Abstract
This study combined in vitro digestion and INT-407 cells to evaluate the bioaccessibility of anthocyanins in the small intestinal epithelial cells. Black soybean, grape, and purple sweet potato were chosen as they have a different anthocyanin composition. After the aqueous extract was digested under in vitro gastric and intestinal conditions, the digested mixture was incubated in the media of INT-407 for 2 h at 37 °C. Low proportion (< 0.3%) of anthocyanins in black soybean and grape passed through cell membranes. Cyanidin-3-O-glucoside and pelargonidin-3-O-glucoside in black soybean and cyanidin-3-O-(6-O-p-coumaroyl)-5-O-diglucoside and delphinidin-3-O-(6-O-p-coumaroyl)-5-O-diglucoside in grape were found inside the cell. However, acylated anthocyanins containing three sugar moieties in purple sweet potato were not detected inside the cell. p-Coumaric acid was detected in the cells incubated with grape, but not in the media. These indicate that chemical structure of anthocyanins affected their cellular uptake and antioxidant activity in INT-407 cells.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-021-00976-y.
Keywords: Anthocyanin, INT-407, Cellular uptake, Antioxidant activity, p-Coumaric acid
Introduction
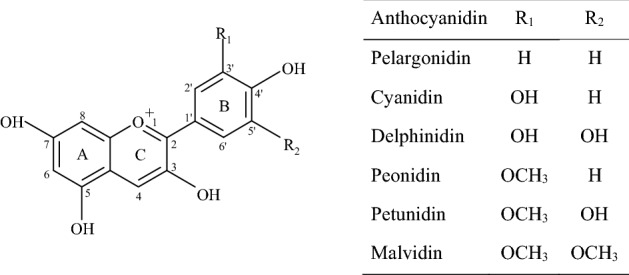
Anthocyanins in fruit and vegetables are responsible for red, blue, or purple color (Khoo et al., 2017). Earlier studies have reported health-promoting properties of anthocyanin-rich food extracts (He and Giusti, 2010; Khoo et al., 2017). Anthocyanins are glycoside forms of anthocyanidins (Fig. 1) that vary in hydroxyl or methoxyl substitution. The glycosylation of hydroxyl groups increases the hydrophilicity of anthocyanins, thus limits a passive diffusion through biological membranes. Nevertheless, anthocyanins are absorbed, distributed, and excreted in the urine as intact forms (Bub et al., 2001; Cao et al., 2001; Matsumoto et al., 2001; McGhie et al., 2003). Suda et al. (2002) found that peonidin-3-(6″-caffeoylsophoroside)-5-glucoside, a major anthocyanin in purple sweet potato, was absorbed directly into rat plasma. In addition, Miyazawa et al. (1999) reported a direct absorption of cyanidin-3-glucoside and cyanidin-3,5-diglucoside in rat and human studies. These indicate that active transport mechanisms may involve in the absorption of anthocyanins in epithelial cells of digestive tract. SGLT1 (sodium-glucose linked transporter) and GLUT2 (glucose transporter) have been suggested as uptake systems of anthocyanins (Faria et al., 2009; Hollman et al., 1999; Kay, 2006; Zou et al., 2014). Several anthocyanins including peonidin-3-glucoside and cyanidin-3-glucoside were found as potential substrates of efflux transporter breast cancer resistance protein (BCRP), thus they may be actively transported out of intestinal tissues and endothelia (Dreiseitel et al., 2009).
Fig. 1.
Chemical structures of anthocyanidins
Anthocyanins are efficiently absorbed from small intestine of humans and rats (McGhie et al., 2003; Miyazawa et al., 1999; Talavéra et al., 2004). In the absorption study of cyanidin-3-glucoside using various tissues of mouse, a jejunum had the highest absorption efficiency of 55%, and a colon showed no absorption (Matuschek et al., 2006). Nevertheless, human large intestinal epithelial cell Caco-2 has been commonly used for in vitro cellular uptake of anthocyanins (Steinert et al., 2008; Yang et al., 2017; Yi et al., 2006). To date, human small intestinal epithelial cell has never been reported for the uptake of anthocyanins.
The absorption of anthocyanins has been reported to be affected by their chemical structures (Miyazawa et al., 1999; Talavéra et al., 2004). McGhie et al. (2003) demonstrated that phenolic aglycone and conjugated sugars in berry fruit were important determinants of anthocyanin absorption in humans and rats. Novotny et al. (2012) found that absorption efficiencies of acylated anthocyanins in purple carrot were lower compared with those of non-acylated anthocyanins in human study. Kurilich et al. (2005) also reported that the level of acylated anthocyanins in urine and plasma was lower than glycosylated forms after ingestion of purple carrot in humans. These suggest that molecular structure of anthocyanins may play a role in their absorption and availability. The absorption efficiency and antioxidant activity of anthocyanins from different sources have never been reported by small intestinal epithelial cell.
Therefore, this study chose black soybean, grape, and purple sweet potato that differ in the composition of anthocyanins and evaluated the absorption of in vitro digested anthocyanins using the INT-407 cell that was originally derived from the jejunum and ileum of a Caucasian embryo. Additionally, antioxidant activity of cell lysates was measured to estimate the availability of anthocyanins that are absorbed in the cells.
Materials and methods
Chemicals
Dimethyl sulfoxide (DMSO), fluorescein sodium salt, Trolox, 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), pepsin, pancreatin, and bile salt were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s phosphate-buffered saline (DPBS) and minimum essential media (MEM) were purchased from Welgene (Gyeongsan, Korea). Human small intestinal cell (INT-407) was obtained from American Type Culture Collection (Manassas, VA, USA). C18 cartridge (Bond Elut, 10 g, particle size 40 μm) was purchased from Agilent Technologies (Diegen, Belgium). Cyanidin-3-O-glucoside, delphinidin-3-O-glucoside, malvidin-3-O-glucoside, pelargonidin-3-O-glucoside, peonidin-3-O-glucoside, and petunidin-3-O-glucoside were purchased from Extrasynthese (Genay Cedex, France). Pierce™ BCA protein assay kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Sample preparation
Black soybean (Glycine max (L.) Merr. cv. Cheongja4ho) was provided from the Department of Southern Area Crop Science (Miryang, Korea) of the National Institute of Crop Science. Campbell Early grape, a hybrid of Vitis labrusca and V. vinifera, was provided by the National Institute of Horticultural and Herbal Science (Wanju, Korea) of the Rural Development Administration. Purple sweet potato (Ipomoea batatas L. cv. Sinjami) was obtained from the Bioenergy Crop Research Center (Muan, Korea) of the National Institute of Crop Science. Instead of organic solvents, distilled water was used as an extracting solvent to reflect the extraction condition of food processing. The grape (1 kg) was frozen, freeze-dried (FDU-1200; EYELA, Tokyo, Japan), and ground using an IKA A10 basic analytical mill (Staufen, Germany) for 1 min. The sweet potatoes (1 kg) were washed, sliced, frozen, freeze-dried, and ground. The pulverized samples were kept at − 35 ºC until extraction.
Each sample was separately optimized for a maximum extraction yield of anthocyanins using response surface methodology. The optimized conditions were a sample-water ratio of 1:54.2 (w:v; g:mL), a hydrochloric acid concentration of 0.359%, and an extraction temperature of 56.8 °C for black soybean; a sample-water ratio of 1:50 (w:v; g:mL), an extraction temperature of 80 °C, and an extraction time of 10 min for grape; and an extraction time of 13 min, an extraction temperature of 75 °C, an amplitude of 25 μm, and a sample-water ratio of 1:40 (w:v; g:mL) by ultrasound-assisted extraction for purple sweet potato. Each extract was kept in the dark at 4 °C and used for in vitro digestion within 24 h.
Anthocyanin analysis
Individual anthocyanins were identified using a Waters® ACQUITY™ ultra-performance liquid chromatograph (UPLC) system (Milford, MA, USA) equipped with a quadruple-time-of-flight-tandem mass spectrometer (Q-TOF–MS/MS) system (SYNAPT™ G2; Waters, Manchester, UK). Anthocyanin separation was performed on a CORTECS™ UPLC C18 (2.1 mm × 100 mm, 1.6 μm; Waters) column with a linear gradient of solvent A (water/formic acid, 99.9:0.1, v/v) and solvent B (acetonitrile/formic acid, 99.9:0.1, v/v). The gradient was programmed as 95% A at 0 min and 0% A at 20 min at a flow rate of 0.3 mL/min. Electrospray ionization was operated in the positive mode at capillary voltage of 3.1 kV for anthocyanins and negative mode at capillary voltage of − 2.5 kV for phenolic acid. The cone voltage was 40 V and cone gas flow was 100 L/h. Desolvation gas flow was 800 L/h at 350 °C and source temperature was 120 °C.
The quantification of anthocyanins was performed by Agilent 1260 Infinity HPLC with a quaternary pump and a diode array detector (Agilent Technologies, Santa Clara, CA, USA) connected with a Kintex C18 column (4.6 mm × 150 mm, 5 μm; Phenomenex, Torrance, CA, USA). The cell lysates incubated with the digested black soybean and grape were analyzed with solvent A (5% formic acid in 75% methanol), solvent B (methanol), and solvent C (water) at a flow rate of 0.6 mL/min with a gradient of 100–60% A, 0–36% B, and 0–4% C for 20 min. The cell lysate obtained from purple sweet potato was separated using solvent A (1% formic acid in water) and solvent B (1% formic acid in methanol) with a mobile program of 80% A at 0 min; 70% A at 20 min; 65% A at 50 min; and 55% A at 60 min. The absorbance was monitored at 530 nm for anthocyanins and 300 nm for phenolic acids. The ranges of calibration curve were 0.2–50 μg/g for cyanidin-3-O-glucoside, 2–100 μg/g for delphinidin-3-O-glucoside, 0.2–5 μg/g for malvidin-3-O-glucoside, 2–50 μg/g for pelargonidin-3-O-glucoside, 2–50 μg/g for peonidin-3-O-glucoside, and 0.2–50 μg/g for petunidin-3-O-glucoside. The contents of diglucoside and acylated forms of anthocyanins were determined from the curve of the corresponding monoglucosylated form. Each compound was confirmed by the comparison with retention time, ultraviolet–visible spectrum of an authentic standard, and earlier published data (De la Cruz et al., 2012; Kim et al., 2012; Lee et al., 2009).
In vitro digestion
The digestion was performed by the method of Bermúdez-Soto et al. (2007), Bouayed et al. (2011), and Mcdougall et al. (2005) with some modifications. In the gastric digestion, each food extract (15 mL) was mixed with 5 mL of pepsin (1,260 unit/mL) in 250 mL amber Erlenmeyer flask, followed by adjustment to pH 2.0 using 1 N sodium bicarbonate (0.2–0.6 mL). The mixture was purged with nitrogen gas (MG-2200; Rikakikai Co., Ltd., Tokyo, Japan) to simulate an anaerobic condition of stomach and then mixed at 120 rpm for 2 h at 37 °C under dark conditions. In the intestinal digestion, the gastric digested mixture (10.25 mL) was neutralized with 1 N sodium bicarbonate (0.05–0.93 mL), followed by the addition of the mixture (5 mL) of pancreatin (4 mg/mL in 0.1 N sodium bicarbonate) and bile salts (25 mg/mL in 0.1 N sodium bicarbonate) and purged with nitrogen gas for 30 s. The mixture was incubated in a shaking water bath at 120 rpm for 2 h at 37 °C under dark conditions. To remove bile salts in the gastrointestinal mixture, the digested solution of 25 mL was loaded onto C18 cartridge (10 g) preconditioned with 50 mL of absolute methanol and 50 mL of distilled water. Bile salt in the digested solution loaded on the column was washed with 100 mL of distilled water. Then, anthocyanins were eluted with 30 mL of absolute methanol and concentrated by a rotary evaporator (SB-1200; EYELA, Tokyo, Japan). The concentrated solution was freeze-dried, dissolved in distilled water of 1 mL, and used for cellular uptake within 24 h. After filtered with 0.45 μm polytetrafluoroethylene (PTFE) syringe filter (Millipore, Milford, MA, USA), it was stored at 4 °C until HPLC analysis.
Cellular uptake
The INT-407 was grown in MEM at 37 °C in 5% carbon dioxide. The cells were seeded in a 6-well plate at a density of 1 × 106 cells/well and grown for 24 h. The mixture obtained from the in vitro digestion was diluted with serum free media until anthocyanin concentration reached 100 μg/g based on total content of individual anthocyanins obtained from HPLC analysis. The culture media in each well was replaced by 1 mL of the media containing anthocyanins. Serum free media (1 mL) was added into the other three wells as a control. After being incubated for 2 h at 37 °C, the media of three different wells were combined, and the obtained cells were washed twice using an ice-cold DPBS buffer to control equal uptake and outflow of anthocyanins from the cells during washing process. The cells were lysed with 100 μL of DMSO and analyzed after being filtered with 0.45 μm PTFE syringe filter (Millipore). After the cell lysate in DMSO was 100-fold diluted with absolute alcohol, anthocyanins and their metabolites were identified using UPLC-MS/MS and quantified with HPLC/DAD. Cellular uptake was calculated as the proportion of anthocyanins inside the cell to anthocyanins that was treated into the well.
Antioxidant activity
Antioxidant activity was determined by oxygen radical absorbance capacity (ORAC) method (Cao et al., 1993). The ten-fold diluted cell lysates (20 μL) in DPBS was mixed with 120 μL of 60 nM fluorescein for 15 min at 37 °C. After 12 mM AAPH (60 μL) was added into the mixture, fluorescence changes in excitation at 485 nm and emission at 520 nm were monitored at an interval of 1 min during 60 min using a Spectramax M2. ORAC value was calculated using the area under the curve (AUC) of the standard Trolox and the samples. Calibration curve was obtained by plotting AUC versus Trolox concentration (0–40 μmol), and the activity was expressed as μmol Trolox equivalent (TE)/L.
Results and discussion
The stability of anthocyanins varies depending on their molecular structure such as anthocyanidin type and glycosylated sugar number (Castañeda-Ovando et al., 2009). Since authentic standards of diglycosylated and acylated anthocyanins were not commercially available, these compounds were identified by the mass spectrometry data obtained in this study as well as reported in the previous studies of black soybean (Lee et al., 2009), grape (De la Cruz et al., 2012), and purple sweet potato (Kim et al., 2012).
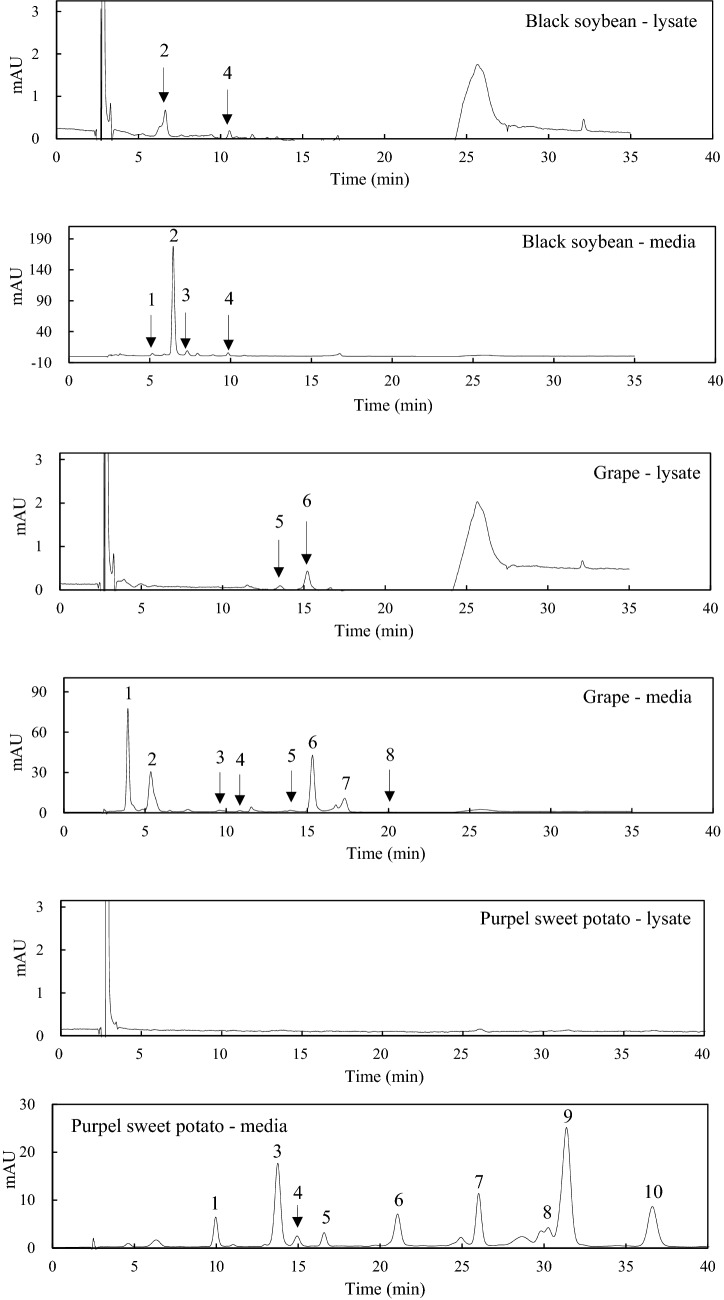
To evaluate the proportion of anthocyanins absorbed inside the cells, the amounts of anthocyanins determined in the media and the cell lysates are presented as total amount (μg) of anthocyanins (Table 1). The in vitro digested black soybeans contained monoglucoside forms of anthocyanins, which included delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, petunidin-3-O-glucoside, and pelargonidin-3-O-glucoside. The digested grape had four diglucosides and four acylated diglucosides of anthocyanins, whereas purple sweet potato contained all acylated anthocyanins. Given that anthocyanins are susceptible to pH, enzymes, oxygen, and antioxidant (Khoo et al., 2017), digestion conditions may affect their stabilities. Therefore, each extract was firstly digested under gastric and intestinal conditions, adjusted to the same concentration of total anthocyanins, and then their uptake was measured using small intestinal INT-407 cell. Despite that the content of cyanidin-3-O-glucoside was about 28-fold higher than pelargonidin-3-O-glucoside, the level of cyanidin-3-O-glucoside inside the cell was 1.3-fold higher than that of pelargonidin-3-O-glucoside (Table 1 and Fig. 2). It can be explained by the reason that more hydrophilic cyanidin-3-O-glucoside had a relatively lower absorption in INT-407 cells since pelargonidin and cyanidin have one hydroxyl and two hydroxyl groups, respectively, in the B-ring of anthocyanidin (Fig. 1). Delphinidin has three hydroxyl groups in the B-ring, and petunidin comprised two hydroxyls and one methoxy group. Moreover, low content of delphinidin-3-O-glucoside and petunidin-3-O-glucoside in digested black soybean might result in the absence of these compounds in the cell lysates. This is similar to the result of Yi et al. (2006), demonstrating that delphinidin-3-glucoside had the lowest uptake in Caco-2 cell monolayer examined with bilberry extract. They found that more hydroxy groups and less methoxy groups had a decreased bioavailability of anthocyanins. Tsuda et al. (1999) reported that cyanidin-3-glucoside was rapidly absorbed as an intact form in male Wistar rats. These suggest that chemical structure of anthocyanins affect their cellular uptake in INT-407 cells.
Table 1.
Total amount of anthocyanins treated into the well, anthocyanins remained in the media after cell removal, and anthocyanins obtained from cell lysates
| Source | Peak | Anthocyanin | Anthocyanin (µg) | ||
|---|---|---|---|---|---|
| Addeda | Mediab | Absorbedc | |||
| Black soybean | |||||
| 1 | Delphinidin-3-O-glucoside | 5.24 | 4.73 | –d | |
| 2 | Cyanidin-3-O-glucoside | 264.63 | 152.13 | 0.48 | |
| 3 | Petunidin-3-O-glucoside | 13.65 | 11.27 | – | |
| 4 | Pelargonidin-3-O-glucoside | 9.49 | 7.48 | 0.37 | |
| Total | 293.03 | 175.61 | 0.85 | ||
| Grape | |||||
| 1 | Delphinidin-3,5-O-diglucoside | 78.07 | 57.87 | – | |
| 2 | Peonidin-3,5-O-diglucoside | 69.65 | 60.42 | – | |
| 3 | Malvidin-3,5-O-diglucoside | 2.74 | 1.98 | – | |
| 4 | Cyanidin-3-O-sophoroside | 20.44 | 3.88 | – | |
| 5 | Delphinidin-3-O-(6-O-p-coumaroyl)-5-O-diglucoside | 10.52 | 3.74 | 0.28 | |
| 6 | Cyanidin-3-O-(6-O-p-coumaroyl)-5-O-diglucoside | 83.49 | 59.81 | 0.46 | |
| 7 | Peonidin-3-O-(6-O-p-coumaroyl)-5-O-diglucoside | 28.35 | 18.20 | – | |
| 8 | Cyanidin-3-O-(6-O-p-coumaroyl)glucoside | 1.07 | 2.90 | – | |
| Total | 294.34 | 208.82 | 0.74 | ||
| Purple sweet potato | |||||
| 1 | Cyanidin-3-p-hydroxybenzoylsophoroside-5-glucoside | 12.33 | 11.25 | – | |
| 2 | Peonidin-3-p-hydroxybenzoylsophoroside-5-glucoside | 5.06 | – | – | |
| 3 | Peonidin-3-caffeoylsophoroside-5-glucoside | 45.66 | 43.40 | – | |
| 4 | Cyanidin-3-(6″-feruloylsophoroside)-5-glucoside | 6.23 | 6.39 | – | |
| 5 | Peonidin-3-(6″-feruloylsophoroside)-5-glucoside | 6.90 | 15.37 | – | |
| 6 | Cyanidin-3-caffeoylsophoroside-5-glucoside | 20.66 | 17.43 | – | |
| 7 | Cyanidin-3-caffeoyl-p-hydroxybenzoylsophoroside-5-glucoside | 43.40 | 22.07 | – | |
| 8 | Peonidin-3-(6″,6″′-dicaffeoylsophoroside)-5-glucoside | 12.61 | 18.17 | – | |
| 9 | Peonidin-3-caffeoyl-p-hydroxybenzoylsophoroside-5-glucoside | 105.90 | 75.54 | – | |
| 10 | Peonidin-3-(6″-caffeoyl-6″′-feruloylsophoroside)-5-glucoside | 33.19 | 36.35 | – | |
| 11 | Peonidin-3-feruloyl-p-hydroxybenzoylsophoroside-5-glucoside | 2.11 | – | – | |
| Total | 294.05 | 245.97 | – | ||
Since authentic standards for diglucosides and acylated forms of anthocyanins were not available, their quantification was achieved by calibration curve obtained from corresponding monoglucoside and expressed as equivalent weight of corresponding monoglucoside
aAmount of anthocyanins in in vitro digested mixture that was treated into the wells
bAmount of anthocyanins remaining in the media of INT-407 cells after the cells were removed
cAmount of anthocyanins determined in the cell lysates
dND (not detected)
Fig. 2.
HPLC–DAD chromatograms of cell lysates from INT-407 cells incubated with in vitro digested of black soybean, purple sweet potato, and grape. Absorbance was monitored at 520 nm for black soybean and grape and 530 nm for purple sweet potato. Peak assignments are presented in Table 1
Of the eight anthocyanins present in digested grape (Table 1), two acylated anthocyanins including cyanidin-3-O-(6-O-p-coumaroyl)-5-diglucoside and delphinidin-3-O-(6-O-p-coumaroyl)-5-diglucoside were found in the cell lysates (Fig. 2), whereas the other diglycosylated anthocyanins were not (Table 2). The digested grape contained eightfold higher content of cyanidin-3-O-(6-O-p-coumaroyl)-5-diglucoside than that of delphinidin-3-O-(6-O-p-coumaroyl)-5-diglucoside. Among anthocyanins absorbed in the INT-147 cells, cyanidin-3-O-(6-O-p-coumaroyl)-5-diglucoside had about two-fold higher level compared with delphinidin-3-O-(6-O-p-coumaroyl)-5-diglucoside. Kurilich et al. (2005) demonstrated that the level of acylated anthocyanins was lower than that of glycosylated anthocyanins in human urine and plasma after ingestion of purple carrot. Steinert et al. (2008) found no significant uptake difference between delphinidin-3-rutinoside and cyanidin-3-rutinoside that were absorbed after 80 min treatment of black currant extract using Caco-2 cells.
Table 2.
Anthocyanins and phenolic acid identified in cell lysates using UPLC-MS/MS
| tR (min) | Molecular ion (m/z) | Fragment ions (m/z) | Compound | |
|---|---|---|---|---|
| Black soybean | ||||
| 6.23 | 449.108 | 287.055 | Cyanidin-3-O-glucoside | |
| 7.97 | 433.112 | 271.060 | Pelargonidin-3-O-glucoside | |
| Grape | ||||
| 4.89 | 162.506 | 118.595, 116.583, 92.633 | p-Coumaric acid | |
| 7.67 | 773.193 | 611.139, 465.101, 303.051 | Delphinidin-3-O-(6-O-p-coumaroyl)-5-O-diglucoside | |
| 8.18 | 757.198 | 595.146, 449.200, 287.057 | Cyanidin-3-O-(6-O-p-coumaroyl)-5-O-diglucoside | |
Anthocyanins and phenolic acids were identified in the positive mode (M+) and negative mode (M−), respectively
The digested purple sweet potato was composed of all acylated anthocyanins (Table 1). No anthocyanin was found in the lysate of INT-407 cells incubated with the digested purple sweet potato (Fig. 2). Absorption of acylated anthocyanins was lower than non-acylated anthocyanins in the human studies (Kurilich et al., 2005; Novotny et al., 2012). Anthocyanins found in purple sweet potato are glycosylated with three sugars as well as acylated with various phenolic acids such as caffeic acid, ferulic acid, or hydroxybenzoic acid. As a result, larger molecular weight and increased hydrophilicity might hinder cellular uptake of anthocyanins in INT-407 cells. Meanwhile, Suda et al. (2002) found peonidin-3-caffeoylsophoroside-5-glucoside in the plasma of male Wistar rats after ingestion of purple sweet potato concentrate. Oki et al. (2006) also reported that an acylated anthocyanin, peonidin-3-caffeoylsophoroside-5-glucoside, was detected in human urine after ingestion of purple sweet potato beverage. Talavéra et al. (2003) reported that anthocyanins were absorbed in the stomach of anesthetized rats. These suggest that other digestive tracts such as gastric and colon may involve in the absorption of acylated anthocyanins in rats and humans.
To evaluate the proportion of anthocyanins absorbed inside the cells, anthocyanins’ amount was expressed as total amount (μg) of the well, the media, and the cell lysates. The recoveries of anthocyanins in each culture media of black soybean, grape, and purple sweet potato were 60%, 71%, and 84%, respectively (Table 1). Aglycones, anthocyanidins, were not detected in both in vitro digested solution and inside the cell, possibly below the detection limit in this study. Anthocyanins exist as the colorless carbinol pseudobase or chalcone pseudobase at the physiological condition of INT-407 cells. Kuntz et al. (2015) reported that anthocyanin concentration decreased significantly over time in the Dulbecco’s modified Eagle’s media without cells, which shows the instability of anthocyanins in the media. In addition, the metabolism of anthocyanins might contribute to the reduction of their concentration. Despite of the same values (≈ 294 μg) of anthocyanins (Table 1), their absorption efficiencies varied depending on dietary sources. Low proportion (about 0.3%) of anthocyanins in black soybean and grape was found to pass through the cell membranes of INT-407 cells after incubation for 2 h. This value is lower than the result of Yi et al. (2006), who reported that transport efficiency in Caco-2 cells was 3–4% of anthocyanins in blueberry extract that contained seven monoglucosylated anthocyanins. Pacheco-Palencia et al. (2010) demonstrated that transported monomeric anthocyanins from açaí fruit ranged from 0.5 to 4.9% in Caco-2 cells. Kuntz et al. (2015) found that recovery rates of cyanidin-3-glucoside, petudinin-3-glucoside, and delphinidin-3-glucoside in Caco-2 cells were 0.03%, 0.01%, and 0.005%, respectively, which suggest that absorption efficiencies were influenced by the structural skeleton. Similarly, the recoveries of cyanidin derivatives were relatively higher than those of other anthocyanins in this study (Table 1). SGLT1 and GLUT2 played a role in the absorption of cyanidin-3-glucoside in a Caco-2 cell monolayer (Zou et al., 2014). On the other hand, 16 anthocyanins and anthocyanidins interacted with the efflux transporter BCRP transporter in vitro (Dreiseitel et al., 2009). Anthocyanins that interfere with BCRP activity may facilitate crossing of the intestinal barriers. Moreover, malvidin and petunidin exhibited bimodal activities, serving as BCRP substrates at low micromolar concentrations and as BCRP inhibitors at higher concentrations. These indicate that the absorption of anthocyanins with different compositions at multiple levels should be further studied to evaluate their bioavailability from the consumption of anthocyanin-rich foods.
Anthocyanins in cells probably affect cellular reactive oxygen species (ROS) levels through direct quenching ROS and by indirectly modulating various cellular events. To evaluate the direct antioxidant activity of anthocyanins absorbed in cells, ORAC assay was employed in this study. Of the cell lysates obtained from INT-407 cells that were incubated with in vitro digested black soybean, grape, and purple sweet potato, grape had a higher antioxidant activity than the other samples (Fig. 3). Total content of anthocyanins inside the cell in grape was lower than that of the black soybean. This suggests that other compounds except anthocyanins may contribute to antioxidant activity of the cell lysate from grape. A new compound peak in the chromatogram monitored at 300 nm was found only in the cell that was treated with grape digestion. UPLC-ESI/MS/MS analysis revealed that the peak was p-coumaric acid with a molecular ion at m/z 162.506 and fragment ions at m/z 118.595, 116.583, and 92.633 in the negative mode (Table 2). In the digested grape, anthocyanins acylated with p-coumaric acid comprised approximately 42% of total anthocyanin content (Table 1). Moreover, p-coumaric acid was not found in the media. Similarly, Nurmi et al. (2009) reported that 18 phenolic acids including p-coumaric acid were found in human urines after bilberry-lingonberry puree and oat cereal supplementation. Vitaglione et al. (2007) also found that protocatechuic acid was the major metabolite of cyanidin-3-glucoside and cyanidin-malonylglucoside in humans after ingestion of blood orange juice. It seems that anthocyanins acylated with p-coumaric acid was absorbed and degraded to form p-coumaric acid. Anthocyanins and p-coumaric acid are known to quench radicals efficiently (Kiliç and Yeşiloğ, 2013; Stintzing et al., 2002). Wang et al. (1997) reported the influence of glycosylation and B-ring structure in anthocyanins on ORAC values. These findings suggest that anthocyanins as well as p-coumaric acid contributed to antioxidant activity to scavenge AAPH radicals examined in this study.
Fig. 3.

Antioxidant activity of the cell lysates obtained from INT-407 cells incubated with in vitro digested black soybean, purple sweet potato, or grape. Values with different letters on the bar are significantly different at p < 0.05
In conclusion, cellular absorption of anthocyanins obtained from the simulated digested black soybean, grape, and purple sweet potato was evaluated using human small intestinal INT-407 cell. Some anthocyanins passed through cell membranes, while acylated anthocyanins with three sugars did not. This indicates that chemical structure of anthocyanins, in particular sugar moiety, affected the small intestinal absorption. Therefore, the information about anthocyanin structure may make it easier to select a functional ingredient in food industry as well as to predict their absorption from the consumption of anthocyanin-rich foods.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03028841).
Declarations
Conflict of interest
Authors declared that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dayeon Ryu, Email: dryu4@naver.com.
Yunkyung Sung, Email: Yunbbang13@gmail.com.
Jungil Hong, Email: hjil@swu.ac.kr.
Eunmi Koh, Email: kohem7@swu.ac.kr.
References
- Bermúdez-Soto MJ, Tomás-Barberán FA, García-Conesa MT. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chemistry. 2007;102:865–874. doi: 10.1016/j.foodchem.2006.06.025. [DOI] [Google Scholar]
- Bouayed J, Hoffmann L, Bohn T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chemistry. 2011;128:14–21. doi: 10.1016/j.foodchem.2011.02.052. [DOI] [PubMed] [Google Scholar]
- Bub A, Watzl B, Heeb D, Rechkemmer G, Briviba K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholised red wine and red grape juice. European Journal of Nutrition. 2001;40:113–120. doi: 10.1007/s003940170011. [DOI] [PubMed] [Google Scholar]
- Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biology and Medicine. 1993;14:303–311. doi: 10.1016/0891-5849(93)90027-R. [DOI] [PubMed] [Google Scholar]
- Cao G, Muccitelli HU, Sánchez-Moreno C, Prior RL. Anthocyanins are absorbed in glycated forms in elderly women: a pharmacokinetic study. American Society for Clinical Nutrition. 2001;73:920–926. doi: 10.1093/ajcn/73.5.920. [DOI] [PubMed] [Google Scholar]
- Castañeda-Ovando A, de Lourdes Pacheco-Hernández M, Páez-Hernández E, Rodríguez JA, Galán-Vidal CA. Chemical studies of anthocyanins: a review. Food Chemistry. 2009;113:859–871. doi: 10.1016/j.foodchem.2008.09.001. [DOI] [Google Scholar]
- De la Cruz AA, Hilbert G, Rivière C, Mengin V, Ollat N, Bordenave L, Decroocq S, Delaunay J-C, Delrot S, Mérillon J-M, Monti J-P, Gomès E, Richard T. Anthocyanin identification and composition of wild Vitis spp. accessions by using LC–MS and LC–NMR. Analytica Chimica Acta. 2012;732:145–152. doi: 10.1016/j.aca.2011.11.060. [DOI] [PubMed] [Google Scholar]
- Dreiseitel A, Oosterhuis B, Vukman KV, Schreier P, Oehme A, Locher S, Hajak G, Sand PG. Berry anthocyanins and anthocyanidins exhibit distinct affinities for the efflux transporters BCRP and MDR1. British Journal of Pharmacology. 2009;158:1942–1950. doi: 10.1111/j.1476-5381.2009.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria A, Pestana D, Azevedo J, Martel F, de Freitas V, Azevedo I, Mateus N, Calhau C. Absorption of anthocyanins through intestinal epithelial cells – Putative involvement of GLUT2. Molecular Nutrition and Food Research. 2009;53:1430–1437. doi: 10.1002/mnfr.200900007. [DOI] [PubMed] [Google Scholar]
- He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annual Review of Food Science and Technology. 2010;1:163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- Hollman PC, Bijsman MN, van Gameren Y, Cnossen EP, de Vries JH, Katan MB. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radical Research. 1999;31:569–573. doi: 10.1080/10715769900301141. [DOI] [PubMed] [Google Scholar]
- Kay CD. Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutrition Research. 2006;19:137–146. doi: 10.1079/NRR2005116. [DOI] [PubMed] [Google Scholar]
- Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food & Nutrition Research. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiliç I, Yeşiloğ Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2013;115:719–724. doi: 10.1016/j.saa.2013.06.110. [DOI] [PubMed] [Google Scholar]
- Kim HW, Kim JB, Cho SM, Chung MN, Lee YM, Chu SM, Che JH, Kim SN, Kim SY, Cho YS, Kim JH, Park HJ. Anthocyanin changes in the Korean purple-fleshed sweet potato, Shinzami, as affected by steaming and baking. Food Chemistry. 2012;130:966–972. doi: 10.1016/j.foodchem.2011.08.031. [DOI] [Google Scholar]
- Kuntz S, Rudloff S, Asseburg H, Borsch C, Fröhling B, Unger F, Dold S, Spengler B, Römpp A, Kunz C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. British Journal of Nutrition. 2015;113:1044–1055. doi: 10.1017/S0007114515000161. [DOI] [PubMed] [Google Scholar]
- Kurilich AC, Clevidence BA, Britz SJ, Simon PW, Novotny JA. Plasma and urine responses are lower for acylated vs nonacylated anthocyanins from raw and cooked purple carrots. Journal of Agricultural and Food Chemistry. 2005;53:6537–6542. doi: 10.1021/jf050570o. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kang NS, Shin S-O, Shin S-H, Lim S-G, Suh D-Y, Baek I-Y, Park K-Y, Ha TJ. Characterisation of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chemistry. 2009;112:226–231. doi: 10.1016/j.foodchem.2008.05.056. [DOI] [Google Scholar]
- Matsumoto H, Inaba H, Kishi M, Tominaga S, Hirayama M, Tsuda T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. Journal of Agricultural and Food Chemistry. 2001;49:1546–1551. doi: 10.1021/jf001246q. [DOI] [PubMed] [Google Scholar]
- Matuschek MC, Hendriks WH, McGhie TK, Reynolds GW. The jejunum is the main site of absorption for anthocyanins in mice. Journal of Nutritional Biochemistry. 2006;17:31–36. doi: 10.1016/j.jnutbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- McDougall GJ, Dobson P, Smith P, Blake A, Stewart D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. Journal of Agricultural and Food Chemistry. 2005;53:5896–5904. doi: 10.1021/jf050131p. [DOI] [PubMed] [Google Scholar]
- McGhie TK, Ainge GD, Barnett LE, Cooney JM, Jensen DJ. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. Journal of Agricultural and Food Chemistry. 2003;51:4539–4548. doi: 10.1021/jf026206w. [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Nakagawa K, Kudo M, Muraishi K, Someya K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3,5-diglucoside, into rats and humans. Journal of Agricultural and Food Chemistry. 1999;47:1083–1091. doi: 10.1021/jf9809582. [DOI] [PubMed] [Google Scholar]
- Novotny JA, Clevidence BA, Kurilich AC. Anthocyanin kinetics are dependent on anthocyanin structure. British Journal of Nutrition. 2012;107:504–509. doi: 10.1017/S000711451100314X. [DOI] [PubMed] [Google Scholar]
- Nurmi T, Mursu J, Heinonen M, Nurmi A, Hiltunen R, Voutilainen S. Metabolism of berry anthocyanins to phenolic acids in humans. Journal of Agricultural and Food Chemistry. 2009;57:2274–2281. doi: 10.1021/jf8035116. [DOI] [PubMed] [Google Scholar]
- Oki T, Suda I, Terahara N, Sato M, Hatakeyama M. Determination of acylated anthocyanin in human urine after ingesting a purple-fleshed sweet potato beverage with various contents of anthocyanin by LC-ESI-MS/MS. Bioscience, Biotechnology, and Biochemistry. 2006;70:2540–2543. doi: 10.1271/bbb.60187. [DOI] [PubMed] [Google Scholar]
- Pacheco-Palencia LA, Mertens-Talcott SU, Talcott ST. In vitro absorption and antiproliferative activities of monomeric and polymeric anthocyanin fractions from açai fruit (Euterpe oleracea Mart.) Food Chemistry. 2010;119:1071–1078. doi: 10.1016/j.foodchem.2009.08.017. [DOI] [Google Scholar]
- Steinert RE, Ditscheid B, Netzel M, Jahreis G. Absorption of black currant anthocyanins by monolayers of human intestinal epithelial Caco-2 cells mounted in using type chambers. Journal of Agricultural and Food Chemistry. 2008;56:4995–5001. doi: 10.1021/jf703670h. [DOI] [PubMed] [Google Scholar]
- Stintzing FC, Stintzing AS, Carle R, Frei B, Wrolstad RE. Color and antioxidant properties of cyanidin-based anthocyanin pigments. Journal of Agricultural and Food Chemistry. 2002;50:6172–6181. doi: 10.1021/jf0204811. [DOI] [PubMed] [Google Scholar]
- Suda I, Oki T, Masuda M, Nishiba Y, Furuta S, Matsugano K, Sugita K, Terahara N. Direct absorption of acylated anthocyanin in purple-fleshed sweet potato into rats. Journal of Agricultural and Food Chemistry. 2002;50:1672–1676. doi: 10.1021/jf011162x. [DOI] [PubMed] [Google Scholar]
- Talavéra S, Felgines C, Texier O, Besson C, Manach C, Lamaison JL, Remesy C. Anthocyanins are efficiently absorbed from the small intestine in rats. Journal of Nutrition. 2004;134:2275–2279. doi: 10.1093/jn/134.9.2275. [DOI] [PubMed] [Google Scholar]
- Talavéra S, Felgines C, Texier O, Besson C, Lamaison JL, Remesy C. Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. Journal of Nutrition. 2003;133:4178–4182. doi: 10.1093/jn/133.12.4178. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Horio F, Osawa T. Absorption and metabolism of cyanidin 3-O-β-D-glucoside in rats. FEBS Letters. 1999;449:179–182. doi: 10.1016/S0014-5793(99)00407-X. [DOI] [PubMed] [Google Scholar]
- Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. Journal of Nutrition. 2007;137:2043–2048. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
- Wang H, Cao G, Prior RL. Oxygen radical absorbing capacity of anthocyanins. Journal of Agricultural and Food Chemistry. 1997;45:304–309. doi: 10.1021/jf960421t. [DOI] [Google Scholar]
- Yang C, Zhang H, Liu R, Zhu H, Zhang L, Tsao R. Bioaccessibility, cellular uptake, and transport of astaxanthin isomers and their antioxidative effects in human intestinal epithelial Caco-2 cells. Journal of Agricultural and Food Chemistry. 2017;65:10223–10232. doi: 10.1021/acs.jafc.7b04254. [DOI] [PubMed] [Google Scholar]
- Yi W, Akoh CC, Fischer J, Krewer G. Absorption of anthocyanins from blueberry extracts by Caco-2 human intestinal cell monolayers. Journal of Agricultural and Food Chemistry. 2006;54:5651–5658. doi: 10.1021/jf0531959. [DOI] [PubMed] [Google Scholar]
- Zou TB, Feng D, Song G, Li HW, Tang HW, Ling WH. The role of sodium-dependent glucose transporter 1 and glucose transporter 2 in the absorption of cyanidin-3-O-β-glucoside in Caco-2 cells. Nutrients. 2014;6:4165–4177. doi: 10.3390/nu6104165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.