the Kolmogorov-Smirnov-Lillifors test to evaluate the normal distribution of the parameters. If the data was not normally distributed, we used the Wilcoxon matched-pair test. To compare the incidence of the ASA nonresponse in the total cohort, we used the Chi-squared test. The incidence of ASA nonresponse within one group was evaluated by the Chi-squared test or the Fisher exact test. The Wilcoxon-Mann-Whitney-test was used as a non-parametric test for the comparison of the aggregometric measurements. Multiple logistic regression analysis and the Spearman rank correlation test were used to investigate a potential correlation of the variables gender, age, platelet and hemoglobin count, and surgery time to ASA nonresponse. To compare three different surgical procedures, we used the unifactorial variance analysis (ANOVA).
Results
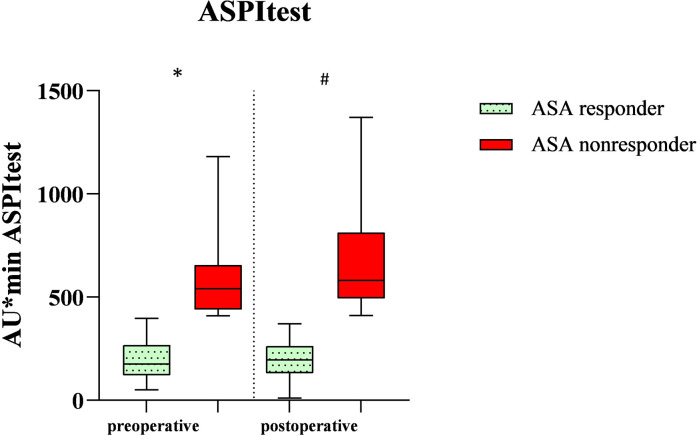
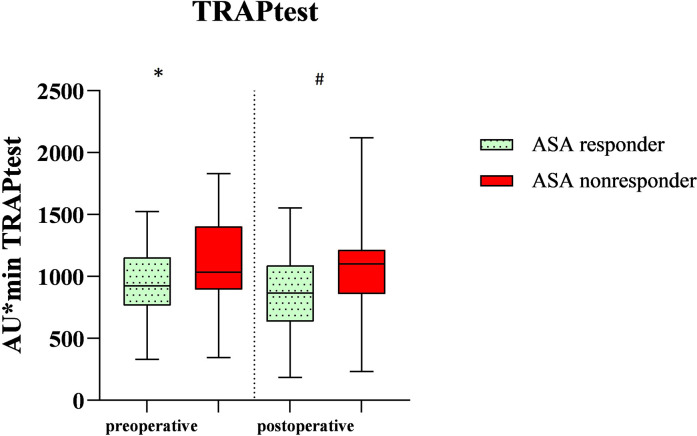
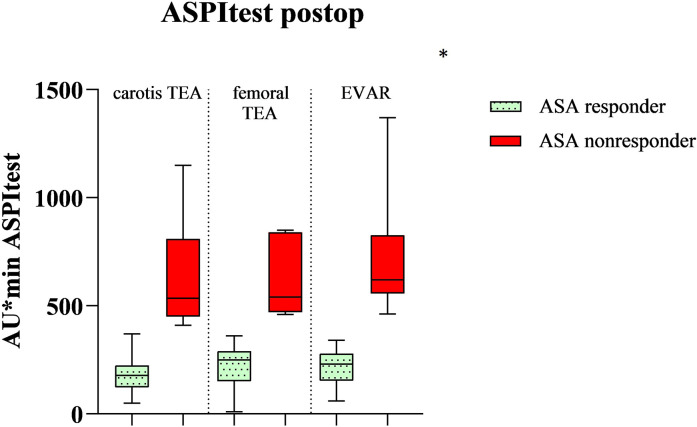
These 70 patients were divided into three different groups. The division into ASA response group and ASA nonresponse group resulted from cut-off values of 400 AU × min in the ASPItest of the Multiplate Analyzer. Patients with a score of ≥400 AU × min were called ASA nonresponders, whereas patients with a score <400 AU × min were called ASA responders. Overall, preoperatively 14 out of the 70 patients (20%) already showed ASA nonresponse. The nonresponder rate postoperatively increased significantly to 35.7% (p = 0.005, n = 25 of 70) in all patients. In the group of patients with carotid thromboendarterectomy, postoperatively, 10 out of 30 patients were classified as ASA nonresponders (33.3%, p = 0.009) while after femoral thromboendarterectomy 25% ASA nonresponders were identified (n = 5 out of 20 patients, p = 0.06). In the EVAR group, 50% of the patients showed an insufficient response to ASA (n = 10 out of 20 patients, p = 0.04) (Figure 1). In Table 1, the demographic data are displayed. Arterial hypertension and diabetes mellitus were defined according to the ESC guidelines, so a blood pressure that was >140/90 mmHg was calculated as arterial hypertension and HbA1c > 6.5% was the definition for diabetes mellitus.11,12 Arterial hypertension (P < .001, rho = 0.44) and diabetes mellitus (p = 0.04, rho = 0.4) significantly correlated with ASA nonresponse: 76% of the postoperative ASA nonresponders had arterial hypertension and 52% of them had diabetes mellitus. We also found that ASA nonresponders had preoperatively and postoperatively a higher platelet count and a lower hemoglobin level, but both findings were not significant. Table 2 shows AUC in the TRAPtest of the ASA nonresponders was preoperatively (p = 0.03) as well as postoperatively (p = 0.02) higher than the AUC in the TRAPtest of the ASA responders. Figures 2 and 3 demonstrate the aggregometric analyzes. ASA nonresponders, both pre- and postoperatively, showed lower means in the ASPItest but also in the TRAPtest. The surgical procedure did not influence the nonresponder rate (p = 0.36) (Figure 4).
Figure 1.
Overview of the trial design and patients. Patients were considered as ASA nonresponders if the AUC was ≥400 AU × min in the ASPItest of the Multiplate Analyzer.
Table 1.
Demographic Data. The Data are Shown as Number (%) or Mean (SD). p < .05 Indicates Significant Difference. rho = Correlation Coefficient. ASA Response and ASA Nonresponse was Defined on Second or Fourth Postoperative for this Table.
| Parameters | ASA response (n = 45) | ASA nonresponse (n = 25) | P value |
|---|---|---|---|
| Gender [male, n (%)] | 37 (82%) | 20 (80%) | .86 |
| Age [years (SD)] | 71 (10) | 68 (9) | .31 |
| Arterial hypertension [n (%)] (total n = 53) | 34 (75.6%) | 19 (76%) | <.001 rho = 0.44 |
| Diabetes [n (%)] (total n = 28) | 15 (33%) | 13 (52%) | .04 rho = 0.39 |
| Platelet count postoperative [/ng (SD)] | 199 (76.4) | 229.4 (87.7) | .15 |
| Hemoglobin postoperative [g/dl (SD)] | 10.6 (1.75) | 10.4 (2.1) | .6 |
| Total procedure time carotis TEA [min] | 126 (23) | 122 (23) | .3 |
| Total procedure time femoral TEA [min] | 179 (28) | 179 (45) | .31 |
| Total procedure time EVAR | 191 (55) | 182 (37) | .1 |
Table 2.
Aggregometric Parameters as the Median. Here is the AU × min for the ASPItest and the TRAPtest Demonstrated. ASA Response (n = 56 Preoperative, n = 45 Postoperative). ASA Nonresponse (n = 14 Preoperative, n = 25 Postoperative). P < .05 Indicates Statistical Significance. AUC in the TRAPtest of the ASA Nonresponders was Preoperatively (p = 0.03) as Well as Postoperatively (p = 0.02) Higher than the AUC in the TRAPtest of the ASA Responders.
| Parameter | ASA response | ASA nonresponse | P value |
|---|---|---|---|
| ASPItest (preop) (AU × min) | 175 | 540 | <.0001 |
| ASPItest (postop) (AU × min) | 196 | 580 | <.0001 |
| TRAPtest (preop) (AU × min) | 915 | 1155 | .04 |
| TRAPtest (postop) (AU × min) | 866 | 1100 | .02 |
Figure 2.
Demonstration of the in-vitro induced platelet aggregation of the total cohort after being stimulated with arachidonic acid (ASPItest) on the first preoperative and second or fourth postoperative day after vascular surgery. *A significant difference between the ASA responders and nonresponders on the first preoperative day (P < .0001). # A significant difference between the ASA responders and nonresponders on the second or fourth postoperative day (P < .0001). ASA response (n = 56 preoperative, n = 45 postoperative). ASA nonresponse (n = 14 preoperative, n = 25 postoperative).
Figure 3.
Demonstration of the in-vitro induced platelet aggregation of the total cohort after being stimulated with thrombin receptor activating peptide (TRAPtest) on the first preoperative and second or fourth postoperative day after vascular surgery. *A significant difference between the ASA responders and nonresponders on the first preoperative day (p 0.04). # A significant difference between the ASA responders and nonresponders on the second or fourth postoperative day (p 0.02). ASA response (n = 56 preoperative, n = 45 postoperative). ASA nonresponse (n = 14 preoperative, n = 25 postoperative).
Figure 4.
Comparison of the three groups in the in-vitro induced platelet aggregation after being stimulated with arachidonic acid (ASPItest) on the second or fourth postoperative day after vascular surgery. *no significant difference in the nonresponder rate between these 3 groups (p 0.36). ASA response (n = 45). ASA nonresponse (n = 10 Carotis TEA, n = 5 femoral TEA, n = 10 EVAR).
Discussion
We here compared the prevalence of ASA nonresponse within a cohort of patients undergoing vascular surgery with the aim to identify risk factors for ASA nonresponse. To date, while most studies have investigated the incidence and outcomes of ASA nonresponse in coronary artery disease (CAD) fewer reports have described ASA response in patients with peripheral artery disease (PAD). 6 In the meta-analysis of Hovens et al., 7 including a total of 42 studies, seven studies investigated the ASA nonresponders rate in PAD patients. Here they found that PAD patients had a mean nonresponder rate comparable to that of the CAD patients of approximately 24%. 7 However, the range of ASA nonresponse differs widely in the literature between <1% to 40 to 60%.6,7 The main reason for this spread may be that there are many different platelet function assays. The here used Multiplate Analyzer is a reliable and solid method for measuring the platelet function.4,6 Our cut-off values for ASA were defined as <400 AU × min in the ASPItest. In our total cohort the prevalence of ASA nonresponders significantly increased from 20% to 35.7% (p = 0.005 ). The rate is influenced by the definition of the cut-off values. While there is no universal cut-off for ASA response and nonresponse in the Multiplate Analyzer cut-off values of <300 AU × min in the ASPItest have been described for aspirin response, and results between 300-690 AU × min are categorized as a partial response whereas >690AU × min is called ASA nonresponse.2,13 So, if we here used a cut-off <300AU × min for our trial, we could expect an even higher amount of ASA nonresponse.
Wand et al. 14 investigated 400 patients who underwent coronary artery bypass grafting (CABG) surgery. In their study 51.3% of patients on the third postoperative day and 71.3% of patients on the fifth postoperative day (p = 0.0049) were ASA nonresponders according to their analysis using Multiplate Analyzer using a cut-off which was also located at 400 AU × min. Their study presented a higher nonresponders rate as compared to our study. In alignment to our data, ASA non-response was significantly higher postoperatively. Indeed there is data that describes postoperative nonresponse. 15 Postoperative nonresponse may be a temporary event. Payne et al. 15 investigated 50 patients who underwent a carotid thromboendarterectomy (CEA). Blood samples were taken preoperatively, intraoperatively, and postoperatively. They concluded that in CEA patients the platelet aggregation was significantly greater towards the end of the operation than preoperatively. This may be caused by increased platelet activity during surgery because of the surgical trauma and the liberation of prothrombotic factors like thrombin. 15 In concordance, we found a higher platelet aggregation in the TRAPtest postoperatively (P < .0001). A drawback of our data is that we did not measure ASA response intraoperatively. Another factor for this temporary ASA nonresponse may be the intraoperative administration of unfractionated heparin. Heparin indirectly leads to an increased level of arachidonic acid so that at the end, there is more arachidonic acid than ASA, and thus arachidonic acid can, to some extent, may escape from the inhibition through ASA.15,16 This may lead to a transient nonresponse postoperatively.
In contrast to Wand et al. 14 we did not find a significant positive correlation between the surgical procedure time and nonresponder rate (p = 0.65). While the highest number of nonresponders was found in the EVAR group with the longest procedure time, the differences between the groups were not significant. As described above, vascular surgery implies vascular trauma which may result in higher platelet activity. This is relevant in carotid artery disease: Robless et al. 17 and Nambi et al. 18 also pointed out that in carotid disease, there is a higher level of P-selectin expression. This, in turn, leads to higher platelet aggregation, which further induces the formation of chemokines and cytokines and thus indirectly perpetuates the atherosclerotic plaque. Temporary postoperative ASA nonresponse is clinically relevant since it may influence the rate of thromboembolic complications.19–21
Lewszuk et al. 21 investigated 66 patients who underwent a carotid endarterectomy and were pre-treated with ASA 75 mg/d. They measured platelet aggregation with the analyzer PFA-100 on the second day after the operation and identified a nonresponder rate of 32% (19 out of 66 patients). Moreover, they also reported a correlation with unstable carotid plaques and ASA nonresponse. This has further consequences as those patients are at higher risk of intracerebral micro-embolisms. Moreover, Mueller et al. 20 investigated 100 patients with peripheral arterial disease who were treated with 100 mg ASA daily and underwent elective percutaneous balloon angioplasty. They detected that vascular obliteration was 87% higher in ASA nonresponsive male patients. This points out that our laboratory ASA nonresponders are at higher risks of clinical thromboembolic outcomes.
A variety of factors can lead to ASA nonresponse. In concordance to other studies, we could find a significant correlation between ASA nonresponse and arterial hypertension. 4 We demonstrated that 19 out of 25 nonresponders (75%) suffered from hypertension. A higher platelet reactivity in hypertensive patients is discussed through elevated shear stress, endothelial disorder, and arterial tone elevation.4,22 Jefferson et al. 23 investigated in a trial 330 patients with a history of myocardial infarction who were treated with a minimum of 81 mg ASA/d. Out of this number, 95 patients (29%) were ASA nonresponders. They discovered that ASA nonresponders were older, and they noticed a correlation between diabetes mellitus, lower HDL levels and ASA nonresponse (p = 0.01). We could also confirm a significant correlation between the existence of diabetes mellitus and ASA nonresponse (p = 0.04, rho = 0.39) as 52% of our nonresponders had diabetes mellitus. No correlation could be found for the patient age. The postoperative analgesia regimen in our trial mainly included NSAIDs and the pyrazolinone metamizole, which may have a supplement effect to the ASA nonresponder rate because of potential medical interference as they both practice their effect via the cyclooxygenase. 14 Last but not least, the omnipresent problem of noncompliance is the most usual cause for ASA nonresponse.24,25
Our study has several limitations. We had a relatively small cohort of patients from one single center. Moreover, we did not take any intraoperative blood samples to calculate the correlation of a higher intraoperative platelet turn. Furthermore, we did not evaluate the information of body weight of the patients. A 2018 metaanalysis from randomized trials evaluated the potential impact of body weight on ASA efficacy and safety which might be a potential confounder to ASA nonresponse. 26 As our study was merely observational without any long-term follow-up, we cannot conclude any direct clinical outcomes for our findings.
Despite the above-mentioned limitations, the present study is of clinical value because it demonstrated a high incidence of preoperative and postoperative ASA nonresponse in patients who underwent vascular surgery. Our ASA nonresponder rate correlated to the known percentage of ASA nonresponders. A variety of factors (treatment adherence, increased postoperative platelet turnover, postoperative analgesia regimen and comorbidities like arterial hypertension and diabetes mellitus) can lead to ASA nonresponse. Though we may not have a direct clinical outcome due to the lack of a follow-up, our laboratory nonresponder rate lets us assume an indirect clinical effect in peripheral arterial disease patients with ASA nonresponse, as we know from the literature that those are at higher risk of cardiovascular events. 27 Our study emphasizes the demand to conduct further studies including a follow-up with peripheral arterial disease patients who are pre-treated with ASA to evaluate the ASA nonresponder rate and direct clinical outcomes.
Appendix
List of Abbreviations
- ASA
acetylsalicylic acid
- CEA
carotid thromboendarterectomy
- EVAR
endovascular aortic repair
- MEA
Multiplate – multiple electrode aggregometry
- ASPItest
arachidonic acid
- TRAPtest
thrombin receptor activating peptide 6
- CAD
coronary artery disease
- PAD
peripheral artery disease
- COX
cyclooxygenase
- HDL
high-density lipoprotein
- NSAID
non-steroidal anti-inflammatory drug
- Preop
preoperative
- Postop
postoperative
Footnotes
Author Contributions: WM, MK and CW designed the study and contributed to the manuscript. AK performed the study and wrote the first draft of thismanuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Alia Uzra Kazimi https://orcid.org/0000-0001-9083-5914
References
- 1.Assadian A, Lax J, Meixner-Loicht U, Hagmüller GW, Bayer PM, Hübl W. Aspirin resistance among long-term aspirin users after carotid endarterectomy and controls: flow cytometric measurement of aspirin-induced platelet inhibition. J Vasc Surg. 2007;45(6):1142-1147. discussion 1147. [DOI] [PubMed] [Google Scholar]
- 2.Łabuz-Roszak B, Pierzchała K, Niewiadomska E, Skrzypek M, Machowska-Majchrzak A. Searching for factors associated with resistance to acetylsalicylic acid used for secondary prevention of stroke. Archives of Medical Science : AMS. 2015;11(1):106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong S, Appleberg M, Ward CM, Lewis DR. Aspirin resistance in cardiovascular disease: a review. European Journal of Vascular and Endovascular Surgery: the Official Journal of the European Society for Vascular Surgery. 2004;27(5):456-465. [DOI] [PubMed] [Google Scholar]
- 4.Ball STE, Taylor R, McCollum CN. Resistance to antiplatelet therapy is associated with symptoms of cerebral ischemia in carotid artery disease. Vasc Endovascular Surg. 2020;54(8):712-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abacı O, Kılıçkesmez KO. Aspirin resistance: where are we now? Anadolu Kardiyoloji Dergisi : AKD=the Anatolian Journal of Cardiology. 2013;13(4):370-373. [DOI] [PubMed] [Google Scholar]
- 6.Guirgis M, Thompson P, Jansen S. Review of aspirin and clopidogrel resistance in peripheral arterial disease. J Vasc Surg. 2017;66(5):1576-1586. [DOI] [PubMed] [Google Scholar]
- 7.Hovens MMC, Snoep JD, Eikenboom JCJ, van der Bom JG, Mertens BJA, Huisman MV. Prevalence of persistent platelet reactivity despite use of aspirin: a systematic review. Am Heart J. 2007;153(2):175-181. [DOI] [PubMed] [Google Scholar]
- 8.Hummel T, Meves SH, Rüdiger K, et al. Prävalenz der ASS-low-response in der Gefäßchirurgie. Chirurg. 2016;87(5):446-454. [DOI] [PubMed] [Google Scholar]
- 9.Snoep JD, Hovens MMC, Eikenboom JCJ, van der Bom JG, Huisman MV. Association of laboratory-defined aspirin resistance with a higher risk of recurrent cardiovascular events: a systematic review and meta-analysis. Arch Intern Med. 2007;167(15):1593-1599. [DOI] [PubMed] [Google Scholar]
- 10.Cardinal DC, Flower RJ. The electronic aggregometer: a novel device for assessing platelet behavior in blood. J Pharmacol Methods. 1980;3(2):135-158. [DOI] [PubMed] [Google Scholar]
- 11.Williams B, Mancia G, Spiering W, et al. ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-3104. [DOI] [PubMed] [Google Scholar]
- 12.Cosentino F, Grant PJ, Aboyans V, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255-323. [DOI] [PubMed] [Google Scholar]
- 13.Calatzis A, Loreth R, Spannagl M. Multiplate ® platelet function analysis-application and interpretation, 2007, https://www.semanticscholar.org/paper/Multiplate-%C2%AE-platelet-function-analysis-application-Calatzis-Loreth/1bd3cf62cb0a199de6bdc79e22434aabdff491d9.
- 14.Wand S, Adam EH, Wetz AJ, et al. The prevalence and clinical relevance of ASA nonresponse after cardiac surgery: a prospective bicentric study. Clinical and Applied Thrombosis/Hemostasis : Official Journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2018;24(1):179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Payne DA, Jones CI, Hayes PD, Webster SE, Ross Naylor A, Goodall AH. Platelet inhibition by aspirin is diminished in patients during carotid surgery: a form of transient aspirin resistance? Thromb Haemostasis. 2004;92(1):89-96. [DOI] [PubMed] [Google Scholar]
- 16.Webster SE, Payne DA, Jones CI, et al. Anti-platelet effect of aspirin is substantially reduced after administration of heparin during carotid endarterectomy. J Vasc Surg. 2004;40(3):463-468. [DOI] [PubMed] [Google Scholar]
- 17.Robless PA, Okonko D, Lintott P, Mansfield AO, Mikhailidis DP, Stansby GP. Increased platelet aggregation and activation in peripheral arterial disease. European Journal of Vascular and Endovascular Surgery : the Official Journal of the European Society for Vascular Surgery. 2003;25(1):16-22. [DOI] [PubMed] [Google Scholar]
- 18.Nambi V, Kimball KT, Bray PF, et al. Differences in responses of platelets to fluid shear stress in patients with peripheral artery disease (PAD) and coronary artery disease (CAD). Platelets. 2009;20(3):199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackerstaff RG, Moons KG, van de Vlasakker CJ, et al. Association of intraoperative transcranial Doppler monitoring variables with stroke from carotid endarterectomy. Stroke. 2000;31(8):1817-1823. [DOI] [PubMed] [Google Scholar]
- 20.Mueller MR, Salat A, Stangl P, et al. Variable platelet response to low-dose ASA and the risk of limb deterioration in patients submitted to peripheral arterial angioplasty. Thromb Haemostasis. 1997;78(3):1003-1007. [PubMed] [Google Scholar]
- 21.Lewszuk AJ, Postuła M, Madycki G, Staszkiewicz W, Opolski G, Eberhardt A. Evaluation of aspirin resistance and the presence of unstable carotid plaque in patients undergoing carotid endarterectomy. Kardiol Pol. 2015;73(4):255-260. [DOI] [PubMed] [Google Scholar]
- 22.Ozben B, Tanrikulu AM, Ozben T, Caymaz O. Aspirin resistance in hypertensive patients. Journal of Clinical Hypertension (Greenwich, Conn.). 2010;12(9):714-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jefferson BK, Foster JH, McCarthy JJ, et al. Aspirin resistance and a single gene. Am J Cardiol. 2005;95(6):805-808. [DOI] [PubMed] [Google Scholar]
- 24.Prabhakaran S, Wells KR, Lee VH, Flaherty CA, Lopes DK. Prevalence and risk factors for aspirin and clopidogrel resistance in cerebrovascular stenting. AJNR Am J Neuroradiol. 2008;29(2):281-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders J, Nambi V, Kimball KT, et al. Variability and persistence of aspirin response in lower extremity peripheral arterial disease patients. J Vasc Surg. 2011;53(3):668-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothwell PM, Cook NR, Michael Gaziano Jet al. et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet (London, England). 2018;392(10145):387, https://www-ncbi-nlm-nih-gov.proxy.ub.uni-frankfurt.de/pmc/articles/PMC6083400/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasotakis G, Pipinos II, Lynch TG. Current evidence and clinical implications of aspirin resistance. J Vasc Surg. 2009;50(6):1500-1510. [DOI] [PubMed] [Google Scholar]