Abstract
Pial arteriovenous fistulas (AVFs) are rare neurovascular malformations. They differ from arteriovenous malformations (AVMs) in that they involve single or multiple feeding arteries, draining directly into a dilated cortical vein with no intervening nidus. Pial and dural AVFs differ in blood supply, as the first originate from pial or cortical arteries and the latter from outside the dural leaflets. Unlike dural AVFs, most of the pial AVFs are supratentorial. The vast majority are congenital, manifesting during infancy. Acquired pial AVFs are significantly rarer and occur after vasculopathy, head trauma, brain surgery, or cerebral vein thrombosis. We describe a unique case of an acquired pial AVF in a 50-year-old man secondary to a cortical vein thrombosis manifesting as a focal-onset seizure with secondary generalization. A cerebral digital subtraction angiography revealed a low-flow pial AVF fed by a postcentral branch of the left middle cerebral artery draining to the superior sagittal sinus via a cortical vein. It also showed a collateral venous circulation adjacent to the previously thrombosed left parietal vein. There was no evidence of an associated dural AVF or venous varix. Endovascular treatment was scheduled three months later, but the angiogram preceding the embolization showed spontaneous and complete closure of the malformation. To our knowledge, this is the first case illustrating acquired pure pial AVF unaccompanied by a dural component following cortical vein thrombosis, eventually resulting in an unprompted closure.
Keywords: Pial arteriovenous fistula, cortical vein thrombosis, magnetic resonance imaging, digital subtracted angiography, endovascular treatment.
Introduction
Pial arteriovenous fistulas (AVFs) are extremely rare, accounting for 1.6% of neurovascular malformations. They differ from the arteriovenous malformations (AVMs) by the absence of intervening nidus, as the supplying pial arteries, which can have one or more pedicles, drain directly into a single cortical vein.1–3 Most of the pial AVFs are supratentorial and of congenital origin, usually presenting in childhood. Acquired pial AVFs develop in adulthood resulting from trauma, brain surgery or cerebral vein thrombosis. 4 We describe herein an exceptionally rare case of acquired pial AVF following cortical vein thrombosis, ultimately resulting in spontaneous and complete closure.
Case report
A 50-year-old man presented to the emergency department with acute numbness and tingling of the right upper limb, followed by a focal-onset right clonic seizure with secondary generalization. A brain magnetic resonance imaging (MRI) showed a left parietal cortical vein thrombosis associated with adjacent venous infarct (Figure 1). Upon admission, the patient was treated with antiepileptic drugs and anticoagulation therapy. The neurological manifestations improved with no recurrent seizures. A complete hereditary and acquired thrombophilia screening was negative, and the treatment was stopped six months later. A follow-up brain MRI performed one year later revealed complete revascularization of the previously thrombosed cortical vein. Two years later, the patient presented with recurring similar episodes of focal seizures with dysarthria. An MRI was performed, showing flow void signals in the left frontoparietal convexity, suggestive of vascular malformation. No signs of recurrent thrombosis were noted (Figure 2). Further study with cerebral digital subtraction angiography (DSA) showed a pial AVF fed by a small postcentral branch of the left middle cerebral artery (MCA) and draining into the superior sagittal sinus (SSS) via a parietal cortical vein. No nidus or venous varix were found. It also displayed a left parietal collateral venous circulation adjacent to the previously thrombosed cortical vein (Figure 3). Selective external carotid artery displayed no associated dural AVF.
Figure 1.
Initial brain magnetic resonance imaging. (a) and (b) Axial T2* and T1 weighted images, respectively, showing an enlarged left parietal cortical vein presenting high T1 and low T2* signals relevant to a cortical vein thrombosis (arrows). (c) and (d) Axial fluid attenuated inversion recovery (FLAIR) and diffusion-weighted images, respectively, showing a hyperintensity FLAIR signal area in the left parietal convexity region associated with restricted diffusion consistent with venous infarct (star).
Figure 2.
Follow-up brain magnetic resonance imaging. (a) Axial T2 weighted image and (b) 3D T1 axial reformat image with maximal intensity projection reconstruction showing multiple dilated vascular structures in the left frontoparietal convexity (black arrows). (c) 3D T1 axial reformat image demonstrating the revascularization of the previously thrombosed left parietal cortical vein (white arrow). (d) Axial fluid attenuated inversion recovery image displaying a hyperintensity area of the left parietal region consistent with the venous infarct’s sequelae (arrowhead).
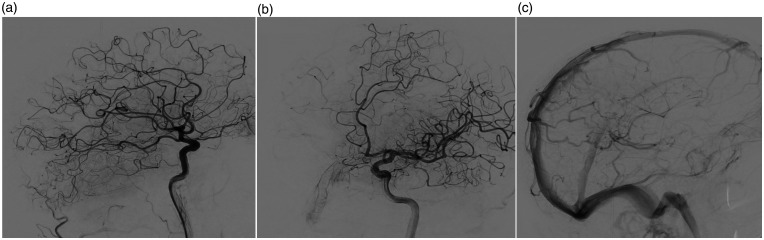
Figure 3.
Initial cerebral digital subtraction angiography of the left internal carotid artery. Lateral (a) and oblique (b) views during the early arterial phase showing an early opacification of a parietal cortical vein (arrowheads) fed directly by a small parietal branch of the left middle cerebral artery (arrow) consistent with pial arteriovenous fistula. (c) Lateral view during the venous phase demonstrating a left parietal collateral venous circulation adjacent to the previously thrombosed cortical vein.
The multidisciplinary committee decided on endovascular embolization of the pial AVF. Three months later, the patient was readmitted to our hospital for endovascular treatment. The cerebral DSA preceding the embolization showed spontaneous and complete regression of the pial AVF (Figure 4).
Figure 4.
Follow-up cerebral digital subtraction angiography of the left internal carotid artery. Lateral (a) and oblique (b) views during the early arterial phase showing the exclusion of the pial arteriovenous fistula. (c) Lateral view during the venous phase demonstrating a substantial decrease of the left parietal collateral venous circulation.
Discussion
This case illustrates an uncommon observation of a pial AVF between a small parietal branch of the left MCA and a cortical vein following cerebral venous thrombosis. To our knowledge, this is the first case of an acquired pure pial AVF unaccompanied by a dural component occurring in the wake of cortical vein thrombosis, eventuating in spontaneous closure.
Newton and Cronqvist 5 classified intracranial AVMs into three categories according to their arterial supply: pure pial, mixed pial-dural, and pure dural. Given the absence of intervening nidus or any dural supply involvement, our case’s most accurate angiographic term is a pure pial AVF. Halbach et al. 1 reported a series of 320 AVMs wherein AVFs accounted for nearly 1.6% of total neurovascular malformations. Most pial AVFs are congenital and usually present in childhood as part of the Rendu–Osler–Weber disease or Klippel–Trenaunay–Weber syndrome. 6 , 7 Acquired pial AVFs are extremely rare. Very few reports have related the occurrence of acquired pial AVF and its association with cortical vein thrombosis.8–10 Current evidence suggests that venous hypertension is the primary mechanism leading to AVFs following head trauma, brain surgery or cerebral vein thrombosis. Experimental venous hypertension induced in rats has been proved to lead to the development of acquired arteriovenous fistulas by stimulating the release of angiogenic factors and/or by prompting microscopic outgrowth of the arteriovenous shunts found within the vasa vasorum of the normal pachymeninges. 4 ,9–12 Various clinical presentations may accompany pial AVFs, such as seizures, haemorrhage, headache, neurological deficit and increased intracranial pressure. The natural course of this disease remains unsettled because of the scarcity of cases worldwide. In our case, it was a distal low-flow fistula, which may explain its spontaneous closure. Hoh et al. 13 suggested that the treatment of high-flow AVFs, especially with venous varix, be based on shunt disconnection by microsurgery or endovascular intervention rather than the resection of the lesion. The choice between these two therapeutic options is at professionals’ discretion by considering their personal experience.
Ozawa et al. 10 reported a pial AVF case with dural supply coming after SSS and the right transverse sinus thrombosis. The authors put forward that retrograde cortical venous drainage resulted in thrombosis propagation to the adjacent cortical vein by obliteration of the connections between the cortical veins and dural sinuses, thus developing a pial AVM. Endovascular treatment followed by surgical removal was accomplished without neurological complications.
Phatouros et al. 9 described a case of pial AVM occurring after an asymptomatic thrombosis of a superficial cerebral vein, which was incidentally discovered during a cerebral angiogram for an aneurysmal subarachnoid haemorrhage. The follow-up cerebral angiogram revealed a pial AVM between a small branch of the left MCA and the previously thrombosed cortical vein with distant dural AVF, which was not present initially. On account of the low-risk nature of the lesion, no endovascular treatment was performed.
Matsubara et al. 8 reported two cases of mixed pial and dural AVFs occurring after dural sinuses thrombosis in patients with protein S deficiency. Local fibrinolysis of the dural sinuses was performed in the acute phase. Mixed pial and dural AVFs had developed during the follow-up. Subsequently, the first patient had a craniotomy after an acute intracerebral haemorrhage. The second patient had embolization of the dural AVF and secondary surgical treatment for persistent shunt patency. The pial AVF came about in the craniotomy’s aftermath and was left untreated, considering it was asymptomatic.
Reported cases of pial AVFs following cerebral venous thrombosis are summarized in Table I.
Table I.
Reported cases of pial AVFs following cerebral vein thrombosis.
| Author | Age/sex | Thrombosed vein | Location of AVF | Type of AVF | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Ozawa et al. 10 (1998) | 39/M | SSS and right transverse sinus | Right parietal | Pial and dural AVFs | Embolization +surgery | Cure |
| Phatouros et al. 9 (1999) | 51/M | Left superficial middle cerebral vein | Left Sylvian fissure | Pial and dural AVFs | None | No cure |
| Matsubara et al. 8 (2014) | 38/M | SSS | Right Sylvian fissure | Pial and dural AVFs | Surgery | Cure |
| 50/M | SSS and right sigmoid sinus | Left parietal | Pial and dural AVFs | None for pial AVF | No cure | |
| Our case (2020) | 50/M | Left parietal cortical vein | Left parietal | Pial AVF | None | Spontaneous exclusion |
M: male; AVF: arteriovenous fistula; SSS: superior sagittal sinus.
In our patient, the cortical vein thrombosis probably gave rise to venous hypertension and secondarily led to an uncommon finding, that is, pial AVF adjacent to the thrombosis site. This AVF was suspected when the MRI detected abnormal flow voids that did not previously exist in the first MRI. The spontaneous closure of the pial AVF was probably due to the low-flow nature of this malformation. Due to the scarcity of reported cases, optimal management is yet to be defined.
We report a unique observation of an acquired pial AVF, manifesting as a complication of cortical vein thrombosis. This case is distinctive by the pure form of the pial AVF and its spontaneous closure.
Supplemental Material
Supplemental material, sj-pdf-1-neu-10.1177_19714009211049080 for Acquired pial arteriovenous fistula secondary to cerebral cortical vein thrombosis: A case report and review of the literature by Skander Sammoud, Nadia Hammami, Dhaker Turki, Fatma Nabli, Samia Ben Sassi, Samir Belal, Cyrine Drissi and Mohamed Ben Hamouda in The Neuroradiology Journal
Footnotes
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Skander Sammoud https://orcid.org/0000-0001-7612-5924
References
- 1.Halbach VV, Higashida RT, Hieshima GB, et al. Transarterial occlusion of solitary intracerebral arteriovenous fistulas. AJNR Am J Neuroradiol 1989; 10: 747–752. [PMC free article] [PubMed] [Google Scholar]
- 2.Lv X, Liang S. Update Onyx embolization for plexiform arteriovenous malformation: Ante-grade drifting technique. Neuroradiol J 2020; 33: 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Liang S, Zhang W, et al. Liquid embolic agent Fe3O4-EVOH for endovascular arteriovenous malformation embolisation: Preliminary evaluation in an in vivo swine rete mirabile model. Neuroradiol J 2020; 33: 306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu J, Shi L, Lv X, et al. Intracranial non-galenic pial arteriovenous fistula: A review of the literature. Interv Neuroradiol 2016; 22: 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology 1969; 93:1071–1078. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Ye X, Gao F, et al. Intracranial pial arteriovenous fistulas: Two case reports. Int J Clin Exp Med 2018; 11: 5269-5274. [Google Scholar]
- 7.Alurkar A, Karanam LSP, Nayak S, et al. Intracranial pial arteriovenous fistulae: Diagnosis and treatment techniques in pediatric patients with review of literature. J Clin Imaging Sci 2016; 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsubara S, Satoh K, Satomi J, et al. Acquired pial and dural arteriovenous fistulae following superior sagittal sinus thrombosis in patients with protein S deficiency: A report of two cases. Neurol Med Chir 2014; 54: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phatouros C C, Halbach VV, Dowd C F, et al. Acquired pial arteriovenous fistula following cerebral vein thrombosis. Stroke 1999; 30: 2487–2490. [DOI] [PubMed] [Google Scholar]
- 10.Ozawa T, Miyasaka Y, Tanaka R, et al. Dural-pial arteriovenous malformation after sinus thrombosis. Stroke 1998; 29: 1721–1724 [DOI] [PubMed] [Google Scholar]
- 11.Terada T, Higashida RT, Halbach VV, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg 1994; 80: 884–889. [DOI] [PubMed] [Google Scholar]
- 12.Herman JM, Spetzler RF, Bederson JB, et al. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg 1995; 83: 539–545. [DOI] [PubMed] [Google Scholar]
- 13.Hoh BL, Putman CM, Budzik RF, et al. Surgical and endovascular flow disconnection of intracranial pial single-channel arteriovenous fistulae. Neurosurgery 2001; 49: 1351–1363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-neu-10.1177_19714009211049080 for Acquired pial arteriovenous fistula secondary to cerebral cortical vein thrombosis: A case report and review of the literature by Skander Sammoud, Nadia Hammami, Dhaker Turki, Fatma Nabli, Samia Ben Sassi, Samir Belal, Cyrine Drissi and Mohamed Ben Hamouda in The Neuroradiology Journal