Abstract
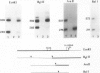
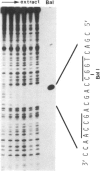
The hepatitis B virus (HBV) surface antigen (HBsAG) is encoded by the S gene under the regulation of a promoter in the pre-S1 region. The S gene promoter does not contain a 'TATA' box-like sequence, but there is a sequence resembling, in part, the late promoter of Simian virus 40 (SV40). In an attempt to study the regulation of the S gene promoter we looked for cellular proteins which bind to this region. We report here that a nuclear protein is tightly bound to the HBV genome at a position approximately 190 bases upstream from the S gene promoter. Evidence is provided to show that (a) this nuclear protein is the nuclear factor I (NF-I) that was previously found to be bound to the inverted terminal repeat of the adenovirus (Ad) DNA and to enhance Ad DNA replication in vitro and (b) this NF-I binding site is required for optimal activity of the S gene promoter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgmeyer U., Nowock J., Sippel A. E. The TGGCA-binding protein: a eukaryotic nuclear protein recognizing a symmetrical sequence on double-stranded linear DNA. Nucleic Acids Res. 1984 May 25;12(10):4295–4311. doi: 10.1093/nar/12.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Will H., Hernandez N., Schaller H. Signals regulating hepatitis B surface antigen transcription. Nature. 1983 Sep 22;305(5932):336–338. doi: 10.1038/305336a0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gronostajski R. M., Adhya S., Nagata K., Guggenheimer R. A., Hurwitz J. Site-specific DNA binding of nuclear factor I: analyses of cellular binding sites. Mol Cell Biol. 1985 May;5(5):964–971. doi: 10.1128/mcb.5.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein U., England B., Tjian R. Characterization of Drosophila transcription factors that activate the tandem promoters of the alcohol dehydrogenase gene. Cell. 1985 Jul;41(3):965–977. doi: 10.1016/s0092-8674(85)80077-5. [DOI] [PubMed] [Google Scholar]
- Hennighausen L., Siebenlist U., Danner D., Leder P., Rawlins D., Rosenfeld P., Kelly T., Jr High-affinity binding site for a specific nuclear protein in the human IgM gene. Nature. 1985 Mar 21;314(6008):289–292. doi: 10.1038/314289a0. [DOI] [PubMed] [Google Scholar]
- Leegwater P. A., van Driel W., van der Vliet P. C. Recognition site of nuclear factor I, a sequence-specific DNA-binding protein from HeLa cells that stimulates adenovirus DNA replication. EMBO J. 1985 Jun;4(6):1515–1521. doi: 10.1002/j.1460-2075.1985.tb03811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegwater P. A., van der Vliet P. C., Rupp R. A., Nowock J., Sippel A. E. Functional homology between the sequence-specific DNA-binding proteins nuclear factor I from HeLa cells and the TGGCA protein from chicken liver. EMBO J. 1986 Feb;5(2):381–386. doi: 10.1002/j.1460-2075.1986.tb04223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNab G. M., Alexander J. J., Lecatsas G., Bey E. M., Urbanowicz J. M. Hepatitis B surface antigen produced by a human hepatoma cell line. Br J Cancer. 1976 Nov;34(5):509–515. doi: 10.1038/bjc.1976.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowock J., Borgmeyer U., Püschel A. W., Rupp R. A., Sippel A. E. The TGGCA protein binds to the MMTV-LTR, the adenovirus origin of replication, and the BK virus enhancer. Nucleic Acids Res. 1985 Mar 25;13(6):2045–2061. doi: 10.1093/nar/13.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J., Rutter W. J. Hybrid hepatitis B virus-host transcripts in a human hepatoma cell. Proc Natl Acad Sci U S A. 1985 Jan;82(1):83–87. doi: 10.1073/pnas.82.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcel C., Louise A., Gervais M., Chenciner N., Dubois M. F., Tiollais P. Transcription of the hepatitis B surface antigen gene in mouse cells transformed with cloned viral DNA. J Virol. 1982 Apr;42(1):100–105. doi: 10.1128/jvi.42.1.100-105.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall L. B., Standring D. N., Laub O., Rutter W. J. Transcription of hepatitis B virus by RNA polymerase II. Mol Cell Biol. 1983 Oct;3(10):1766–1773. doi: 10.1128/mcb.3.10.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins D. R., Rosenfeld P. J., Wides R. J., Challberg M. D., Kelly T. J., Jr Structure and function of the adenovirus origin of replication. Cell. 1984 May;37(1):309–319. doi: 10.1016/0092-8674(84)90327-1. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Bourgeois S., Cohn M. The lac repressor-operator interaction. 3. Kinetic studies. J Mol Biol. 1970 Nov 14;53(3):401–417. doi: 10.1016/0022-2836(70)90074-4. [DOI] [PubMed] [Google Scholar]
- Shaul Y., Rutter W. J., Laub O. A human hepatitis B viral enhancer element. EMBO J. 1985 Feb;4(2):427–430. doi: 10.1002/j.1460-2075.1985.tb03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y., Ziemer M., Garcia P. D., Crawford R., Hsu H., Valenzuela P., Rutter W. J. Cloning and analysis of integrated hepatitis virus sequences from a human hepatoma cell line. J Virol. 1984 Sep;51(3):776–787. doi: 10.1128/jvi.51.3.776-787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Hennighausen L., Battey J., Leder P. Chromatin structure and protein binding in the putative regulatory region of the c-myc gene in Burkitt lymphoma. Cell. 1984 Jun;37(2):381–391. doi: 10.1016/0092-8674(84)90368-4. [DOI] [PubMed] [Google Scholar]
- Standring D. N., Rutter W. J., Varmus H. E., Ganem D. Transcription of the hepatitis B surface antigen gene in cultured murine cells initiates within the presurface region. J Virol. 1984 May;50(2):563–571. doi: 10.1128/jvi.50.2.563-571.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Ziemer M., Garcia P., Shaul Y., Rutter W. J. Sequence of hepatitis B virus DNA incorporated into the genome of a human hepatoma cell line. J Virol. 1985 Mar;53(3):885–892. doi: 10.1128/jvi.53.3.885-892.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W., Tyndall C., Lupton S., Kamen R. Polyoma virus DNA replication requires an enhancer. Nature. 1984 Nov 15;312(5991):242–246. doi: 10.1038/312242a0. [DOI] [PubMed] [Google Scholar]