Abstract
Mass spectrometry imaging investigations of tissues infected with agents that require high-security biocontainment, such as Mycobacterium tuberculosis, have been limited due to incompatible sterilization techniques. Here we describe an on-slide heat sterilization method that enables mass spectrometry imaging investigations of pharmaceuticals, lipids, and metabolites in infected tissue samples outside of biocontainment. An evaluation of different temperatures and incubation times determined that 100 °C for 1 h was essential to sterilize 5 times the bacterial burden observed in tuberculosis (TB) cavity sections. Laser-capture microdissection combined with liquid chromatography with tandem mass spectrometry quantitation, in addition to mass spectrometry imaging, showed that no degradation was observed following the on-slide heat sterilization protocol for a variety of drug classes covering a range of physicochemical properties. Utilizing the tissue mimetic model, we demonstrated that the detection of lipid and metabolite ions was not impacted by heat sterilization and that, for several metabolites, the on-slide heat sterilization method improved the sensitivity when compared to control samples. An application of the on-slide heat sterilization to M. tuberculosis infected tissue enabled the first detection and spatial distribution of lipids indicative of a lysosomal storage disease phenotype within TB granuloma macrophages, in addition to the differential distribution of metabolites central to the fatty acid oxidation pathway. These initial investigations detected a pronounced heterogeneity within the cellular regions and necrotic cores of individual TB granulomas and across different evolving granulomas. This study provides the framework for mass spectrometry imaging investigations of high-threat pathogens outside of biocontainment.
Keywords: heat sterilization, MALDI, mass spectrometry imaging, Mycobacterium tuberculosis, TB granuloma, drug distribution, lipidomics, metabolomics
Introduction
To study some of the world’s most deadly pathogens and toxins that pose a serious threat to human health, research must be conducted in specialized biocontainment laboratories.1−3 These biocontainment laboratories are designated as biosafety level 3 or 4 (BSL-3 or -4) depending on the severity of the threat to life and the availability of treatment options.3 For example, pathogens handled in BSL-4 laboratories are a high risk, causing life-threatening disease, and there are currently no available treatment options.4,5 BSL-4 pathogens include viruses that cause hemorrhagic fevers such as Ebola and the Marburg virus.5,6 Pathogens handled in BSL-3 laboratories pose serious or potentially life-threatening disease for which treatment options are available. Examples of BSL-3 pathogens include Mycobacterium tuberculosis (tuberculosis (TB)),7Bacillus anthracis (anthrax),8 and Yersinia pestis (plague).9 The mechanisms of pathogen infection, host–pathogen interactions, host–response, and therapeutic penetration at infection sites remain poorly understood, as the level of containment required for their research greatly limits the types of studies that can be performed.
Mass spectrometry imaging (MSI) is the only technique that can provide untargeted spatial information on the distribution of endogenous biomolecules and exogenous therapeutics during tissue and cell infection studies.7,10−13 Recent technological advances that encompass MS instrument design and commercialization of novel laser systems have enabled researchers to obtain the spatial localization of lipids, metabolites, and pharmaceuticals at the single-cell and subcellular level.14−17 These new developments are fundamental to the establishment of accurate PK/PD models and to the understanding of host–pathogen interactions that would aid in the identification of new therapeutic targets.
MSI investigation of infections caused by high-containment pathogens, such as M. tuberculosis, is currently limited due to incompatible inactivation/sterilization techniques and issues associated with the housing of high-end instrumentation in biocontainment.18 Current pathogen sterilization techniques include chemical solutions,19−22 irradiation,23,24 and heat treatment.25−27 Chemical solutions include formalin,21 hypochlorite,22 hydrogen peroxide,20 and organic solvents19 that act in a time- and contact-dependent manner. Chemical solution-based methods are generally not suitable for downstream MSI studies, as they modify compounds within the cells (formalin), contaminate the tissue with small molecular weight compounds (hypochlorite), and can delocalize or cause “leeching” of lipid, metabolite, and pharmaceuticals from the tissue into the solution.21,28,29 γ-Irradiation, mainly using a cobalt-60 source, is an established sterilization method for high-threat pathogens.23,24 This technique, along with ultraviolet irradiation, can cause dose-dependent oxidation and degradative effects of both endogenous and exogenous compounds.30−32 Additionally, very few laboratories in the world have the equipment necessary for γ-irradiation. This is due to both the health/safety and environmental concerns associated with such instrumentation.33,34 Thermal sterilization was first utilized for a histological fixation of tissue cubes in the 1970s and is a commonly used technique to inactivate heat-sensitive high-threat pathogens.25−27 Currently, one study has utilized heat sterilization for a downstream MSI analysis of both viral and bacterial biocontainment pathogens.35 This protocol utilizes the commercial Denator T1 Stabilizor, which rapidly heats whole organs or biopsy specimens. The rapid heating renders the organs very fragile; the tissue crumbles upon sectioning and requires adapted workflows.36 Additional loss in tissue morphology was also observed with this method, which may have contributed to its lack of application for MSI analysis of high-threat pathogens.
Using M. tuberculosis as a proof-of-concept, we developed a simple and cost-effective on-slide heat sterilization method to overcome the current restrictions for an MSI analysis of high-containment pathogens. By sectioning infected tissue onto slides prior to heating, we mitigated the issues observed with the prior heat sterilization method and successfully maintained tissue integrity and cellular morphology. We highlight the diverse applications of our new protocol for the analysis of multiple antimicrobial drug classes covering a range of physicochemical properties, in addition to lipids and metabolites that inform on immunometabolism and a sphingolipid storage disease phenotype within the TB granuloma. An application of our method identified lipid and metabolite heterogeneity that was dependent upon the individual TB granuloma. This method can be readily adapted for use worldwide to study all heat-sensitive BSL-3 pathogens that require biocontainment.
Experimental Section
Materials and Reagents
9-Aminoacridine (9-AA) was purchased from Sigma-Aldrich. 2,5-Dihydroxybenzoic acid (DHB), hematoxylin and eosin stains, all high-performance liquid chromatography (HPLC)-grade solvents, and the Superfrost Plus slides were purchased from the Fisher Scientific Company. Rifampin was purchased from Gold Biotechnology. Kanamycin sulfate was purchased from Fisher Scientific. Rifapentine and rifabutin were purchased from Sigma-Aldrich. Bedaquiline was provided by Janssen Research and Development.
Mycobacterium tuberculosis Heat Sterilization and Validation
All steps were conducted within a biosafety cabinet (BSC) in a BSL-3 facility. M. tuberculosis HN878 was grown in the Middlebrook 7H9 liquid medium (10% ADC [bovine albumin, dextrose, beef catalase], supplemented with 0.2% glycerol and 0.05% Tween 80). Aliquots (100 μL) of a concentrated culture were placed on presterilized standard microscope glass slides and spread with loops. A total of 16 slides were prepared and air-dried. A Boekel Scientific Slide Moat Slide Hybridizer was transferred into a BSC and prewarmed to the desired temperatures. Eight slides were transferred to the slide warmer and incubated at 85 °C for 30 min, 95 °C for 20 min, or 100 °C for a period of 1 h. The remaining eight slides (controls) were left standing at room temperature. After the incubation period, the heat–treated slides were allowed to come to room temperature. 100 microliters of phosphate-buffered saline (PBS) supplemented with 0.01% Tween 80 was added to each slide (control and heat-treated), and sterile loops were used to agitate the films of dehydrated bacterial culture. The suspensions were pipetted up and down to further disperse bacterial clumps. An aliquot of 20 μL of the cell suspension from the control slides was then transferred out and serially diluted in PBS + Tween 80. Dilutions from 104 to 107 were plated onto the Middlebrook 7H11 agar (supplemented with 0.5% glycerol and 10% oleic albumin dextrose catalase (OADC)) on quadrant plates (25 μL per quad). Cell suspensions from each heat-treated slide were transferred in their entirety to a whole 7H11 agar plate. An additional 100 μL of PBS + Tween 80 was added to each of these slides, pipetted up and down, and transferred onto the same agar plates to ensure that all residual bacterial cells were accounted for. Agar plates from control and heat-treated slides were incubated at 37 °C for three and six weeks, respectively. Colony forming units (CFU) were calculated to provide the number of viable bacteria per slide. The sample sterility was qualified by the absence of colony growth on 7H11 for up to six weeks of incubation at 37 °C.
Mycobacterium tuberculosis Infection and Treatment
All rabbit infection studies were performed in BSL-3 facilities in accordance with National Institutes of Health (NIH) guidelines for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committees of the New Jersey Medical School, Rutgers University, NJ, and Hackensack Meridian Health, NJ.
Female New Zealand White rabbits (Millbrook Farm), weighing 2.2–2.6 kg, were infected with Mtb HN878 using a nose-only aerosol exposure system as previously described.37 Starting at 12–16 weeks postinfection rabbits received doxycycline in their diet as described.38 For the kanamycin study, 25 mg/kg was administered via an intramuscular injection, and for the pretomanid, 20 mg/kg was administered via an oral gavage.
Female-specific pathogen-free C3HeB/FeJ mice aged 8–10 weeks were purchased from Jackson Laboratories. An aerosol infection with Mtb Erdman strain (TMCC 107) and a subsequent bedaquiline (BDQ) treatment (25 mg/kg administered via an oral gavage) has been previously reported.39
Preparation of Tissue Mimetic Models
Liver tissue mimetics were prepared according to the previously published protocol.40 Briefly, liver tissue was homogenized and spiked with the following drug compounds—rifampicin, rifapentine, rifabutin, and pretomanid—at concentrations ranging from 0 to 40 μg/g of tissue. The mimetic samples were mixed, vortexed, and spun down, and 200 μL of each was frozen in a 3 mL syringe running serially from control to high concentration. These were then snap-frozen and placed in a −80 °C freezer until analyzed.
Tissue Sectioning and Heat Sterilization
The tissue mimetics and infected TB lesions were cryo-sectioned at 10 μm for mass spectrometry imaging and at 25 μm for laser-capture microdissection (LCM) using a Leica CM 1860 cryostat (Leica Biosystems) and thaw-mounted onto Superfrost Plus slides or poly(ethylene terephthalate) (PET) membrane slides, respectively (Fisher Scientific Company). Both the tissue mimetic model and the Mtb-infected lung tissue dosed with BDQ were sectioned into n = 3 technical replicates for all studies. Biological replicates were used for the rabbit Mtb-infected lung tissue. Slides were separated into control and heat-treated cohorts. For the heat treatment, slides were incubated in a Slide Moat slide heater (Boekel Scientific) at 100 °C for 1 h as mentioned above.
LCM-LC-MS/MS Quantitation
A region-specific drug quantitation was performed using LCM liquid chromatography with tandem mass spectrometry quantitation (LC-MS/MS) as previously described.41 Briefly, 3 × 106 μm2 regions of mimetic, caseum, and normal lung were dissected and collected in polymerase chain reaction (PCR) tubes for a liquid–liquid extraction and LC-MS/MS quantitation. LC-MS/MS analysis was performed on a Sciex QTRAP 6500+ triple-quadrupole mass spectrometer coupled to a Shimadzu Nexera X2 UHPLC system. Chromatographic separation was performed on an Agilent Zorbax SB-C8 column (2.1 × 30 mm; particle size, 3.5 μm) using a reverse-phase gradient elution of 0.1% formic acid (FA) in Milli-Q water (mobile phase A) and 0.1% FA in acetonitrile (mobile phase B) for Rifampicin, rifapentine, rifabutin and pretomanid. For Doxycycline and Kanamycin, 0.1% heptafluorobutyric acid (HFBA) was added to each mobile phase. Multiple-reaction monitoring (MRM) of precursor/fragment transitions in electrospray positive-ionization mode was used to quantify the analytes. Data processing was performed using Analyst software (ver. 1.7.1; Applied Biosystems Sciex). Technical triplicates (n = 3) were quantified for each drug.
Mass Spectrometry Imaging
The matrix deposition was optimized based on the analytes of interest. For bedaquiline samples, 25 mg/mL 2,5-dihydroxybenzoic acid (DHB) matrix (50% methanol, 0.1% trifluoroacetic acid added) was applied to the tissue using HTX M5 sprayer (HTX Technologies LLC) with the following settings: 60 μL/min flow rate; 60 °C nozzle temperature; 10 p.s.i. nitrogen (69 KPa); 3 mm track spacing; 1200 mm/min velocity; crisscross pattern; 30 passes. For rifamycins samples, 10 mg/mL 9-aminoacridine (9-AA) matrix (tetrohydrofuran/2-propanol/H2O = 4.5:4.5:1) was applied to the tissue using an HTX M5 sprayer (HTX Technologies LLC) with the following settings: 160 μL/min flow rate; 60 °C nozzle temperature; 10 p.s.i. nitrogen (69 KPa); 2.75 mm track spacing; 1200 mm/min velocity; crisscross pattern; 14 passes.
Matrix-assisted laser desorption/ionization (MALDI) imaging data were acquired using a Bruker SolariX 7T FT-ICR mass spectrometer (Bruker Daltonics) equipped with a smartbeam II Nd:YAG laser (355 nm). The instrument was operated in the negative ion mode for the analysis of the mimetic samples and in the positive ion mode for TB lesion samples. Data were acquired over a mass range of m/z 150–3000 using the small laser setting, at a frequency of 2 kHz, and a 50 μm raster size. An estimated resolving power of 99 000 at m/z 400 was achieved with a transient time of 0.7340 s. All instrument parameters were optimized based on the drug compounds under investigation, the endogenous molecules of interest, and the matrix used for analysis. Following the MSI data acquisition the tissue sections were washed to remove the matrix and stained with hematoxylin and eosin (H&E) using the manufacturers protocol (Thermo Fisher). The stained slides were then scanned using a Pannoramic DESK II DW scanner (3DHISTECH).
MALDI MSI Data Analysis
The SCiLS Lab MVS, ver. 2020a Pro (Bruker Daltonics), was utilized for all MALDI MSI data analysis. Data from Mtb-infected tissue studies were directly overlaid with their corresponding H&E images for a correlation with the histopathology. Data presented were not normalized, and the weak denoising function was used during the image generation. For the analyses of lipids and metabolites from TB-infected lung lesions, a probabilistic latent semantic analysis with a deterministic initialization was performed evaluating 5, 7, and 10 components. For a tentative assignment of lipids and metabolites, spectral lists were imported into the Lipid Maps (https://www.lipidmaps.org) and HMDB (https://hmdb.ca) databases using the batch search functions.
Results and Discussion
Validation of On-Slide Heat Sterilization
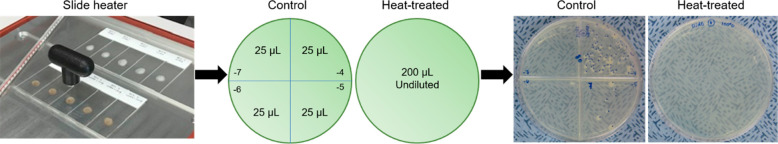
Tuberculosis cavitary caseum carries the highest bacterial burden at ∼109 bacteria/g.42 It was thus estimated that a 10 μm thick section of a large lesion, weighing 0.7–1 mg, would harbor a maximum of ∼106 bacteria. Therefore, the heat sterilization method was validated for 5 × 106 Mtb bacterium/slide. Heat sterilization temperatures and times were initially performed according to those previously published for Mtb.43,44 Slides were incubated at 85 and 95 °C for 30 and 20 min, respectively, and at 100 °C for 1 h. Following heat treatment at 85 and 95 °C, Mtb growth was observed after three weeks of incubation, while heating at 100 °C for 1 h resulted in a complete sterilization . A schematic of the heat sterilization workflow with representative colony growth data from the control slides and the slides incubated at 100 °C for 1 h is shown in Figure 1. The control plates contained Mtb colonies in two quadrants (104 to 105) after three weeks of incubation at 37 °C, whereas no Mtb growth was observed on the eight plates from the heat-treated cell suspension following six weeks of incubation at 37 °C (Figure 1, Figure S1, and Table S1). The on-slide heat sterilization method was approved by the Institutional Biosafety Committee of Hackensack Meridian Health prior to all subsequent investigations.
Figure 1.
Schematic of the on-slide heat sterilization and validation for M. tuberculosis. Twenty microliters of the Mtb cell suspension from the control glass slides were serially diluted (104–107), and 25 μL of each dilution were plated in quadrants onto Middlebrook 7H11 agar; the entire cell suspension from the heat-treated slides were plated out (middle panel). Mtb growth was observed in two quadrants of the control plates following three weeks of incubation, whereas no growth was observed on the plates containing the heat-treated cell suspension (right panel).
LCM-LC-MS/MS Drug Quantitation from Heat-Sterilized Tissue Sections
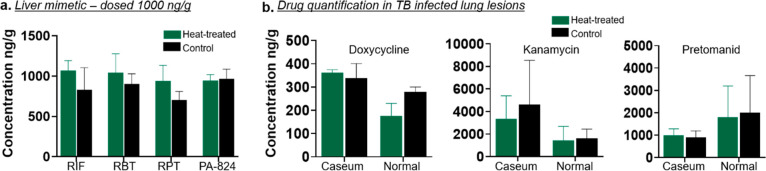
To evaluate the effect of the on-slide heat sterilization method for the detection of pharmaceutical compounds and to ensure no degradation occurred, initial investigations used the tissue mimetic model spiked with known concentrations of several drug compounds.40 This model can accurately evaluate the effects of heat treatment as it provides a homogeneous tissue with known drug concentrations and thus avoids issues that may arise due to section-to-section variability and differences in pharmacokinetic profiles. As this method is applied to tissue sections and not whole tissue, we utilized LCM-LC-MS/MS for drug quantitation from regions dissected from tissue sections, as previously published.45 Rifampicin, and its structural analogues rifapentine and rifabutin, along with pretomanid were selected for the mimetic studies. Rifampicin is the pillar of front-line TB treatment; rifabutin is in clinical use in TB-HIV (HIV = human immunodeficiency virus) patients owing to its reduced drug–drug interactions compared to rifampicin, and rifapentine is mostly used for the treatment of latent TB. Pretomanid is the most recently Food and Drug Administration (FDA)-approved TB drug for the treatment of multidrug and extensively drug-resistant TB infections in combination with bedaquiline and linezolid.46 These are essential TB drugs, and therefore being able to determine their penetration and cell-specific accumulation in TB lung lesions is of vital importance. The results from the comparison of control versus heat-treated mimetic sections spiked with rifampicin, rifapentine, rifabutin, and pretomanid at 1000 ng/g of tissue are shown in Figure 2a, demonstrating that heating the tissue sections at 100 °C for 1 h did not cause degradation of these drugs, as no significant differences in drug concentrations were observed. The results also demonstrate the accuracy of the LCM-LC-MS/MS method in the quantification of drugs extracted from dissected tissue regions.
Figure 2.
(a) LCM-LC-MS/MS quantification of antimicrobial drug compounds in heat-treated vs control liver mimetic tissue sections dosed with 1000 ng/g of tissue. (b) LCM-LC-MS/MS quantification of antimicrobial drug compounds in heat-treated vs control TB lung lesions. RIF, rifampicin; RBT, rifabutin; RPT, rifapentine; PA-824, pretomanid.
To further demonstrate the application of our method for drug quantitation in actual TB-infected tissues, we next performed LCM-LC-MS/MS quantitation of doxycycline (used to achieve a silencing of tet-promoter genetic constructs in Mtb in vivo),38 kanamycin (a key second-line TB drug), and pretomanid in heat-treated and control Mtb-infected lung lesions. The concentration of these antibiotics did not differ in the caseum and normal lung of TB lesions following a heat treatment when compared to the control sections (Figure 2b). The larger error bars shown in Figure 2b compared with those of Figure 2a are attributed to the use of biological replicates and differences in drug penetration between the different TB granulomas taken from different animals. These results further demonstrate that our on-slide heat sterilization method did not cause degradation of a drug analyzed in the mimetic (pretomanid) and two additional drugs (doxycycline and kanamycin). Together, these studies provide evidence for the successful application of the heat-sterilization method in the detection of multiple drug classes spanning different physicochemical properties. The physicochemical properties of each drug class is presented in Table S2 and were adapted from a previously published study.47
MSI Analysis of Drug Compounds from Heat-Sterilized Tissue Sections
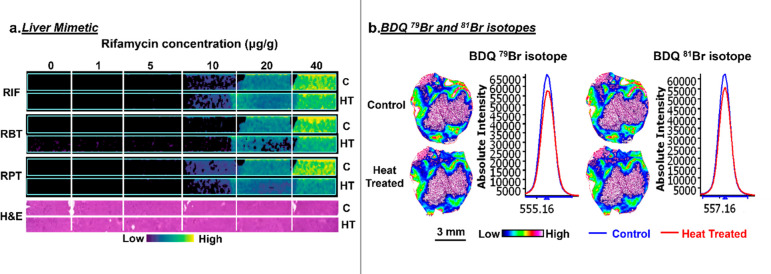
Having established the validity of our method for drug-like small molecules using LC-MS/MS quantitation, we next investigated the impact of heat sterilization on the extraction, detection, and spatial distribution of pharmaceuticals by mass spectrometry imaging. Initial studies focused on the liver mimetic model dosed with known concentrations of rifamycins. The results from the MSI analysis of the mimetic model demonstrate minimal differences in the signal intensity of rifampicin and rifapentine in heat-treated compared to control sections (Figure 3a). Rifabutin was detected with slightly lower signal intensity in the heat-treated section compared to the control. As the LC-MS-MS studies demonstrated no loss in quantitation following the heat treatment for this drug, the differences observed here are believed to be attributed to extraction following heat treatment, which could be mitigated with further optimization of the sample preparation and matrix deposition. In all instances the limit of detection (LOD) was not affected by the heat-sterilization method, which remained at 10 μg/g for rifampicin and rifapentine and at 20 μg/g for rifabutin.
Figure 3.
(a) MALDI MS images of the antimicrobial rifamycin compounds in control and heat-treated liver mimetic sections. (b) The MSI images and average spectra of the BDQ 79Br and 81Br isotopes. BDQ, bedaquiline; C, control; HT, heat-treated.
The heat-sterilization method was next applied to the imaging of bedaquiline, a drug used to treat multidrug-resistant TB (MDR-TB) infection, in a TB-infected mouse lesion. The control and heat-treated samples for both bedaquiline isotopes (79Br and 81Br) demonstrated a similar ion intensity and distribution (Figure 3b). A high-resolution H&E stain of the heat-treated tissue section is shown in Figure S2 demonstrating the histologically distinct tissue subcompartments: caseum (necrotic core), cellular rim, and normal lung. In both control and heat-treated tissue sections, the BDQ accumulation was mapped to the histologically normal lung and cellular regions surrounding the caseum of the TB granuloma. BDQ was not detected in the caseum region of the control or heat-treated sections, as it was below the limit of detection for MALDI MSI. These results are in agreement with the previously reported MALDI MSI analysis of this sample and correlate with the known reduced penetration of BDQ into caseous regions of the TB granuloma core.39,48 The slight differences observed in the regional signal intensity are believed to arise from differences in the pathology between the two sections, and this is shown in more detail in Figures S3 and S4.
MSI Analysis of Lipids and Metabolites from Heat Sterilized Tissue Mimetic Sections
Lipids and metabolites play essential roles in immune cell signaling, cell recruitment to sites of infection, and immune cell effector functions.49,50 Pathogens also utilize lipids to gain entry into cells during infection and modulate the lipid and metabolite cellular machinery to enable survival.51,52 Understanding the lipidomic and metabolic alterations during host–pathogen interactions and the host response to infection is essential to aid in the development of more efficacious therapeutics.
To ensure the analysis of comparative tissue histology, we utilized the tissue mimetic model from the rifamycin study to evaluate the impact of heat sterilization on the detection of lipids and metabolites. For this study, the data analysis focused on the mass range of m/z 150–1000. Tentatively assigned lipid and metabolite ions detected during the negative ion mode analyses of the liver mimetic sections following heat sterilization are shown in Tables S3 and S4. A lipidomic comparison of heat-treated and control samples demonstrated minimal alterations in the signal intensity of individual lipid ions following a heat treatment (Figure S5). This is further exemplified by the intensity box plots presented in Figure S6. These were obtained from regions of interest (ROIs) consisting of ∼2500 spectra within the mimetic tissue of control and heat-treated sections. Representative lipid classes from each subgroup detected demonstrate similar trends in ion intensity across all species presented.
Unlike the detection of lipid ions, several metabolites were detected with an increased signal intensity following heat treatment when compared to the control mimetic sections. Representative MS images for several metabolite classes are presented in Figure S7, and the spread in intensity for several classes is again demonstrated using box plots (Figure S8). The increased detection sensitivity in the heat-treated samples is believed to be due to the reduced degradation of these molecules afforded by a reduction in enzyme activity. This observation has been previously reported for metabolite and protein ions detected by MSI in heat-stabilized/treated versus control nonheated samples.53,54 Additionally, several metabolites (e.g., adenosine mono-, di- and triphosphate (AMP, ADP, ATP) and uridine mono- and diphosphate (UMP, UDP)) in the heat-treated samples displayed increased signal intensity in the first 1–2 mimetic layers compared to the remaining layers. This is believed to be attributed to the order of freezing. Fast-freezing has been shown to impact the detection sensitivity of these metabolites.55 The first 1–2 layers were frozen more rapidly than the remaining mimetic layers and thus experienced less enzymatic degradation resulting in better detection sensitivity. This difference was more apparent in the heat-treated sections due the enhanced detection sensitivity in these samples. A few metabolites were detected with a lower signal intensity in the heat-treated samples compared to the control sections. This was more prominently observed during the detection and analysis of glutathione (GSH) and oxidized glutathione (GSSH), indicating a possible degradation of these metabolites following a heat treatment.
MSI Analysis of Lipids and Metabolites from Mycobacterium tuberculosis Infected Lung Lesions
The granuloma is the pathological hallmark of tuberculosis and is histologically well-characterized.56 The lesion contains a central acellular necrotic core that is more commonly called the caseum, which is predominantly formed from necrotic macrophages, and in some instances neutrophils. This caseous necrotic core is surrounded by heterogeneous populations of macrophages and dendritic cells, in which foamy macrophages are the predominant cell type. These are encased in a lymphocyte-rich cellular region. The heterogeneity of granulomas span varying morphological architecture and phenotypes that far exceed this simplified histological characterization.57 The success of Mtb is largely dictated by the host response to infection at the individual granuloma level, and these lesions can either contain the bacilli and become sterile or progress to the active disease. For this reason, host-directed therapy (HDT) approaches are becoming a central research focus aimed at eradicating TB.58 One of the critical factors hindering the development of HDTs is a poor understanding of the host–pathogen interactions within the granuloma and how this leads to disease progression or containment. Following the validation of heat sterilization for the analysis of lipids and metabolites by MSI, we next applied the method to study M. tuberculosis infected lung tissue sections outside of biocontainment.
Probabilistic latent semantic analysis (PLSA) was utilized to automatically extract the underlying trends in the data.59 This computational algorithm reduces the thousands of mass spectra collected across the tissue section and presents them as score images based on their spatial distribution within the tissue. The score images presented in Figures S9–S11 show the main spatial features of TB lesion sections, based on the mass spectra detected in each tissue microenvironment, which is presented alongside the score images. The score images correspond well with the histological presentation of these sections, as demonstrated by comparing the PLSA images to the histological images in Figure S12. These images also demonstrate that heat sterilization maintained the histological tissue morphology and cellular integrity. As Mtb infection begins with alveolar macrophages and the formation of cellular and necrotic lesions is the result of this initial infection, we focused on the lipids and metabolites detected within the caseous regions and the foamy macrophages surrounding the caseum.60−63 Interestingly, while samples 1 and 2 demonstrate a clear spectral separation for both the caseous and foamy macrophage regions (score images 1 and 2), for sample 3 only the caseum was clearly separated (score image 1). This is further demonstrated in the single-ion images for these profiles in the forthcoming sections.
The most abundant ions detected within the caseous and cellular regions of each TB granuloma were identified as sphingomyelin species (SM). The spatial distribution of individual SMs demonstrated distinct accumulation within the necrotic and foamy macrophage cellular regions of each TB granuloma analyzed (Figure 4). This heterogeneous accumulation was dependent upon the carbon chain lengths and levels of unsaturation in the acyl chains of the SMs detected. This is evident by comparing the MSI data to its corresponding H&E-stained section in Figure 4. For example, the individual ion intensity maps demonstrate that SM 34:0 and 34:1 accumulated with a higher abundance in the cellular regions with a lower signal intensity detected within the caseous regions, while SMs with longer carbon chains and higher levels of unsaturation were detected in the cellular and caseous regions, in addition to a lower signal intensity in the uninvolved lung parenchyma. The intensity and distribution of the SM species detected were also different for the different lesion sizes analyzed within the same sections and in different biological sections, as can be observed by comparing the MSI data with the H&E-stained sections presented for samples 1–3. SMs were recently shown to be critical for phagocytic uptake and thus the host cell entry that initiates intracellular infection in alveolar macrophages.51 The accumulation of SM and cholesterol in foamy macrophages, coupled with the ability of Mtb to prevent lysosomal maturation, is a phenotype that mimics the profile of the lysosomal storage disease, Niemann-Pick.64,65 A recent in vitro study supported this notion by demonstrating that an infection of macrophages with Mtb or a supplementation of the media with mycolic acids produced a Niemann-Pick C phenotype.66 This may be a potential new avenue for the development of novel host-directed therapeutics based on lysosomal storage disease phenotypes.
Figure 4.
MS images of SMs of three heat-inactivated TB-infected rabbit lung samples (1–3). All species are in [M + H]+ ion form.
The next most-abundant ions detected during the positive ion mode analysis of the necrotic and cellular regions of TB granulomas were cholesterol, triglycerides (TAGs), and acylcarnitines (CARs). A heterogeneous distribution was also observed for these lipids, dependent upon the acyl chain length and the size and nature of the TB granuloma, as shown in Figure 5. Dysregulated lipid metabolism is a hallmark of human TB granuloma development, and the accumulation of TAGs and cholesterol within these cells has been well-documented.67 The TAG and cholesterol-forming intracellular lipid inclusion bodies that reside in the cytoplasm of infected cells are known to be a source of nutrients for Mtb.52,67−71 The functional relevance of the different acyl chains that correlate to the differential distribution of these lipids remains to be defined. Acylcarnitines are crucial metabolic intermediates in the fatty acid β-oxidation (FAO) pathway for the transport of long-chain fatty acids across mitochondrial membranes for energy production.72 Dysregulated lipid and metabolic profiles of macrophages following infection has been associated with cell-specific permissiveness to infection based on macrophage phenotype.73,74 Alveolar macrophages were shown to be more permissive to infection and utilized FAO, while interstitial macrophages were more restrictive and utilized glycolysis. Several groups have also reported a switch to aerobic glycolysis during an Mtb infection in cells of the innate immune system.75−77 These previous reports documenting immune cell metabolism and phenotype during an Mtb infection centered on single-cell in vitro studies or single-cell isolation following in vivo studies.78,79 This is the first study mapping the spatial distribution of cholesterol, TAGs, and FAO metabolites within TB granulomas, and our results demonstrate that lesion-specific heterogeneity exists within the cellular rim and caseum and that this distribution is dependent on the individual granuloma.
Figure 5.
MS images of cholesterol, CARs, and TAGs of three heat-inactivated TB-infected rabbit lung samples (1–3). TAGs are in [M + Na]+ ion form; all other species are in [M + H]+ ion form.
Molecular Heterogeneity within the Cellular Rim and Caseum Is Granuloma-Specific
The three biological replicates utilized in this study demonstrated a profound diversity in the accumulation and spatial distribution of SMs, cholesterol, TAGs, and CARs. This heterogeneity was observed in an individual lesion, across the different lesions (samples 1–3), and within neighboring lesions in the same sections (samples 2 and 3). This heterogeneity is further evidence in the example higher-magnification H&E regions and corresponding MSI data presented in Figure 6. TB granuloma sample 1 contained a large lesion that demonstrated a difference in the signal intensity in the foamy macrophages surrounding the necrotic caseating center. TB granuloma sample 2 contained a large lesion and several smaller lesions of varying sizes. These lesions show heterogeneity in the signal intensity of the lipids detected within the foamy macrophage cellular rims of individual lesions and across the different neighboring lesions. In contrast to the distribution of lipids detected in samples 1 and 2, TB granuloma sample 3 displayed a much higher accumulation within the necrotic core of the larger lesion in this sample (Figures 4–6) when compared to the smaller lesion and the lipid profiles of the sections from samples 1 and 2. This lesion did not differ greatly in size when compared to the smaller lesions in sample 2 that displayed a heterogeneous distribution in the cellular rim with a low signal intensity detected within the necrotic caseating cores. Understanding the host–pathogen interactions and host–pathogen metabolism that drives these differences in relation to their functional impact is of vital importance in understanding the drivers of granuloma formation and resolution at the individual granuloma level.80 This will aid in the development of more efficacious therapeutics.
Figure 6.
Higher-magnification H&E images of caseous and foamy macrophage TB granuloma regions. MS images merged with histology display cholesterol, CARs, SMs, and TAGs. The scale bars for the higher-magnification regions of the TB-infected rabbit lung samples 1–3 are 100, 300, and 300 μm, respectively.
Conclusion
We present an on-slide heat-sterilization method that enables mass spectrometry imaging of tissue sections infected with the high-risk BSL-3 pathogen, M. tuberculosis, outside of biocontainment. The diverse applications afforded by our method enable the investigation of pharmaceuticals at sites of infection to correlate with efficacy and sterilizing capabilities as well as lipid and metabolite distribution to elucidate the host–pathogen microenvironment, characterize the host-response to infection, and determine the mechanisms of infection that will aid in the identification of new therapeutic pathways. Future studies will use this method to fully elucidate the lipidomic and metabolic heterogeneity within the developing and developed TB granulomas. This method could also be adapted and applied to all heat-sensitive BSL-3 pathogens and toxins that pose serious threats to human life.
Acknowledgments
This work has been supported by the Bill and Melinda Gates Foundation OPP1174780 (V.D.) and Federal Funds for a shared instrument grant from the National Institutes of Health, Department of Health and Human Services (S10OD023524). The authors thank A. Lenaerts and the Colorado State University TB group for the BDQ mouse samples.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jasms.1c00205.
Images of agar plates and H&E stained samples, lipid species and metabolites in liver tissue mimetic sections, component analysis of samples, CFU data (PDF)
Author Contributions
C.L.C. conceived the method. J.P.S. designed and executed the validation studies for Mtb heat sterilization. V.D. designed the Mtb infection and pharmaceutical experiments. M.D.Z., F.Y., and H.W. quantified all study drugs in mimetic and lung tissue. N.W. and C.L.C. designed all MSI studies. N.W. carried out all tissue MSI data acquisition, processing, and presentation of figures. All authors wrote the method section. N.W. and C.L.C. wrote the manuscript, V.D. edited and improved the manuscript, and all authors contributed to the final version.
The authors declare no competing financial interest.
Supplementary Material
References
- Byrne G.; Cole K. S. Emerging infections, biosecurity and public health. Pathog. Dis. 2014, 71 (2), 93. 10.1111/2049-632X.12198. [DOI] [PubMed] [Google Scholar]
- Patterson A.; Fennington K.; Bayha R.; Wax D.; Hirschberg R.; Boyd N.; Kurilla M. Biocontainment laboratory risk assessment: perspectives and considerations. Pathog. Dis. 2014, 71 (2), 102–108. 10.1111/2049-632X.12162. [DOI] [PubMed] [Google Scholar]
- Chosewood L. C.; Wilson D. E.. Biosafety in microbiological and biomedical laboratories ,6th ed.; Centers for Disease Control and Prevention, 2009. [Google Scholar]
- Logue J.; Crozier I.; Jahrling P. B.; Kuhn J. H. Post-exposure prophylactic vaccine candidates for the treatment of human Risk Group 4 pathogen infections. Expert Rev. Vaccines 2020, 19 (1), 85–103. 10.1080/14760584.2020.1713756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifflett K.; Marzi A. Marburg virus pathogenesis - differences and similarities in humans and animal models. Virol. J. 2019, 16 (1), 1–12. 10.1186/s12985-019-1272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H.; Sprecher A.; Geisbert T. W. Ebola. N. Engl. J. Med. 2020, 382 (19), 1832–1842. 10.1056/NEJMra1901594. [DOI] [PubMed] [Google Scholar]
- Prideaux B.; Dartois V.; Staab D.; Weiner D. M.; Goh A.; Via L. E.; Barry C. E. 3rd; Stoeckli M. High-sensitivity MALDI-MRM-MS imaging of moxifloxacin distribution in tuberculosis-infected rabbit lungs and granulomatous lesions. Anal. Chem. 2011, 83 (6), 2112–8. 10.1021/ac1029049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney E. A. S.; Beatty M. E.; Taylor T. H.; Weyant R.; Sobel J.; Arduino M. J.; Ashford D. A. Inactivation of Bacillus anthracis spores. Emerging Infect. Dis. 2003, 9 (6), 623–627. 10.3201/eid0906.020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. R.; Shah S. R.; Alfaro T. M. Development of a rapid viability polymerase chain reaction method for detection of Yersinia pestis. J. Microbiol. Methods 2019, 162, 21–27. 10.1016/j.mimet.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prideaux B.; Via L. E.; Zimmerman M. D.; Eum S.; Sarathy J.; O’Brien P.; Chen C.; Kaya F.; Weiner D. M.; Chen P. Y.; Song T.; Lee M.; Shim T. S.; Cho J. S.; Kim W.; Cho S. N.; Olivier K. N.; Barry C. E. 3rd; Dartois V. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat. Med. 2015, 21 (10), 1223–7. 10.1038/nm.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme H. E.; Meikle L. M.; Wessel H.; Strittmatter N.; Swales J.; Thomson C.; Nilsson A.; Nibbs R. J. B.; Milling S.; Andren P. E.; Mackay C. L.; Dexter A.; Bunch J.; Goodwin R. J. A.; Burchmore R.; Wall D. M. Mass spectrometry imaging identifies palmitoylcarnitine as an immunological mediator during Salmonella Typhimurium infection. Sci. Rep. 2017, 7 (1), 2786–2786. 10.1038/s41598-017-03100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Macedo C. S.; Anderson D. M.; Pascarelli B. M.; Spraggins J. M.; Sarno E. N.; Schey K. L.; Pessolani M. C. MALDI imaging reveals lipid changes in the skin of leprosy patients before and after multidrug therapy (MDT). J. Mass Spectrom. 2015, 50 (12), 1374–1385. 10.1002/jms.3708. [DOI] [PubMed] [Google Scholar]
- Lee A.; Prideaux B.; Zimmerman M.; Carter C.; Barat S.; Angulo D.; Dartois V.; Perlin D. S.; Zhao Y. Penetration of Ibrexafungerp (formerly SCY-078) at the site of infection in an Intra-abdominal candidiasis mouse model. Antimicrob. Agents Chemother. 2020, 64, 1–11. 10.1128/AAC.02268-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarelli M. K.; Pirkl A.; Moellers R.; Grinfeld D.; Kollmer F.; Havelund R.; Newman C. F.; Marshall P. S.; Arlinghaus H.; Alexander M. R.; West A.; Horning S.; Niehuis E.; Makarov A.; Dollery C. T.; Gilmore I. S. The 3D OrbiSIMS-label-free metabolic imaging with subcellular lateral resolution and high mass-resolving power. Nat. Methods 2017, 14 (12), 1175–1183. 10.1038/nmeth.4504. [DOI] [PubMed] [Google Scholar]
- Bhandari D. R.; Coliva G.; Fedorova M.; Spengler B. Single Cell Analysis by High-Resolution Atmospheric-Pressure MALDI MS Imaging. Methods Mol. Biol. 2020, 2064, 103–111. 10.1007/978-1-4939-9831-9_8. [DOI] [PubMed] [Google Scholar]
- Barré F. P.; Paine M. R.; Flinders B.; Trevitt A. J.; Kelly P. D.; Ait-Belkacem R.; Garcia J. o. P.; Creemers L. B.; Stauber J.; Vreeken R. J.; et al. Enhanced sensitivity using MALDI imaging coupled with laser postionization (MALDI-2) for pharmaceutical research. Anal. Chem. 2019, 91 (16), 10840–10848. 10.1021/acs.analchem.9b02495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien T.; Bessler S.; Dreisewerd K.; Soltwisch J. J. A. C. Transmission-Mode MALDI Mass Spectrometry Imaging of Single Cells: Optimizing Sample Preparation Protocols. Anal. Chem. 2021, 93 (10), 4513–4520. 10.1021/acs.analchem.0c04905. [DOI] [PubMed] [Google Scholar]
- Jahrling P. B.; Keith L.; St Claire M.; Johnson R. F.; Bollinger L.; Lackemeyer M. G.; Hensley L. E.; Kindrachuk J.; Kuhn J. H. The NIAID Integrated Research Facility at Frederick, Maryland: a unique international resource to facilitate medical countermeasure development for BSL-4 pathogens. Pathog. Dis. 2014, 71 (2), 213–218. 10.1111/2049-632X.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrda G.; Boniewska-Bernacka E.; Man D.; Barchiewicz K.; Słota R. The effect of organic solvents on selected microorganisms and model liposome membrane. Mol. Biol. Rep. 2019, 46 (3), 3225–3232. 10.1007/s11033-019-04782-y. [DOI] [PubMed] [Google Scholar]
- Kaspari O.; Lemmer K.; Becker S.; Lochau P.; Howaldt S.; Nattermann H.; Grunow R. Decontamination of a BSL 3 laboratory by hydrogen peroxide fumigation using three different surrogates for B acillus anthracis spores. J. Appl. Microbiol. 2014, 117 (4), 1095–1103. 10.1111/jam.12601. [DOI] [PubMed] [Google Scholar]
- Hackett M. J.; McQuillan J. A.; El-Assaad F.; Aitken J. B.; Levina A.; Cohen D. D.; Siegele R.; Carter E. A.; Grau G. E.; Hunt N. H.; et al. Chemical alterations to murine brain tissue induced by formalin fixation: implications for biospectroscopic imaging and mapping studies of disease pathogenesis. Analyst 2011, 136 (14), 2941–2952. 10.1039/c0an00269k. [DOI] [PubMed] [Google Scholar]
- Lineback C. B.; Nkemngong C. A.; Wu S. T.; Li X.; Teska P. J.; Oliver H. F. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrobial Resistance Infection Control 2018, 7 (1), 1–7. 10.1186/s13756-018-0447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B. D. The Sterways process: a new approach to inactivating viruses using gamma radiation. Biologicals 1998, 26 (2), 125–130. 10.1006/biol.1998.0132. [DOI] [PubMed] [Google Scholar]
- Feldmann F.; Shupert W. L.; Haddock E.; Twardoski B.; Feldmann H. Gamma irradiation as an effective method for inactivation of emerging viral pathogens. Am. J. Trop. Med. Hyg. 2019, 100 (5), 1275–1277. 10.4269/ajtmh.18-0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayers C. Histological fixation by microwave heating. J. Clin. Pathol. 1970, 23 (3), 273–275. 10.1136/jcp.23.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Asselt E. D.; Zwietering M. H. A systematic approach to determine global thermal inactivation parameters for various food pathogens. Int. J. Food Microbiol. 2006, 107 (1), 73–82. 10.1016/j.ijfoodmicro.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Ahnoff M.; Cazares L. H.; Sköld K. Thermal inactivation of enzymes and pathogens in biosamples for MS analysis. Bioanalysis 2015, 7 (15), 1885–1899. 10.4155/bio.15.122. [DOI] [PubMed] [Google Scholar]
- Cingolani M.; Cippitelli M.; Froldi R.; Gambaro V.; Tassoni G. Detection and quantitation analysis of cocaine and metabolites in fixed liver tissue and formalin solutions. J. Anal. Toxicol. 2004, 28 (1), 16–19. 10.1093/jat/28.1.16. [DOI] [PubMed] [Google Scholar]
- Carter C. L.; Jones J. W.; Farese A. M.; MacVittie T. J.; Kane M. A. Inflation-fixation method for lipidomic mapping of lung biopsies by matrix assisted laser desorption/ionization–mass spectrometry imaging. Anal. Chem. 2016, 88 (9), 4788–4794. 10.1021/acs.analchem.6b00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannhorn A.; Ling S.; Powell S.; McCall E.; Maglennon G.; Jones G. N.; Pierce A. J.; Strittmatter N.; Hamm G.; Barry S. T.; et al. Evaluation of UV-C Decontamination of Clinical Tissue Sections for Spatially Resolved Analysis by Mass Spectrometry Imaging (MSI). Anal. Chem. 2021, 93 (5), 2767–2775. 10.1021/acs.analchem.0c03430. [DOI] [PubMed] [Google Scholar]
- Sun W. Q.; Leung P. Calorimetric study of extracellular tissue matrix degradation and instability after gamma irradiation. Acta Biomater. 2008, 4 (4), 817–826. 10.1016/j.actbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Harrell C. R.; Djonov V.; Fellabaum C.; Volarevic V. Risks of using sterilization by gamma radiation: the other side of the coin. Int. J. Med. Sci. 2018, 15 (3), 274–279. 10.7150/ijms.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage E.; Shikazono N. Radiation-induced clustered DNA lesions: Repair and mutagenesis. Free Radical Biol. Med. 2017, 107, 125–135. 10.1016/j.freeradbiomed.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Pavlopoulou A.; Savva G. D.; Louka M.; Bagos P. G.; Vorgias C. E.; Michalopoulos I.; Georgakilas A. G. Unraveling the mechanisms of extreme radioresistance in prokaryotes: lessons from nature. Mutat. Res., Rev. Mutat. Res. 2016, 767, 92–107. 10.1016/j.mrrev.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Cazares L. H.; Van Tongeren S. A.; Costantino J.; Kenny T.; Garza N. L.; Donnelly G.; Lane D.; Panchal R. G.; Bavari S. Heat fixation inactivates viral and bacterial pathogens and is compatible with downstream MALDI mass spectrometry tissue imaging. BMC Microbiol. 2015, 15, 1–11. 10.1186/s12866-015-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R. J. A.; Nilsson A.; Borg D.; Langridge-Smith P. R. R.; Harrison D. J.; Mackay C. L.; Iverson S. L.; Andrén P. E. Conductive carbon tape used for support and mounting of both whole animal and fragile heat-treated tissue sections for MALDI MS imaging and quantitation. J. Proteomics 2012, 75 (16), 4912–4920. 10.1016/j.jprot.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Subbian S.; Tsenova L.; Yang G.; O’Brien P.; Parsons S.; Peixoto B.; Taylor L.; Fallows D.; Kaplan G. Chronic pulmonary cavitary tuberculosis in rabbits: a failed host immune response. Open Biol. 2011, 1 (4), 1–14. 10.1098/rsob.110016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengenbacher M.; Zimmerman M. D.; Sarathy J. P.; Kaya F.; Wang H.; Mina M.; Carter C.; Hossen M. A.; Su H.; Trujillo C.; et al. Tissue distribution of doxycycline in animal models of tuberculosis. Antimicrob. Agents Chemother. 2020, 64 (5), 1–11. 10.1128/AAC.02479-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin S. M.; Prideaux B.; Lyon E. R.; Zimmerman M. D.; Brooks E. J.; Schrupp C. A.; Chen C.; Reichlen M. J.; Asay B. C.; Voskuil M. I.; Nuermberger E. L.; Andries K.; Lyons M. A.; Dartois V.; Lenaerts A. J. Bedaquiline and Pyrazinamide Treatment Responses Are Affected by Pulmonary Lesion Heterogeneity in Mycobacterium tuberculosis Infected C3HeB/FeJ Mice. ACS Infect. Dis. 2016, 2 (4), 251–267. 10.1021/acsinfecdis.5b00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry J. A.; Groseclose M. R.; Fraser D. D.; Castellino S. Revised preparation of a mimetic tissue model for quantitative imaging mass spectrometry. Protocol Exchange 2018, 1 (104), 1–20. 10.1038/protex.2018.104. [DOI] [Google Scholar]
- Zimmerman M.; Lestner J.; Prideaux B.; O’Brien P.; Dias-Freedman I.; Chen C.; Dietzold J.; Daudelin I.; Kaya F.; Blanc L.; Chen P.-Y.; Park S.; Salgame P.; Sarathy J.; Dartois V. Ethambutol partitioning in tuberculous pulmonary lesions explains its clinical efficacy. Antimicrob. Agents Chemother. 2017, 61 (9), 1–12. 10.1128/AAC.00924-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarathy J. P.; Via L. E.; Weiner D.; Blanc L.; Boshoff H.; Eugenin E. A.; Barry C. E. 3rd; Dartois V. A. Extreme Drug Tolerance of Mycobacterium tuberculosis in Caseum. Antimicrob. Agents Chemother. 2018, 62 (2), 1–11. 10.1128/AAC.02266-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig C.; Seagar A.; Watt B.; Forbes K. The efficacy of the heat killing of Mycobacterium tuberculosis. J. Clin. Pathol. 2002, 55 (10), 778–779. 10.1136/jcp.55.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabiiti W.; Azam K.; Esmeraldo E.; Bhatt N.; Rachow A.; Gillespie S. H. Heat inactivation renders sputum safe and preserves Mycobacterium tuberculosis RNA for downstream molecular tests. J. Clin. Microbiol. 2019, 57 (4), 1–8. 10.1128/JCM.01778-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M.; Blanc L.; Chen P.-Y.; Dartois V.; Prideaux B. Spatial quantification of drugs in pulmonary tuberculosis lesions by laser capture microdissection liquid chromatography mass spectrometry (LCM-LC/MS). J. Visualized Exp. 2018, (134), 1–7. 10.3791/57402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keam S. J. Pretomanid: first approval. Drugs 2019, 79 (16), 1797–1803. 10.1007/s40265-019-01207-9. [DOI] [PubMed] [Google Scholar]
- Lakshminarayana S. B.; Huat T. B.; Ho P. C.; Manjunatha U. H.; Dartois V.; Dick T.; Rao S. P. Comprehensive physicochemical, pharmacokinetic and activity profiling of anti-TB agents. J. Antimicrob. Chemother. 2015, 70 (3), 857–67. 10.1093/jac/dku457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarathy J. P.; Liang H.-p. H.; Weiner D.; Gonzales J.; Via L. E.; Dartois V. An in vitro caseum binding assay that predicts drug penetration in tuberculosis lesions. J. Visualized Exp. 2017, (123), 1–6. 10.3791/55559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek R. D.; Gerriets V. A.; Jacobs S. R.; Macintyre A. N.; MacIver N. J.; Mason E. F.; Sullivan S. A.; Nichols A. G.; Rathmell J. C. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011, 186 (6), 3299–3303. 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S.; Ernst O.; Avni D.; Athamna M.; Philosoph A.; Arana L.; Ouro A.; Hoeferlin L. A.; Meijler M. M.; Chalfant C. E.; Gómez-Muñoz A.; Zor T. Exogenous ceramide-1-phosphate (C1P) and phospho-ceramide analogue-1 (PCERA-1) regulate key macrophage activities via distinct receptors. Immunol. Lett. 2016, 169, 73–81. 10.1016/j.imlet.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niekamp P.; Guzman G.; Leier H. C.; Rashidfarrokhi A.; Richina V.; Pott F.; Barisch C.; Holthuis J. C.; Tafesse F. G. Sphingomyelin biosynthesis is essential for phagocytic signaling during Mycobacterium tuberculosis host cell entry. mBio 2021, 12 (1), 1–19. 10.1128/mBio.03141-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. E.; Pandey A. K.; Gilmore S. A.; Mizrahi V.; McKinney J. D.; Bertozzi C. R.; Sassetti C. M. Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem. Biol. 2012, 19 (2), 218–27. 10.1016/j.chembiol.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatherwick E. Q.; Svensson C. I.; Frenguelli B. G.; Scrivens J. H. Localisation of adenine nucleotides in heat-stabilised mouse brains using ion mobility enabled MALDI imaging. Int. J. Mass Spectrom. 2013, 345, 19–27. 10.1016/j.ijms.2013.02.004. [DOI] [Google Scholar]
- Goodwin R. J.; Lang A. M.; Allingham H.; Borén M.; Pitt A. R. Stopping the clock on proteomic degradation by heat treatment at the point of tissue excision. Proteomics 2010, 10 (9), 1751–1761. 10.1002/pmic.200900641. [DOI] [PubMed] [Google Scholar]
- Mulder I. A.; Esteve C.; Wermer M. J.; Hoehn M.; Tolner E. A.; van den Maagdenberg A. M.; McDonnell L. A. Funnel-freezing versus heat-stabilization for the visualization of metabolites by mass spectrometry imaging in a mouse stroke model. Proteomics 2016, 16 (11–12), 1652–1659. 10.1002/pmic.201500402. [DOI] [PubMed] [Google Scholar]
- Lenaerts A.; Barry C. E. 3rd; Dartois V. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol. Rev. 2015, 264 (1), 288–307. 10.1111/imr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena A. M.; Fortune S. M.; Flynn J. L. Heterogeneity in tuberculosis. Nat. Rev. Immunol. 2017, 17 (11), 691–702. 10.1038/nri.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmandas E.; Eckold C.; Böhme J.; Koeken V.; Marzuki M. B.; Blok B.; Arts R. J. W.; Chen J.; Teng K. W. W.; Ratter J.; Smolders E. J.; Van den Heuvel C.; Stienstra R.; Dockrell H. M.; Newell E.; Netea M. G.; Singhal A.; Cliff J. M.; Van Crevel R. Metformin Alters Human Host Responses to Mycobacterium tuberculosis in Healthy Subjects. J. Infect. Dis. 2019, 220 (1), 139–150. 10.1093/infdis/jiz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanselmann M.; Kirchner M.; Renard B. Y.; Amstalden E. R.; Glunde K.; Heeren R. M. A.; Hamprecht F. A. Concise representation of mass spectrometry images by probabilistic latent semantic analysis. Anal. Chem. 2008, 80 (24), 9649–9658. 10.1021/ac801303x. [DOI] [PubMed] [Google Scholar]
- Warsinske H. C.; DiFazio R. M.; Linderman J. J.; Flynn J. L.; Kirschner D. E. Identifying mechanisms driving formation of granuloma-associated fibrosis during Mycobacterium tuberculosis infection. J. Theor. Biol. 2017, 429, 1–17. 10.1016/j.jtbi.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila J. T.; Ojo O. O.; Kepka-Lenhart D.; Marino S.; Kim J. H.; Eum S. Y.; Via L. E.; Barry C. E. 3rd; Klein E.; Kirschner D. E.; Morris S. M. Jr; Lin P. L.; Flynn J. L. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J. Immunol. 2013, 191 (2), 773–84. 10.4049/jimmunol.1300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S.; Cilfone N. A.; Mattila J. T.; Linderman J. J.; Flynn J. L.; Kirschner D. E. Macrophage polarization drives granuloma outcome during Mycobacterium tuberculosis infection. Infect. Immun. 2015, 83 (1), 324–38. 10.1128/IAI.02494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.; Luo Q.; Guo Y.; Chen J.; Xiong G.; Peng Y.; Ye J.; Li J. Mycobacterium tuberculosis-Induced Polarization of Human Macrophage Orchestrates the Formation and Development of Tuberculous Granulomas In Vitro. PLoS One 2015, 10 (6), 1–16. 10.1371/journal.pone.0129744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yañez M. J.; Marín T.; Balboa E.; Klein A. D.; Alvarez A. R.; Zanlungo S. Finding pathogenic commonalities between Niemann-Pick type C and other lysosomal storage disorders: Opportunities for shared therapeutic interventions. Biochim. Biophys. Acta, Mol. Basis Dis. 2020, 1866 (10), 1–19. 10.1016/j.bbadis.2020.165875. [DOI] [PubMed] [Google Scholar]
- Schulze H.; Sandhoff K. Lysosomal lipid storage diseases. Cold Spring Harbor Perspect. Biol. 2011, 3 (6), 1–19. 10.1101/cshperspect.a004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineran P.; Lloyd-Evans E.; Lack N. A.; Platt N.; Davis L. C.; Morgan A. J.; Höglinger D.; Tatituri R. V. V.; Clark S.; Williams I. M.; Tynan P.; Al Eisa N.; Nazarova E.; Williams A.; Galione A.; Ory D. S.; Besra G. S.; Russell D. G.; Brenner M. B.; Sim E.; Platt F. M. Pathogenic mycobacteria achieve cellular persistence by inhibiting the Niemann-Pick Type C disease cellular pathway. Wellcome Open Res. 2016, 1, 1–30. 10.12688/wellcomeopenres.10036.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J.; Maamar H.; Deb C.; Sirakova T. D.; Kolattukudy P. E. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 2011, 7 (6), 1–16. 10.1371/journal.ppat.1002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.; VanderVen B. C.; Fahey R. J.; Russell D. G. Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J. Biol. Chem. 2013, 288 (10), 6788–800. 10.1074/jbc.M112.445056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderVen B. C.; Fahey R. J.; Lee W.; Liu Y.; Abramovitch R. B.; Memmott C.; Crowe A. M.; Eltis L. D.; Perola E.; Deininger D. D.; Wang T.; Locher C. P.; Russell D. G. Novel inhibitors of cholesterol degradation in Mycobacterium tuberculosis reveal how the bacterium’s metabolism is constrained by the intracellular environment. PLoS Pathog. 2015, 11 (2), 1–20. 10.1371/journal.ppat.1004679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Ramirez M. D.; Aguilar-Ayala D. A.; Garcia-Morales L.; Rodriguez-Peredo S. M.; Badillo-Lopez C.; Rios-Muniz D. E.; Meza-Segura M. A.; Rivera-Morales G. Y.; Leon-Solis L.; Cerna-Cortes J. F.; Rivera-Gutierrez S.; Helguera-Repetto A. C.; Gonzalez-y-Merchand J. A. Cholesterol plays a larger role during Mycobacterium tuberculosis in vitro dormancy and reactivation than previously suspected. Tuberculosis (Oxford, U. K.) 2017, 103, 1–9. 10.1016/j.tube.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Russell D. G.; VanderVen B. C.; Lee W.; Abramovitch R. B.; Kim M.-J.; Homolka S.; Niemann S.; Rohde K. H. Mycobacterium tuberculosis wears what it eats. Cell Host Microbe 2010, 8 (1), 68–76. 10.1016/j.chom.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q.; Zeng F.; Liu X.; Wang Q.; Deng F. Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis. 2016, 7 (5), 1–9. 10.1038/cddis.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L.; Nazarova E. V.; Tan S.; Liu Y.; Russell D. G. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 2018, 215 (4), 1135–1152. 10.1084/jem.20172020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L.; Nazarova E. V.; Russell D. G. Mycobacterium tuberculosis: bacterial fitness within the host macrophage. Bacteria Intracellularity 2020, 127–138. 10.1128/9781683670261.ch9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming B. M.; Pacl H. T.; Steyn A. J. C. Relevance of the Warburg Effect in Tuberculosis for Host-Directed Therapy. Front. Cell. Infect. Microbiol. 2020, 10, 576596–576596. 10.3389/fcimb.2020.576596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson L. E.; Sheedy F. J.; Palsson-McDermott E. M.; Triglia D.; O’Leary S. M.; O’Sullivan M. P.; O’Neill L. A.; Keane J. Cutting Edge: Mycobacterium tuberculosis Induces Aerobic Glycolysis in Human Alveolar Macrophages That Is Required for Control of Intracellular Bacillary Replication. J. Immunol. 2016, 196 (6), 2444–9. 10.4049/jimmunol.1501612. [DOI] [PubMed] [Google Scholar]
- Shi L.; Salamon H.; Eugenin E. A.; Pine R.; Cooper A.; Gennaro M. L. Infection with Mycobacterium tuberculosis induces the Warburg effect in mouse lungs. Sci. Rep. 2016, 5, 18176. 10.1038/srep18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. L.; Lamprecht D. A.; Mandizvo T.; Jones T. T.; Naidoo V.; Addicott K. W.; Moodley C.; Ngcobo B.; Crossman D. K.; Wells G.; Steyn A. J. C. Compromised Metabolic Reprogramming Is an Early Indicator of CD8(+) T Cell Dysfunction during Chronic Mycobacterium tuberculosis Infection. Cell Rep. 2019, 29 (11), 3564–3579. 10.1016/j.celrep.2019.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L.; Jiang Q.; Bushkin Y.; Subbian S.; Tyagi S. Biphasic Dynamics of Macrophage Immunometabolism during Mycobacterium tuberculosis Infection. mBio 2019, 10 (2), 1–19. 10.1128/mBio.02550-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marakalala M. J.; Raju R. M.; Sharma K.; Zhang Y. J.; Eugenin E. A.; Prideaux B.; Daudelin I. B.; Chen P. Y.; Booty M. G.; Kim J. H.; Eum S. Y.; Via L. E.; Behar S. M.; Barry C. E. 3rd; Mann M.; Dartois V.; Rubin E. J. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat. Med. 2016, 22 (5), 531–8. 10.1038/nm.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.