Abstract
Introduction
The risk of bleeding associated with transjugular kidney biopsies is unclear, and which patients are the best candidates for this route is unknown.
Methods
This was a retrospective cohort study comparing proportion of bleeding associated with transjugular versus percutaneous native kidney biopsies in all patients in France in the 2010–2019 period. Major bleeding at day 8 (i.e., blood transfusions, hemorrhage/hematoma, angiographic intervention, nephrectomy) and risk of death at day 30 were assessed, and we used a bleeding risk score initially developed for the percutaneous route.
Results
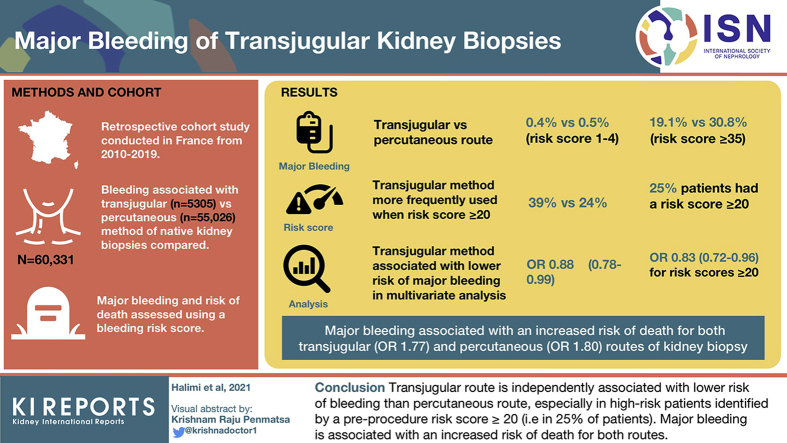
Our analysis included 60,331 patients (transjugular route: 5305; percutaneous route: 55,026 patients). The observed proportion of major bleeding varied widely (transjugular vs. percutaneous): 0.4% versus 0.5% for the lowest risk scores (0–4) to 19.1% versus 30.8% for the highest risk scores (≥35). Transjugular was more frequently used than percutaneous route (39% vs. 24%) when the risk score was ≥20 (15,133/60,331; 25% of all patients). Transjugular was associated with a lower risk of major bleeding than percutaneous route in multivariate analyses (odds ratio [OR]: 0.88 [0.78–0.99]), especially for scores ≥20 (OR: 0.83 [0.72–0.96], (i.e., 25% of patients). Major bleeding was associated with an increased risk of death both for transjugular (OR: 1.77 [1.00–3.14]) and percutaneous (OR: 1.80 [1.43–2.28]) routes.
Conclusions
The transjugular route is independently associated with a lower risk of bleeding than the percutaneous route, especially in high-risk patients identified by a preprocedure risk score ≥20 (i.e., 25% of patients). Major bleeding is associated with an increased risk of death for both routes.
Keywords: bleeding score, epidemiology, kidney biopsy, percutaneous, transjugular
Graphical abstract
See Commentary on Page 2535
Kidney biopsies provide important diagnostic information and guide optimal management of patients with kidney abnormalities. The risk of major bleeding associated with percutaneous kidney biopsies is highly variable.1,2 To guide the decision of kidney biopsy and the choice of the most adequate procedure, a preprocedure major bleeding score was recently developed in 50,000 patients undergoing a percutaneous kidney biopsy.2
The percutaneous route is preferred in most patients, but the need for alternative techniques remains for several reasons. First, although accurate histological diagnosis is crucial to identify the cause of renal diseases and propose the most accurate treatments, the percutaneous route is impossible in some patients. Second, patients who most need accurate histological diagnosis are often critically ill, including patients with acute kidney injury, abnormal renal function, vasculitis, thrombotic microangiopathy, thrombocytopenia, and anemia, and these comorbid conditions constitute major risks factors for bleeding after percutaneous kidney biopsy.2 Third, the risk of major bleeding associated with percutaneous kidney biopsy is unacceptably high in some patients, and this risk can now be anticipated in many patients using a score predicting the risk of major bleeding2: with this information in hand, many physicians and patients may refuse the risk of percutaneous kidney biopsy. Finally, the dilemma between performing a percutaneous kidney biopsy in patients with a high risk of major bleeding (associated with a subsequent 2-fold risk of death2) or ignoring the cause of the kidney disease with the accepted consequence of a higher risk of short-term end-stage kidney disease is unacceptable for ethical reasons.1
Transjugular kidney biopsy was first reported in the early 1990s.3 This technique is appealing because no difference in the diagnosis yield was found in 400 patients who had a transjugular kidney biopsy versus those who had a percutaneous kidney biopsy.4 More recent data from the same center reported similar results.5 However, the exact risk of major bleeding is unknown because it derives from a few, mostly small case studies, which they may be vulnerable to publication and selection biases.4, 5, 6, 7, 8 The rate of major complications seem to vary from 0% to 25%.4, 5, 6, 7, 8 It is currently difficult to know whether the high risk of major bleeding associated with the transjugular route reported in some case series is due to patient selection or to the procedure itself.4, 5, 6, 7, 8
In the present study, we assessed the risk of major bleeding of patients who had a transjugular kidney biopsy in France from 2010 to 2019 and compared it with patients who had a percutaneous kidney biopsy during the same period. We assessed whether the recently published major bleeding risk score could be applied to patients who had a transjugular kidney biopsy and whether it could identify patients who could benefit most from the transjugular route.
Methods
Study Design
This longitudinal cohort study was based on the national hospitalisation database covering hospital care from the entire French population. The data for all patients admitted in hospital in France from 1 January 2008 to 31 December 2019, in whom a native kidney biopsy was performed, were collected from the national medico-administrative PMSI database (“programme de médicalisation des systèmes d’information,” i.e., medicalized information system program), which was inspired by the US Medicare system.2
Briefly, this program includes more than 98% of the French population (67 million people) from birth (or immigration) to death (or emigration). Routinely collected medical information includes the principal and secondary diagnoses according to the International Classification of Diseases, Tenth Revision (ICD-10).2,9, 10, 11, 12, 13
The study was conducted retrospectively, and because patients were not involved in its conduct, there was no impact on their care. Ethical approval was not required because all data were anonymized. The French Data Protection Authority granted access to the PMSI data. Procedures for data collection and management were approved by the Commission Nationale de l’Informatique et des Libertés (CNIL), the independent national ethical committee protecting human rights in France, which ensures that all information is kept confidential and anonymous, in compliance with the Declaration of Helsinki. This study requires neither information nor consent of the included individuals. Access to linked anonymous file in the PMSI databases was approved by the CNIL (MR-005 registration number 0415141119).2
Patient Selection
We restricted the analysis to patients admitted after 2010 because this allowed us to obtain at least 2 years of past events to define comorbidities since 2008. Patients who had a transjugular kidney biopsy according to ICD-10 codes (Supplementary Table 1: ICD-10 code JAHH002); they were compared with patients who had a percutaneous native kidney biopsy (Supplementary Table 1: ICD-10 codes JAHB001, JAHJ006, JAGJ007) in France during the 2010–2019 period. Of note, data regarding patients who had a percutaneous kidney biopsy during the 2010–2018 period have already been reported.2
Major Bleeding and Risk of Death After Biopsy
Major bleeding (blood transfusion; ICD-10 codes FELF011, JAFA023), hematoma/hemorrhage (ICD-10 code T810), angiographic intervention (ICD-10 codes EDSF003, EDSF008), and nephrectomy (ICD-10 codes JAFA002, JAFA023) during an 8-day period after kidney biopsy was ascertained by diagnosis code (Supplementary Table 1). For the risk of death associated with major bleeding after biopsy, a 30-day period was considered.
Collected Data
Demographic, cardiovascular and metabolic conditions
Patient information (demographics, comorbidities, medical history, and events during hospitalization or follow-up) was described using data collected in the hospital records. For each hospital stay, combined diagnoses at discharge were obtained. Each variable was identified using ICD-10 codes (Supplementary Table 1). We also used the Charlson Comorbidity Index and the Claims-Based Frailty Indicator to assess patient clinical status.2,14,15 Because the information was based on codes, there were no missing values. Cardiovascular and metabolic conditions of interest included hypertension, diabetes, obesity, heart failure, valve diseases, coronary artery disease, smoking, dyslipidemia, stroke, vascular disease, and atrial fibrillation.
Kidney diagnoses known at the time of biopsy
These parameters included reported history of acute kidney failure, glomerular disease, vascular or hypertensive kidney disease, diabetic kidney disease, autoimmune diseases, vasculitis, thrombotic microangiopathy, hematological-related kidney diseases, amyloidosis, and other kidney diseases (Supplementary Table 1).
Other relevant parameters
We collected information regarding history of alcohol-related diagnoses, lung diseases, liver diseases, cancer within the years preceding the biopsy, thrombocytopenia, and anemia. Medications including antiplatelet agents and anticoagulants were not available.
Statistical Analyses
Data are presented as mean and SD for quantitative parameters and percentages for categorical parameters, respectively. Patients who had major bleeding complications (blood transfusion, hematoma/hemorrhage, angiographic intervention, or nephrectomy) within 8 days of the biopsy were compared with other patients using Student’s t or chi-square test as appropriate. Multivariable logistic regressions were used, and results were expressed as odds ratio (OR) and 95% confidence interval (CI).
The proportion of major bleeding in patients who had a transjugular kidney biopsy and those who had a percutaneous kidney biopsy was compared. We assessed whether our previously published major bleeding risk score initially developed in patients with percutaneous kidney biopsy (Table 1)2 could be applied in patients with transjugular kidney biopsy. The proportion of patients who had a transjugular versus percutaneous kidney biopsy as well as the proportion of major bleeding in both groups were compared according to this score. Receiver operating characteristic curves were constructed and areas under the curve (c-indexes) with 95% CIs were calculated to evaluate the predictive ability of major bleeding events after kidney biopsy of the score; these were compared using the DeLong test.
Table 1.
Major bleeding risk score
| Components of the score∗ | Points |
|---|---|
| 5 | |
| Age | |
| 45–60 | 1 |
| 61–71 | 2 |
| >71 | 3 |
| Charlson Comorbidity Index | |
| 2—4 | 1 |
| 5–6 | 2 |
| >6 | 3 |
| Frailty index | |
| 1.5–4.4 | 1 |
| 4.5–9.5 | 2 |
| >9.5 | 3 |
| Sex (male) | –1 |
| Dyslipidemia | –1 |
| Obesity | –1 |
| Anaemia | 8 |
| Thrombocytopenia | 2 |
| Cancer within preceding years | 2 |
| Abnormal renal function | 2 |
| Acute renal failure | 4 |
| Glomerular disease | –1 |
| Vascular or hypertensive renal disease | –1 |
| Diabetic kidney disease | –1 |
| Autoimmune disease | 2 |
| Vasculitis | 5 |
| Hematological-related renal disease | 2 |
| Thrombotic microangiopathy | 4 |
| Amyloidosis | –2 |
| Other renal presentation | –1 |
Score: from 0 (minimal score) to 41 (maximal score) points
= sum of the points + 5
Results
Baseline Characteristics
Overall, 60,331 patients were included in the present study: 5305 (8%) patients had a transjugular kidney biopsy; 55,026 (92%) patients who had a percutaneous native kidney biopsy (95% of whom were previously described2) were used as controls.
Patients who had a transjugular kidney biopsy had similar age and gender compared with patients with percutaneous kidney biopsy but had more frequent serious comorbid conditions such as malnutrition, cancer, elevated Charlson and frailty index, anemia, thrombocytopenia, liver disease, vascular disease, coronary artery disease, atrial fibrillation, and stroke (Table 2).
Table 2.
Baseline characteristics
| Tranjugular kidney biopsy |
Percutaneous kidney biopsy |
P value | |
|---|---|---|---|
| n = 5305 | n = 55,026 | ||
| Age, years | 58 ± 17 | 58 ± 17 | 0.53 |
| Sex (male) | 3172 (60) | 33,523 (61) | 0.11 |
| Charlson Comorbidity Index | 5.9 ± 3.3 | 4.6 ± 2.8 | <0.0001 |
| Frailty index | 9.4 ± 8.4 | 7.1 ± 7.7 | <0.0001 |
| Comorbid conditions | |||
| Hypertension | 3399 (64) | 29,392 (53) | <0.0001 |
| Diabetes mellitus | 1589 (30) | 12,376 (23) | <0.0001 |
| Obesity | 1154 (22) | 8626 (16) | <0.0001 |
| Heart failure | 952 (18) | 5827 (11) | <0.0001 |
| Valve disease | 338 (6) | 2454 (5) | <0.0001 |
| Coronary artery disease | 975 (18) | 5486 (10) | <0.0001 |
| Vascular disease | 811 (15) | 5889 (11) | <0.0001 |
| Atrial fibrillation | 661 (13) | 4839 (9) | <0.0001 |
| Ischemic stroke | 208 (4) | 1006 (2) | <0.0001 |
| Smoking | 650 (12) | 5565 (10) | <0.0001 |
| Dyslipidemia | 1477 (28) | 10,257 (19) | <0.0001 |
| Malnutrition | 632 (12) | 4865 (9) | <0.0001 |
| Alcohol-related diagnoses | 705 (13) | 3689 (7) | <0.0001 |
| Lung disease | 738 (14) | 5749 (10) | <0.0001 |
| Liver disease | 945 (18) | 3345 (6) | <0.0001 |
| Anaemia | 1863 (35) | 13,382 (24) | <0.0001 |
| Thrombocytopenia | 675 (13) | 3854 (7) | <0.0001 |
| History of cancer | 1423 (27) | 13,264 (24) | <0.0001 |
| Kidney diagnoses at the time of biopsy | |||
| Abnormal renal function | 2367 (45) | 17,566 (32) | <0.0001 |
| Acute renal injury | 2447 (46) | 16,671 (30) | <0.0001 |
| Glomerular disease | 1673 (32) | 18,018 (33) | 0.07 |
| Vascular or hypertensive-related kidney disease | 517 (10) | 4751 (9) | 0.01 |
| Diabetic kidney disease | 435 (8) | 3974 (7) | 0.01 |
| Autoimmune kidney disease | 537 (10) | 3307 (6) | <0.0001 |
| Vasculitis | 123 (2) | 2335 (4) | <0.0001 |
| Hematological-related kidney disease | 111 (2) | 1716 (3) | <0.0001 |
| Thrombotic microangiopathy | 250 (5) | 969 (2) | <0.0001 |
| Amyloidosis | 47 (0.9) | 204 (0.4) | <0.0001 |
| Other diagnosis | 56 (1) | 688 (1) | 0.22 |
Values are in n (%).
Risk of Major Bleeding
Transjugular route was associated with a more frequent major bleeding than percutaneous route (7% vs. 5%, P < 0.001). Specifically, transjugular route was associated with more frequent blood transfusion (5.4% vs. 4.8%, P = 0.003) and angiographic intervention (0.8% vs. 0.4%, P < 0.001), but not with more nephrectomy (0% vs 0.1%, P = 0.07), than percutaneous route (Table 3).
Table 3.
Proportion of bleeding in transjugular versus percutaneous kidney biopsy
| Tranjugular kidney biopsy |
Percutaneous kidney biopsy |
P value | |
|---|---|---|---|
| n = 5305 | n = 55,026 | ||
| Bleeding events day 0–8 | |||
| Any major event | 354 (7) | 2991 (5) | 0.0002 |
| Blood transfusion | 301 (6) | 2614 (5) | 0.003 |
| Angiographic intervention | 43 (0.8) | 216 (0.4) | <0.0001 |
| Hemorrhage/hematoma | 29 (0.5) | 273 (0.5) | 0.62 |
| Nephrectomy | 0 (0.0) | 33 (0.1) | 0.07 |
Preprocedure Bleeding Risk Score and Observed Risk of Major Bleeding After Transjugular or Percutaneous Kidney Biopsy
Area under the receiver operating characteristic curve of the major bleeding risk score initially developed for percutaneous kidney biopsy was 0.750 (0.727–0.774; continuous score) in patients with transjugular kidney biopsy (8-level score: 0.746 [0.723–0.770]; Figure 1a and b).
Figure 1.
Major bleeding risk score receiver operating characteristic (ROC) curve. ROC curves are presented using the continuous score (a) and the 8-level score (b). Area under curve (AUC) of the bleeding risk score was good in patients with transjugular kidney biopsies but was significantly lower than in patients with percutaneous kidney biopsies (continuous score: 0.750 [0.727–0.774] vs. 0.801 [0.793–0.808], P < 0.001); 8-level score: 0.746 [0.723–0.770] vs. 0.793 [0.786–0.801], P < 0.001).
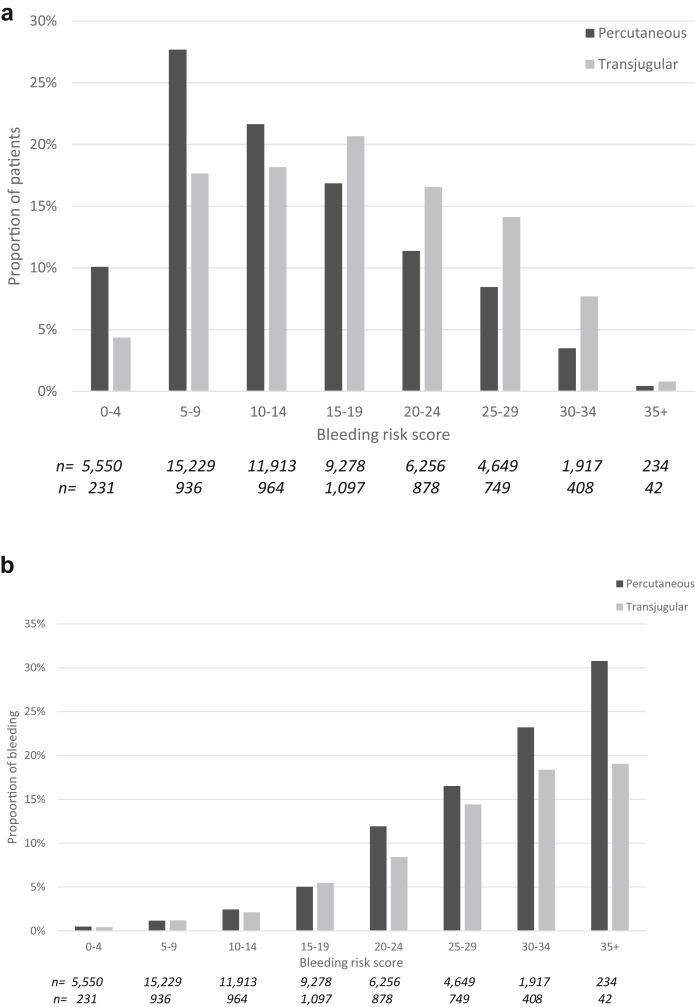
The distribution of the preprocedure major bleeding risk score was different according to the route of biopsy (transjugular vs. percutaneous: the average score was [mean ± SD] 17.2 ± 8.2 vs. 13.7 ± 7.9; (P < 0.001), and patients with the greater scores being more likely to have a transjugular route (P < 0.001; Figure 2a). The proportion of patients with a score ≥20 (15,133/60,331; 25% of all patients) was greater for the transjugular than the percutaneous route (39% vs. 24%, P < 0.001).
Figure 2.
(a) Proportion of patients who had a transjugular versus a percutaneous kidney biopsy according to the preprocedure bleeding risk score. The proportion of transjugular versus percutaneous kidney biopsy is shown in relation to the number of points of the bleeding risk score (from 0–4 to ≥35). The distribution of the route of biopsy (transjugular vs. percutaneous) differed according to the bleeding risk. The percentages of transjugular versus percutaneous kidney biopsy according to the risk score were 4.4% versus 10.1% (score = 0–4); 17.6% versus 27.7% (score = 5–9); 18.2%–21.7% (score = 10–14); 20.7% vs. 16.9% (score = 15–19); 16.6% vs. 11.4% (score = 20–24); 14.1% vs. 8.5% (score = 25–29); 7.7% vs. 3.5% (score = 30–34); 0.8% vs. 0.4% (score ≥35) (P < 0.001). (b) Proportion of major bleeding according to preprocedure bleeding risk score. The proportion of major bleeding (blood transfusion, hemorrhage/hematoma, angiography intervention, or nephrectomy) is shown in relation to the number of points of the preprocedure bleeding risk score (from 0–4 to ≥35) in patients with transjugular and percutaneous kidney biopsy. The proportion of major bleeding was significantly lower in transjugular than in percutaneous kidney biopsy for score ≥20: 9.2% versus 13.5% (score = 20–24); 16.8% versus 19.8% (score = 25–29); 22.5% versus 30.2%; (score = 30–34); 23.5% versus 44.4% (score ≥35) (overall P < 0.001). For lower scores, the proportion of major bleeding was similar in the 2 groups (0.4% vs. 0.5% [score = 0–4]; 1.2% vs. 1.2% [score = 5–9]; 2.1% vs. 2.5% [score = 10–14]; 5.5% vs. 5.3% [score = 15–19]).
The observed proportion of major bleeding differed according to the route of biopsy (percutaneous: 0.5%–30.8%; transjugular: 0.4%–19.1%) and the preprocedure major bleeding risk score (Figure 2b). It was similar in both groups when the risk score was <20 (2.8% vs. 2.3%, P = 0.09) but was significantly greater for the percutaneous route when the risk score was ≥20 (16% vs. 13%, P = 0.001) (Figure 2b).
Risk of Major Bleeding After Biopsy for Transjugular and Percutaneous Kidney Biopsy
Parameters associated with the risk of major bleeding including frailty index, Charlson index, age, gender, obesity, abnormal kidney function, anemia, thrombocytopenia, cancer, acute kidney injury, diabetic kidney disease, autoimmune-related kidney disease, vasculitis, and thrombotic microangiopathy in univariate analyses for both routes (Table 4).
Table 4.
Risk factors of major bleeding after kidney biopsy: univariate and multivariable analyses
| Univariate |
P value | Multivariable |
P value | |
|---|---|---|---|---|
| OR, 95% CI | OR, 95% CI | |||
| Transjugular route (vs percutaneous) | 1.24 (1.11–1.39) | <0.0001 | 0.88 (0.78–1.00) | 0.04 |
| Age, years | 1.31 (1.27–1.36) | <0.0001 | 1.06 (1.02–1.10) | 0.006 |
| Sex (male) | 1.50 (1.45–1.55) | <0.0001 | 1.09 (1.04–1.14) | 0.001 |
| Charlson Comorbidity Index | 1.74 (1.69–1.80) | <0.0001 | 1.25 (1.20–1.30) | <0.0001 |
| Frailty index | 0.77 (0.72–0.83) | <0.0001 | 0.83 (0.77–0.90) | <0.0001 |
| Comorbid conditions | ||||
| Hypertension | 1.27 (1.18–1.36) | <0.0001 | 0.88 (0.81–0.96) | 0.003 |
| Diabetes mellitus | 1.16 (1.07–1.26) | <0.0001 | 0.92 (0.83–1.01) | 0.09 |
| Obesity | 2.29 (2.10–2.50) | <0.0001 | 1.08 (0.98–1.20) | 0.14 |
| Heart failure | 2.16 (1.91–2.45) | <0.0001 | 1.16 (1.00–1.33) | 0.04 |
| Valve disease | 1.38 (1.25–1.53) | <0.0001 | 1.00 (0.88–1.13) | 1.00 |
| Coronary artery disease | 1.34 (1.21–1.48) | <0.0001 | 0.90 (0.80–1.01) | 0.08 |
| Vascular disease | 2.00 (1.82–2.20) | <0.0001 | 1.05 (0.94–1.18) | 0.37 |
| Atrial fibrillation | 1.34 (1.08–1.67) | 0.009 | 0.87 (0.69–1.10) | 0.24 |
| Ischemic stroke | 1.20 (1.07–1.33) | 0.001 | 1.01 (0.89–1.15) | 0.85 |
| Smoking | 1.01 (0.93–1.10) | 0.81 | 0.90 (0.81–1.00) | 0.04 |
| Dyslipidemia | 0.86 (0.78–0.95) | 0.003 | 0.76 (0.68–0.85) | <0.0001 |
| Malnutrition | 2.56 (2.34–2.81) | <0.0001 | 1.05 (0.95–1.16) | 0.37 |
| Alcohol-related diagnoses | 1.54 (1.37–1.72) | <0.0001 | 0.96 (0.83–1.11) | 0.58 |
| Lung disease | 2.11 (1.97–2.26) | <0.0001 | 1.30 (1.20–1.41) | <0.0001 |
| Liver disease | 1.55 (1.41–1.71) | <0.0001 | 0.92 (0.83–1.03) | 0.13 |
| Anaemia | 1.67 (1.49–1.87) | <0.0001 | 0.95 (0.82–1.09) | 0.44 |
| Thrombocytopenia | 6.34 (5.89–6.83) | <0.0001 | 3.43 (3.15–3.73) | <0.0001 |
| History of cancer | 2.68 (2.43–2.95) | <0.0001 | 1.26 (1.13–1.40) | <0.0001 |
| Kidney diagnoses at the time of biopsy | ||||
| Abnormal renal function | 1.67 (1.55–1.80) | <0.0001 | 1.20 (1.09–1.32) | <0.0001 |
| Acute renal injury | 4.63 (4.30–4.98) | <0.0001 | 2.11 (1.94–2.30) | <0.0001 |
| Glomerular disease | 0.69 (0.64–0.75) | <0.0001 | 0.76 (0.69–0.83) | <0.0001 |
| Vascular or hypertensive kidney disease | 0.84 (0.73–0.96) | 0.008 | 0.82 (0.71–0.95) | 0.006 |
| Diabetic kidney disease | 0.79 (0.68–0.91) | 0.002 | 0.69 (0.58–0.81) | <0.0001 |
| Auto-immune kidney disease | 1.42 (1.25–1.61) | <0.0001 | 1.32 (1.14–1.52) | <0.0001 |
| Vasculitis | 3.72 (3.33–4.17) | <0.0001 | 2.41 (2.12–2.74) | <0.0001 |
| Hematological-related kidney disease | 2.62 (2.27–3.01) | <0.0001 | 1.34 (1.15–1.57) | <0.0001 |
| Thrombotic microangiopathy | 4.20 (3.63–4.87) | <0.0001 | 1.90 (1.61–2.24) | <0.0001 |
| Amyloidosis | 1.01 (0.59–1.73) | 0.98 | 0.74 (0.42–1.30) | 0.30 |
| Other diagnosis | 1.20 (0.90–1.61) | 0.21 | 0.72 (0.53–0.97) | 0.03 |
CI, confidence interval; OR, odds ratio.
Transjugular route was associated with a greater risk in crude analyses (OR: 1.24 [1.11–1.39], P < 0.001) but a significantly lower risk of major bleeding than percutaneous route after adjustments on the bleeding risk factors (OR: 0.88 [0.78–0.99], P = 0.04; Table 4).
When patients were analyzed according to their preprocedure bleeding risk score, the risk of major bleeding was lower in patients who had a transjugular versus percutaneous kidney biopsy for scores ≥20 (OR: 0.83 [0.72–0.96], P = 0.01) and similar for lower scores (OR: 1.01 [0.80–1.26], P = 0.9) in multivariate analyses.
Of note, no center effect was detected for the risk of bleeding in patients in whom transjugular biopsy was performed (Supplementary Table 2).
Risk of Death After Biopsy: Association With Major Bleeding for Transjugular and Percutaneous Kidney Biopsy
Overall, 683/66,331 (1.1%) patients died (564/55,026 (1%) in patients who had a percutaneous kidney biopsy versus 119/5305 (2.2%) in patients who had a transjugular kidney biopsy).
Major bleeding was an independent risk factor for death for both transjugular and percutaneous routes in crude (OR: 2.30 [1.42–4.06] and 3.13 [2.53–3.88], respectively) and multivariate (OR: 1.77 [1.00–3.14] and 1.80 [1.43–2.28], respectively) analyses (Table 5 and 6).
Table 5.
Risk factors of death at day 30 after transjugular kidney biopsy
| Transjugular kidney biopsy | Univariate |
P value | Multivariable |
P value |
|---|---|---|---|---|
| OR, 95%CI | OR, 95%CI | |||
| Major bleeding after biopsy | 2.30 (1.42–4.06) | 0.001 | 1.77 (1.00–3.14) | 0.05 |
| Age (per 1 quartile) | 1.35 (1.13–1.60) | 0.001 | 1.44 (1.16–1.80) | 0.001 |
| Sex (male) | 1.57 (1.05–2.32) | 0.03 | 1.20 (0.78–1.84) | 0.40 |
| Charlson Comorbidity Index (per 1 quartile) | 1.37 (1.15–1.64) | <0.0001 | 0.77 (0.59–1.01) | 0.06 |
| Frailty index (per 1 quartile) | 1.41 (1.18–1.69) | <0.0001 | 1.08 (0.87–1.34) | 0.50 |
| History of cardiovascular and metabolic diseases | ||||
| Hypertension | 0.62 (0.43–0.90) | 0.01 | 0.50 (0.32–0.77) | 0.002 |
| Diabetes mellitus | 0.72 (0.47–1.10) | 0.12 | 0.57 (0.34–0.96) | 0.04 |
| Obesity | 1.22 (0.80–1.85) | 0.36 | 1.63 (1.01–2.62) | 0.05 |
| Heart failure | 2.87 (1.97–4.18) | <0.0001 | 2.71 (1.70–4.33) | <0.0001 |
| Valve disease | 1.36 (0.70–2.62) | 0.36 | 0.74 (0.36–1.53) | 0.42 |
| Coronary artery disease | 1.38 (0.90–2.12) | 0.14 | 0.98 (0.55–1.76) | 0.96 |
| Vascular disease | 1.34 (0.84–2.12) | 0.22 | 1.42 (0.77–2.62) | 0.26 |
| Atrial fibrillation | 2.00 (1.29–3.12) | 0.002 | 1.17 (0.69–2.00) | 0.55 |
| Stroke | 0.63 (0.20–1.99) | 0.43 | 0.66 (0.20–2.21) | 0.50 |
| Smoker | 1.12 (0.65–1.90) | 0.69 | 0.82 (0.46–1.48) | 0.52 |
| Dyslipidemia | 0.65 (0.41–1.02) | 0.06 | 0.72 (0.43–1.22) | 0.22 |
| History of other comorbid conditions | ||||
| Denutrition | 1.80 (1.13–2.86) | 0.01 | 0.98 (0.58–1.64) | 0.93 |
| Alcohol-related disease | 3.31 (2.24–4.89) | <0.0001 | 1.37 (0.80–2.34) | 0.25 |
| Lung disease | 1.58 (1.00–2.49) | 0.05 | 1.04 (0.63–1.74) | 0.87 |
| Liver disease | 4.61 (3.19–6.65) | <0.0001 | 4.25 (2.54–7.13) | <0.0001 |
| Anaemia | 1.45 (1.00–2.09) | 0.05 | 0.84 (0.55–1.31) | 0.45 |
| Thrombocytopenia | 1.31 (0.80–2.16) | 0.28 | 0.63 (0.36–1.09) | 0.10 |
| History of cancer | 2.09 (1.44–3.01) | <0.0001 | 1.97 (1.23–3.07) | 0.003 |
| Renal presentations | ||||
| Abnormal renal function | 0.70 (0.48–1.02) | 0.06 | 0.65 (0.43–1.00) | 0.05 |
| Acute renal injury | 4.53 (2.90–7.06) | <0.0001 | 3.39 (2.05–5.61) | <0.0001 |
| Glomerular disease | 0.57 (0.37–0.89) | 0.01 | 1.01 (0.62–1.63) | 0.97 |
| Vascular or hypertensive disease | 0.40 (0.16–0.99) | 0.05 | 0.64 (0.25–1.67) | 0.36 |
| Diabetic kidney disease | 0.49 (0.20–1.19) | 0.12 | 0.63 (0.23–1.72) | 0.37 |
| Autoimmune disease | 0.47 (0.20–1.06) | 0.07 | 0.89 (0.37–2.15) | 0.80 |
| Vasculitis | 0.35 (0.05–2.54) | 0.30 | 0.30 (0.04–2.26) | 0.24 |
| Hematological-related renal disease | 0.39 (0.05–2.83) | 0.35 | 0.33 (0.04–2.46) | 0.28 |
| Thrombotic microangiopathy | 0.89 (0.36–2.18) | 0.79 | 1.13 (0.43–2.96) | 0.80 |
| Amyloidosis | 4.16 (1.47–11.78) | 0.007 | 2.95 (0.98–8.87) | 0.06 |
| Other diagnoses | 0.79 (0.11–5.76) | 0.82 | 0.78 (0.10–5.99) | 0.81 |
CI, confidence interval; OR, odds ratio.
Table 6.
Risk factors of death at day 30 after percutaneous kidney biopsy
| Percutaneous kidney biopsy | Univariate |
p value | Multivariable |
p value |
|---|---|---|---|---|
| OR, 95%CI | OR, 95%CI | |||
| Major bleeding after biopsy | 3.13 (2.53–3.88) | <0.0001 | 1.80 (1.43–2.28) | <0.0001 |
| Age (per 1 quartile) | 1.96 (1.81–2.14) | <0.0001 | 1.63 (1.47–1.80) | <0.0001 |
| Gender (male) | 1.40 (1.19–1.62) | <0.0001 | 1.21 (1.01–1.43) | 0.04 |
| Charlson comorbidity index (per 1 quartile) | 1.79 (1.66–1.92) | <0.0001 | 1.05 (0.94–1.16) | 0.41 |
| Frailty index (per 1 quartile) | 1.67 (1.55–1.79) | <0.0001 | 1.11 (1.02–1.21) | 0.02 |
| History of cardiovascular and metabolic diseases | ||||
| Hypertension | 1.25 (1.06–1.46) | 0.006 | 0.84 (0.70–1.00) | 0.05 |
| Diabetes mellitus | 1.10 (0.92–1.32) | 0.28 | 0.76 (0.61–0.94) | 0.01 |
| Obesity | 1.07 (0.87–1.31) | 0.55 | 0.97 (0.78–1.21) | 0.78 |
| Heart failure | 3.86 (3.28–4.56) | <0.0001 | 2.18 (1.78–2.66) | <0.0001 |
| Valve disease | 1.96 (1.49–2.58) | <0.0001 | 0.76 (0.56–1.03) | 0.08 |
| Coronary artery disease | 2.61 (2.18–3.13) | <0.0001 | 1.46 (1.17–1.83) | 0.001 |
| Vascular disease | 1.75 (1.43–2.14) | <0.0001 | 0.92 (0.73–1.17) | 0.51 |
| Atrial fibrillation | 2.85 (2.36–3.43) | <0.0001 | 1.08 (0.87–1.34) | 0.48 |
| Stroke | 1.93 (1.29–2.89) | 0.001 | 1.15 (0.75–1.75) | 0.53 |
| Smoker | 1.36 (1.08–1.70) | 0.008 | 1.02 (0.80–1.32) | 0.86 |
| Dyslipidemia | 1.22 (1.01–1.46) | 0.04 | 0.86 (0.69–1.05) | 0.14 |
| History of other comorbid conditions | ||||
| Denutrition | 2.82 (2.34–3.40) | <0.0001 | 1.32 (1.07–1.61) | 0.008 |
| Alcohol-related disease | 2.43 (1.97–3.01) | <0.0001 | 1.22 (0.93–1.60) | 0.14 |
| Lung disease | 2.34 (1.94–2.82) | <0.0001 | 1.22 (0.99–1.50) | 0.06 |
| Liver disease | 3.56 (2.94–4.30) | <0.0001 | 2.49 (1.96–3.17) | <0.0001 |
| Anaemia | 2.18 (1.86–2.54) | <0.0001 | 1.00 (0.84–1.21) | 0.97 |
| Thrombocytopenia | 1.56 (1.22–1.99) | <0.0001 | 0.74 (0.57–0.97) | 0.03 |
| History of cancer | 4.38 (3.75–5.12) | <0.0001 | 2.78 (2.32–3.34) | <0.0001 |
| Renal presentations | ||||
| Abnormal renal function | 0.98 (0.83–1.15) | 0.76 | 0.62 (0.52–0.75) | <0.0001 |
| Acute renal injury | 3.29 (2.82–3.86) | <0.0001 | 2.05 (1.70–2.46) | <0.0001 |
| Glomerular disease | 0.47 (0.38–0.57) | <0.0001 | 0.72 (0.59–0.89) | 0.002 |
| Vascular or hypertensive disease | 0.41 (0.27–0.61) | <0.0001 | 0.49 (0.32–0.74) | 0.001 |
| Diabetic kidney disease | 0.66 (0.47–0.94) | 0.02 | 0.72 (0.49–1.07) | 0.10 |
| Autoimmune disease | 0.67 (0.46–0.98) | 0.04 | 1.08 (0.73–1.59) | 0.71 |
| Vasculitis | 1.40 (1.00–1.96) | 0.05 | 1.25 (0.88–1.79) | 0.21 |
| Hematological-related renal disease | 1.37 (0.93–2.02) | 0.11 | 0.67 (0.45–1.01) | 0.06 |
| Thrombotic microangiopathy | 1.44 (0.91–2.28) | 0.12 | 1.01 (0.62–1.63) | 0.98 |
| Amyloidosis | 4.59 (2.56–8.24) | <0.0001 | 2.49 (1.35–4.59) | 0.004 |
| Other diagnoses | 0.61 (0.25–1.47) | 0.27 | 0.37 (0.15–0.90) | 0.03 |
CI, confidence interval; OR, odds ratio.
Other independent risks of death included older age, history of malnutrition, heart failure, liver disease, cancer, and acute kidney injury in both groups (Table 5 and 6).
Discussion
In the present study, the risk of major bleeding was assessed in a cohort of more than 60,000 patients. The risk of major bleeding was highly variable both for transjugular and percutaneous routes. After adjustment on the bleeding risk factors, the transjugular procedure was associated with a lower risk of major bleeding than the percutaneous procedure, especially when the major bleeding risk score was score ≥20 (i.e., in 25% of patients). Major bleeding after biopsy was associated with an increased risk of death for both routes.
The observed risk of major bleeding associated with the transjugular kidney biopsy was 6.7% but varied from 0.4% (lowest risk score) to 23.5% (highest risk score) in the present study. The risk of major bleeding derived from small case series varies from 0% to 20% in the United States.7,8,16 Two small case series reported a risk of 24.6% and 11.9%, respectively, and capsular perforation requiring embolization occurred in some patients in the United Kingdom.17,18 Rychlik et al. reported a 26.9% risk of major bleeding in Czech Republic.19 However, the largest series come from France, and the reported major bleeding risk rate in several hundred patients varied from 0% to 10% according to patient selection.3, 4, 5,20 Nevertheless, the risk of selection and publication bias may occur in case series. In contrast, we report the result of a nationwide study in which our results reflect real-life practice in the 155 French centers where transjugular procedures were performed during the 2010–2019 period.
Patients selected for a transjugular kidney biopsy had more frequent risk factors of bleeding (such as low platelet count, liver disease, and anemia) and more frequent serious comorbid conditions than patients who had a percutaneous kidney biopsy. Medications including antiplatelet agents and anticoagulants were not available in our study, but it is likely that some of them were still receiving antiplatelet agents or anticoagulants.4,6,21 In the seminal paper of Sam et al., many patients who had a transjugular kidney biopsy had severe clotting disorders and serious comorbid conditions.3 Similar patient characteristics were reported in more recent case series.3, 4, 5,7,8,16, 17, 18, 19, 20
The proportion of major bleeding was greater for the transjugular than the percutaneous route in crude analyses but appeared significantly lower after adjustments. In the most recent review of 1321 transjugular kidney biopsies, the rate of bleeding was 4.5% but varied from 0% to 25%.22
The use of our bleeding risk score originally developed for percutaneous kidney biopsies did shed some light on this issue.2 The area under the receiver operating characteristic curve was 0.750, allowing us to use it in this population. We were able to quantify the preprocedure the risk of major bleeding associated with biopsy. As expected, the distribution of the score was different in the 2 groups, patients with the higher scores being more likely to have a transjugular biopsy. This score was also used to identify which patients could benefit most from the transjugular route. The observed proportion of major bleeding after biopsy was significantly lower for the transjugular route when the preprocedure major bleeding risk score was ≥20 (i.e., 25% of patients who had a kidney biopsy). This score may therefore be useful as a practical tool for patients and physicians to facilitate shared decision-making and guide the choice of the most adequate procedure when both techniques are available. The transjugular kidney biopsies were performed in 155 centers in France during the 2010–2019 period, indicating a large availability of this procedure. However, percutaneous kidney biopsies were performed in 627 centers in France within the same period, indicating a more widespread availability of the percutaneous procedure. Of note, In 2010, the activity was described in a survey of 73 units in France: transjugular kidney biopsies were used in 45% of nephrology units (1%–5% in 33.8% units, 5%–10% in 6.8%, 10%–20% in 4.1% units, and >20% in 1.4%).23 Whether different figures would be found in the United States or in other countries is unknown, but the availability of this procedure is probably a key issue in many countries for reasons of personnel, time, cost, and need for experienced operators.1,6,21
Finally, we have previously shown that major bleeding after percutaneous kidney biopsy is associated with an increased risk of death. The present data confirm this finding and extend it to the transjugular route.
The strength of the present study derives from its size and design. It represents by far the largest study focused on this issue. We included all patients who had a percutaneous and a transjugular native kidney biopsy. There is no selection bias and essentially no missing data. In a previous paper, this score was robust and internally validated using a bootstrap procedure.2 The crude risk of bleeding associated with transjugular kidney biopsy was higher compared with percutaneous kidney biopsy; however, this finding was completely explained by a higher bleeding baseline risk of these patients. In multivariable analysis, the bleeding risk appeared slightly lower in this transjugular route, especially for high-risk patients, so that we can now identify using this score.
This study has also some limits. Several parameters including biological data, size of gauge, presence of liver biopsy, experience and specialty of physicians (nephrologist vs. radiologist), and indication of transfusion were not available for analysis, and our data derived from administrative codes. However, our goal was to assess the risk of major bleeding in real-life conditions. We did not have information regarding the use of antiplatelet agents or anticoagulants. Some minor bleedings were certainly missed. We did not assess the yield of transjugular versus percutaneous kidney biopsy. However, it was showed that 87% to 96% of all samples had 8 glomeruli or more (median: 23) and contained renal cortex in 256 patients with transjugular biopsy performed for systemic lupus erythematosus and antiphospholipid antibody syndrome patients5: these results suggest that the yield of transjugular kidney biopsies is very good.4,5,21 The role of anemia before biopsy as a risk factor for blood transfusion has been reported. Whether it is an independent risk of bleeding is unsure.24 Finally, we excluded from the analysis kidney transplant recipients.
In conclusion, the risk of bleeding varies widely and the risk of bleeding is associated with an increased risk of death. The risk of bleeding can be assessed using a preprocedure score. Patients selected for the transjugular route are at high risk of major bleeding, explaining the greater observed proportion of major bleeding in patients with transjugular biopsy versus percutaneous biopsy. Multivariable analyses indicate that the transjugular route is associated with a lower risk of major bleeding than percutaneous route, and this was especially true for major bleeding risk scores ≥20 (i.e., 25% of all patients who had a kidney biopsy). These findings may have consequences on the interest and diffusion of this technique in the nephrology community.
Author Contributions
JMH: design, data analysis, and writing. LF and AB: data analysis and writing. PG, AG, JG, NG, CB, HL, BS: writing. JH: data management and analysis.
Footnotes
Table S1. ICD 10 codes.
Table S2. Major bleeding events in patients with transjugular biopsy by quartile of center volume.
Figure S1. Bleeding risk score receiver operating characteristic (ROC) curve for identifying the risk of blood transfusion, angiographic intervention, or nephrectomy during an 8 day-period after kidney biopsy. (A) Transjugular and percutaneous kidney biopsy (continuous score). (B) Transjugular and percutaneous kidney biopsy (8-level risk score).
Supplementary Material
Table S1. ICD 10 codes.
Table S2. Major bleeding events in patients with transjugular biopsy by quartile of center volume.
Figure S1. Bleeding risk score receiver operating characteristic (ROC) curve for identifying the risk of blood transfusion, angiographic intervention, or nephrectomy during an 8 day-period after kidney biopsy. (A) Transjugular and percutaneous kidney biopsy (continuous score). (B) Transjugular and percutaneous kidney biopsy (8-level risk score).
References
- 1.Hogan J.J., Mocanu M., Berns J.S. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol. 2016;11:354–362. doi: 10.2215/CJN.05750515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halimi J., Gatault P., Longuet H. Major bleeding and risk of death after percutaneous native kidney biopsies. A French nationwide cohort study. Clin J Am Soc Nephrol. 2020;15:1587–1594. doi: 10.2215/CJN.14721219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mal F., Meyrier A., Callard P. Transjugular renal biopsy. Lancet. 1990;335:1512–1513. doi: 10.1016/0140-6736(90)93040-v. [DOI] [PubMed] [Google Scholar]
- 4.Cluzel P., Martinez F., Bellin M.F. Transjugular versus percutaneous renal biopsy for the diagnosis of parenchymal disease: comparison of sampling effectiveness and complications. Radiology. 2000;215(3):689–693. doi: 10.1148/radiology.215.3.r00ma07689. [DOI] [PubMed] [Google Scholar]
- 5.Nielly H., Mathian A., Cazenave M. Safety and effectiveness of transjugular renal biopsy for systemic lupus erythematosus and antiphospholipid antibody syndrome patients taking antithrombotics. Nephrol Dial Transplant. 2020;35:1721–1729. doi: 10.1093/ndt/gfz085. [DOI] [PubMed] [Google Scholar]
- 6.Zhuo L., Wang H., Chen D. Alternative renal biopsies: past and present. Int Urol Nephrol. 2018;50:475–479. doi: 10.1007/s11255-017-1668-x. [DOI] [PubMed] [Google Scholar]
- 7.Sam R., Leehey D.J., Picken M.M. Transjugular renal biopsy in patients with liver disease. Am J Kidney Dis. 2001;37:1144–1151. doi: 10.1016/s0272-6386(01)99000-6. [DOI] [PubMed] [Google Scholar]
- 8.Misra S., Gyamlani G., Swaminathan S. Safety and diagnostic yield of transjugular renal biopsy. J Vasc Interv Radiol. 2008;19:546–551. doi: 10.1016/j.jvir.2007.12.447. [DOI] [PubMed] [Google Scholar]
- 9.Lorgis L., Cottenet J., Molins G. Outcomes after acute myocardial infarction in HIV-infected patients: analysis of data from a French nationwide hospital medical information database. Circulation. 2013;127:1767–1774. doi: 10.1161/CIRCULATIONAHA.113.001874. [DOI] [PubMed] [Google Scholar]
- 10.Fauchier L., Clementy N., Pelade C. Patients with ischemic stroke and incident atrial fibrillation: a nationwide cohort study. Stroke. 2015;46:2432–2437. doi: 10.1161/STROKEAHA.115.010270. [DOI] [PubMed] [Google Scholar]
- 11.Fauchier L., Chaize G., Gaudin A.F., Predictive ability of HAS-BLED, HEMORR2HAGES, and ATRIA bleeding risk scores in patients with atrial fibrillation A French nationwide cross-sectional study. Int J Cardiol. 2016;217:85–91. doi: 10.1016/j.ijcard.2016.04.173. [DOI] [PubMed] [Google Scholar]
- 12.Bisson A., Bodin A., Clementy N. Prediction of incident atrial fibrillation according to gender in patients with ischemic stroke from a nationwide cohort. Am J Cardiol. 2018;121(4):437–444. doi: 10.1016/j.amjcard.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen V., Michel M., Eltchaninoff H. Implementation of transcatheter aortic valve replacement in France. J Am Coll Cardiol. 2018;71:1614–1627. doi: 10.1016/j.jacc.2018.01.079. [DOI] [PubMed] [Google Scholar]
- 14.Charlson M., Szatrowski T.P., Peterson J., Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 15.Segal J.B., Chang H.Y., Du Y. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care. 2017;55:716–722. doi: 10.1097/MLR.0000000000000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott K.C., Musio F.M., Chung E.M. Transjugular renal biopsy in high-risk patients: an American case series. BMC Nephrol. 2002;3:5. doi: 10.1186/1471-2369-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson B.C., Kingdon E., Johnston M. Transjugular kidney biopsy. Am J Kidney Dis. 2004;43:651–662. doi: 10.1053/j.ajkd.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 18.See T.C., Thompson B.C., Howie A.J. Transjugular renal biopsy: our experience and technical considerations. Cardiovasc Intervent Radiol. 2008;31:906–918. doi: 10.1007/s00270-008-9308-6. [DOI] [PubMed] [Google Scholar]
- 19.Rychlik I., Petrtyl J., Tesar V., Transjugular renal biopsy Our experience with 67 cases. Kidney Blood Press Res. 2001;24:207–212. doi: 10.1159/000054229. [DOI] [PubMed] [Google Scholar]
- 20.Jouet P., Meyrier A., Mal F. Transjugular renal biopsy in the treatment of patients with cirrhosis and renal abnormalities. Hepatology. 1996;24:1143–1147. doi: 10.1002/hep.510240527. [DOI] [PubMed] [Google Scholar]
- 21.Meyrier A., Transjugular renal biopsy Update on hepato-renal needlework. Nephrol Dial Transplant. 2005;20:1299–1302. doi: 10.1093/ndt/gfh866. [DOI] [PubMed] [Google Scholar]
- 22.St Jeor J.D., Reisenauer C.J., Andrews J.C. Transjugular renal biopsy bleeding risk and diagnostic yield: a systematic review. J Vasc Interv Radiol. 2020;31:2106–2112. doi: 10.1016/j.jvir.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bollee G., Martinez F., Moulin B. Renal biopsy practice in France: results of a nationwide study. Nephrol Dial Transplant. 2010;25:3579–3585. doi: 10.1093/ndt/gfq254. [DOI] [PubMed] [Google Scholar]
- 24.Palsson R., Short S.A.P., Kibbelaar Z.A. Bleeding complications after percutaneous native kidney biopsy: results from the Boston Kidney Biopsy Cohort. Kidney Int Rep. 2020;5:511–518. doi: 10.1016/j.ekir.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.