Abstract
Brazilian traditional medicine has explored the antiviral properties of many plant extracts, including those from the Brazilian pepper tree, Schinus terebinthifolius. In the present study, we investigated the chemical composition and anti-mayaro virus (MAYV) activity of S. terebinthifolius fruit. Extensive virucidal activity (more than 95%) was detected for the ethyl acetate extract and the isolated biflavonoids. From the ethyl acetate extract of Schinus terebinthifolius fruits, two bioflavonoids were isolated ((2S, 2″S)-2,3,2″,3″-tetrahydroamentoflavone and agathisflavone), which showed strong virucidal activity against Mayaro virus. Furthermore, several other compounds like terpenes and phenolics were identified by hyphenated techniques (GC–MS, LC–MS and HPLC–UV), as well as by mass spectrometry. Immunofluorescence assay confirmed antiviral activity and transmission electron microscopy revealed damage in viral particles treated with biflavonoids. The data suggest the direct action of the extract and the biflavonoids on the virus particles. The biflavonoids tetrahydroamentoflavone and agathisflavone had strong virucidal activity and reduced MAYV infection.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13337-021-00698-z.
Keywords: Antiviral, Biflavonoids, Schinus terebinthifolius, Virus mayaro
Introduction
Fruits of the Brazilian pepper tree (Schinus terebinthifolius Raddi) are used in international cuisine as condiments, and in traditional medicine for their antimicrobial activity [1–27]. Due to high demand, the plant is commercially cultivated in the state of Espirito Santo, Brazil. To add value to this locally grown product, the biological properties of the fruit extracts have been investigated, with particular focus on the antimicrobial activity of its secondary metabolites, such as the biflavonoids [4–17].
Arboviruses, such as Mayaro virus (MAYV) are transmitted by mosquitoes into susceptible hosts, and are major public health concern [11]. In the last decade there were epidemics of MAYV in the Brazilian states of Mato Grosso (2012) [28] and Goiás (2014–2016) [2]. MAYV, an arbovirus of the Togaviridae family (genus Alphavirus), is closely related to Chikungunya and other human alphaviruses [15]. An enveloped virus, whose RNA genome is single positive polarity type, exhibits icosahedral symmetry of 70 nm diameter. Its genome has approximately 11 kbp coding for two polyproteins that are cleaved into non-structural proteins (nsP1, nsP2, snP3 and nsP4) and structural proteins (C, E2, E3, 6k, E1). The Mayaro disease consists of a mild or moderate fever of abrupt onset and short duration, chills, and pain of the muscles, joints, and head. Mosquitoes of Haemagogus sp. and Aedes sp. act as vectors of MAYV in rural and urban areas, respectively [5]. A recent finding shows the potential for MAYV transmission by the urban vectors Aedes aegypti and Aedes albopictus thus contributing to the classification of the MAYV as an emerging virus, having a potential for inclusion in the urban cycle, as occurred recently with the Chikungunya virus [13–25]. Fruits of Schinus terebinthifolius are a known source of biflavonoids [3], with natural antiviral activity [12].
In this work, the biological activity and antiviral activity of the biflavonoids and extracts from S. terebinthifolius, against the emerging alphavirus, Mayaro virus (MAYV) is evaluated. The physicochemical properties of the extracts and the direct action on the viral particles were also uncovered. This study makes significant progress toward mitigating the risk of emerging diseases transmitted by arboviruses.
Materials and methods
General experimental procedures
Optical rotations were measured on an Acatec PD-5000 digital polarimeter. Circular dichroism spectra of biflavonoids were obtained with a Chirascan™ CD spectrometer (Applied Photophysics, UK). 1H-NMR, APT, HSQC, and HMBC NMR spectra with MeOD or DMSO as solvents, and TMS as internal standard, were recorded on Bruker DRX 400 and 500 MHz spectrometers. EI-MS was obtained using a Shimadzu QP5050 GC–MS on a DB-5 column (30 m × 0.25 mm and film thickness of 0.25 µm, J&W Scientific) with an ionizing energy of 70 eV. LC-DAD-MS analysis was performed on an ultra-high-performance liquid chromatography (Shimadzu) coupled to a Bruker compact Q-Tof high resolution mass spectrometer (HRMS) controlled by Compass Data Analysis 4.2. Bruker compact Q-Tof was run at mode ESI(-), 4.5 kV capillary voltage, 500 V set end-plate offset, 2000 V charging voltage, nebulizer gas pressure of 5.5 bar, dry heater at 220 °C, dry gas at 12.0 L/min, and a mass scan range of m/z 50–1500 for MS. Separation was performed on a Shimpack ODS3 column (100 × 2.0 mm). The flow was 0.3 mL/min with a gradient of formic acid 0.1% (phase A) and acetonitrile (phase B) as mobile phases. The gradient ranges from 3 to 70% of phase A for a duration of 16 min. ESI-MS was obtained on a mass spectrometer (Model 9.4 T Solarix, Bruker Daltonics, Bremen, Germany), which was set to operate in negative ion mode, ESI(-), over a mass range of m/z 200–1300. The parameters of the ESI(-) source were as follows: nebulizer gas pressure of 0.5–1.0 bar, capillary voltage of 3–3.5 kV, and transfer capillary temperature of 250 °C. The mass spectrum was processed using the Compass Data Analysis software package (Bruker Daltonics, Bremen, Germany). A resolving power of m/Δm50% ≅ 500,000 provided unambiguous molecular formula assignments for singly charged molecular ions (where Δm50% is the full peak width at half-maximum peak height of m/z ≅ 400, at a mass accuracy of < 1 ppm). Cellulose acetate 20% (Sigma-Aldrich), XAD-16 (Sigma-Aldrich), and Sephadex LH-20 (GE Healthcare Bio-Sciences AB®) were used as stationary phases for column chromatography.
Plant identification and collection
Schinus terebinthifolius fruits were obtained from farmers in São Mateus, Espírito Santo, Brazil, in April 2012. The plant material was identified by taxonomist Helio de Queiroz Boudet Fernandes using morphological features. The voucher specimen (No. 41895) was deposited in the Biology Museum Mello Leitão (MBML), Espírito Santo, Brazil.
Preparation of the extracts
Air-dried fruits (500 g) were crushed in a blender in 70% ethanol. The mixture was transferred to an Erlenmeyer flask and allowed to stand for 15 days at temperature of 25 °C. After drying, the ethanol extract weighed 54 g. It was suspended in water (500 mL) to be partitioned with dichloromethane (three extractions of 300 mL each) and ethyl acetate (three extractions of 300 mL each) in a 1 L separatory funnel. Finally, 15.2 g of the dichloromethane extract and 11.9 g of the ethyl acetate extract were obtained.
Isolation of biflavonoids
The ethyl acetate fraction (2.0 g) was chromatographed on a Sephadex LH-20 open column using MeOH as the mobile phase. The obtained fractions (numbered till 50) were developed on a silica gel thin-layer chromatography (TLC) plate and sprayed with Natural Product-polyethylene glycol (NP/PEG) to detect flavonoids in fractions 23–30 (observed as yellow spots). These fractions were pooled (440 mg) and subjected to a second Sephadex LH-20 open column chromatography, this time with MeOH:H2O (7:3), as the mobile phase. From the fractions 23–34 and fractions 38–46, biflavonoids (2S, 2″S)-2,3,2″,3″-tetrahydroamentoflavone (THA; 300 mg) and agathisflavone (AAF; 20 mg) were obtained, respectively.
Phytochemical analyses
The dichloromethane extract was analyzed by gas chromatography (GC–MS). Homologous series of n-alkanes (from C-11 to C-32) were injected simultaneously to calculate the Kovats indices. The ethyl acetate extract was analyzed by liquid chromatography with DAD/MS detector (LC-DAD-QTOF-MS) and mass spectrometry with (-)-ESI FT-ICR-MS.
Cell culture
Vero cells were grown in Dulbecco’ modified Eagle’s medium (DMEM; Gibco-Life Technologies, Carlsbad, CA, USA), 7.5% NaHCOз, supplemented with 5 mL of the solution 100 unit/mL penicillin and 100 µg/mL streptomycin (Sigma-Aldrich, St. Louis, MO, USA), 5.0% fetal bovine serum (Gibco-Life Technologies, Carlsbad, CA, USA) and maintained at 37 °C with 5.0% CO2.
Mayaro virus
Vero cells were infected with the Mayaro virus (ATCC VR-66, lineage TR 4675) with a multiplicity of infection (MOI) of 0.01, in the fifth passage, with a viral titer of 5 × 107 pfu (plaque forming units)/mL. The supernatant containing the virus was then aliquoted and stored at −70 °C.
Cytotoxicity determination
The cytotoxicity of the samples was determined by the incorporation of neutral red by living cells. This was followed by fixing with 20% formaldehyde in phosphate-buffered saline (PBS) 150 mM pH 7.2 (v/v), extraction with 50% methanol and 10% acetic acid, and quantifying with a spectrophotometer at a wavelength of 490 nm.
Stock samples
The stock solutions had a concentration of 5.5 mg/mL for the dichloromethane extract, 2.5 mg/mL for each biflavonoid, and 2.5 mg/mL for the ethyl acetate extract diluted in dimethylsulfoxide (DMSO) (the final concentration of DMSO used in the tests was less than 0.001%). There is no observed cytotoxicity for cells treated with 0.001% DMSO (data not shown). The preparations were stored at -20 °C and added to the culture medium of the cells at the indicated concentrations.
Viral inhibition by extracts obtained from S. terebinthifolius
Confluent Vero cells in 24-well microplates were infected at MOI of 0.01 for 1 h. After adsorption, the culture medium was added with or without the substances at the indicated concentrations. After 24 h, supernatants of cell cultures were collected for determination of viral titer. Vero cells were cultured in 96-well microplates (TPP, USA). Supernatants collected from the infectivity inhibition experiments were added in serial dilutions to the cells. Tissue Culture Infective Dose (TCID50) was calculated according to the Reed-Muench method [18]. The TCID50 experiments were performed in 96 well plates in quadruplicate technique and three independent experiments.
Effect of the test substances on the first cycle of replication
Confluent monolayers of Vero cells were infected with MAYV. After adsorption with viruses at MOI 1.0, for 1 h, cells were incubated for 4 h with two concentrations of the substances: 4 µg/mL and 30 µg/mL each, for the ethyl acetate extract and THA, and 14 µg/mL and 30 µg/mL for AAF. Incubation was also performed with the crude extract at the concentrations of 15 µg/mL and 100 µg/mL.
Virucidal activity
To detect virucidal activity, 100 PFU of MAYV was incubated for 1 h at 37 °C with twice the value of the test substances that achieved the highest inhibition activity. After the incubation period, the viral suspension was added to confluent Vero cell monolayers in 6-well plates for 1 h at 37 °C. After adsorption, the viral suspension was removed and culture medium with 2% carboxymethylcellulose (CMC) was added. Plaques were counted after an incubation period of 48 h at 37 °C. Cells that were not pre-incubated were treated with the test substances and viruses at the same concentration, and these were used as controls. The experiments by plaque assays were performed in 6 well plates in duplicate technique and three independent experiments.
Immunofluorescence
For immunofluorescence analysis, infected Vero cells in 24-well plates were washed with PBS and fixed with 4% paraformaldehyde for 15 min at room temperature. Cells were permeabilized with 0.1% Triton X-100 in PBS for 10 min. They were blocked for 15 min at 25 °C with a solution that contained 3 × rinsing with 3% BSA in PBS with fish gelatin (PBSA). Fixed cells were incubated for 2 h at 25 °C with the primary antibody (anti-HA) diluted in blocking solution. The secondary antibody (Cy3-conjugated anti-mouse IgG) was diluted in blocking solution and incubated with the cells for 1 h at 25 °C, followed by dilution with 546 Phalloidin blocking solution (Invitrogen) for 40 min at 25 °C. The cells were washed five times with PBSA between all antibody treatments. Finally, cells were treated with the DAPI Prolongold (Invitrogen). Fluorescence was analyzed microscopically with an Automated Fluorescence Microscope (Olympus, Tokyo, Japan) and the cells were photographed with a digital camera DP70 (Olympus, Tokyo, Japan).
Transmission electron microscopy (TEM)
MAYV was purified by ultra-centrifugation in a tartrate two-step gradient (15% and 35%) at 24,000 rpm (sw-28 rotor) in a Beckman ultracentrifuge. The resulting virus band was collected and incubated with the test substances, THA, AAF, and the ethyl acetate extract for 1 h at 37 °C. Untreated purified MAYV was used as control. The viruses were then placed on 400-mesh carbon grids for 1 min. Then the grids were washed three times and contrasted with 1% uranyl acetate. Finally, the material was visualized under a transmission electron microscope.
Data analysis
One-way ANOVA and Dunnett's multiple comparisons test were performed with Prism 7 (GraphPad Software). All significant values had p-values less than 0.05.
Results and discussion
Phytochemical analyses
The chemical profile of the dichloromethane extract obtained by GC–MS (Figure S1 of Supplementary Material) revealed that sesquiterpenoids (57%) and steroids/triterpenoids (38%) constituted the major classes of natural compounds, while alkylphenols (5%) comprised a smaller proportion. Sesquiterpenoids and monoterpenoids are known constituents of the essential oils of the fruits and leaves of S. terebinthifolius [21]. The compounds were identified by spectral comparison with the NIST library and by the Kovats indices calculated from injection of n-alkanes under identical chromatographic conditions. Table S1 (see Supplementary Material) lists the identified compounds. The most common sesquiterpenoids were α-, β-, γ-, and δ-cadinene, and the most common steroids were β- and γ-sitosterol. Ursonic aldehyde and ursonic aldehyde 3-acetate were the most common triterpenoids. Among the alkylphenols, m-tridecanoilphenol and three isomeric m-pentadecanoilphenols were detected.
The ethyl acetate extract was subjected to DAD-UV-HPLC analysis (Figure S2 of Supplementary Material). It displayed a phenolic profile typical of the Schinus species. Phenolic acids, gallotannins and biflavonoids were detected [4]. When the detector was focused on 254 nm, biflavones were detected at 22.9 min (UV, 272, 332 nm) and 23.8 min (UV, 269, 334 nm), and a biflavonone was detected at 24.3 min (UV, 289 nm) (Figure S3 of Supplementary Material). When analyzed by LC-QTOF-MS, these compounds showed [M-H]− peaks for biflavones at 537.0820 and 537.0812, and at 541.1131 for the biflavonone (Figure S4 of Supplementary Material). Agathisflavone (AAF) and amentoflavone were identified as the two biflavones, and tetrahydroamentoflavone (THA) was identified as the biflavonone [4]. In addition to biflavonoids, gallotannins were observed by LC-QTOF-MS. Several peaks with [M-H]− at 331 correspond to galloylglucose isomers, and those at 325 correspond to galloylshikimic acid derivatives. They also presented as [M + (M-H)] peaks at 663 and 651 in the clusters produced in the ionization source [4]. Ethyl gallate ([M-H]− 197.0450) was the major phenolic acid detected (with a retention time of 6.08 min in DAD-UV-HPLC), and gallic acid ([M-H]− 169.0137) was detected as a minor peak. The (-)-ESI-FT-ICR MS spectrum of the ethyl acetate extract (Figure S5 of Supplementary Material) showed additional classes of compounds, like acid triterpenes and their glycosides. Major molecular formulas were C30H45O3 [M-H]− = 453.33790, and C30H47O3 [M-H]− = 455.35354, which correspond to Z- and E-masticadienoic acid, and Z-Schinol, respectively [16]. Table S2 (Supplementary Material) lists the (-)-ESI-FT-ICR-MS data for the ethyl acetate extract. Molecular formulas as well as MS/MS fragments were obtained. The presence of glycosides of acid triterpenes and esters of acid triterpenes with methylgallate in S. terebinthifolius fruits is described for the first time in this study.
Various spectroscopic methods, including ESIMS, NMR (1H, APT, HSQC, HMBC), and circular dichroism aided in the identification of (2S, 2″S)-2,3,2″,3″-tetrahydroamentoflavone (termed Compound 1; the biflavonone) and agathisflavone (termed Compound 2; the biflavone) (see Figure S6 of Supplementary Material). They were previously identified in S. terebinthifolius fruits [21]. Biflavonoids are known to be present in Anacardiaceae [23]. Compound 1, ESI–MS [M-H +]- 541, was revealed to be a dimer (biflavonone) of two naringenin moieties interconnected by a chemical bond between C-3′ (ring B) and C-8″ (ring D). Such a bond was shown to exist using HMBC correlations among 106.7 (C-8″) and 6.81 (H-5′, J4), 7.28 (H-2′, J3) and 5.94 (H-6″, J3) as well as 121.17 (C-3′) and 5.94 (H-6″, J4) and 6.81 (H-5′, J3). Absolute configuration 2S, 2″S was determined by circular dichroism, with a positive cotton effect at 327 nm (n → π* transition), and a negative cotton effect at 288 nm (π → π*). Compound 2, ESI–MS [M-H +]- 539 was identified as a biflavone of two apigenin moieties. Attachment between rings A and D of each flavone occurred through C-6 (103.7 ppm) and C-8″ (99.46 ppm). HMBC J3 correlations between OH-5 (13.31 ppm) and C-6 (103.7 ppm) and between OH-5″ (13.05 ppm) and C-6″ (98.92 ppm) strongly corroborate the proposed structure.
Cell viability
Toxicity assays for the dichloromethane and ethyl acetate extracts and for the biflavonoids from the fruits of S. terebinthifolius were performed in Vero cell cultures. Table 1 shows the concentrations of the test substances for 50% and 90% cell viability (CC50 and CC90, respectively). Only the dichloromethane extract exhibited 100% viability at the maximum tested concentration of the extract. AAF was more cytotoxic than THA (Figure S7 of Supplementary Material).
Table 1.
Cytotoxicity and anti-MAYV activity of dichloromethane extract, biflavonoids and ethyl acetate extract
| Samples | aCC50 | aCC90 | bIC50 | bIC90 | cIS50 | cIS90 | RP |
|---|---|---|---|---|---|---|---|
| Tetrahidroamentoflavone | 270 ± 8 | 96 ± 3 | 4.5 ± 0.1 | 8.1 ± 0.2 | 60 | 12 | 7.5 |
| Agathisflavone | 63 ± 2 | 5.7 ± 0.2 | 5.1 ± 0.2 | 8.8 ± 0.3 | 12 | 1 | 1.5 |
| Ethyl acetate extract | 294 ± 9 | 72 ± 2 | 3.0 ± 0.1 | 5.4 ± 0.2 | 98 | 13 | 12.3 |
| Dichloromethane extract | > 5000 | > 5000 | 830 ± 25 | 1409 ± 99 | > 6 | > 4 | > 0.75 |
| Ribavirin | 523 ± 11 | 215 ± 7 | 63 ± 4 | 113 ± 8 | 8 | 2 | – |
aConcentration for 50% and 90% cell viability, in µg/mL
bConcentration for 50% and 90% inhibition of virus production, in µg/mL
cSelectivity Index = CC50/IC50
dRelative Potency = IS50(substance)/IS50(Ribavirin)
Antiviral activity
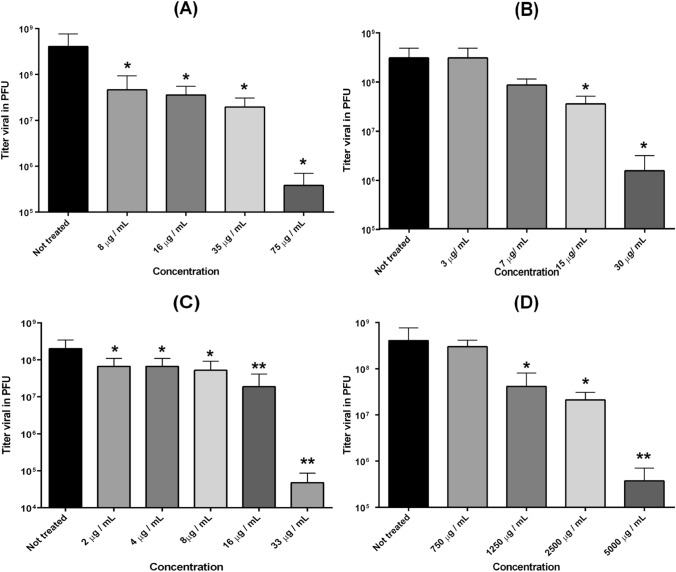
Antiviral activity of the test substances was assessed as the ability to inhibit MAYV replication in Vero cells (Fig. 1). Infected cells were treated with different concentrations of the test substances. All of them exhibited a strong antiviral effect when compared to ribavirin, a known antiviral used in the treatment of hepatitis C, respiratory syncytial virus, and other viral infections. The antiviral activity was dose-dependent, with more than 95% inhibition at the highest nontoxic concentrations tested (Table 1). The ethyl acetate extract showed a selectivity index (SI) of 53 (Table 1). The biflavonoid THA had the highest SI of 70. The results indicate that all tested substances have potential antiviral activity in concentrations that are nontoxic for Vero cells.
Fig. 1.
Antiviral activity of ethyl acetate and biflavonoids on MAYV replication. a Tetrahydroamentoflavone; b agasthisflavone; c ethyl acetate extract; d dichloromethane extract. Vertical bars represent standard deviation and the * represents a significant difference between the conditions
Effect during the first cycle of replication
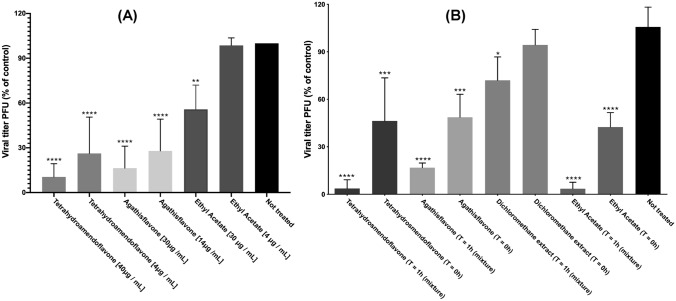
The inhibition of the viral titer during the first replication cycle of MAYV was studied using two different concentrations of each test substance (Fig. 2a). After adsorption with MAYV at an MOI of 1.0 for 1 h, cells were incubated for 4 h, which is the average time required for a full replication cycle of an Alphavirus [7]. All tested substances inhibited the viral titer in these conditions.
Fig. 2.
a Effect during the first cycle of replication. Confluent monolayers of Vero cells were treated with two different concentrations of ethyl acetate extract, aroeira tetrahydroamentoflavone and agasthisflavone. Data are presented as mean % virus yield (compared to untreated controls) ± SD. b Virucidal activity. 100 PFU MAYV was treated with tetrahydroamentoflavone, agasthisflavone, aroeira oil and ethyl acetate extract. Data are presented as mean % virus yield (compared to untreated controls) ± SD. For T = 1 h, virus and substance were incubated for 1 h prior to infection). For T = 0 h, virus and substance were added at the infection time (no previous incubation)
Virucidal activity
Virucidal effects can be inferred if the mechanism of antiviral action is related to the loss of infectivity of the viral particles by direct action of the test substances. To assay their virucidal potential, the test substances were incubated 1:1 with 100 pfu of MAYV. Incubation period was either 1 h, in which the mixture was incubated for 1 h prior to infection, or none (the virus and test substances were mixed at the time of infection). Viruses were treated with 60 µg/mL of the ethyl acetate extract and AAF, 80 µg/mL of THA, and 2500 g/mL of the dichloromethane extract (in oil). The data show that the ethyl acetate extract, THA and AAF had significant virucidal effect as shown in Fig. 2b.
Immunofluorescence
Immunofluorescence analysis was used to confirm the antiviral activity of these substances. The mechanism of antiviral activity could be similar to that of the virucidal activity, involving the loss of infectivity of viral particles treated with the test substances. Therefore, Vero cells were treated with the ethyl acetate extract, THA and AAF, and incubated with MAYV as in the virucidal experiment. A significant inhibition was observed on virus replication as shown in Figure S8 of Supplementary Material.
Transmission electron microscopy
The virucidal effect was also observed by TEM (Fig. 3). Viruses that were treated with the test substances presented a larger number of defective particles. They displayed structures that resembled membrane extrusions from the virus particles and debris from disrupted virus particles.
Fig. 3.
a Purified Mayaro virus particles; b Purified Mayaro virus particles incubated at 37 °C for 1 h; c Purified Mayaro virus particles treated with tetrahydroamentoflavone; d Purified Mayaro virus particles treated with agasthisflavone; e Purified Mayaro virus particles treated with ethyl acetate extract. Bars, 100 nm
This is the first report of antiviral activity for extracts of S. terebinthifolius plants. This paper shows that natural extracts from S. terebinthifolius plants inhibited MAYV replication in Vero cells in a dose-dependent manner, and at concentrations at which there were no toxic effects to the cells.
The antiviral effect of substances extracted such as flavonoids, isoflavonoids, and their derivatives have shown therapeutic potential in a variety of viral infections in different virus-cell systems. These include the Japanese encephalitis virus, hepatitis B virus, herpes simplex virus Type 1 (HSV-1), parainfluenza-3 virus (PI-3), adenovirus, bovine viral diarrhea virus, human immunodeficiency virus (HIV), rotavirus, influenza virus, and MAYV [27, 1].
The action of substances derived from S. terebinthifolius was evaluated in the first replicative cycle of MAYV. Thus, a reduction in viral titer due to the presence of THA and AAF was observed. This implied that the mechanisms involved in the antiviral activity of these compounds are fast-acting and are not the result of defective virus production due to a large number of replication cycles.
Tests were carried out with the dichloromethane extract, the ethyl acetate extract, THA, and AAF. The results indicated the presence of virucidal activity in all substances tested. The largest effect was observed for the ethyl acetate extract and THA. These also caused reduced infection intensity after 8 h, as observed in the immunofluorescence assays. TEM observations indicated that THA interacted with the MAYV particle, damaging its structure. This suggests that virucidal activity, in the form of direct targeting of the virus particle, is one of the mechanisms of action for these flavonoids.
The results presented herein show that the biflavonoids, THA and AAF, possess antiviral activity against an important re-emerging alphavirus. These molecules also had strong virucidal activity, were able to reduce the intensity of MAYV infection and could possibly also affect other alphaviruses. They are, therefore, potential candidates for future therapeutic use. Ethyl acetate extract also had high selectivity index (90) and approximately 98% virucidal activity. In conclusion this plant has great potential as a source of antiviral substances. The data presented show that these substances have good antiviral activity against the alphavirus MAYV and are excellent candidates for future studies with other enveloped RNA viruses.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação de Amparo à Pesquisa do Estado do Espirito Santo (FAPES), Instituto Nacional de Ciência e Tecnologia de Biologia Estrutural e Bioimagem (INBEB).
Declaration
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andres A, Donovan SM, Kuhlenschmidt MS. Soy isoflavones and virus infections. J Nutr Biochem. 2009;20:563–569. doi: 10.1016/j.jnutbio.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunini S, França DDS, Silva JB, Silva LN, Silva FPA, Spadoni M, Rezza G. High frequency of Mayaro virus IgM among febrile patients, Central Brazil. Emerg Infect Dis. 2017;23:1025–1026. doi: 10.3201/eid2306.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covington CL, Junior FMS, Silva JHS, Kuster RM, de Amorim MB, Polavarapu PL. Atropoisomerism in biflavones: the absolute configuration of (−)-agathisflavone via chiroptical spectroscopy. J Nat Prod. 2016;79:2530–2537. doi: 10.1021/acs.jnatprod.6b00395. [DOI] [PubMed] [Google Scholar]
- 4.Feuereisen MM, Hoppe J, Zimmermann BF, Weber F, Schulze-Kaysers N, Schieber A. Characterization of phenolic compounds in Brazilian pepper (Schinus terebinthifolius Raddi) exocarp. J Agric Food Chem. 2014;62:6219–6226. doi: 10.1021/jf500977d. [DOI] [PubMed] [Google Scholar]
- 5.Figueiredo LTM. Serious disease outbreaks caused by viruses transmitted by Aedes aegypti in Brazil. Rev Soc Bras Med Trop. 2016;49:265–266. doi: 10.1590/0037-8682-0209-2016. [DOI] [PubMed] [Google Scholar]
- 6.Figueiredo MLG, Figueiredo LTM. Emerging alphaviruses in the americas: Chikungunya and Mayaro. Rev Soc Bras Med Trop. 2014;47:677–683. doi: 10.1590/0037-8682-0246-2014. [DOI] [PubMed] [Google Scholar]
- 7.Gould EA, Coutard B, Malet H, Morin B, Jamal S, Weaver S, Gorbalenya A, Moureau G, Baronti C, Delogu I, Forrester N, Khasnatinov M, Gritsun T, de Lamballerie X, Canard B. Understanding the alphaviruses: recent research on important emerging pathogens and progress towards their control. Antiviral Res. 2009;87:111. doi: 10.1016/j.antiviral.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong HJ, Ryu YB, Park SJ, Kim JH, Kwon HJ. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg Med Chem. 2009;17:6816–6823. doi: 10.1016/j.bmc.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Huang H, Feng M, Zhou W, Shi X. In vitro and in vivo antihepatitis b virus activities of a plant extract from Geranium carolinianum l. Antiviral Res. 2008;79:114–120. doi: 10.1016/j.antiviral.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 10.de Lima MR, de Souza Luna J, dos Santos AF, de Andrade MC, Sant'Ana AE, Genet JP, Marquez B, Neuville L, Moreau N. Anti-bacterial activity of some Brazilian medicinal plants. J Ethnopharmacol. 2006;105:137–147. doi: 10.1016/j.jep.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Lima-Camara TN. Emerging arboviruses and public health challenges in Brazil. Rev Saúde Pública. 2016;50:1–7. doi: 10.1590/S1518-8787.2016050006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin YM, Flavin MT, Schure R, Chen FC, Sidwell R, Barnard DI, Huffmann JH, Kern ER. Antiviral activities of biflavonoids. Planta Med. 1999;65:120–125. doi: 10.1055/s-1999-13971. [DOI] [PubMed] [Google Scholar]
- 13.Long KC, Ziegler SA, Thangamani S, Hausser NL, Kochel TJ, Higgs S, Tesh RB. Experimental transmission of Mayaro virus by Aedes aegypti. Am J Trop Med Hyg. 2011;85:750–757. doi: 10.4269/ajtmh.2011.11-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Melo Júnior EJM, Raposo MJ, Lisboa Neto JA, Diniz MFA, Marcelino Júnior CAC, Sant’Ana AEG. Medicinal plants in the healing of dry socket in rats: Microbiological and microscopic analysis. Phytomedicine. 2002;9:109–116. doi: 10.1078/0944-7113-00087. [DOI] [PubMed] [Google Scholar]
- 15.Mezencio JMS, de Souza W, Fonseca MEF, Rebello MA. Ultrastructural study of Mayaro virus replication in BHK-21 cells. Arch Virol. 1990;114:229–235. doi: 10.1007/BF01310751. [DOI] [PubMed] [Google Scholar]
- 16.Morais TR, da Costa-Silva TA, Tempone AG, Borborema SET, Scotti MT, de Sousa RM, Araujo AC, de Oliveira A, de Morais SA, Sartorelli P, Lago JH. Antiparasitic activity of natural and semi-synthetic tirucallane triterpenoids from Schinus terebinthifolius (Anacardiaceae): structure/activity relationships. Molecules. 2014;19:5761–5776. doi: 10.3390/molecules19055761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orhan DD, Ozcelik B, Ozgen S, Ergun F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol Res. 2009;165:496–504. doi: 10.1016/j.micres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Epidemiol. 1938;27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 19.Santa-Cecília FV, Vilela FC, da Rocha CQ, Dias DF, Cavalcante GP, Freitas LA, dos Santos MH, Giusti-Paiva A. Anti-inflammatory and antinociceptive effects of Garcinia brasiliensis. J Ethnopharmacol. 2011;133:467–473. doi: 10.1016/j.jep.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 20.dos Santos AE, Kuster RM, Yamamoto KA, Salles TS, Campos R, de Meneses MDF, Soares MR, Ferreira D. Quercetin and quercetin 3-O-glycosides from Bauhinia longifolia (Bong.) Steud. show anti-Mayaro virus activity. Parasit Vectors. 2014;7:130–136. doi: 10.1186/1756-3305-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.dos Santos CA, de Souza Alves M, Da Silva LCP, dos Santos PD, Sanches MN, de Almeida DS, de Souza MAA. Volatiles composition and extraction kinetics from Schinus terebinthifolius and Schinus molle leaves and fruit. Braz J Pharmacog. 2015;25:356–362. doi: 10.1016/j.bjp.2015.07.003. [DOI] [Google Scholar]
- 22.Spindola KCW, Simas NK, Salles TS, de Meneses MDF, Sato A, Ferreira D, Romão W, Kuster RM. Anti-Mayaro virus activity of Cassia australis extracts (Fabaceae, Leguminosae) Parasit Vectors. 2014;7:537–543. doi: 10.1186/s13071-014-0537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wannan BS, Waterhouse JT, Gadek PA, Quinn CJ. Biflavonyls and the affinities of Blepharocarya. Biochem Syst Ecol. 1985;13:105–108. doi: 10.1016/0305-1978(85)90066-3. [DOI] [Google Scholar]
- 24.Weniger B, Vonthron-Sénécheau C, Kaiser M, Brun R, Anton R. Comparative antiplasmodial, leishmanicidal and antitrypanosomalactivities of several biflavonoids. Phytomedicine. 2006;13:176–180. doi: 10.1016/j.phymed.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Wiggins K, Eastmond B, Alto BW. Transmission potential of Mayaro virus in Florida Aedes aegypti and Aedes albopictus mosquitoes. Med Vet Entomol. 2018;32:436–442. doi: 10.1111/mve.12322. [DOI] [PubMed] [Google Scholar]
- 26.Zakaryan H, Arabyan E, Oo A, Zandi K. Flavonoids: promising natural compounds against viral infections. Arch Virol. 2017;162:2539–2551. doi: 10.1007/s00705-017-3417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z, Zhang Y, Ding XR, Chen SH, Yang J, Wang XJ, Jia GL, Chen HS, Bo XC, Wang SQ. In vitro and in vivo anti-hepatitis B virus activities of a plant extract from Geranium carolinianum L. Antiviral Res. 2008;79:114–120. doi: 10.1016/j.antiviral.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Zuchi N, Heinen LB, Santos MA, Pereira FC, Slhessarenko RD. Molecular detection of Mayaro virus during a dengue outbreak in the state of Mato Grosso, Central-West Brazil. Mem Inst Oswaldo Cruz. 2014;109:20–823. doi: 10.1590/0074-0276140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.