Abstract
Background
Colorectal cancer (CC) is the third most common cancer in the world. Annona reticulata (AR) also known as bullock's heart, is a traditional herb. AR leaf extract was initially investigated for its anti-bacterial, anti-inflammatory, anti-malarial, anti-helminthic, anti-stress, and wound healing properties. Only a few in vitro cancer studies have been conducted on AR. Although few studies have linked AR leaf extract to many cancers, comprehensive studies addressing regulation, biological functions, and molecular mechanisms leading to CC pathogenesis are clearly lacking.
Objectives
The present study aimed to explore the antioxidant and anti-cancer potentials of AR leaf extract in CC.
Materials and methods
The MTT assay was used to test the anti-proliferative activity of AR leaf extract in vitro on the HCT116 cell line. Qualitative and quantitative phytochemical characterization was carried out using gas chromatography: mass spectrometry (GC–MS). 1,2-dimethylhydrazine (DMH) was used to establish CC model in female Wistar rats. The acute toxicity of AR leaf extract was tested in accordance with OECD guidelines. Aberrant Crypt Foci (ACF) count, organ index, and hematological estimations were used to screen for in vivo anti-cancer potential. The antioxidant activity of colon homogenate was determined.
Results
The alcoholic leaf extract (IC50, 0.55 μg/ml) was found to be more potent than the aqueous extract. Using GC–MS, a total of 108 compounds were quantified in the alcoholic leaf extract. The LD 50 value was found to be safe at a dose of 98.11 mg/kg of body weight. AR alcoholic leaf extract significantly (p < 0.05) decreased ACF count and normalized colon length/weight ratio. AR leaf extract increased RBC, hemoglobin and platelets levels. The AR alcoholic leaf extract reduced the DMH-induced tumors and significantly (p < 0.05) increased the activity of endogenous antioxidant enzymes such as catalase, reduced glutathione, superoxide dismutase, and decreased the lipid peroxidase activity. AR leaf extract reduced the inflammation caused by DMH and helped to repair the colon's damaged muscle layers.
Conclusion
Based on the findings from the present study, it can be concluded that the alcoholic leaf extract of AR has antioxidant and anti-proliferative properties and can aid in the prevention of CC development and dysplasia caused by DMH.
Keywords: Annona reticulata; 1,2-Dimethylhydrazine; Colorectal cancer; Antioxidant; Anticancer
1. Introduction
Colorectal cancer (CC) is the third most common cancer in the world. In developing countries, the incidence and mortality rate of CC are rapidly increasing. According to the Globocan 2020 report, the incidence and mortality rate in Asia are 49.6% and 51.4%, respectively, with a 5 years prevalence of 1,436,272 (47.2%) (https://gco.iarc.fr/). The prevalence of this cancer is expected to rise to 60% by 2030 [1]. Approximately 41% of all CC occurs in the proximal colon, 22% in the distal colon, and 28% in the rectum [2]. Alcohol consumption, smoking, obesity, and meat consumption have all been identified as risk factors for CC. In addition, genetic and environmental factors play a role in the etiology of CC [3]. This cancer typically begins as a single mutation in a cell and progresses until it is detectable or as a polyp, at which point the polyp can develop into a life-threatening cancer [4]. Surgery, chemotherapy, radiotherapy, hormone therapy, immunotherapy, and newer targeted therapies are used to diagnose CC. Despite the fact that these treatments improve the outcome. There were some drawbacks and side-effects recorded; side-effects were associated with the use of chemotherapies, such as oxaliplatin (dysesthesias and renal dysfunction) or irinotecan (nausea, alopecia, and bone marrow suppression), fluorouracil (stomatitis, diarrhea, neutropenia), etc. Even if treatments allow for long survival, long-term side-effects such as gastrointestinal problems, urinary incontinence, sensory neuropathy, and sexual dysfunction have been increasing for many years. People rely heavily on traditional medicines in addition to modern medicines.
Global herbal medicine is one of the most important aspects of traditional medicine, and it is also used in cancer treatment. Approximately 70% of the world's population uses medicinal plants as a promising potential source of the bioactive anti-cancer molecule. When vinca alkaloids were discovered in the 1950s, it became possible to extract anti-cancer drugs from plants [5]. Plant-derived phytochemicals such as terpenoids, glycosides, phenolics, flavonoids, and alkaloids exhibit meticulous physiological results. These effects often include positive beneficial effects [6]. In most cases, herbal treatment does not depend on the individual, age, or gender.
Annona reticulata L. (AR) is one such herbal source for anti-cancer activity, belonging to Annonaceae family. It is also known as bullock's heart, custard apple, netted custard apple, wild-sweetsop (English) and locally popular as ‘Ramphal’ in Karnataka, India. Traditionally, the plant has been used to treat bacterial infection, parasite and worm infestations, cardiac problems, dysentery, epilepsy, ulcer, hemorrhage, constipation, dysuria, as insecticide and in fever. It is also used as an anti-inflammatory, anti-malarial, anti-helminthic, anti-stress, wound healing agent, and in the treatment of diabetes, diarrhea, and dysentery [7,8]. An in vitro study stated that the ethanolic root extract of AR has strong anti-proliferative activity against human cancer cell lines such as HeLa, K-562, A-549 and MDA-MB [9]. Methanolic leaf extract of AR inhibited the growth of MCF-7, A549, SCC9, and HCT116 cells in a dose-dependent manner. The extract is reported to show cell cycle arrest at the G2/M phase in A549 and MCF-7 cells [10]. Another study found that AR methanolic extract was cytotoxic to colon and liver cancer cells and may have anti-cancer properties [11]. A study identified the phytochemical constituent of methanolic leaf extract of AR. They emphasized the presence of important phytochemicals such as amino acids, alkaloids, glycosides, flavonoids, steroids, proteins, triterpenoids, and phenolic compounds. The same study discovered that AR has anti-cancer activity against hepatoma (HEPG2) and human lung cancer (Hop65) cell lines [12]. According to a recent review of AR, the isolated constituent catechin and methanolic extract of AR demonstrated excellent free-radical scavenging activity as well as antioxidant activity. Acetogenins are abundant in the Annonaceae plant family. The presence of acetogenins may be responsible for AR methanol extract's anti-cancer properties [13]. Ganesh et al. investigated the acute toxicity of AR leaf extract on Swiss albino mice and found that the leaf extract is non-toxic and non-allergic, with no significant toxic effect observed. They also found that AR leaf extract 2000 mg/kg body weight is safe for oral administration [14]. Although few studies have linked AR to many cancers, comprehensive studies addressing regulation, biological functions, and molecular mechanisms leading to CC pathogenesis are clearly lacking. Thus, investigations are necessary to understand the mechanism action of AR in CC cell lines. In vivo studies are also needed to understand the potential impact of AR. As a result, the current study was designed to investigate the anti-cancer effect of AR leaf extract on the 1,2-dimethylhydrazine (DMH) induced CC rat model, which might relate pharmacological significance to the ethnobotanical claims of the local population.
2. Materials and methods
The current research has been approved by the Institutional Animal Ethics Committee (IAEC), Kasturba Medical College, Manipal Academy of Higher Education (MAHE) Manipal. The AR leaf was used in this study and it was collected in Parkala, Udupi district, Karnataka, India, in January 2018. The plant was authenticated by Ms. Usharani S Suvarna, Associate Professor and Head of Department of Botany, Mahatma Gandhi Memorial (MGM) College, Udupi, Karnataka, India (MGMC/2018-19/1). The availability of the plant name has been checked and confirmed with https://mpns.science.kew.org/
2.1. Reagents and chemicals
DMH has been purchased from TCI Chemicals, Chennai, India. CC cell line, Human colon cancer cell line (HCT116) was procured from NCCS, Pune and sub-cultured according to the standard protocol (http://www.atcc.org/).
2.2. Preparation of extract
The leaves were completely washed and dried under the shade and coarsely powdered using a blender. The powder was packed into two different thimbles within the Soxhlet extractor/apparatus. It was then macerated with aqueous and absolute alcohol in two different Soxhlet apparatus. Extraction/cycles were performed until the solvent became colorless. Once the extraction was completed, the solvent was removed and placed in a lyophilizer to remove the moisture content. The dried extract was stored in a desiccator until use.
2.3. Cell culture
HCT116 cells were maintained in DMEM medium supplemented with 10% Fetal Bovine Serum (FBS). In presence of 5% CO2, the cells were grown at 37 °C in a humidified incubator. Every 2–3 days, the media was replaced with fresh media. Once it reached the confluency of 80–90%, cell was trypsinized and seeded in different plates for the various experiments. After 24hr, the media was removed, and cells were exposed to different concentrations of AR for 48hr.
2.4. Cell viability study by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
To determine cell viability, an enzyme-based colorimetric assay was used. The MTT assay is most commonly used to estimate the cytotoxic potential of the crude extract, which can be determined using the IC50 value. Initially, 0.1 × 105 HCT116 cells/well were seeded into a 96-well sterile tissue culture plate and allowed to attach for 24hr. Cells were then exposed to various concentrations of AR extract and incubated at 37 °C for 48 hr. After incubation, 20 μl of MTT reagent (Stock 5 mg/ml in PBS and pH −7.4) was added to the cells and incubated for 4hr at 37 °C. The medium and MTT were then aspirated, and to stabilize the formazan crystals, 100 μl of 100% DMSO was added to each well, and the plate absorbance was measured at 540 nm.
2.5. Phytochemical characterization
2.5.1. Qualitative analysis
To identify the preliminary active phytochemicals in AR's alcoholic leaf extract. The test was performed using standard procedures [15,16].
2.5.2. Quantitative analysis
The phytochemical investigation of alcoholic leaf extract of AR has been performed using Shimadzu gas chromatography: mass spectrometry (GCMS-QP2010S) instrument. The GCMS conditions were, RTX5 column with a length of 30 m and internal diameter was 0.25 mm, and the film thickness was 0.25 micron. The column oven initial 60 °C, injection mode was split, and its ratio was 4.0. Ion sources were 200 °C and temperature at the interface was 280 °C. For analysis, 1 μl of the sample was injected and the flow rate of 1 ml/min was maintained. MS values from the range of 40–500 m/z were identified. For compound recognition and conformation, the obtained MS spectrum was aligned with the NIST library. The study was performed at Analytical Research and Metallurgical Laboratories Pvt. Ltd. (ARML), Bengaluru, India.
2.6. Animal procurement
Female Wistar rats inbred at Central Animal Research Facility (CARF), MAHE, Manipal. Animals were maintained at temperature 23 ± 3 °C, 14:10 hrs dark and light cycle for adaptation to the experimental environment, and controlled humidity conditions. The animals were housed in sterile polypropylene cages with bedding made of fully clean/sterile paddy husk. Animal care and handling have been carried out in accordance with the guidelines put forth by the Institutional Animal Ethics Committee (IAEC), KMC-Manipal, MAHE-Manipal who approved the study conducted and the clearance certificate was provided with numbers: IAEC/KMC/40/2017.
2.7. Acute toxicity study
In order to assess the safety and to determine a safe dose of a drug, an acute toxicity analysis was carried out in compliance with recommendations of the Organization for Economic Co-operation and Development (OECD No. 425). The fractions to be tested were given to female Wistar rats who had fasted overnight. The leaf extract was given as a suspension in 0.25 percent carboxymethyl cellulose (CMC), and the dosage was determined based on the bodyweight of Wistar rats. After administration, the animals were constantly monitored for 30 min, 1 hr, 4 hr, 24 hr, and up to 14 days. The animals were then examined for gross behavioral changes using Irwin's table and a tolerable drug dose was chosen based on acute toxicity (LD50) [16].
2.8. Carcinogen preparation
The methods for inducing carcinogens with DMH are well established and have already been published. DMH was dissolved in 1 mM EDTA and the pH was adjusted to 6.5 with 1 M sodium hydroxide. Before each dosing, the DMH solution was freshly prepared. The carcinogen was injected intraperitoneally (i.p) once a week for the first 10 weeks at a dosage of 20 mg/kg and 30 mg/kg for the next 10 weeks during the 20-week induction period [16,17].
2.9. Experimental setup
The female Wistar rats were divided into five groups, each with six rats.
Group I: Healthy control = Healthy control.
Group II: DMH control = Disease control (DMH control).
Group III: Standard = Disease and 5-Fluorouracil (10 mg/kg, i.p).
Group IV: Treatment 1 = Disease and treatment with AR-5mg/kg, p.o.
Group V: Treatment 2 = Disease and treatment with AR-10 mg/kg, p.o.
2.10. Experimental procedure
Following carcinogen induction, group III received the standard drug 5-Flurouracil (10 mg/kg, i.p) once a week, while, groups IV and V received AR leaf extract 5 mg/kg and 10 mg/kg, p.o. daily respectively for 21 days [16,17].
2.11. Aberrant crypt foci (ACF) count
The experimental animals were euthanized, and a portion of the colon was dissected, opened into a longitudinal section, and washed with saline. The specimen was then fixed flat on filter paper for 24 h with 10% of formalin buffered. The colon was then stained for 5 min with 0.1% of methylene blue dye and washed with PBS, specimens were observed under a microscope with a magnification of 40× and ACF numbers were counted and measured as number/cm2 and the images were taken under a microscope.
2.12. Colon length/weight ratio
The dissected entire colon length was measured in centimeters (cm) and weighted in grams, and the ratio was calculated as follows:
| Ratio of colon length/weight = length by cm/weight in grams |
2.13. Spleen and liver index
The spleen and liver, two major organs, were weighed. The organ index was calculated based on the animal's body weight:
| Organ index (liver/spleen) = (organ weight in grams/animal body weight in grams) |
2.14. Hematological estimation
Blood was collected from the euthanized animals via retro-orbital sinus puncture and 500 μl of blood was collected in a microcentrifuged tube containing 50 μl of 10% EDTA as an anti-coagulant. A veterinary blood cell counter was used to test the blood samples for hematological parameters (Model: PCE-210VET, ERMA INC, TOKYO).
2.15. Estimation of antioxidant markers
Following the 21st dose of treatment, all animals were euthanized and the colon was isolated and weighed. A 10% colon tissue homogenate was prepared using a homogenizer and ice-cold potassium chloride (150 mM) (Model: Yamato L.S.GL.H-21, Japan). The homogenized colon has been used for estimation antioxidants such as catalase (CAT) [18], reduced glutathione (GSH) [19], superoxide dismutase (SOD) [20], and lipid peroxidase (LPO/MDA) [21]. The methods for estimating antioxidants are well-established and have already been published.
2.16. Histopathological study
After the end of the study, the colonic tissue was dissected out and stored in 10% formalin, and tissue blocks were prepared. A thin section of tissue was stained with hematoxylin and eosin stain, and images were captured using a 4× magnification microscope, and samples were examined.
2.17. Statistical analysis
GraphPad Prism 5.03 Version (Graph Pad Software Inc., La Jolla, CA, USA) was used for data analysis and One-way variance analysis (ANOVA) was used to compare results between groups, followed by Tukey's post hoc test for statistically significant result. P < 0.05 was considered statistically significant.
3. Results
3.1. Cell viability study by MTT
AR aqueous and alcoholic leaf extracts were tested for anti-proliferative activity against HCT116 cell line at different concentrations. The IC50 values of aqueous and alcoholic leaf extract of AR on HCT116 was found to be >7.25 μg/ml and 0.55 μg/ml respectively. The AR alcoholic leaf extract was found to be more potent than aqueous extract.
3.2. Phytochemical characterization
3.2.1. Qualitative analysis
The alcoholic leaf extract tested positive for flavonoids, terpenoids, saponins, tannins, quinine, carbohydrate, and steroids but negative for phenols and glycosides (Table 1).
Table 1.
Qualitative analysis of alcoholic leaf extract of AR.
| S. No | Phytoconstituents | Result |
|---|---|---|
| 01 | Terpenoids | Present |
| 02 | Flavonoids | Present |
| 03 | Phenols | Absent |
| 04 | Saponins | Present |
| 05 | Tannins | Present |
| 06 | Quinine | Present |
| 07 | Glycosides | Absent |
| 08 | Carbohydrates | Present |
| 09 | Steroids | Present |
3.2.2. Quantitative analysis
The GC–MS method was used to quantify the contents of AR's alcoholic leaf extract. A total of 108 compounds were identified from the large percentage area of components listed in Supplementary Table 1.
GC–MS profiling of the extracts revealed the presence of a total of 108 peaks (Fig. 1). Based on peak area (%), 50 major compounds were listed in Supplementary Table 1. These compounds reported to exhibit important biological functions. Some of major components such as gamma-Sitosterol (15.86%), Phytol (7.64%), Stigmasterol (7.25%), Ergost-5-en-3-ol, (3 beta)- (5.88%), 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (5.46%), 9-Octadecenoic acid, 1,2,3-propanetriyl ester, (E,E,E)- (5.10%), Stigmast-4-en-3-one (4.94%), Tetrapentacontane (4.35%), l-(+)-Ascorbic acid 2,6-dihexadecanoate (4.34%), were detected (Supplementary Table 1).
Fig. 1.
GC–MS chromatogram for alcoholic leaf extract of AR. Legend: GC–below in MS chromatogram, contents from the alcoholic leaf extract of AR. A total of 108 compounds were recorded and represented in peaks. The X-axis represents R. time and Y-axis the percentage of area.
3.3. Acute toxicity study
Acute toxicity of AR alcoholic leaf extract was tested in Wistar rats and the LD50 value was found to be safe at a dose of 98.11 mg/kg of body weight, with no lethality or serious visible toxicity. Based on these findings, 1/20th and 1/10th of the LD50 which corresponds to 5 mg/kg and 10 mg/kg respectively, of alcoholic leaf extract were chosen as treatment doses.
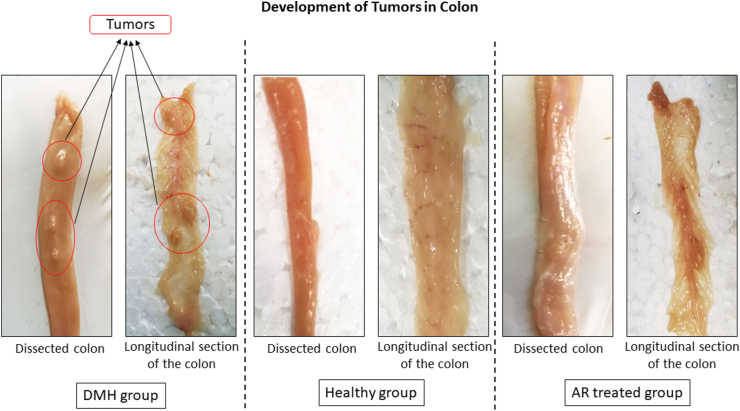
3.4. Tumor development
The tumors were developed in the colon region after 20 weeks of intraperitoneal DMH induction, and the tumor size was reduced after treatment with alcoholic leaf extract of AR (Fig. 2).
Fig. 2.
Showing the development of tumors in various groups. Legend: DMH induced tumors in the colon region, AR leaf extract inhibited tumor formation, and the healthy group had no tumors.
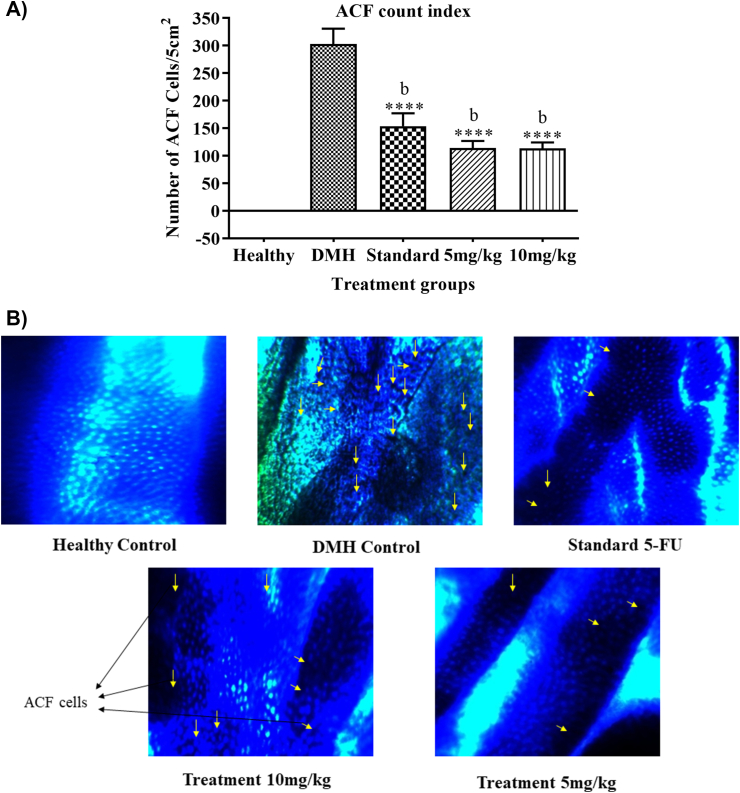
3.5. ACF count
DMH treatment showed ACF production in the colon (302.83 ± 27.83). Standard (153.7 ± 23.8) and AR treatment groups 5 mg/kg (113.7 ± 13.15) and 10 mg/kg (113.2 ± 11.02) showed a significant (p < 0.05) decrease in ACF counts as compared to DMH Control group (Fig. 3).
Fig. 3.
A) Graph represents the effect of AR alcoholic leaf extract on the ACF count index in different groups. B) ACF pathology figures. Legend: all the values are mean ± SEM of six samples, where a∗∗∗p < 0.001 compared to Healthy (no ACF formation observed in healthy animals), b∗∗∗p < 0.001 compared to DMH control.
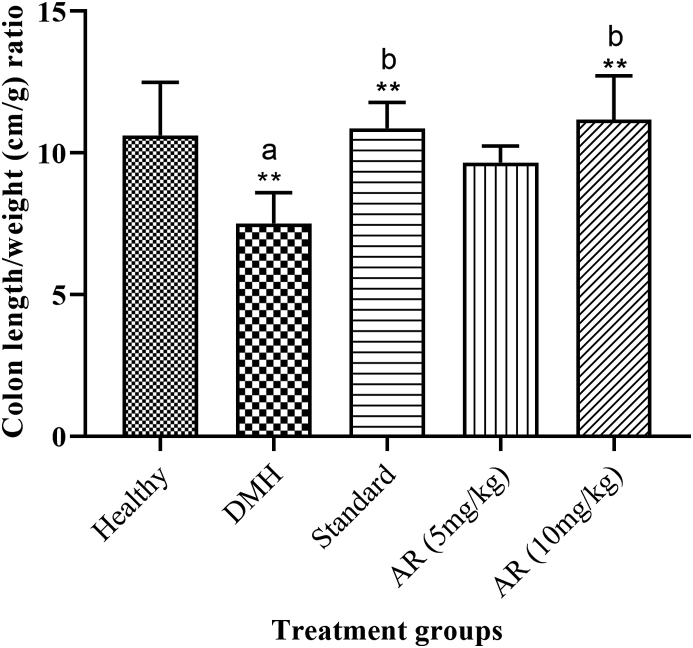
3.6. Colon length/weight ratio
There was a significant (p < 0.05) reduction in the colon length/weight ratio in the DMH (7.51 ± 0.44) control group as compared to healthy group (10.62 ± 0.76). Standard (10.86 ± 0.37) and AR treatment 10 mg/kg (11.18 ± 0.63) significantly (p < 0.05) normalized the length/weight ratio of the colon as compared to disease control (Fig. 4).
Fig. 4.
Effect of AR leaf extract on colon length/weight ratio. Legend: values are expressed as mean ± SEM of six samples, where a∗∗p < 0.01 compared to healthy control, b∗∗p < 0.01 compared to DMH control.
3.7. Spleen and liver index
There were no major variations observed between treated and control groups (Table 2).
Table 2.
Showing the effect of AR leaf extract AR on the spleen and liver index.
| Treatment groups | Spleen index (Mean ± SEM) | Liver index (Mean ± SEM) |
|---|---|---|
| Healthy control | 0.38 ± 0.016 | 0.028 ± 0.000 |
| DMH control | 0.42 ± 0.022 | 0.078 ± 0.047 |
| Standard (5FU) | 0.37 ± 0.015 | 0.030 ± 0.001 |
| AR 5 mg/kg | 0.38 ± 0.011 | 0.028 ± 0.001 |
| AR 10 mg/kg | 0.044 ± 0.074 | 0.035 ± 0.004 |
Legend: Values are expressed as Mean ± SEM of five samples.
3.8. Hematological examination
DMH control showed a significant (p < 0.05) decrease in RBC and hemoglobin (Hb) levels as compared to normal group. Standard and AR leaf extract showed a significant (p < 0.05) improvement in RBC, Hb and platelets, whereas there was no considerable difference was observed in the WBC count (Table 3).
Table 3.
Showing the effect of AR leaf extract AR on haematological parameters.
| Treatment groups | WBC (cells/mm3) | RBC (cells/mm3) | % Hb (g/dl) | Platelets (cells/μl) |
|---|---|---|---|---|
| Healthy control | 3800 ± 385.86 | 12,281,667 ± 1,106,068 | 13.55 ± 0.125 | 82666.67 ± 7760.298 |
| DMH control | 4066.6 ± 668.99 | 11,402,667 ± 1,271,870 | 10.36 ± 1.563a | 123333.3 ± 5605.553a |
| Standard (5FU) | 3675 ± 151.84 | 13,071,667 ± 492604.2 | 12.85 ± 0.313b | 136666.7 ± 4825.856b |
| AR 5 mg/kg | 3400 ± 598.88 | 14,188,333 ± 922239.4b | 12.77 ± 0.326b | 145666.7 ± 5211.099a |
| AR 10 mg/kg | 3500 ± 186.18 | 14,336,667 ± 525048.1b | 12.94 ± 0.431b | 143666.7 ± 6747.016a |
Legend: All values are expressed in Mean ± SEM, where ap < 0.05 compared to healthy control, bp < 0.05 compared to DMH control.
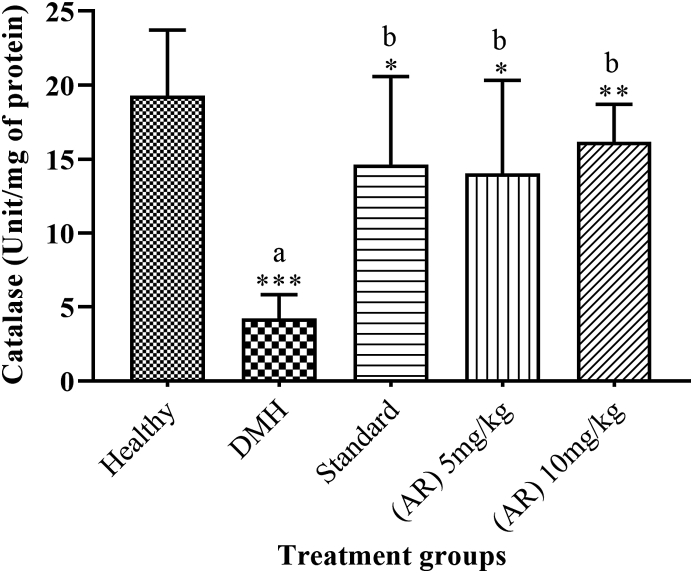
3.9. Catalase activity (CAT)
DMH control group (4.22 ± 0.655) showed a significant (p < 0.05) reduction in CAT activity as compared to healthy group (19.3 ± 1.80). Standard (14.63 ± 2.434) and AR leaf extract showed a significantly (p < 0.05) increased in the CAT activity in both treatment groups of 5 mg/kg (14.02 ± 2.57), and 10 mg/kg (16.22 ± 2.54) groups as compared to DMH group (Fig. 5).
Fig. 5.
Impact of AR leaf extract on CAT activity. Legend: values are expressed as mean ± SEM of six samples, where a∗∗∗p < 0.001 compared to Healthy control, b∗p < 0.05 compared to DMH control, b∗∗p < 0.01 compared to DMH control.
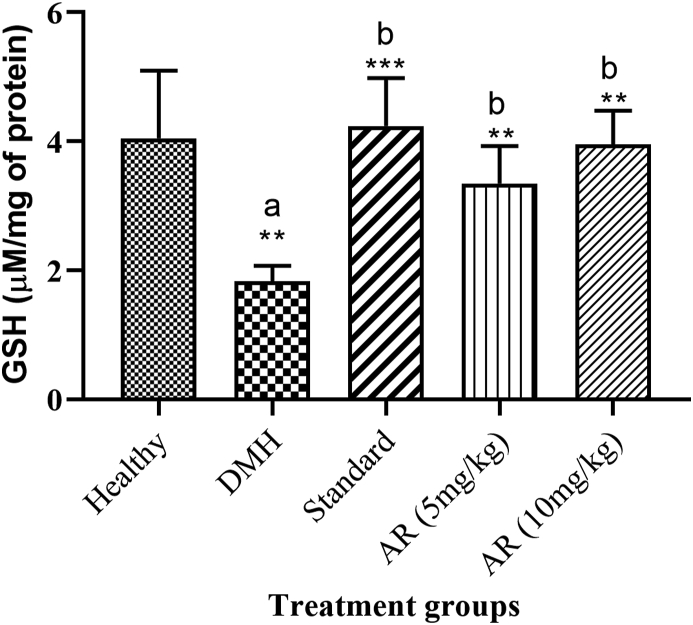
3.10. Reduced glutathione (GSH)
GSH level was found to be decreased in DMH control (1.830 ± 0.362) group when compared to healthy control (4.04 ± 0.429). Standard (4.23 ± 0.302) and AR leaf extract showed significantly increased in GSH activity in both treatment groups 5 mg/kg (3.34 ± 0.236), and 10 mg/kg (3.95 ± 0.522) compared to DMH control group (Fig. 6).
Fig. 6.
Impact of AR leaf extract on GSH activity. Legend: values are expressed as mean ± SEM of six samples, where a∗∗p < 0.05 compared to Healthy control, b∗∗∗p < 0.001 compared to DMH control, b∗∗p < 0.01 compared to DMH control.
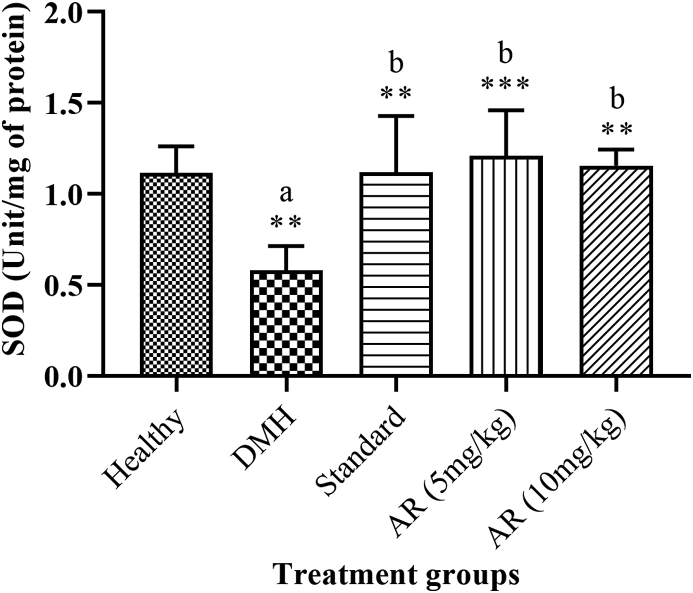
3.11. Superoxide dismutase (SOD)
DMH (0.58 ± 0.055) control group showed a significant (p < 0.05) reduction in SOD activity as compared to healthy control (1.11 ± 0.059). Standard (1.11 ± 0.125) and AR leaf extract 5 mg/kg (1.20 ± 0.102) and 10 mg/kg (1.15 ± 0.087) showed a significant (p < 0.05) increases in SOD activity as compared to DMH control group (Fig. 7).
Fig. 7.
Impact of AR leaf extract on SOD activity. Legend: values are expressed as Mean ± SEM of six samples, where a∗∗p < 0.01 compared to Healthy control, b∗∗p < 0.01 compared to DMH control, b∗∗∗p < 0.001 compared to DMH control.
3.12. Lipid peroxidase (LPO)
DMH (1167.6 ± 159.70) group showed a significant (p < 0.05) increases in LPO activity as compared to healthy group (404.0 ± 28.30). Standard (676.5 ± 72.6) and both AR leaf extract 5 mg/kg (590.3 ± 62.03) and 10 mg/kg (730.1 ± 39.97) showed a significant (p < 0.05) reduction in the LPO activity as compared to DMH control group (Fig. 8).
Fig. 8.
Impact of AR leaf extract on LPO activity. Legend: values are expressed as mean ± SEM of six samples, where a∗∗p < 0.01 compared to healthy control, b∗p < 0.05 compared to DMH control, b∗∗p < 0.01 compared to DMH control.
3.13. Histopathological examination
DMH induction caused the severity of inflammation, hyperproliferative cells and damage of muscle layers of the colon in DMH group (Fig. 9(c) & (d)) as compared to healthy group (Fig. 9(a) & (b)). The alcoholic leaf extract of AR decreased the inflammation formed due to DMH and recovering of damaged muscle layers was observed in both AR 5 mg/kg (Fig. 9(g) & (h)), and 10 mg/kg treated groups (Fig. 9(i) & (j)) compared to the DMH control group (Fig. 9(c) & (d)).
Fig. 9.
Histopathological examination of colon. Legend: (a) & (b) are healthy control group, (c) & (d) are DMH control group, (e) & (f) are standard- 5flurouracil, (g) & (h) are treatment with 5 mg/kg and (i) & (j) are treatment with 10 mg/kg. Objective used 4×.
4. Discussion
Studies have shown that AR has anti-bacterial, anti-viral, and anti-cancerous properties. Suresh et al. showed that AR root extract inhibited the growth of human cancer cell lines A-549, K-562, HeLa, and MDA-MB [22]. AR leaf extract exhibited cytotoxicity against HT-29 cell lines indicating that it is a potent anti-cancer agent [23]. AR also showed anti-cancer activity against HCT116, MCF7, A549, and SCC9 cell lines [10]. Our current study also demonstrated the cytotoxicity of alcoholic leaf extract of AR against HCT116 cell lines. Biological active phytochemicals such as saponins, flavonoids, steroids, terpenoids, tannins, quinine, and carbohydrates have been shown to have anti-cancer, antioxidant, and anti-microbial activity [[24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]]. In this study, we also confirmed the presence of phytochemical constituents like saponins, flavonoids, terpenoids, tannins, quinine, carbohydrates, and steroids in the alcoholic leaf extract of AR through quantitative analysis performed by GC–MS. From the major percentage area of the components listed, a total of 108 compounds were identified. The identified compounds have anti-cancer activity against a variety of cancers such as Gamma-Sitosterol, which has been shown to have anti-cancer activity against the human MCF-7 and A549 cell lines [36]. Phytol-inhibited cell proliferation and promoted autophagy in MCF-7 and human gastric adenocarcinoma AGS cells [37]. Other constituents observed were stigmasterol chemopreventive activity in cancer [38], Ergost-5-en-3-ol [anti-cancer in gastric, lung, and ovarian cancer [[39], [40], [41], [42]]], 3,7,11,15-Tetramethyl-2-hexadecen-1-ol [anti-cancer [43]], 9-Octadecenoic acid, 1,2,3-propanetriyl ester, (E,E,E)-compound (cytotoxic on human epidermoid larynx carcinoma (Hep-2) cell line) [44], stigmast-4-en-3-one [anti-cancer effect on the MG-63 cell line [45]].
To investigate the safety of AR in animals, acute oral toxicity was performed, where LD50 of the extract was found to be 98.5 mg/kg body weight. Thus, 1/20th and 1/10th doses of the extract's LD50 value were chosen as treatment doses which were 5 mg/kg and 10 mg/kg respectively in CC model in animals. The lowest LD50 indicated toxic nature of the extract.
ACF is known to be the earliest neoplastic lesion in the progression of CC, and it is one of the biomarkers used to diagnose CC [46]. Patients with an elevated number of adenomas have also reported a large amount of ACF [47]. Studies indicated that genetic alteration in CC may contribute to the development of malignant tumors [48]. According to the researchers, individuals with adenomas or CC have significantly increased histological progression, ACF number, and size. In our study, we observed an increase in the number of ACFs in the DMH-treated group compared to the AR-treated group and a significant (p < 0.05) decrease in ACF in all treated groups. Bodyweight is an important marker of CC in rats. Several studies have reported a change in the bodyweight of the CC rat; significantly lower colon length of azoxymethane (AOM) and dextran sulfate sodium (DSS)-inducing CC mice as compared to healthy mice [49]. Wistar rats with DSS-induced colitis had a lower length/weight ratio of the colon [50]. The development of adenomas and adenocarcinomas reduces the length/weight ratio of the colon. The length/weight ratio of the colon is reduced in the DMH induced CC model [16]. In this study, we observed that DMH significantly reduced the length/weight ratio of the colon in the DMH control group when compared to the healthy group and that alcoholic leaf extract of AR significantly (p ≤ 0.05) increased the length/weight ratio of the colon at both doses of 5 mg/kg and 10 mg/kg compared to the DMH control.
Tumor growth, manifest pathological changes in the organism, and prognostic factors for disease development and treatment all cause changes in blood values. Thus, a study of the macro-functional status of blood cells in rats during DMH-induced CC revealed a link between the hemolysis mechanism and oxidative stress. As a result, oxidative products are increased and antioxidant enzymes such as SOD, GSH, and CAT activity in cells are altered [51]. Lee Y-J et al. found a positive correlation between WBC and CC, indicating that an enhanced level of WBC increases the risk of CC incidence and mortality in both men and women [52]. According to studies, RBC lysis occurs during tumor development, and microscopic bleeding has been observed in the colon and related digestive tract in CC, which may be a reason to lower RBC levels in CC [53]. In the current study, it was observed that the RBC level in the DMH control group decreased while the WBC level increased when compared to the healthy group whereas, alcoholic leaf extract of AR lowers the WBC levels and significantly (p ≤ 0.05) increased RBC levels in both doses of 5 mg/kg and 10 mg/kg when compared to the DMH control group. In a recent study, erythrocytes and Hb levels were measured in CC patients and anemia, often normocytic, followed by microcytic conditions was also observed. The Hb count was reduced due to a tumor, and low blood Hb was observed as a result of systemic inflammation [54]. In the current study, we observed that there is a significant decrease in Hb count at p < 0.05 in the DMH control group (10.36 ± 1.563) compared to the healthy control (13.55 ± 0.125) and the treatment with AR leaf extract showed a significant increase in Hb count at both doses of 5 mg/kg (12.27 ± 0.326) and 10 mg/kg (12.28 ± 0.431) p < 0.05 compared to the DMH group. Cancer cell and platelet interactions have been the subject of extensive research for many years. Recent research has linked a high platelet count and thrombocytosis to a poor prognosis in many cancer patients, including those with colorectal cancer [55]. A study reported that platelet count and plateletcrit (Pct) were increased in CC patients compared to healthy subjects [56]. Xu-Dong Rao et al. have stated that increased platelet counts are a negative association predictor of survival in both primary CC and resectable colorectal liver metastases [57]. Our current study shows that the DMH group has higher platelet levels (123333.3 ± 5605.5) than the healthy group (82666.6 ± 7760.2), but there is no significant difference between the AR treated groups and the DMH group.
Histopathological results showed that DMH caused severe inflammation, hyperproliferative cells, and muscle layer damage in the DMH control group compared to healthy control. Treatment with alcoholic leaf extract of AR reduces the inflammation caused by DMH and improves muscle damage recovery at doses of 5 mg/kg and 10 mg/kg compared to the DMH control.
CAT, SOD, and GSH are endogenous enzymes with antioxidant activity that help the body maintain normal free radical levels. These antioxidants play an important role in maintaining the body's health. These enzyme activities decrease as cancer progresses, resulting in an increase in free radical levels [58]. The antioxidant defence system in CC tissue is determined by SOD activity. The SODs convert superoxide radicals into hydrogen peroxide (H2O2) and a molecule of oxygen (O2), whereas CAT degrades H2O2 into O2 and water. Two lethal species, superoxide radical and hydrogen peroxide, are converted into water [59].
Many studies have found that CAT activity decreases in DMH-treated rats during the development of CC [[60], [61], [62]]. Another study found that CAT is losing its ability to protect against free radical damage [63]. Our current study also showed a strong resemblance to the previous study, with a significant decrease in CAT activity (p ≤ 0.05) in DMH control compared to a healthy control group, whereas AR with both doses of 5 mg/kg and 10 mg/kg showed a significant increase in CAT activity (p ≤ 0.05). Many studies have found that DMH reduces the activity of antioxidants such as SOD and CAT [[64], [65], [66], [67]]. Several studies have also found significantly higher levels of MDA in DMH-treated rats [64,65]. In our study, we found that the activity of SOD and CAT was significantly reduced in DMH-treated rats compared to other groups, while MDA activity was significantly higher in DMH-treated colon tissue. Following treatment with AR leaf extract, SOD, CAT, and MDA activity returned to normal in comparison to the healthy control groups, but there was no significant difference in GSH activity.
5. Conclusion
The current study was carried out to investigate the anti-cancer efficacy of AR alcoholic leaf extract. The AR extract had the greatest anti-proliferative activity against the human HCT116 cell line, which may be due to the presence of major phytochemical constituents such as flavonoids, triterpenoids, gamma-sitosterol, phytol, stigmasterol, ergost-5-en-3-ol, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, 9-Octadecenoic acid, and 1,2,3-propanetriyl ester. The in vivo study provided evidence for the protective effect of AR alcoholic leaf extract in the DMH-induced CC rat model. The leaf extract reduced the DMH-induced tumors and increased the activity of endogenous antioxidant enzymes such as CAT, SOD, and GSH, while decreasing MDA levels. AR alcoholic leaf extract was effective in reducing DMH-induced inflammation and dysplasia. Thus, based on the current study's findings, it can be concluded that the alcoholic leaf extract of AR has antioxidant and anti-proliferative properties and can aid in the prevention of CC development and dysplasia caused by DMH. AR extract has shown to be effective against CC. Further research on the isolation of specific components of the leaf extract responsible for the anti-cancer activity can be conducted to increase specificity.
Source(s) of funding
This research work was funded by Kasturba Medical College, Manipal Academy of Higher Education (Accts/2017-18/PGR116)
Conflict of interest
None.
Authors contributions
Vijetha Shenoy Belle and Nitesh kumar conceived the study, reviewed the manuscript and approved submission. Nadeem Khan G performed the study and written the manuscript. Abhijna Ballal and Divya Datta helped during study performing and writing the manuscript.
Acknowledgments
We thank Kasturba Medical College, Manipal Academy of Higher Education (MAHE) (Accts/2017-18/PGR116) for financial support and Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education (MAHE) for providing the necessary facilities to perform this study.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2021.05.010.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Qualitative analysis of alcoholic leaf extract of AR.
References
- 1.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2.Cheng L., Eng C., Nieman L.Z., Kapadia A.S., Du X.L. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol Cancer Clin Trials. 2011;34:573–580. doi: 10.1097/COC.0b013e3181fe41ed. [DOI] [PubMed] [Google Scholar]
- 3.Kuipers E.J., Grady W.M., Lieberman D., Seufferlein T., Sung J.J., Boelens P.G., et al. Colorectal cancer. Nat Rev Dis Prim. 2015;1:1–51. doi: 10.1038/nrdp.2015.65.COLORECTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labianca R., Beretta G.D., Kildani B., Milesi L., Merlin F., Mosconi S., et al. Colon cancer. Crit Rev Oncol Hematol. 2010;74:106–133. doi: 10.1016/j.critrevonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Moudi M., Go R., Yien C.Y.S., Nazre M. Vinca alkaloids. Int J Prev Med. 2013;4:1231–1235. [PMC free article] [PubMed] [Google Scholar]
- 6.Shukla S., Mehta A. Anticancer potential of medicinal plants and their phytochemicals: a review. Rev Bras Bot. 2015;38:199–210. doi: 10.1007/s40415-015-0135-0. [DOI] [Google Scholar]
- 7.Wen W., Lin Y., Ti Z. Antidiabetic, antihyperlipidemic, antioxidant, anti-inflammatory activities of ethanolic seed extract of Annona reticulata L. In streptozotocin induced diabetic rats. Front Endocrinol. 2019;10:1–15. doi: 10.3389/fendo.2019.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thang T.D., Kuo P.C., Huang G.J., Hung N.H., Huang B.S., Yang M.L., et al. Chemical constituents from the leaves of annona reticulata and their inhibitory effects on NO production. Molecules. 2013;18:4477–4486. doi: 10.3390/molecules18044477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suresh H., Shivakumar B., Hemalatha K., Heroor S., Hugar D., Sambasiva Rao K.R. In vitro antiproliferative activity of Annona reticulata roots on human cancer cell lines. Pharmacogn Res. 2011;3:9. doi: 10.4103/0974-8490.79109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajarajan P., Senthilkumar R. Assessment of antineoplastic potential of Annona muricata Linn. in human cancer cell lines. Int J Pharma Bio Sci. 2015;6:B1101–B1109. [Google Scholar]
- 11.Mondal S., Mondal N., Mazumder U. In vitro cytotoxic and human recombinant caspase inhibitory effect of Annona reticulata leaves. Indian J Pharmacol. 2007;39:253–254. doi: 10.4103/0253-7613.37279. [DOI] [Google Scholar]
- 12.Gingine A.P., Mandge S.V.J.P. In vitro evaluation of anticancer activity of methnaolic extract of Annona reticulata linn. (Ramphal) leaves on different human cancer cell lines. J Anal Pharm Res. 2016;3 doi: 10.15406/japlr.2016.03.00087. [DOI] [Google Scholar]
- 13.Yadav K., Nagarathna P.K.M., Moria D., Lou M., Bhutia G. Review article on anticancer and anti-oxidant activity of leaves of Annona reticulata on EAC induced mammary. Tumor. 2018;8:4–11. [Google Scholar]
- 14.Siva Ganesh M., Radhika J. Acute toxicity study of ethanolic extract of apium leptophyllum pers in Swiss albino mice. Int J Res Pharm Sci. 2019;10:3750–3754. doi: 10.26452/ijrps.v10i4.1764. [DOI] [Google Scholar]
- 15.Gul R., Jan S.U., Faridullah S., Sherani S., Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia Indigenous to Balochistan. Sci World J. 2017;2017 doi: 10.1155/2017/5873648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chari K.Y., Polu P.R., Shenoy R.R. An appraisal of pumpkin seed extract in 1, 2-dimethylhydrazine induced colon cancer in wistar rats. J Toxicol. 2018;2018 doi: 10.1155/2018/6086490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prasad V.G., Reddy N., Francis A., Nayak P.G., Kishore A., Nandakumar K., et al. Sambar, an Indian dish prevents the development of dimethyl hydrazine-induced colon cancer: a preclinical study. Pharmacogn Mag. 2016;12:S441–S445. doi: 10.4103/0973-1296.191454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aebi H. [13] Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 19.Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. BBA Gen Subj. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 20.Satomi A., Murakami S., Hashimoto T., Ishida K., Matsuki M. 1995. tissue: correlation with malignant intensity; pp. 177–182. [DOI] [PubMed] [Google Scholar]
- 21.Devasagayam T.P.A., Boloor K.K., Ramasarma T. Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J Biochem Biophys. 2003;40:300–308. [PubMed] [Google Scholar]
- 22.Suresh H.M., Shivakumar B., Hemalatha K., Heroor S.S., Hugar D.S., Rao K.R.S.S. In vitro antiproliferativeactivity of Annona reticulata roots on human cancer cell lines. Pharmacogn Res. 2011;549 doi: 10.4103/0974-8490.79109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shivanna L.M., Urooj A. Apoptotic effects of Annona reticulata leaves extract in HT-29 cell lines. Asian J Biol Sci. 2019;12:820–831. doi: 10.3923/ajbs.2019.820.831. [DOI] [Google Scholar]
- 24.Ali S., Khan M.R., Sajid M., Zahra Z. Phytochemical investigation and antimicrobial appraisal of Parrotiopsis jacquemontiana (Decne) Rehder. BMC Complement Altern Med. 2018;18:1–15. doi: 10.1186/s12906-018-2114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yildirim I., Kutlu T. Anticancer agents: saponin and tannin. Int J Biol Chem. 2015;9:332–340. doi: 10.3923/ijbc.2015.332.340. [DOI] [Google Scholar]
- 26.Man S., Gao W., Zhang Y., Huang L., Liu C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia. 2010;81:703–714. doi: 10.1016/j.fitote.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Yu Z., Zhang T., Zhou F., Xiao X., Ding X., He H., et al. Anticancer activity of saponins from Allium chinense against the B16 melanoma and 4T1 breast carcinoma cell. Evid Based Complement Altern Med. 2015;2015 doi: 10.1155/2015/725023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoppil R.J., Bishayee A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J Hepatol. 2011;3:228–249. doi: 10.4254/wjh.v3.i9.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhandari J., Muhammad B.T., Thapa P., Shrestha B.G. Study of phytochemical, anti-microbial, anti-oxidant, and anti-cancer properties of Allium wallichii. BMC Complement Altern Med. 2017;17:1–9. doi: 10.1186/s12906-017-1622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iqbal J., Abbasi B.A., Mahmood T., Kanwal S., Ali B., Shah S.A., et al. Plant-derived anticancer agents: a green anticancer approach. Asian Pac J Trop Biomed. 2017;7:1129–1150. doi: 10.1016/j.apjtb.2017.10.016. [DOI] [Google Scholar]
- 31.Vijayalakshmi A., Kumar P.R., Sakthi Priyadarsini S., Meenaxshi C. In vitro antioxidant and anticancer activity of flavonoid fraction from the aerial parts of Cissus quadrangularis Linn. against human breast carcinoma cell lines. J Chem. 2013;2013 doi: 10.1155/2013/150675. [DOI] [Google Scholar]
- 32.Al-Rimawi F., Rishmawi S., Ariqat S.H., Khalid M.F., Warad I., Salah Z. Anticancer activity, antioxidant activity, and phenolic and flavonoids content of wild Tragopogon porrifolius plant extracts. Evid Based Complement Altern Med. 2016;2016 doi: 10.1155/2016/9612490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Islam M.S., Rahi M.S., Jahangir C.A., Rahman M.H., Jerin I., Amin R., et al. In vivo anticancer activity of Basella alba leaf and seed extracts against Ehrlich's Ascites Carcinoma (EAC) cell line. Evidence-based Complement. Altern Med. 2018:2018. doi: 10.1155/2018/1537896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freeman 乳鼠心肌提取. Physiol Behav. 2018;176:139–148. HHS public access. [Google Scholar]
- 35.Khan T., Ali M., Khan A., Nisar P., Jan S.A., Afridi S., et al. Anticancer plants: a review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules. 2020;10 doi: 10.3390/biom10010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundarraj S., Thangam R., Sreevani V., Kaveri K., Gunasekaran P., Achiraman S., et al. γ-Sitosterol from Acacia nilotica L. induces G2/M cell cycle arrest and apoptosis through c-Myc suppression in MCF-7 and A549 cells. J Ethnopharmacol. 2012;141:803–809. doi: 10.1016/j.jep.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Song Y., Cho S.K. Phytol induces apoptosis and ROS-mediated protective autophagy in human gastric adenocarcinoma AGS cells. Biochem Anal Biochem. 2015;4:4. doi: 10.4172/2161-1009.1000211. [DOI] [Google Scholar]
- 38.Ali H., Dixit S., Ali D., Alqahtani S.M., Alkahtani S., Alarifi S. Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma. Drug Des Devel Ther. 2015;9:2793–2800. doi: 10.2147/DDDT.S83514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sianipar N.F., Purnamaningsih R., Rosaria Bioactive compounds of fourth generation gamma-irradiated Typhonium flagelliforme Lodd. mutants based on gas chromatography-mass spectrometry. IOP Conf Ser Earth Environ Sci. 2016;41 doi: 10.1088/1755-1315/41/1/012025. [DOI] [Google Scholar]
- 40.McCann S.E., Freudenheim J.L., Marshall J.R., Graham S. Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J Nutr. 2003;133:1937–1942. doi: 10.1093/jn/133.6.1937. [DOI] [PubMed] [Google Scholar]
- 41.De Stefani E., Boffetta P., Ronco A.L., Brennan P., Deneo-Pellegrini H., Carzoglio J.C., et al. Plant sterols and risk of stomach cancer: a case-control study in Uruguay. Nutr Cancer. 2000;37:140–144. doi: 10.1207/S15327914NC372_4. [DOI] [PubMed] [Google Scholar]
- 42.Mendilaharsu M., De Stefani E., Deneo-Pellegrini H., Carzoglio J., Ronco A. Phytosterols and risk of lung cancer: a case-control study in Uruguay. Lung Cancer. 1998;21:37–45. doi: 10.1016/s0169-5002(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 43.Swantara M.D., Rita W.S., Suartha N., Agustina K.K. Anticancer activities of toxic isolate of Xestospongia testudinaria sponge. Vet World. 2019;12:1434–1440. doi: 10.14202/vetworld.2019.1434-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jalill A., Dh R., Jalill A. GC-MS analysis of Calendula officinalis and cytotoxic effects of its flower crude extract on human epidermoid larynx carcinoma (HEP-2) World J Pharm Pharm Sci. 2014;3(4):237–275. [Google Scholar]
- 45.James J., Thomas J. Anticancer activity of micro-algae extract on human cancer cell line (Mg-63) Asian J Pharm Clin Res. 2019;12:139. doi: 10.22159/ajpcr.2019.v12i1.28652. [DOI] [Google Scholar]
- 46.Tetsuji Takayama S., Katsuki, Yasuo Takahashi M., Ohi, Nojiri Shuichi, Sakamaki Sumio, Junji Kato K., et al. Abe Rrant Cryp T F Oci of T He C Olon as precurs ors of a D enoma a Nd C a Ncer aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med. 1998;339:1277–1284. doi: 10.1056/NEJM199810293391803. [DOI] [PubMed] [Google Scholar]
- 47.Yokota T., Sugano K., Kondo H., Saito D., Sugihara K., Fukayama N., et al. Detection of aberrant crypt foci by magnifying colonoscopy. Gastrointest Endosc. 1997;46:61–65. doi: 10.1016/S0016-5107(97)70212-8. [DOI] [PubMed] [Google Scholar]
- 48.Orlando F.A., Tan D., Baltodano J.D., Khoury T., Gibbs J.F., Hassid V.J., et al. NIH Public Access. 2014;98:207–213. doi: 10.1002/jso.21106.Aberrant. [DOI] [Google Scholar]
- 49.Jeong J.K., Chang H.K., Park K.Y. Inhibitory effects of meju prepared with mixed starter cultures on azoxymethane and dextran sulfate sodium-induced colon carcinogenesis in mice. J Carcinog. 2012;11 doi: 10.4103/1477-3163.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajendiran V., Natarajan V., Devaraj S.N. Anti-inflammatory activity of Alpinia officinarum hance on rat colon inflammation and tissue damage in DSS induced acute and chronic colitis models. Food Sci Hum Wellness. 2018;7:273–281. doi: 10.1016/j.fshw.2018.10.004. [DOI] [Google Scholar]
- 51.Byelinska I.V., Lynchak O.V., Rybalchenko T.V., Yablonska S.V., Bahurynska O.M., Rybalchenko V.K. Morphofunctional parameters of blood cells of a rat with 1 , 2 dimethylhydrazine induced colon. Carcinogenesis. 2015;49:158–164. doi: 10.3103/S0095452715030044. [DOI] [PubMed] [Google Scholar]
- 52.Lee Y.-J., Lee H.-R., Nam C.-M., Hwang U.-K., Jee S.-H. White blood cell count and the risk of colon cancer. Yonsei Med J. 2006;47:646–656. doi: 10.3349/ymj.2006.47.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irving A.A., Yoshimi K., Hart M.L., Parker T., Clipson L., Ford M.R., et al. The utility of Apc-mutant rats in modeling human colon cancer. Dis Model Mech. 2014;7:1215–1225. doi: 10.1242/dmm.016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Väyrynen J.P., Tuomisto A., Väyrynen S.A., Klintrup K., Karhu T., Mäkelä J., et al. Preoperative anemia in colorectal cancer: relationships with tumor characteristics, systemic inflammation, and survival. Sci Rep. 2018;8:1–11. doi: 10.1038/s41598-018-19572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riedl J., Pabinger I., Ay C. Platelets in cancer and thrombosis. Hämostaseologie. 2014;34:54–62. doi: 10.5482/HAMO-13-10-0054. [DOI] [PubMed] [Google Scholar]
- 56.Karagöz B., Sücüllü I., Ö Sayan, Bilgi O., Tuncel T., Filiz A.I., et al. Platelet indices in patients with colorectal cancer. Cent Eur J Med. 2010;5:365–368. doi: 10.2478/s11536-009-0077-7. [DOI] [Google Scholar]
- 57.Rao X.D., Zhang H., Xu Z.S., Cheng H., Shen W., Wang X.P. Poor prognostic role of the pretreatment platelet counts in colorectal cancer: a meta-analysis. Medicine. 2018;97:1–7. doi: 10.1097/MD.0000000000010831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamiza O.O., Rehman M.U., Tahir M., Khan R., Khan A.Q., Lateef A., et al. Amelioration of 1,2 dimethylhydrazine (DMH) induced colon oxidative stress, inflammation and tumor promotion response by tannic acid in wistar rats. Asian Pac J Cancer Prev. 2012;13:4393–4402. doi: 10.7314/APJCP.2012.13.9.4393. [DOI] [PubMed] [Google Scholar]
- 59.Weydert C.J., Cullen J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ansil P.N., Prabha S.P., Nitha A., Latha M.S. Chemopreventive EFFECT of Amorphophallus campanulatus (Roxb.) blume tuber against aberrant crypt foci and cell proliferation in 1, 2-dimethylhydrazine induced colon carcinogenesis. Asian Pac J Cancer Prev. 2013;14:5331–5339. doi: 10.7314/APJCP.2013.14.9.5331. [DOI] [PubMed] [Google Scholar]
- 61.Martín Mateo M.C., Martín B., Beneit M.S., Rabadán J. Catalase activity in erythrocytes from colon and gastric cancer patients. Influence of nickel, lead, mercury, and cadmium. Biol Trace Elem Res. 1997;57:79–90. doi: 10.1007/BF02803872. [DOI] [PubMed] [Google Scholar]
- 62.Skrzydlewska E. Lipid peroxidation and antioxidant status in colorectal cancer. World J Gastroenterol. 2005;11:403. doi: 10.3748/wjg.v11.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayo J.C., Tan D.X., Sainz R.M., Lopez-Burillo S., Reiter R.J. Oxidative damage to catalase induced by peroxyl radicals: functional protection by melatonin and other antioxidants. Free Radic Res. 2003;37:543–553. doi: 10.1080/1071576031000083206. [DOI] [PubMed] [Google Scholar]
- 64.Hussein Aziza S.A., Abdel-Aal S.A., Mady H.A. Chemopreventive effect of curcumin on oxidative stress, antioxidant status, DNA fragmentation and caspase-9 gene expression in 1,2-dimethylhydrazine-induced colon cancer in rats. Am J Biochem Mol Biol. 2014;4:22–34. doi: 10.3923/ajbmb.2014.22.34. [DOI] [Google Scholar]
- 65.Lokeshkumar B., Sathishkumar V., Nandakumar N., Rengarajan T., Madankumar A., Balasubramanian M.P. Anti-oxidative effect of myrtenal in prevention and treatment of colon cancer induced by 1, 2-dimethyl hydrazine (DMH) in experimental animals. Biomol Ther. 2015;23:471–478. doi: 10.4062/biomolther.2015.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manju V., Balasubramaniyan V., Nalini N. Rat colonic lipid peroxidation and antioxidant status: the effects of dietary luteolin on 1,2-dimethylhydrazine challenge. Cell Mol Biol Lett. 2005;10:535–551. [PubMed] [Google Scholar]
- 67.Ghadi F.E. Selenium as a chemopreventive agent in experimentally induced colon carcinogenesis. World J Gastrointest Oncol. 2009;1:74. doi: 10.4251/wjgo.v1.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Qualitative analysis of alcoholic leaf extract of AR.