Abstract
Background:
Electronic cigarette use has escalated rapidly in recent years, particularly among youth. Little is known about the genetic influences on e-cigarette use.
Aims:
To determine whether genetic risk for regular use of combustible cigarettes or for number of cigarettes smoked per day confers risk for ever e-cigarette use or frequency of e-cigarette use.
Design, setting, participants, and measurements:
We used data from 9,541 young adults from the Spit for Science longitudinal cohort study (2011–2019). Polygenic scores (PGS) of regular combustible cigarette use (PGS-RCU) and cigarettes per day (PGS-CPD) were constructed using summary statistics from the two largest available GWAS meta-analysis of European ancestry and East Asian ancestry of combustible cigarette use and used to test whether the PGS of RCU or CPD predicted lifetime e-cigarette use and frequency of past 30-day e-cigarette use in a diverse sample of young adults of African (AFR), Admixed American (AMR), East Asian (EAS), European (EUR), and South Asian (SAS) ancestry.
Findings:
The PGS-RCU was associated with lifetime e-cigarette use in the EUR sample (OR:1.27, 95% CI:1.19–1.36, p=7.53×10−12) but not in the other subsamples (p’s>0.12). This association remained significant after excluding regular combustible cigarette smokers (OR:1.21, 95% CI:1.12–1.31, p=3.36×10−6). The PGS-CPD was not significantly associated with lifetime e-cigarette use and neither the PGS-RCU nor the PGS-CPD were associated with frequency of e-cigarette use in the past 30-days in any of the subsamples.
Conclusions:
Genetic factors associated with regular combustible cigarettes use are associated with ever e-cigarette use in young adults. We did not find evidence for shared genetic factors influencing heaviness of use of combustible cigarettes and current e-cigarette use frequency.
Introduction
Use of e-cigarettes has increased rapidly over the last several years, especially amongst youth. The prevalence of past 30-day e-cigarette use has more than doubled from 2017 to 2019 among both 12th graders (11% to >25%) and young adults (6.5% to 15%) (1,2). This increase is noteworthy, as early exposure to nicotine through e-cigarette use may increase risk for combustible cigarette smoking (3), nicotine dependence (4), adverse health effects (5,6) and other drug use. A recent meta-analysis showed that individuals with lifetime e-cigarette use were 3.5 times more likely to try combustible cigarettes than those who had never used e-cigarettes (7). Intent to try combustible cigarettes was nearly twice as high among individuals with lifetime e-cigarette use compared to those who have never used e-cigarettes (8). It is not known if the higher rate of combustible cigarette use among those who use e-cigarettes is due to nicotine exposure and/or dependence, a common genetic liability to use e-cigarettes and other combustible tobacco products, or both.
Initial evidence suggests that the increased risk of combustible cigarette use amongst e-cigarette users could be due to a shared genetic liability for nicotine use (9,10). A recently published twin study (11) also provides initial evidence for a genetic liability to e-cigarette initiation and shared genetic liability with combustible cigarette initiation. These findings require replication in further studies of genetic influences on e-cigarette use, including molecular genetic studies addressing these phenotypic associations and latent or molecular genetic studies in individuals of non-European ancestry.
Since the introduction of e-cigarettes to the consumer market, research into the genetics of nicotine use has focused on factors influencing combustible cigarette use. Combustible cigarettes and e-cigarettes share the common addictive and psychoactive ingredient, nicotine. Twin and family studies have shown a significant role of genetic influences on both combustible cigarette initiation and maintenance (12,13). Further, the strength of genetic influences on smoking initiation and quantity of combustible cigarettes smoked increases over the lifespan from adolescence to young adulthood (14). Further, genetic factors that are identified by studying combustible cigarette initiation are shared with regular smoking and nicotine dependence (15–19). However, the role of genetic factors on nicotine use via e-cigarettes remain largely unknown. Establishing the degree to which genetic factors influence e-cigarette initiation can provide important insight into the etiology of nicotine dependence. Further, the study of e-cigarette initiation in young adulthood, provides a unique opportunity to study the role of genetic factors particularly when genetic influences are expected to be increasing during life course development (14,20).
Genome-wide association studies (GWAS) of nicotine use have tested the effects of millions of genetic variants and their results complement those of twin and family studies. GWAS of combustible cigarette use have consistently identified genetic markers in genes which code for nicotinic acetylcholine receptors (nACHRs), which respond to nicotine (21–23). A recent GWAS from the GWAS & Sequencing Consortium of Alcohol and Nicotine (GSCAN) identified 467 variants associated with a range of combustible cigarette smoking behaviors, including initiation of regular smoking and cigarettes per day (22). However, compared to studies of combustible cigarette use, current GWAS of e-cigarette use do not have the appropriate sample sizes with sufficient power to detect statistically significant associations. Instead, it is possible to generate polygenic scores (PGS, i.e., a weighted aggregated score of the genetic effects across the genome) using summary statistics from a very large discovery sample with sufficient statistical power to detect associations (e.g., GSCAN) and applying it in other smaller samples. This PGS can then be used to test for associations with nicotine use outcomes.
The study of PGS for combustible cigarette use also provides a unique opportunity to test the degree to which genetic factors associated with combustible cigarette use are similarly associated with e-cigarette use. For example, Allegrini and colleagues (9) reported that a PGS for cigarettes per day was positively associated with lifetime e-cigarette use in a sample of Dutch adults ages 18–88 (M=45, SD=16), but only among ex-cigarette smokers. We build on these results in several important ways. First, we examine associations between PGSs of combustible cigarette use (regular use and cigarettes per day) from the largest GWAS to date on e-cigarette use in an ancestrally diverse sample. As such, ours is the first study to investigate associations between combustible cigarette use and e-cigarette use in individuals of non-European ancestry. Second, we characterize ever e-cigarette use and past 30-day frequency of e-cigarette use in young adults, ages 18–36 (M at study start = 18.59) since genetic factors shared between regular combustible cigarette use and ever e-cigarette use remain largely unexplored for this developmental period. Testing these associations will increase our knowledge regarding overlapping molecular genetic liability between combustible cigarette and e-cigarette use.
Methods
Sample
Data were from the Spit for Science (S4S) study, a longitudinal cohort study with five cohorts enrolled to date (24). Participants were incoming college freshman who completed online assessments of college behavioral health (25,26) and provided a saliva sample for DNA analysis. This analysis includes data from all five available cohorts with genetic and phenotypic data (N=11,147. This study was reviewed and approved by the appropriate Institutional Review Board.
Outcome Measures
In S4S data, binary variables represented if the participant had ever used e-cigarettes in their lifetime, if they had ever been a regular combustible cigarette smoker in their lifetime, operationalized as ever use of 100 cigarettes or more (21,22,27), or if they had endorsed daily combustible cigarette use in the past 30 days. Frequency of e-cigarette use in the past 30 days was represented ordinally (“None”, “Once or twice”, “A few days”, “A couple of days a week”, “Three times a week”, “Most days of the week”, “Daily or almost daily”). For each variable, data was used from the survey wave where the participant reported the most frequent or heaviest use. See Supplemental Methods and Supplemental Tables S1 and S2 for additional description of the variables and their endorsement rates.
Genotyping and imputation
Cohorts 1–3 were genotyped on the Affymetrix BioBank array, Cohort 4 on the Smokescreen Array, and Cohort 5 on the Affymetrix Axiom Precision Medicine Research Array. Quality control measures followed protocols similar to those used by the Psychiatric Genomic Consortium (PGC; further described in Webb et al. (28) and the Supplemental Methods). Imputation was conducted for each array using SHAPEIT2 (29), IMPUTE2 (30), and the 1000 genomes (1KGP) phase 3 reference panel (all 26 populations) (31,32). 1KGP was used as an external reference (31,32) for S4S genetic based ancestry assignments (African [AFR], admixed from the Americas [AMR], East Asian [EAS], European [EUR], and South Asian [SAS]) as described in Peterson et al. (33) and in the Supplemental Methods.
Creation of PGS score
GWAS summary statistics for regular combustible cigarette use and cigarettes per day were obtained from GSCAN and the BioBank Japan Project (BBJ) (22,23) to construct the PGS. The GSCAN summary statistics excluded 23andMe due to pre-established access restriction agreements. Specifics on the data cleaning and filtering of pre and post GWAS for the GSCAN and BBJ summary statistics can be found in Liu et al. (22) and Matoba et al. (23) respectively. These summary statistics, and their respective phenotypes, were chosen because they were the largest published GWAS at the time of the analyses. Larger sample sizes in the GWAS translates to more precise effect size estimates in the summary statistics which in turn yields polygenic scores that explain a greater amount of variance in the phenotype of the GWAS.
Polygenic scores were then created from the filtered GWAS summary statistics in S4S using the PRS-CSx method (34) (additional details in Supplemental Methods). Combining GWAS summary statistics from multiple ancestry groups has been shown to increase the predictive value of the PGS even when testing in ancestries not included in the PGS creation (34). A regular combustible cigarette use polygenic score (PGS-RCU) was created from the GWAS of initiation of regular smoking and a cigarettes per day polygenic score (PGS-CPD) was created from the GWAS of cigarettes per day. These polygenic scores were used as the main predictors of interest in the analyses.
Data analysis plan
Analyses were conducted separately within each ancestry group. Chi-square tests were used to assess differences between subsamples in rates of e-cigarette and combustible cigarette use. The 10 ancestry-specific principal components, cohort, and two binary variables denoting genotyping batch were included in all analyses as covariates. Logistic regression models were fit to the data to test the association between each polygenic score (PGS-RCU & PGS-CPD) and lifetime e-cigarette use. Ordinal regressions were used to test the associations between each PGS and frequency of past 30-day e-cigarette use. Odds ratios (ORs) and 95% confidence intervals (CI) are presented for all logistic and ordinal regression results. Estimates of variance explained (Nagelkerke’s pseudo R2) by the PGS were obtained by subtracting the pseudo R2 estimates of the model with covariates only from the full model including the PGS. The analytic plan was not pre-registered on Open Science Framework and results should be considered exploratory (See Supplemental Material for a priori power calculations).
Results
Participants
The S4S sample used in the current analyses is comprised of 9,541 participants of AFR (21.3%), AMR (12.5%), EAS (9.7%), EUR (47.8%), and, or SAS (8.6%) ancestry across five cohorts. The mean age at study start was 18.59 (SD=0.58), and the sample was 61.9% female. Additional descriptive information on the current sample can be found in Dick et al. (24). Of the 7,510 who received questions about e-cigarette use, approximately 41% (n=3,090) of participants endorsed lifetime e-cigarette use, 45.3% (n=1,402) of whom had used e-cigarettes in the past 30 days. Table 1 shows the rates of e-cigarette and cigarette use by ancestry amongst those who answered the e-cigarette questions. Chi-square tests revealed significant (ps<0.0001) differences in proportions of use by ancestry across the outcomes listed in Table 1 (see Supplemental Materials S3 for full tabulation of rates of use).
Table 1.
E-cigarette and Combustible Cigarette Endorsement Rates by Ancestry Group
| Ever ECIG Use | Past 30-day ECIG Use | Lifetime Regular CIG Use | Daily CIG Use | Lifetime Regular CIG Use/ Ever ECIG Use | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | %1 | N | %2 | N | %1 | N | %3 | N | %2 | |
| AFR (n=2,034) | 419 | 20.6 | 146 | 34.8 | 79 | 3.9 | 46 | 58.2 | 61 | 14.6 |
| AMR (n=1,196 | 469 | 38.5 | 233 | 49.7 | 170 | 14.2 | 73 | 42.9 | 133 | 28.4 |
| EAS (n=928) | 250 | 26.9 | 119 | 47.6 | 50 | 5.4 | 38 | 76.0 | 41 | 16.4 |
| EUR (n=4,562) | 1,701 | 37.3 | 795 | 46.9 | 776 | 17 | 512 | 66.0 | 685 | 40.3 |
| SAS (n=821) | 249 | 30.3 | 123 | 49.4 | 73 | 8.9 | 34 | 46.6 | 50 | 20.1 |
Note. Ever ECIG = number and proportion of participants who have ever tried e-cigarettes. Past 30-day ECIG Use = number and proportion of participants who have used e-cigarettes in the past 30 days. This was modeled as a categorical variable representing frequency of e-cigarette use in the past 30 days. Regular CIG Use = number and proportion of participants who have had 100 or more cigarettes in their lifetime. Daily CIG Use = number and proportion of participants who used cigarettes daily in the past 30 days. Lifetime Regular CIG User/ Ever ECIG Use = number and proportion of participants who have both used CIGs 100 or more times and have ever used e-cigarettes out of the full sample. The percentages for the first and second columns are out of the total sample while the percentages for the third and fourth columns are out of the individuals who have ever used e-cigarettes.
Denominator is total sample.
Denominator is ever ECIG use.
Denominator is Lifetime Regular CIG Use.
Predicting lifetime e-cigarette use
The PGS-CPD was not significantly associated with lifetime e-cigarette use in any ancestral group (ORs: 0.95–1.04, p’s>0.50, see S4 for full model results). The PGS-RCU was associated with lifetime e-cigarette use in the EUR sample (OR: 1.27, 95% CI: 1.19–1.36, p=7.53×10−12, Figure 1). The full model (S5), including the PGS-RCU, S4S cohort, 10 ancestry specific PCs, and genotyping batch variables, explained 2.70% of the variance in lifetime e-cigarette use (AUC=0.58) and the PGS-RCU explained 1.83%. The PGS-RCU had a reduced although non-significant effect with lifetime e-cigarette use in the AMR (OR: 1.11, 95% CI: 0.97–1.28, p=0.12) and EAS samples (OR: 1.09, 95% CI: 0.94–1.28, p=0.25). The PGS-RCU showed no association with lifetime e-cigarette use in the AFR (OR: 1.02, 95% CI: 0.91–1.14, p=0.74) or SAS (OR: 0.99, 95% CI: 0.85–1.17, p=0.96) samples.
Figure 1 – Associations between PGS and e-cigarette or combustible cigarette use outcomes.
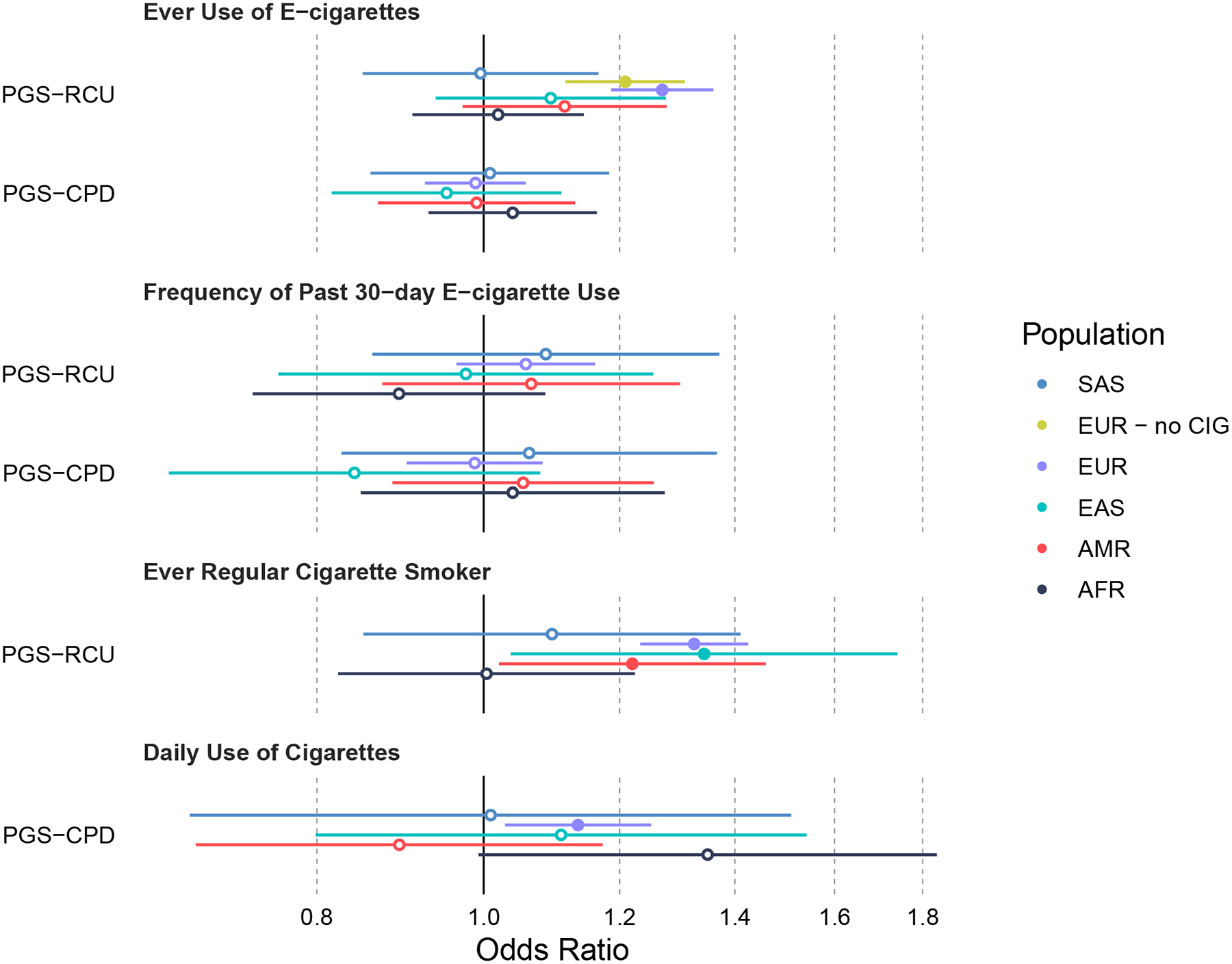
Forest plot with odds ratios and 95% confidence intervals for the associations between polygenic scores (PGS) and e-cigarette and cigarette use. PGS-RCU = polygenic score for regular combustible cigarette use. PGS-CPD = polygenic score for cigarettes per day. EUR – no CIG = EUR sample with regular combustible cigarette smokers removed.
As a sensitivity analysis, we tested whether the PGS-RCU was associated with lifetime e-cigarette use in never regular users of combustible cigarettes in the EUR sample. After excluding 905 lifetime regular combustible cigarette users, we found the PGS-RCU remained associated with lifetime e-cigarette use (OR: 1.21, 95% CI: 1.12–1.31, p=3.36×10−6).
Predicting frequency of past 30-day e-cigarette use
Neither PGS-RCU (ORs: 0.89–1.09, p’s>0.23), nor the PGS-CPD (ORs: 0.95–1.06, p’s>0.53) were associated with frequency of past 30-day e-cigarette use in any subsample (Figure 1, see table S6 and S7 for full model results).
Predicting combustible cigarette use
As an internal control, we tested whether the PGS-RCU was associated with regular combustible cigarette use and whether the PGS-CPD was associated with daily combustible cigarette use. In the EUR sample, the PGS-RCU predicted lifetime regular combustible cigarette use (OR: 1.33, 95% CI: 1.23–1.43, p=2.23×10−14; Figure 1). The full model explained 4.53% of the variance in lifetime regular combustible cigarette use (AUC=0.61) and the PGS-RCU explained 1.96%. In the EAS sample, the PGS-RCU predicted lifetime regular combustible cigarette use (OR: 1.34, 95% CI: 1.04–1.74, p=0.025). The full model explained 7.84% of the variance in lifetime regular combustible cigarette use (AUC=0.69) and the PGS-RCU explained 1.29%. In the AMR sample, the PGS-RCU predicted lifetime regular combustible cigarette use (OR: 1.22, 95% CI: 1.02–1.46, p=0.029). The full model explained 6.59% of the variance in lifetime regular combustible cigarette use (AUC=0.66) and the PGS-RCU explained 0.70%. PGS-RCU was not associated with lifetime regular combustible cigarette use (p’s>0.48) in the AFR and SAS samples. The PGS-CPD was a significant predictor of daily combustible cigarette use (OR: 1.13, 95% CI: 1.03–1.25, p=0.01) in the EUR sample. The full model explained 2.1% of the variance in daily combustible cigarette use (AUC=0.57) and the PGS-CPD explained 0.38%. The PGS-CPD was not associated with daily combustible cigarette use in any of the non-European subsamples (p’s>0.06; see tables S8 and S9 for full model results).
Discussion
This study applied summary statistics from the largest meta-analysis GWAS of combustible cigarette use (22,23) among individuals of European or East Asian ancestry into a young adult sample of European ancestry. Using this young adult sample, we demonstrated that a PGS of lifetime regular combustible cigarette use was associated with ever use of e-cigarettes. This result is consistent with previous studies (10) and remained significant after excluding regular combustible cigarette smokers from the sample. This result suggests a shared genetic liability for the earliest stages of e-cigarette and combustible cigarette use. In contrast, the PGS for cigarettes per day showed no effect on lifetime e-cigarette use or frequency of e-cigarette use in the past month, despite being well powered to detect an effect in the sample of European ancestry. Additionally, neither PGS was significantly associated with any e-cigarette use measure in the smaller non-European ancestry samples, highlighting the need for future studies with larger sample sizes and among ancestrally diverse populations (35,36).
The finding of increased ever use of e-cigarettes in individuals of European ancestry with a higher genetic loading for regular combustible cigarette use has important implications. There has been a drastic reduction in the number of adolescents and young adults who use combustible cigarettes in recent decades through marketing and intervention campaigns focused on changing youth attitudes towards combustible cigarette use (1,37–40). Our results suggest that young people with a genetic liability to combustible cigarette use are at increased risk for lifetime e-cigarette use. Early e-cigarette use may lead to subsequent combustible cigarette use (7,8) which could significantly impact the advances made in the last several decades in reducing combustible cigarette use in youth and the overall population. This makes it difficult to determine if e-cigarette use is a pathway to combustible cigarette use or if e-cigarette use may be a diversion from combustible cigarette use. More research is needed on longitudinal trajectories of e-cigarette use and related outcomes fully understand the relationship between e-cigarette use in youth and the risk of subsequent combustible cigarette use.
The lack of an association between the PGS for cigarettes per day and any e-cigarette measure in any sample indicates there may be a much weaker genetic liability between heavier combustible cigarette use and lifetime or frequency of e-cigarette use. This indicates that the knowledge we have gained from research into combustible cigarette etiology may not directly translate to a similar understanding for e-cigarette use. The results reported here suggest that there is a set of genetic factors shared between combustible cigarette use and e-cigarette use, as well as a set of genetic factors specific to each product. Additionally, the identification of these genetic factors may be dependent on the stage of product use severity.
Our findings differ from Allegrini et al. (9) who found no association between a PGS for regular combustible cigarette use and lifetime e-cigarette use, but found an association between a PGS for cigarettes per day and lifetime e-cigarette use in prior users of combustible cigarettes. Although our sample sizes of European ancestry were similar (N of Allegrini et al. = 4,050; N of current EUR sample = 4,562), there are several notable differences between the analyses. First, Allegrini et al., (9) used summary statistics from an older GWAS of smoking initiation and cigarettes per day from the Tobacco and Genetics Consortium (21). Second, the prevalence rates of lifetime e-cigarette use in Allegrini et al., were very low compared to the current study, with only 4.7% of the sample reporting lifetime e-cigarette use. While the data for Allegrini et al. was collected around the same time as S4S (2013–2014), participants in Allegrini et al. were older (M=37.3, SD=13.3). In addition, the overlap between e-cigarette users and combustible cigarette users in Allegrini et al. was very high, with 70.5% of e-cigarette users being current combustible cigarette smokers. Thus, Allegrini et al. appears to have captured a sample with more nicotine experience, while in the S4S sample, there was a greater number of e-cigarette users who had never been regular combustible cigarette smokers.
Our study is the first to examine the associations between combustible cigarette PGS and e-cigarette use in non-European ancestry samples. In contrast to the EUR sample in our study and others, we did not detect any association between combustible cigarette PGS and any measure of e-cigarette use in any non-European sample. Several factors should be considered when interpreting these results. First, cross-ancestry PGS associations often result in reduced effect sizes compared to analyses where the discovery and target sample are from the same ancestry (41). This effect is especially pronounced in samples of African ancestry (~50% reduction in effect size relative to samples of European ancestry) (41). We observed this in the current study in the reduction of odds ratios for the non-significant association between PGS-RCU and regular combustible cigarette smoking in the AFR, and SAS samples and reduced effect size in the AMR sample (Figure 1). These effect sizes are likely to be further reduced when examining cross-trait and cross-ancestry associations as in this study. Therefore, while no statistically significant association was detected between combustible cigarette PGS from a European and East Asian discovery sample and e-cigarette use in samples of non-European ancestry, this is not necessarily strong evidence that there is no relationship between genetic markers that are associated with combustible cigarette and e-cigarette use in these groups. Summary statistics from a GWAS in individuals of diverse ancestry with sufficient statistical power are critically needed.
In the AFR and EAS samples, there was a very low rate of overlap in e-cigarette use and combustible cigarette use, and a low rate of regular combustible cigarette use compared to the AMR or EUR sample, consistent with previous studies (42,43). The low phenotypic overlap between combustible cigarette use and e-cigarette use in the AFR and EAS samples seen in our study suggests that these individuals are not using e-cigarettes to reduce their combustible cigarette use, but instead may be new nicotine users whose product of choice is e-cigarettes. Therefore, these individuals may not show a strong genetic liability to combustible cigarette use and either environmental or genetic factors specific to e-cigarettes may be driving their use.
Limitations
Results of the current study should be viewed in the context of the following limitations. Our results in the samples of non-European ancestry were limited due to the lack of well powered GWAS of combustible cigarette use in samples of these ancestries. Our cross-trait, cross-ancestry analyses provide preliminary data on the potential shared genetic influences on combustible cigarette and e-cigarette use. However, well powered GWAS in non-European or non-East Asian samples are needed to fully understand the relationship between combustible cigarette and e-cigarette use in groups other than those of European or East Asian ancestry. Second, we were unable to measure concentration of nicotine in the e-cigarette products that participants were using, which meant we were unable to test whether the association of PGS-RCU and e-cigarette use varied as a function of nicotine concentration. The nicotine concentration of e-cigarette products can vary widely, with some products containing much greater amounts of nicotine than combustible cigarettes (44), and others containing no nicotine at all. It will be important for future studies to test the extent to which nicotine concentration may moderate genetic influences on e-cigarette use. Relatedly, our findings center on ever e-cigarette use which is one of many potential e-cigarette phenotypes of interest. As larger datasets become available that contain both genetic data and e-cigarette use this work can be expanded to mirror our knowledge of the genetic influences the progression of combustible cigarette use. Finally, our study focused on college-aged young adults, but e-cigarette use is now highly prevalent in middle and high school students (1) making replication of these findings in secondary school age groups, who are at greatest risk for negative outcomes and transitioning to combustible cigarette use, critically important.
Conclusion
A polygenic score for regular combustible cigarette smoking was associated with ever use of e-cigarettes in college students of European ancestry, providing initial evidence of the genetic factors underlying e-cigarette experimentation, without conducting a GWAS of e-cigarette use, which would require sample sizes of ~1 million that are currently unavailable. Therefore, well powered polygenic scores of regular combustible cigarette smoking may be used in future studies to quantify the genetic influences on e-cigarette experimentation to better understand how genes shape early e-cigarette use behavior in the absence of an e-cigarette specific GWAS or alternative approaches for heritability estimation.
Supplementary Material
Acknowledgements
MEC is supported by the Louis V. Gerstner III Research Scholar Award from the Gerstner Family Foundation. JG is supported by R01DA042043 from the National Institute on Drug Abuse. REP is supported by The Brain & Behavior Research Foundation NARSAD grant 28632 P&S Fund. RMS is supported by K23DA042946 from the National Institute on Drug Abuse. HMM was supported by DA025109 from the National Institute on Drug Abuse. AEE is supported by K24DA030443 from the National Institute on Drug Abuse.
Spit for Science has been supported by Virginia Commonwealth University, P20 AA017828, R37AA011408, K02AA018755, P50 AA022537, and K01AA024152 from the National Institute on Alcohol Abuse and Alcoholism, and UL1RR031990 from the National Center for Research Resources and National Institutes of Health Roadmap for Medical Research. This research was also supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number U54DA036105 and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA. Data from this study are available to qualified researchers via dbGaP (phs001754.v2.p1). We would like to thank the Spit for Science participants for making this study a success, as well as the many University faculty, students, and staff who contributed to the design and implementation of the project.
Declaration of Competing Interests:
AEE has received research grant support to her institution from Pfizer Inc, Forum Pharmaceuticals, and GSK, consultation fees from Charles River Analytics, and honoraria for advisory work from Pfizer, and Kaurna Pharmaceuticals in the past 5 years for work unrelated to this project. All other authors declare no conflicts of interest.
References
- 1.Miech R, Johnston L, O’Malley P, Bachman J, Schulenberg J, Patrick M. Monitoring the future national survey results on drug use, 1975–2018: volume I, secondary school students. 2019. [Google Scholar]
- 2.Schulenberg J, Johnston L, O’Malley P, Bachman J, Miech R, Patrick M. Monitoring the Future national survey results on drug use, 1975–2019: Volume II, college students and adults ages 19–60. 2020. [Google Scholar]
- 3.Spindle TR, Hiler MM, Cooke M, Eissenberg T, Kendler KS, Dick D. Electronic Cigarette Use and Uptake of Cigarette Smoking: A Longitudinal Examination of U.S. College Students. Addict Behav. 2017. Apr;67:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel EA, Prochaska JJ, Ramo DE, Andres J, Rubinstein ML. Adolescents’ E-Cigarette Use: Increases in Frequency, Dependence, and Nicotine Exposure Over 12 Months. J Adolesc Health. 2019. Jun 1;64(6):770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djordjevic MV, Stellman SD, Zang E. Doses of Nicotine and Lung Carcinogens Delivered to Cigarette Smokers. JNCI J Natl Cancer Inst. 2000. Jan 19;92(2):106–11. [DOI] [PubMed] [Google Scholar]
- 6.United States Surgeon General. The Health Consequences of Smoking -- 50 Years of progress: A Report of the Surgeon General: (510072014-001) [Internet]. American Psychological Association; 2014. [cited 2021 Jan 1]. Available from: http://doi.apa.org/get-pe-doi.cfm?doi=10.1037/e510072014-001
- 7.Soneji S, Barrington-Trimis JL, Wills TA, Leventhal AM, Unger JB, Gibson LA, et al. Association Between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017. Aug 1;171(8):788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bunnell RE, Agaku IT, Arrazola RA, Apelberg BJ, Caraballo RS, Corey CG, et al. Intentions to Smoke Cigarettes Among Never-Smoking US Middle and High School Electronic Cigarette Users: National Youth Tobacco Survey, 2011–2013. Nicotine Tob Res. 2015. Feb 1;17(2):228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allegrini AG, Verweij KJH, Abdellaoui A, Treur JL, Hottenga J-J, Willemsen G, et al. Genetic Vulnerability for Smoking and Cannabis Use: Associations With E-Cigarette and Water Pipe Use. Nicotine Tob Res. 2019. May 21;21(6):723–30. [DOI] [PubMed] [Google Scholar]
- 10.Khouja JN, Wootton RE, Taylor AE, Smith GD, Munafò MR. Association of genetic liability to smoking initiation with e-cigarette use in young adults: A cohort study. PLOS Med. 2021. Mar 18;18(3):e1003555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prom-Wormley EC, Clifford JS, Cooke ME, Cecilione J, Maes HH, Do E, et al. The Genetic and Environmental Influences Contributing to the Association between Electronic and Conventional Cigarette Initiation. Nicotine Tob Res [Internet]. 2020. Oct 6 [cited 2020 Dec 10];(ntaa201). Available from: 10.1093/ntr/ntaa201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98(1):23–31. [DOI] [PubMed] [Google Scholar]
- 13.Maes HH, Prom-Wormley E, Eaves LJ, Rhee SH, Hewitt JK, Young S, et al. A Genetic Epidemiological Mega Analysis of Smoking Initiation in Adolescents. Nicotine Tob Res. 2017. Apr 1;19(4):401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Do EK, Prom-Wormley EC, Eaves LJ, Silberg JL, Miles DR, Maes HH. Genetic and Environmental Influences on Smoking Behavior across Adolescence and Young Adulthood in the Virginia Twin Study of Adolescent Behavioral Development and the Transitions to Substance Abuse Follow-Up. Twin Res Hum Genet Off J Int Soc Twin Stud. 2015. Feb;18(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behav Genet. 1999;29:383–93. [DOI] [PubMed] [Google Scholar]
- 16.Broms U, Silventoinen K, Madden PAF, Heath AC, Kaprio J. Genetic Architecture of Smoking Behavior: A Study of Finnish Adult Twins. Twin Res Hum Genet. 2006. Feb 1;9(1):64–72. [DOI] [PubMed] [Google Scholar]
- 17.Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PAF. Estimating Two-Stage Models for Genetic Influences on Alcohol, Tobacco or Drug Use Initiation and Dependence Vulnerability in Twin and Family Data. Twin Res. 2002. Apr 1;5(2):113–24. [DOI] [PubMed] [Google Scholar]
- 18.Maes H, Sullivan P, Bulik C, Neale M, Prescott C, Eaves L, et al. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol Med. 2004. Nov 1;34:1251–61. [DOI] [PubMed] [Google Scholar]
- 19.Morley KI, Lynskey MT, Madden PAF, Treloar SA, Heath AC, Martin NG. Exploring the inter-relationship of smoking age-at-onset, cigarette consumption and smoking persistence: genes or environment? Psychol Med. 2007. Sep;37(9):1357–67. [DOI] [PubMed] [Google Scholar]
- 20.Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and Environmental Influences on Alcohol, Caffeine, Cannabis, and Nicotine Use From Early Adolescence to Middle Adulthood. Arch Gen Psychiatry. 2008. Jun 2;65(6):674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furberg H, Kim Y, Dackor J, Boerwinkle E, Franceschini N, Ardissino D, et al. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010. May;42(5):441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019. Feb;51(2):237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matoba N, Akiyama M, Ishigaki K, Kanai M, Takahashi A, Momozawa Y, et al. GWAS of smoking behaviour in 165,436 Japanese people reveals seven new loci and shared genetic architecture. Nat Hum Behav. 2019. May;3(5):471–7. [DOI] [PubMed] [Google Scholar]
- 24.Dick D, Nasim A, Edwards AC, Salvatore J, Cho SB, Adkins A, et al. Spit for Science: launching a longitudinal study of genetic and environmental influences on substance use and emotional health at a large US university. Front Genet [Internet]. 2014. [cited 2020 Oct 26];5. Available from: https://www.frontiersin.org/articles/10.3389/fgene.2014.00047/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. Apr 1;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019. Jul;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bondy SJ, Victor JC, Diemert LM. Origin and use of the 100 cigarette criterion in tobacco surveys. Tob Control. 2009. Aug;18(4):317. [DOI] [PubMed] [Google Scholar]
- 28.Webb BT, Edwards AC, Wolen AR, Salvatore JE, Aliev F, Riley BP, et al. Molecular Genetic Influences on Normative and Problematic Alcohol Use in a Population-Based Sample of College Students. Front Genet [Internet]. 2017. [cited 2020 Oct 28];8. Available from: https://www.frontiersin.org/articles/10.3389/fgene.2017.00030/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delaneau O, Zagury J-F, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods. 2013. Jan;10(1):5–6. [DOI] [PubMed] [Google Scholar]
- 30.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009. Jun;5(6):e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015. Oct 1;526(7571):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sudmant PH, Rausch T, Gardner EJ, Handsaker RE, Abyzov A, Huddleston J, et al. An integrated map of structural variation in 2,504 human genomes. Nature. 2015. Oct 1;526(7571):75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson RE, Edwards AC, Bacanu S-A, Dick DM, Kendler KS, Webb BT. The utility of empirically assigning ancestry groups in cross-population genetic studies of addiction. Am J Addict. 2017. Aug;26(5):494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruan Y, Feng Y-CA, Chen C-Y, Lam M, Initiatives SGA, Sawa A, et al. Improving Polygenic Prediction in Ancestrally Diverse Populations. medRxiv. 2021. Jan 2;2020.12.27.20248738. [Google Scholar]
- 35.Peterson RE, Kuchenbaecker K, Walters RK, Chen C-Y, Popejoy AB, Periyasamy S, et al. Genome-wide Association Studies in Ancestrally Diverse Populations: Opportunities, Methods, Pitfalls, and Recommendations. Cell. 2019. Oct 17;179(3):589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wojcik GL, Graff M, Nishimura KK, Tao R, Haessler J, Gignoux CR, et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature. 2019. Jun;570(7762):514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrelly MC, Duke JC, Nonnemaker J, MacMonegle AJ, Alexander TN, Zhao X, et al. Association Between The Real Cost Media Campaign and Smoking Initiation Among Youths — United States, 2014–2016. MMWR Morb Mortal Wkly Rep. 2017. Jan 20;66(2):47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang L-L, Lazard AJ, Pepper JK, Noar SM, Ranney LM, Goldstein AO. Impact of The Real Cost Campaign on Adolescents’ Recall, Attitudes, and Risk Perceptions about Tobacco Use: A National Study. Int J Environ Res Public Health. 2017. Jan;14(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the Future National Survey Results on Drug Use, 1975–2018. Volume II, College Students & Adults Ages 19–60 [Internet]. Institute for Social Research. Institute for Social Research; 2019. [cited 2020 Dec 10]. Available from: https://eric.ed.gov/?id=ED599071
- 40.Vallone D, Cantrell J, Bennett M, Smith A, Rath JM, Xiao H, et al. Evidence of the Impact of the truth FinishIt Campaign. Nicotine Tob Res. 2018. Apr 2;20(5):543–51. [DOI] [PubMed] [Google Scholar]
- 41.Duncan L, Shen H, Gelaye B, Meijsen J, Ressler K, Feldman M, et al. Analysis of polygenic risk score usage and performance in diverse human populations. Nat Commun. 2019. Jul 25;10(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garrett BE, Dube SR, Winder C, Caraballo RS, Prevention C for DC and. Cigarette smoking—United States, 2006–2008 and 2009–2010. MMWR Surveill Summ. 2013;62(Suppl 3):81–4. [PubMed] [Google Scholar]
- 43.Pampel FC. Racial Convergence in Cigarette Use from Adolescence to the Mid-Thirties. J Health Soc Behav. 2008. Dec;49(4):484–98. [PMC free article] [PubMed] [Google Scholar]
- 44.Rao P, Liu J, Springer ML. JUUL and Combusted Cigarettes Comparably Impair Endothelial Function. Tob Regul Sci. 2020. Jan 1;6(1):30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


