Abstract
SARS-CoV-2 transmission remains a global problem which exerts a significant direct cost to public health. Additionally, other aspects of physical and mental health can be affected by limited access to social and exercise venues as a result of lockdowns in the community or personal reluctance due to safety concerns. Swimming pools reopened in the UK on April 12th 2021, but the effect of swimming pool water on inactivation of SARS-CoV-2 has not yet been directly demonstrated. Here we demonstrate that chlorinated water which adheres to UK swimming pool guidelines is sufficient to reduce SARS-CoV-2 infectious titre by at least 3 orders of magnitude.
Keywords: SARS-CoV-2, Coronavirus, Chlorine, Swimming pools, Water, Inactivation
Graphical abstract
1. Introduction
SARS-CoV-2, the causative agent of the COVID-19 pandemic, continues to transmit globally and makes quantifying the risks involved in different settings of great importance as societies attempt to return to normal. Airborne transmission is accepted as the primary route of spread of SARS-CoV-2 (Greenhalgh et al., 2021; Lednicky et al., 2020; van Doremalen et al., 2020) but many have proposed waterborne transmission particularly through wastewater as a secondary route. SARS-CoV-2 RNA has been detected in wastewater around the world (Ahmed et al., 2020; La Rosa et al., 2020) and live SARS-CoV-2 virus can be isolated from faecal material (Xiao et al., 2020) suggesting that the faecal-oral and/or faecal-nasal route of transmission could present a viable risk to humans (Cahill and Morris, 2020). Other human coronaviruses such as 229E are inactivated after 4 days in wastewater and 10 days in tap water at 23 °C (Gundy et al., 2009) and SARS-CoV, the causative agent of the 2003 SARS outbreak, persists for 2–3 days in wastewater and faeces (Wang et al., 2005).
Chlorine is often used to disinfect water as it has broad activity against human pathogens (CDC, 2012) and it has been demonstrated that a residual free chlorine level of 0.5 mg l−1 results in complete inactivation of SARS-CoV over the 30 min timeframe tested (Wang et al., 2005). While efforts have been made to use in vitro results to model the potential risk of faecal-oral transmission through global water systems (Shutler et al., 2021), the survival of SARS-CoV-2 specifically in chlorinated swimming pools has not yet been investigated.
Outbreaks of respiratory viruses such as adenoviruses, and enteric viruses such as enteroviruses, Hepatitis A and noroviruses which can transmit by the faecal-oral route are sometimes linked to swimming pools but this is often due to improper maintenance of chlorine levels (Bonadonna and La Rosa, 2019; 2000). In the UK between June 2020 and July 2021, most commercial pools adhered to guidelines for treatment with a chlorine based disinfectant to maintain a free chlorine level of 1.5–3 mg l−1 or parts per million (ppm), with a pH range of 7.0 – 7.6 as the availability of active free chlorine decreases with increasing pH (2020). Since the removal of lockdown measures in the UK these guidelines have been revised to include a free chlorine level of 2.0 ppm when the pH is between 7.2 and 7.4 (2021). Here, by treating SARS-CoV-2 with swimming pool water which conforms to UK guidelines we demonstrate at least a 3-log10 reduction in infectious titre.
2. Results
2.1. Generating SARS-CoV-2 virus stocks suitable for inactivation testing
Virus stocks of SARS-CoV-2 for use in infectivity assays are generally generated by infection of a permissive cell line such as Vero and harvesting of virus in highly buffered cell culture medium. However, we observed in preliminary experiments that when testing the chlorine levels in water samples before and after adding a 1:1000 vol of buffered cell culture medium, free chlorine levels fell below the limit of detection of the equipment used. In contrast, adding a 1:100 vol of an unbuffered saline solution resulted in a negligible drop in free chlorine levels in the water sample. The buffering capacity of the virus stock itself in cell culture medium would make it difficult to observe inactivation at the desired free chlorine and pH levels during testing. To overcome this issue, we generated a stock of SARS-CoV-2 virus in unbuffered solution. By infecting Caco-2 and Vero cells with a SARS-CoV-2 B1 lineage virus at a multiplicity of 0.01 plaque forming units (pfu) cell−1, extensively washing off and replacing the growth medium with saline solution 24 h before harvest at 3 days post-infection, we were able to generate stocks of infectious virus with reduced buffering capacity. To further minimise the effect of the non-viral constituents of the stock, such as cellular components which would exert a chlorine demand on the water samples tested, a 1:100 dilution of virus in normal saline was used in all inactivation tests.
2.2. Inactivation of SARS-CoV-2 by chlorinated water
Water was collected from swimming pools in volumes of up to 1 litre and transported to the laboratory on the same day. The water was tested for free chlorine and pH levels onsite and upon arrival at the laboratory and adjusted to a range of values. A 10 µl volume of the SARS-CoV-2 virus stock generated in Caco-2 cells was then added to duplicate water samples in a total volume of 1 ml (1:100 dilution of virus stock), incubated for 30 s at room temperature before quenching the chlorine with a one-tenth volume of 10X cell culture medium. Residual virus infectivity in the samples was then titrated on Vero cells by TCID50 assay commonly used to measure survival of viruses after treatment (Chin et al., 2020; Gundy et al., 2009; Payment and Trudel, 1979). In each experiment the same virus stock was incubated for 30 s at a 1:100 dilution in PBS as a control.
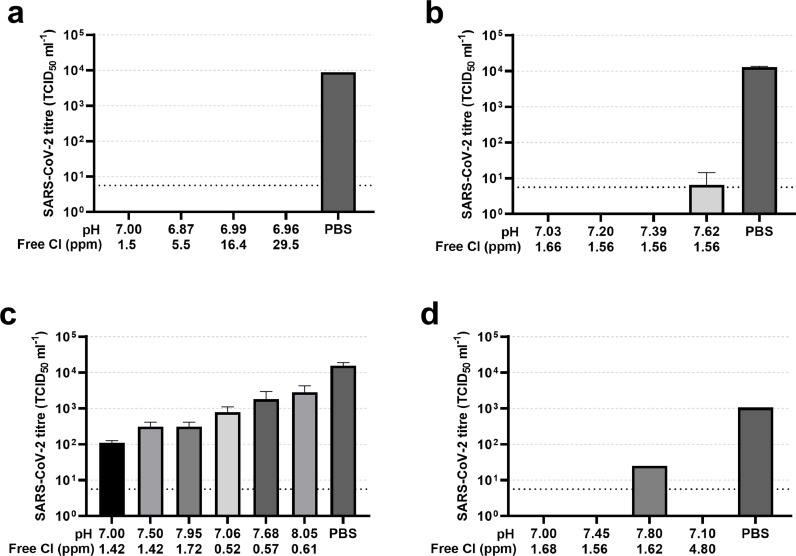
Firstly, the effect of a range of increasing free chlorine levels in water starting at the minimum 1.5 ppm recommended in UK swimming pools until July 2021 were tested (Fig. 1 a). A low pH of approximately pH 7.0 was used to give the best chance of observing virus inactivation as availability of free chlorine is maximized at lower pH. Under these conditions no detectable virus infectivity remained after 30 s demonstrating at least a 3-log10 reduction in infectious titre compared to the PBS control where approximately 1 × 104 TCID50 ml−1 of virus was measured (Fig. 1a). We next measured residual SARS-CoV-2 infectivity in conditions with higher pH while keeping the free chlorine level at approximately 1.5 ppm. Inactivation was observed to undetectable levels in all conditions except at the elevated pH of 7.62 at which low levels of virus infectivity were still observed at the threshold of detection of the assay. This inactivation equated to 3-log10 decreased infectivity compared to the control (Fig. 1b). To demonstrate the interaction between the variables of pH and free chlorine in causing inactivation of SARS-CoV-2 infectivity, swimming pool water samples either at (1.42–1.72 ppm) or below (0.52–0.61 ppm) the UK recommended free chlorine levels were modified to pHs of approximately 7, 7.5 and 8. This resulted in only partial inactivation of the virus infectivity and revealed the importance of both chlorine levels and pH to achieve inactivation. (Fig. 1c). Finally, we generated a further stock of the SARS-CoV-2 lineage B.1 virus in unbuffered saline in Vero cells and tested it against water at 3 pH levels at chlorine levels of 1.56 – 1.68 ppm. The new stock had a lower titre resulting in a yield of 1 × 103 TCID50 ml−1 from the PBS control condition. Nonetheless full inactivation equating to a greater than 2 log10 drop in infectivity was observed at pH 7.00 and pH 7.45, and even at pH 7.80 the infectivity was decreased more than 50-fold (Fig. 1d).
Fig. 1.
Exposure to chlorinated water inactivates SARS-CoV-2. Water samples taken from a swimming pool were modified in the laboratory to a range of pH and free chlorine values. A known amount of infectious SARS-CoV-2 was added to duplicate water samples in a volume of 1 ml, incubated for 30 s at room temperature and any remaining infectious virus then titrated by TCID50 on Vero cells. Residual virus titres are shown as the mean and standard deviation of duplicate TCID50 ml−1 values. Successive experiments were performed with varying free chlorine levels (a), varying pH (b), a range of both pH and free chlorine levels (c), and an independent preparation of virus at a range of pH and chlorine levels (d). A PBS control was included in each experiment to validate the infectivity of the virus input. Lower pH and higher free chlorine levels resulted in greater inactivation of SARS-CoV-2. A pH of no more than 7.4 and free chlorine above 1.5 parts per million (ppm) resulted in at least a 3-log10 reduction in viral titre. Dotted line indicates the lower limit of detection of the assay.
3. Conclusion
Swimming pools have reopened in the UK as of April 12th 2021 and therefore present locations of possible COVID-19 transmission. The likelihood of transmission events occurring in shared areas such as changing rooms can be minimised with social distancing and hygiene measures around the pool but different variables affect any risk associated with time spent in the water. Chlorination of swimming pool water has been used for decades to mitigate any onwards transmission of pathogens between swimmers. However, since the causative agent of COVID-19, the betacoronavirus named SARS-CoV-2, only emerged in late 2019, inactivation of SARS-CoV-2 by chlorinated water has not yet been directly demonstrated prior to this study. Since viruses cannot replicate outside of a host, a transmission event via swimming pool water would require that virus emitted directly from a bather reached another at a sufficient infectious dose. Firstly, emitted virus will be greatly diluted before this occurs, potentially below a minimal infectious dose. In addition, if chlorinated water is directly viricidal against SARS-CoV-2, the likelihood of infectious virus being transmitted in swimming pool water will be further lowered. Demonstrating this may be important in increasing public confidence in returning to pools. Here we demonstrate that inactivation of SARS-CoV-2 in chlorinated swimming pool water is dependant on free chlorine and pH levels with increased inactivation at higher free chlorine and lower pH. We show that 30 s contact time at room temperature with water of a pH of no more than 7.4 and free chlorine above 1.5 mg l−1 (ppm) resulted in at least a 3-log10 reduction in viral titre within 30 s (Fig. 1). These levels are within the recommendations for swimming pools from June 2021 to July 2021 of the pandemic in the UK of at least 1.5 ppm free chlorine at pH 7.0, 2.0 ppm at pH 7.4 and 2.7 ppm at pH 7.6 (2020). The newly revised UK guidelines that swimming pools at pH 7.2 – 7.4 should have a minimum free chlorine level of 2.0 ppm is also supported by our observation that 1.5 ppm is adequate at pH 7.4 (2021). We found here that some residual virus was detected after treatment with water above pH 7.4 even when at least 1.5 ppm free chlorine was present.
A limitation of this study is that we did not test survival of SARS-CoV-2 contained within mucus or saliva mixed with swimming pool water. Also SARS-CoV-2 has been demonstrated to be heat labile with rapid inactivation above 65 °C and prolonged survivability at 4 °C, showing the importance of water temperature in assessing inactivation and the risk presented by water systems (Abraham et al., 2020; Chin et al., 2020; Shutler et al., 2021). It is recommended that pools in the UK are kept at 26 °C-32 °C (2021) and while we did not measure or determine the effect of temperature in this study, our pool water samples at the time of testing would be at this temperature or have decreased to closer to ambient temperature, particularly in the small volumes used. However, it is possible that pools below 26 °C would permit survival of SARS-CoV-2 at the chlorine and pH levels we tested.
Further we were only able to test reduction of a virus stock with starting infectivity around 1 × 104 TCID50 ml−1 due to the limited replication of SARS-CoV-2 in the laboratory and the need to use a minimal volume of virus material during testing. Nonetheless, this viral challenge of 1 × 104 infectious particles equates to approximately 5 × 107 total virus particles as measured by RT-qPCR to detect virus genomes. This corresponds to a cycle threshold (Ct) value of ∼21 which is greatly in excess of the viral load typically detected in the upper respiratory tract of asymptomatic people, with an average Ct of 31.15 (Ra et al., 2021). The route by which any residual virus in swimming pool water might infect another swimmer is not clear. SARS-CoV-2 is transmitted in the air and also by direct inoculation. There is also a potential faecal-oral route of transmission for SARS-CoV-2 (Cahill and Morris, 2020; Guo et al., 2021).
Our findings on the susceptibility of SARS-CoV-2 to inactivation by swimming pool water underscore the importance for those who maintain swimming pools to adhere to UK guidelines for chlorination, and this should give confidence in the safety of bathers when in the water. Finally, we stress that swimmers should continue to adhere to locally recommended social distancing rules both in and out of the water.
4. Methods
4.1. Cells and viruses
African green monkey kidney (Vero) cells (Nuvonis Technologies) were maintained in OptiPRO SFM (Life Technologies) containing 2X GlutaMAX (Gibco). Human epithelial colorectal adenocarcinoma (Caco-2) cells were maintained in DMEM, 20% foetal calf serum, 1% non-essential amino acids, 1% penicillin-streptomycin. SARS-CoV-2 linage B.1 isolate hCoV-19/England/IC19/2020 (EPI_ISL_475572) was diluted in cell growth medium and used to infect confluent cells at a multiplicity of 0.01 pfu cell−1 and incubated at 37 °C, 5% CO2. Growth medium was removed 2 days post infection, the cell sheet washed twice with saline solution (ddH2O, 0.9% NaCl) and replaced with saline solution. After a further 24 hrs virus supernatant was harvested and clarified by centrifugation.
4.2. Water samples
Swimming pool water samples were collected from pools at approximately 28 °C in London, UK by dipping a sterile bottle approx. 30 cm below the surface of the pool, and then tested onsite and upon arrival at the laboratory. Free chlorine and pH levels were tested using a MD 100 photometer (Lovibond) to the manufacturer's instructions for tests in Fig. 1a–c and a PoolTest 25 (Palintest) for the test in Fig. 1d. Consistent with methods used in swimming pools in the UK (2021), chlorine levels of the water samples were increased by addition of sodium hypochlorite and pH was increased by addition of sodium carbonate or decreased using sodium bisulphate before retesting. Inactivation experiments were performed within 30 min of water sample preparation to minimise decay of chlorine levels.
4.3. Inactivation testing and titration of residual virus by TCID50 assay
Treatment of SARS-CoV-2 with water samples was carried out as described in the text. In short 10 µl of virus stock was added to 990 µl of water sample, incubated for 30 s at room temperature before addition of 110 µl of 10X MEM. Titration of residual virus was performed by TCID50 assay on Vero cells using cytopathic effect as the readout for infectious virus (Payment and Trudel, 1979). In short, a half-log10 dilution series of each sample was performed and 4 replicates of each dilution transferred to 96-well plates of Vero cells, incubated for 1 hr at 37 °C, 5% CO2 and replaced with cell growth medium. After 4 days, cells were stained with crystal violet and scored for either an intact, stained cell sheet or the absence of cells due to virus-induced cytopathic effect. For each condition, the Spearman-Karber method was used to calculate the 50% tissue culture infectious dose (TCID50) of the residual virus.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Alex Blackwell (AB) works for Water Babies as their Group Head of Facilities and Operational Management. Water Babies is a swim school franchise network that hires pools to provide swimming lessons. Water Babies does not manufacture or sell swimming pool(s), equipment or disinfection products. AB sourced funding for this project from Swim England, Royal Life Saving Society UK (RLSS UK) and the Spatex Foundation. AB acts as the Lifeguard advisor for the RLSS UK and is also a member of the Pool Water Treatment Advisory Group - Industry Forum.
Acknowledgement
We would like to thank Water Babies, Swim England, SPATEX Foundation and RLSS UK for their help, support and funding for this work. Special thanks to Ian Ogilvie (CMIOSH, Independent Pool Consultant), Richard Lamburn (Swim England), Ellen Meyer (Sigura), Rachel Chalmers (Public Health Wales), John Lee (PWTAG), Hannah Smith (Water Babies) and Jared Crockford (Kingfisher Environmental) for providing technical expertise.
References
- Abraham J.P., Plourde B.D., Cheng L. Using heat to kill SARS-CoV-2. Rev. Med. Virol. 2020;30 doi: 10.1002/rmv.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W.…Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna L., La Rosa G. A review and update on waterborne viral diseases associated with swimming pools. Int. J. Environ. Res. Public Health. 2019;16 doi: 10.3390/ijerph16020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill N., Morris D. Recreational waters – a potential transmission route for SARS-CoV-2 to humans? Sci. Total Environ. 2020;740 doi: 10.1016/j.scitotenv.2020.140122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2012). Effectiveness on pathogens | the safe water system | CDC. Retrieved August 16, 2021, from https://www.cdc.gov/safewater/effectiveness-on-pathogens.html.
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-.L., Chan M.C.W.…Poon L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1):e10. doi: 10.1016/s2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh T., Jimenez J.L., Prather K.A., Tufekci Z., Fisman D., Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021;397:1603–1605. doi: 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1(1):10–14. doi: 10.1007/S12560-008-9001-6. [DOI] [Google Scholar]
- Guo M., Tao W., Flavell R.A., Zhu S. Potential intestinal infection and faecal–oral transmission of SARS-CoV-2. Nat. Rev. Gastroenterol. Hepatol. 2021;18:269–283. doi: 10.1038/s41575-021-00416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L.…Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lednicky J.A., Lauzard M., Fan Z.H., Jutla A., Tilly T.B., Gangwar M.…Wu C.Y. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMS. (2000). Guidelines for safe recreational-water environments final draft for consultation Vol. 2: swimming pools, spas and similar recreational-water environments. Retrieved from http://www.who.int/water_sanitation_health/bathing/bathing2/es/.
- Payment P., Trudel M. Methods and techniques in teaching. Teach. Psychol. 1979;6(3):170–179. doi: 10.1207/s15328023top0603_13. [DOI] [Google Scholar]
- PWTAG. (2020). Swimming pool technical operation after COVID-19 shutdown | PWTAG. Retrieved from https://www.pwtag.org/swimming-pool-technical-operation-after-covid-19-shutdown/.
- PWTAG. (2021). PWTAG code of practice | Pool water treatment advisory group. Retrieved from https://www.pwtag.org/code-of-practice/.
- Ra S.H., Lim J.S., Kim G.U., Kim M.J., Jung J., Kim S.H. Upper respiratory viral load in asymptomatic individuals and mildly symptomatic patients with SARS-CoV-2 infection. Thorax. 2021;76(1):61–63. doi: 10.1136/thoraxjnl-2020-215042. [DOI] [PubMed] [Google Scholar]
- Shutler J.D., Zaraska K., Holding T., Machnik M., Uppuluri K., Ashton I.G.C.…Dahiya R.S. Rapid assessment of SARS-CoV-2 transmission risk for fecally contaminated river water. ACS ES&T Water. 2021;1(4):949–957. doi: 10.1021/acsestwater.0c00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N.…Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/nejmc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-W., Li J.-S., Jin M., Zhen B., Kong Q.-X., Song N.…Li J.-W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126(1):171. doi: 10.1016/J.JVIROMET.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Sun J., Xu Y., Li F., Huang X., Li H.…Zhao J. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerging Infect. Dis. 2020;26(8):1920–1922. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]