Summary
Conidial pigment is an important virulence factor in Aspergillus fumigatus, a human fungal pathogen. The biosynthetic gene cluster for 1,8-dihydroxynaphthalene (DHN)-melanin in A. fumigatus consists of six genes, alb1, ayg1, arp1, arp2, abr1, and abr2. In contrast to black DHN-melanin fungi such as Magnaporthe grisea, the polyketide synthase Alb1p in A. fumigatus produces naphthopyrone YWA1 instead of 1,3,6,8-THN (T4HN) and YWA1 is converted to T4HN by Ayg1p. The yeast transformant expressing Alb1p and Arp1p dehydratase produced an unknown compound which was identified to be a novel angular naphthopyrone named YWA3 formed from YWA1. In addition, the amount of YWA3 produced was much more than that of YWA2 formed by non-enzymatic dehydration from YWA1. To further analyze the reaction in vitro, Arp1p was overexpressed in E. coli and purified. Kinetic analysis revealed Km value of Arp1p for YWA1 to be 41 μM which is comparable with that of Ayg1p for YWA1 in conversion to T4HN. The complex structure modelling well explained the mechanism of YWA3 generation by the dehydration of angular YWA1 by Arp1p. These results indicated the possibility that polymerization of angular naphthopyrone YWA3 but not YWA2 could be involved in the characteristic bluish-green conidial pigmentation of A. fumigatus.
Introduction
Aspergillus fumigatus, a ubiquitous fungus, can cause allergy, noninvasive colonization, or life-threatening invasive pulmonary aspergillosis (Latgé and Chamilos, 2019). Pigmentation of A. fumigatus conidia has been shown to contribute not only to the survival and longevity of the fungal propagules in the environment but also to its pathogenicity (Stappers et al., 2018).
Studies of conidial pigment biosynthesis in A. fumigatus have uncovered a unique 1,8-dihydroxynaphthalene (DHN)-melanin pathway rather different from those of brown or black fungi (Tsai et al., 2001). In A. fumigatus, a heptaketide naphthopyrone, YWA1, produced by Alb1p polyketide synthase (PKS) (Watanabe et al., 2000), is converted to 1,3,6,8-tetrahydroxynaphthalene (T4HN) by a chain-shortening enzyme, Ayg1p (Fujii et al., 2004). T4HN is then converted to DHN by sequential reduction and dehydration reactions by Arp2p and Arp1p, respectively. Finally, polymerization catalyzed by laccase(s) Abr1p and/or Abr2p leads to formation of DHN-melanin. However, characteristic bluish-green pigmentation of A. fumigatus conidia has not been explained by DHN-melanin alone and thought that some other YWA1 derived pigmentation could contribute to the bluish-green color (Fig. 1).
Fig. 1. Proposed biosynthesis of A. fumigatus spore pigments and its biosynthetic gene cluster.

YWA3 was identified in this study. The dashed arrow indicates its possible involvement in A. fumigatus spore pigment biosynthesis.
YWA1 was first identified in A. nidulans as a product of WA PKS (Watanabe et al., 1999) and subsequently confirmed as a product of Alb1p PKS by means of heterologous expression in A. oryzae (Watanabe et al., 2000). A linear naphthopyrone, YWA2 formed from YWA1 by non-enzymatic dehydration was also detected in A. oryzae expressing WA PKS and Alb1p (Watanabe et al., 2000).
In order to characterize the complete DHN-melanin biosynthetic process, a reconstitution system was developed step by step in Saccharomyces cerevisiae. In the course of our yeast expression experiment, we noticed the production of a novel angular naphthopyrone compound YWA3 which was from the dehydration of YWA1 by the Arp1p dehydratase. To characterize the Arp1p enzymatic reaction, Arp1p was also overexpressed in E. coli and analyzed in vitro.
Materials and methods
Host yeast strain
Saccharomyces cerevisiae strain INVSc1 (MATa/MATα his3Δ1 leu2 trp1-289 ura3-52) was obtained from Invitrogen. Aspergillus nidulans phosphopantetheinyl transferase gene npgA (Mootz et al., 2002) cloned from the A. nidulans FGSC4 was placed under the GPD promoter in the yeast expression plasmid p424GPD (Mumberg et al., 1994) to construct p424-npgA. The npgA expression cassette containing GPD promoter, npgA and CYC terminator, was cleaved from p424-npgA by digestion with KpnI and SacI, which was then ligated in KpnI and SacI sites in the integration vector pAUR101 (Takara Bio, Japan) (Hashida-Okada et al., 1998) to obtain pAUR-npgA. The pAUR-npgA plasmid was then linearized by BstPI digestion and introduced into S. cerevisiae INVSc1 by lithium acetate method (Gietz et al., 1995). The yeast was selected for aureobasidin resistance (Takesako et al., 1993) and a strain, INVSc1-npgA, expressing NpgAp constitutively under GPD promoter was obtained.
Construction of yeast expression plasmids
Aspergillus fumigatus B-5233, a clinical isolate that produces conidia with a bluish-green color, was the source of cDNAs of alb1, ayg1, arp1, and arp2 which are involved in conidial pigment biosynthesis (Tsai et al. 1999). The plasmids pYES-DEST52 (Invitrogen), p425GALL (Mumberg et al., 1994), and pESC-HIS (Stratagene) were used to construct expression plasmids pYES-alb1, p425-ayg1, pESC-arp1, pESC-arp2 and pESC-arp2/1, respectively. To express Alb1p, the full-length alb1 cDNA was amplified by LA Taq polymerase (Takara Bio) with primers attB-alb-N2 and attB-alb-C, which had attB1 and attB2 sequences at their 5’ ends, respectively, for GATEWAY cloning (Invitrogen). The amplified fragment was submitted to BP reaction with donor vector pDONR 201 to form entry plasmid, which was then used in an LR reaction with pYES-DEST52 destination vector (Invitrogen). The resulting expression plasmid pYES-alb1 was used to transform S. cerevisiae INVSc1-npgA.
For Ayg1p expression, the full-length ayg1 cDNA was amplified by Phusion DNA polymerase (New England Biolab) from the pT7 Blue plasmid containing the ayg1 cDNA (Fujii et al. 2004), using the forward primer ayg1-N and the reverse primer ayg1-C. The resulting PCR product was digested with SpeI and PstI, and then ligated into SpeI/PstI-digested p425 GALL to construct p425-ayg1.
The plasmids pESC-arp2, pESC-arp1 and pESC-arp2/1 were constructed as follows. The full-length cDNAs of arp1 and arp2 were amplified by Phusion DNA polymerase (New England Biolab) from the plasmids pGC37-6 and pGC37-11 containing the arp1 and arp2 cDNAs (Tsai et al. 1997, Tsai et al. 1999), using primer pairs arp1-N and -C, arp2-N and -C, respectively. After digestion with EcoRI and SacI, the arp1 cDNA fragment was ligated into EcoRI/SacI sites of pESC-HIS to form pESC-arp1. The BamHI and ApaI digested arp2 cDNA fragment was cloned into BamHI/ApaI sites of pESC-HIS to yield pESC-arp2. In the same way, both arp1 and arp2 cDNAs were cloned into pESC-HIS to construct pESC-arp2/1.
Yeast transformation
S. cerevisiae (SC) dropout semisynthetic medium was used for selection of S. cerevisiae transformants. Transformation of S. cerevisiae INVSc1-npgA was conducted using Frozen-EZ Yeast Transformation kit (Zymo Research). Yeast cells harboring transformed plasmid(s) were initially cultured in the SC dropout medium overnight at 30°C on a 220 rpm shaker. For expression under GAL promoter, cells were further cultured for 2 days in SC medium containing 2% galactose and 1% raffinose in place of glucose.
Instrumental analysis
1H-NMR and 13C-NMR spectra were obtained with JNM-A500 and JNM-ECA 500 instruments (JEOL, Japan) and mass spectra were obtained with SX-102A apparatus (JEOL, Japan). LC/MS analysis was carried out by using Micro TOF focus-WS (Brucker Daltonics) with Agilent 1100 LC system using Electrospray Ionization with Cadenza CD-C18 column (2.0 i.d. × 150 mm; Imtakt Co. Ltd.) maintained at 40 °C. The solvent mixtures were A) water/acetic acid (99/1) and B) acetonitrile/acetic acid (99/1). The elution was performed with a linear gradient of 10% B to 50% B in the first 20 min and then from 50% B to 100% B up to 25 min at 0.2 mL/min flow rate with photodiode array detection.
HPLC analyses were carried out by using a Tosoh 8020 CCPM pump (Tosoh, Japan) connected to a reversed-phase column (Tosoh ODS-80Ts, 4.6 i.d. × 150 mm) maintained at 40 °C. The solvent mixtures were A) water/acetic acid (99/1) and B) acetonitrile/acetic acid (99/1). The elution was performed with a linear gradient of 10% B to 50% B in the first 20 min and then from 50% B to 100% B up to 25 min at 0.8 mL/min flow rate with photodiode array detection.
Isolation of YWA3
After 2 days incubation of induction culture, the yeast cells were collected by centrifugation and extracted with ethyl acetate. From 1 L induction culture of S. cerevisiae INVSc1-npgA/pYES-alb1+pESC-arp1 transformants, 46 mg of yellow compound was isolated by silica gel column chromatography. Chemical structure of YWA3 was determined by NMR and MS analyses. Spectral data of YWA3 are shown in Table S4 and Fig. S8–S13.
Overexpression and purification of Arp1p-HT
E. coli expression plasmid pET-arp1HT was constructed as follows. The arp1 cDNA with His-tag sequence and enterokinase recognition site at N-terminus was amplified using Arp1-N (NcoI) and Arp1-C (BamHI) primers by Phusion DNA polymerase (New England Biolab). After digestion with NcoI and BamHI, arp1 cDNA fragment was inserted into NcoI site and BamHI site of pET-3d (Novagen). Thus constructed pET-arp1HT was transformed into E. coli BL21-Codon Plus(DE3)-RIPL (Stratagene). The transformant was precultured in LB medium containing carbenicillin and chloramphenicol (50 μg/mL each) for overnight at 37°C. The 1 ml of the overnight culture was inoculated into 200 mL LB medium containing carbenicillin and chloramphenicol and grown to an A600 of 0.5 at 37°C. The culture was cooled to 25°C, and expression was induced with 0.1 mM IPTG and incubated for additional 6 h at 25°C. The cells harvested by centrifugation were resuspended in ice-cold 9 mL equilibration buffer (potassium phosphate buffer, pH 7.5, 150 mM NaCl, 20 mM imidazole, 30% glycerol). After cell disruption by sonication, the Arp1p-HT soluble fraction was obtained by centrifugation and then purified using HIS-select Nickel Affinity Gel (Sigma).
Arp1p enzyme assay
Arp1p enzyme activity was assayed spectrophotometrically as follows: Ten microliters of 1 mM YWA1 dissolved in ethylene glycol monomethyl ether and 50 μL of the enzyme solution were added to 440 μL of 50 mM potassium phosphate buffer, pH 6.0. Based on the absorption shift of YWA1 λmax at 406 nm to YWA3 λmax at 373.5 nm, the decrease of A406 was monitored at 30°C. Reaction velocities were calculated based on the A406 molar absorption difference coefficient of substrate YWA1 to product YWA3 (Δε=4950). (Fig. S1)
When scytalone was used as the substrate, reaction velocities were calculated based on the A352 molar absorption difference coefficient of substrate scytalone to product 1,3,8-tetrahydroxynaphthalene (T3HN) Δε=4100 was used.
Modeling of complex structures
The X-ray structure of Magnaporthe grisea scytalone dehydratase (SDH) in complex with a scytalone analog (PDB ID: 4STD) was taken from the PDB databank. For Arp1p, a homology model was created based on the chain A of 4STD using the SWISS-MODEL workspace (Waterhouse et al., 2018). After processing the chain A of each protein structure appropriately, docking calculations of scytalone were performed. For all structures obtained from the docking calculations, energy minimization calculations for scytalone and its surrounding residues (residues within 5 Å of the substrate) were performed. The most stable complex structure that is valid as the pre-reaction complex structure was selected for each target protein. The obtained scytalone-SDH complex model and scytalone-Arp1p complex model are shown in the upper left and lower left of Fig. 5. Next, by superposing angular YWA1 on scytalone in the complex models, the valid initial configurations of angular YWA1 in SDH and Arp1p were created (Arp1p and SDH were already superposed when the homology model was created). Subsequently, the energy minimization calculations for angular YWA1 and its surrounding residues were performed. The obtained angular YWA1-SDH complex model and angular YWA1-Arp1p complex model are shown in the upper right and lower right of Fig. 5. The Protein Preparation Wizard, Glide and Prime (Schrödinger Release 2021–2: Protein Preparation Wizard; Epik, Schrödinger, LLC, New York, NY, 2021; Impact, Schrödinger, LLC, New York, NY; Prime, Schrödinger, LLC, New York, NY, 2021; Glide, Schrödinger, LLC, New York, NY, 2021) available from the Schrödinger suite 2021–2 were used for protein structure preparation, docking calculation, and energy minimization calculation, respectively. At the protein structure preparation stage, His-85 and His-110 in SDH and His-84 and His-109 in Arp1p were fixed to HID form. In the docking calculation, Glide SP mode docking was used and a water molecule between Tyr-30 and Tyr-50 in SDH, acting as a general acid for the carbonyl group of the substrate, was kept and treated as a part of the protein. The energy minimization was performed using Prime MM-GBSA method (VSGB solvation model). All calculations were performed using OPLS_2005 force field. To obtain four complex structure models, binding free energy ΔGbind between the protein and the substrate were calculated as the measure of binding affinity (Table 1). The value includes energetic penalty due to strain of both the substrate and the protein from the free non-complexed state to the complexed state. The details of the protein structure preparation, docking and energy minimization calculations for scytalone are in the supplementary information.
Fig. 5. Predicted binding models of substrates in the active sites of SDH and Arp1p.

Hydrogen bonds are shown in dotted lines. The structures were visualized by PyMOL (The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC.).
Table 1.
Binding affinities and the Nε…HR distances of complex models.
| ΔGbind (kcal/mol) | Nε…HR distancea (Å) | |
|---|---|---|
| scytalone-SDH | −64.04 | 2.52 |
| scytalone-Arp1p | −47.09 | 2.53 |
| angular YWA1-SDH | −45.13 | 2.81 |
| angular YWA1-Arp1p | −50.75 | 2.84 |
Nε atom of His-85 was used for SDH and His-84 for Arp1p complex models.
Results and discussion
In order to reconstitute A. fumigatus DHN-melanin biosynthesis system in yeast, the expression plasmids pYES-alb1, p425-ayg1, pESC-arp1, pESC-apr2, and pESC-arp2/1 were transformed into the host strain S. cerevisiae INVSc1-npgA. The S. cerevisiae INVSc1-npgA was prepared by genomic integration of A. nidulans npgA phosphopantetheinyltransferase gene under the GPD promoter at aur1 gene (Heidler et al., 1995) of S. cerevisiae INVSc1. As expected, S. cerevisiae INVSc1-npgA transformed with pYES-alb1 produced naphthopyrone YWA1 as a main product together with YWA2, which is a linear naphthopyrone formed from YWA1 by non-enzymatic dehydration (Watanabe et al., 2000). (Fig. 2 A-2)
Fig. 2. HPLC analysis of products of S. cerevisiae INVSc1-npgA transformants.
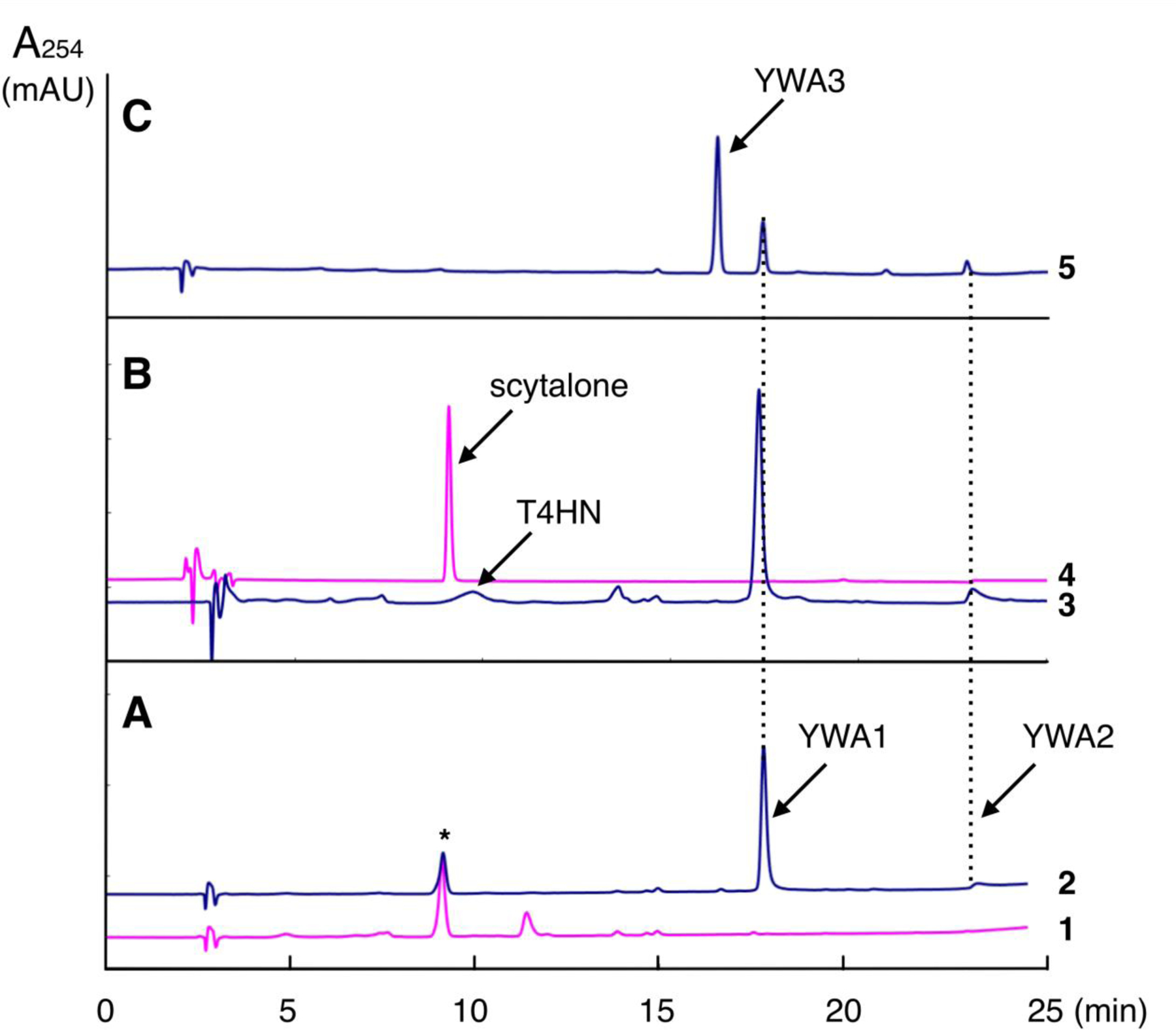
1: S. cerevisiae INVSc1-npgA/pYES-DEST52 (control transformant), 2: S. cerevisiae INVSc1-npgA/pYES-alb1, 3: S. cerevisiae INVSc1-npgA/pYES-alb1+p424-ayg1+pESC-arp2, 4: authentic scytalone, 5: S. cerevisiae INVSc1-npgA/pYES-alb1+pESC-arp1. *Background peak was observed in the control transformant.
The pYES-alb1 and p425-ayg1 were co-transformed into yeast (S. cerevisiae INVSc1-npgA/pYES-alb1+p425-ayg1) and T4HN production was detected in the derived yeast strain (Fig. S2). However, further addition of pESC-arp2 (S. cerevisiae INVSc1-npgA/pYES-alb1+p425-ayg1+pESC-arp2) to express Arp2p T4HN reductase failed to produce scytalone (Fig. 2 B-3).
By addition of Arp2p and Arp1p co-expression plasmid pESC-arp2/1 (S. cerevisiae INVSc1-npgA/pYES-alb1+p425-ayg1+pESC-arp2/1) also failed to produce scytalone but produced T4HN and an unknown compound. This unknown product was also observed in the transformant expressing alb1, ayg1, and arp1 but not arp2 (S. cerevisiae INVSc1-npgA/pYES-alb1+p425-ayg1+pESC-arp1). Co-expression of alb1 and arp1 without ayg1 (S. cerevisiae INVSc1-npgA/pYES-alb1+pESC-arp1) also produced the same unknown compound. These results confirmed that Alb1p product YWA1 was converted by Arp1p to the unknown compound named YWA3 (Fig. 2 C-5). The amount of YWA3 produced was much more than the linear naphthopyrone YWA2 born non-enzymatically from YWA1.
From 1 L induction culture of the S. cerevisiae INVSc1-npgA/pYES-alb1+pESC-arp1 co-transformant, 46 mg of yellow product was isolated by silica gel column chromatography. NMR analyses identified it to be an angular naphthopyrone formed from YWA1 by dehydration. This compound YWA3 was identified to be didesmethyl derivative of flavasperone (Lund et al., 1953; Gorst-Allman et al., 1980). (Fig. 3)
Fig. 3.
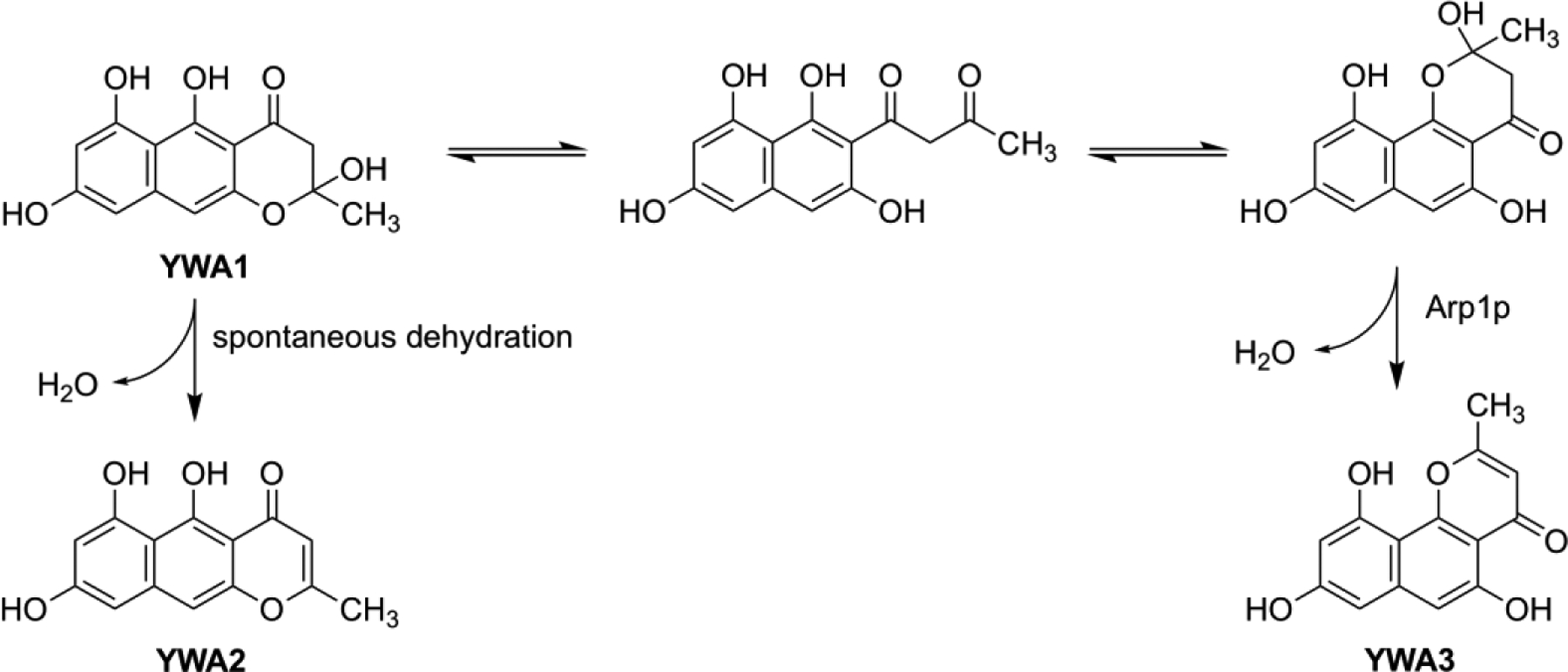
Structure of YWA3 and its formation from YWA1 via open side-chain intermediate.
To further characterize the role of Arp1p for this dehydrative conversion, Arp1p with His-tag (Arp1p-HT) was overexpressed in E. coli and purified by Ni-affinity column (Fig. 4). By gel filtration, Arp1p-HT was mainly eluted as trimer of 65 kDa (Fig. S3). The purified Arp1p-HT converted YWA1 to YWA3 in a time-dependent manner as shown in Fig. S4.
Fig. 4. SDS-PAGE analysis of Arp1p-HT purification fractions.

Lane 1, standard protein markers; lane 2, E. coli BL21-Codon Plus (DE3)-RIPL/pET-3d soluble fraction; 3, E. coli BL21-Codon Plus (DE3)-RIPL/pET-arp1HT soluble fraction; 4, Arp1p-HT nickel-affinity purified fraction.
Optimum pH of Arp1p reaction to convert YWA1 to YWA3 was found to be pH 6.0 and significant non-enzymatic formation of YWA2 from YWA1 was observed at lower pH 4.5~5.5. By kinetic analysis using 1.8 μM Arp1p-HT and 10~80 μM YWA1 in 50 mM potassium phosphate buffer, pH 6.0, at 30°C, Km=41 μM, Vmax=8.5 μM/min, and kcat=0.080 s−1 were obtained (Fig. S5). When scytalone was used as a substrate, Km=15.7 μM, Vmax=3.0 μM/min, and kcat=2.7 s−1 were determined (Fig. S6). These data indicated that scytalone is a more favorable substrate than YWA1.
It is likely that in A. fumigatus, YWA1 is a common substrate for both Ayg1p and Arp1p. Since Ayg1p can convert YWA1 to T4HN and Km value for YWA1 was previously reported to be 44 μM (Fujii et al., 2004), which is comparable with that of Arp1p for YWA1, it is possible that combination of T4HN-derived and YWA3-derived pigmentation is involved in the formation of characteristic bluish-green conidia in A. fumigatus.
In order to clarify whether angular YWA1 can be a substrate for Arp1p from a structural point of view, we modeled the complex structure of angular YWA1 and Arp1p. For comparison, models of scytalone-Arp1p complex, scytalone-SDH complex, and angular YWA1-Arp1p complex were also created (Fig. 5). For the four models, the distance between the pro-R hydrogen atom at the α-position of the carbonyl group of the substrate to which a water molecule is bound as a general acid and the N atom at the ε-position of the nearby histidine was measured (Table 1). This is because a hydrogen atom at the α-position must be extracted in order for the β-elimination reaction of substrate to occur, and in the case of SDH, the pro-R hydrogen atom is extracted by His-85 (Jordan et al., 2000). In Arp1p, His-84 is expected to play the role of His-85 in SDH. According to Table 1, angular YWA1 has a slightly longer but about the same distance both in SDH and Arp1p than scytalone. This means that angular YWA1, like scytalone, can form a pre-reaction complex structure that is capable of β-elimination reaction in both enzymes. However, there were differences in the relative binding affinities between the two substrates for each enzyme. The binding affinity of scytalone to SDH is higher (−64.04 kcal/mol) than that of angular YWA1 (−45.13 kcal/mol), whereas for Arp1p, scytalone and angular YWA1 have almost the same binding affinity (−47.09 kcal/mol and −50.75 kcal/mol, respectively) (Table 1). This is consistent with the experimental results that the main substrate of SDH is scytalone and the substrate of Arp1p is both scytalone and angular YWA1. There is a 50% identity of amino acid sequences between SDH and Arp1p. However, one of the key amino acid Ser-129 in SDH is not conserved but substituted with Ala-128 in Arp1p (Fig. S7). Therefore, scytalone is stable in SDH because it can form a hydrogen bond with Ser-129, whereas in Arp1p, it cannot form a hydrogen bond because it is Ala-128 and the stability is reduced. In the case of angular YWA1, it cannot form a hydrogen bond with Ser-129 in SDH, but rather causes structural distortion to avoid collision with Ser-129. On the other hand, in Arp1p, Ser-129 is replaced with Ala-128, which is one heavy atom shorter, thus reducing the destabilization caused by the collision with angular YWA1. In addition, Ala-127 of SDH is replaced by the smaller Gly-126 in Arp1p (Fig. S7), also reducing the destabilization of angular YWA1 in Arp1p. Hence, due to both the decrease in the stability of scytalone and the decrease in the instability of angular YWA1 in Arp1p, the two substrates have almost the same binding affinity in Arp1p. Angular YWA1 can be a substrate for Arp1p to the same extent as scytalone. Thus, the complex structure modelling well supported that the experimentally observed compound YWA3 is generated by the dehydration of angular YWA1 by Arp1p.
In conclusion, we carried out construction of A. fumigatus DHN-melanin biosynthesis in yeast expressing alb1, ayg1, arp2 and arp1. Although it is not clear why expression of arp2 coding for possible T4HN reductase failed in our yeast system to produce scytalone, a novel angular naphthopyrone named YWA3 formation was observed from YWA1 by Arp1p dehydratase. These results indicated the possibility that polymerization of angular naphthopyrone YWA3 could be involved in characteristic bluish-green conidial pigmentation in A. fumigatus.
Supplementary Material
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP18018009, JP20018024 and JP17H03995 to I.F. from the Ministry of Education, Science, Sports and Culture, Japan.
H-FT, YCC and KJK-C were supported by the Division of Intramural Research (DIR), NIAID, NIH.
Footnotes
Conflicts of interest. None declared.
References
- Basarab GS, Steffens JJ, Wawrzak Z, Schwartz RS, Lundqvist T, and Jordan DB (1999) Catalytic mechanism of scytalone dehydratase: site-directed mutagenesis, kinetic isotope effects, and alternate substrates. Biochem 38: 6012–6024. [DOI] [PubMed] [Google Scholar]
- Fujii I, Yasuoka Y, Tsai H-F, Chang YC, Kwon-Chung KJ, and Ebizuka Y (2004) Hydrolytic polyketide shortening by Ayg1p, a novel enzyme involved in fungal melanin biosynthesis. J Biol Chem 279: 4613–44620. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH, Willems AR, and Woods RA (1995) Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 15: 355–360. [DOI] [PubMed] [Google Scholar]
- Gorst-Allman CP, Steyn PS, and Rabie CJ (1980) Structural elucidation of the nigerones, four new naphthopyrones from cultures of Aspergillus niger. J Chem Soc Perkin Trans 1 2474–2479. [Google Scholar]
- Hashida-Okada T, Ogawa A, Kato I, and Takesako K (1998) Transformation system for prototrophic industrial yeasts using the AUR1 gene as a dominant selection marker. FEBS Lett 425: 117–122. [DOI] [PubMed] [Google Scholar]
- Heidler SA, and Radding JA (1995) The AUR1 Gene in Saccharomyces cerevisiae encodes dominant resistance to the antifungal agent aureobasidin A (LY295337). Antimicrob Agents Chemother 39: 2765–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan DB, Zheng Y-J, Locket BA, Basarab GS (2000) Stereochemistry of the enolization of scytalone by scytalone dehydratase. Biochem 39, 2276–2282. [DOI] [PubMed] [Google Scholar]
- Latgé J-P, and Chamilos G (2019) Aspergillus fumigatus and aspergillosis in 2019. Clin Microbiol Rev 33: e00140–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund NA, Robertson A, and Whalley WB (1953) The chemistry of fungi. Part XXI. Asperxanthone and a preliminary examination of aspergillin. J Chem Soc 2434–2439. [Google Scholar]
- Mootz HD, Schörgendorfer K, and Marahiel MA (2002) Functional characterization of 4’-phosphopantetheinyl transferase genes of bacterial and fungal origin by complementation of Saccharomyces cerevisiae lys5. FEMS Microbiol Lett 213:51–57. [DOI] [PubMed] [Google Scholar]
- Mumberg D, Müller R, and Funk M (1994) Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acid Res 22:5767–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman SB, and Wunsch CD (1970) A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol 48: 443–53. [DOI] [PubMed] [Google Scholar]
- Stappers MHT, Clark AE, Aimanianda V, Bidula S, Reid DM, Asamaphan P, et al. (2018) Recognition of DHN-melanin by a C-type lectin receptor is required for immunity to Aspergillus. Nature 555: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesako K, Kuroda H, Inoue T, Haruna F, Yoshikawa Y, and Kato I (1993) Biological properties of aureobasidin A, depsipeptide antifungal antibiotic. J Antibiot 46:1414–1420. [DOI] [PubMed] [Google Scholar]
- Tsai H-F, Washburn RG, Chang YC, and Kwon-Chung KJ (1997) Aspergillus fumigatus arp1 modulates conidial pigmentation and complement deposition. Mol Microbiol 26:175–183. [DOI] [PubMed] [Google Scholar]
- Tsai H-F, Wheeler MH, Chang YC, and Kwon-Chung KJ (1999) A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol 181:6469–6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H-F, Fujii I, Watanabe A, Wheeler MH, Chang YC, Yasuoka Y, Ebizuka Y, and Kwon-Chung KJ (2001) Pentaketide melanin biosynthesis in Aspergillus fumigatus requires chain-length shortening of a heptaketide precursor. J Biol Chem 276:29292–29298. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Fujii I, Sankawa U, Mayorga ME, Timberlake WE, and Ebizuka Y (1990) Re-identification of Aspergillus nidulans wA gene to code for a pentaketide synthase of naphthopyrone. Tetrahedron Lett 40: 91–94. [Google Scholar]
- Watanabe A, Fujii I, Tsai H-F, Chang YC, Kwon-Chung KJ, and Ebizuka Y (2000) Aspergillus fumigatus alb1 encodes naphthopyrone synthase when expressed in Aspergillus oryzae. FEMS Microbiol Lett 192: 39–44. [DOI] [PubMed] [Google Scholar]
- Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, and Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46(W1), W296–W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


